Abstract
BRAFV600E is a common mutation in papillary thyroid carcinoma (PTC) correlated with aggressive features. Our objective was to assess the feasibility and accuracy of a novel RNA-based blood assay to identify individuals with a high-risk tumor mutation in patients with PTC. Patients with benign or malignant thyroid disorders were included between September 2013 and July 2014 before either thyroidectomy (n = 62) or treatment of recurrent or metastatic PTC (n = 8). RNA was isolated from peripheral blood lymphocytes and reverse transcribed and followed by two rounds of nested PCR amplification with a restriction digest specific for wild-type BRAF. BRAFV600E levels were quantified with standardization curves. Circulating BRAFV600E levels were compared with BRAF mutation status from surgical pathologic DNA-based tissue assays. Testing characteristics and receiving-operator curve using tissue results as the gold standard were assessed. Matched blood and tissue assays for BRAFV600E were performed on 70 patients with PTC (stages I to IV, n = 48) or other (n = 22) thyroid tumors. Sixty-three percent of PTC patients tested positive for BRAFV600E with conventional tissue assays on surgical specimens. The correlation between the RNA-based blood assay and tissue BRAF status was 0.71. PTC patients harbor detectable BRAFV600E circulating tumor cells. This blood assay is feasible and has potential as a biomarker for prognosis, surveillance, clinical decision making, and assessment of treatment response to BRAF-targeted therapies.
Thyroid cancer affects >530,000 individuals in the United States, and its incidence is increasing faster than any other cancer.1 Thyroid cancer is the fifth most commonly diagnosed cancer in women (http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2013, last accessed July 14, 2015). Given that the number of deaths attributable to papillary thyroid cancer (PTC) is relatively stable, we are likely diagnosing a large number of patients that would otherwise not have become symptomatic or died as a result of their thyroid cancer.2 Distinguishing those patients who may require more aggressive interventions from patients who may need less aggressive treatment would therefore be of great benefit.
Conventional thyroid cancer risk-stratification algorithms do not integrate mutational status as a predictor of risk.3, 4, 5 BRAFV600E, an activating mutation present in approximately one-half of PTCs, is highly specific for PTC among patients with thyroid nodules and is correlated with aggressive tumor features, recurrence of disease, loss of radioactive iodine (RAI) avidity, and increased mortality.6, 7, 8, 9, 10, 11, 12 It remains controversial if prophylactic central cervical lymph node dissection improves outcomes in patients with PTC; however, given that BRAFV600E+ patients were shown to have higher rates of central compartment metastases, some experts recommend the use of BRAF status to guide extent of initial surgery.13, 14, 15, 16, 17, 18, 19, 20 Moreover, the knowledge of BRAF status may be clinically actionable, because it can guide the extent of initial surgery (lobectomy versus total thyroidectomy and consideration of central lymphadenectomy), approach to imaging during surveillance (RAI scan versus positron emission tomography-computed tomography),3 and adjuvant therapy. Furthermore, BRAF-targeted therapies for advanced thyroid cancers are being evaluated in clinical trials.21, 22, 23
Currently, fine-needle aspiration (FNA) or tissue biopsy is required for BRAF molecular testing and immunohistochemistry with anti-BRAFV600E antibodies (Abs).24 Traditional tissue assays are considered less sensitive because of the potential for background tissue contamination. Compared with a routine blood draw, the FNA procedure, processing, and interpretation is costly and more challenging for patients. In addition, a blood-based assay would allow for easy access to serial, quantitative analysis to assess treatment effect and as a potential biomarker of recurrence. Our group has previously developed a highly sensitive blood-based BRAFV600E assay in patients with melanoma.25
Here, we report the feasibility of an RNA-based blood assay for the identification of individuals with a high-risk tumor mutation, BRAFV600E, that previously could only be assessed invasively. We specifically hypothesize that an RNA-based blood BRAFV600E assay will be able to detect BRAFV600E from circulating tumor cells in patients with PTC. A sensitive blood-based BRAFV600E assay would provide an inexpensive and less-invasive mechanism for risk stratification, surveillance, and longitudinal assessment of treatment response. Ultimately, we believe that a rapid and easily ascertainable blood test for tumor BRAFV600E status may enable more targeted and resource-efficient management of patients with PTC.
Materials and Methods
Patient Selection
Under approval by the Partners Human Research Committee Institutional Review Board at the Massachusetts General Hospital, patients with benign (n = 22) and malignant (n = 48) thyroid disorders undergoing initial curative surgery or treatment of recurrent disease were enrolled between September 2014 and July 2014. After informed consent was obtained, a 5-mL sample of peripheral blood was obtained from each patient before surgery or before initiation with BRAF-inhibitor therapy for two patients with iodine-refractory metastatic disease and two patients after initiation of chemotherapy.
Protocol
The protocol in detail (reproduced from Panka et al25 with permission from American Association for Cancer Research) is as follows.
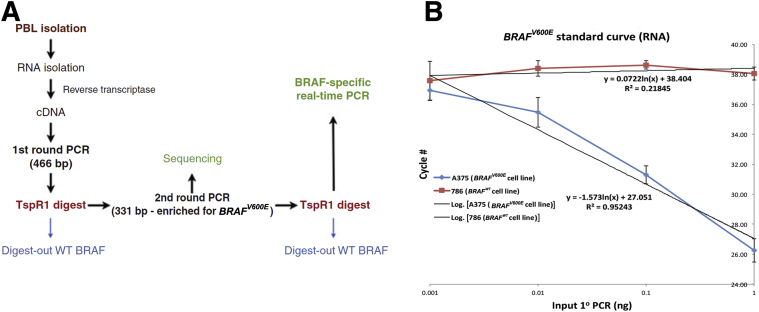
Peripheral blood lymphocytes (PBLs) were isolated by Ficoll density centrifugation from each patient's pretreatment blood sample. These samples were stored in freezing medium (95% fetal calf serum with 5% DMSO) at −80°C. The BRAFV600E assay followed the protocol previously reported (Figure 1A).25 Briefly, RNA from Ficoll purified PBLs was isolated by the Trizol method (Invitrogen, Grand Island, NY) and (3 μg) reverse transcribed to cDNA by standard methods using M-MLV reverse transcriptase (Invitrogen) and oligo (dt)15 (Promega, Madison, WI). The cDNA was subjected to real-time PCR for 18S RNA to normalize the quantity, as well as quality of the input RNA before the next step (ABI for oligo/probe set, Grand Island, NY). The equilibrated cDNA was PCR amplified using PCR master mix (Promega) and oligonucleotides [5′-CCATATCATTGAGACCAAATTTGAGATG-3′ (forward) and 5′-GGCACTCTGCCATTAATCTCTTCATGG-3′ (reverse)] that produced a product of 466 bp including the mutation site at position 600. The PCR conditions were 94° for 2 minutes followed by 40 cycles of 94° for 1 minute, 60° for 2 minutes and 72° for 2 minutes with a final incubation of 72° for 7 minutes. After clean up using a nucleospin extract column (Clontech, Mountain View, CA), a portion of the PCR product was digested with TSPR1 (restriction site = NNCASTGNN; New England Biolabs, Beverly, MA) at 65° for 16 hours. Only wild-type (WT) BRAF was digested by this enzyme. This digestion was added to reduce the amount of contaminating normal BRAF from surrounding and infiltrating normal tissue in the blood samples. A 1/100 dilution of the TSPR1 digested material was then PCR amplified a second time using nested oligonucleotides 5′-ACGCCAAGTCAATCATCCACAGAG-3′ and 5′-CCGTACCTTACTGAGATCTGGAGACAGG-3′ producing a product of 331 bp, which was enriched in PCR products containing the position 600 mutation. The conditions of the PCR were the same as the first PCR except the amplification was 45 cycles for PBLs instead of 40 cycles. After a second cleanup using a nucleo-spin extract column, the DNA (1/1000 dilution) was digested again with TspR1 and then subjected to a BRAFV600E real-time PCR as described. The annealing and extension temperature was adjusted to 64° resulting in a more favorable amplification of the mutant as compared to the WT templates than was reported. To further favor the mutant over the WT product, a 33-fold excess of the reverse (common sequence in mutant and WT) to forward (exact match for mutant and 1 base mismatch for WT sequences) primers were used in the real-time PCR assay.
Figure 1.
A: Schematic of the BRAFV600E assay. B: Standard curve of the BRAF assay used for quantification. The equation representative of the best fit line for the BRAFV600E (lower equation) and the wild-type (WT) BRAF (upper equation) are shown. Experiments were used to calculate the standard deviations of the a375 (BRAFV600E hemizygous cell line) and 786 (BRAF wild-type cell line), respectively. n = 5 and 3 experiments. Panel A was reproduced from Panka et al25 with permission from American Association for Cancer Research.
Purified BRAFV600E first round PCR product with a known concentration was also run through the assay and was used to create a standard curve. Using the standard curve the amount of end product was determined. The RNA-based assay can reliably detect as low as 10 pg of BRAFV600E and has a 100-fold increased sensitivity compared to the WT PCR product (Figure 1B). Oligonucleotides were custom synthesized from Invitrogen (Carlsbad, CA) and Sigma (St. Louis, MO).
Assay development, testing, and validation were detailed previously.26
Tissue-Based BRAF Analysis
Patients with PTC had BRAF mutational analysis on tumor tissue as part of standard of care via SNaPshot (Massachusetts General Hospital Cancer Center Translational Research Laboratory).27, 28 For PTC patients in whom mutational status was not obtained at the discretion of the surgeon and for the benign lesions, BRAFV600E mutation was sequenced from the 10-μm sections cut from formalin-fixed, paraffin-embedded tissue blocks. Tumor was verified in selected blocks by a pathologist (P.M.S.). The genomic DNA was isolated from the tissue sections with the use of QIAamp DNA FFPE tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. This DNA is quantified and used in the PCR reaction with the use of primers forward, 5′-TCATAATGCTTGCTCTGATAGGA-3′, and reverse, 5′-GGCCAAAAATTTAATCAGTGGA-3′, to amplify a 200-bp product of the 15th exon region around the BRAFT1799A mutation with the use of high-fidelity Platinum PCR super mix (Invitrogen). The PCR product was later purified with QIAquick PCR purification kit (Qiagen) and sequenced with the forward PCR primer by the DNA core facility at Massachusetts General Hospital with the use of ABI3730XL DNA Analyzer. The data were visualized and analyzed with Finch TV software (Geospiza, Seattle, WA).
Thyroglobulin
Thyroglobulin levels were quantified for all patients by a commercial assay used in our institution (Mayo Medical Laboratories New England, Andover, MA).
Statistical Analysis
Clinical variables were chosen on the basis of established demographic and pathologic risk factors for decreased thyroid cancer-free survival. American Joint Committee on Cancer tumor, node, metastasis stage was calculated for each patient. For comparisons between BRAFV600E and WT groups, pathologic variables were only considered present if they were specifically described in the final pathology report (as per convention). Univariate comparisons of categorical variables were analyzed with Fisher's exact test, and continuous variables were assessed by Student's t-test or Wilcoxon rank sum test for nonparametric data. Comparisons of BRAF expression according to tissue mutational status and demographic and pathologic characteristics were based on linear regression models of natural log (BRAF). Tumor diameter, tumor volume, and age were considered as continuous variables. Statistical significance was defined as P < 0.05; no adjustments were made for multiple comparisons. With the use of tissue results as the gold standard, proportion of true positives (ie, sensitivity) and true negatives (ie, specificity) of the blood test in predicting the tissue results were calculated at various positivity thresholds. Likelihood ratios (sensitivity/1 − specificity) were calculated, and a receiver-operating curve was produced. An a priori decision was made to maximize specificity for determining the positivity criterion.
Results
Patient Characteristics
Seventy patients were enrolled in the study. Final surgical pathologic diagnoses were as follows: PTC (n = 48) (Table 1), follicular adenoma (n = 9), Hurthle cell neoplasm (n = 4), multinodular goiter (n = 7), minimally invasive follicular carcinoma (n = 1), and medullary thyroid carcinoma (n = 1). None of the patients had known melanoma or colorectal adenocarcinoma at enrollment. Patients with cancer had American Joint Committee on Cancer tumor, node, metastasis stages I to IV, including four patients with locally recurrent disease and four patients with iodine-refractory distant metastatic disease undergoing chemotherapy (with three of four patient with distant metastases undergoing treatment at the time of the blood draw, two BRAFV600E+ patients with BRAF-inhibitor therapy, and one BRAFWT patient with recent treatment with a multitargeted tyrosine-kinase inhibitor). Compared with BRAFWT PTC patients, patients with BRAFV600E tumors on the basis of traditional tissue assays were more often men (P = 0.03) and had more lymphovascular (P ≤ 0.01) and capsular (P = 0.02) invasion, indicating more aggressive tumors. Consistent with prior reports, a 63% prevalence of BRAFV600E mutation was found within the PTC patients with conventional tissue assays.
Table 1.
Clinical and Pathologic Characteristics of Patients
| Characteristic | All PTC patients (n = 48) | PTC patients by BRAFV600E status (n = 48) |
||
|---|---|---|---|---|
| V600E (n = 30) | Wild-type (n = 18) | P value | ||
| Demographic characteristic | ||||
| Age, years, means ± SD | 53 ± 16 | 55 ± 15 | 51 ± 18 | 0.47 |
| Male, n (%) | 15 (31) | 13 (43) | 2 (11) | 0.03∗ |
| Primary tumor characteristics | ||||
| Tumor diameter, cm, median (IQR) | 1.8 (1.3–2.5) | 1.8 (1.4–2.6) | 1.8 (1.1–3.1) | 0.83 |
| Multifocality, n (%) | 28 (74) | 21 (84) | 7 (54) | 0.06 |
| Lymphovascular invasion, n (%) | 23 (61) | 21 (84) | 2 (15) | <0.01∗ |
| Capsular invasion, n (%) | 27 (71) | 21 (84) | 6 (46) | 0.02∗ |
| Extrathyroidal extension, n (%) | 11 (29) | 8 (32) | 3 (23) | 0.71 |
| Cervical lymph node status | ||||
| Central lymph node(s)+, n (%) | 16 (33) | 13 (43) | 3 (17) | 0.07 |
| Lateral lymph node(s)+, n (%) | 9 (19) | 6 (20) | 3 (17) | 0.77 |
| AJCC TNM stage | ||||
| I, n (%) | 24 (50) | 16 (53) | 8 (44) | 0.76 |
| II, n (%) | 4 (8) | 2 (7) | 2 (11) | |
| III, n (%) | 12 (25) | 8 (27) | 4 (22) | |
| IV, n (%) | 8 (17) | 4 (13) | 4 (22) | |
AJCC, American Joint Committee on Cancer; TNM, tumor, node, metastasis.
Statistically significant.
Two patients had false-positive tissue testing for BRAFV600E (with the use of surgical pathologic diagnosis as the gold standard). The indication for surgery in both of these patients was suspicious FNA biopsy (ie, suspicious for follicular neoplasm) with associate 30% risk of malignancy. Pathologic review (P.M.S.) of these two patients confirmed their diagnoses. The first patient, diagnosed as having a unilateral multinodular goiter with dominant adenomatous nodule (serum BRAF level, 7.2 pg), had only undergone thyroid lobectomy. The second patient (serum BRAF level, 7.2 pg) was subsequently diagnosed and treated for malignant melanoma.
Correlation of Circulating BRAFV600E Levels with Patient and Tumor Characteristics
Linear regression analysis with demographic and pathologic predictors was performed in two subsets of patients: those in the cohort with PTC (n = 48) (Table 2) and those with PTC who were BRAFV600E+ by conventional tissue assay (n = 30). Neither age nor sex was associated with blood BRAFV600E levels. Of the primary tumor characteristics, tissue BRAF positivity (P = 0.007) and central cervical lymph node positivity (P = 0.003) were correlated with circulating BRAFV600E levels in the first cohort (all PTC). Largest tumor diameter, tumor volume, and American Joint Committee on Cancer tumor, node, metastasis stage were not correlated with assay levels. Patients with benign disease had median serum BRAF levels 3.0 pg (interquartile range, 0.8 to 5.8 pg). The BRAF levels of patients who had a final pathologic diagnosis of PTC and were BRAFV600E+ had a median blood BRAFV600E level of 17.3 pg (interquartile range, 1.9 to 127.5 pg), whereas in patients who had a PTC and were BRAF− the median blood BRAFV600E level was 2.0 pg (interquartile range, 0.5 to 4.4 pg).
Table 2.
Correlation of Circulating ln(BRAFV600E) Levels and Other Covariates in PTC Patients (n = 48)
| Variable | Coefficient | SE | Univariate R2 | P value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years | 0.041 | 0.024 | 0.061 | 0.096 |
| Female | 0.263 | 0.906 | 0.002 | 0.772 |
| Primary tumor characteristics | ||||
| Tissue BRAFV600E | 2.260 | 0.801 | 0.148 | <0.01∗ |
| Maximum tumor diameter, cm | −0.063 | 0.357 | 0.001 | 0.860 |
| Tumor volume, cm3 | −0.059 | 0.104 | 0.010 | 0.567 |
| Multifocality | 0.456 | 0.968 | 0.006 | 0.640 |
| Lymphovascular invasion | 1.048 | 0.872 | 0.038 | 0.237 |
| Capsular invasion | 0.446 | 0.944 | 0.006 | 0.639 |
| Extrathyroidal extension | −0.421 | 0.969 | 0.005 | 0.666 |
| Cervical lymph node status | ||||
| Central lymph node(s)+ | 0.703 | 0.885 | 0.014 | <0.01∗ |
| Lateral lymph node(s)+ | 0.744 | 1.071 | 0.010 | 0.491 |
| AJCC-TNM stage | 0.514 | 0.346 | 0.046 | 0.144 |
AJCC, American Joint Committee on Cancer; TNM, tumor, node, metastasis.
Statistically significant.
Four patients in the study had distant metastatic disease, three of whom were BRAFV600E+ by tissue testing. One patient with widely metastatic disease to the lung, liver, bone, and brain had a blood BRAFV600E level of 39,976 pg [thyroglobulin (Tg), 0.2 ng/mL]. This patient had been on treatment with BRAF-inhibitor therapy at the time of the blood draw for over a year, yet had progressive disease. The other two BRAFV600E+ patients by tissue testing with distant metastatic disease had blood BRAFV600E levels of 8.2 pg (Tg, 68 ng/mL) and 442 pg (Tg Ab, >3000 IU/mL). Both of these samples were drawn before planned initiation with BRAF-inhibitor therapy. The last patient, with BRAFWT tumor (blood BRAFV600E level, 4.5 pg; Tg, 4193 ng/mL) had been treated with multitargeted receptor tyrosine kinase inhibitor, pazopanib, up until 3 months before the blood draw.
Testing Characteristics
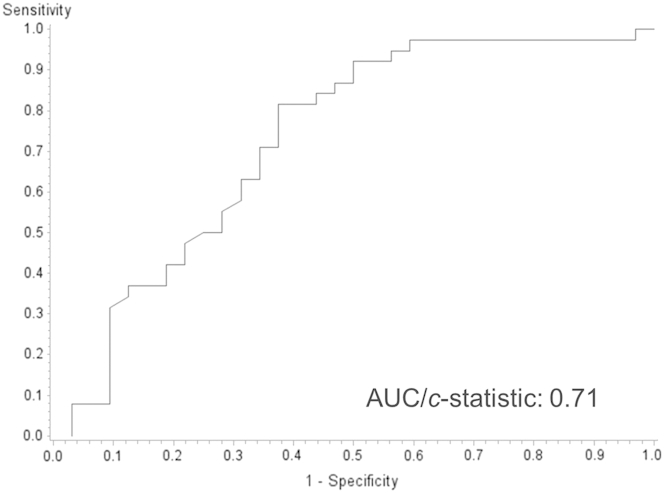
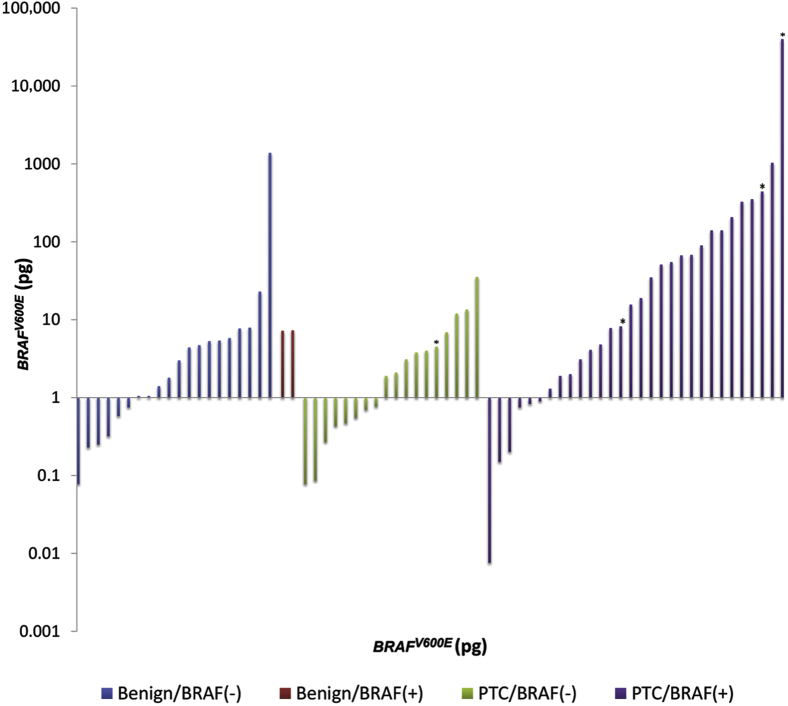
With the use of the tissue BRAF result as the gold standard, likelihood ratios were calculated, and a receiver-operating characteristic curve for the blood assay predicting tissue BRAF mutational status was constructed (Figure 2 and Table 3). The area under the curve was 0.71, indicating moderate correlation of tests, assuming the tissue results are true. Correlation of tissue and blood assays with final surgical pathologic diagnosis is illustrated in Figure 3. As expected, the use of stricter (ie, higher) levels of circulating BRAFV600E decreased the sensitivity and increased the specificity. For example, the figure illustrates that most malignant PTC patients that were tissue typed as BRAFV600E+ had circulating BRAFV600E levels in large excess of 10 pg. However, patients with benign tumor or patients with malignant tumors lacking the BRAFV600E mutation rarely had circulating BRAFV600E levels >10 pg.
Figure 2.
Receiver-operating analysis with each of the 70 circulating BRAFV600E levels as a possible positivity criterion in predicting the gold standard (tissue BRAF testing). AUC, area under the curve.
Table 3.
Sensitivity, Specificity, and Likelihood Ratio for Positive Tissue BRAF Test in Various Thresholds
| BRAFV600E positivity threshold, pg | Sensitivity, % | Specificity, % | LR+ |
|---|---|---|---|
| 5 | 62.5 | 71.1 | 2.2 |
| 10 | 50.0 | 86.8 | 3.9 |
| 20 | 43.8 | 92.1 | 5.5 |
With the use of tissue results as the gold standard, proportion of true-positive (ie, sensitivity) and true-negative (ie, specificity) results of the blood test in predicting the tissue results were calculated at various positivity thresholds. Likelihood ratios (sensitivity/1 − specificity) were calculated, and a receiver-operating curve was produced.
LR+, likelihood ratio for positive tissue BRAF test.
Figure 3.
Circulating levels of BRAFV600E by pathologic diagnosis (benign, left; malignant, right) and tissue mutational status. Benign/BRAF− indicates a tumor that was read as either an adenoma or a benign hyperplastic lesion that is also negative for BRAFV600E by conventional tissue testing. Benign/BRAF+ indicates pathologically negative, tissue BRAFV600E+. Asterisk indicates patients with known distant metastatic disease.
Discussion
Clinical Implications and Utility of BRAFV600E Assay
Patients with PTC harboring the BRAFV600E mutation have more aggressive tumors and a reduced disease-free and overall survival.6, 7, 9, 13 Moreover, the BRAFV600E mutation is found in one-half of patients with PTC, making it a potentially useful biomarker in a large subgroup of thyroid cancer patients. Currently, BRAF status on the basis of the surgical pathology specimen is used by some clinicians to risk-stratify patients for recurrence.13 Others have argued the use of BRAF mutational status from preoperative FNA specimens to guide extent of thyroidectomy (ie, lobectomy versus total thyroidectomy) and whether to perform prophylactic central lymphadenectomy.14, 15, 29, 30, 31, 32 Although a prospective trial to test the benefit of BRAF status to guide extent of surgery on outcome was not performed, we believe that BRAF status, in addition to other clinicopathologic risk factors, can be helpful in clinical decision making.
Traditional assays used to test BRAF require harvesting cytologic or pathologic tissue. The results of these tests are limited by the lack of quantitative measurements and the potential for high background noise from surrounding stromal tissue.33 In this study, we report the feasibility of an RNA-based assay for detection of BRAFV600E from circulating tumor cells. This assay has a number of advantages over current mutational testing and has potential clinical applications. In particular, blood-based biomarker detection has the advantage of allowing for serial measurements of circulating BRAFV600E that can be correlated with disease presence and/or severity over time. BRAF+ tumors have more aggressive features and, in most series, have a higher incidence of disease recurrence.7, 8, 13, 34, 35 Treatment of BRAF+ recurrences are more difficult, however, because BRAF+ tumors have impairment of the Na+/I− symporter necessary for RAI uptake. Increased uptake of RAI after BRAFi treatment in RAI-refractory patients was shown.11, 36, 37 The ability to test circulating BRAF levels during treatment with BRAFi to assess response to treatment and to predict redifferentiation would be of great value in guiding continued treatment and choice of imaging. Finally, serial measurement of BRAFV600E may be particularly useful in patients with Tg-Abs (present in approximately 20% of PTC patients) in which Tg (conventional biomarker for PTC after thyroidectomy) levels are not useful as a biomarker.38 Indeed, of the eight patients undergoing treatment for metastatic disease (ie, patients we had Tg levels available), one BRAF+ patient undergoing treatment with BRAFi had elevated Tg Ab, making Tg levels useless as a biomarker. This same patient had a blood BRAF level of 442 pg, highlighting the application in this subset of patients. In addition, a small subset of patients with aggressive tumors ceases making Tg, rendering this assay potentially useful. This is illustrated by the one patient with known distant metastases who has a blood BRAF level of 39,976.0 pg but an almost undetectable Tg (0.2 ng/mL).
Improved Sensitivity with RNA-Based Assay
Our assay can detect mutant BRAF amid excess BRAFWT. Previous blood-based assays for BRAF in melanoma patients were reported; however, they used DNA rather than RNA for the assay.39, 40, 41, 42 DNA is the preferred substrate for molecular testing in general because of its stability during handling in comparison with RNA. Vallachi et al43 reviewed several BRAFV600E assays with the use of DNA as a substrate in metastatic melanomas. Most the assays were able to detect mutant BRAF at a frequency of 0.1% to 1% relative to BRAFWT. Huang et al44 examined formalin-fixed PTC tissue with the use of a DNA amplification refractory mutation system assay and were able to detect mutant BRAF at a frequency of 0.5% relative to BRAFWT. Reported DNA-based assays for BRAFV600E in PTC patients have not been sensitive. Cradic et al,45 only detected circulating BRAFV600E in 8 of 42 of the tumor-positive PTC patients during postoperative follow-up. Zane et al46 did not identify any mutant BRAF in cell-free (ie, plasma-based assay) DNA in 46 patients with tissue-positive PTC. In addition to having exponentially higher copy numbers of BRAF mRNA, digestion of BRAFWT with TspRI in our assay further increased the sensitivity for detection. We have performed a direct comparison of usage of RNA with DNA as substrates in this assay. On the basis of the data from the standard curves BRAFWT is not detected in this assay with the use of up to 1 ng of DNA from the first round of PCR. The best fit lines of the BRAFV600E and BRAFWT intersect at 1 pg but are statistically significantly different at 10 pg (P < 0.03), which represents the lower limit of detection of the BRAFV600E. We therefore concluded that the BRAFV600E can be statistically detected at a frequency of 10 pg V600E in 1000 pg of BRAFWT or 1%. By comparison, following the same procedure except for the oligonucleotides for the first two PCRs and with the use of genomic DNA as a substrate and a statistically significant lower limit of 10 pg, the assay could only detect the mutant at a frequency of 3% relative to the BRAFWT, a difference that in some patient samples prevented the detection of the mutant BRAF in the blood (D.J.P., personal communication; data not shown). The use of RNA and digestion of BRAFWT enhances the sensitivity of our assay.
Importantly, we have shown that circulating BRAFV600E is detectable even in patients with minimal tumor burden. Even in the 10 patients without extrathyroidal extension or lymph node metastases and tumors <2 cm (T1 tumors) that were BRAF+ on tissue testing, 6 had blood BRAF levels >10 pg (all were detectable). In comparison with the population of patients with PTC in the United States, our cohort had more patients with stage III and IV tumors. However, reflective of national trends, most patients in this study had small tumors (<2.0 cm) and were female. We found a relatively high prevalence of BRAFV600E mutation in our group, perhaps accounted for by the over-representation of patients with more aggressive tumors. This mutation is common and highly specific for PTC among patients with thyroid nodules. Tissue BRAFV600E status in our study was correlated with male patients, lymphovascular invasion, and capsular invasion. When correlating the blood BRAF levels with the same clinical and pathologic variables, these three variables were not correlated. As expected, good correlation was found between tissue BRAF status and level of circulating BRAFV600E.
Although the assay appears feasible, findings warrant discussion. Of concern were the two patients that had a final pathologic diagnosis of multinodular goiter with BRAFV600E+ tissue testing (blood BRAF levels were 7.2 and 7.4 pg). Both patients had surgery for FNA biopsies that were suspicious for follicular neoplasm which portends a 30% risk of malignancy. On review of the surgical pathology for these two patients it was noted that one patient had a papillary microcarcinoma (1 mm in diameter) that may have been the source of BRAFV600E in both the tissue and the blood. BRAFV600E is detectable even in papillary thyroid microcarcinomas (PTC < 1 cm).47, 48, 49 No further intervention is required for this patient. The situation for the second patient is less clear. Although the surgical pathology does not reveal any PTC (unless missed by the pathologist), the patient was subsequently diagnosed and treated for malignant melanoma, potentially explaining the elevated blood level but not the thyroid tissue result. In addition, this patient had only a thyroid lobectomy. The circulating BRAFV600E could potentially be caused by an occult PTC in the contralateral lobe. As with our prior work in a group of patients with malignant melanoma, wherein mean blood BRAFV600E levels were detectable but low (ie, BRAFWT, 1.7 pg; controls, 1.2 pg; BRAFV600E, 50.3 pg), we found detectable circulating BRAFV600E in patients with benign disease.25 Finally, one patient had a thyroid lobectomy for a Hurthle cell neoplasm and a BRAFV600E level of 1384 pg; a possibility of a BRAFV600E+ tumor in the contralateral unresected lobe or occult melanoma, benign nevi, or serrated colorectal adenoma or colorectal carcinoma, all known to have BRAFV600E mutations, cannot be eliminated.25, 50, 51, 52, 53 Outliers may be considered for evaluation for occult cancers.
Determining the Optimal Positivity Criterion
The receiver-operating characteristic analysis and area under the curve (c-statistic = 0.71) indicates a moderate ability of our RNA-based blood assay to predict the BRAF status as assessed by DNA-based conventional tissue assays. As shown in Figures 2 and 3, increasing thresholds for a positive blood test increases the specificity (ie, low false-positive rate). However, prevalence of disease (in this case prevalence of BRAFV600E in the PTC population) and the consequences of false-positive and false-negative results should be considered when choosing a positivity criterion. Given the minimal impact on clinical care with a false-negative result and that a false-positive result could result in potential unwarranted treatment, we would choose to optimize specificity (ie, a higher threshold). Unlike premalignant nevi, in which BRAFV600E has been detected, BRAFV600E is thought to be highly specific for PTC in thyroid nodules.43
Study Limitations
In assessing the accuracy of the blood assay, we used tissue BRAF status as the gold standard, making the assumption that this test is perfect. Although we are left without an alternative gold standard by which to assess the accuracy of the blood assay, it is possible that the blood assay is more sensitive and specific than the tissue samples, given the potential for high background from sampling of stromal areas of the tumor normally surrounding thyroid in the tissue specimens. In addition, we cannot entirely exclude the possibility that detectable circulating levels of BRAFV600E are from an undiagnosed dermal or colorectal disorder. None of the patients in our study had known concomitant cancers at the time of enrollment, and one patient subsequently was diagnosed with a malignant melanoma (within months of thyroidectomy).
Conclusions
We have shown that an RNA-based BRAFV600E is feasibly detectable in the blood patients with PTC, even with low tumor burden. This assay has a number of potential clinical applications. Given that BRAFV600E has not been found in benign thyroid tissue or adenomas, high blood BRAFV600E levels could be used as a preoperative adjunct to FNA to guide initial extent of surgery. A highly specific, less costly blood test could be an alternative to other costly, send-out molecular diagnostics. Thyroid cancer is a common disease with a clinical need for improved risk stratification for most patients who do well. Next, by quantifying the change in levels after surgery for BRAF+ patients clinicians could assess completeness of surgery, residual disease in Tg Ab+ patients, need for or utility of adjuvant RAI, or response to adjuvant BRAF-targeted therapy. Moreover, in the small but substantial group of patients destined to do poorly, there is an enrichment of BRAF mutations. Thus, the ability to accurately detect and follow BRAF levels over time as a biomarker for recurrence and response to BRAF inhibitors would be of great clinical value. Specifically, we previously reported the accuracy of this assay in a cohort of patients with melanoma wherein we were able to show that patient BRAF levels declined after treatment with BRAF-inhibitor therapy and that serial measurements allowed biochemical detection before structural disease.25 Cradic et al45 found a correlation with detectable BRAF in patients with known residual PTC. Moreover, should BRAF-targeted therapy prove beneficial in patients with BRAF-mutant advanced thyroid cancer, the assay may be useful as an early predictor of response to therapy.
Footnotes
Supported by NIH National Cancer Institute Program in Cancer Outcomes Research Training grant R25CA092203 (C.C.L.); NIH National Cancer Institute grants K07CA177900-01 (C.C.L.) and R01CA149738-01A1 (S.P.); Massachusetts General Hospital Department of Surgery (C.C.L.); Massachusetts General Hospital American Cancer Society Institutional Research grant (C.C.L.); The Egan Memorial Melanoma Translational Research Fund (R.J.S.); the Conquer Cancer Foundation (R.J.S.); and the Clinical Investigator Training Program, Harvard Medical School and Massachusetts Institute of Technology (R.J.S.).
Disclosures: None disclosed.
References
- 1.Chen A.Y., Jemal A., Ward E.M. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 2.Ahn H.S., Kim H.J., Welch H.G. Korea's thyroid-cancer “epidemic”–screening and overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 3.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper D.S., Doherty G.M., Haugen B.R., Kloos R.T., Lee S.L., Mandel S.J., Mazzaferri E.L., McIver B., Pacini F., Schlumberger M., Sherman S.I., Steward D.L., Tuttle R.M. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 4.Hay I.D., Bergstralh E.J., Goellner J.R., Ebersold J.R., Grant C.S. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1057. discussion 1057–1058. [PubMed] [Google Scholar]
- 5.Sanders L.E., Cady B. Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Arch Surg. 1998;133:419–425. doi: 10.1001/archsurg.133.4.419. [DOI] [PubMed] [Google Scholar]
- 6.Elisei R., Viola D., Torregrossa L., Giannini R., Romei C., Ugolini C., Molinaro E., Agate L., Biagini A., Lupi C., Valerio L., Materazzi G., Miccoli P., Piaggi P., Pinchera A., Vitti P., Basolo F. The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab. 2012;97:4390–4398. doi: 10.1210/jc.2012-1775. [DOI] [PubMed] [Google Scholar]
- 7.Kim T.H., Park Y.J., Lim J.A., Ahn H.Y., Lee E.K., Lee Y.J., Kim K.W., Hahn S.K., Youn Y.K., Kim K.H., Cho B.Y., Park do J. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer. 2012;118:1764–1773. doi: 10.1002/cncr.26500. [DOI] [PubMed] [Google Scholar]
- 8.Prescott J.D., Sadow P.M., Hodin R.A., Le L.P., Gaz R.D., Randolph G.W., Stephen A.E., Parangi S., Daniels G.H., Lubitz C.C. BRAF V600E status adds incremental value to current risk classification systems in predicting papillary thyroid carcinoma recurrence. Surgery. 2012;152:984–990. doi: 10.1016/j.surg.2012.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing M., Alzahrani A.S., Carson K.A., Viola D., Elisei R., Bendlova B., Yip L., Mian C., Vianello F., Tuttle R.M., Robenshtok E., Fagin J.A., Puxeddu E., Fugazzola L., Czarniecka A., Jarzab B., O'Neill C.J., Sywak M.S., Lam A.K., Riesco-Eizaguirre G., Santisteban P., Nakayama H., Tufano R.P., Pai S.I., Zeiger M.A., Westra W.H., Clark D.P., Clifton-Bligh R., Sidransky D., Ladenson P.W., Sykorova V. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kebebew E., Weng J., Bauer J., Ranvier G., Clark O.H., Duh Q.Y., Shibru D., Bastian B., Griffin A. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007;246:466–470. doi: 10.1097/SLA.0b013e318148563d. discussion 470–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riesco-Eizaguirre G., Gutierrez-Martinez P., Garcia-Cabezas M.A., Nistal M., Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr Relat Cancer. 2006;13:257–269. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing M., Alzahrani A.S., Carson K.A., Shong Y.K., Kim T.Y., Viola D., Elisei R., Bendlova B., Yip L., Mian C., Vianello F., Tuttle R.M., Robenshtok E., Fagin J.A., Puxeddu E., Fugazzola L., Czarniecka A., Jarzab B., O'Neill C.J., Sywak M.S., Lam A.K., Riesco-Eizaguirre G., Santisteban P., Nakayama H., Clifton-Bligh R., Tallini G., Holt E.H., Sykorova V. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33:42–50. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joo J.Y., Park J.Y., Yoon Y.H., Choi B., Kim J.M., Jo Y.S., Shong M., Koo B.S. Prediction of occult central lymph node metastasis in papillary thyroid carcinoma by preoperative BRAF analysis using fine-needle aspiration biopsy: a prospective study. J Clin Endocrinol Metab. 2012;97:3996–4003. doi: 10.1210/jc.2012-2444. [DOI] [PubMed] [Google Scholar]
- 15.Howell G.M., Nikiforova M.N., Carty S.E., Armstrong M.J., Hodak S.P., Stang M.T., McCoy K.L., Nikiforov Y.E., Yip L. BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann Surg Oncol. 2013;20:47–52. doi: 10.1245/s10434-012-2611-0. [DOI] [PubMed] [Google Scholar]
- 16.Xing M., Clark D., Guan H., Ji M., Dackiw A., Carson K.A., Kim M., Tufaro A., Ladenson P., Zeiger M., Tufano R. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J Clin Oncol. 2009;27:2977–2982. doi: 10.1200/JCO.2008.20.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yip L., Nikiforova M.N., Carty S.E., Yim J.H., Stang M.T., Tublin M.J., Lebeau S.O., Hodak S.P., Ogilvie J.B., Nikiforov Y.E. Optimizing surgical treatment of papillary thyroid carcinoma associated with BRAF mutation. Surgery. 2009;146:1215–1223. doi: 10.1016/j.surg.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Barbaro D., Incensati R.M., Materazzi G., Boni G., Grosso M., Panicucci E., Lapi P., Pasquini C., Miccoli P. The BRAF V600E mutation in papillary thyroid cancer with positive or suspected pre-surgical cytological finding is not associated with advanced stages or worse prognosis. Endocrine. 2014;45:462–468. doi: 10.1007/s12020-013-0029-5. [DOI] [PubMed] [Google Scholar]
- 19.Lee K.C., Li C., Schneider E.B., Wang Y., Somervell H., Krafft M., Umbricht C.B., Zeiger M.A. Is BRAF mutation associated with lymph node metastasis in patients with papillary thyroid cancer? Surgery. 2012;152:977–983. doi: 10.1016/j.surg.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutenhefner S.E., Marui S., Santos A.B., de Lima E.U., Inoue M., Neto J.S., Shiang C., Fukushima J.T., Cernea C.R., Friguglietti C.U. BRAF: a tool in the decision to perform elective neck dissection? Thyroid. 2013;23:1541–1546. doi: 10.1089/thy.2012.0304. [DOI] [PubMed] [Google Scholar]
- 21.Dadu R., Shah K., Busaidy N.L., Waguespack S.G., Habra M.A., Ying A.K., Hu M.I., Bassett R., Jimenez C., Sherman S.I., Cabanillas M.E. Efficacy and tolerability of vemurafenib in patients with BRAF(V600E) -positive papillary thyroid cancer: M.D. Anderson Cancer Center off label experience. J Clin Endocrinol Metab. 2015;100:E77–E81. doi: 10.1210/jc.2014-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falchook G.S., Millward M., Hong D.S., Naing A., Piha-Paul S., Waguespack S.G., Cabanillas M., Sherman S.I., Ma B., Curtis M., Goodman V., Kurzrock R. BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid cancer. Thyroid. 2015;25:71–77. doi: 10.1089/thy.2014.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim K.B., Cabanillas M.E., Lazar A.J., Williams M.D., Sanders D.L., Ilagan J.L., Nolop K., Lee R.J., Sherman S.I. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF(V600E) mutation. Thyroid. 2013;23:1277–1283. doi: 10.1089/thy.2013.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dvorak K., Aggeler B., Palting J., McKelvie P., Ruszkiewicz A., Waring P. Immunohistochemistry with the anti-BRAF V600E (VE1) antibody: impact of pre-analytical conditions and concordance with DNA sequencing in colorectal and papillary thyroid carcinoma. Pathology. 2014;46:509–517. doi: 10.1097/PAT.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panka D.J., Buchbinder E., Giobbie-Hurder A., Schalck A.P., Montaser-Kouhsari L., Sepehr A.P., Lawrence D.P., McDermott D.F., Cohen R., Carlson A., Wargo J.A., Merritt R., Seery V.J., Hodi F.S., Gunturi A., Frederick D., Atkins M.B., Iafrate A.J., Flaherty K.T., Mier J.W., Sullivan R.J. Clinical utility of a blood-based BRAF(V600E) mutation assay in melanoma. Mol Cancer Ther. 2014;13:3210–3218. doi: 10.1158/1535-7163.MCT-14-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panka D.J., Mier J.W., Sullivan R.J. Assaying for BRAF V600E in tissue and blood in melanoma. Methods Mol Biol. 2014;1102:117–136. doi: 10.1007/978-1-62703-727-3_8. [DOI] [PubMed] [Google Scholar]
- 27.Halait H., Demartin K., Shah S., Soviero S., Langland R., Cheng S., Hillman G., Wu L., Lawrence H.J. Analytical performance of a real-time PCR-based assay for V600 mutations in the BRAF gene, used as the companion diagnostic test for the novel BRAF inhibitor vemurafenib in metastatic melanoma. Diagn Mol Pathol. 2012;21:1–8. doi: 10.1097/PDM.0b013e31823b216f. [DOI] [PubMed] [Google Scholar]
- 28.Su Z., Dias-Santagata D., Duke M., Hutchinson K., Lin Y.L., Borger D.R., Chung C.H., Massion P.P., Vnencak-Jones C.L., Iafrate A.J., Pao W. A platform for rapid detection of multiple oncogenic mutations with relevance to targeted therapy in non-small-cell lung cancer. J Mol Diagn. 2011;13:74–84. doi: 10.1016/j.jmoldx.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia Y., Yu Y., Li X., Wei S., Zheng X., Yang X., Zhao J., Xia T., Gao M. Diagnostic value of B-RAF(V600E) in difficult-to-diagnose thyroid nodules using fine-needle aspiration: systematic review and meta-analysis. Diagn Cytopathol. 2014;42:94–101. doi: 10.1002/dc.23044. [DOI] [PubMed] [Google Scholar]
- 30.Lee J.W., Koo B.S. The prognostic implication and potential role of BRAF mutation in the decision to perform elective neck dissection for thyroid cancer. Gland Surg. 2013;2:206–211. doi: 10.3978/j.issn.2227-684X.2013.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C., Lee K.C., Schneider E.B., Zeiger M.A. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab. 2012;97:4559–4570. doi: 10.1210/jc.2012-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathur A., Weng J., Moses W., Steinberg S.M., Rahbari R., Kitano M., Khanafshar E., Ljung B.M., Duh Q.Y., Clark O.H., Kebebew E. A prospective study evaluating the accuracy of using combined clinical factors and candidate diagnostic markers to refine the accuracy of thyroid fine needle aspiration biopsy. Surgery. 2010;148:1170–1176. doi: 10.1016/j.surg.2010.09.025. discussion 1176–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller C.J., Cheung M., Sharma A., Clarke L., Helm K., Mauger D., Robertson G.P. Method of mutation analysis may contribute to discrepancies in reports of (V599E)BRAF mutation frequencies in melanocytic neoplasms. J Invest Dermatol. 2004;123:990–992. doi: 10.1111/j.0022-202X.2004.23468.x. [DOI] [PubMed] [Google Scholar]
- 34.Elisei R., Ugolini C., Viola D., Lupi C., Biagini A., Giannini R., Romei C., Miccoli P., Pinchera A., Basolo F. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 35.Xing M., Westra W.H., Tufano R.P., Cohen Y., Rosenbaum E., Rhoden K.J., Carson K.A., Vasko V., Larin A., Tallini G., Tolaney S., Holt E.H., Hui P., Umbricht C.B., Basaria S., Ewertz M., Tufaro A.P., Califano J.A., Ringel M.D., Zeiger M.A., Sidransky D., Ladenson P.W. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 36.Mian C., Barollo S., Pennelli G., Pavan N., Rugge M., Pelizzo M.R., Mazzarotto R., Casara D., Nacamulli D., Mantero F., Opocher G., Busnardo B., Girelli M.E. Molecular characteristics in papillary thyroid cancers (PTCs) with no 131I uptake. Clin Endocrinol (Oxf) 2008;68:108–116. doi: 10.1111/j.1365-2265.2007.03008.x. [DOI] [PubMed] [Google Scholar]
- 37.Rothenberg S.M., McFadden D.G., Palmer E.L., Daniels G.H., Wirth L.J. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2014;21:1028–1035. doi: 10.1158/1078-0432.CCR-14-2915. [DOI] [PubMed] [Google Scholar]
- 38.Lupoli G.A., Okosieme O.E., Evans C., Clark P.M., Pickett A.J., Premawardhana L.D., Lupoli G., Lazarus J.H. Prognostic significance of thyroglobulin antibody epitopes in differentiated thyroid cancer. J Clin Endocrinol Metab. 2015;100:100–108. doi: 10.1210/jc.2014-2725. [DOI] [PubMed] [Google Scholar]
- 39.Board R.E., Ellison G., Orr M.C., Kemsley K.R., McWalter G., Blockley L.Y., Dearden S.P., Morris C., Ranson M., Cantarini M.V., Dive C., Hughes A. Detection of BRAF mutations in the tumour and serum of patients enrolled in the AZD6244 (ARRY-142886) advanced melanoma phase II study. Br J Cancer. 2009;101:1724–1730. doi: 10.1038/sj.bjc.6605371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniotti M., Vallacchi V., Rivoltini L., Patuzzo R., Santinami M., Arienti F., Cutolo G., Pierotti M.A., Parmiani G., Rodolfo M. Detection of mutated BRAFV600E variant in circulating DNA of stage III-IV melanoma patients. Int J Cancer. 2007;120:2439–2444. doi: 10.1002/ijc.22598. [DOI] [PubMed] [Google Scholar]
- 41.Pinzani P., Santucci C., Mancini I., Simi L., Salvianti F., Pratesi N., Massi D., De Giorgi V., Pazzagli M., Orlando C. BRAFV600E detection in melanoma is highly improved by COLD-PCR. Clin Chim Acta. 2011;412:901–905. doi: 10.1016/j.cca.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Yancovitz M., Yoon J., Mikhail M., Gai W., Shapiro R.L., Berman R.S., Pavlick A.C., Chapman P.B., Osman I., Polsky D. Detection of mutant BRAF alleles in the plasma of patients with metastatic melanoma. J Mol Diagn. 2007;9:178–183. doi: 10.2353/jmoldx.2007.060135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallachi V., Rivoltini L., Rodolfo M. InTech; Rijeka, Croatia: 2011. BRAF V600E Mutated Gene Variant as a Circulating Molecular Marker in Metastatic Melanoma Patients. [Google Scholar]
- 44.Huang T., Zhuge J., Zhang W.W. Sensitive detection of BRAF V600E mutation by Amplification Refractory Mutation System (ARMS)-PCR. Biomark Res. 2013;1:3. doi: 10.1186/2050-7771-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cradic K.W., Milosevic D., Rosenberg A.M., Erickson L.A., McIver B., Grebe S.K. Mutant BRAF(T1799A) can be detected in the blood of papillary thyroid carcinoma patients and correlates with disease status. J Clin Endocrinol Metab. 2009;94:5001–5009. doi: 10.1210/jc.2009-1349. [DOI] [PubMed] [Google Scholar]
- 46.Zane M., Agostini M., Enzo M.V., Casal Ide E., Del Bianco P., Torresan F., Merante Boschin I., Pennelli G., Saccani A., Rubello D., Nitti D., Pelizzo M.R. Circulating cell-free DNA, SLC5A8 and SLC26A4 hypermethylation, BRAF(V600E): a non-invasive tool panel for early detection of thyroid cancer. Biomed Pharmacother. 2013;67:723–730. doi: 10.1016/j.biopha.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Niemeier L.A., Kuffner Akatsu H., Song C., Carty S.E., Hodak S.P., Yip L., Ferris R.L., Tseng G.C., Seethala R.R., Lebeau S.O., Stang M.T., Coyne C., Johnson J.T., Stewart A.F., Nikiforov Y.E. A combined molecular-pathologic score improves risk stratification of thyroid papillary microcarcinoma. Cancer. 2012;118:2069–2077. doi: 10.1002/cncr.26425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walczyk A., Kowalska A., Kowalik A., Sygut J., Wypiorkiewicz E., Chodurska R., Pieciak L., Gozdz S. The BRAFV600E mutation in papillary thyroid microcarcinoma: does the mutation have an impact on clinical outcome? Clin Endocrinol. 2014;80:899–904. doi: 10.1111/cen.12386. [DOI] [PubMed] [Google Scholar]
- 49.Zheng X., Wei S., Han Y., Li Y., Yu Y., Yun X., Ren X., Gao M. Papillary microcarcinoma of the thyroid: clinical characteristics and BRAF(V600E) mutational status of 977 cases. Ann Surg Oncol. 2013;20:2266–2273. doi: 10.1245/s10434-012-2851-z. [DOI] [PubMed] [Google Scholar]
- 50.Poynter J.N., Elder J.T., Fullen D.R., Nair R.P., Soengas M.S., Johnson T.M., Redman B., Thomas N.E., Gruber S.B. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res. 2006;16:267–273. doi: 10.1097/01.cmr.0000222600.73179.f3. [DOI] [PubMed] [Google Scholar]
- 51.Taube J.M., Begum S., Shi C., Eshleman J.R., Westra W.H. Benign nodal nevi frequently harbor the activating V600E BRAF mutation. Am J Surg Pathol. 2009;33:568–571. doi: 10.1097/PAS.0b013e31818a64fb. [DOI] [PubMed] [Google Scholar]
- 52.Morimoto T., Mitomi H., Saito T., Takahashi M., Murakami T., Sakamoto N., Yao T., Watanabe S. Distinct profile of HIF1alpha, PTCH, EphB2, or DNA repair protein expression and BRAF mutation in colorectal serrated adenoma. J Gastroenterol Hepatol. 2014;29:1192–1199. doi: 10.1111/jgh.12553. [DOI] [PubMed] [Google Scholar]
- 53.Stefanius K., Ylitalo L., Tuomisto A., Kuivila R., Kantola T., Sirnio P., Karttunen T.J., Makinen M.J. Frequent mutations of KRAS in addition to BRAF in colorectal serrated adenocarcinoma. Histopathology. 2011;58:679–692. doi: 10.1111/j.1365-2559.2011.03821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]