Abstract
Background:
The risk of lymph node positivity (LN+) in rectal cancer is a parameter that impacts therapeutic recommendations. We aimed to quantify the effect of younger age on LN+ in rectal cancer.
Methods:
Using the Surveillance, Epidemiology, and End Results (SEER) database, patients with rectal cancer diagnosed between 1988 and 2008 were identified. Patients were stage I-III, without preoperative radiotherapy, at least one lymph node examined, and a standard rectal cancer operation performed. The association of age and LN+ status was examined with logistic regression separately for each T stage, adjusting for multiple covariates. Poisson regression was used to evaluate age and number of positive lymph nodes (LNs). All statistical tests were two-sided.
Results:
Fifty-six thousand seventy-six patients were identified, including 1194 (2.1%) patients age 20 to 39 years at diagnosis and 4199 (7.5%) patients age 40 to 49 years (defined as young). For each T stage, LN+ was inversely associated with age (all P < .001). For T1, T2, and T3, age remained predictive of LN+ status after adjustment for number of LNs examined and other covariates (P < .001 for each stage). Adjusted odds ratios (ORs) for LN+ for age 20 to 39 vs 60 to 69 were: T1: 1.97 (95% confidence interval [CI] = 1.36 to 2.86); T2: 1.48 (95% CI = 1.13 to 1.95); T3: 1.30 (95% CI = 1.10 to 1.53). Young age was a statistically significant predictor of an increased number of LNs positive for stage T2 (P = .042) and T3 (P = .002).
Conclusion:
In this large national dataset, young age at diagnosis is associated with an increased risk of LN+. This finding merits further investigation and may ultimately impact treatment decision-making for young early-stage patients.
Rectal cancer is a disease with an estimated incidence of 40 340 cases in the United States in 2013 (1). This incidence is skewed toward the elderly, with the median age at diagnosis being 65 years (2). However, it has recently been reported that the incidence of rectal cancer in adults under age 40 years is increasing, with increasing rates under age 40 years (3). In that study, the annual percent change between 1984 and 2005 was 3.8 percent from 0.39 per 100 000 to 0.85 per 100 000, representing an approximate doubling of the incidence in this group (3).
A well-recognized clinical observation holds that the same surgical procedure on a younger patient will typically result in a larger number of lymph nodes to be examined pathologically. This was demonstrated in colorectal cancer specimens by Ostadi et al. (4), who showed that each additional year of patient age was associated with retrieval of 0.1 fewer nodes (4). A similar finding was seen in Sarli et al. (5). This trend has also been seen in breast cancer, where a greater number of total lymph nodes removed has been seen in younger breast cancer patients undergoing sentinel lymph node biopsy and axillary dissection (6,7).
In staging rectal cancer patients, the evaluation of the lymph nodes for metastatic disease is critical. Finding metastatic disease in the regional lymphatics can change the treatment recommendations for a patient (8). In our clinical experience, we noted LN+ being anecdotally higher in younger patients, particularly in those with earlier T stage. Known clinical factors that predict for LN+ include: higher T stage, higher-grade histology, and the number of lymph nodes examined (9,10). Increased number of lymph nodes examined has been associated with improved survival (11,12). Examination of 12 lymph nodes has been endorsed as the minimum required for accurate identification of early-stage colorectal cancers (13,14). However, the impact of age at diagnosis on LN+, independent of number of nodes examined, has not been reported. We examined a national database to investigate this association.
Methods
Patient Selection
Rectal and rectosigmoid cancer patient records were obtained from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Database. The SEER Program collects and publishes cancer incidence and survival data from 17 population-based cancer registries, covering more than 25% of the population in the United States. The SEER database for the years 1988 to 2008 was queried to identify eligible patients. Primary site labels “C19.9-Rectosigmoid junction” and “C20.9-Rectum, NOS” were used. Pathologically staged patients with nonmetastatic adenocarcinoma over the age of 19 years were included. Patients who received radiotherapy prior to surgery were excluded, to eliminate the effect of preoperative radiation on lymph node harvest and positivity. Only patients with at least one lymph node examined were included. All patients were required to have a standard rectal cancer operation, based on the SEER coded description of surgical procedure. Local excision or local destruction procedures were excluded because of the lack of expectation of obtaining lymph nodes with this type of procedure. Other excluded patients were those with surgery types characterized as “pull-through resection WITH sphincter preservation (eg, Turnbull’s and Swenson’s operations, Soave’s submucosal resection, Altemeier’s operation and Duhamel’s operation)”. All patients undergoing total colectomies were excluded because of the influence of this more extensive operation on lymph node retrieval. Patients with unknown T stage, surgery type, or number of lymph nodes positive were excluded.
Patient demographics (race, sex, age at diagnosis, year of diagnosis), tumor characteristics (histologic grade, extension of primary tumor invasion, number of lymph nodes examined and positive for metastatic disease), and type of surgery were included. T stage, categorized as T1, T2, T3, and T4, is based on the SEER extent of disease (EOD) extension codes for cases from 1988 to 2003, and the American Joint Committee on Cancer–derived T stage for cases from 2004 to 2008. These years were chosen because of the lack of specific staging information prior to 1988 and the most recent data available at the time of the initial analysis (2011). Type of surgery was categorized as upper rectal cancer surgery (eg, low anterior resection), lower rectal cancer surgery (eg, abdominoperineal resection), or en bloc resection/exenteration.
Outcome Measures and Statistical Analysis
The primary study outcome was LN+ status. Covariates included age, number of LNs examined (LNE), year of diagnosis, type of surgery, tumor grade, sex, and race. Age was included as a categorical variable using 10-year intervals, except for ages 20 to 39 years, because of the smaller number of cases. All analyses were stratified by T stage. Differences in patient characteristics by age were determined using Chi-square tests. Trends in the median number of LNE by age were estimated using quantile regression models. Trends in LN+ with age were evaluated with Cochran Armitage trend tests and further stratified by number of LNE (1–11, and 12+). In univariate logistic regression analyses with LN+ as the outcome, the number of LNE was most predictive of LN+ as a log-transformed variable, and therefore was utilized in such a way in multivariable analyses (MVAs). However, results were similar when included as a linear or categorical variable. Logistic regression MVAs were performed for each T stage, with LN+ status as the outcome and age (10–20 year intervals, as above), number of LNE (log transformed), year of diagnosis (in 3-year intervals), type of surgery (3 categories), grade (I, II, III, IV, or unknown), sex, and race (white, black, other) as covariates. We assessed interaction of age with each covariate by including an interaction term in the covariate-adjusted model. Results are reported as odds ratios with 95% confidence intervals using age 60 to 69 years as the reference category.
In a secondary analysis, we examined the relationship of age at diagnosis and number of positive LNs in those who were node positive. We assessed whether age was associated with the number of positive LNs, using Poisson regression. Results of the Poisson regressions are presented as rate ratios, again using age 60 to 69 years as the reference category. This provides an adjusted estimate of the ratio of the mean number of positive LNs in a specified age group relative to the age 60 to 69 years age group. In initial analysis examining the mean and variance of the number of positive LNs, we found evidence of overdispersion, which is common in count data. To adjust for this, we estimated confidence intervals with robust standard errors, which relaxes the assumption that the variance is equal to the mean. For covariate adjustment, we considered the same variables as in the logistic regression models. Because of small cell sizes, tumor grade was collapsed into three categories: I, II, and III/IV/unknown. We assessed interaction of age with each covariate with an interaction term in the covariate-adjusted model. We further explored this with graphical analysis of the number of LNE as a categorical variable that indicated that the age association differed in the groups with less than 12 LNE vs those with 12 or higher. In the covariate-adjusted multivariable model, we accounted for the interaction by including a categorical variable for age group–LNE group. We accounted for this interaction in the covariate-adjusted model by including a categorical variable for age-type of surgery, using age 60 to 69 years–upper resection as the reference group (the largest age-surgery subgroup). Analyses were done using SAS statistical analysis software, version 9.2. All statistical tests were two-sided, with a 5% type I error (15,16,17).
Results
Descriptive Statistics
We identified 56 076 patients who met eligibility criteria for our study. Overall, 20 845 patients, or 37.2% of the patient population, had at least one lymph node positive. Table 1 summarizes the patient, tumor, and surgical characteristics. Only 2.1% (n = 1194) of patients were under age 40 years, with 7.5% (n = 4199) between age 40 and 49 years and 17.8% between age 50 and 59 years. The greatest proportion was T3 (56.4%) and grade II (73.1%). The overwhelming majority underwent an operation classified as upper resection (77.4%), with 18.2% undergoing a lower resection and the remaining 4.4% receiving an en bloc resection or exenteration.
Table 1.
Patient and tumor characteristics by age at diagnosis*
| Characteristic | Age at diagnosis, y | ||||||
|---|---|---|---|---|---|---|---|
| 20–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | ||
| No. (%) | Percent (within age group) | ||||||
| Age at diagnosis, y | |||||||
| 20–39 | 1194 (2.1) | ||||||
| 40–49 | 4199 (7.5) | ||||||
| 50–59 | 9964 (17.8) | ||||||
| 60–69 | 14 480 (25.8) | ||||||
| 70–79 | 16 496 (29.4) | ||||||
| 80+ | 9743 (17.4) | ||||||
| Race | |||||||
| White | 47 270 (84.3) | 77.3 | 78.9 | 81.3 | 83.4 | 86.0 | 89.1 |
| Black | 3609 (6.4) | 8.9 | 8.8 | 8.1 | 6.8 | 5.7 | 4.2 |
| Other | 5197 (9.3) | 13.8 | 12.3 | 10.6 | 9.9 | 8.4 | 6.7 |
| Sex | |||||||
| Male | 31 397 (56.0) | 49.8 | 52.9 | 58.6 | 61.6 | 56.6 | 46.0 |
| Female | 24 679 (44.0) | 50.3 | 47.1 | 41.4 | 38.4 | 43.4 | 54.0 |
| T stage | |||||||
| T1 | 8694 (15.5) | 16.5 | 16.1 | 18.1 | 17.2 | 15.1 | 10.7 |
| T2 | 13 557 (24.2) | 21.1 | 22.5 | 23.7 | 24.6 | 25.0 | 23.7 |
| T3 | 31 615 (56.4) | 57.1 | 56.8 | 54.5 | 54.6 | 56.4 | 60.7 |
| T4 | 2210 (3.9) | 5.3 | 4.6 | 3.7 | 3.6 | 3.6 | 4.9 |
| Grade | |||||||
| I | 4581 (8.2) | 7.7 | 7.6 | 8.2 | 8.6 | 7.9 | 8.2 |
| II | 40 994 (73.1) | 68.7 | 72.1 | 73.1 | 72.8 | 73.2 | 74.3 |
| III | 7881 (14.1) | 17.8 | 15.9 | 14.1 | 13.5 | 13.9 | 13.9 |
| IV | 250 (0.4) | 0.8 | 0.6 | 0.4 | 0.4 | 0.5 | 0.4 |
| Unknown | 2370 (4.2) | 4.9 | 3.9 | 4.2 | 4.7 | 4.5 | 3.2 |
| Surgery type | |||||||
| APR | 10 221 (18.2) | 15.4 | 15.2 | 15.8 | 18.9 | 20.1 | 18.1 |
| EBR/exenter | 2468 (4.4) | 10.4 | 6.0 | 4.3 | 4.1 | 4.0 | 4.2 |
| Upper resection | 43 387 (77.4) | 74.2 | 78.8 | 79.9 | 77.0 | 75.9 | 77.7 |
* Characteristics differ by age group, Chi-square tests; all P < .0001. APR = abdominal perineal resection; EBR = en bloc resection.
The median number of LNE increased by T stage (median LNE = 7, 9, 11, and 12 for T1, T2, T3, and T4 tumors, respectively). Within each T stage, the median LNE decreased with increasing age (Table 2). For LN+, the proportion of patients with at least one lymph node positive was 11.4%, 24.0%, 48.8%, and 52.9% for T1, T2, T3, and T4 tumors, respectively. Within each T stage, LN+ rates decreased with increasing age (within T stage, P trend < .001). The youngest patients had the highest LN+ rates within each T stage (Table 3). In univariate analyses, the LN+ rates for age 20 to 39 years were statistically significantly higher than patients age 60 to 69 years (reference group) for stage T1, T2, and T3 (Table 4).
Table 2.
Number of LN examined by age and stage
| Age Group, y |
T stage T1 | T stage T2 | T stage T3 | T stage T4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median LNE | 25th, 75th percentile | N | Median LNE | 25th, 75th percentile | N | Median LNE | 25th, 75th percentile | N | Median LNE | 25th, 75th percentile | |
| All | 8694 | 7 | 4, 13 | 13 557 | 9 | 5, 14 | 31 615 | 11 | 7, 17 | 2210 | 12 | 7, 18 |
| 20–39 | 197 | 12 | 8, 21 | 252 | 13 | 8, 21 | 682 | 16 | 10, 23 | 63 | 16 | 11, 28 |
| 40–49 | 677 | 9 | 5, 15 | 943 | 11 | 7, 17 | 2385 | 13 | 9, 19 | 194 | 15 | 10, 21 |
| 50–59 | 1801 | 8 | 5, 14 | 2363 | 10 | 6, 16 | 5427 | 12 | 8, 18 | 373 | 14 | 9, 20 |
| 60–69 | 2490 | 7 | 4, 12 | 3563 | 9 | 5, 14 | 7909 | 11 | 6, 16 | 518 | 11 | 6, 17 |
| 70–79 | 2484 | 6 | 3, 12 | 4126 | 8 | 5, 13 | 9300 | 10 | 6, 15 | 586 | 10 | 6, 16 |
| 80+ | 1045 | 7 | 4, 11 | 2310 | 8 | 5, 14 | 5912 | 10 | 6, 15 | 476 | 11 | 6, 17 |
| Qreg P* | <.0001 | <.0001 | <.0001 | <.0001 | ||||||||
* Qreg = Trend estimated using quantile regression model, within each stage. LNE = no. lymph nodes examined.
Table 3.
Lymph node positivity and age within T stage groups
| Age Group, y |
T stage T1 | T stage T2 | T stage T3 | T stage T4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | LN-positive | N | LN-positive | N | LN-positive | N | LN-positive | |||||
| No. (%) | No. (%) | No. (%) | No. (%) | |||||||||
| All | 8694 | 989 (11.4) | 13 557 | 3251 (24.0) | 31 615 | 15 437 (48.8) | 2210 | 1168 (52.9) | ||||
| 20–39 | 197 | 44 (22.3) | 252 | 93 (36.9) | 682 | 414 (60.7) | 63 | 38 (60.3) | ||||
| 40–49 | 677 | 114 (16.8) | 943 | 293 (31.1) | 2385 | 1381 (57.9) | 194 | 113 (58.2) | ||||
| 50–59 | 1801 | 238 (13.2) | 2363 | 653 (27.6) | 5427 | 2908 (53.6) | 373 | 213 (57.1) | ||||
| 60–69 | 2490 | 268 (10.8) | 3563 | 896 (25.1) | 7909 | 3904 (49.4) | 518 | 276 (53.3) | ||||
| 70–79 | 2484 | 228 (9.2) | 4126 | 868 (21.0) | 9300 | 4290 (46.1) | 586 | 299 (51.0) | ||||
| 80+ | 1045 | 97 (9.3) | 2310 | 448 (19.4) | 5912 | 2540 (43.0) | 476 | 229 (48.1) | ||||
| P* | <.0001 | <.0001 | <.0001 | .0008 | ||||||||
* P trend from Cochran Armitage trend test for lymph node positivity and age within T stage. LN = lymph node.
Table 4. Association of age and LN positivity
| OR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| T stage T1 | T stage T2 | T stage T3 | T stage T4 | |||||
| Age at diagnosis, y | Unadjusted | Adjusted for covariates* | Unadjusted | Adjusted for covariates* | Unadjusted | Adjusted for covariates* | Unadjusted | Adjusted for covariates* |
| 20–39 vs 60–69 | 2.39 (1.67 to 3.41) | 1.97 (1.36 to 2.86) | 1.74 (1.34 to 2.28) | 1.48 (1.13 to 1.95) | 1.59 (1.35 to 1.86) | 1.30 (1.10 to 1.53) | 1.33 (0.78 to 2.27) | 1.12 (0.65 to 1.95) |
| 40–49 vs 60–69 | 1.68 (1.32 to 2.13) | 1.55 (1.21 to 1.97) | 1.34 (1.15 to 1.57) | 1.22 (1.04 to 1.44) | 1.41 (1.29 to 1.55) | 1.26 (1.15 to 1.39) | 1.22 (0.88 to 1.71) | 1.14 (0.80 to 1.60) |
| 50–59 vs 60–69 | 1.26 (1.05 to 1.52) | 1.19 (0.98 to 1.44) | 1.14 (1.01 to 1.28) | 1.09 (0.96 to 1.22) | 1.18 (1.11 to 1.27) | 1.13 (1.05 to 1.21) | 1.17 (0.89 to 1.53) | 1.11 (0.84 to 1.46) |
| 60–69 (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 70–79 vs 60–69 | 0.84 (0.70 to 1.01) | 0.85 (0.71 to 1.03) | 0.79 (0.71 to 0.88) | 0.79 (0.71 to 0.88) | 0.88 (0.83 to 0.93) | 0.90 (0.84 to 0.95) | 0.91 (0.72 to 1.16) | 0.92 (0.72 to 1.18) |
| 80+ vs 60–69 | 0.85 (0.66 to 1.08) | 0.85 (0.66 to 1.09) | 0.72 (0.63 to 0.81) | 0.73 (0.64 to 0.83) | 0.77 (0.72 to 0.83) | 0.80 (0.75 to 0.86) | 0.81 (0.63 to 1.04) | 0.84 (0.64 to 1.09) |
| P* | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | .0429 | .3591 |
* Chi-square P values for age group variable in each logistic regression model. All statistical tests were two-sided. CI = confidence interval; Ref = referent; OR = odds ratio.
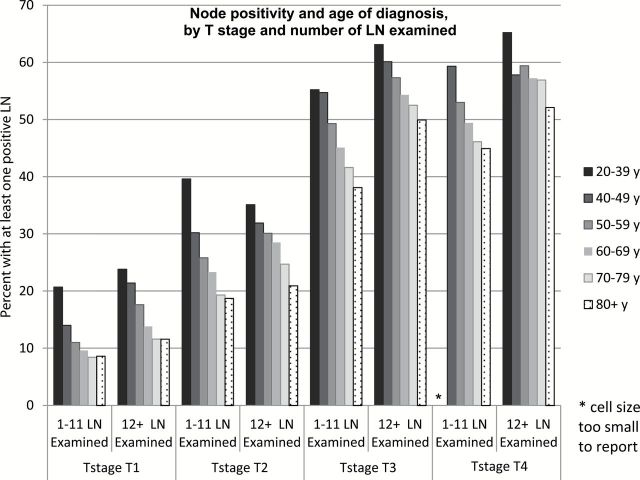
Figure 1 presents the LN+ rate by age within stage, further stratified by number of LNE (<12 and 12+ LNE). Within T stage and LNE group, the inverse association between age and LN+ remained statistically significant (P < .001), with the exception of T4, LNE 12+ (P = .093).
Figure 1.
Node positivity and age of diagnosis, by T stage and number of lymph nodes examined. LN = lymph node.
Multivariable Analysis of Lymph Node Positivity
We used multivariable logistic regression to examine whether the association between age at diagnosis and LN+ was independent of other known risk factors. The adjusted model included number of LNE (log transformed), year of diagnosis, surgery type, grade, sex, and race. With these covariates, age remained a statistically significant predictor of LN+ for stages T1, T2, and T3 (table 4). Patients younger than age 40 years when diagnosed were more likely to show LN+ compared with the reference age 60 to 69 years, with adjusted odds ratios (ORs) for age 20 to 39 years vs age 60 to 69 years of: T1, (odds ratio [OR] = 1.97, 95% confidence interval [CI] = 1.36 to 2.86), T2 (OR = 1.48, 95% CI = 1.13 to 1.95), and T3 (OR = 1.30, 95% CI = 1.10 to 1.53). In the multivariable models (one for each T stage), covariates including number of LNE, histologic grade, and race were statistically significant predictors of LN+ in all four models (data not shown).
We further examined the impact of age by looking at the number of positive LNs in node-positive patients. Table 5 shows the mean number of positive LNs by age group within T stage. For each T stage, the average number of positive LNs was highest in the youngest age group, and statistically significantly higher than the reference age 60 to 69 years group (unadjusted for covariates). In multivariable analyses, adjusting for number of LNE (log transformed) and other covariates, the mean number of positive LNs differed by age for T2 (P = .042) and T3 (P = .002). The adjusted mean number of positive LNs for age 20 to 39 years was 13% higher compared with the 60 to 69 years age group.
Table 5.
Association of age and number of positive LNs in node-positive patients*
| Age at diagnosis, y | No. | No. Pos LNs | Estimates from Poisson regression models† | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | RR‡ (95% CI) | P§ | ||||
| T1: stratification by number of LNE | |||||||
| <12 LNs examined (n = 605) | .98 | ||||||
| 20–39 | 19 | 1.58 | 1.02 | 0.95 (0.71 to 1.25) | |||
| 40–49 | 59 | 1.75 | 1.36 | 1.06 (0.87 to 1.29) | |||
| 50–59 | 13 | 1.68 | 1.27 | 1.00 (0.86 to 1.17) | |||
| 60–69 | 173 | 1.69 | 1.18 | 1 (Ref) | |||
| 70–79 | 156 | 1.66 | 1.04 | 1.01 (0.88 to 1.16) | |||
| 80+ | 68 | 1.62 | 0.90 | 0.97 (0.83 to 1.14) | |||
| 12+ LNs examined (n = 384) | .026 | ||||||
| 20–39 | 25 | 3.96 | 4.05 | 1.40 (0.87 to 2.25) | |||
| 40–49 | 55 | 2.31 | 1.96 | 0.82 (0.58 to 1.18) | |||
| 50–59 | 108 | 2.70 | 2.95 | 1.00 (0.72 to 1.39) | |||
| 60–69 | 95 | 2.83 | 3.85 | 1 (Ref) | |||
| 70–79 | 72 | 1.85 | 1.62 | 0.65 (0.46 to 0.94) | |||
| 80+ | 29 | 2.86 | 3.53 | 1.00 (0.59 to 1.71) | |||
| T2: no stratification (n = 3257) | .042 | ||||||
| 20–39 | 93 | 3.44 | 2.91 | 1.13 (0.95 to 1.34) | |||
| 40–49 | 293 | 3.11 | 2.96 | 1.09 (0.97 to 1.23) | |||
| 50–59 | 653 | 2.72 | 2.64 | 1.01 (0.92 to 1.10) | |||
| 60–69 | 896 | 2.57 | 2.41 | 1 (Ref) | |||
| 70–79 | 868 | 2.44 | 2.35 | 0.94 (0.87 to 1.03) | |||
| 80+ | 448 | 2.26 | 2.50 | 0.89 (0.79 to 1.00) | |||
| T3: stratification by type of surgery | |||||||
| APR surgery (n = 3102) | .079 | ||||||
| 20–39 | 57 | 7.98 | 9.28 | 1.41 (1.11 to 1.80) | |||
| 40–49 | 223 | 5.04 | 5.40 | 1.02 (0.89 to 1.16) | |||
| 50–59 | 522 | 5.10 | 4.93 | 1.12 (1.01 to 1.23) | |||
| 60–69 | 830 | 4.23 | 4.33 | 1 (Ref) | |||
| 70–79 | 964 | 4.44 | 5.11 | 1.08 (0.98 to 1.18) | |||
| 80+ | 506 | 4.31 | 4.47 | 1.03 (0.93 to 1.15) | |||
| EBR/exenter surgery (n = 625) | .76 | ||||||
| 20–39 | 46 | 5.46 | 4.68 | 0.94 (0.64 to 1.38) | |||
| 40–49 | 79 | 4.52 | 5.33 | 0.91 (0.65 to 1.27 | |||
| 50–59 | 105 | 4.31 | 5.58 | 0.94 (0.67 to 1.31) | |||
| 60–69 | 135 | 4.34 | 8.11 | 1 (Ref) | |||
| 70–79 | 168 | 3.63 | 3.24 | 0.92 (0.72 to 1.18) | |||
| 80+ | 92 | 4.36 | 4.84 | 1.12 (0.83 to 1.51) | |||
| Upper resection surgery (n = 11 710) | <.0001 | ||||||
| 20–39 | 311 | 5.11 | 4.44 | 1.00 (0.91 to 1.11) | |||
| 40–49 | 1079 | 4.87 | 4.83 | 1.11 (1.04 to 1.18) | |||
| 50–59 | 2281 | 4.28 | 4.36 | 1.01 (0.96 to 1.06) | |||
| 60–69 | 2939 | 4.08 | 4.21 | 1 (Ref) | |||
| 70–79 | 3158 | 3.86 | 4.05 | 0.98 (0.93 to 1.03) | |||
| 80+ | 1942 | 3.56 | 3.73 | 0.92 (0.87 to 0.97) | |||
| T4: no stratification (n = 1168) | .23 | ||||||
| 20–39 | 38 | 7.05 | 5.69 | 1.25 (0.97 to 1.61) | |||
| 40–49 | 113 | 4.65 | 4.38 | 0.98 (0.80 to 1.21) | |||
| 50–59 | 213 | 4.76 | 5.04 | 0.99 (0.83 to 1.17) | |||
| 60–69 | 276 | 4.33 | 4.48 | 1 (Ref) | |||
| 70–79 | 299 | 4.47 | 4.39 | 1.03 (0.89 to 1.20) | |||
| 80+ | 229 | 4.76 | 4.25 | 1.15 (0.99 to 1.34) | |||
* Statistically significant interactions with age required further stratification by number of lymph nodes examined (T1 only) and by type of surgery (T3 only). CI = confidence interval; LNE = No. lymph nodes examined; Ref = referent; RR = rate ratio.
† Covariates include log (number of lymph nodes examined), year of diagnosis (categorical variable in 3-year intervals), grade (I, II, III/IV/unknown), sex, race (3 categories: white, black, other). Surgery (APR, EBR/exent, upper resection) included for stages T1, T2, and T4.
‡ Rate ratio compares mean number of positive LNs for each age group relative to the reference group (age 60–65 years) after adjusting for covariates.
§ P value corresponds to the Score statistic Chi-square test for the age variable in the multivariable model after adjusting for other covariates. All statistical tests were two-sided.
We assessed interaction of age with each covariate with an interaction term in the covariate-adjusted model. For T2 and T4, none of the age interactions were statistically significant. For T1, there was a statistically significant interaction between age and number of LNE. We further explored this with graphical analysis of number of LNE as a categorical variable that indicated that the age association differed in the groups with less than 12 LNE compared with those with 12 or higher. For T3, there was a statistically significant interaction between age and type of surgery. The detailed analyses can be found in Supplementary Tables 1 and 2 (available online).
Discussion
Multiple factors are well known to influence the risk of LN+ in rectal cancer patients. These include T stage, histologic grade, and number of lymph nodes examined. However, we are unaware of any previous studies examining LN+ as a function of age in rectal cancer patients. We undertook this study to investigate this question.
Our study showed that younger patients have an increased risk of lymph node metastasis when examined within T stage cohorts. This finding persists on multivariable analyses, including potential covariates such as those listed above. As the analysis adjusts for an increased number of lymph nodes examined, this is not simply a function of younger patients with more easily identifiable nodal tissue. While lymph nodes would not be visible through typical methods of screening for CRC, it is possible that screening would enrich the older population for earlier-stage patients. However, as our data were all compared with patients of the same T stage, this does not explain our results. This finding lends more support to the conclusion that rectal cancer in younger patients may have an increased predisposition for nodal metastasis.
One possible explanation for our results is that there is a biological difference in the rectal cancers of younger patients that is either because of genetic differences in the host or the tumor. One study has found that younger patients have more adenocarcinomas with mucinous features and signet ring histologies (18). Poorly differentiated histology has also been found in greater frequency in younger patients. There are also data suggesting that patients with CRC under age 50 years have an increasing mortality over time, whereas those over age 50 years are exhibiting a decrease in mortality (19). A series identifying 75 CRC patients under the age of 40 years with tumors predominantly in the rectum or sigmoid colon compared these to a control group of older patients (20). Tumors from the younger patients showed more adverse histological features such as signet ring differentiation, perineural invasion, and venous invasion. They also found increased presentation with or development of metastatic disease in younger patients (45% vs 25%). Local recurrence was more common among younger patients as well (15% vs 0%). These data suggest that CRC in younger patients may behave biologically more aggressively than in older patients and fit with our findings.
In order to be most clinically useful, it would be important to translate these findings to the preoperative setting, where management recommendations for a patient with a T1-2 rectal cancer would vary depending on lymph node status; neoadjuvant chemoradiation would only be recommended for patients with nodal involvement. If patients with LN+ undergo immediate radical surgery without neoadjuvant chemoradiotherapy, this would be expected to lead to increased local recurrence and toxicity rates, as compared with preoperative therapy (13). Clinical staging, typically done with endorectal ultrasound or MRI, is not available on the patients in our analyzed dataset, thereby limiting the direct application of these data to clinical practice. Therefore, this study should not be interpreted as justification for a change in management of younger patients. However, it may be prudent to ensure that a thorough clinical investigation of lymph node–bearing regions is performed prior to surgical resection for younger patients. Additionally, these data may affect the preoperative workup for younger patients with early-stage disease being considered for local excision, as this procedure does not include lymph node dissection. A recent study has shown increasing use of local excision for higher-risk rectal cancers that do not meet guidelines for this more limited surgery (14). The current study suggests that these patients may be at an increased risk of harboring occult metastatic disease in the regional lymph nodes.
There are limitations to our study that must be considered. The SEER database is a valuable resource that contains large numbers of patient files, but the possibility exists that some of these files have been miscoded. Nevertheless, this miscoding would be expected to be random and not introduce any systematic bias. The surgery that these patients received varied depending on the location of the tumor and other clinical factors, in addition to the experience and judgment of each patient’s surgeon. These were accounted for in the multivariable analysis, but it is possible that the clinical approach to a young patient may be more aggressive than one who is older, introducing systematic bias. Finally, the staging in these patients is pathologic and should be relatively standardized. However, it is possible that some specimens were misclassified.
In conclusion, our study demonstrates increased rates of LN+ in younger patients with rectal cancer, after accounting for other known predictive factors. This persists in stages T1-3, and young patients who have LN+ have higher lymph node ratios. These findings warrant further investigation and could impact the aggressiveness of nodal staging in younger patients with rectal cancer.
Funding
This publication was supported by grant number P30 CA006927 from the National Cancer Institute, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Supplementary Material
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J Clin. 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015.
- 3. Meyer JE, Narung T, Schnoll-Sussman FH, et al. Increasing Incidence of Rectal Cancer in Patients Aged Younger Than 40 years. Cancer. 2010;116:4354–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ostadi MA, Harnish JL, Stegienko S, et al. Factors affecting the number of lymph nodes retrieved in colorectal cancer specimens. Surg Endosc. 2007;21 (12):2142–2146. [DOI] [PubMed] [Google Scholar]
- 5. Sarli L, Bader G, Iusco D, et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. 2005;41 (2):272–279. [DOI] [PubMed] [Google Scholar]
- 6. Port ER, Patil S, Stempel M, et al. Number of lymph nodes removed in sentinel lymph node-negative breast cancer patients is significantly related to patient age and tumor size: a new source of bias in morbidity assessment? Cancer. 2010;116 (8):1987–1991. [DOI] [PubMed] [Google Scholar]
- 7. Schaapveld M, de Vries EG, van der Graaf WT, et al. The prognostic effect of the number of histologically examined axillary lymph nodes in breast cancer: stage migration or age association? Ann Surg Oncol. 2006;13 (4):465–474. [DOI] [PubMed] [Google Scholar]
- 8. National Comprehensive Cancer Network (NCCN). NCCN Clinical Pratice Guidelines in Oncology. Rectal Cancer Version 2.2015, 12/09/14; National Comprehensive Cancer Network. [DOI] [PMC free article] [PubMed]
- 9. Glasgow SC, Bleier JIS, Burgart LJ, et al. Meta-analysis of histopathological features of primary colorectal cancers that predict lymph node metastases. J Gastrointest Surg. 2012;16:1019–1028. [DOI] [PubMed] [Google Scholar]
- 10. Baxter NN, Ricciardi R, Simunovic M, et al. An evaluation of the relationship between lymph node number and staging in pT3 colon cancer using population-based data. Dis Colon Rectum. 2010;53 (1):65–70. [DOI] [PubMed] [Google Scholar]
- 11. Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19 (1):157–163. [DOI] [PubMed] [Google Scholar]
- 12. Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary analysis of intergroup trial INT-0089. J Clin Oncol. 2003;21 (15):2912–2919. [DOI] [PubMed] [Google Scholar]
- 13. Sauer R, Becker H, Hohenberger W, et al. Preopreative versus Postoperative Chemoradiotherapy for Rectal Cancer. N Engl J Med. 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 14. Stitzenberg KB, Sanoff HK, Penn DC, et al. Practice Patterns and Long-Term Survival for Early Stage Rectal Cancer. J Clin Oncol. 2013;31 (34):4276–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agresti A. Categorical Data Analysis. Hoboken NJ: John Wiley & Sons; 2013. [Google Scholar]
- 16. Cameron AC, Trivedi PK. Microeconometrics Using Stata. College Station, TX: Stata Press; 2009. [Google Scholar]
- 17. SAS Data Analysis Examples, Poisson Regression. UCLA: Statistical Consulting Group; http://www.ats.ucla.edu/stat/ dae/poissonreg. [Google Scholar]
- 18. You YN, Xing Y, Feig BW, et al. Young-Onset Colorectal Cancer: Is It Time to Pay Attention? Arch Int Med. 2012;172:287–289. [DOI] [PubMed] [Google Scholar]
- 19. Ahnen DJ, Wade SW, Jones WF, et al. The Increasing Incidence of Young-Onset Colorectal Cancer: A Call to Action. Mayo Clin Proc. 2014;89:216–224. [DOI] [PubMed] [Google Scholar]
- 20. Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25:1128–1139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.