Abstract
Background.
Higher cardiorespiratory fitness (CRF) is cross-sectionally associated with more conserved brain volume in older age, but longitudinal studies are rare. This study examined whether higher midlife CRF was prospectively associated with slower atrophy, which in turn was associated with higher late-life CRF.
Methods.
Brain volume by magnetic resonance imaging was determined annually from 1994 to 2003 in 146 participants (M baseline age = 69.6 years). Peak oxygen uptake on a treadmill yielded estimated midlife CRF in 138 and late-life CRF in 73 participants.
Results.
Higher midlife CRF was associated with greater middle temporal gyrus, perirhinal cortex, and temporal and parietal white matter, but was not associated with atrophy progression. Slower atrophy in middle frontal and angular gyri was associated with higher late-life CRF, independent of CRF at baseline magnetic resonance imaging.
Conclusions.
Higher midlife CRF may play a role in preserving middle and medial temporal volumes in late adulthood. Slower atrophy in middle frontal and angular gyri may predict late-life CRF.
Key Words: Cardiovascular, Epidemiology, Neuroimaging.
The intriguing relationship between increased cardiorespiratory fitness (CRF) and improved brain function, in particular executive control function and memory, is supported in human observational studies and small intervention studies (1–3). However, the mechanisms for brain structures involved in this relationship are not well understood, as little information is available on how brain structural changes are related to CRF.
Initial neuroimaging studies largely rely on cross-sectional designs. Current evidence shows higher CRF is associated with more conserved gray matter (GM) and white matter (WM) volumes (4–9), increased cortical thickness (9), fewer WM lesions (10), and greater WM integrity (11–14) in cognitively healthy older adults. Small intervention studies suggest aerobic exercise increases brain volumes in selected brain areas (15,16) and increases in fitness from walking are associated with increases in WM integrity (17). Brain regions strongly associated with CRF tend to be localized in prefrontal and medial temporal areas important for executive function and memory. These brain areas are sensitive to physical exercise because of their watershed localization. The watershed areas receive dual blood supply and are located in the end of the circulation, which are vulnerable to a reduced cerebral blood flow and highly susceptible to changes in blood oxygenation levels (18,19). A few studies in cognitively healthy older adults are unable to detect any association between fitness and brain volumes (20,21). To date, only one study sought to examine changes in CRF in relation to brain atrophy over a 2-year period in older adults and showed slower decline in fitness tended to be associated with less brain atrophy (22).
The major limitation of cross-sectional studies is that they are ill-fitted to establish causal relationships. The relationship between CRF and brain volume can be bidirectional. First, higher CRF levels may lead to greater brain volumes in areas important to executive control function and memory. In animal models, exercise triggers neurogenesis in the brain by inducing growth factors cascades and stimulating synaptic plasticity (23). Second, brain atrophy may precede future CRF levels through declined cognitive function and worsened motor planning. Specifically, brain atrophy leads to cognitive decline, which is associated with mobility decline (24). Also, brain atrophy in middle frontal and inferior parietal lobes is critically involved with motor awareness and planning for an intended action (25). These two pathways may converge to influence subsequent motor activity. Understanding the longitudinal sequence of events may contribute to establish the mechanism of this relationship between fitness and brain volume changes.
This study examined the associations of midlife and late-life CRF with longitudinal changes in regional brain volume in late adulthood in a sample of 146 community-dwelling older adults from the Baltimore Longitudinal Study of Aging (BLSA) neuroimaging study. It was hypothesized that higher midlife CRF would be associated with greater volumes in prefrontal and medial temporal lobes and slower atrophy in these regions. It was also hypothesized that slower atrophy in frontal and parietal lobes would be associated with higher late-life CRF.
Methods
Study Population
This study includes 146 participants in the neuroimaging study of the BLSA (26). Participants agreed to receive an annual brain magnetic resonance imaging (MRI) starting from 1994 and were prospectively followed till 2003 (M baseline age = 69; 58% male). They also agreed to receive neuropsychological testing, a neurological exam, and interval medical history during the visits. To be included in the study, participants had to be in good general health with exclusionary criteria of mild cognitive impairment (MCI), dementia, stroke, Parkinson’s disease, epilepsy, other neurological conditions, severe cardiovascular disease, severe pulmonary disease, or metastatic cancer at study entry. All participants were reviewed at a consensus conference following death or, during life if their Blessed Information Memory Concentration score was 3 or above, their informant or subject Clinical Dementia Rating score was 0.5 or above or the dementia questionnaire was abnormal (27). Dementia was diagnosed by consensus diagnostic conferences using Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised criteria for dementia and the National Institute of Neurological and Communicative Disorders and Stroke—Alzheimer’s Disease and Related Disorders Association criteria for Alzheimer’s disease (28) using neuropsychological diagnostic tests and clinical data. The diagnosis of dementia required evidence of a progressive cognitive syndrome, including memory decline. Exclusion criteria were used to allow a representative sample of aging BLSA participants.
Based on standardized consensus diagnostic procedures for the BLSA (29), 11 of 146 were diagnosed with MCI over the course of the study and 8 out of those 11 went on to develop dementia. As this study focused on brain volume changes in normal aging, data at and after the age of onset of dementia were excluded from the analysis. Approximately half of the participants were hypertensive, and 85% were controlled by medication.
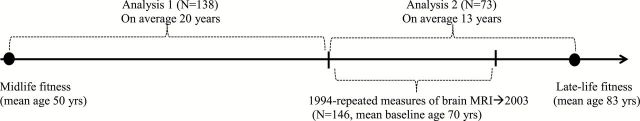
Of the 146, 138 participants had midlife CRF estimated by repeated measures over time. The time lag between midlife (ie, age 50) and baseline brain MRI (ie, mean age 69.6) was on average 20 years (ranged from 6 to 36 years). Of the 146, 73 participants had late-life CRF measured at one time point. The time lag between baseline brain MRI (ie, mean age 69.6) and late-life CRF (ie, mean age 83) was on average 13 years (ranged from 10 to 19 years) (Figure 1). Of the 73, 63 had CRF at baseline brain MRI (with a range of minus or plus three years apart from baseline MRI). Late-life CRF was measured after the last brain MRI. The research staff who processed brain MRI images were blinded to CRF status of the participants. Studies were approved by the local institutional review boards. All participants provided written informed consent before each assessment.
Figure 1.
Timeline for the analyses of cardiorespiratory fitness and longitudinal changes in brain volume from the Baltimore Longitudinal Study of Aging.
MRI Protocol and Markers
MRI scans were obtained on a General Electric Signa 1.5 tesla scanner (Milwaukee, WI) using a high-resolution volumetric spoiled-grass gradient recalled echo axial series. Spoiled gradient recalled echo images were acquired to obtain volumes of GM and WM. The acquisition parameters were as follows: echo time = 5ms, repetition time = 35ms, field of view = 24cm, flip angle = 45°, matrix = 256×256, number of excitations = 1, voxel dimensions = 0.94×0.94×1.5mm3.
MRI imaging was preprocessed using a previously validated method (30). In brief, images were corrected for head tilt and rotation, and reformatted parallel to the anterior–posterior commissure plane. Extracranial tissue was removed using a semiautomated procedure followed by manual editing. Then, images were segmented into GM, WM, and cerebrospinal fluid. Regional GM and WM were identified using a template-based deformation approach using an ICBM-coregistered standard MRI as the template and a hierarchical elastic matching algorithm for deformation and regions of interest determination (31). All images were normalized individually to the same template. Voxel-based analysis utilized the regional analysis of volumes examined in normalized space approach (32), whereby regional values of tissue density maps reflect the amount of respective tissue in the vicinity of a voxel. Intracranial volume (ICV) was determined using the template warping algorithm modified for head image registration.
Regional GM and WM volumes throughout the entire brain were examined in this study, including frontal gyrus (superior, middle, inferior, medial), parietal gyrus (superior, supramarginal, angular), temporal lobe (superior, middle, inferior, parahippocampal gyrus, entorhinal cortex, perirhinal cortex, hippocampus), occipital gyrus (superior, middle, inferior, and occipito-temporal), and frontal, parietal, temporal, and occipital WM.
Cardiorespiratory Fitness
CRF was measured by a maximal treadmill exercise test using a modified Balke protocol (33). Oxygen consumption was measured throughout the test with the constant speed of 3.0 mph for women and 3.5 mph for men and the increased grade by 3% every 2 minutes until voluntary exhaustion. Oxygen consumption was recorded every 30 seconds and the highest value was used as peak VO2 in mL/kg/min. Midlife CRF was estimated at age 50 by repeated measures of peak VO2 over time. Late-life CRF was measured on average 13 years after baseline brain MRI.
To account for the precision of midlife CRF prediction, the weights were then generated as the inverse of square of standard errors (ie, for ith subject the weight is w i = 1/σi 2) that can be used in all of the following regression analyses. Specifically, estimated midlife CRF values with higher precision were given greater weights in the regression analyses than those with lower precision. The weights depend on a number of factors including the number and frequency of repeat measurements, proximity of the actual measurement to age 50 and within-individual variability relative to between-individual variability.
To estimate midlife CRF, a linear mixed effects model was applied on the entire BLSA longitudinal sample (N = 2,053; a total of 5,345 measures from 1975 to 2013) with peak VO2 as the dependent variable to derive Best Linear Unbiased Estimates (BLUE) of midlife CRF. Fixed effects in the model included intercept, age, age2, and their interactions with sex and race. Random effects included intercept, age, and age2. This model is flexible and powerful in that it allows nonlinear CRF trajectories both at the individual and population levels. Furthermore, the model utilized all available peak VO2 data, including longitudinal assessments, to derive estimates of an individual’s peak VO2 at age 50. The predicted CRF at age 50 for each individual and the corresponding standard error were obtained at this stage.
Other Measures of Interest
Other measures of interest included race, education, midlife, and late-life body mass index (BMI) and midlife blood pressure. BMI was calculated as weight divided by the square of height in kg/m2 where height and weight were measured at each visit. Blood pressure was the average of two measures at each visit. Midlife BMI and blood pressure were estimated using the same approach of estimating midlife CRF.
Statistical Analysis
Univariate correlations between CRF and sample characteristics were examined using t-tests or Pearson correlation coefficient as appropriate. Cross-sectional associations between CRF and brain volume at baseline MRI were examined using partial correlation analysis controlling for ICV, baseline age, and sex (N = 63). Associations of longitudinal changes in brain volume with midlife and late-life CRF were examined using linear mixed effects model which accounted for the correlations for repeated measures (SAS v. 9.3; SAS Institute, Cary, NC). Regional brain volume was modeled as the dependent variable. Midlife or late-life CRF, interval (ie, the time period in years between baseline MRI and each follow up visit), interaction of CRF with interval, age, sex, their interaction with interval, and ICV were modeled as fixed effects. The intercept and interval were modeled as random effects.
In analysis 1, the full model to test the association of brain volume changes with midlife CRF: Brain volume = β 0 + β 1 × interval + β 2 × midlife peak VO2 + β 3 × (midlife peak VO2×interval) + β 4 × age + β 5 × sex + β 6 × ICV + β 7 × (interval × age) + β 8 × (interval × sex). In analysis 1, we did not adjust for time lag between midlife and baseline MRI because the adjustment for the time lag (age at baseline MRI minus 50) was the same as the adjustment for age at baseline MRI. Adjustment for both would introduce overadjustment. In analysis 2, the model was additionally adjusted for time lag between baseline MRI and time of late-life CRF. In order to further understand the temporal sequence between brain volume changes and late-life CRF, CRF at baseline MRI was further adjusted in a subsample (N = 63).
Education and BMI at midlife and late-life CRF were adjusted as covariates because of their known associations with CRF. In analysis 1, midlife blood pressure was also adjusted in the model, because midlife blood pressure was associated with hippocampal atrophy (34).
Additional sensitivity analyses were repeated in participants who had at least two MRI scans (N = 130 and 69 in two analyses, respectively), and were also repeated in participants who did not yet experience MCI or dementia over the course of the study (N= 129 in analysis 1. In analysis 2, all 73 participants were free of MCI or dementia over the course of the study. Because this was an exploratory analysis, results were reported as significant at p < .05.
Results
Table 1 describes sample characteristics in midlife and late-life, respectively. In midlife, men had higher CRF than women (p < .001), and Caucasian had higher CRF than African American (p < .001). Higher midlife CRF was correlated with lower midlife BMI (r = −.227, p < .05), but was not correlated with education (r = −.088, p > .05). Men had higher late-life CRF than women (p < .05). There were no racial differences in late-life CRF (p > .05). Higher CRF was correlated with lower BMI (r = −.363, p < .05), but was not correlated with education (r = .018, p > 0.05). Higher CRF was cross-sectionally associated with greater perirhinal cortex after adjustment for ICV (r = 0.288, p = .023). This association was not significant after further controlling for age and sex (r = 0.222, p = .088). Univariate associations between CRF and other regional volumes were not significant after adjustment for ICV (p > .05 for all).
Table 1.
Sample Characteristics
| Analysis 1 (N = 138) | Analysis 2 (N = 73) | |
|---|---|---|
| Age at baseline MRI, y | 69.6±7.9 | 66.3±6.0 |
| Female | 59 (41.8) | 35 (47.9) |
| Caucasian | 126 (89.4) | 61 (83.6) |
| Education, y | 17±7 | 17±2 |
| BMI, kg/m2* | 25.3±3.3 | 27.3±4.6 |
| Systolic blood pressure, mmHg* | 126±12 | 120±13 |
| Diastolic blood pressure* | 82±7 | 61±8 |
| Peak VO2, mL/kg/min* | 32.3±4.8 | 18.3±4.7 |
Notes: Values were mean ± SD or N (%) as noted.
*Measures were in midlife of the analysis 1 and late-life of the analysis 2, respectively.
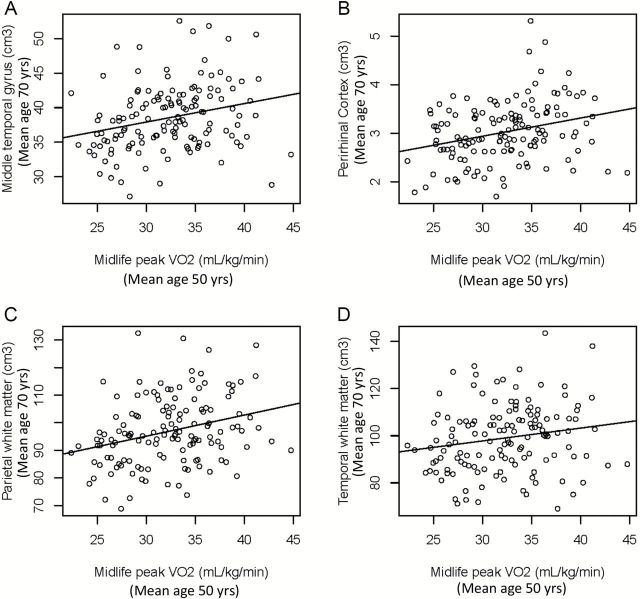
Higher midlife CRF was prospectively associated with greater middle temporal gyrus and perirhinal cortex and greater parietal and temporal WM at baseline MRI, independent of age at baseline MRI, sex, ICV, and effects of age and sex on the rate of decline in brain volume (Table 2; Figure 2). The associations between midlife CRF and the rate of decline in these regions were not significant. The associations of midlife CRF with other regional volumes at baseline MRI or the rate of decline were not significant (Table 2).
Table 2.
Associations Between Midlife Peak VO2 and Brain Volume Changes (N = 138)
| Peak VO2 | Peak VO2× Interval | |
|---|---|---|
| β (SE) | β (SE) | |
| Gray matter | ||
| Frontal gyrus | ||
| Superior | .029 (.049) | −.002 (.005) |
| Middle | .131 (.084) | .003 (.008) |
| Inferior | .049 (.057) | .007 (.008) |
| Medial | .064 (.054) | .005 (.006) |
| Parietal gyrus | ||
| Superior | .093 (.073) | −.001 (.007) |
| Supramarginal | .067 (.039) | −.006 (.004) |
| Angular | .064 (.055) | .004 (.005) |
| Temporal gyrus | ||
| Superior | .022 (.068) | .004 (.006) |
| Middle | .157 (.077)* | −.005 (.009) |
| Parahippocampal | .006 (.009) | .000 (.001) |
| Entorhinal cortex | −.007 (.010) | −.000 (.001) |
| Perirhinal cortex | .036 (.014)* | −.000 (.002) |
| Inferior | .038 (.049) | .004 (.005) |
| Hippocampus | .001 (.014) | −.001 (.001) |
| Occipital gyrus | .019 (.177) | .003 (.011) |
| White matter | ||
| Frontal | .666 (.341) | −.026 (.024) |
| Parietal | .397 (.187)* | −.025 (.015) |
| Temporal | .466 (.219)* | −.028 (.019) |
| Occipital | .134 (.126) | −.022 (.018) |
Notes: Models were adjusted for age at baseline MRI, sex, ICV, interval × age, and interval × sex.
*p < .05.
Figure 2.
Scatterplots of the associations between midlife cardiorespiratory fitness and middle temporal gyrus (a), perirhinal cortex (b), parietal white matter (c), and temporal white matter (d) on average 20 y later.
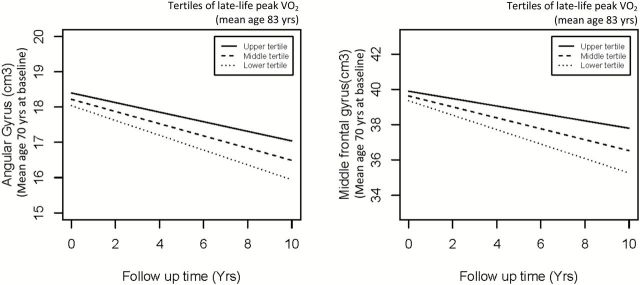
Greater inferior frontal gyrus and perirhinal cortex at baseline MRI were associated with higher late-life CRF, independent of covariates (Table 3, Model 1). The associations between the rate of decline in these regions and late-life CRF were not significant (Table 3, Model 1). Slower rates of decline in angular and superior temporal gyri were associated with higher late-life CRF, independent of covariates (Table 3, Model 1) (Figure 3). The associations of volumes in angular and superior temporal gyri at baseline MRI with late-life CRF were not significant (Table 3, Model 1). After further adjustment for CRF at baseline MRI, slower rate of decline in middle frontal angular gyri was significantly associated with higher late-life CRF (Table 3, Model 2). Because the associations of either midlife or late-life CRF with subregions of occipital gyrus were not significant, results were shown as occipital GM volume as a whole.
Table 3.
Associations Between Brain Volume Changes and Late-Life Peak VO2
| Model 1 (N = 73) | Model 2 (N = 63) | |||
|---|---|---|---|---|
| Peak VO2 | Peak VO2 × interval | Peak VO2 | Peak VO2 × interval | |
| β (SE) | β (SE) | β (SE) | β (SE) | |
| Gray matter | ||||
| Frontal gyrus | ||||
| Superior | −.023 (.064) | .003 (.006) | −.069 (.078) | .004 (.007) |
| Middle | .100 (.112) | .018 (.009) | .043 (.140) | .024 (.010)* |
| Inferior | .142 (.070)* | .009 (.010) | .035 (.085) | .020 (.011) |
| Medial | .046 (.058) | .008 (.006) | .001 (.069) | .011 (.008) |
| Parietal gyrus | ||||
| Superior | .041 (.092) | .005 (.008) | −.024 (.116) | .010 (.009) |
| Supramarginal | .082 (.053) | .001 (.005) | .112 (.069) | .001 (.005) |
| Angular | .046 (.075) | .011 (.005)* | .148 (.091) | .015 (.006)* |
| Temporal gyrus | ||||
| Superior | −.051 (.087) | .017 (.007)* | .112 (.069) | .001 (.005) |
| Middle | .131 (.102) | −.004 (.011) | .072 (.128) | .005 (.012) |
| Parahippocampal | .017 (.011) | .000 (.001) | .019 (.014) | −.000 (.001) |
| Entorhinal cortex | .008 (.014) | .001 (.001) | .006 (.020) | .002 (.001) |
| Perirhinal cortex | .042 (.017)* | .001 (.002) | .027 (.023) | .002 (.002) |
| Inferior | −.010 (.065) | .007 (.005) | .035 (.085) | .020 (.011) |
| Hippocampus | −.011 (.016) | .001 (.001) | −.024 (.021) | .001 (.001) |
| Occipital gyrus | .167 (.112) | .012 (.024) | .218 (.225) | .003 (.014) |
| White matter | ||||
| Frontal | .347 (.450) | .029 (.028) | .001 (.525) | .025 (.030) |
| Parietal | .174 (.247) | −.015 (.018) | .145 (.302) | −.024 (.019) |
| Temporal | .239 (.261) | −.004 (.020) | .144 (.319) | −.015 (.022) |
| Occipital | .254 (.154) | −.020 (.021) | .264 (.187) | −.040 (.023) |
Notes: Model 1: adjusted for age at baseline MRI, sex, ICV, interval × age, interval × sex, and time lag; Model 2: adjusted for covariates in model 1 and peak VO2 at baseline MRI.
*p < .05.
Figure 3.
Atrophy in angular gyrus and middle frontal gyrus in tertiles of late-life cardiorespiratory fitness level, after adjustment for covariates, and cardiorespiratory fitness level at baseline magnetic resonance imaging.
Adjustment for education, BMI, or blood pressure did not substantially change the results. In additional sensitivity analyses, results remained similar among participants with at least two MRI scans. In analysis 1, results remained similar in participants free of MCI or dementia over the course of the study (data not shown).
Discussion
This study investigated whether regional brain volume changes in late adulthood are related to midlife and late-life CRF. Findings suggest that higher midlife CRF may play an important role in preserving middle and medial temporal areas in late adulthood. Slower atrophy in middle frontal and angular gyri may predict higher CRF in late life.
The longitudinal data on brain volume allowed us to examine brain atrophy in relation to CRF. The time course from midlife to late life provided a unique view in understanding the spatial distribution of gray and white matter linked to midlife and late life CRF. With the application of weights, midlife CRF was precisely modeled in the linear mixed effects model. Participants with more CRF measures over time and with more visits close to age 50 contribute more in the model compared to those with fewer measures and fewer visits close to age 50.
Brain areas that showed strong associations with midlife CRF were localized in middle and medial temporal areas, which fell into vascular watershed areas. This is consistent with previous cross-sectional studies showing that higher CRF is associated with greater volumes in middle and medial temporal areas (4,5,7,8,15). Considerable animal studies have suggested that perirhinal cortex, one of the adjacent cortical regions surrounding hippocampus, provides direct and indirect projections to the hippocampus (35). The major cortical inputs from perirhinal cortex to entorhinal cortex and to the route where hippocampal formation can affect other cortical regions indicate the critical role of perirhinal cortex in the medial temporal lobe (36). Further, exercise elevates brain-derived neurotropic factor gene expression in perirhinal cortex in both adult and aged rat brains (37). We also found positive associations of midlife CRF with temporal and parietal WM. Previous observational study and small intervention study reported a similar pattern of the positive effect of CRF on WM tracts connecting frontal and parietal lobes (4,15). Importantly, we observed that these positive associations appeared maintain as the associations with rates of decline in these regions were not significant. Findings suggest building higher CRF early in life may be beneficial for brain health in late adulthood and this beneficial effect appear to be maintained over time.
In this study, older adults in their late sixties with slower atrophy in middle frontal and angular gyri showed higher late-life CRF. These regions also belonged to watershed areas. Because these associations were independent of the CRF level at baseline MRI, they may suggest that such prospective associations were not accounted by prior higher fitness level. A possible cause–effect relationship may be hypothesized. These findings need to be replicated in large samples to examine whether atrophy in middle frontal and angular gyri predicts motor activity.
Greater inferior frontal and perirhinal cortex at baseline MRI and slower atrophy in superior temporal gyrus were also associated with higher late-life CRF. However, these associations were attenuated after further adjustment for CRF at baseline MRI. Although inferior frontal and superior temporal gyri have been previously shown to be associated with CRF in cross-sectional studies (6,15), the attenuated associations in the present study suggest that the observed associations may be explained by prior higher CRF, which is associated with higher late-life fitness level.
We did not find an association of CRF with the hippocampus. The hippocampus was identified as an important subcortical region correlated with fitness from prior cross-sectional studies (but not all) and found to be responsive to physical exercise in one small intervention study (16). Cross-sectional studies targeting hippocampal volume in relation to fitness in cognitively healthy older adults have yielded mixed results. Two studies observed a significant association of fitness with hippocampal volume (5,7), while one study did not find a significant association with hippocampal volume (21). Previous studies focused on older adults in their mid-sixties with more women than men, while the sample in our study was slightly older who were in their late sixties with more men. The null findings in the present study may also be limited by the small sample size and also by the lack of contemporary data.
We should interpret the results of the prediction of longitudinal regional brain volume changes from midlife CRF with caution, because there are no brain volume measures in midlife. It is possible that participants who had higher midlife CRF would have greater brain volumes in midlife, which would lead to greater brain volume in late life. Future studies are needed to investigate whether prior CRF precedes brain atrophy independent of brain structure at the time of prior CRF. Second, although this study focused on brain atrophy in normal aging excluded data on and after the age of onset of dementia, the trajectories of brain structural changes in those who developed mild cognitive impairment and dementia may be different from those who did not yet experience cognitive impairment over the course of the study. However, it is not likely to be the case because results from the sensitivity analyses with those who did not experience MCI or dementia over the course of the study remained similar. Future studies should future examine the longitudinal associations between fitness and brain atrophy in large samples with cognitive impairment or dementia. Because this was an exploratory analysis, we chose to analyze the relationship of midlife and late-life CRF with brain atrophy in each region. This approach required testing multiple comparisons. With the application of correction for multiple comparisons, we would have missed most of the associations. Even with our current approach, findings indicated the presence of networks related to midlife and late-life CRF.
In conclusion, higher midlife CRF may play an important role in preserving GM volume localized in middle and medial temporal lobes as well as temporal and parietal WM in late adulthood. Slower atrophy in middle frontal and angular gyri may predict higher late-life CRF level. Future studies are needed to study whether midlife CRF precedes brain atrophy, independent of midlife brain volume.
Funding
This research was supported by the Intramural Research Program of the National Institute on Aging. C.D. was supported in part by National Institutes of Health grant R01AG149-71.
References
- 1. Kramer AF, Colcombe SJ, McAuley E, Scalf PE, Erickson KI. Fitness, aging and neurocognitive function. Neurobiol Aging. 2005;26(suppl 1):124–127. [DOI] [PubMed] [Google Scholar]
- 2. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. [DOI] [PubMed] [Google Scholar]
- 3. Etnier JL, Nowell PM, Landers DM, Sibley BA. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res Rev. 2006;52:119–130. [DOI] [PubMed] [Google Scholar]
- 4. Colcombe SJ, Erickson KI, Raz N, et al. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. [DOI] [PubMed] [Google Scholar]
- 5. Erickson KI, Prakash RS, Voss MW, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. :10.1002/hipo.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon BA, Rykhlevskaia EI, Brumback CR, et al. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825–838. :10.1111/j.1469-8986.2008.00676.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McAuley E, Szabo AN, Mailey EL, et al. Non-exercise estimated cardiorespiratory fitness: associations with brain structure, cognition, and memory complaints in older adults. Ment Health Phys Act. 2011;4:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinstein AM, Voss MW, Prakash RS, et al. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav Immun. 2012;26:811–819. :10.1016/j.bbi.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alosco ML, Brickman AM, Spitznagel MB, et al. Poorer physical fitness is associated with reduced structural brain integrity in heart failure. J Neurol Sci. 2013;328:51–57. :10.1016/j.jns.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sen A, Gider P, Cavalieri M, et al. Association of cardiorespiratory fitness and morphological brain changes in the elderly: results of the Austrian Stroke Prevention Study. Neurodegener Dis. 2012;10:135–137. :10.1159/000334760 [DOI] [PubMed] [Google Scholar]
- 11. Marks BL, Katz LM, Styner M, Smith JK. Aerobic fitness and obesity: relationship to cerebral white matter integrity in the brain of active and sedentary older adults. Br J Sports Med. 2011;45:1208–1215. :10.1136/bjsm.2009.068114 [DOI] [PubMed] [Google Scholar]
- 12. Marks BL, Madden DJ, Bucur B, et al. Role of aerobic fitness and aging on cerebral white matter integrity. Ann N Y Acad Sci. 2007;1097:171–174. [DOI] [PubMed] [Google Scholar]
- 13. Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59:1514–1523. 10.1016/j.neuroimage.2011.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tseng BY, Gundapuneedi T, Khan MA, et al. White matter integrity in physically fit older adults. Neuroimage. 2013;82:510–516. :10.1016/j.neuroimage.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. [DOI] [PubMed] [Google Scholar]
- 16. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–3022. :10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Voss MW, Heo S, Prakash RS, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp. 2012;34:2972–2985. doi:10.1002/hbm.22119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pantano P, Baron JC, Lebrun-Grandie P, Duquesnoy N, Bousser MG, Comar D. Regional cerebral blood flow and oxygen consumption in human aging. Stroke. 1984;15:635–641. [DOI] [PubMed] [Google Scholar]
- 19. Lu H, Xu F, Rodrigue KM, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21:1426–1434. :10.1093/cercor/bhq224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. :10.1212/01.wnl.0000317094.86209.cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Honea R, Thomas GP, Harsha A, et al. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:188–197. :10.1097/WAD.0b013e31819cb8a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vidoni ED, Honea RA, Billinger SA, Swerdlow RH, Burns JM. Cardiorespiratory fitness is associated with atrophy in Alzheimer’s and aging over 2 years. Neurobiol Aging. 2012;33:1624–1632. :10.1016/j.neurobiolaging.2011.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaliman P, Párrizas M, Lalanza JF, Camins A, Escorihuela RM, Pallàs M. Neurophysiological and epigenetic effects of physical exercise on the aging process. Ageing Res Rev. 2011;10:475–486. :10.1016/j.arr.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 24. Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014. :10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berti A, Bottini G, Gandola M, et al. Shared cortical anatomy for motor awareness and motor control. Science. 2005;309:488–491. [DOI] [PubMed] [Google Scholar]
- 26. Shock NW, Greulich RC, Andres R, et al. Normal human aging: the Baltimore Longitudinal Study of Aging. Washington, DC: US Government Printing Office; 1984. [Google Scholar]
- 27. Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O’Brien RJ. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol. 2008;64:168–176. :10.1002/ana.21413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 29. Driscoll I, Resnick SM, Troncoso JC, An Y, O’Brien R, Zonderman AB. Impact of Alzheimer’s pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60:688–695. [DOI] [PubMed] [Google Scholar]
- 30. Davatzikos C, Genc A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14:1361–1369. [DOI] [PubMed] [Google Scholar]
- 31. Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21:1421–1439. [DOI] [PubMed] [Google Scholar]
- 32. Goldszal AF, Davatzikos C, Pham DL, Yan MX, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22:827–837. [DOI] [PubMed] [Google Scholar]
- 33. Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. [DOI] [PubMed] [Google Scholar]
- 34. Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. [DOI] [PubMed] [Google Scholar]
- 35. Naber PA, Witter MP, Lopez da Silva FH. Perirhinal cortex input to the hippocampus in the rat: evidence for parallel pathways, both direct and indirect. A combined physiological and anatomical study. Eur J Neurosci. 1999;11:4119–4133. [DOI] [PubMed] [Google Scholar]
- 36. Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. [DOI] [PubMed] [Google Scholar]
- 37. Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. :10.1016/j.tics.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]