Abstract
Influenza B viruses split into 2 distinct lineages in the early 1980s, commonly named the Victoria and Yamagata lineages. There are few data on the comparative epidemiology of Victoria- and Yamagata-lineage viruses. In 2007–2011, we enrolled 75 and 34 households containing index patients with acute respiratory illness who tested positive for Yamagata- and Victoria-lineage viruses, respectively, from outpatient clinics in Hong Kong, China. These index patients and their household contacts were followed up for 7–10 days. We examined overall risk of polymerase chain reaction–confirmed infection among household contacts and the risk of secondary infection within households using an individual-based hazard model that accounted for tertiary transmission and infections occurring outside the household. We found that for Victoria-lineage viruses, the risk of within-household infection among household contacts aged ≤15 years was significantly higher (risk ratio = 12.9, 95% credibility interval: 4.2, 43.6) than that for older household contacts, while for Yamagata-lineage viruses, the risk of within-household infection for household contacts did not differ by age. Influenza B Yamagata- and Victoria-lineage viruses have similar characteristics in terms of viral shedding and clinical illness. The mechanisms underlying these epidemiologic differences deserve further investigation.
Keywords: disease transmission, influenza, influenza B virus, Victoria lineage, Yamagata lineage
Influenzavirus is a major respiratory pathogen which causes epidemics worldwide every year. Currently, human influenza A(H1N1), influenza A(H3N2), and influenza B viruses are the most common variants circulating in humans globally. Influenza B viruses isolated since the early 1980s can be classified into 2 lineages, Victoria (B/Victoria/2/87-like) and Yamagata (B/Yamagata/16/88-like), which are distinct antigenically and genetically (1). Yamagata-lineage viruses became the dominant influenza B viruses in the 1990s (2, 3). Between 1991 and 2000, Victoria-lineage viruses were identified only in eastern Asia (2, 3). However, Victoria-lineage viruses reemerged outside of eastern Asia around 2001–2002 (4), and since then they have cocirculated with Yamagata-lineage viruses globally (5).
Influenza B epidemics are associated with considerable disease burden, including morbidity, hospitalization, and mortality, in persons of all ages, but particularly at the extremes of age, while infections are thought to be most common in school-age children (6–8). Two hospital-based studies of influenza-like illness and acute respiratory infections found that patients infected with Victoria-lineage viruses were significantly younger than those infected with Yamagata-lineage viruses (9, 10). In a recent study, Vijaykrishna et al. (11) reported age-specific differences in the patterns of Victoria and Yamagata infections based on surveillance data, and they estimated that the effective reproductive number was higher for the Victoria-lineage viruses than for the Yamagata-lineage viruses. However, few studies have compared the epidemiologic characteristics of influenza B Victoria- and Yamagata-lineage viruses at the individual level, including potential differences in their transmission dynamics in the community.
We conducted a household-based study of influenza transmission in 2007–2011 in Hong Kong, a subtropical city in southern China. The study was initially designed to study the effectiveness of nonpharmaceutical interventions in preventing influenza transmission in households from February 2007 through June 2009 (12–14), and it evolved into an observational study of influenza transmission dynamics in households from July 2009 through September 2011 (15, 16). In the present study, we focused on the households of index patients with confirmed cases of influenza B virus infection and compared the epidemiology of Victoria- and Yamagata-lineage viruses in terms of virus shedding, clinical illness, and transmissibility within households.
METHODS
Subjects
We used a case-ascertained study design (17) to study influenza transmission in Hong Kong households. We recruited participants from 2007 through 2011. We enrolled patients who reported at least 2 symptoms of acute respiratory illness (ARI)—temperature ≥37.8°C, cough, headache, sore throat, or myalgia—with symptom onset within 48 hours and who lived with at least 2 other individuals, none of whom had reported ARI symptoms during the previous 14 days. Study participants were recruited from a network of public and private outpatient clinics in Hong Kong. Enrolled patients who tested positive for influenza A or B on a rapid test (QuickVue Influenza A+B test; Quidel Corporation, San Diego, California) were termed “index cases” and were followed up, along with their household members, for 7–10 days.
In 2007, 2008, and the first half of 2009 (January through June), we randomly allocated households to 3 groups to estimate the effectiveness of surgical face masks and hand hygiene in preventing influenza transmission within households (12, 13). In the summer of 2009, we provided all households with a simplified hand hygiene intervention and observed the transmission dynamics of the novel H1N1pdm09 virus in comparison with cocirculating seasonal influenza viruses (16). In 2010–2011, households were not provided with any intervention and were followed up to observe transmission.
Participating households were visited within 36 hours of index case recruitment, and 2 or 3 additional visits were scheduled at 3-day intervals. A digital tympanic thermometer was provided, and all household members were requested to record their body temperature and the presence or absence of systemic and respiratory signs and symptoms in symptom diaries once daily until the final home visit. During each home visit, pooled nasal and throat swabs were collected from all household members, regardless of illness.
Ethics
Written consent was obtained from all participants who were ≥18 years of age, and proxy written consent for participants younger than 18 years of age was obtained from their parents or legal guardians. Additional written assent was obtained from participants aged 7–17 years. The study protocol was approved by the Institutional Review Board of the University of Hong Kong.
Laboratory methods
In 2007, all nasal and throat swabs collected were cultured on Madin-Darby canine kidney cells to detect influenza A and B viruses. A subset of specimens were also tested by reverse transcription polymerase chain reaction (RT-PCR) for influenza A and B viruses, including specimens from index patients and symptomatic household contacts who were negative by virus culture (13). From 2008 onwards, all nasal and throat swabs collected were tested by quantitative RT-PCR assay to detect the presence of influenza A or B virus and determine molecular viral loads, as described previously (12). We defined laboratory-confirmed infection with influenza B Victoria- or Yamagata-lineage viruses as a positive result by viral culture or RT-PCR or upon testing of one or more nasal or throat swabs collected during recruitment or follow-up. Yamagata and Victoria lineages were differentiated using real-time polymerase chain reaction (18, 19).
Statistical analysis
We calculated standardized daily scores for 3 groups of signs and symptoms—systemic signs and symptoms (temperature ≥37.8°C, headache, and myalgia), upper respiratory symptoms (sore throat and runny nose), and lower respiratory symptoms (cough and phlegm)—by adding up the total number of signs and symptoms that were present and dividing by the highest possible score (3, 2, and 2, respectively). We plotted average symptom scores and the geometric mean viral load upon RT-PCR assay for index cases according to time since the onset of ARI, which was defined as the first day when the participant reported at least 2 of the 7 signs or symptoms listed above. We defined time to alleviation of total symptoms as the time from symptom onset to the first day on which the total symptom score was 0. The time at which viral shedding ceased was defined as occurring within the interval between the last positive laboratory result and the first negative laboratory result. We compared the times to alleviation of any symptoms using Kaplan-Meier estimates. The times from symptom onset to cessation of viral shedding were compared using nonparametric estimates.
We defined the overall risk of laboratory-confirmed infection among household contacts as the proportion of household contacts who developed laboratory-confirmed influenza B virus infection during the follow-up period in households of index cases testing positive for influenza B Victoria- or Yamagata-lineage viruses. This has also been called the crude secondary attack risk (or rate) in other studies (17). We used an individual-based hazard model to characterize influenza transmission dynamics within households and to estimate the associations for factors affecting transmission (15, 20). Under this model, the overall risk of infection among household contacts depended on the risk of person-to-person transmission within the household, as well as the risk of infection from outside the household, which we assumed to be constant over the short-term follow-up period of each household (approximately 1 week) and the same across different households (15). The risk of secondary infection within the household was assumed to depend on time since illness onset in other infected persons in the household. We used the model to estimate the association of household contact age with the risk of transmission and to estimate the serial interval distribution.
We fitted the model using the Markov chain Monte Carlo method to estimate the posterior distributions of parameter estimates, permitting estimation of 95% credibility intervals for the overall risk of infection in household contacts, and the association of age with the risk of infection. Households in which 1 or more household contacts had laboratory-confirmed infection with influenza B viruses at the baseline home visit (i.e., potential co–index cases) were excluded from the analysis of infection risk among household contacts. All statistical analyses were conducted using R, version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria) and MATLAB 7.8.0 (MathWorks Inc., Natick, Massachusetts).
We calculated the sample size as follows. If we assumed the ratio of participants aged >15 years to participants aged ≤15 years to be 4:1, with a sample size of 88 household contacts, we would have 80% power to detect at least a 30% difference in the risk of secondary infection between the two groups. With a sample size of 28 household contacts in the households of index cases with Yamagata-lineage virus and 14 household contacts in the households of index cases with Victoria-lineage virus, we would have 80% power to detect at least a 43% difference in the risk of secondary infection between the two groups.
RESULTS
From 2007 through 2011, we followed up 109 households which included 109 index cases confirmed by RT-PCR or viral culture to have an infection with influenza B Yamagata- or Victoria-lineage virus at the clinic visit, plus their 339 household contacts. Of the 109 index cases, 75 were infected with Yamagata-lineage virus and 34 with Victoria-lineage virus. Sixty-seven (89%) of the 75 index cases with Yamagata-lineage virus infection were enrolled in 2007 and 2008, and 26 (76%) of the 34 index cases with Victoria-lineage virus infection were enrolled in 2008 (see Web Figure 1, available at http://aje.oxfordjournals.org/). Seventy-three (67%) of the 109 index cases and 56 (17%) of the 339 household contacts were children aged ≤15 years (Table 1). The median number of household members was 4. The characteristics of index cases with Yamagata- and Victoria-lineage infections and their household contacts were similar in terms of age, sex, the allocation of intervention arms, receipt of seasonal influenza vaccine in the past year, and receipt of antiviral treatment (Table 1).
Table 1.
Characteristics of Index Cases With Laboratory-Confirmed Influenza B Victoria- or Yamagata-Lineage Virus Infection and Their Household Contacts, Hong Kong, China, 2007–2011
| Characteristic | All Households |
P Value | Households Without Co–Index Cases |
P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yamagata |
Victoria |
Yamagata |
Victoria |
|||||||
| No. | % | No. | % | No. | % | No. | % | |||
| Index Cases | ||||||||||
| Total | 75 | 100 | 34 | 100 | 63 | 100 | 28 | 100 | ||
| Year(s) | 0.001 | <0.001 | ||||||||
| 2007 | 17 | 23 | 0 | 0 | 16 | 25 | 0 | 0 | ||
| 2008 | 50 | 67 | 26 | 76 | 41 | 65 | 21 | 75 | ||
| 2009–2011 | 8 | 11 | 8 | 24 | 6 | 10 | 7 | 25 | ||
| Age, years | 0.48 | 0.61 | ||||||||
| ≤5 | 11 | 15 | 4 | 12 | 8 | 13 | 3 | 11 | ||
| 6–15 | 36 | 48 | 22 | 65 | 31 | 49 | 18 | 64 | ||
| 16–49 | 24 | 32 | 7 | 21 | 21 | 33 | 6 | 21 | ||
| ≥50 | 4 | 5 | 1 | 3 | 3 | 5 | 1 | 4 | ||
| Male sex | 29 | 39 | 15 | 44 | 0.74 | 25 | 40 | 14 | 50 | 0.10 |
| Interventiona | 0.36 | <0.01 | ||||||||
| Yes | 38 | 51 | 19 | 56 | 29 | 46 | 18 | 64 | ||
| No | 37 | 49 | 15 | 44 | 34 | 54 | 10 | 36 | ||
| Vaccination in the past yearb | 0.32 | 0.15 | ||||||||
| No | 68 | 92 | 29 | 85 | 58 | 92 | 24 | 86 | ||
| Yes | 6 | 8 | 5 | 15 | 5 | 8 | 4 | 14 | ||
| Prescribed antiviral treatmentb | 0.07 | |||||||||
| No | 46 | 70 | 27 | 79 | 0.42 | 40 | 73 | 23 | 82 | |
| Yes | 20 | 30 | 7 | 21 | 15 | 27 | 5 | 18 | ||
| Household Contacts | ||||||||||
| Total | 227 | 100 | 112 | 100 | 181 | 100 | 94 | 100 | ||
| Year | <0.001 | <0.001 | ||||||||
| 2007 | 44 | 19 | 0 | 0 | 37 | 20 | 0 | 0 | ||
| 2008 | 157 | 69 | 86 | 77 | 124 | 69 | 71 | 76 | ||
| 2009–2011 | 26 | 11 | 26 | 23 | 20 | 11 | 23 | 24 | ||
| Age, yearsb | 0.83 | 0.78 | ||||||||
| 0–5 | 13 | 6 | 6 | 5 | 7 | 4 | 4 | 4 | ||
| 6–15 | 25 | 11 | 12 | 11 | 21 | 12 | 10 | 11 | ||
| 16–49 | 143 | 63 | 75 | 67 | 111 | 63 | 62 | 66 | ||
| ≥50 | 42 | 19 | 19 | 17 | 38 | 21 | 18 | 19 | ||
| Male sex | 78 | 34 | 37 | 33 | 0.67 | 62 | 34 | 31 | 33 | 0.71 |
| Interventiona | 0.96 | <0.001 | ||||||||
| Yes | 122 | 54 | 60 | 54 | 87 | 48 | 57 | 61 | ||
| No | 105 | 46 | 52 | 46 | 94 | 52 | 37 | 39 | ||
| Vaccination in the past year | 0.09 | 0.38 | ||||||||
| No | 197 | 87 | 101 | 90 | 156 | 86 | 83 | 88 | ||
| Yes | 30 | 13 | 11 | 10 | 25 | 14 | 11 | 12 | ||
a In 2007, 2008, and the first half of 2009 (January through June), households were randomly allocated to 3 groups for estimation of the effectiveness of surgical face masks and hand hygiene in preventing influenza transmission within households.
b Some index cases and household contacts had missing values for this characteristic.
Of the 109 households followed up, 18 households with 64 household contacts were excluded from the analysis of household transmission because more than 1 household member was positive for influenza at the initial home visit, complicating assessment of transmission dynamics (Table 1). The characteristics of index cases and their household contacts from the households without co–index cases for Yamagata- and Victoria-lineage viruses were similar in terms of age, sex, receipt of seasonal influenza vaccine in the past year, and receipt of antiviral treatment. However, the proportions of index cases and their household contacts allocated to intervention arms were significantly different for Yamagata- and Victoria-lineage viruses. Of the remaining 275 household contacts in 91 households, 25 contacts from 25 households were confirmed to have infections with influenza B virus at the second or third home visit. Of these 25 household contacts with influenza B virus infections, 14 infections were caused by Yamagata-lineage virus and 11 by Victoria-lineage virus, and in every case the lineage identified in the household contact matched the lineage identified in the corresponding index case. Of 181 household contacts, we had missing information on age for 4. For the remaining 177 household contacts from 63 households with index cases having Yamagata-lineage virus infections, 11 of 149 household contacts aged >15 years (7%) and 3 of 28 household contacts aged ≤15 years (11%) were infected with Yamagata-lineage virus. Of the 94 household contacts from 28 households with index cases having Victoria-lineage virus infections, 3 of 80 household contacts aged >15 years (4%) and 8 of 14 household contacts aged ≤15 years (57%) were infected with Yamagata-lineage virus (Web Table 1).
Overall risks of infection among household contacts were 11.6% (95% credibility interval (CrI): 6.3, 20.0) for household contacts of index cases with Victoria-lineage virus and 7.6% (95% CrI: 3.3, 13.6) for household contacts of index cases with Yamagata-lineage virus (Table 2). The majority (84.0%, 95% CrI: 56.0, 100.0) of infections among household contacts were estimated to have occurred within the household rather than to have been acquired outside the household. There was a very significant difference between lineages in age-specific risk of secondary infection within the household—compared with household contacts aged >15 years, those aged ≤15 years had a 13 times' higher risk of infection within the household for Victoria-lineage virus (risk ratio = 12.9, 95% CrI: 4.2, 43.6) but no significant difference in risk of infection within the household for Yamagata-lineage virus (risk ratio = 1.0, 95% CrI: 0.2, 3.3). The estimated risks of secondary infection for contacts aged ≤15 years were 39.7% (95% CrI: 12.3, 69.7) and 6.9% (95% CrI: 1.5, 19.2) for Victoria and Yamagata viruses, respectively (P = 0.03 for difference). The estimated risks of secondary infection for contacts aged >15 years were 3.7% (95% CrI: 1.0, 8.6) and 7.1% (95% CrI: 3.1, 13.0) for Victoria and Yamagata viruses, respectively (P = 0.32 for difference). We did not have sufficient data to estimate serial interval distributions separately for each lineage, but we estimated the serial interval for influenza B overall to be 3.6 days (95% confidence interval: 2.9, 4.2), with a standard deviation of 1.4 days (95% confidence interval: 1.0, 1.7).
Table 2.
Overall Risk of Infection With Influenza B Yamagata- and Victoria-Lineage Viruses and Risk of Secondary Infection Within the Household Among Household Contacts, by Age of Household Contacts, Hong Kong, China, 2007–2011
| Risk Measure and Age of Household Contacts |
Yamagata-Lineage Viruses |
Victoria- Lineage Viruses |
P Value | ||
|---|---|---|---|---|---|
| Infection Risk,a % | 95% CrIb | Infection Risk,a % | 95% CrIb | ||
| Overall risk of infection among household contacts (any age) | 7.6 | 3.3, 13.6 | 11.6 | 6.3, 20.0 | 0.33 |
| Risk of secondary infection within the household | |||||
| Contacts aged ≤15 years | 6.9 | 1.5, 19.2 | 39.7 | 12.3, 69.7 | 0.03 |
| Contacts aged >15 years | 7.1 | 3.1, 13.0 | 3.7 | 1.0, 8.6 | 0.32 |
Abbreviation: CrI, credibility interval.
a All risks of infection among household contacts were estimated using the individual-based hazard model.
b All 95% CrIs were calculated using the Markov chain Monte Carlo method.
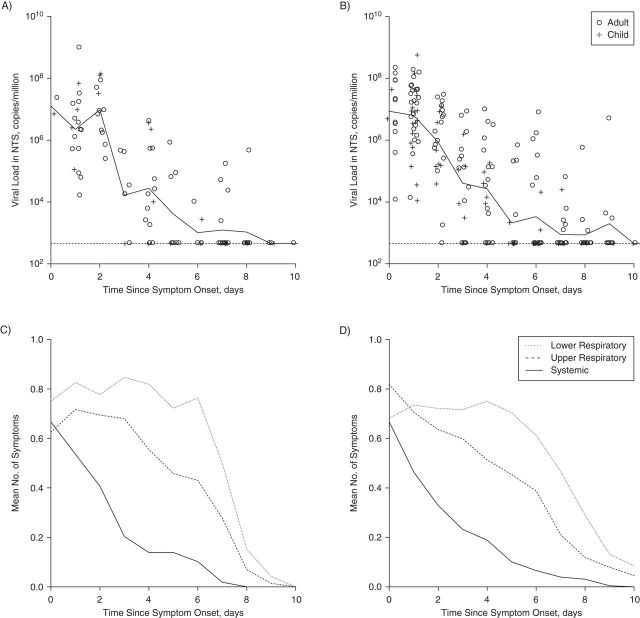
Regardless of the lineage, molecular viral shedding in most index cases had ceased by 9–10 days of illness (Figure 1). The patterns in viral shedding upon RT-PCR assay and the course of systemic signs and symptoms, upper respiratory symptoms, and lower respiratory symptoms were similar for index cases with Yamagata-lineage infection and those with Victoria-lineage infection (Figure 1). The median durations of viral shedding after symptom onset were 4 days for both Yamagata- and Victoria-lineage viruses (P = 0.32; Figure 2A). For both Yamagata- and Victoria-lineage infections, the median duration of the presence of any symptoms was 8 days (P = 0.13), and systematic symptoms ceased after 8–9 days of illness (Figure 2B).
Figure 1.
Patterns of viral shedding and course of illness among index patients in households with cases of influenza B virus infection, Hong Kong, China, 2007–2011. Panels A and B show the geometric mean viral load (solid lines) upon reverse transcription polymerase chain reaction (RT-PCR) assay for index cases with influenza B Victoria-lineage virus and those with influenza B Yamagata-lineage virus, respectively. The lower limit of detection for the RT-PCR assay was approximately 900 copies per mL (horizontal dashed lines). Panels C and D show the mean numbers of lower respiratory, upper respiratory, and systemic symptoms in index cases with influenza B Victoria-lineage and Yamagata-lineage virus, respectively. The mean numbers of symptoms were calculated from a composite of 3 groups of influenza signs and symptoms and were rescaled to the range 0–1, with higher scores indicating a greater number of symptoms. In all panels, the onset of acute respiratory illness was defined as the self-reported day of illness onset prior to recruitment into the study. NTS, nasal and throat swab.
Figure 2.
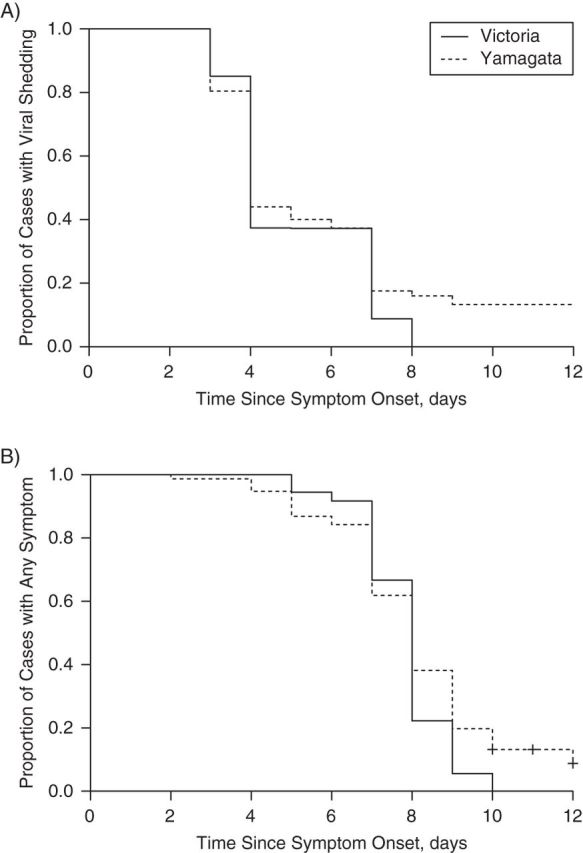
Estimates (Kaplan-Meier estimators) of time to cessation of viral shedding (A) and resolution of all symptoms (B) among index patients in households with cases of influenza B virus infection, Hong Kong, China, 2007–2011.
Web Figure 2 shows the patterns of viral shedding and symptom scores throughout the course of illness in household contacts infected with Yamagata- or Victoria-lineage virus. Because of the small size of this sample, we could not make precise comparisons, but for both Yamagata- and Victoria-lineage viruses, respiratory symptom scores persisted at a higher level for 5 days after ARI onset. Systematic symptom scores peaked at ARI onset or 1 day after ARI onset. In both lineages, the levels of molecular viral shedding were variable.
DISCUSSION
In this study, we found that influenza B Yamagata- and Victoria-lineage viruses had different age-specific patterns in risks of secondary infection within the household, although the overall risks of infection were not significantly different for Yamagata-lineage and Victoria-lineage viruses. Specifically, we found there was a much greater risk of secondary infection among household contacts aged ≤15 years than among household contacts aged >15 years for Victoria-lineage viruses but not for Yamagata-lineage viruses. The higher age-specific risk of secondary infection in children that we identified for Victoria-lineage viruses was similar to the observations for influenza A (H1N1)pdm09 and seasonal influenza in previous studies of influenza A virus transmission in households during the 2009 pandemic period and seasonal interpandemic periods (21–23). However, we did not find a similar pattern for Yamagata-lineage viruses, where we found similar risks of secondary infection for children and adults. This is consistent with previous reports of significant differences in the age distribution of cases infected with the two influenza B-lineage viruses, with Victoria-lineage viruses infecting younger people on average than Yamagata-lineage viruses (9, 10).
The differences we identified between Victoria- and Yamagata-lineage viruses could be explained by children having less immunity against Victoria-lineage viruses, faster evolution of Victoria-lineage viruses, and inherent differences in the transmissibility of the two lineages. Recent analysis of influenza B genomic and epidemiologic data from Australia and New Zealand showed that in 2008 the Victoria lineage exhibited a significantly higher effective reproductive number than the Yamagata lineage, coinciding with the year in which a new variant of the Victoria lineage was first detected (B/Brisbane/60/2008-like viruses) (11). We could not find other published reports on the relative prevalence of the two lineages in Hong Kong in 2007–2011. However, studies carried out in Taiwan and mainland China found that the representative viruses were B/Florida/4/2006-like for Yamagata-lineage viruses in 2007–2011 and B/Brisbane/60/2008-like for Victoria-lineage viruses in 2008–2011, respectively (24–29). B/Brisbane/60/2008-like viruses evolved from reassortants and had a hemagglutinin gene (HA) from Victoria-like viruses and a neuraminidase gene (NA) from Yamagata-like viruses (10). Reassortment could play an important role in the evolution of influenza B virus to enhance viral fitness, perhaps by placing gene segments, particularly the HA and NA segments, in new and advantageous genetic backgrounds (5). The Victoria-lineage viruses experienced faster antigenic drift than the Yamagata-lineage viruses (11).
Lower levels of Victoria-lineage virus antibodies than of Yamagata-lineage virus antibodies among children were reported in several vaccine studies during 2008–2011 (30–32). Additionally, influenza B Yamagata-lineage viruses may boost more robust cross-lineage antibody responses than Victoria-lineage viruses, which has been observed in humans and mice (32–34). However, unlike the study in Australia and New Zealand that showed a difference in the overall effective reproductive number between the two lineages in 2008 (11), we found a significantly higher risk of secondary infection within the household for the Victoria-lineage viruses among household contacts aged ≤15 years in 2007–2011, which could be related to different designs, outcome measures, and timelines between the two studies. The Australia–New Zealand study also showed that the overall effective reproductive number of the Victoria-lineage viruses dropped gradually after 2008 (11).
Index cases infected with Yamagata-lineage viruses and those infected with Victoria-lineage viruses had similar characteristics in terms of viral shedding and clinical illness (Figures 1 and 2). We also examined virus shedding and illness in secondary cases, but the numbers of participants were small and it was difficult to discern patterns (Web Figure 2). The median duration of viral shedding for index cases with Victoria- and Yamagata-lineage viruses in this study was similar to that (range, 3–9 days) for medically attended patients with laboratory-confirmed influenza virus infection (35–40). Our estimate of median illness duration of 8 days was similar to previously reported estimates ranging from 7 days to 11 days (22, 41–43).
Previous estimates of the serial interval for influenza have ranged from 1 day to 6 days, with most estimates falling between 2.5 and 4 days (17, 22, 44, 45). Our estimated serial interval for influenza B Yamagata- and Victoria-lineage viruses of 3.6 days falls within that range. The serial interval can be influenced by influenza type/subtype, the transmissibility of index cases, contact patterns within households, the susceptibility of contacts, transmission dynamics in the community, and the incubation period (22, 45, 46). We did not have a sufficient sample size to compare the serial intervals between the two lineages of influenza B virus in this study.
Our study had a number of limitations. First, it involved ascertainment of index cases from outpatient clinics, with a rapid diagnostic test used for screening, which could have led to bias in the selection of the index cases. Second, during the study period of 5 years, the transmissibility and herd immunity of the circulating influenza B Victoria- and Yamagata-lineage viruses could have varied across years; furthermore, we had allocated face masks and hand hygiene interventions to some households in the first half of our study, when most of the cases were enrolled. We found no evidence that random allocation of face masks or hand hygiene interventions reduced the risk of influenza B transmission in households (12), and there was no significant association of the interventions with influenza B transmission when they were incorporated in our individual-based transmission model, but we cannot exclude the possibility that these interventions slightly led to bias in our estimates of the secondary infection risks. Third, households with more than 1 individual concurrently infected with influenza, as confirmed by RT-PCR or viral culture at the first home visit, were excluded from the study. This may have led to exclusion of cases who were infected with more transmissible strains for which secondary infection might have occurred before the first visit, or exclusion of index cases that had generally more susceptible household contacts. Fourth, we assumed the risks of infection from outside the household to be identical for all households in the individual-based model (for various reasons), although this is unlikely. However, the risk of infection from outside the household was estimated to be very low, and our separate study of virus sequence data confirmed that in these types of household transmission studies, almost all of the infections among household contacts can be attributed to infection from the index case (47). Finally, the small sample size did not allow us to identify the associations of other factors with the risk of infection.
In conclusion, in the household setting, children had a much higher risk of infection with influenza B Victoria-lineage virus than did older people, while the risk of infection did not vary significantly by age for Yamagata-lineage virus. We also found that children had a higher risk of infection with Victoria-lineage virus than with Yamagata-lineage virus. However, infections with influenza B Yamagata- and Victoria-lineage viruses were associated with similar patterns of viral shedding and clinical illness. The mechanisms underlying these epidemiologic differences deserve further investigation.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: WHO Collaborating Centre for Infectious Disease Epidemiology and Control, School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong Special Administrative Region (SAR), China (Cuiling Xu, Tim K. Tsang, Vicky J. Fang, Rita O. P. Fung, Dennis K. M. Ip, Gabriel M. Leung, J. S. Malik Peiris, Benjamin J. Cowling); Department of Microbiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China (Kwok-Hung Chan); Mathematical Modelling of Infectious Diseases Unit, Institut Pasteur, Paris, France (Simon Cauchemez); and Centre of Influenza Research, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China (J. S. Malik Peiris).
This study was supported by a commissioned grant from theHealth and Medical Research Fund of theHong Kong SAR Health, Welfare and Food Bureau; by the USNational Institute of Allergy and Infectious Diseases (NIAID) (contract HHSN26620070000 5C); byAsian Development Bank grant N01-AI-70005 (NIAID Centers for Excellence in Influenza Research and Surveillance); by theHarvard Center for Communicable Disease Dynamics (US National Institute of General Medical Sciences grant U54 GM088558); and by theResearch Grants Council of the Hong Kong SARUniversity Grants Committee (project T11-705/14N).
We thank the physicians, nurses, and staff members at the participating study centers for facilitating recruitment; the dedicated team of health-care workers who conducted the home visits; and Kit Man Chan, Dr. Calvin Cheng, Anita Li, Winnie Lim, Ho Yuk Ling, Loretta Mak, Lam Yiu Pong, Dr. Lincoln Lau, Tom Lui, Tong Hok Leung, Sara Poon, and Teresa So for research support.
The funders played no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
D.K.M.I. has received research funding from F. Hoffmann-La Roche Ltd. (Basel, Switzerland). J.S.M.P. has received research funding from Crucell N.V. (Leiden, the Netherlands). G.M.L. has received consulting honoraria from Janssen Pharmaceutica (Beerse, Belgium). B.J.C. has received research funding from MedImmune Inc. (Gaithersburg, Maryland) and Sanofi Pasteur (Lyon, France) and consults for Crucell N.V.
REFERENCES
- 1.Rota PA, Wallis TR, Harmon MW, et al. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology. 1990;1751:59–68. [DOI] [PubMed] [Google Scholar]
- 2.Nerome R, Hiromoto Y, Sugita S, et al. Evolutionary characteristics of influenza B virus since its first isolation in 1940: dynamic circulation of deletion and insertion mechanism. Arch Virol. 1998;1438:1569–1583. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa N, Kubota R, Maeda A, et al. Heterogeneity of influenza B virus strains in one epidemic season differentiated by monoclonal antibodies and nucleotide sequences. J Clin Microbiol. 2000;389:3467–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw MW, Xu X, Li Y, et al. Reappearance and global spread of variants of influenza B/Victoria/2/87 lineage viruses in the 2000–2001 and 2001–2002 seasons. Virology. 2002;3031:1–8. [DOI] [PubMed] [Google Scholar]
- 5.Chen R, Holmes EC. The evolutionary dynamics of human influenza B virus. J Mol Evol. 2008;666:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan PK, Chan MC, Cheung JL, et al. Influenza B lineage circulation and hospitalization rates in a subtropical city, Hong Kong, 2000–2010. Clin Infect Dis. 2013;565:677–684. [DOI] [PubMed] [Google Scholar]
- 7.Feng L, Shay DK, Jiang Y, et al. Influenza-associated mortality in temperate and subtropical Chinese cities, 2003–2008. Bull World Health Organ. 2012;904:279–288B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson DR, Heffernan RT, Paladini M, et al. Monitoring the impact of influenza by age: emergency department fever and respiratory complaint surveillance in New York City. PLoS Med. 2007;48:e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sočan M, Prosenc K, Učakar V, et al. A comparison of the demographic and clinical characteristics of laboratory-confirmed influenza B Yamagata and Victoria lineage infection. J Clin Virol. 2014;611:156–160. [DOI] [PubMed] [Google Scholar]
- 10.Tan Y, Guan W, Lam TT-Y, et al. Differing epidemiological dynamics of influenza B virus lineages in Guangzhou, southern China, 2009–2010. J Virol. 2013;8722:12447–12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijaykrishna D, Holmes EC, Joseph U, et al. The contrasting phylodynamics of human influenza B viruses. eLife. 2015;4:e05055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowling BJ, Chan K-H, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;1517:437–446. [DOI] [PubMed] [Google Scholar]
- 13.Cowling BJ, Fung RO, Cheng CK, et al. Preliminary findings of a randomized trial of non-pharmaceutical interventions to prevent influenza transmission in households. PLoS One. 2008;35:e2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowling BJ, Ip DK, Fang VJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Nat Commun. 2013;4:1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsang TK, Cauchemez S, Perera RA, et al. Association between antibody titers and protection against influenza virus infection within households. J Infect Dis. 2014;2105:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowling BJ, Chan KH, Fang VJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;36223:2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau LL, Nishiura H, Kelly H, et al. Household transmission of 2009 pandemic influenza A (H1N1): a systematic review and meta-analysis. Epidemiology. 2012;234:531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang N, Fang S, Wang T, et al. Applicability of a sensitive duplex real-time PCR assay for identifying B/Yamagata and B/Victoria lineages of influenza virus from clinical specimens. Appl Microbiol Biotechnol. 2012;932:797–805. [DOI] [PubMed] [Google Scholar]
- 19.Biere B, Bauer B, Schweiger B. Differentiation of influenza B virus lineages Yamagata and Victoria by real-time PCR. J Clin Microbiol. 2010;484:1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cauchemez S, Donnelly CA, Reed C, et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med. 2009;36127:2619–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nukiwa-Souma N, Burmaa A, Kamigaki T, et al. Influenza transmission in a community during a seasonal influenza A(H3N2) outbreak (2010–2011) in Mongolia: a community-based prospective cohort study. PLoS One. 2012;73:e33046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrie JG, Ohmit SE, Cowling BJ, et al. Influenza transmission in a cohort of households with children: 2010–2011. PLoS One. 2013;89:e75339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugimoto JD, Borse NN, Ta ML, et al. The effect of age on transmission of 2009 pandemic influenza A (H1N1) in a camp and associated households. Epidemiology. 2011;222:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su WJ, Shao PL, Liu MT, et al. Low seroprotection against preseasonal influenza local strains in children might predict the upcoming epidemic influenza strains. Clin Infect Dis. 2010;512:171–176. [DOI] [PubMed] [Google Scholar]
- 25.Qi SX, Han GY, Liu YF. Virological surveillance and molecular characteristics of influenza B viruses in Hebei Province during 2004–2008 [in Chinese] Zhongguo Yi Miao He Mian Yi. 2009;151:27–30. [PubMed] [Google Scholar]
- 26.Liu YZ, Zhao X, Huang YW, et al. Analysis of genetic features of influenza B virus in Hunan Province from 2007 to 2010 [in Chinese] Zhonghua Yu Fang Yi Xue Za Zhi. 2012;463:258–263. [PubMed] [Google Scholar]
- 27.Mao HY, Zhou M, Zhang YJ, et al. Genetic variation of the hemagglutinin and neuraminidase of influenza B viruses isolated in Zhejiang Province during 1999–2010 [in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2011;324:376–381. [PubMed] [Google Scholar]
- 28.Huang WJ, Tan MJ, Lan Y, et al. Virological characterization of influenza B virus in mainland China during 2011–2012 [in Chinese] Bing Du Xue Bao. 2013;291:32–38. [PubMed] [Google Scholar]
- 29.Chinese Center for Disease Control and Prevention. Chinese Influenza and Human Avian Influenza Weekly Report [in Chinese]. (Issue 11, total issue 23). http://www.cnic.org.cn/uploadfile/2014/1128/20141128112032445.pdf. Published August 19, 2009. Updated August 19, 2009. Accessed October 10, 2014. [DOI] [PMC free article] [PubMed]
- 30.Ng S, Fang VJ, Ip DK, et al. Humoral antibody response after receipt of inactivated seasonal influenza vaccinations one year apart in children. Pediatr Infect Dis J. 2012;319:964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Domachowske JB, Pankow-Culot H, Bautista M, et al. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3–17 years. J Infect Dis. 2013;20712:1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langley JM, Carmona Martinez A, Chatterjee A, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine candidate: a phase III randomized controlled trial in children. J Infect Dis. 2013;2084:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skowronski DM, Hamelin ME, Janjua NZ, et al. Cross-lineage influenza B and heterologous influenza A antibody responses in vaccinated mice: immunologic interactions and B/Yamagata dominance. PLoS One. 2012;76:e38929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skowronski DM, Hottes TS, De Serres G, et al. Influenza B/Victoria antigen induces strong recall of B/Yamagata but lower B/Victoria response in children primed with two doses of B/Yamagata. Pediatr Infect Dis J. 2011;3010:833–839. [DOI] [PubMed] [Google Scholar]
- 35.Cao B, Li X-W, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;36126:2507–2517. [DOI] [PubMed] [Google Scholar]
- 36.Leung YH, Lim WL, Wong MH, et al. Delayed oseltamivir treatment is associated with longer viral shedding of pandemic (H1N1) 2009 virus. Epidemiol Infect. 2012;1405:814–817. [DOI] [PubMed] [Google Scholar]
- 37.Li CC, Wang L, Eng HL, et al. Correlation of pandemic (H1N1) 2009 viral load with disease severity and prolonged viral shedding in children. Emerg Infect Dis. 2010;168:1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hien TT, Boni MF, Bryant JE, et al. Early pandemic influenza (2009 H1N1) in Ho Chi Minh City, Vietnam: a clinical virological and epidemiological analysis. PLoS Med. 2010;75:e1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu H, Liao Q, Yuan Y, et al. Effectiveness of oseltamivir on disease progression and viral RNA shedding in patients with mild pandemic 2009 influenza A H1N1: opportunistic retrospective study of medical charts in China. BMJ. 2010;341:c4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhattarai A, Villanueva J, Palekar RS, et al. Viral shedding duration of pandemic influenza A H1N1 virus during an elementary school outbreak—Pennsylvania, May–June 2009. Clin Infect Dis. 2011;52(suppl 1):S102–S108. [DOI] [PubMed] [Google Scholar]
- 41.Cauchemez S, Carrat F, Viboud C, et al. A Bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Stat Med. 2004;2322:3469–3487. [DOI] [PubMed] [Google Scholar]
- 42.Petrie JG, Ohmit SE, Johnson E, et al. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis. 2011;2039:1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng S, Cowling BJ, Fang VJ, et al. Effects of oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin Infect Dis. 2010;505:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowling BJ, Fang VJ, Riley S, et al. Estimation of the serial interval of influenza. Epidemiology. 2009;203:344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levy JW, Cowling BJ, Simmerman JM, et al. The serial intervals of seasonal and pandemic influenza viruses in households in Bangkok, Thailand. Am J Epidemiol. 2013;17712:1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klinkenberg D, Nishiura H. The correlation between infectivity and incubation period of measles, estimated from households with two cases. J Theor Biol. 2011;2841:52–60. [DOI] [PubMed] [Google Scholar]
- 47.Poon LL, Chan KH, Chu DK, et al. Viral genetic sequence variations in pandemic H1N1/2009 and seasonal H3N2 influenza viruses within an individual, a household and a community. J Clin Virol. 2011;522:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.