Abstract
Introduction:
It is unknown how the timing between doses might affect nicotine’s impact on neural activity. Our objective was to examine how the interdose interval affects nicotine’s impact on resting-state functional connectivity (rsFC).
Materials and Methods:
Adult male Sprague-Dawley rats were administered nicotine daily (0.4mg/kg) over 6 days while control animals received saline vehicle. Functional magnetic resonance imaging was used to measure rsFC before and after a challenge dose of nicotine (0.4mg/kg) delivered for the first time and 3, 6, 12, or 24hr after the previous dose.
Results:
As the interval between nicotine doses increased from 3 to 24hr, the strength of rsFC increased in some circuits, particularly the nucleus accumbens and prefrontal circuits, and decreased in others, namely the interpeduncular nucleus, hippocampus, caudoputamen, retrosplenial cortex, ventral tegmental, and the insular circuits.
Conclusions:
These data indicate that the effect that nicotine has on the brain is affected by the amount of time that has passed since the previous dose. The effect on rsFC of cumulative doses is not additive. This may have important implications for the study of nicotine addiction as it implies that the same dose of nicotine might have a different impact on the brain depending on the time elapsed from the previous exposure.
Introduction
Heavy smokers can experience a greater impact from the first cigarette of the day even though the concentration of nicotine in the blood is lower after the first cigarette than it is throughout the rest of the day.1,2 A potential explanation for this paradox is that smoking the first morning cigarette relieves withdrawal symptoms after an overnight abstinence in heavy smokers. Another possibility is that some tolerance is lost after an overnight abstinence.3 Some 4 decades ago, it was demonstrated that a single dose of nicotine could produce a transient acute tolerance in rats that was evident when challenge doses were delivered 2 or 4hr after an initial dose, but not after 8hr.4,5 These studies suggest that nicotine may have a different impact on the brain depending on the interdose interval. It is possible that nicotine received from the first cigarette of the day may have a different impact than that from subsequent cigarettes, or given the wide range of temporal patterns in daily cigarette consumption, that each cigarette may produce a different impact.
In order to evaluate the effect on brain function of the timing between nicotine doses, we investigated resting-state functional connectivity (rsFC), a measure of the degree to which neural activity in different brain regions is coordinated. We compared rsFC in response to challenge doses of nicotine given at different intervals after the previous dose.
Materials and methods
All experiments were approved by the Institution for Animal Care and Use Committee at the University of Massachusetts Medical School and carried out in accordance with the guidelines published by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats weighing 250–350g were obtained from Harlan Laboratories, Inc. and housed in pairs for the duration of testing (n = 54 in total). The housing environment was maintained at 22–24 °C with a reversed 12-hr light–12-hr dark schedule (lights on at 21:00 and off at 09:00). Food and water were provided ad libitum.
Drugs
Nicotine hydrogen tartrate (Sigma) was dissolved in saline, pH-adjusted to 7.2±0.2, and delivered at a dose of 0.4mg/kg (base) subcutaneously. Sterile saline was used in an equivalent volume.
Four experimental groups received six daily nicotine injections (0.4mg/kg) and were randomly assigned to receive a challenge injection at the same dose delivered either 3 (n = 12), 6 (n = 11), 12 (n = 10), or 24 (n = 13) hr after the 6th daily dose of nicotine. This challenge dose was delivered while the animals were being imaged. As a within-subject control, in the same imaging session, the animals also initially received a saline injection. To control for the effects of handling and injections, a control group (n = 8) instead received six daily doses of saline followed on the 7th day by a challenge injection of nicotine (same 0.4mg/kg dose as the other groups).
Acclimation for Awake Imaging
A validated procedure was utilized to acclimate rats to magnetic resonance imaging (MRI) restraint and noise.6 Rats were anesthetized with isoflurane and EMLA cream (Lidocaine 2.5% and Prilocaine 2.5% Cream, Hi-Tech Pharmacal Co., Inc.) was applied topically to minimize pain from mechanical restraint. The animals were then secured in a Plexiglas stereotaxic head holder using plastic ear bars. Animals were then placed into a black opaque tube “mock scanner” and exposed to recorded scanner noises. Animals were acclimated for 8 days, one session per day. The time of acclimation increased from 15min on the first day to 90min on days 6, 7, and 8, with an increment of 15min per day.
Animal Preparation for Imaging
Animals were briefly anesthetized using isoflurane. The head was fitted into a head restrainer with a built-in coil, with the incisors secured over a bite bar. The nose was secured with a nose clamp, and ear bars were positioned inside the head holder with adjustable screws fitted into lateral sleeves. The body of the animal was then placed into a body restrainer. Isoflurane was removed after this setup and the restraining system was positioned in the magnet for imaging under awake conditions. Following signal optimization, imaging sessions started approximately 15min after animals were placed in the magnet.
Magnetic Resonance Imaging
MRI was performed on a 4.7 T/40cm horizontal magnet equipped with a Biospec Bruker console (inner diameter 12cm). A surface coil (inner diameter: 2.3cm) developed in house was used for brain imaging.
For each rat, anatomical images were obtained using Rapid Acquisition Relaxation-Enhanced (RARE) sequence with the following parameters: relaxation time = 3,000ms, RARE factor = 8, echo time = 12ms, resolution matrix = 256×256, field of view = 32×32mm, slice number = 18, slice thickness = 1mm.
Three subsequent functional scans were performed using Echo-Planar Imaging (EPI) sequence with the following parameters: relaxation time = 1 s, echo time = 30ms, flip angle = 60°, resolution matrix = 64×64, field of view = 32×32mm, slice thickness = 1mm. The first EPI (EPI1) had a number of repetitions = 1,200, and the last two EPIs (EPI2 and EPI3) had a number of repetitions of 1,800. EPI1 was acquired at “resting state” for 20min, where no drug was administered. Each of the last two EPIs (EPI2 and EPI3) took 30min, in which 1min of baseline period was followed by subcutaneous saline and nicotine injections, respectively, followed by 29min of continuous data acquisition. Each injection took about 5 s.
Preprocessing of Imaging Data
The imaging data were preprocessed using Medical Image Visualization and Analysis (MIVA, http://ccni.wpi.edu/), Matlab 2010b (The Mathworks Inc.), and SPM8 (Wellcome Department of Cognitive Neurology). EPI1 and the last 20min of EPI2 and EPI3 was used for rsFC analysis. Each of the three 20-min resting-state scans was then divided into six sessions containing 200 repetitions each.
All EPI sessions were first aligned and coregistered to a fully segmented rat brain atlas in MIVA. After registration, all sessions went through the following preprocessing steps: motion correction, spatial smoothing (full-width-half maximum = 1mm), and 0.002–0.1 Hz band-pass filtering. Data sets with excessive motion (greater than 0.2mm in each dimension) were discarded (six sessions in total: one, two, two, and one from four different scans, respectively). The time course for each individual voxel was further corrected for head movement by regression on the six motion parameters (translations and rotations) estimated in the procedure of motion correction. For rsFC, the global signal was estimated by averaging the time courses of all voxels inside the whole-brain mask. The ventricle and white matter signal was estimated by averaging the time courses of all voxels inside the ventricle and white matter and then regressed out from time courses for connectivity analysis.7
rsFC Analysis
rsFC was evaluated using region of interest (ROI)-based correlational analysis on a voxel-by-voxel basis.7–9 Fourteen ROIs were chosen to be seeds focusing on the reward circuit. These included (number of EPI voxels) the orbital frontal area (74), prelimbic cortex (38), infralimbic cortex (14), anterior cingulate cortex (57), nucleus accumbens (43), ventral tegmental area (14), insula (149), amygdala (69), retrosplenial cortex (86), hippocampus (329), caudoputamen (323), substantia nigra (22), medial habenula (4), and the interpeduncular nucleus (5) (Figure 1).
Figure 1.
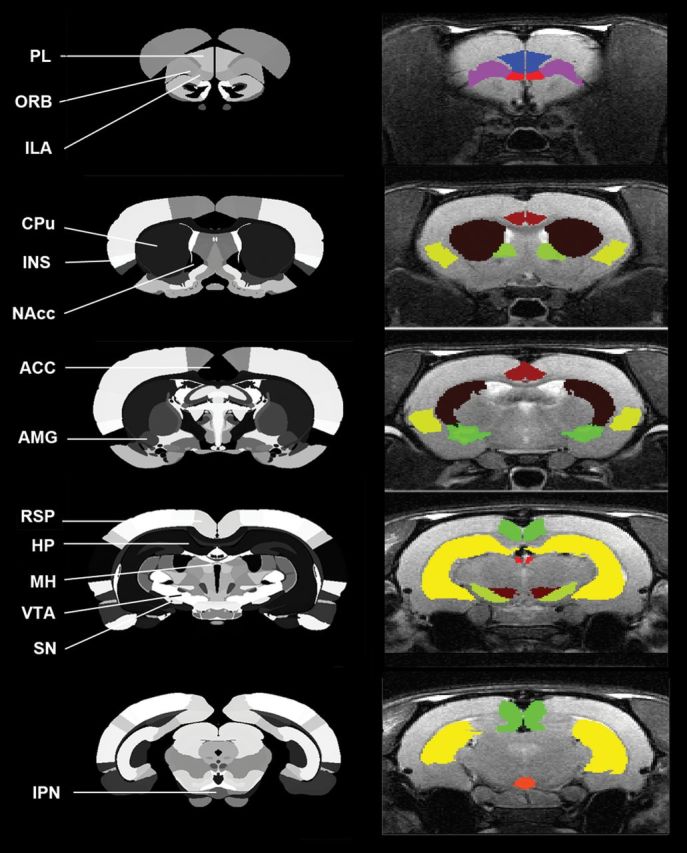
Regions of interest (ROIs) used in functional connectivity analysis. Fourteen ROIs are labeled on the atlas on the left side, including the orbital frontal area (ORB), prelimbic area (PL),10 infralimbic area (ILA), anterior cingulate cortex (ACC), nucleus accumbens (NAcc),11 ventral tegmental area (VTA), insula (INS),3 amygdala (AMG), retrosplenial cortex (RSP), hippocampus (HP), caudoputamen (CPu), substantia nigra (SN), medial habenula (MH), and the interpeduncular nucleus (IPN). Right side shows these ROIs mapped on the magnetic resonance imaging of a typical subject.
For each animal, regionally averaged time courses from all voxels inside the ROI regions were used as time courses to correlate. Pearson cross-correlation coefficients between each of the two ROI time courses were calculated. Correlation coefficients (CCs) were transformed using Fisher’s z transformation. Z scores from sessions were averaged and then transformed back to CCs to produce representative rsFC of the animal.
As the representation of rsFC between ROIs, CCs were compared before and after the animals received saline (i.e., between EPI1 and EPI2), as well as before and after nicotine (i.e., EPI2 and EPI3) within each group using a paired t-test (significance level p < .05). Representative CCs were also compared among all five groups (saline control, 3, 6, 12, and 24hr) using single-factor analysis of variance with a significance level of p <.05, where warranted by Tukey–Kramer post hoc comparisons (Matlab function, version 2010b).
Based on the ROI-based results, three recurring regions were chosen as “seeds” to further examine whole-brain rsFC: prefrontal area (prelimbic and infralimbic cortices), nucleus accumbens, and the anterior cingulate cortex. Seed-based rsFC maps were compared before and after the nicotine challenge (p < .001, uncorrected).
Results
Table 1 summarizes how the change in rsFC produced by a dose of nicotine was influenced by the time lapsed since the previous dose. The observed changes in rsFC differed when doses were spaced at 3, 6, 12, and 24hr. The saline injection had no significant effect on rsFC in any group (EPI1 vs. EPI2).
Table 1.
Circuits in Which a Challenge Dose of Nicotine Increased or Decreased the Strength of rsFC (p < .05)
| Group | Increased rsFC | Decreased rsFC |
|---|---|---|
| 3 hr | Nucleus accumbens–retrosplenial cortex | Caudoputamen–medial habenula |
| Orbital frontal area–prelimbic area | ||
| 6 hr | Anterior cingulate cortex–amygdala | Hippocampus–ventral tegmental area |
| Amygdala–retrosplenial cortex | Ventral tegmental area–amygdala | |
| Insula–ventral tegmental area | ||
| Insula–medial habenula | ||
| Insula–orbital frontal area | ||
| Orbital frontal area–substantia nigra | ||
| 12 hr | Nucleus accumbens–retrosplenial cortex | Anterior cingulate cortex–interpeduncular nucleus |
| Retrosplenial cortex–anterior cingulate cortex | ||
| 24 hr | Nucleus accumbens–amygdala | Nucleus accumbens–infralimbic area |
| Infralimbic area–caudoputamen | ||
| Ventral tegmental area–interpeduncular nucleus | ||
| Interpeduncular nucleus–retrosplenial cortex | ||
| Orbital frontal area–medial habenula |
Note. The groups indicate the number of hours expired between the administration of the nicotine challenge dose and the previous dose. rsFC = resting-state functional connectivity.
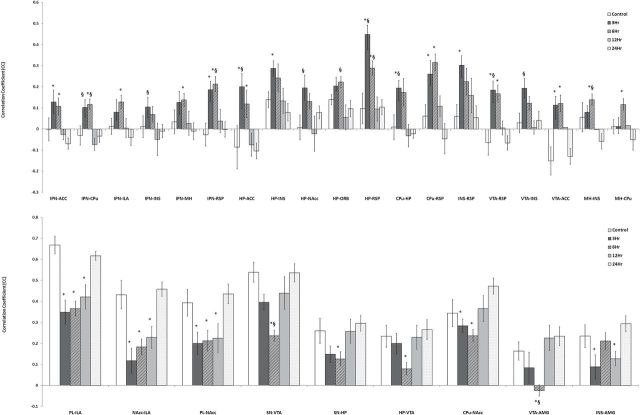
Figure 2 shows 28 regional connectivities in which intergroup differences in the strength of rsFC were detected when the nicotine challenge was administered at various time points after the previous dose. There were no significant differences in rsFC in any circuit when comparing the 24-hr group with the control group (first dose of nicotine). Significant group differences in rsFC involved the anterior cingulate, infralimbic, prelimbic, orbitofrontal, and retrosplenial cortices, as well as amygdala, caudoputamen, hippocampus, insula, interpeduncular nucleus, medial habenula, nucleus accumbens, substantia nigra, and ventral tegmental area. In comparison to rsFC in the 24-hr group, several circuits showed increased strength of rsFC when the intervals between nicotine doses were shorter, while in other circuits shorter intervals between doses were associated with decreased strength of rsFC. Shorter interdose intervals were associated with stronger rsFC involving the interpeduncular nucleus with six regions (retrosplenial cortex, insula, anterior cingulate cortex, infralimbic cortex, caudoputamen, and medial habenula; Figure 2a, left). Likewise, rsFC of the hippocampus with the anterior cingulate cortex, caudoputamen, insula, nucleus accumbens, orbital frontal area, and retrosplenial cortex was also stronger with shorter interdose intervals (Figure 2a, right). In contrast, rsFC of the hippocampus with the substantia nigra and the ventral tegmental area was weaker with shorter intervals between doses (Figure 2b). Circuits involving the nucleus accumbens with the infralimbic and prelimbic cortices also showed markedly decreased rsFC with shorter interdose intervals (Figure 2b).
Figure 2.
Region of interest (ROI)-based resting-state functional connectivity (rsFC). Four experimental groups of rats received six daily doses of nicotine and challenged on Day 6 with the usual daily dose followed by a second dose delivered 3, 6, 12, or 24hr later, while the control group received six daily doses of saline followed by a nicotine injection 24hr later. The graphs depict the strength of rsFC (correlation coefficient) between pairs of ROI, error bars being the SE. (a) pairs of regions where rsFC decreased as dose intervals increased from 3 to 24hr, and (b) pairs of regions were rsFC increased or partially increased as dose intervals increased. *Significantly different from the 24-hr group (p < .05); §Significantly different from the 12-hr group (p < .05). Statistics were warranted by Tukey–Kramer post hoc multiple comparison. ACC, anterior cingulate cortex; AMG, amygdala; CPu, caudoputamen; HP, hippocampus; ILA, infralimbic area; INS, insula; IPN, interpeduncular nucleus; MH, medial habenula; NAcc, nucleus accumbens; ORB, orbital frontal area; PL, prelimbic area; RSP, retrosplenial cortex; SN, substantia nigra; VTA, ventral tegmental area.
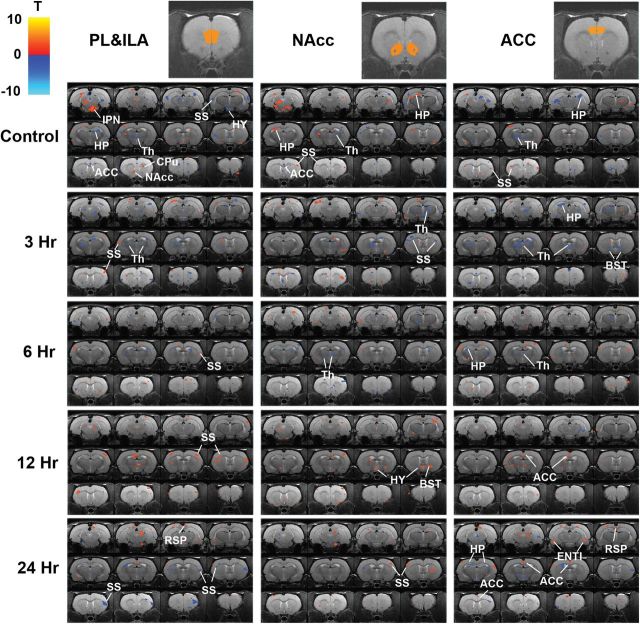
Figure 3 presents the maps depicting rsFC changes in response to the nicotine challenge for all groups using three seed regions: the prefrontal area, nucleus accumbens, and anterior cingulate cortex. Comparing across groups, the control and 3- and 6-hr groups showed predominantly decreased rsFC while in the 12- and 24-hr groups, areas with increased strength of rsFC were more common. The control group showed decreased anterior cingulate cortex connectivity with the prefrontal areas and nucleus accumbens (in response to their first exposure to nicotine), but this was not seen in any of the four experimental groups. In the control group, rsFC between the prefrontal area and the somatosensory area was decreased. As the interdose interval increased from 3 to 24hr, rsFC between these areas switched from increased connectivity (3, 6, and 12hr) to decreased connectivity (24hr). There was decreased nucleus accumbens–thalamus and anterior cingulate cortex–thalamus rsFC in the control and in 3- and 6-hr groups, but not in the 12- and 24-hr groups.
Figure 3.
Seed-based resting-state functional connectivity (rsFC). A control group received a dose of nicotine after six daily doses of saline. The experimental groups received six daily doses of nicotine and were challenged on Day 6 with the usual daily dose followed by a second dose delivered 3, 6, 12, or 24hr later. Top row shows the position of the three seed regions. Maps depicting nicotine-induced rsFC change are superimposed on a standard brain anatomy. Warm color means increased rsFC, and cold color means decreased rsFC (p < .001, uncorrected. p < .02 for display purpose only). ACC = anterior cingulate cortex; BST = bed nucleus stria terminalis; CPu = caudoputamen; HP = hippocampus; ILA = infralimbic area; IPN = interpeduncular nucleus; NAcc = nucleus accumbens; ORB = orbital frontal area; PL = prelimbic area; RSP = retrosplenial cortex; Th = thalamus; SS = somatosensory cortex; HY = hypothalamus; ENTI = entorhinal area.
Discussion
The interval between the challenge dose and the previous dose of nicotine had significant effects on rsFC in many greater limbic system structures including the amygdala, anterior cingulate cortex, caudoputamen, hippocampus, infralimbic area, insula, interpeduncular nucleus, medial habenula, nucleus accumbens, orbital frontal area, prelimbic area, retrosplenium, substantia nigra, and ventral tegmental area. rsFC in many of these areas is affected by nicotine in humans, such as the anterior cingulate cortex, insula, caudate, putamen, and the prefrontal cortex.12,13 The site exhibiting functional connectivity alterations with the widest number of other regions is the interpeduncular nucleus. Interestingly, this brain region reportedly plays a key role in nicotine withdrawal symptoms (reviewed in Dani et al.14).
The possibility that the data reflect random variation is reduced by the observation that as the interdose interval was increased, a progressive increase in the strength of rsFC was observed in some circuits while a progressive decrease was observed in other circuits. Also arguing against random variation is the large number of limbic system structures that showed sensitivity to the interdose interval. The observation that rsFC was strengthened in some circuits and simultaneously decreased in others argues against the possibility that the data reflect global changes in blood flow.
The pattern of some of the results suggests acute and chronic tolerance.4,5 The first dose of nicotine in naive animals decreased rsFC between the prefrontal area and the somatosensory area. At interdose intervals of 3, 6, and 12hr, nicotine increased rsFC in this circuit, but at an interdose interval of 24hr, a decrease in rsFC was again observed. This suggests a transient tolerance to this action of nicotine that was present for at least 12hr after exposure but was gone by 24hr. The control group showed decreased anterior cingulate cortex connectivity with the prefrontal areas and nucleus accumbens which was not seen in any of the four experimental groups which had received daily injections of nicotine prior to the challenge dose. This suggests that prior exposure to nicotine might have induced a chronic tolerance to this effect.
It is worth mentioning that no significant difference was found in nicotine-challenged rsFC between the control group and the 24-hr group. This was surprising as interdose intervals of 24hr have been demonstrated to have a sensitizing effect on blood oxygen level dependence (BOLD) reactivity in rats.15 No sensitization of the rsFC response to nicotine was observed.
We can only speculate in regard to mechanisms that might account for the differences in rsFC observed when the challenge dose was delivered at 3, 6, 12, or 24hr after the previous dose in comparison to a control group which received the first dose of nicotine. With a half-life of 1hr in rats, nicotine levels would be negligible 6 or 12hr after a single dose.16 However, we cannot rule out the possibility that longer-lived metabolites of nicotine might have a lingering effect.
While large and sustained exposures to nicotine can trigger an upregulation of nicotinic acetylcholine receptors (nAChRs) that can persist for weeks, intermittent moderate doses trigger a more transient upregulation in nAChRs measured in hours.17,18 It is possible that differences in the number of nAChRs present in various brain regions at different time intervals after the last dose contribute to differences in responses.19
Another potential mechanism involves the desensitization of nAChRs.20 The many subtypes of nAChRs are distributed throughout the brain with different nAChR subtypes being found in higher concentrations in some regions than others.21 nAChR subtypes are known to differ in relation to the concentration of nicotine required to desensitize them, in how quickly they desensitize, in how deeply they become desensitized, and in the amount of time required to restore normal functioning.19,22–24 A dose of nicotine triggers an augmented outflow of dopamine in the nucleus accumbens in animals that have been sensitized to nicotine.25 This effect was observed at 2 and 7 days after the termination of a nicotine infusion but could not be produced when the challenge dose was delivered while animals were receiving an ongoing infusion of nicotine.25 The experimenters postulated that desensitization of nAChRs during chronic exposure prevented the sensitized dopamine response. When doses of nicotine are delivered at 24-hr intervals, any desensitizing effect from the previous dose has likely worn off.20 The regional differences in rsFC observed when nicotine was delivered at 3, 6, 12, and 24hr after the previous dose in the current study might reflect regional differences in the distribution of nAChR subtypes and their varying sensitivity to desensitization. Another possible factor would be prolonged nAChR occupancy by nicotine.26
There appear to be very few animal studies that examine the effects of manipulating nicotine dosing intervals.4,5 Kenny and Markou examined the effect of 21 days of nicotine self-administration on intracranial self-stimulation thresholds in rats.27 One group was allowed only 1hr of access to nicotine each day while the other was allowed 12hr. Although it was not intentional, this design provided 23hr of abstinence between self-administration sessions for one group and 12hr for the other. Although the group with a 23-hr interval between exposures obtained much less nicotine than the group with a 12-hr interval between exposures (0.38 vs. 1.36mg/kg/day), the impact on the intracranial self-stimulation threshold was significantly greater in the 23-hr abstinence group. This supports the idea that the interval between doses may be critical to the impact nicotine has on the brain.
A limitation of our investigation is that we studied the effect of only one dose strength (0.4mg/kg) and route of administration (subcutaneous). Although this is the most common dose used in nicotine sensitization studies,28 different outcomes might be seen with different doses. Also, we studied interdose intervals of 3–24hr, future studies could examine the effects of interdose intervals of less than 3hr or greater than 24hr. Another limitation is that we were unable to completely control for multiple comparisons in the seed-based analysis but used a threshold for significance of <.001 instead of <.05. We were unable to examine the BOLD reactivity due to movement artifacts. Human studies will be required to determine if the interdose interval is an important consideration in humans. As rsFC does not measure neural activity in absolute terms, we cannot determine if a dosing interval of 24hr produces a greater absolute impact than more frequent dosing. However, our data strongly support the proposition that the timing of nicotine doses influences the impact on the brain, and that the impact may be different when cigarettes are widely spaced than when they are smoked one after another.
Conclusions
It has been commonly assumed that a dose of nicotine will have the same effect regardless of when it is delivered in relation to previous doses. This is especially evident in early human imaging studies in which the timing of the last cigarette smoked prior to the experiment was rarely controlled or reported. The data from the current study indicate that the interval between doses may influence nicotine’s impact on the brain and should be controlled and reported in experimental studies.
Our results may have important implications for the development of nicotine addiction which can start when adolescents are smoking only two cigarettes per week.29 With wider intervals between doses, rsFC in response to nicotine decreased in some circuits while it increased in others. Our data suggest that the nicotine delivered by a cigarette might impact a nondaily smoker differently than a daily smoker. It cannot be assumed that the effects of nicotine observed in experiments involving adult daily smokers can be extrapolated to nondaily smokers. This has important implications for the interpretation of past studies and the design of future studies, especially those with adolescent smokers.
Funding
This work was supported by National Institutes of Health (NIH) grant R01 DA 021846 and R01 DA 025690.
Declaration of Interests
None declared.
References
- 1. Jarvik M, Killen JD, Varady A, Fortmann SP. The favorite cigarette of the day. J Behav Med. 1993;16:413–422. [DOI] [PubMed] [Google Scholar]
- 2. Benowitz NL, Kuyt F, Jacob P., 3rd Circadian blood nicotine concentrations during cigarette smoking. Clin Pharmacol Ther. 1982;32:758–764. [DOI] [PubMed] [Google Scholar]
- 3. Perkins KA, Grobe JE, Mitchell SL, et al. Acute tolerance to nicotine in smokers: lack of dissipation within 2 hours. Psychopharmacology. 1995;118:164–170. [DOI] [PubMed] [Google Scholar]
- 4. Stolerman IP, Fink R, Jarvik ME. Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacologia. 1973;30:329–342. [DOI] [PubMed] [Google Scholar]
- 5. Stolerman IP, Bunker P, Jarvik ME. Nicotine tolerance in rats: role of dose and dose interval. Psychopharmacologia. 1974;34:317–324. [DOI] [PubMed] [Google Scholar]
- 6. King JA, Garelick TS, Brevard ME, et al. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liang Z, Li T, King J, Zhang N. Mapping thalamocortical networks in rat brain using resting-state functional connectivity. NeuroImage. 2013;83C:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang Z, King J, Zhang N. Uncovering intrinsic connectional architecture of functional networks in awake rat brain. J Neurosci. 2011;31:3776–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang N, Rane P, Huang W, et al. Mapping resting-state brain networks in conscious animals. J Neurosci Methods. 2010;189:186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Valentini P, De Sole P, De Luca D, et al. Decreased chemiluminescence in leukocyte adhesion deficiency presenting with recurrent sepsis, amoebiasis and Candida albicans urinary tract infection. Minerva Med. 2006;97:437–442. [PubMed] [Google Scholar]
- 11. Cermelli C, Cenacchi V, Beretti F, Pezzini F, Luca DD, Blasi E. Human herpesvirus-6 dysregulates monocyte-mediated anticryptococcal defences. J Med Microbiol. 2006;55(Pt 6):695–702. [DOI] [PubMed] [Google Scholar]
- 12. Huang W, King J, Ursprung W, et al. The development and expression of physical nicotine dependence corresponds to structural and functional alterations in the anterior cingulate-precuneus pathway. Brain Behav. 2014;4:408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cole D, Beckmann C, Long C, Mathews P, Durcan M, Beaver J. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. NeuroImage. 2010;52:590–599. [DOI] [PubMed] [Google Scholar]
- 14. Dani JA, Jenson D, Broussard JI, De Biasi M. Neurophysiology of nicotine addiction. J Addict Res Ther. 2011;S1:001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Z, DiFranza JR, Wellman RJ, Kulkarni P, King JA. Imaging brain activation in nicotine-sensitized rats. Brain Res. 2008;1199:91–99. [DOI] [PubMed] [Google Scholar]
- 16. Kyerematen GA, Taylor LH, deBethizy JD, Vesell ES. Pharmacokinetics of nicotine and 12 metabolites in the rat. Application of a new radiometric high performance liquid chromatography assay. Drug Metab Dispos. 1988;16:125–129. [PubMed] [Google Scholar]
- 17. Govind A, Walsh H, Green W. Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J Neurosci. 2012;32:2227–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker LK, Mao D, Chi H, et al. Intermittent nicotine exposure upregulates nAChRs in VTA dopamine neurons and sensitises locomotor responding to the drug. Eur J Neurosci. 2013;37:1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–1097. [DOI] [PubMed] [Google Scholar]
- 20. Quick MW, Lester RAJ. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. [DOI] [PubMed] [Google Scholar]
- 21. Feduccia AA, Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors: neuroplastic changes underlying alcohol and nicotine addictions. Front Mol Neurosci. 2012;5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Picciotto MR, Addy N, Mineur Y, Brunzell D. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. [DOI] [PubMed] [Google Scholar]
- 24. Grady SR, Wageman CR, Patzlaff NE, Marks MJ. Low concentrations of nicotine differentially desensitize nicotinic acetylcholine receptors that include alpha5 or alpha6 subunits and that mediate synaptosomal neurotransmitter release. Neuropharmacology. 2012;62:1935–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benwell ME, Balfour DJ, Birrell CE. Desensitization of the nicotine-induced mesolimbic dopamine responses during constant infusion with nicotine. Br J Pharmacol. 1995;114:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brody A, Mandelkern M, London E, et al. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. [DOI] [PubMed] [Google Scholar]
- 28. DiFranza JR, Wellman RJ. Sensitization to nicotine: how the animal literature might inform future human research. Nicotine Tob Res. 2007;9:9–20. [DOI] [PubMed] [Google Scholar]
- 29. DiFranza J, Savageau J, Fletcher K, et al. Symptoms of tobacco dependence after brief intermittent use -The Development and Assessment of Nicotine Dependence in Youth-2 Study. Arch Pediatr Adolesc Med. 2007;161:704–710. [DOI] [PubMed] [Google Scholar]