Abstract
Phthalate esters (PEs) constitute a large class of compounds that are used for many consumer product applications. Many of the C2–C7 di-ortho PEs reduce fetal testicular hormone and gene expression levels in rats resulting in adverse effects seen later in life but it appears that relatively large reductions in fetal testosterone (T) levels and testis gene expression may be required to adversely affect reproductive development (Hannas, B. R., Lambright, C. S., Furr, J., Evans, N., Foster, P. M., Gray, E. L., and Wilson, V. S. (2012). Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: a targeted RT-PCR array approach for defining relative potency. Toxicol. Sci. 125, 544–557). The objectives of this study were (1) to model the relationships between changes in fetal male rat plasma testosterone (PT), T levels in the testis (TT), T production (PROD), and testis gene expression with the reproductive malformation rates, and (2) to quantify the “biologically relevant reductions” (BRRs) in fetal T necessary to induce adverse effects in the offspring. In the fetal experiment, Harlan Sprague-Dawley rats were dosed with dipentyl phthalate (DPeP) at 0, 11, 33, 100, and 300 mg/kg/day from gestational days (GD) 14–18 and fetal testicular T, PT levels, and T Prod and gene expression were assessed on GD 18. In the postnatal experiment, rats were dosed with DPeP from GD 8–18 and reproductive development was monitored through adulthood. The dose-response curves for TT levels (ED50 = 53 mg/kg) and T PROD (ED50 = 45 mg/kg) were similar, whereas PT was reduced at ED50 = 19 mg/kg. When the reductions in TPROD and Insl3 mRNA were compared with the postnatal effects of in utero DPeP, dose-related reproductive alterations were noted when T PROD and Insl3 mRNA were reduced by >45% and 42%, respectively. The determination of BRR levels may enable risk assessors to utilize fetal endocrine data to help establish points of departure for quantitative risk assessments.
Keywords: anti-androgen, risk assessment, fetal male rat endocrine, dipentyl phthalate
Phthalate esters (PEs) constitute a large class of plasticizer compounds that are widely used for many consumer product applications. Many of the C2–C7 di-ortho PEs (DioPEs) reduce fetal male testicular testosterone production (T PROD) and gene expression levels resulting in adverse effects that are seen only after birth (Furr et al., 2014). More than 25–30 genes have been shown to date to display reduced expression levels in the fetal testis after exposure to PEs, including genes involved in steroid hormone synthesis and transport, Insl3 hormone synthesis (Hannas et al., 2012), and cholesterol synthesis (Gray et al., in preparation; Johnson et al., 2011). A recent study from our laboratory (Hannas et al., 2011a) demonstrated that the PE ED50 values for anogenital distance (AGD) at birth and retained nipples in infant male rats were 3–5-fold higher than the ED50 value for reductions in testosterone production and testis gene expression with dipentyl phthalate (DPeP) being the most potent of the active PEs. Taken together, these results indicate that relatively large reductions in T PROD and gene expression may be required to induce biologically relevant alterations of postnatal reproductive development.
These fetal endocrine alterations result in a suite of postnatal alterations known collectively as the Phthalate Syndrome (PS) (Gray and Foster, 2003). PS has a unique phenotype that differs from the phenotype seen in males exposed to androgen receptor (AR) antagonists like flutamide (McIntyre et al., 2001; Miyata et al., 2002), vinclozolin (Gray et al., 1994; Hellwig et al., 2000; van Ravenzwaay et al., 2013), procymidone (Hosokawa et al., 1993; Ostby et al., 1999), or pyrifluquinazon (Yasunaga et al., 2013). PS includes abnormalities in androgen-dependent and Insl3-dependent tissues. Androgen-dependent tissues that are affected in F1 males include the epididymis, seminal vesicle, ventral prostate, vas deferens, AGD, female-like nipple retention and, at the higher dosage levels, hypospadias. Reduced Insl3 hormone levels result in gubernacular cord agenesis or hypoplasia and elongation and testis non-descent. In contrast, AR antagonists-like flutamide and vinclozolin, for example, can also induce testis non-descent but these chemicals do not induce gubernacular cord agenesis.
In addition to the in utero effects on reproductive tract development in the male rat, the active DioPEs also cause uterine and vaginal malformations in F1 females (Hannas et al., 2013), low maternal plasma progesterone and spontaneous abortions at mid pregnancy (Gray et al., 2006), perinatal mortality, reduced postnatal growth, skeletal malformations, and anophthalmia.
The objectives of this study were (1) to model the relationship between changes in fetal male rat plasma testosterone (PT), T levels in the testis (TT), T PROD, and testis gene expression with the postnatal reproductive alterations in F1 males, and (2) to describe “biologically relevant reductions” (BRRs) in the fetal endocrine measures (ie, how much of a change is necessary to induce malformations in the male offspring). We also examined the relationship between mRNA for the peptide hormone Insl3 and testis descent since this hormone is critical for normal development of the gubernacular cord during sexual differentiation.
In the fetal experiment study, pregnant Harlan Sprague-Dawley (SD) rats were dosed by oral gavage on gestational days (GD) 14–18 with DPeP and fetal testosterone levels were assessed on GD 18. In the postnatal study, pregnant rats were dosed with DPeP from GD 8 to 18, in order to include the periods of major organogenesis and sexual differentiation. The data from these 2 experiments were combined in order to determine the percent reductions in T PROD, TT, and PT to the dose-response curves for the postnatal effects of in utero DPeP using logistic regression models to (1) determine the shape of the curves (T level vs postnatal effect) and (2) interpolate the reductions in T levels required to induce postnatal changes using logistic regression models. Our studies have focused on the measurement of T PROD rather than PT or TT levels because T PROD is more precise and reproducible within and between experiments and the measurement of T PROD is less resource intensive than TT or PT, enabling us to execute studies in a shorter time frame.
MATERIALS AND METHODS
Animals
Timed-pregnant SD rats were purchased from Harlan Laboratories (Indianapolis, Indiana). Rats were shipped to EPA on GD 1. The presence of a positive sperm plug was considered GD 0. Animals were housed individually in clear, polycarbonate cages (20 × 25 × 47 cm3) lined with laboratory-grade heat-treated pine shavings (Northeastern Products, Warrensburg, New York), with a 14:10 light/dark photoperiod (lights off at 19:00) at 20°C –24°C. Animals were fed NIH 07 breeding diet for rats and water from a municipal supply (Durham, North Carolina), filtered at 5 µm ad libitum. These studies were conducted under protocols approved by the National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee at a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Chemicals
DPeP was obtained from Sigma (CAS # 131-18-0, lot # 1431420). Purity was verified as >99% by GC with flame ionization detection by the vendor. The vehicle used to deliver DPeP was laboratory grade corn oil (Sigma; CAS 8001-30-7; lot # 126K0117; cat # C-8267) at 2.5 ml/kg body weight. All other chemicals used for this study were of analytical grade purchased from Fisher Scientific (Pittsburg, Philadelphia).
Fetal Experiments with DPeP Administered on GD 14–18
Experimental design
Pregnant Harlan SD rats were gavaged daily on GD 14–18 with 0, 11, 33, 100, or 300 mg DPeP/kg/day in corn oil to compare the dose-related reductions on fetal testis T Prod with those reported by Hannas et al. (2011a) and to determine if plasma T and testis T levels were similarly reduced.
Fetal necropsy
Approximately 2–4 h after the last dosage was administered on the morning of GD 18, the rat dams were euthanized by exsanguination, the uterus was removed and the number of fetuses (live and dead) and resorptions were counted and recorded. Fetal necropsies were performed within a 2-h window between 08:30 am and 10:30 am. Once removed from the uterus, fetuses were euthanized, fetal blood was collected, and testes were removed using a dissecting microscope.
Fetal plasma collection
Fetuses were removed from the uterus and blood was collected from the jugular vein using heparinized capillary tubes. Blood was blown from the capillary tube with a fine-tip disposable transfer pipet, and transferred into a 0.65-ml (siliconized) microcentrifuge tube. Blood samples from all males in each litter were pooled and stored on ice until centrifugation. The pooled blood samples were then centrifuged at 3000 rpm at 4°C for 10 min. The plasma was removed using a 10-µl pipet, placed in a new tube, and stored at −80°C until assayed for T by RIA using Coat-a-Count Kits (Siemens, Los Angeles, California) according to the protocol provided by the manufacturer. Fetal plasma T levels are based upon the 7–9 litters/dose group.
Ex vivo testicular T production (T PROD)
One testis per male from 3 males per litter was used to measure ex vivo T PROD levels (numbers of litters are 16, 11, 10, 12, and 14 in the 0, 11, 33, 100, and 300 mg/kg/day groups, respectively). Paired testes from other males in some of these litters (up to 3 males per litter) were used for determinations of fetal testis weight (5–6 litters/dose group) and extracted testicular T levels (7–8 litters/dose group). Testicular T PROD was measured as previously described (Furr et al., 2014) in media using a Coat-a-Count radioimmunoassay (RIA) kit for total testosterone according to the protocol provided by the manufacturer (Siemens Healthcare Diagnostics, Deerfield, Illinois). The intra-assay coefficient of variation was 3.1% (based on variability of the standard curve). The interassay coefficient of variation was 13.7%. Cross reactivity with DHT was 3.2%. The limit of detection for T was 0.2 ng/ml.
Fetal testis gene expression using 96 well QPCR custom arrays
Fetal testis gene expression was measured using 96 gene custom-designed QPCR arrays (described in detail Hannas et al., 2011b, 2012). We have found that phthalates that disrupt testosterone levels also reduce the expression of 10–12 genes on these arrays including those coding for proteins involved in steroid transport, and testosterone and Insl3 hormone synthesis (including Cyp11b1, StAR. Cyp11a1, Hsd3b, Cyp17a1, Scarb1, Insl3, Inha, Cyp11b2, Lhcgr, and Dhcr7). Among the DioPEs, several of these genes on our arrays typically display ED50 values that are similar to (StAR, Scarb1, and Cyp17a1) or below (Cyp11b1) the ED50 value for T Prod.
Fetal testicular T extraction
Testosterone was extracted from the testes collected on GD 18. Paired testes were placed in glass tubes (12 × 75 mm2) (n = 3 per litter), the testes in the glass tubes were flash frozen with dry ice and stored at −80°C. Detailed extraction methods are provided in the Supplementary files.
Postnatal Experiment with DPeP administered on GD 8–18
Experimental design
Pregnant Harlan SD rats were gavaged daily on GD 8–18 with 0, 11, 33, 100, or 300 mg/kg/day DPeP in corn oil with 5 dams/dose group. The maternal data and F1 male offspring up to weaning on postnatal day (PND) 24 were described by Hannas et al. (2011a). The exposures started on GD 8 rather than GD 14, when dosing started in the fetal study, so we could compare the dose responses for effects on F1 male reproductive development induced during the masculinizing window with other adverse effects that are induced earlier in gestation than is disruption of male sexual differentiation; effects that might turn out to be relevant to a hazard assessment of the chemical. For example, we have found that in utero phthalate administration during organogenesis results in F1 female reproductive tract malformations, skeletal malformations, and other adverse effects, effects not seen if phthalate exposure is restricted to the period of sexual differentiation (Hannas et al., 2013). In addition, exposure to a phthalates from GD 8 to 13 does not affect F1 male reproductive tract development (Carruthers and Foster, 2005; Hannas et al., 2013) which is consistent with the fact that the fetal testis is not producing measurable testosterone at this stage of development (Feldman and Bloch, 1978; Gangnerau and Picon, 1987; Majdic et al., 1998; Warren et al., 1973). Furthermore, early gestational exposure to phthalates like DPeP would not be expected to accumulate in tissues because the majority of the chemical and its metabolites are likely excreted within 24 h, similar to the better studied phthalates DBP and DEHP (Kluwe, 1982).
The continuation of the F1 offspring study from weaning through adult life is reported here. The AGD (PND 2) and infant male nipple retention (PND) 13 data from this study were reported by Hannas et al. (2011a) and are shown here for comparison to the prenatal T PROD, PT and TT data, and the postnatal effects seen later in life.
Male and female offspring were weaned on PND 24 and housed in groups of 2–3 per cage by sex until necropsy. Male offspring were checked daily from 37 to 55 days of age to determine the age and weight at “puberty” (preputial separation—PPS) and both male (n = 98 total) and female (n = 103 total) offspring were necropsied beginning at 120 days of age.
F1 females were examined for gross malformations (including anophthalmia, absent vaginal opening or vaginal canal, uterine agenesis and hydrometrocolpos and hydronephrosis; all traits seen in the female rat—female PS). Weights of the pituitary, uterus, ovaries, kidneys, and liver were recorded from 2 females per litter (since organ weights were unaffected by in utero exposure to DPeP these measurements were not taken from all F1 females).
In F1 males, body, glans penis, seminal vesicle, ventral prostate, testis, epididymal, levator ani-bulbocavernosus muscles, Cowper’s glands, kidney, and liver weights were recorded along with any gross malformations. In addition, gubernacular cord lengths were measured with a micrometer. Gubernacular abnormalities were either complete absence or elongation of a hypoplastic cord. We defined “abnormal”—elongated gubernacular cords as those longer than 17 mm, a value that exceeds the control mean by several standard deviations. Control gubernacular cord in this study averaged ∼9.5 mm with a standard deviation of ∼1.2 mm.
Testes and epididymal tissues were preserved in Bouin’s fixative, placed in 70% ethanol after 24 h and sent to Experimental Pathology Laboratories, Inc., Durham, North Carolina (EPL Project No. 431-603) where they were embedded in paraffin, sectioned at 4–6 μm, stained using hematoxylin and eosin and histopathological analyses were conducted by a board certified pathologist.
Since several of the male and female F1 rats in the high dose group developed malocclusion of the incisors due to obvious skull abnormalities, skulls were frozen and shipped to Skulls Unlimited International, INC., Oklahoma City, Oklahoma. At the taxidermist, skulls were individually tagged for identification, cleaned using flesh-eating dermestid beetles (Dermestes maculatus) and returned for gross examination and morphometric analysis of the length and width of the left and right zygomatic arch. Since this structure was obviously asymmetrical in the affected animals it provided a quantitative descriptor of the gross skeletal malformation.
Statistics
Data analysis was performed using 1-way ANOVA through the General Linear Model procedure (PROC GLM) in the Statistical Analysis System (SAS, SAS Institute, Cary, North Carolina). If the overall ANOVA was significant at (P < .05) the significant differences between control and treated groups were determined by a post hoc 2-tailed t test (LSMEANS on SAS) between litter means. Fetal T PROD data were analyzed using litter mean values generated from the individual testis incubations from males within a litter. The fetal T PROD data from this study were pooled with DPeP dose-response data collected earlier (Furr et al., 2014) and these pooled results were used to model the relationship between T PROD and the postnatal consequences of in utero DPeP exposure. The effects of in utero DPeP exposure on F1 male rat postnatal reproductive development were also analyzed using litter means rather than individual animal values.
Dose-response curves from all experiments were analyzed using untransformed and transformed (to percent of control) data in a non-linear 4 parameter logistic (4PL) regression model (sigmoidal fit with variable slope Prism GraphPad 5.01 software, GraphPad Software, Inc., La Jolla, California). For logistic regression analyses, the control dose value was set to 1 mg/kg/day rather than 0 mg/kg/day so the control data would be included in the analysis as Prism converts the dose to log10 values and the top of the models were constrained to 100% and the bottom to 0%.
In addition, we used both the 4PL and 5 (5PL) logistic regression models to describe the relationship between the fetal testosterone measures (T Prod on the X-axis rather than the DPeP dose) and the postnatal effects of DPeP to estimate by interpolation the BRR in fetal T PROD (BRRT); BRRT being the estimated reductions in T levels resulting in postnatal alterations in the 2%, 5%, 10%, 25%, 50%, 75%, and 95% of the male offspring. The BRRT values were estimated using a 4PL which assumes curve symmetry on both sides of the ED50 as well as a 5PL parameter model which includes a parameter for asymmetry of the slope above and below the ED50 value. The 4PL logistic regression model was also used to define the BRR in Insl3 mRNA with DPeP-induced increases in gubernacular cord abnormalities and undescended testes in F1 male rats.
RESULTS
Fetal Study
Maternal and litter effects
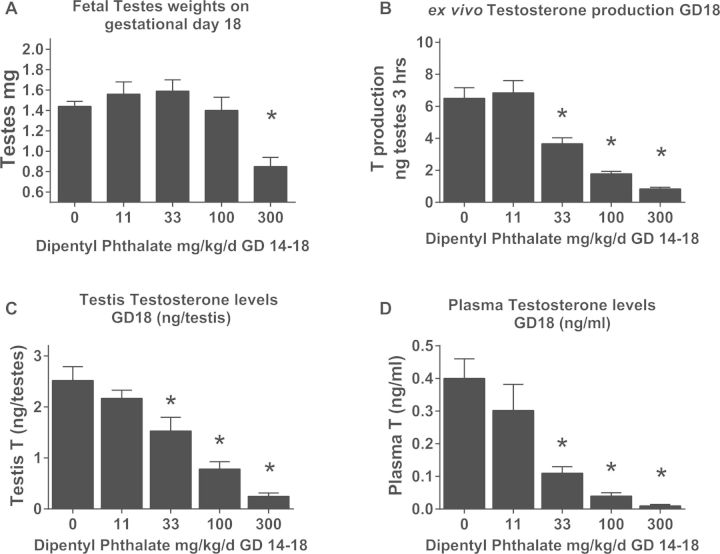
DPeP did not induce any signs of overt maternal toxicity at any dosage levels in this study. Maternal weight gain was slightly reduced in the highest dose group by ∼10 g compared with controls (Table 1) during the 5-day dosing period (GD 14–18) whereas maternal weight was not significantly reduced from 333 g in control dams to 321 g at 300 mg DPeP/kg. In utero exposure to DPeP during GD 14–18 did not significantly alter the total numbers of fetuses, the numbers of resorbed fetuses, or the number of dead fetuses (Table 1). During the fetal necropsies, the descent of the testes from the renal area to the inguinal region was noticeably delayed in males from most litters in the high DPeP dose group. In addition, paired testes weight was significantly reduced in this group (Figure 1A) and partially descended, small testes were noted in 2 males in one litter exposed to 33 mg DPeP/kg/day.
TABLE 1.
Maternal, Fetal and Neonatal Effects of Gestational Administration of Dipentyl Phthalate (DPeP)
| Dose mg/kg/day | 0 | 11 | 33 | 100 | 300 | F value |
|---|---|---|---|---|---|---|
| Maternal and fetal effects of administration of DPeP on days 14–18 of pregnancy | ||||||
| Number of litters | 8 | 9 | 7 | 9 | 9 | |
| Maternal weight GD 18 | 333 ± 8.7 | 328 ± 8.6 | 339 ± 8.5 | 337 ± 7.9 | 321 ± 6.9 | NS |
| Maternal weight gain GD 18 | 49.5 ± 5.2 | 43.6 ± 4.0 | 47.3 ± 2.9 | 49.6 ± 2.3 | 37.9 ± 4.7* | NS |
| Number fetuses | 12.8 ± 1.3 | 9.2 ± 1.7 | 12.4 ± 1.2 | 12.3 ± 0.7 | 10.0 ± 1.5 | NS |
| Number resorbed | 0 ± 0 | 1.8 ± 1.7 | 0.14 ± 0.14 | 0.33 ± 0.17 | 1.0 ± 0.9 | NS |
| Number dead | 0.12 ± 0.12 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | NS |
| Maternal and neonatal effects of administration of DPeP on days 8–18 of pregnancy | ||||||
| Number of litters | 5 | 5 | 5 | 5 | 5 | |
| Maternal weight (g) GD 18 | 318.2 ± 12.9 | 317.6 ± 5.5 | 304.3 ± 5.5 | 323.1 ± 4.4 | 306.7 ± 7.6 | NS |
| Maternal weight gain (g) GD 8–18 | 74.9 ± 4.4 | 74.7 ± 4.5 | 69.9 ± 5.1 | 72.7 ± 1.5 | 55.1 ± 4.4 ** | p < 0.02 |
| % Pup Survival to 2 days of agea | 94.4 ± 3.4 | 87.2 ± 3.1 | 80.2 ± 7.5 | 67.2 ± 8.0* | 48.4 ± 15.4** | p < 0.02 |
| Male AGD (mm) at 2 days of agea | 3.73 ± 0.07 | 3.59 ± 0.08 | 3.67 ± 0.13 | 3.32 ± 0.14* | 2.7 ± 0.04** | p < 0.0001 |
| Male body weight (g) at 2 days of agea | 7.77 ± 0.11 | 8.04 ± 0.18 | 8.02 ± 0.40 | 7.54 ± 0.56 | 6.94 ± 0.31 | NS |
| Female AGD (mm) at 2 days of age | 1.75 ± 0.07 | 1.83 ± 0.06 | 1.90 ± 0.07 | 1.75 ± 0.04 | 1.74 ± 0.13 | NS |
| Female body weight (g) at 2 days of age | 7.30 ± 0.09 | 7.60 ± 0.20 | 7.60 ± 0.30 | 7.28 ± 0.49 | 6.50 ± 0.20 | NS |
Values are means ± Standard errors; *P < .05, **P < .01 different from control by t test
aOriginally reported by Hannas et al. (2011a).
Survival = (100 − mortality); mortality = (implants − number of live 2-day-old pups).
FIG. 1.
In utero exposure to DPeP (GD 14–18) reduces (A fetal testicular weight (data represents litter means ± standard errors of the means of 5–6 litters per group, ex vivo fetal testicular T production (T PROD, B) with 10–16 litters, extracted fetal testicular T (C) levels with 5–6 litters per group and fetal plasma T levels (D) with litters. (*P < .05).
Ex vivo fetal testicular T production
Administration of DPeP during the critical period of sexual differentiation (GD 14–18) significantly reduced fetal ex vivo T PROD on GD 18 in a dose-dependent fashion at 33 mg/kg/day and higher (Figure 1B). These results are similar to the fetal T PROD values reported previously (Furr et al., 2014; Hannas, et al., 2011a). The ED50 for T PROD following a 5-day exposure to DPeP in this study was 45 mg DPeP/kg/day. We also analyzed T PROD without constraining the bottom of the 4PL model to 0% (since T PROD generally plateaus at about 10%–15% of control). When modeled without the bottom of the curve constrained the ED50 for T PROD was ∼32 mg DPeP/kg/day and the bottom of the curve plateaued at ∼15% of control T PROD.
Extracted fetal testicular T (TT)
Testosterone extracted from the testis of GD 18 fetal male fetuses also was significantly decreased in a dose-dependent manner at doses of 33 mg/kg/day or higher (P < .05) (Figure 1C). The ED50 for TT levels was 53 mg DPeP/kg/day.
Fetal plasma T (PT)
In addition to reduced ex vivo T production and extracted TT levels, in utero exposure to DPeP significantly reduced circulating PT at doses of 33 mg/kg/day or higher (P < .05) (Figure 1D) (PT ED50 = 19 mg DPeP/kg/day).
Fetal T PROD, PT, and TT compared
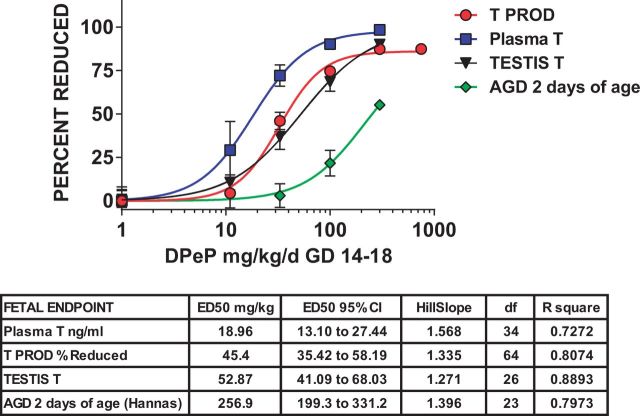
In summary, the dose-response curves for TT levels (ED50 = 53 mg/kg) and T PROD (ED50 = 45 mg/kg) were similar, whereas PT was reduced at lower dose levels (ED50 = 19 mg/kg). A comparison of the T PROD model with the PT model indicated that they do differ significantly from one another (P > .01). The dose-response curves with logistic regression parameters for these 4 fetal endpoints, along with AGD at 2 days of age from the postnatal study, are shown in Figure 2.
FIG. 2.
Sigmoidal logistic regression analyses of fetal testosterone production (T PROD), and plasma and testis testosterone (T) levels demonstrates that the ED50 values for these measures are ∼5–12-fold lower than is the ED50 value for AGD at 2 days of age in male rats exposed to DPeP in utero.
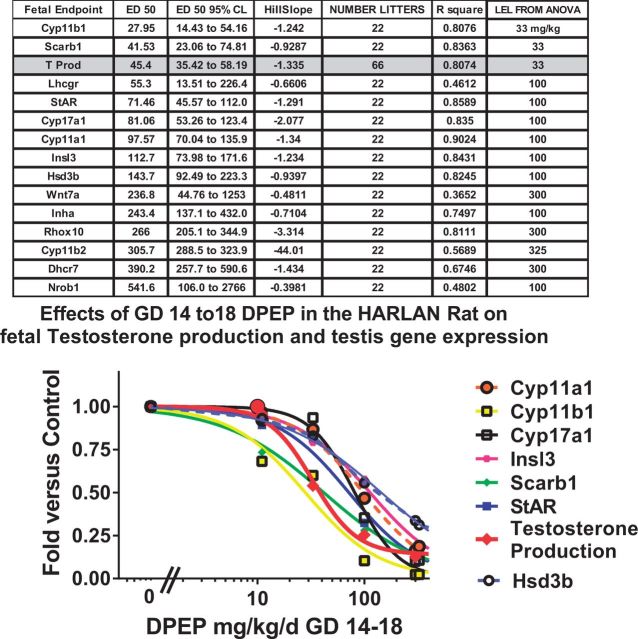
Fetal testis gene expression
Administration of DPeP during the critical period of sexual differentiation (GD 14–18) significantly (P < .01) reduced gene expression of the suite of genes typically affected by anti-androgen DioPES in the fetal testes (Figure 3). The affected genes included those coding for proteins involved in steroid transport, and steroid hormone, and Insl3 hormone. Expression of the mRNA for all these genes on GD 18 was reduced in a dose-dependent fashion with ED50 values as low as 28 mg DPeP/kg/day (Cyp11b1) compared with 45 mg DPeP/kg/day for T PROD. mRNA for several genes (Scarb, Lhcgr, and Cyp17a1) displayed ED50 values that were within the 95% confidence limits of the ED50 value for T PROD. The ED50 value for the DPeP induced reduction in testis mRNA for the Insl3 gene was ∼112 mg/kg/day.
FIG. 3.
Logistic regression analyses of fetal testosterone production (T PROD) and the mRNA for 13 genes with reduced expression levels as a consequence of in utero DPeP exposure. Values in the headings are all derived from the 4PL regression model (constrained to 0 and 1.0) except the LEL from the ANOVA (followed by LSMEANS) which is the lowest dose of DPeP that significantly (P < .05) reduced the gene expression level versus the control.
Postnatal Study with DPeP
Maternal and F1 offspring effects
Effects previously described in Hannas et al. (2011a) are presented for comparison to the new postnatal data beyond 14 days of age from the same study.
Similar to the results of the fetal study, in the postnatal study DPeP did not induce overt maternal toxicity or significantly reduce maternal weight during dosing in any group whereas maternal weight gain was significantly (P < .02) reduced in the highest dose group (Table 1) during the dosing period (GD 8–18). In utero exposure to DPeP during GD 8–18 significantly reduced the numbers of live pups at birth and decreased the fetal/neonatal survival [% survival = (100 − (100*(number of maternal implants − number of live pups at 2 days of age)/number of implants))] at 100 and 300 mg DPeP/kg (Table 1) (Hannas et al., 2011a).
AGD was reduced significantly in 2-day-old male rat pups at 100 and 300 mg/kg/day (Figure 2; Table 1) whereas female AGD and male and female body weights (Table 1) were not reduced by DPeP treatment at any dose level at this age (also reported by Hannas et al., 2011a). Body weight at weaning at 24 days of age was significantly reduced in the high-dose group. A few of these males weighed only ∼60% of control body weight (Figure 4a).
FIG. 4.
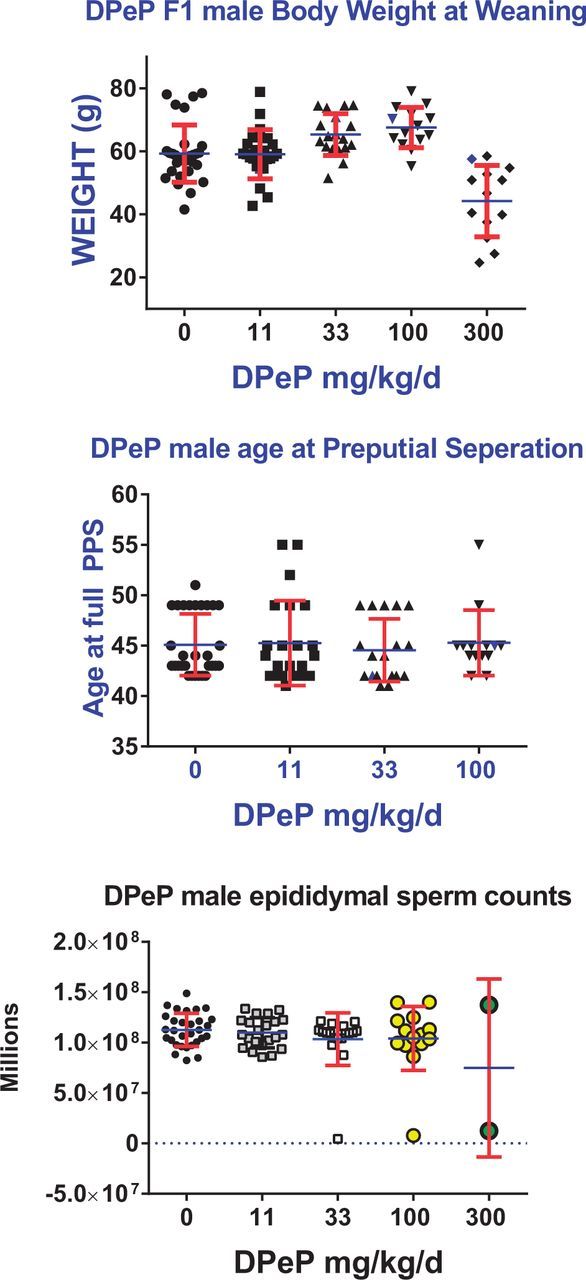
In utero exposure to DPeP on GD 8–18 reduced weaning weight in F1 male rat offspring at 300 mg/kg/day (A). The age at PPS (puberty) was not delayed in males without penile malformations (B) Epididymal sperm numbers in males with an intact epididymis were reduced in a few males in the 33, 100, and 300 mg/kg/day dose groups (C). Data are individual animal values from 4 to 5 litters/ dose group. Points on the graph represent the values from individual males in each dose group.
Age and body weight at PPS (PPS-puberty) in male rats
In the high-dose group, the onset and completion of PPS was delayed by in utero DPeP treatment (Figure 4b). Ten of 13 high-dose males had not attained full PPS by 55 days of age and in 8 of the 10 males, PPS had not begun by 55 days of age. These results were due to the fact that these animals had hypospadias rather than a true delay in male “puberty”. Two males in the 11 mg/kg/day dose group also had not completed PPS by 55 days of age; however, these males did not display penile malformations. Body weight at full PPS was not affected in animals that attained this landmark by 55 days of age.
Male rat necropsy data collected at 6–7 months of age
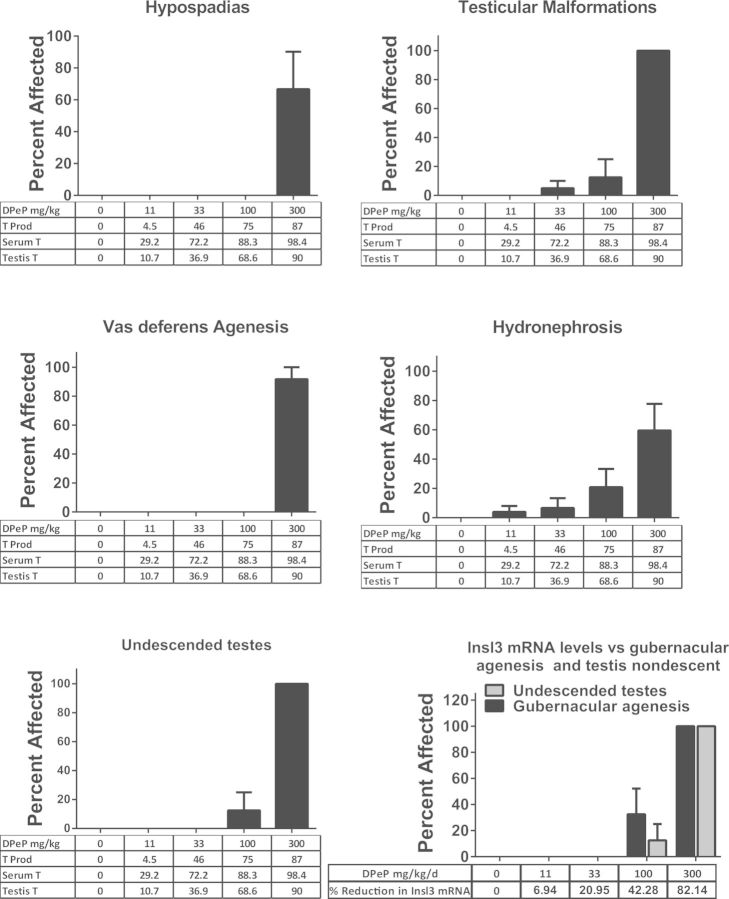
In this phase of the study, we necropsied 30 (5), 23 (5), 18 (5), 14 (4), and 13 (4) F1 males (litters) in the 0, 11, 33, 100, and 300 mg DPeP/kg/day, respectively. All of the F1 males in the 300 mg/kg/day dose group were characterized as displaying combinations of malformations or lesions associated with PS (Table 2); over 90% displayed gubernacular cord agenesis, agenesis of the vas deferens, and agenesis of all of a segment of the epididymis and 50% displayed penile hypospadias (Table 2; Figure 5).
TABLE 2.
In Utero Exposure to DPeP Induces Gross Abnormalities in the Reproductive and Urogenital Tracts of the Male Rat Offspring
| DPeP dose mg/kg/d | 0.0 | 11.0 | 33.0 | 100.0 | 300.0 |
| Number of litters | 5.0 | 5.0 | 5.0 | 4.0 | 4.0 |
| Number of males examined | 30.0 | 23.0 | 18.0 | 14.0 | 13.0 |
| Percent (number) of individual F1 male rats affected | |||||
| Retained nipples | 0 | 0 | 0 | 14.3 (2) | 69.2 (9) |
| Hypospadias | 0 | 0 | 0 | 0 | 53.8 (7) |
| Agenesis of vas deferns | 0 | 0 | 0 | 0 | 92.3 (12) |
| Small ventral prostate | 0 | 0 | 0 | 0 | 15.4 (2) |
| Agenesis or abnormal seminal vesicle | 0 | 0 | 0 | 0 | 69.2 (9) |
| Abnormal testis | 0 | 0 | 5.6 (1) | 7.1 (1) | 100 (13) |
| Agenesis of epididymis | 0 | 0 | 0 | 7.1 (1) | 92.3 (12) |
| Agenesis or elongated gubernaculums | 0 | 0 | 0 | 35.7 (5) | 100 (13) |
| Undescended testis | 0 | 0 | 0 | 7.1 (1) | 100 (13) |
| Phthalate syndrome | 0 | 0 | 5.6 (1) | 42.8 (6) | 100 (13) |
| Hydronephrosis | 0 | 4.3 (1) | 5.6 (1) | 14.3 (2) | 53.8 (7) |
The table presents the percentage of F1 males (on an individual rather than a litter basis), displaying gross reproductive and kidney abnormalities following in utero exposure to DPeP. In contrast to the data in this table, the figures in this study display litter mean values for the malformations. In the 2 high DPeP dose groups, males often displayed more than one malformation per male.
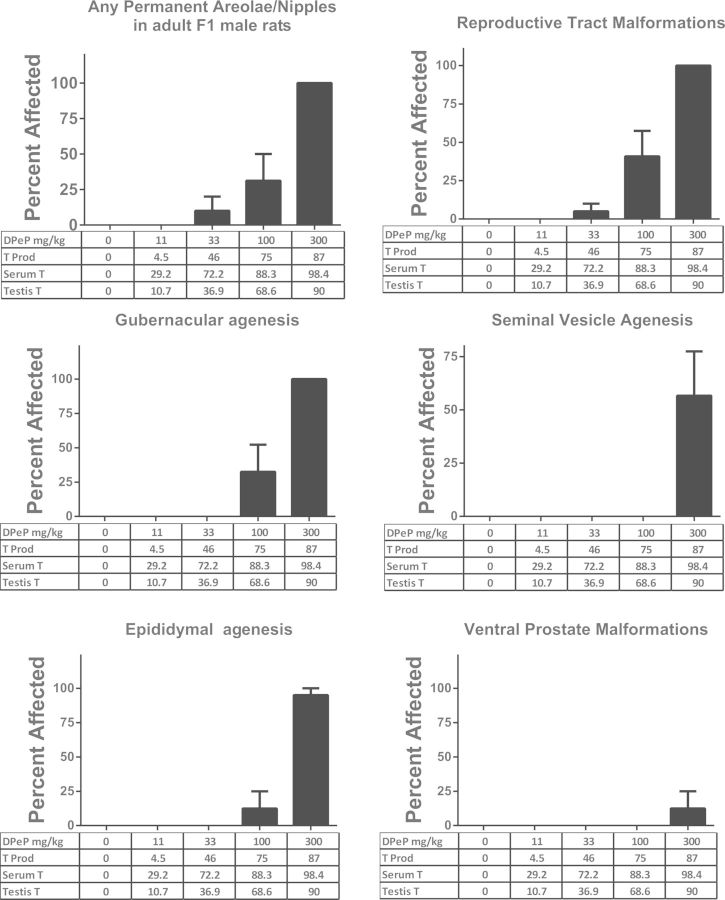
FIG. 5.
DPeP-induced gross malformations in several androgen-dependent tissues (examined at necropsy) in F1 males. The percent affected for each effect is shown on the Y-axes graphed with dose and the percent reductions in fetal testosterone production (T PROD), PT and testis testosterone on the X-axes (except undescended testes and gubernacular agenesis). The incidence of undescended testes and gubernacular agenesis are also graphed with DPeP dose and percent of control Insl3 mRNA levels on the X-axis since normal development of the fetal gubernacular cord is dependent upon the Insl3 peptide hormone.
In the 100 mg/kg/day dose group, F1 males displayed mildly to severely elongated gubernacular cords or gubernacular agenesis (36% total of which one displayed complete agenesis), retained female-like nipples (albeit faint, 14%), and epididymal agenesis and testicular abnormalities (7%). In addition, 5.6% (one male) of F1 males exposed to 33 mg DPeP/kg in utero displayed severe bilateral testicular atrophy. In summary, 0%, 0%, 5.6%, 42.8%, and 100% of the F1 males displayed some PS lesion in the 0, 11, 33, 100, and 300 mg DPeP mg/kg/day dose groups, respectively (Figure 5).
The androgen-dependent tissue malformations seen at 33 mg DPeP/kg/day were associated with reductions in fetal T PROD, plasma T levels, and testis T of 46%, 72%, and 37%, respectively (Figure 5).
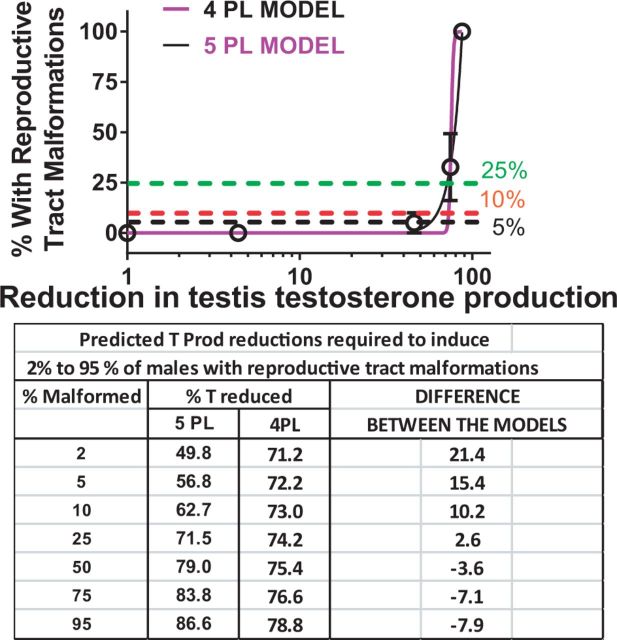
Although the 4PL and 5PL model fits do not differ significantly from one another, the estimated reductions in T PROD required to produce malformations do differ. For example, a 5% malformation rate was predicted to result from a 57% reduction in T PROD with the 5PL model and 72% reduction with the 4PL model (Figure 6). While both models have relatively high R2 values of ∼89%, the slopes are “ambiguous” because they are quite steep and there are few points on the linear portion of the curves. The 4PL logistic regression models of the relationship between Insl3 mRNA expression and gubernacular development and testis non-descent also displayed a R2 values >85% with steep, ambiguous slopes.
FIG. 6.
The percent of F1 males with reproductive tract malformations per dose group and percent reduction in fetal testis testosterone production was fit to 2 logistic regression models; the 4PL parameter model which assumes symmetry on both sides of the ED50 and a 5PL parameter model which includes an additional parameter to estimate asymmetry. This was done in order to try to provide a better fit in the region of the curve with low malformations rates. As seen in the accompanying table, the predictions of the amount of reduction in testosterone production differs considerably at low malformations rates. Further research is required to enable us to determine which model is the “best” model of the T PROD versus malformation data. BRR T PROD values interpolated from the 4PL and 5PL parameter logistic regression curves. The dashed lines represent where the 25%, 10%, and 5% malformation rates intersect the curve and suggest that the predictions of the % reduction in T PROD required to induce malformations differ by as much as 21%. The parameters of the 4PL model include the top, bottom, ED50, and slope of the curve whereas the 5PL model also estimates the degree of asymmetry of the curve.
F1 male reproductive organ weights and body and liver weight all were reduced by in utero DPeP exposure; however, the effects were generally significant only in the high-dose group (Table 3). In addition, three of 13 males (23%) exposed in utero to 300 mg DPeP/day displayed malocclusion as a result of the skull malformations (Figs. 7A and B).
TABLE 3.
Adult F1 Male Rat Necropsy
| DPeP dose (mg/kg/day) |
|||||
|---|---|---|---|---|---|
| 0 | 11 | 33 | 100 | 300 | |
| Body weight (g) | |||||
| Mean | 487.6 | 472.5 | 481.0 | 493.5 | 405.5 |
| SE | 11.64 | 14.04 | 13.03 | 20.75 | 5.98 |
| N | 5 | 5 | 5 | 4 | 4 |
| Liver weight (g) | |||||
| Mean | 16.5 | 16.0 | 16.4 | 16.3 | 13.1 |
| SE | 0.61 | 0.91 | 0.28 | 0.82 | 0.76 |
| N | 5 | 5 | 5 | 4 | 4 |
| Cowper’s glands (mg) | |||||
| Mean | 102.6 | 96.2 | 99.8 | 109.2 | 89.2 |
| SE | 5.47 | 5.74 | 4.04 | 4.92 | 11.52 |
| N | 5 | 5 | 5 | 4 | 4 |
| Glans penis (mg) | |||||
| Mean | 103.1 | 105.5 | 103.1 | 103.2 | 87.9 |
| SE | 1.46 | 2.16 | 1.74 | 2.93 | 6.54 |
| N | 5 | 5 | 5 | 4 | 2 |
| Levator–ani-bulbocavernosus muscles (g) | |||||
| Mean | 1.15 | 1.11 | 1.09 | 1.07 | 0.45 |
| SE | 0.036 | 0.064 | 0.017 | 0.051 | 0.104 |
| N | 5 | 5 | 5 | 4 | 4 |
| Seminal vesicle (g) | |||||
| Mean | 1.73 | 1.62 | 1.68 | 1.60 | 0.64 |
| SE | 0.081 | 0.065 | 0.047 | 0.116 | 0.282 |
| N | 5 | 5 | 5 | 4 | 4 |
| Caput corpus epididymis (mg) | |||||
| Mean | 0.35 | 0.34 | 0.34 | 0.34 | 0.01 |
| SE | 0.010 | 0.007 | 0.010 | 0.036 | 0.013 |
| N | 5 | 5 | 5 | 4 | 4 |
| Cauda epididymis (mg) | |||||
| Mean | 0.262 | 0.255 | 0.241 | 0.226 | 0.016 |
| SE | 0.009 | 0.005 | 0.004 | 0.024 | 0.016 |
| N | 5 | 5 | 5 | 4 | 4 |
| Paired testes (g) | |||||
| Mean | 4.42 | 4.04 | 3.90 | 4.05 | 1.13 |
| SE | 0.44 | 0.11 | 0.19 | 0.39 | 0.09 |
| N | 5 | 5 | 5 | 4 | 4 |
| Ventral prostate (mg) | |||||
| Mean | 691.2 | 626.1 | 705.1 | 659.0 | 442.5 |
| SE | 32.80 | 42.53 | 47.82 | 68.72 | 68.90 |
| N | 5 | 5 | 5 | 4 | 4 |
| Left gubernacular cord (mm) | |||||
| Mean | 9.31 | 9.37 | 10.04 | 10.25 | |
| SE | 0.37 | 0.28 | 0.54 | 0.77 | |
| N | 5 | 5 | 5 | 4 | 0 A |
| Right gubernacular cord (mm) | |||||
| Mean | 9.89 | 10.88 | 10.77 | 14.41 | |
| SE | 0.37 | 0.17 | 0.43 | 3.43 | |
| N | 5 | 5 | 5 | 4 | 0 A |
Shaded values in bold indicate values that differ significantly from control at the P < .01 level. n refers to the numbers of litters. “A” indicates that none of the males in the high-dose group had a gubernacular cord.
FIG. 7.
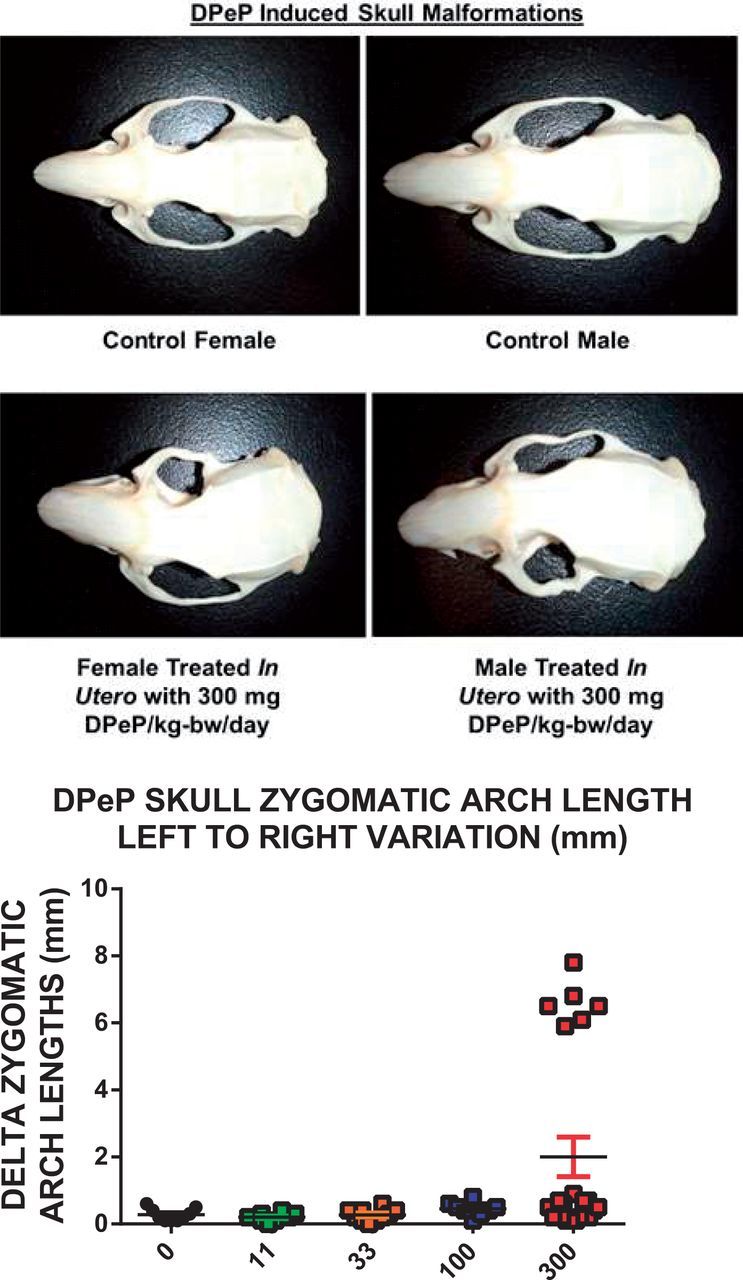
Examples of the malformed skulls of F1 males and females exposed to 300 mg DPeP/kg are shown in the photographs with a control male and female skull and a treated male and female skull. The graph presents one skull measure that quantifies this lesion—“Delta zygomatic arch lengths (mm)” which represents the absolute value of the length of one arch subtracted from the other.
Female rat necropsy data collected at 3–4 months of age
Three of 10 females exposed in utero to 300 mg DPeP/day also displayed malocclusions and skull malformations (Figure 7A) and 1 of 10 females in this dose group displayed reproductive tract malformations (uterus unicornis with ipsilateral hydrometrocolpos). In addition, 300 mg DPeP/day permanently reduced F1 female body and liver weights but had no effect on kidney, pituitary, or ovarian weights (data not shown).
DISCUSSION
Results of this study and other studies (Hannas et al., 2011a, 2012), taken together, demonstrate that in utero exposure to DPeP disrupts fetal testicular endocrine function and induces PS reproductive tract malformations in F1 male rats at doses lower than most other DioPEs. The ED50s for the postnatal effects are lower than that seen with DEHP (Blystone et al., 2010; Gray et al., 2009) or DBP (Hotchkiss et al., 2010; Mylchreest et al., 1998), or other DioPEs that disrupt fetal male rat endocrine function and postnatal reproductive development.
Testis T PROD, extracted testis T, and plasma T all were all significantly reduced at 33 mg DPeP/kg/day with plasma T having a slightly lower ED50 than T PROD or extracted testis T. In addition to reducing T levels in the fetus, DPeP also reduced the mRNA expression levels for genes involved in steroid transport and steroid and Insl3 hormone synthesis; these are the same genes that are affected by other “active” DioPEs (Hannas et al., 2011b). Furthermore, the relative sensitivity of the mRNA levels reduced by in utero DPeP exposure is very similar to that reported for other phthalates. In contrast to the endocrine alterations in the fetal testis, fetal testis weight on GD 18 was only reduced at 300 mg/kg/day, the highest dose group tested.
In the postnatal study, the most sensitive effects to in utero DPeP were the percent of F1 male rats displaying PS reproductive tract malformations with 5.6% of the males (1 male) displaying abnormal testis morphology at 33 mg/kg/day, retained-female-like areolae/nipples (2 of 14 F1 males), and hydronephrosis (1 male each exposed to 11 or 33 mg/kg/day). The incidence and severity of the PS increases with DPeP dose with effects at 33 mg DPeP/kg/day being associated with 46%, 37%, and 72% reductions in testis T PROD, extracted testis T and plasma T levels, respectively. In addition, pup survival was reduced from 94% in controls to 82% in the 33 DPeP mg/kg/day dose group. While none of these effects by themselves were statistically significant, taken together they could be considered biologically relevant since they are dose-related and typical outcomes after in utero exposure to other PEs like DBP and DEHP. Statistically significant increases in postnatal abnormalities were noted when T Prod, PT, and TT were reduced by 65%, 86%, and 69%, respectively, and all F1-treated males displayed PS malformations when these 3 measures of T were reduced by 85% or higher.
When the reductions in Insl3 mRNA were compared with the rates of gubernacular abnormalities and undescended testes, dose-related reproductive alterations were noted when Insl3 mRNA was reduced by >42%. We did detect partially undescended, small testes during the fetal necropsies in males from 1 litter exposed to 33 mg DPeP/kg/day (a dose that reduces Insl3 mRNA by ∼20%); however, this effect could have been a transient effect and no gubernacular cord alterations were noted in the postnatal study in this dose group.
The fact that such large reductions in fetal T levels are required to induce postnatal reproductive alterations is similar to the seminiferous tubular fluid (STF) T dose-response required to maintain spermatogenesis in the adult rat testis (Zirkin et al., 1989). Zirkin et al. (1989) reported that “Complete spermatogenesis was maintained despite an 80% reduction in the STF T concentration from control values to ∼13 ng/ml. The ability of the testis to maintain complete spermatogenesis was extremely sensitive to further decreases in STF T concentration. Thus, reduction of the STF T concentration from ∼13 to 9 ng/ml resulted in a reduction in the number of advanced spermatids that were maintained in the testis from ∼275 × 106 to 150 × 106. Reduction of the STF T concentration to ∼4 ng/ml resulted in a further reduction in the number of advanced spermatids per testis to about 45 × 106. Taken together, these data support the contention that there is far more T present within the seminiferous tubules of intact rat testes than is required to maintain quantitatively normal spermatogenesis”.
It is also possible that our estimate of the reductions in T levels overestimates the degree to which T is reduced throughout the dosing period. We are taking a single measure of these T levels on GD 18, the last day of dosing, at a time point when T PROD is maximally affected (about 4 h after the last dose). Therefore, the actual area under the curve for T PROD over the 5-day dosing period may not be as dramatically reduced as it is at GD 18 shortly after dosing since the level of T Prod is not constant during this developmental stage. T PROD increases by ∼3-fold from GD 16 to 18 (Hannas et al., 2011a) and, in addition, we were unable to detect any testis T PROD on GDs 14 and 15 using this method.
In addition to the reproductive effects in F1 male rats from in utero DPeP treatment, male and female pup survival also was reduced in a dose-related manner (P < .05 at 100 and 300 mg/kg/day; Table 1) and some F1 male and female rats in the high-dose group displayed skull defects, malocclusion of the incisors (Figure 7) and some females displayed uterine malformations. These results suggest that toxicity assessments for chemicals in this class should not focus solely on disruption of endocrine-mediated alterations of sexual differentiation in the male rat reproductive tract.
Our studies to date on PEs have focused on using a broad dose range of each chemical in order to obtain accurate ED50 values for the in utero effects on fetal T PROD, fetal testis gene expression, and alterations of the male reproductive tract later in life. Since the associations between T PROD and the postnatal effects are quite steep there is considerable uncertainty about the shape of these relationships at lower malformation rates. With this in mind, future studies are being planned to focus on BRR T PROD reductions associated with malformations in the 5%–25% range to reduce the uncertainty (variance) in the BRR T PROD levels associated with malformations at this portion of the curve.
This study is one of about 10 studies that we are conducting with DioPEs and other chemicals that reduce fetal T PROD and testis gene expression on GD 18 in which we are comparing these alterations with the malformation rates in male offspring. In addition to our own transgenerational studies, we are examining the association between reductions in fetal T PROD from our studies with studies from other laboratories on the postnatal effects of in utero DBP (Hannas et al., 2011a; Mylchreest and Foster, 2000; Mylchreest et al., 1998, 1999, 2000), DEHP (Blystone et al., 2010) and diisooctyl phthalate (Saillenfait et al., 2013). It is our intent to determine the variability of the statistical association between reduced fetal T PROD (as measured herein) and the adverse postnatal effects of this class of endocrine disruptors.
The fact that the 4PL and 5PL statistical models provide somewhat different predictions of the BRRT values in the low range of the percent malformed highlights the need for studies with more dose groups in this range in order to provide better estimates of reductions in T required to induce low rates of postnatal abnormalities that exceed the control values. Comparing the relationship between fetal T PROD and the adverse postnatal effects of chemicals across several studies will increase the determination of the BRR T levels in the fetal male rat and enable us to quantify the uncertainty in our predictions of the levels of malformations that result from specific reductions in fetal T PROD.
Supplementary Material
ACKNOWLEDGMENTS
We thank Brandy Riffle, Nicola Evans, Mary Cardon, and Phillip Hartig for the excellent technical assistance with several phases of this research project. We also thank Drs Allen Davis and Vicki Sutherland for their thorough and constructive reviews of the manuscript.
FUNDING
Supported in part by NIH NTP/NIEHS IA RW7592285501-1.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
REFERENCES
- Blystone C. R., Kissling G. E., Bishop J. B., Chapin R. E., Wolfe G. W., Foster P. M. (2010). Determination of the di-(2-ethylhexyl) phthalate NOAEL for reproductive development in the rat: importance of the retention of extra animals to adulthood. Toxicol. Sci. 116, 640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers C. M., Foster P. M. (2005). Critical window of male reproductive tract development in rats following gestational exposure to di-n-butyl phthalate. Birth Defects Res. 74, 277–285. [DOI] [PubMed] [Google Scholar]
- Feldman S. C., Bloch E. (1978). Developmental pattern of testosterone synthesis by fetal rat testes in response to luteinizing hormone. Endocrinology 102, 999–1007. [DOI] [PubMed] [Google Scholar]
- Furr J. R., Lambright C. S., Wilson V. S., Foster P. M., Gray L. E., Jr (2014). A short-term in vivo screen using fetal testosterone production, a key event in the phthalate adverse outcome pathway, to predict disruption of sexual differentiation. Toxicol. Sci. 140, 403–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangnerau M. N., Picon R. (1987). Onset of steroidogenesis and differentiation of functional LH receptors in rat fetal testicular cultures. Arch. Androl. 18, 215–224. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Foster P. M. D. (2003). Significance of experimental studies for assessing adverse effects of endocrine-disrupting chemicals. Pure Appl. Chem. 75, 2125–2141. [Google Scholar]
- Gray L. E., Jr, Barlow N. J., Howdeshell K. L., Ostby J. S., Furr J. R., Gray C. L. (2009). Transgenerational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicol. Sci. 110, 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L. E., Jr, Laskey J., Ostby J. (2006). Chronic di-n-butyl phthalate exposure in rats reduces fertility and alters ovarian function during pregnancy in female Long Evans hooded rats. Toxicol. Sci. 93, 189–195. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Ostby J. S., Kelce W. R. (1994). Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation of the male rat. Toxicol. Appl. Pharmacol. 129, 46–52. [DOI] [PubMed] [Google Scholar]
- Hannas B. R., Furr J., Lambright C. S., Wilson V. S., Foster P. M., Gray L. E., Jr (2011a). Dipentyl phthalate dosing during sexual differentiation disrupts fetal testis function and postnatal development of the male Sprague-Dawley rat with greater relative potency than other phthalates. Toxicol. Sci. 120, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas B. R., Howdeshell K. L., Furr J. (2013). In utero phthalate effects in the female rat: a model for MRKH syndrome. Toxicol. Lett. 223, 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas B. R., Lambright C. S., Furr J., Evans N., Foster P. M., Gray E. L., Wilson V. S. (2012). Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: a targeted RT-PCR array approach for defining relative potency. Toxicol. Sci. 125, 544–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas B. R., Lambright C. S., Furr J., Howdeshell K. L., Wilson V. S., Gray L. E., Jr (2011b). Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol. Sci. 123, 206–216. [DOI] [PubMed] [Google Scholar]
- Hellwig J., van Ravenzwaay B., Mayer M., Gembardt C. (2000). Pre- and postnatal oral toxicity of vinclozolin in Wistar and Long-Evans rats. Regul. Toxicol. Pharmacol. 32, 42–50. [DOI] [PubMed] [Google Scholar]
- Hosokawa S., Murakami M., Ineyama M., Yamada T., Koyama Y., Okuno Y., Yoshitake A., Yamada H., Miyamoto J. (1993). Effects of procymidone on reproductive organs and serum gonadotropins in male rats. J. Toxicol. Sci. 18, 111–124. [DOI] [PubMed] [Google Scholar]
- Hotchkiss A. K., Rider C. V., Furr J., Howdeshell K. L., Blystone C. R., Wilson V. S., Gray L. E., Jr (2010). In utero exposure to an AR antagonist plus an inhibitor of fetal testosterone synthesis induces cumulative effects on F1 male rats. Reprod. Toxicol. (Elmsford, N.Y) 30, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., McDowell E. N., Viereck M. P., Xia J. Q. (2011). Species-specific dibutyl phthalate fetal testis endocrine disruption correlates with inhibition of SREBP2-dependent gene expression pathways. Toxicol. Sci. 120, 460–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluwe W. M. (1982). Overview of phthalate ester pharmacokinetics in mammalian species. Environ. Health Perspect. 45, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdic G., Saunders P. T., Teerds K. J. (1998). Immunoexpression of the steroidogenic enzymes 3-beta hydroxysteroid dehydrogenase and 17 alpha-hydroxylase, C17,20 lyase and the receptor for luteinizing hormone (LH) in the fetal rat testis suggests that the onset of Leydig cell steroid production is independent of LH action. Biol. Reprod. 58, 520–525. [DOI] [PubMed] [Google Scholar]
- McIntyre B. S., Barlow N. J., Foster P. M. (2001). Androgen-mediated development in male rat offspring exposed to flutamide in utero: permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicol. Sci. 62, 236–249. [DOI] [PubMed] [Google Scholar]
- Miyata K., Yabushita S., Sukata T., Sano M., Yoshino H., Nakanishi T., Okuno Y., Matsuo M. (2002). Effects of perinatal exposure to flutamide on sex hormones and androgen-dependent organs in F1 male rats. J. Toxicol. Sci. 27, 19–33. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Cattley R. C., Foster P. M. D. (1998). Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicol. Sci. 43, 47–60. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Foster P. M. (2000). DBP exerts its antiandrogenic activity by indirectly interfering with androgen signaling pathways. Toxicol. Appl. Pharmacol. 168, 174–175. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Sar M., Cattley R. C., Foster P. M. (1999). Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol. Appl. Pharmacol. 156, 81–95. [DOI] [PubMed] [Google Scholar]
- Mylchreest E., Wallace D. G., Cattley R. C., Foster P. M. (2000). Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol. Sci. 55, 143–151. [DOI] [PubMed] [Google Scholar]
- Ostby J., Kelce W. R., Lambright C., Wolf C. J., Mann P., Gray L. E., Jr (1999). The fungicide procymidone alters sexual differentiation in the male rat by acting as an androgen-receptor antagonist in vivo and in vitro. Toxicol. Ind. Health 15, 80–93. [DOI] [PubMed] [Google Scholar]
- Saillenfait A. M., Sabate J. P., Robert A., Cossec B., Roudot A. C., Denis F., Burgart M. (2013). Adverse effects of diisooctyl phthalate on the male rat reproductive development following prenatal exposure. Reprod. Toxicol. (Elmsford, N.Y.) 42, 192–202. [DOI] [PubMed] [Google Scholar]
- van Ravenzwaay B., Kolle S. N., Ramirez T., Kamp H. G. (2013). Vinclozolin: a case study on the identification of endocrine active substances in the past and a future perspective. Toxicol. Lett. 223, 271–279. [DOI] [PubMed] [Google Scholar]
- Warren D. W., Haltmeyer G. C., Eik-Nes K. B. (1973). Testosterone in the fetal rat testis. Biol. Reprod. 8, 560–565. [DOI] [PubMed] [Google Scholar]
- Yasunaga R., Ikuta J., Murata Y., Inoue K., Koga H., Masaki T., Tamura H. (2013). Ligand-independent androgen receptor antagonism caused by the newly developed pesticide pyrifluquinazon (PFQ). Reprod. Toxicol. (Elmsford, N.Y.) 35, 1–6. [DOI] [PubMed] [Google Scholar]
- Zirkin B. R., Santulli R., Awoniyi C. A., Ewing L. L. (1989). Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 124, 3043–3049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.