Abstract
BACKGROUND
Increased arterial stiffness leads to increased pulsatile load on the heart. We investigated associations of components of pulsatile load with a measure of left ventricular (LV) systolic function—global longitudinal strain (GLS), in a community-based cohort ascertained based on family history of hypertension.
METHODS
Arterial tonometry and echocardiography with speckle tracking were performed in 520 adults with normal LV ejection fraction (EF) (age 67±9 years, 70% hypertensive) to quantify measures of pulsatile load (characteristic aortic impedance (Zc), total arterial compliance (TAC), and augmentation index (AI)) and GLS. The associations of log-Zc, log-TAC, and AI with GLS were assessed using sex-specific z-scores for each measure of arterial load.
RESULTS
In univariable analyses, higher Zc was associated with worse GLS (less negative) and higher TAC and AI were associated with better GLS (all P < 0.001). In a multivariable model including age, sex, heart rate (HR), LVEF, mean arterial load (systemic vascular resistance), and measures of pulsatile load, Zc remained associated with GLS (β = 0.28, P < 0.001), while the associations of TAC and AI were no longer significant (P > 0.5). Additional adjustment for cardiovascular risk factors and history of coronary heart disease and stroke did not attenuate the association of Zc with GLS; Zc, sex, HR, LVEF remained associated with GLS after stepwise elimination (all P < 0.001).
CONCLUSIONS
Greater proximal aortic stiffness, as manifested by a higher Zc, is independently associated with worse LV longitudinal function.
Keywords: aortic characteristic impedance, arterial load, arterial–ventricular interaction, blood pressure, hypertension, left ventricular deformation, left ventricular function, speckle tracking echocardiography.
Pulsatile arterial load is mainly determined by large conduit artery stiffness.1 Impaired buffer function of the aorta can increase aortic impedance and pressure, which in turn, affects normal ventricular–arterial coupling. This can lead to myocardial ischemia,2 left ventricular (LV) diastolic dysfunction,3,4 LV remodeling,5 and eventually adverse clinical events.6,7 LV peak strain, a measure of global systolic function, is altered before overt LV dysfunction.8 Whether hemodynamic determinants of pulsatile arterial load—aortic characteristic impedance, arterial compliance, or wave reflection,9,10 are associated with LV deformation has not been fully studied. Identifying the most vulnerable vector of LV multidirectional deformation in response to chronic alteration in arterial load will lead to a better understanding of arterial–ventricular interaction and improve assessment of risk for adverse cardiovascular outcomes.
Of the 3 vectors (longitudinal, circumferential, and radial) of LV deformation, LV global longitudinal strain (GLS) is the most robust prognostic marker in different populations.8 We hypothesized that (i) higher pulsatile arterial load is associated with worse GLS (less negative); (ii) various components of pulsatile load are differentially associated with LV deformation; and (iii) GLS is the primary vector of LV strain associated with pulsatile arterial load. To test these hypotheses, we performed Doppler, 2-dimensional transthoracic and speckle tracking echocardiography (2D-STE), and arterial tonometry in a community-based cohort ascertained based on family history of hypertension.
METHODS
Study participants
The study was approved by the Mayo Institutional Review Board. This was a cross-sectional study, comprising study participants from Genetic Epidemiology Network of Arteriopathy (GENOA) study. The design of the GENOA study has been described previously.11 Briefly, it is one of the 4 “genetic networks” in the NHLBI Family Blood Pressure Program, a community-based study to identify genetic influence on the blood pressure (BP) and target-organ damage due to hypertension, including pathophysiologic changes in cardiovascular system. From September 2009 to December 2011, 660 participants completed noninvasive vascular and echocardiographic assessment and comprised the sample for this study.
Baseline demographics, medical history, and medications were ascertained from a standard questionnaire administrated by a study coordinator. Height was measured by stadiometer and weight by an electronic balance. Resting systolic and diastolic BP were measured with a random zero sphygmomanometer. Hypertension was defined as either systolic BP ≥140mm Hg or diastolic BP ≥90mm Hg at the study visit, or a previous diagnosis of hypertension and current treatment with antihypertensive agents. Diabetes was considered present if a participant had fasting glucose level ≥126mg/dl or was receiving treatment with insulin or oral agents. Smoking (ever) was defined as having smoked more than 100 cigarettes in the past. Fasting glucose and creatinine were measured by standard enzymatic methods. Estimated glomerular filtration rate (eGFR) was calculated by the Modification of Diet in Renal Disease formula12: 186 × serum creatinine−1.154 × age−0.203(× 0.742 if female) and chronic kidney disease was defined as eGFR < 60ml/min 1.73 m2).
Echocardiographic measurements and LV deformation analysis
Comprehensive 2D-transthoracic echocardiographic assessment was performed by experienced diagnostic cardiac sonographers using standardized protocols of the Mayo Clinic echocardiography laboratory and interpreted by a physician with level III training in echocardiography. LV wall thickness, volume, diameters, and ejection fraction were obtained by standard methods.13
Three-beat cine-loop clips (frame rate >40 frames/s) of the apical long, 2- and 4 chamber views, parasternal short axis view at mid-papillary level were archived as standard Digital Images and Communications in Medicine (DICOM) images, and then analyzed off-line using Syngo Velocity Vector Imaging software (Syngo US workstation, Siemens Medical Solutions USA, Malvern, PA) to assess LV peak longitudinal, circumferential and radial strain. The LV endocardium was traced with 10–12 points, starting and ending at the atrioventricular valve annulus. Less negative strain values indicate worse LV systolic function. A positive value of radial strain indicates myocardial thickening. GLS was the average of peak strain from 3 apical views.
Pressure-flow analyses
All tonometry data were acquired on the day of the echocardiographic exam, with participants in the supine position with simultaneous ECG recording. Off-line pressure-flow analyses were performed using a custom-designed software capable of Fourier analysis (Cardiovascular Engineering, Norwood, MA) as previously described.14,15 Briefly, arterial tonometry of right carotid, and brachial arteries was performed by using a custom transducer (Cardiovascular Engineering, Norwood, MA). Carotid waveforms were signal-averaged using the R wave on the ECG as the point of reference and calibrated by brachial diastolic BP and mean BP. Aortic characteristic impedance (Z c) was calculated in the time domain by dividing increase in the pressure by the corresponding increase in aortic volume flow in early systole (time-interval between when foot-flow is identified and when flow reaches 95% of its peak). Total arterial compliance (TAC) was calculated during the latter two-thirds of diastole using the area method.16 Systemic vascular resistance (SVR) was calculated by dividing mean arterial pressure (mm Hg) by cardiac output (l/min) and then multiplying by 80 to convert to dyn s cm5.17 Augmentation index (AI) was defined as the ratio of augmented pressure over central pulse pressure.15
STATISTICAL ANALYSES
Data were expressed as mean (SD) or median (quartiles) for continuous variables and as percentages for categorical variables. Zc, TAC, SVR were natural log-transformed to reduce skewness. Given previously reported sex differences in hemodynamic load,3,18,19 sex-specific z scores of 4 measures of arterial load were used in regression analyses. The associations of measures of arterial load with LV strain were assessed by univariable regression analyses followed by multivariable regression analyses that included age, sex, heart rate, LV ejection fraction (LVEF), and all measures of load as covariates. Additional adjustment for hypertension, diabetes, smoking, chronic kidney disease, history of coronary heart disease, or stroke was also performed. Stepwise regression analyses were performed to identify the component of arterial load with the strongest association with LV strain, using backward elimination and minimal Bayes information criteria. Statistical significance was set as P < 0.05. All analyses were performed by using JMP (SAS Institute, Cary, NC). Intraclass correlation coefficient (ICC) with 95% confidence intervals (CI) was calculated using MedCalc software (version 12.4, Belgium).
Reproducibility of speckle tracking measures
Intra- and interobserver variability in measurements of speckle tracking were assessed in 30 randomly selected participants. Intra- and interobserver ICC (95% CI) in 2 measurements were: 0.94 (0.88–0.97) and 0.92 (0.84–0.96) for GLS; 0.96 (0.92–0.98) and 0.96 (0.86–0.96) for peak circumferential strain; 0.88 (0.76–0.94) and 0.83 (0.66–0.92) for peak radial strain. Bland–Altman plot for comparisons between 2 measures are shown in Supplementary Figure 1.
RESULTS
Participant characteristics
Of 660 participants who completed both noninvasive vascular and echocardiography evaluation, 11 had prosthetic valves, and 131 had LV images inadequate for strain analysis. This resulted in a sample size of 518 for the analyses. Participant characteristics at baseline are summarized in Table 1. The means of LVEF and LV GLS were in the normal ranges.
Table 1.
Participant characteristics
| n = 518 | Mean (SD) or median (IQR) or N (%) |
|---|---|
| Age, years | 66 (9.3) |
| Women, (%) | 317 (61%) |
| Systolic BP, mm Hg | 138 (18) |
| Diastolic BP, mm Hg | 70 (9) |
| Hypertension, % | 398 (77) |
| Diabetes, % | 97 (19) |
| Chronic kidney disease, % | 68 (13) |
| Smoker, % | 222 (43) |
| History of cardiovascular disease, % | 76 (15) |
| Echocardiographic measures | |
| LV mass index, g/m2 | 93 (77–105) |
| LV ejection fraction, % | 63 (6) |
| LA volume index, ml/m2 | 29 (23–42) |
| LV GLS, % | −18.0 (2.1) |
| Peak circumferential strain, % | −27.3 (5.1) |
| Peak radial strain, % | 34.9 (10.1) |
| Arterial load | |
| TAC, ml/mm Hg | 1.4 (1.1–1.9) |
| Zc, dyne-s/cm5 | 188 (145–288) |
| Augmentation index, % | 13.8 (6.6–22.5) |
| SVR, dyn s cm5 | 1,472 (1,264–1,713) |
| Medications | |
| ACEI/ARB, % | 236 (45) |
| Beta blocker, % | 210 (40) |
| Calcium channel blocker, % | 104 (20) |
| Diuretics, % | 258 (50) |
History of cardiovascular disease was defined as history of myocardial infarction, coronary revascularization, or stroke.
Abbreviations: AI, augmentation index; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin II-receptor blocker; GLS, lobal peak longitudinal strain; SVR, systemic vascular resistance; TAC, total arterial compliance; Zc, aortic characteristic impedance.
Association of measures of pulsatile load with LV GLS
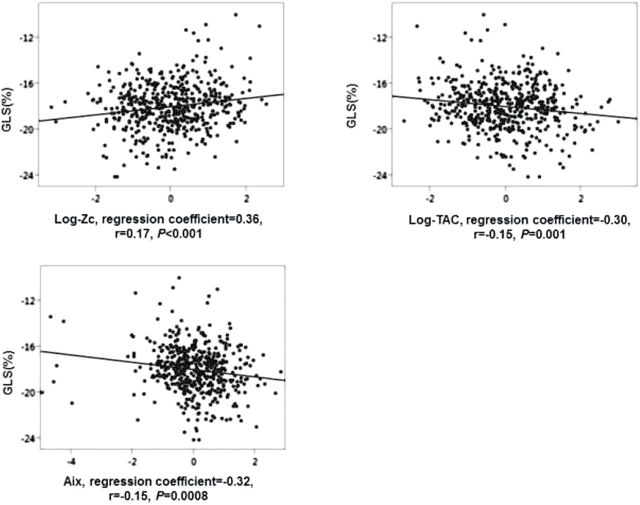
The measures of pulsatile arterial load were associated with GLS in univariable regression analyses (Figure 1); with higher Zc corresponding to worse GLS, and higher TAC and AI corresponding to better GLS. The associations remained significant after adjustment for age and sex. Adjustment for heart rate (HR) attenuated the association of AI with GLS while the association of Zc and TAC remained significant (Table 2).
Figure 1.
Univariate association and correlation of measures of pulsatile load with LV GLS. Z scores of log-Zc, log-TAC and AI were used. Abbreviations: AI, augmentation index; GLS, global longitudinal strain; TAC, total arterial compliance; Zc, aortic characteristic impedance.
Table 2.
Association of pulsatile arterial load with LV GLS
| Measures of pulsatile arterial load | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Log-Zc | 0.34 (<0.001) | 0.31 (<0.001) | 0.28 (0.002) |
| Log-TAC | −0.28 (0.002) | −0.21 (0.02) | −0.10 (0.3) |
| AI | −0.31 (<0.001) | −0.10 (0.1) | −0.17 (0.06) |
Associations indicated percent change in GLS corresponding to one SD increase in measures of load. Value expressed as regression coefficient (P value). Model 1 adjusted for age, sex; Model 2 adjusted for age, sex, HR; Model 3 adjusted for age, sex, HR, mean load-(SVR), and LVEF. Bold values expressed as regression coefficient (P value).
Abbreviations: AI, augmentation index; GLS, global peak longitudinal strain; SVR, systemic vascular resistance; TAC, total arterial compliance; Zc, aortic characteristic impedance.
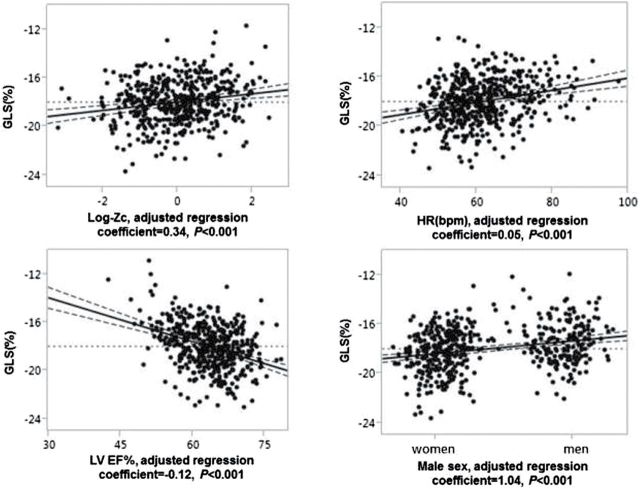
In a multivariable regression model that included age, sex, HR, LVEF, mean arterial load-(SVR), and 3 measures of pulsatile load (Table 3), Zc remained associated with GLS while the associations of TAC and AI were no longer statistically significant. The association of Zc with GLS remained significant after further adjustment for hypertension, diabetes, smoking, chronic kidney disease, history of coronary heart disease, or stroke (β = 0.27, P < 0.001). After stepwise backward elimination from a model including all the covariates listed above and measures of arterial load, 4 variables—Zc, sex, HR, LVEF remained associated with GLS (all P < 0.001, Figure 2). The mean impairment in GLS was 0.34% per 0.4 increase in log-Zc after adjustment for sex, HR, and LVEF.
Table 3.
Multivariate regression analysis of load with LV GLS
| GLS (adjusted R 2 = 0.31) | |||
|---|---|---|---|
| Independent variable | β | Standardized β | P value |
| Age (years) | 0.01 | 0.03 | 0.5 |
| Sex (male) | 1.04 | 0.25 | <0.001 |
| Heart rate (bpm) | 0.05 | 0.23 | <0.001 |
| LVEF, % | −0.11 | −0.36 | <0.001 |
| log-SVR | 0.19 | 0.08 | 0.05 |
| Log-Zc | 0.32 | 0.12 | <0.001 |
| Log-TAC | 0.10 | 0.05 | 0.4 |
| AI | −0.12 | −0.06 | 0.2 |
Associations indicated 1% change in GLS corresponding to per SD change in measures of load. Values expressed as regression coefficient β and standardized coefficient β for each variable in multivariate linear regression analysis. Standardized β showed how many standard deviation (SD) changes in GLS could be explained by per SD change in measures of pulsatile load.
Abbreviations: AI, augmentation index; GLS, global peak longitudinal strain; SVR, systemic vascular resistance; TAC, total arterial compliance; Zc, aortic characteristic impedance.
Figure 2.
Leverage plot to show multivariable adjusted association with GLS after controlling for other covariates. Abbreviations: bpm, beat per minute; GLS, global longitudinal strain; HR, heart rate; Zc, aortic characteristic impedance.
We did not find measures of pulsatile load to be associated with LVEF or TAC to be associated with peak radial strain. Higher TAC was associated with better peak circumferential strain after adjustment for age, sex, and LVEF (β = −0.48, P = 0.04). Further adjustment for HR attenuated the association (P = 0.1). Higher AI was associated with better peak radial strain after adjustment for age, sex, HR, and LVEF (β = 1.12, P = 0.03). However, the association was no longer statistically significant after further adjustment for cardiovascular risk factors (P > 0.05).
Given that medication use may affect the associations of measures of arterial load with GLS, we analyzed associations of ACEI/ARB, beta-blocker, calcium channel blockers, and diuretics use with GLS (Supplementary Table 1). Only ACEI/ARB use was associated with GLS. Measures of pulsatile arterial load remained associated with GLS after adjustment for ACEI/ARB use (Supplementary Table 2). Zc remained associated with GLS after adjustment for all covariates and ACEI/ARB use (β = 0.22, P = 0.03).
To assess whether measures of pulsatile load were associated with GLS independent of central or peripheral BP, we assessed associations with GLS after adjustment for peripheral SBP, DBP PP, central SBP, DBP, and PP, respectively. All associations remained significant (Supplementary Table 3). Zc remained associated with GLS after adjustment for covariates and BP measures (Supplementary Table 4).
Correlation of measures of LV strain and LVEF
A scatterplot sharing show correlations between measures of LV strain and with LVEF is shown in Figure 3. Measures of peak strain in longitudinal, circumferential, and radial directions were correlated, and a better LV strain was correlated with higher LVEF. The correlation of GLS with LVEF was stronger than that of peak circumferential or radial strain with LVEF, based on by Pearson’s r and 95% CI.
Figure 3.
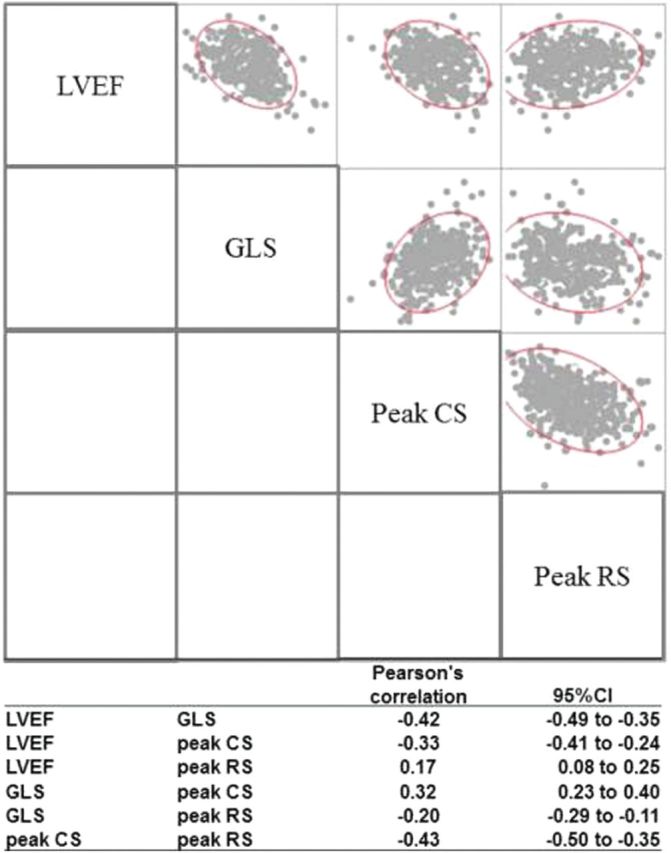
A scatterplot matrix of correlation between measures of LV strain and with LVEF, with Pearson’s correlation r, and 95% confidence interval. Abbreviations: CS, LV peak circumferential strain; GLS, LV global longitudinal strain; RS, LV peak radial strain.
In multivariable regression analyses, the 3 vectors of peak strain explained 21% of overall variation in LVEF (P < 0.001 for the model). GLS and peak circumferential strain remained associated with LVEF (both P < 0.001) while the association of peak radial strain with LVEF was attenuated (P = 0.9). Given the correlation among the 3 vectors of LV strain, we assessed the association of Zc with GLS after additional adjustment for peak circumferential or radial strain to assess whether they were potential confounders. The association of Zc with GLS remained significant after adjusting for all covariates. In contrast, the association of AI with peak radial strain (P = 0.07) and the association of TAC with peak circumferential strain (P = 0.1) were attenuated after adjustment for GLS.
DISCUSSION
The major findings of our study were that: (i) higher pulsatile load, especially a higher Zc, was associated with worse GLS; (ii) GLS was the mediator of associations of load with LV strain in other directions. Thus, increased proximal aortic stiffness may have an adverse effect on LV longitudinal function in older adults without overt LV dysfunction.
Zc is determined by aortic root stiffness and aortic diameter. It is a measure of the magnitude of pulsatile load during early systole in the absence of wave reflection.10 A greater Zc reflects mismatch between aortic flow and area. Mitchell et al.19 and O’Rourke et al.20 both reported Zc as an important determinant of pulsatile load in older individuals in 2 large population-based studies. Zc was increased in patients with chronic heart failure compared to age-matched controls21 and inversely correlated with peak systolic tissue velocity.22,23 LV deformation is a measure of ventricular function increasingly used in clinical practice as impaired LV deformation, especially in the longitudinal direction, has been reported in different disease settings.8 Previous studies reported the association of Zc with LV hypertrophy and concentric remodeling,24,25 and microvascular damage.26 Our results suggest that Zc may influence LV mechanics as well.
TAC and AI are 2 other measures of pulsatile load, with one representing global arterial compliance and the other representing wave reflection. TAC is the relative increase in volume over pressure increase in the aorta during mid to late systole.1 Strain is the summation of deformation mainly determined by the volume change in the ventricle. We found that TAC was associated with LV systolic function in longitudinal and circumferential directions, 2 of the main vectors contributing to LV ejection, consistent with previous studies using tissue Doppler or MR imaging.23,27 Adjustment for GLS attenuated the association of TAC or AI with LV strain, suggesting GLS as a primary vector in the association of load with LV deformation. The association of AI with GLS and TAC with peak circumferential strain was not independent of HR, suggesting that HR influences the association of mid-late arterial load with LV deformation, in contrast to the association of early-systolic load (Zc) with GLS.
Three prior studies evaluated the association of measures of pulsatile load with regional LV deformation in community-based cohorts using tissue Doppler or MR. Sakiewicz et al.28 found higher pulse pressure to be associated with better radial strain but not with longitudinal strain in young adults (n = 324, mean age 40 years, 57% women, 48% hypertensive). Russo et al.29 reported greater arterial wave reflection to be associated with lower longitudinal strain in older community-dwellers (n = 301, mean age 8.3 years, 64.1% women, 65% hypertensive). Fernandes et al.27 found higher carotid arterial compliance to be associated with better mid-wall circumferential strain derived from MR imaging in participants from Multi-Ethnic Study of Atherosclerosis (n = 1,100, mean age 66.3 years, 54% men, 44% hypertensive). Differences in imaging modalities and patient characteristics may explain differences in the results between the studies.
The association of higher AI with better GLS could be due to several possible factors. First, recent studies suggest that AI is influenced by LV function.30–32 A vasodilator such as nitroglycerin decreases AI and stroke volume simultaneously,30 suggesting a positive correlation between AI and preload/LV output. Better GLS correlates with greater relative volume change from end diastole to end systole. Thus, a smaller volume change that is associated with lower AI may lead to worse GLS. Second, higher heart rate was associated with both worse GLS and lower AI. When we adjusted for heart rate, the association of AI with GLS was no longer statistically significant. Whether chronic increase in AI can impair LV contractility will need further investigation.
Our study is the first to report that proximal aortic stiffness measured by Zc is associated with LV GLS and GLS is the primary vector of deformation that is associated with chronic alteration in afterload. Our results suggest that proximal aortic stiffness may impair GLS and lead to arterial-ventricular “decoupling.”
LIMITATIONS
This was a cross-sectional study and the majority of our participants were older adults with hypertension. Patients with poor image quality were likely to have comorbidites, such as obesity or chronic obstructive pulmonary disease. Those who were able to participate and complete functional studies were more likely to be less sick compared with those who were unable to participate. Our results may not be generalizable to adults with greater burden of comorbidity or to younger or normotensive people. In addition, we assessed arterial and ventricular function only at baseline, so our study precludes drawing inferences regarding causality between greater arterial load and LV deformation, including the impact of chronic increase in AI on GLS. We only measured LV strain in longitudinal, circumferential and radial directions, which explained only 21% of variation in LVEF.
In conclusion, in older adults without overt LV dysfunction, higher pulsatile load, especially proximal aortic stiffness measured by Zc, was associated with worse LV global longitudinal function. These results suggest that increased Zc may impair LV function and predispose to adverse outcomes. Follow-up studies are needed to investigate whether: (i) longitudinal change in LV strain corresponds to changes in arterial load during cardiovascular disease progression and (ii) Zc can be target of therapy to improve LV strain and maintain an optimal VA interaction with aging or in the setting of hypertension.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURES
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
We thank Hongmei Xia, MD for assistance with the interobserver agreement analyses for STE. This work was supported by grants HL89354 and M01 RR00585 from the National Institutes of Health.
REFERENCES
- 1. Mitchell GF. Arterial stiffness and wave reflection in hypertension: pathophysiologic and therapeutic implications. Curr Hypertens Rep 2004; 6:436–441. [DOI] [PubMed] [Google Scholar]
- 2. Assmann G, Cullen P, Evers T, Petzinna D, Schulte H. Importance of arterial pulse pressure as a predictor of coronary heart disease risk in PROCAM. Eur Heart J 2005; 26:2120–2126. [DOI] [PubMed] [Google Scholar]
- 3. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex Differences in Arterial Stiffness and Ventricular-Arterial Interactions. J Am Coll Cardiol 2013; 61:2573–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension 2012; 60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hinderliter AL, Sherwood A, Blumenthal JA, Light KC, Girdler SS, McFetridge J, Johnson K, Waugh R. Changes in hemodynamics and left ventricular structure after menopause. Am J Cardiol 2002; 89:830–833. [DOI] [PubMed] [Google Scholar]
- 6. Mitchell GF, Arnold JM, Dunlap ME, O’Brien TX, Marchiori G, Warner E, Granger CB, Desai SS, Pfeffer MA. Pulsatile hemodynamic effects of candesartan in patients with chronic heart failure: the CHARM Program. Eur J Heart Fail 2006; 8:191–197. [DOI] [PubMed] [Google Scholar]
- 7. Gordin D, Wadén J, Forsblom C, Thorn L, Rosengård-Bärlund M, Tolonen N, Saraheimo M, Harjutsalo V, Groop PH. Pulse pressure predicts incident cardiovascular disease but not diabetic nephropathy in patients with type 1 diabetes (The FinnDiane Study). Diabetes Care 2011; 34:886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blessberger H, Binder T. Two dimensional speckle tracking echocardiography: clinical applications. Heart 2010; 96:2032–2040. [DOI] [PubMed] [Google Scholar]
- 9. Mitchell GF. Triangulating the peaks of arterial pressure. Hypertension 2006; 48:543–545. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell GF. Clinical achievements of impedance analysis. Med Biol Eng Comput 2009; 47:153–163. [DOI] [PubMed] [Google Scholar]
- 11. Kullo IJ, Turner ST, Kardia SL, Mosley TH, Jr, Boerwinkle E, de Andrade M. A genome-wide linkage scan for ankle-brachial index in African American and non-Hispanic white subjects participating in the GENOA study. Atherosclerosis 2006; 187:433–438. [DOI] [PubMed] [Google Scholar]
- 12. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 13. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004; 43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 15. Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation 2010; 122:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Z, Brin KP, Yin FC. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol 1986; 251:H588–H600. [DOI] [PubMed] [Google Scholar]
- 17. Fuster V. Hurst’s the heart, 11th edn. McGraw-Hill, Medical Publishing Division: New York, 2004. [Google Scholar]
- 18. Zamani P, Bluemke DA, Jacobs DR, Jr, Duprez DA, Kronmal R, Lilly SM, Ferrari VA, Townsend RR, Lima JA, Budoff M, Segers P, Hannan P, Chirinos JA. Resistive and pulsatile arterial load as predictors of left ventricular mass and geometry: the multi-ethnic study of atherosclerosis. Hypertension 2015; 65:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension 2008; 51:1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Namasivayam M, McDonnell BJ, McEniery CM, O’Rourke MF. Does wave reflection dominate age-related change in aortic blood pressure across the human life span? Hypertension 2009; 53:979–985. [DOI] [PubMed] [Google Scholar]
- 21. Mitchell GF, Tardif JC, Arnold JM, Marchiori G, O’Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension 2001; 38:1433–1439. [DOI] [PubMed] [Google Scholar]
- 22. Weber T, O’Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens 2008; 21:1194–1202. [DOI] [PubMed] [Google Scholar]
- 23. Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol 2007; 50:1570–1577. [DOI] [PubMed] [Google Scholar]
- 24. Chirinos JA, Segers P, Raina A, Saif H, Swillens A, Gupta AK, Townsend R, Emmi AG, Jr, Kirkpatrick JN, Keane MG, Ferrari VA, Wiegers SE, St John Sutton MG. Arterial pulsatile hemodynamic load induced by isometric exercise strongly predicts left ventricular mass in hypertension. Am J Physiol Heart Circ Physiol 2010; 298:H320–H330. [DOI] [PubMed] [Google Scholar]
- 25. Pucci G, Hametner B, Battista F, Wassertheurer S, Schillaci G. Pressure-independent relationship of aortic characteristic impedance with left ventricular mass and geometry in untreated hypertension. J Hypertens 2015; 33:153–160. [DOI] [PubMed] [Google Scholar]
- 26. Mitchell GF. Increased aortic stiffness: an unfavorable cardiorenal connection. Hypertension 2004; 43:151–153. [DOI] [PubMed] [Google Scholar]
- 27. Fernandes VR, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, McClelland R, Hundley G, Pearson G, O’Leary DH, Bluemke DA, Lima JA. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2008; 28:194–201. [DOI] [PubMed] [Google Scholar]
- 28. Sakiewicz W, Kuznetsova T, Kloch-Badelek M, D’Hooge J, Ryabikov A, Kunicka K, Swierblewska E, Thijs L, Jin Y, Loster M, Malyutina S, Stolarz-Skrzypek K, Kawecka-Jaszcz K, Narkiewicz K, Staessen JA. Tissue Doppler indexes of left ventricular systolic function in relation to the pulsatile and steady components of blood pressure in a general population. J Hypertens 2012; 30:403–410. [DOI] [PubMed] [Google Scholar]
- 29. Russo C, Jin Z, Takei Y, Hasegawa T, Koshaka S, Palmieri V, Elkind MS, Homma S, Sacco RL, Di Tullio MR. Arterial wave reflection and subclinical left ventricular systolic dysfunction. J Hypertens 2011; 29:574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fok H, Guilcher A, Li Y, Brett S, Shah A, Clapp B, Chowienczyk P. Augmentation pressure is influenced by ventricular contractility/relaxation dynamics: novel mechanism of reduction of pulse pressure by nitrates. Hypertension 2014; 63:1050–1055. [DOI] [PubMed] [Google Scholar]
- 31. Paglia A, Sasso L, Pirozzi F, Iannuzzi A, Carlomagno A, Abete P, Petretta M, Bonaduce D. Arterial wave reflections and ventricular-vascular interaction in patients with left ventricular systolic dysfunction. Int Heart J 2014; 55:526–532. [DOI] [PubMed] [Google Scholar]
- 32. Sweitzer NK, Hetzel SJ, Skalski J, Velez M, Eggleston K, Mitchell GF. Left ventricular responses to acute changes in late systolic pressure augmentation in older adults. Am J Hypertens 2013; 26:866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.