SUMMARY
Proper establishment of synapses is critical for constructing functional circuits. Interactions between presynaptic neurexins and postsynaptic neuroligins coordinate the formation of synaptic adhesions. An isoform code determines the direct interactions of neurexins and neuroligins across the synapse. However, whether extracellular linker proteins can expand such a code is unknown. Using a combination of in vitro and in vivo approaches, we found that hevin, an astrocyte-secreted synaptogenic protein, assembles glutamatergic synapses by bridging neurexin-1α and neuroligin-1B, two isoforms that do not interact with each other. Bridging of neurexin-1α and neuroligin-1B via hevin is critical for the formation and plasticity of thalamocortical connections in the developing visual cortex. These results show that astrocytes promote the formation of synapses by modulating neurexin/neuroligin adhesions through hevin secretion. Our findings also provide an important mechanistic insight into how mutations in these genes may lead to circuit dysfunction in diseases such as autism.
INTRODUCTION
Neuronal communication flows through highly specialized cell junctions called synapses. Trans-synaptic adhesions between presynaptic neurexins (Nrx) and postsynaptic neuroligins (NL) are critical for the formation and maturation of excitatory and inhibitory synapses (Baudouin and Scheiffele, 2010). Mutations in Nrxs and NLs are linked to many neurological disorders, including autism, schizophrenia, depression and addiction, highlighting the importance of this trans-synaptic link in normal brain function (Sudhof, 2008).
Nrxs are coupled to presynaptic vesicle release machinery, whereas NLs are linked to postsynaptic adaptor proteins and neurotransmitter receptors. Thus, Nrx-NL pairs coordinate the organization and alignment of the pre and postsynaptic specializations (Craig and Kang, 2007). In humans, there are three Neurexin and five Neuroligin genes. Each Neurexin gene produces two isoforms, the long Nrxα and the short Nrxβ. β-isoforms interact strongly with NLs, whereas α-isoforms have weak affinity towards NLs, in particular NL1 with a B-splice site insertion (NL1B) (Boucard et al., 2005; Chih et al., 2006; Graf et al., 2004). Nrxα and NL1B isoforms are far more abundantly expressed in the brain (Schreiner et al., 2015). Recruitment of Nrxαs, but not Nrxβs, is key for the rapid induction of presynaptic release machinery at the sites of new axo-dendritic contacts (Lee et al., 2010), and NL1 is critical for recruiting NMDA type glutamate receptors (NMDAR) to excitatory synapses (Budreck et al., 2013; Chubykin et al., 2007). These observations suggested the presence of synaptic linkers that align postsynaptic NMDARs with presynaptic release machinery via bridging of incompatible Nrxα and NL1B.
Astrocytes, the most abundant glial cells of the brain, control synapse formation by secreting synaptogenic factors, including hevin/SPARCL1 (Kucukdereli et al., 2011; Risher et al., 2014). Hevin is a glycoprotein localized to the synaptic clefts of excitatory synapses (Johnston et al., 1990). Down-regulation of hevin has been reported in neurological disorders such as autism and depression (Purcell et al., 2001; Zhurov et al., 2012), and copy number variations (CNV), polymorphisms and mutations in SPARCL1 are linked to autism and schizophrenia (De Rubeis et al., 2014; Jacquemont et al., 2006; Kahler et al., 2008). Hevin strongly stimulates excitatory synapse formation in the retinal ganglion cell (RGC) cultures and Hevin-null mice (Hevin KO) have fewer and immature excitatory synapses at the superior colliculus (SC), the synaptic target of RGCs in vivo (Kucukdereli et al., 2011). Moreover, in the developing cortex, hevin is required for proper thalamocortical synaptogenesis and the maturation of dendritic spine structures (Risher et al., 2014).
Despite hevin’s fundamental role in excitatory synapse development in rodents and its potential association with neurological disorders in humans, how hevin induces synaptogenesis is unknown. Here, we show that hevin organizes pre and postsynaptic specializations and induces thalamocortical synaptogenesis by bridging Nrx1α and NL1. Moreover, in the developing visual cortex astrocyte-secreted hevin is required for the plasticity of thalamocortical connections. These results reveal that astrocytes alter the trans-synaptic interactions between NLs and Nrxs via hevin and thus modulate the formation and plasticity of excitatory synapses. Because hevin (SPARCL1), Nrx1α (NRXN1) and NL1 (NLGN1) are all genetically linked to neurological disorders, our results also provide mechanistic insights into how mutations in these genes may lead to synaptic dysfunction in disease.
RESULTS
Hevin Organizes Pre and Postsynaptic Specializations
Hevin induces the formation of ultrastructurally normal, but postsynaptically silent synapses (i.e. lacking AMPA receptors) between RGCs (Kucukdereli et al., 2011). However, hevin-induced synapses are presynaptically active as shown by live staining with luminal synaptotagmin antibody (Figure S1A–B). Moreover, hevin strongly enhances NMDA currents in autaptic RGC cultures suggesting it potentiates NMDA receptor function (Figure S1C–K). Because hevin affects both pre and postsynapses in vitro and in vivo we postulated that hevin organizes pre and postsynaptic specializations.
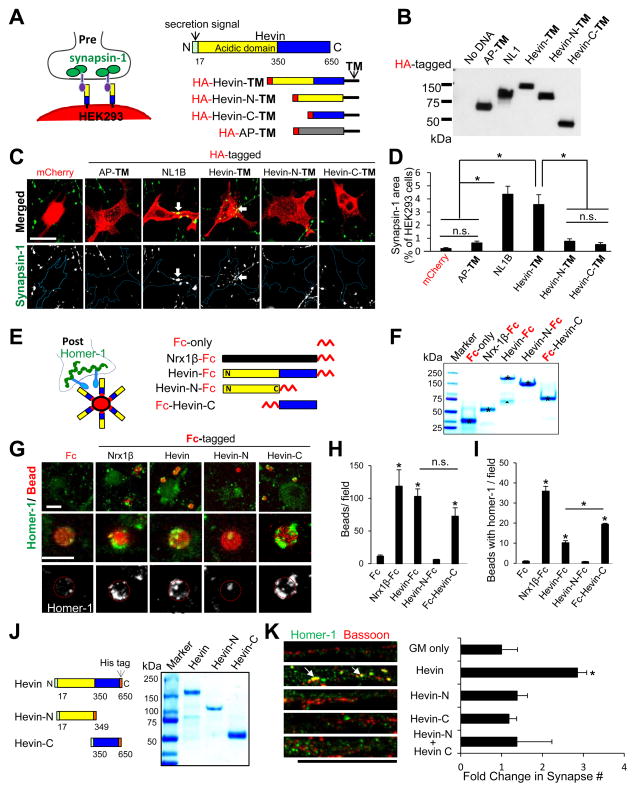
To determine whether hevin organizes presynaptic structures, we utilized a “hemisynapse” assay (Scheiffele et al., 2000). We engineered a membrane-anchored hevin construct (Hevin-TM), expressed it in HEK293 cells, and then co-cultured these cells with purified RGCs (Figures 1A–D). Hevin-TM strongly induced clustering of presynaptic synapsin-1 puncta onto HEK293 cells (Figure 1C–D), but two negative controls: cells expressing 1) cytoplasmic mCherry or 2) alkaline phosphatase tethered to the cell membrane with the same TM-anchor did not (AP-TM, Figure 1A–D).
Figure 1. Hevin organizes pre and postsynaptic specializations.
(A) RGC-HEK293 co-culture assay: Trans-membrane-anchored (TM) and HA-tagged hevin and its fragments were expressed in HEK293 cells co-cultured with RGCs. (B) Western blot analysis of the HEK293 expression of HA-tagged constructs. (C) Representative images of synapsin-1 (green) clustering (arrow) on HA-tagged TM constructs expressed HEK293 cells (red). Scale bar= 20μm. (D) Quantification of the hemisynapse assay (n=15–18 cells/condition) as the area of synapsin-1 on HEK293 cells (% HEK293 cell area). (E) Schematic of the Fc-tagged proteins and homer-1-clustering in RGC-bead assay. (F) SDS-PAGE analysis of pure Fc-tagged proteins (asterisk). In the Hevin-Fc lane a naturally occurring hevin cleavage product is also detected (triangle). (G) Representative images of RGCs in the postsynaptic bead-clustering assay. Scale bar= 5μm. Quantification of the number of beads/field (H) orbeads with homer-1 clusters/field (I)(n=10–15 fields/condition). (J) Purified full-length hevin or hevin fragments (5μg/lane). (K) (Left) Representative images of RGC dendrites treated with growth media (GM) only, 80nM hevin, or hevin fragments showing synapses as co-localization of bassoon and homer-1 (arrows). Scale bar= 20μm. (Right) Quantification of co-localized synaptic puncta/cell as fold change normalized to GM condition (n>20 cells/condition). For all graphs: *p<0.05, one way ANOVA followed by Fisher’s LSD Post-Hoc test, error bars ±SEM.See also Figure S1.
Hevin has two distinct domains: a unique N-terminal acidic region followed by a SPARC (Secreted Protein Acidic and Rich in Cysteines) homology region (Figure 1A). Metalloprotease ADAMTS4 cleaves hevin at amino acid 350, to produce N- and C-terminal fragments, which are found in the brain at low levels (Weaver et al., 2010). We tested presynaptic clustering ability of TM-tagged N- or C-terminal hevin fragments (Hevin-N-TM (17–349), Hevin-C-TM (350–650)) and found that neither induces synapsin-1 clustering onto HEK293 cells (Figure 1A–D). Our findings show that hevin organizes presynaptic specializations and cleavage of hevin at amino acid 350 impairs its ability to organize presynapses.
Next, we tested whether hevin organizes postsynaptic specializations. We cultured RGCs with magnetic beads coated with Fc-tagged full-length hevin or hevin-truncation constructs (Figure 1E–F). Hevin-Fc beads efficiently bound to neuronal processes and significantly induced homer-1 clustering compared to Fc-coated control beads (Figure 1G–I). Deletion of the C-terminus of hevin eliminated these functions (Hevin-N-Fc). In contrast, the C-terminal region of hevin (Fc-Hevin-C) efficiently bound to RGCs and strongly clustered homer-1 (Figure 1G–I). These results show that the C-terminus of hevin is sufficient to interact with neuronal processes and induce postsynaptic clustering. Collectively, our results show that hevin organizes both pre and postsynaptic specializations.
To further understand the mechanism of hevin-induced synapse formation, we tested whether hevin truncation constructs (Figure 1J) were synaptogenic in RGC cultures. RGCs, cultured as a pure neuronal population, form few synapses as determined by the co-localization of pre and postsynaptic markers (e.g. bassoon and homer-1, respectively, Figure 1K). Addition of hevin induces a ~3 fold increase in synapse number (Figure 1K, arrows). Even though the C-terminus of hevin (Hevin-C) organizes postsynaptic specializations (Figure 1G–I), neither Hevin-C alone, nor the co-application of Hevin-N and Hevin-C together mimicked full-length hevin’s synaptogenic effect (Figure 1K). These data suggest that simultaneous organization of pre and postsynaptic sites by hevin is required for its synaptogenic effect.
Nrx1α and NLs are the Pre and Postsynaptic Receptors for Hevin
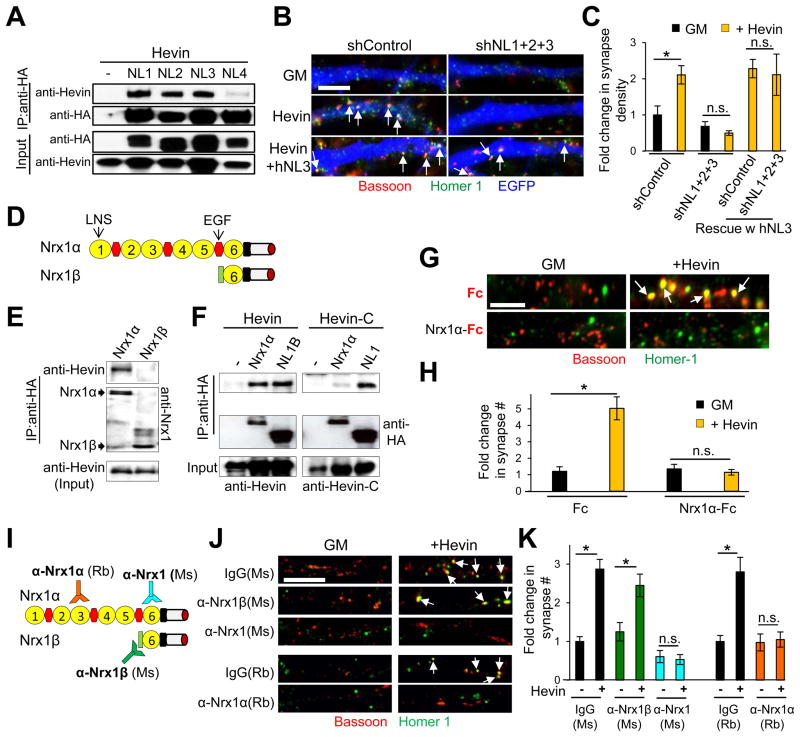
Similar to hevin, presynaptic Nrxs and postsynaptic NLs organize pre and postsynaptic specializations, respectively (Graf et al., 2004; Scheiffele et al., 2000). Thus, we postulated that hevin interacts with these receptors to induce synapse formation. There are four neuroligins (NL1–4) in rodents. We found that hevin co-immunoprecipitates (co-IP) with NL1, 2, and 3 (Figure 2A). Knockdown of NLs 1–3 in RGCs using small hairpin RNAs (shRNA) completely abolished hevin-induced synaptogenesis in vitro, which was rescued by co-expression of shRNA-resistant human NL3 (hNL3, Figures 2B–C and S2A). Individual knockdown of NLs 1–3 or in dual combinations all significantly reduced hevin-induced synaptogenesis (Figure S2B). These results show that NLs 1–3 are required for hevin-induced synapse formation in vitro.
Figure 2. NLs and Nrx1α are synaptic receptors for hevin.
(A) Hevin co-immunoprecipitates (co-IP) with HA-tagged NLs in HEK293 cells. (B) Representative images of shRNA-transfected dendrite stretches (blue). Synapses are labeled as co-localized bassoon (red) and homer-1 (green) puncta. (C) Quantification of fold change in the density of synapses made onto transfected neurons as fold change normalized to the shControl-no hevin condition (n>40 cells per condition).(D) Domain structures of Nrx1αand Nrx1β. (E) Hevin co-IP with HA-tagged Nrx1α, but not with Nrx1β in HEK293 cells. (F) Co-IP of Hevin-C fragment with Nrx1α and NL1. (G) Representative images of RGC dendrites treated with hevin and Fc-tagged proteins stained with bassoon (red) and homer-1 (green). Scale bar= 5μm. (H) Quantification of fold change in synapse number/cell normalized to the Fc-only treatment (3.2±0.76 synapses in Fc-only condition, n=20 cells/condition). For (C) and (H) *p<0.05; Student’s t-test; n.s., not significant, error bars±SEM. (I) Schematic presentation of the epitope locations for anti-Nrx1 antibodies. (J) Representative images of RGC dendrites with bath-applied Nrx1α-recognizing antibodies with or without hevin. (K) Quantification of fold change in synapse number/cell (n>28 cells/condition, *p<0.05; one way ANOVA followed by Fisher’s LSD Post-Hoc test, error bars±SEM). Fold change is calculated by normalizing to the number of synapses/cell in respective Ms or Rb control antibody-only treated condition (2.72±0.97 and 3.36±0.16 synapses in Ms and Rb antibody-only condition, respectively). Scale bars= 5μm. See also Figure S2.
We next tested whether hevin interacts with Nrx1 isoforms (Figure 2D). We found that hevin co-IP specifically with long Nrx1α but not with short Nrx1β (Figure 2E). Hevin did not co-IP with Nrx2α and only weakly interacted with Nrx3α (Figure S2C), suggesting that hevin mainly interacts with Nrx1α. Additionally, the C-terminal domain of hevin, which interacts with NL1, had diminished interactions with Nrx1α (Figure 2F).
We found that hevin interacts in solution with Fc-tagged soluble Nrx1α extracellular domain (ECD, Figure S2D–F). Bath application of the soluble Nrx1α-Fc with hevin at 1:1 molar ratio blocked hevin-induced synaptogenesis in vitro (Figure 2G–H), suggesting that the Nrx1α-hevin interaction is important for hevin’s synaptogenic function. Next, we tested whether antibodies against Nrx1 ECD (Figure 2I and S2G) can block hevin-induced synapse formation. A mouse monoclonal antibody recognizing α and β Nrx1 or a rabbit polyclonal antibody against an epitope specific for Nrx1α blocked hevin-induced synaptogenesis (Figure 2J–K). In contrast, a Nrx1β-specific monoclonal antibody did not block hevin-induced synaptogenesis (Figure 2J–K). Taken together, these data indicate that Nrx1α is the presynaptic receptor for hevin’s synaptogenic function.
Identification of a Region within Hevin that Mediates Receptor Interactions and Synaptogenic Activity
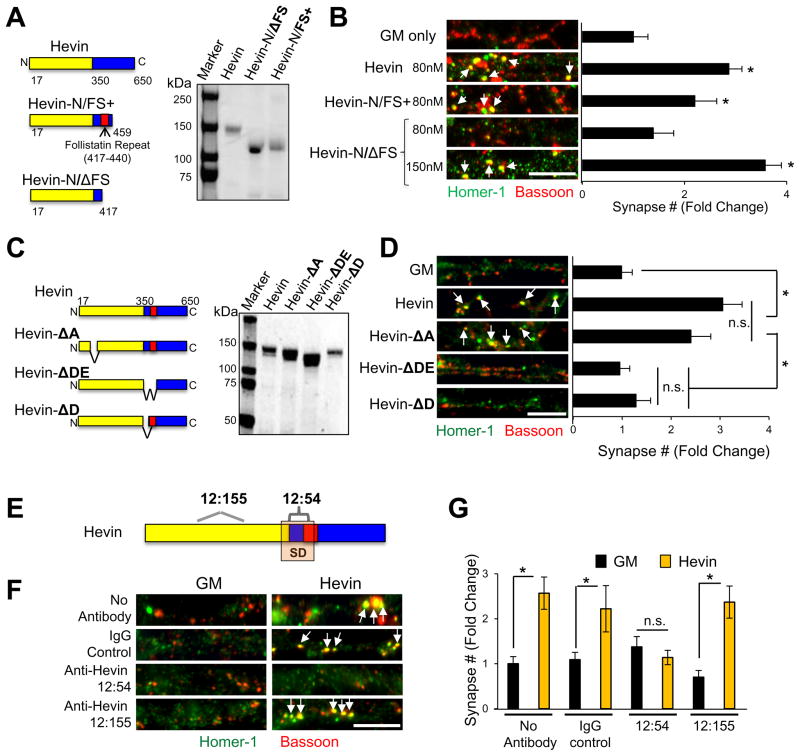
Cleavage of hevin at amino acid 350 eliminates its synaptogenic function (Figure 1K). Thus, we postulated that a region surrounding the cleavage site is critical for hevin’s synaptogenic interactions. Consistent with this, a hevin truncation construct spanning amino acids 17–459 (Hevin-N/FS+) induced synapse formation (Figure 3A–B), and interacted efficiently with Nrx1α and NL1 (Figure S3A). In contrast, non-synaptogenic Hevin-N (Figure 1K) did not interact with Nrx1α and weakly interacted with NL1B (Figure S3A). Moreover, a hevin fragment (17–417) lacking the follistatin-like domain (Hevin-N/ΔFS) was synaptogenic only at higher concentrations (Figure 3A–B and S3B) and interacted weakly with Nrx1α and NL1B (Figure S3A). Taken together, these data show that the first 459 amino acids of hevin mediate its synaptogenic activity and interact with both Nrx1α and NL1B efficiently. The follistatin-like domain of hevin is not essential for its synaptogenic function, but plays a role in strengthening hevin-receptor interactions. Our findings also show that the region around amino acids 350–417 of hevin is critical for its synaptogenic activity.
Figure 3. Identification of a region within hevin that is critical for its receptor interactions and synaptogenic activity.
(A) Domain organization and SDS-PAGE analysis of hevin and two synaptogenic hevin fragments. (B) Representative images of RGC dendrites treated with GM or recombinant hevin proteins. Synapses are labeled as the co-localization of bassoon (red) and homer-1 (green) puncta (arrows). (C) Schematics and SDS-PAGE analysis of hevin and hevin deletion mutants. (D) Representative images of RGC dendrites treated with GM, hevin or hevin deletion mutants, arrows point to co-localized synaptic puncta. (B and D) Data is presented as fold change in synapse number/cell compared to GM (n>35 cells). (E) Schematic of hevin’s putative synaptogenic region (SD) and epitopes for the two rat monoclonal antibodies against hevin. (F) Representative images from dendrites of RGCs treated with GM or hevin in the presence of serotype matched control rat antibody (IgG-Control) or anti-Hevin 12:155 or anti-Hevin 12:54. (G) Quantification of fold changes in synapse number/cell relative to GM condition (3.25±0.84 synapses/cell, n>20 cells/condition). *p<0.05, one way ANOVA followed by Fisher’s LSD Post-Hoc test error bars±SEM. Scale bars= 5μm. See also Figure S3.
Next we mapped two putative Nrx1α and NL1B interaction sites within amino acids 350–440 of hevin by using a peptide array (Figure S3C–D). We named these sites as D and E, based on their location on the arrays (Figure S3C). We produced three hevin-deletion mutants: 1) Hevin-ΔDE (lacking 351–440), 2) Hevin-ΔD (lacking 351–372), and 3) Hevin-ΔA (lacking 88–115, removing another putative interaction site for Nrx1α) (Figure 3C and S3D). We found that Hevin-ΔA was synaptogenic like hevin; however, Hevin-ΔD and Hevin-ΔDE did not induce synapse formation in vitro (Figure 3D) even at higher concentrations (Figure S3E). This result shows that the putative Nrx1α and NL1B interaction sites within 351–440 are critical for hevin’s synaptogenic function.
Consistent with this, a rat monoclonal antibody against an epitope within amino acids 368–419 of hevin (anti-hevin 12:54) blocked hevin’s synaptogenic function, when co-applied to RGCs with hevin (Figure 3E–G). Another rat anti-hevin antibody (12:155), with epitopes within the N-terminal acidic domain of hevin (epitopes: 117–123 and 245–251, Figure 3E–G) did not block hevin-induced synaptogenesis. Collectively, these results show that a region at the cusp of hevin’s N- and C-terminal domains is critical for hevin’s synaptogenic function.
Hevin Bridges Nrx1α and NL1B Transcellularly
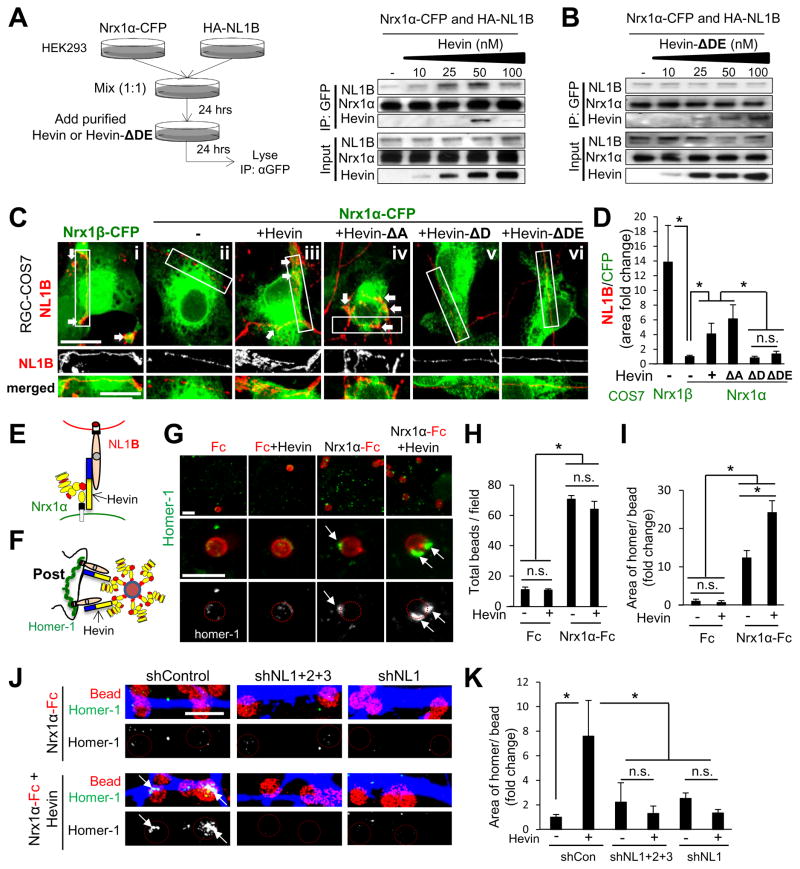
Nrx1α interacts strongly with the A-site containing NL1, but weakly with the B-site containing splice variant, NL1B (Chih et al., 2006; Siddiqui et al., 2010). As hevin interacts with both Nrx1α and NL1B, hevin might bridge these two receptors and enable their interaction. In agreement, hevin facilitated the co-immunoprecipitation of Nrx1α and NL1B in a concentration-dependent manner (Figure 4A), whereas neither the weak interaction between Nrx2α and NL1B nor the strong interaction between Nrx1α and NL1A were altered by hevin (Figure S4A–B). Interestingly, tripartite complex formation is impaired at hevin concentrations beyond its optimal synaptogenic dose (>100nM, Figure S3E), and the non-synaptogenic Hevin-ΔDE did not facilitate the interaction between Nrx1α and NL1B (Figure 4B). These data suggest that hevin enhances the interaction between Nrx1α and NL1B via its synaptogenic region.
Figure 4. Hevin bridges interaction-incompatible Nrx1α and NL1B transcellularly.
(A) Triple co-IP strategy: HEK293 cells were transfected with Nrxα or NL1 isoforms and cells were mixed in the presence of various hevin or Hevin-ΔDE concentrations. Western blot analyses of the effects of hevin (A) or Hevin-ΔDE (B) on Nrx1α/NL1B interactions. (C) Representative images of the co-clustering of CFP-tagged α or β Nrx1-expressing COS7 cells (green) co-cultured with HA-NL1B expressing RGCs (red). Scale bar= 10μm. (D) Quantification of NL1B clustering onto Nrx1-expressing COS7 cells. Data presented as fold change of the ratios of NL1B area per Nrx1-expressing cell area (n=15–38 cells per condition) normalized to Nrx1α-CFP expressing cells in GM condition. (E) Hevin can bridge incompatible Nrx1α and NL1B transcellularly. (F) Schematic presentation of the Nrx1α-bead assay. (G) Representative images of analyzed fields (top) or single beads (middle and bottom) showing homer-1 (green) clustering on Nrx1α-Fc-coated beads (red) in the presence or absence of hevin. Scale bar= 5μm. Quantification of the number of beads/field (H) and fold chance in the area of homer-1 clusters per bead (I) (n=10–15 fields/condition) normalized to the Nrx1α-Fc beads without hevin condition. (J) Representative images of shRNA-transfected dendrites (blue) stained for homer-1 (green) and beads (red circles). Scale bar= 5μm. (K) Quantification of the area of homer-1 clusters per bead (n=10 fields/condition) as fold change normalized to the shControl no hevin condition. *p<0.05 one way ANOVA followed by Fisher’s LSD Post-Hoc test, error bars±SEM. See also Figure S4 and S5.
RGCs and their synaptic target SC predominantly express the interaction incompatible Nrx1α and NL1B (Figure S4C–D). Therefore, we tested whether hevin bridges Nrx1α and NL1B transcellularly, using an “RGC-COS7 co-culture assay”. COS7 cells expressing CFP-tagged (green) Nrx1α or Nrx1β were co-cultured with HA-NL1B-expressing RGCs (red) (Figure 4C). NL1B-expressing neurites strongly interacted with Nrx1β-CFP-expressing COS7 cells (Figure 4Ci and D, arrows), but did not with Nrx1α-CFP-expressing cells (Figure 4Cii and D). However, Nrx1α-CFP-expressing COS7 cells recruited an interaction-compatible postsynaptic partner, LRRTM2 (Figure S4E). Application of soluble hevin strongly promoted the clustering of NL1B onto Nrx1α-CFP-expressing COS7 cells (Figure 4Ciii and D), showing that hevin bridges the interaction-incompatible Nrx1α and NL1B transcellularly (Figure 4E). Similarly, in a “HEK293 cell aggregation” assay hevin induced aggregation of NL1B and Nrx1α-expressing cells (Figure S4F–H, arrow), but did not alter the aggregation of compatible NL1A and Nrx1α-expressing cells (Figure S4H).
To determine if hevin’s synaptogenic activity depends on its ability to bridge Nrx1α and NL1B, we tested hevin deletion mutants in the same transcellular bridging assays. Synaptogenic Hevin-ΔA efficiently bridged Nrx1α and NL1B in both assays, whereas non-synaptogenic Hevin-ΔD and -ΔDE did not (Figure 4Civ–vi and D and Figure S4I–J). In summary, our data show that hevin can bridge incompatible Nrx1α and NL1B transcellularly and that bridging is required for its synaptogenic activity. These results reveal that hevin may act as a trans-synaptic linker to assemble excitatory synapses.
Hevin Recruits NL1 and Associated Postsynaptic Proteins to Excitatory Synapses
Collectively our results suggest that hevin may facilitate the recruitment of NL1-associated proteins, such as PSD95 and homer-1, by bridging Nrx1α and NL1. To test this, we added Nrx1α-Fc coated beads onto the cultured RGCs in the presence or absence of hevin (Figure 4F). These beads bound to RGC somas and dendrites (Figure 4G). Hevin did not alter bead attachment efficiency (Figure 4G–H), but strongly enhanced (2–8 fold) the homer-1 recruitment to the beads (Figure 4G–K). Silencing NLs 1–3 or only NL1 in RGCs, abolished hevin’s recruitment of homer-1 by Nrx1α (Figure 4J–K). In sum, these results show that hevin organizes postsynaptic structures by linking Nrx1α and NL1.
These data indicate that hevin recruits NL1 and NL1-associated proteins to synapses. In agreement, NL1, PSD95, homer-1 and the NMDAR subunits NR1 and NR2B levels were significantly reduced in the postsynaptic membranes of Hevin KO cortices compared to wildtype (WT) (Figure S5A). Moreover, hevin and NL1 co-localize in the mouse cortex (Figure S5B–C) and NL1 staining is greatly reduced in the upper cortical layers (1–4) of Hevin KO mice (Figure S5D). The reduction in NL1 is not due to a gross morphological abnormality in the cortices of Hevin KOs, because these mice have normal neuronal distribution and outgrowth in the cortex (Figure S5E) (Risher et al., 2014).
The presynaptic vesicular glutamate transporters VGluT1 and VGluT2 mark two major classes of cortical excitatory synapses, the intracortical and thalamocortical, respectively (Figure 5A). NL1 puncta are closely apposed to subsets of VGluT1 and VGluT2 terminals in WT mice (Figure S5F and S5K), but Hevin KO cortices have a severe loss of NL1 puncta and NL1/VGluT apposition in the upper cortical layers (Figure S5G–J and S5L). The abundance of NL1 and VGluT proteins are not significantly different in WT and littermate KO mice (Figure S5M–N). Collectively, these data show that hevin is required for the proper localization of NL1 to cortical excitatory connections.
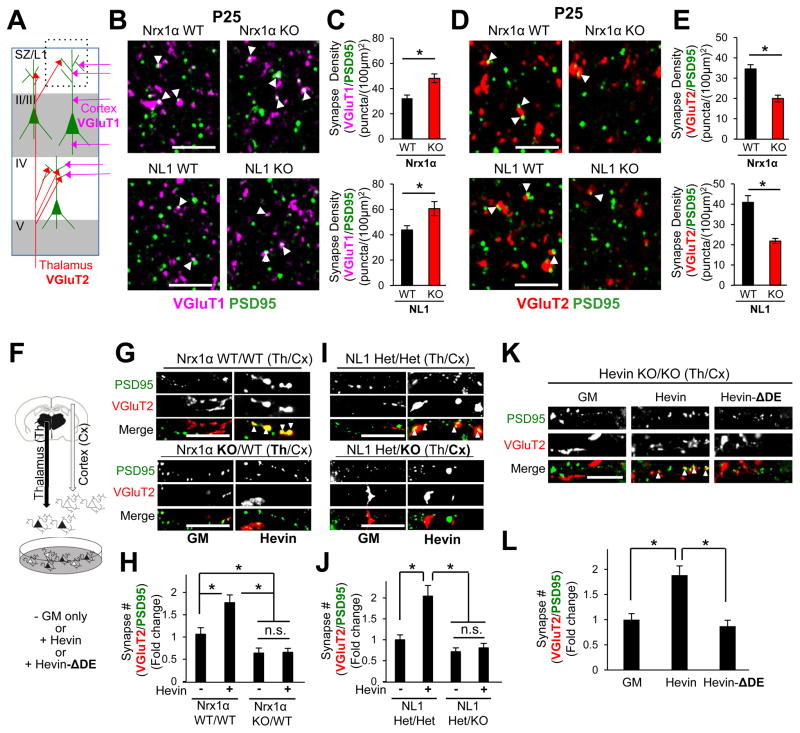
Figure 5. Nrx1α and NL1 are required for thalamocortical synapse formation.
(A) Excitatory synaptic circuitry within the mouse V1 cortex. Synapse density analysis was performed in the synaptic zone (SZ/L1, dashed black box). (B and D) Representative zoomed-in images of P25 Nrx1α (top) or NL1 (bottom) WT and KO V1 cortices stained for (B) VGluT1 (magenta) and PSD95 (green) or (D) VGluT2 (red) and PSD95. Scale bar= 5μm. Quantification of (C) VGluT1/PSD95 or (E) VGluT2/PSD95 synapse density in Nrx1α (top) or NL1 (bottom) littermate WT and KO mice (n=3 mice/genotype). Data are represented as mean synaptic density per (100μm)2±SEM. *p<0.05, Student’s t-test. (F) Schematic of thalamocortical co-culture experiments. Representative images of dendrite segments treated with or without hevin (80nM) from (G) WT cortical (Cx) neurons co-cultured with Nrx1α WT or KO thalamic (Th) neurons or (I) NL1 HET or KO cortical (Cx) neurons co-cultured with NL1 HET thalamic (Th) neurons. Co-localization of VGluT2 (red) and PSD95 (green) mark thalamocortical synapses (arrowheads). Quantification of fold change in VGluT2/PSD95 positive synapse number/cell normalized to the number of synapses (H) in Nrx1α WT/WT (Cx/Th) cultures without Hevin or (J) in NL1 HET/HET (Cx/Th) cultures without Hevin. (K) Representative images of dendrite segments from cortical neurons in thalamocortical co-cultures prepared from Hevin KO mice with or without hevin or Hevin-ΔDE (80nM). (L) Quantification of VGluT2/PSD95 synapse numbers/cell normalized to GM condition. (H, J and L) n>30 cells/condition, *p<0.05; one way ANOVA followed by Fisher’s LSD Post-Hoc test, error bars±SEM). See also Figure S6.
Hevin Assembles Thalamocortical Synapses by Bridging Nrx1α and NL1
Hevin is required for proper cortical synaptic connectivity in mice. Hevin KOs display a profound loss of VGluT2+ thalamocortical synapses, which is offset by an increase in VGluT1+ intracortical connections (Risher et al., 2014). Similar to Hevin KO mice, both Nrx1α and NL1 KOs had significantly higher numbers of VGluT1+ synapses (Figure 5B–C and S6A–B), coupled with a strong reduction in VGluT2+ synapses compared to their WT littermates (Figure 5D–E and S6C–D). The reduced thalamocortical synapse numbers in Nrx1α and NL1 KOs are not due to diminished axonal innervation of V1 by thalamic inputs from the dorsal lateral geniculate nucleus (dLGN) (Figure S6E–G). The total number of glutamatergic excitatory synapses was not significantly different between Nrx1α or NL1 KO mice compared to their littermate controls (Figure S6H–I). These findings show that Nrx1α and NL1 are required for proper thalamocortical synapse formation, suggesting that hevin induces thalamocortical synaptogenesis via its interactions with Nrx1α and NL1.
In a purified cortical and thalamic neuron co-culture system (Figure 5F) hevin specifically induces VGluT2+ thalamocortical synapse formation onto cortical neurons (Risher et al., 2014). Interestingly, thalamic neurons predominantly express Nrx1α, whereas cortical neurons make NL1B (Figure S6L–M). Thus, hevin may induce thalamocortical synaptogenesis by bridging Nrx1α and NL1B. In agreement, we found that when the thalamic neurons lack Nrx1α, hevin cannot induce VGluT2+ synapse formation (Figure 5G–H). Concordantly, the lack of NL1 in cortical neurons abolished hevin-induced thalamocortical synapse formation in vitro (Figure 5I–J). Moreover, Hevin-ΔDE, which cannot bridge Nrx1α and NL1B, did not induce thalamocortical synaptogenesis in co-cultures prepared from Hevin KOs (Figure 5K–L). Taken together these data show that hevin-induced thalamocortical synaptogenesis requires Nrx1α in thalamic and NL1 in cortical neurons and requires hevin’s ability to bridge these two receptors transcellularly.
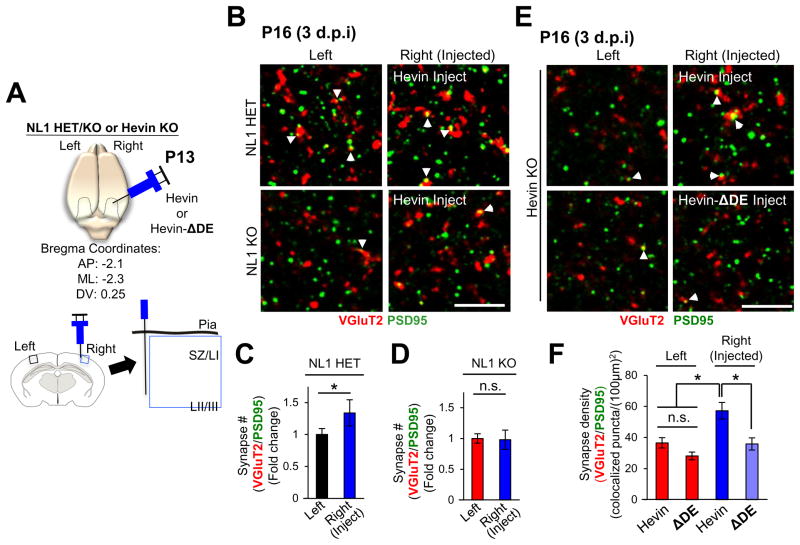
Reduced thalamocortical connectivity in Hevin KOs can be rescued by injection of purified hevin into the cortex (Risher et al., 2014). Using the same assay, we found that hevin could not induce thalamocortical synaptogenesis when injected to NL1 KOs, though it could trigger a significant synaptogenic response in littermate HETs (Figure 6A–D).
Fig 6. Hevin induces thalamocortical synapse formation in vivo by bridging NL1 and Nrx1α.
(A) Schematics of in vivo hevin or Hevin-ΔDE injection experiments into the cortices of P13 NL1 HET/KO or Hevin KO mice at the indicated bregma coordinates. Imaging and analysis for VGluT2/PSD95 was limited to the SZ just outside of the injection site (blue box). (B and E) Representative zoomed-in images of synaptic staining in left (uninjected) and right (injected) hemispheres of (B) NL1 HET and KO littermates or (E) Hevin KOs. Thalamocortical synapses are visualized by the VGlut2/PSD95 colocalization (arrowheads). Scale bar= 5μm. (C–D) Quantification of VGluT2/PSD95 synapses in NL1 HET or KO brains. Data presented as fold change in synapse number normalized to the number of synapses of the uninjected (left) hemisphere. *p<0.05, Student’s t-test; n.s., not significant, n=3 mice/genotype. Synapse density in the left (uninjected) cortices of NL1 HETs (78.87±6.96 VGluT2/PSD95 puncta/(100μm)2) and KOs (48.88±3.01 VGluT2/PSD95 puncta/(100μm)2) were significantly different from each other (p = 0.0002) (F) Quantification of thalamocortical synapse densities in Hevin KOs injected with hevin or Hevin-ΔDE.Data are presented as mean synaptic density per (100 μm)2±SEM. *p<0.05, one way ANOVA followed by Fisher’s LSD Post-Hoc test.
To determine if bridging of Nrx1α and NL1 by hevin is critical for thalamocortical synapse formation in vivo, we injected pure hevin or Hevin-ΔDE into the cortices of Hevin KOs. Importantly, hevin, but not Hevin-ΔDE, strongly induced the formation of thalamocortical synapses (Figure 6E–F). Taken together our results demonstrate that hevin induces thalamocortical synapse formation by bridging its pre and postsynaptic receptors, Nrx1α and NL1, respectively.
Astrocyte-Secreted Hevin is Required for Ocular Dominance Plasticity
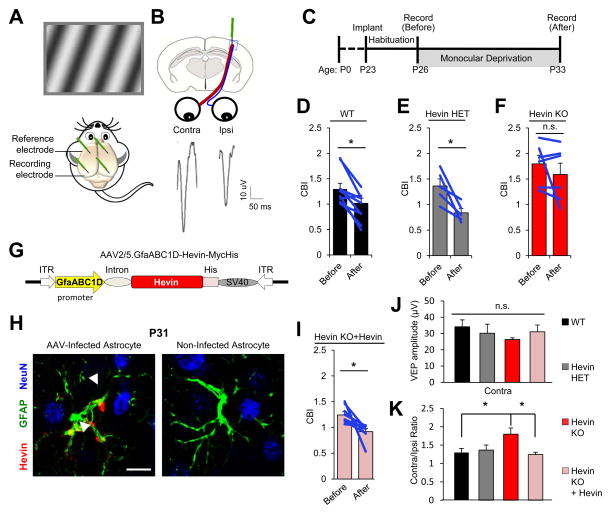
Ocular dominance plasticity (ODP) is a model of cortical plasticity based on visual experience-dependent functional and anatomical changes in V1 cortex during a critical period of development. Stimulating each eye elicits responses in the binocular zone of V1 (V1B) that can be recorded as visually evoked field potentials (VEPs) (Figure 7A). Most V1B neurons have a bias towards the thalamic inputs that relay information from the contralateral eye (Figure 7B). The ratio of VEP amplitudes triggered by the contralateral eye versus the ipsilateral eye produces a contralateral bias index (CBI), which in rodents is typically between 1.2–2.5 (Frenkel and Bear, 2004; Porciatti et al., 1999). During a critical period in development, monocular deprivation (MD) of the contralateral eye reduces CBI (Sawtell et al., 2003). This phenomenon, known as ODP, is dependent on NMDARs (Bear et al., 1990; Roberts et al., 1998).
Figure 7. Astrocyte-Secreted Hevin is required for Ocular Dominance Plasticity (ODP).
(A) Mice were implanted with recording electrodes in layer 4 of the V1B cortex. A visual stimulus is displayed to mice while visually evoked potentials (VEPs) are recorded. (B) Cartoon schematic and VEP tracings from independent eye stimulations. (C) Experimental timeline used to study ODP. (D–F) Contralateral bias indices (CBI) before and after monocular deprivation (MD). Data are presented as mean CBI±SEM. *p<0.05; paired t-test; n.s., not significant. (G) AAV2/5-GfaABC1D-Hevin-myc-His viral construct used for astrocyte-specific rescue of hevin expression. (H) Hevin (red) expression is driven specifically in astrocytes (GFAP+, green), but not in neurons (NeuN+, blue). Scale bar= 10μm. (I) Astrocyte-specific hevin expression fully rescues ODP in Hevin KOs. Data are presented as mean CBI±SEM. *p<0.05; paired t-test. (J) Absolute VEP amplitude does not differ between all groups. (K) Comparison of CBIs before MD. Data are presented as mean CBI±SEM. (J–K) *p<0.05; one way ANOVA followed by Fisher’s LSD Post-Hoc test.
Because hevin induces the formation of thalamocortical synapses and because Hevin KOs have reduced levels of synaptic NR1 subunit (Figure S5A), an essential component for NMDAR function, here we tested whether Hevin KOs have impaired ODP. After 7 days of MD, CBIs were significantly reduced in WT and Hevin HETs as expected (Figure 7D–E). However, CBIs did not change in Hevin KOs (Figure 7F), showing that hevin is required for ODP.
To test whether hevin expression by developing cortical astrocytes is critical for ODP, we injected Hevin KO mice with Adeno-Associated Viruses (AAV), that express hevin specifically in the astrocytes, into V1B at P3 (Figure 7G–H). Remarkably, hevin expression by V1B astrocytes fully rescued ODP in Hevin KO mice (Figure 7I). While the amplitude of VEPs of the contralateral eye was not significantly different between groups (Figure 7J), we noted a higher contralateral bias in Hevin KOs, which returned to normal levels after hevin expression was rescued in astrocytes (Figure 7K).
Taken together, our findings show that the lack of hevin leads to deficits in neuronal plasticity during cortical development. Re-expression of hevin, specifically in astrocytes of V1B, is sufficient to rescue the plasticity defect in Hevin KO mice, demonstrating that secretion of hevin by astrocytes is critical for the assembly of thalamocortical circuits amenable to experience-dependent plasticity.
DISCUSSION
Astrocyte-Secreted Hevin Induces Synapse Formation by Bridging Nrx1α and NL1B
During brain development astrocytes actively provide signals that control synaptic connectivity (Chung et al., 2015). Here we show that astrocyte-secreted hevin is a trans-synaptic linker that bridges presynaptic Nrx1α with postsynaptic NL1B. This way, hevin organizes both pre and postsynaptic specializations and aligns them across the synapse. Importantly, these results show that astrocytes can control the formation and maturation of synaptic contacts by altering the interaction code between pre and postsynaptic cell adhesion proteins. Astrocytes thereby modulate the abundance of specific trans-synaptic adhesion complexes and their associated receptors at synapses. Through hevin, astrocytes orchestrate the assembly of excitatory trans-synaptic adhesion complexes that contain Nrx1α and NL1, into larger adhesions, strengthening the synaptic junctions (Figure S7). This organization is critical for the formation and plasticity of thalamocortical synapses in the developing cortex.
How Does Trans-synaptic Bridging of Nrx1α and NL1B by Hevin Control Synapse Formation and Plasticity?
In addition to their trans-synaptic interactions, Nrx1α recruits presynaptic release machinery to the synapse (Lee et al., 2010; Missler et al., 2003); whereas, NL1 recruits NMDARs to postsynapses (Chubykin et al., 2007). By trans-synaptically linking Nrx1α and NL1B, hevin could align the neurotransmitter release machinery with the NMDARs. This alignment might be critical for the recruitment of NMDARs to the synapse, because the levels of NR1 and NR2B at postsynaptic membranes were severely reduced in the Hevin KOs, and hevin strongly enhanced NR2B-containing NMDAR currents in autaptic RGC cultures. NL1 recruits NR1-subunit of NMDARs through extracellular interactions (Budreck et al., 2013), but how Nrx1α/hevin/NL1 interaction specifically recruits NR2B-containing NMDARs to the synapses remains to be elucidated.
The necessity of hevin for proper synaptic recruitment of NMDARs may also underlie its requirement for cortical plasticity. NMDAR is crucial for activity-dependent plasticity, by acting as a “coincidence detector” (Bourne and Nicoll, 1993; Huang et al., 2005). ODP (Bear et al., 1990; Roberts et al., 1998) as well as binocular recovery from monocular deprivation (Krahe and Medina, 2010) require this feature of NMDAR function. Thus, by bridging Nrx1α and NL1, hevin may recruit NMDARs to thalamocortical synapses enabling plasticity of these connections.
There was a modest, but significant, increase in the CBI of the Hevin KOs. A sharp reduction in ipsilateral inputs leads to a severe increase in CBI, which can affect the development of retinotopy (Smith and Trachtenberg, 2007). However, this seems unlikely here as the CBIs of Hevin KOs are in the range of what is commonly seen in mice (Coleman et al., 2009; Frenkel and Bear, 2004; Porciatti et al., 1999), which exhibit ODP. Moreover, re-expression of hevin in astrocytes by AAVs rescued this difference in CBI as well as the ODP.
How Do Nrx1α-Hevin-NL1 Interactions Dynamically Regulate Synaptic Connectivity?
Fine astrocyte processes, positioned next to synaptic clefts, can detect neurotransmitter release in nearby synapses. It is thought that in response to changes in synaptic activity patterns, astrocytes signal back to the synapse to modulate transmission or alter plasticity (Chung et al., 2015). It is plausible that hevin is provided to the synaptic clefts of certain synapses in an activity-dependent manner to specifically bridge Nrx1α and NL1B. Future studies investigating how hevin secretion is regulated and whether synaptic activity can change hevin abundance in the brain are necessary.
Besides directional release from astrocytes, hevin’s activity may be regulated by metalloproteases that cleave the protein into N- and C-terminal fragments (Weaver et al., 2010). Our findings show that this cleavage renders hevin inactive by impairing its ability to link Nrx1α and NL1B. Moreover, glial cells express a close homolog of hevin, called SPARC. SPARC is not synaptogenic, but blocks the synaptogenic activity of hevin (Kucukdereli et al., 2011). SPARC expression is altered by sensory experience (Tropea et al., 2006). Therefore, changes in SPARC abundance could dynamically regulate hevin activity. How SPARC blocks hevin-induced synaptogenesis remains to be elucidated.
Role of Astrocyte-Neuron Signaling via Hevin in Neurological Disorders
Alterations in synaptic development and function underlie the pathophysiology of many neurological disorders, including addiction, depression, schizophrenia and autism. Therefore, an important implication of our findings is that perturbations in the Nrx1α-hevin-NL1 interaction could play a critical role in the synaptic pathologies seen in these disorders. In agreement with this, hevin (SPARCL1), Nrx1α (NRXN1) and NL1 (NLGN1) are linked to these diseases.
In a study on the molecular mechanisms of resilience, the injection of viruses that express hevin reversed the effects of social defeat in mice by promoting resilience and acted in an antidepressant-like fashion (Vialou et al., 2010). Moreover, hevin mRNA is one of the most downregulated transcripts in the brains of suicide victims compared to age-matched controls and hevin expression is reduced in the brains of depressed individuals (Zhurov et al., 2012), indicating that modulation of Nrx1α-hevin-NL1 interactions may provide fruitful new avenues for treatment or prevention of these mood disorders.
A comprehensive whole-genome sequencing of autistic individuals, identified SPARCL1 gene mutations (ranking 35th among 107 high risk genes identified) (De Rubeis et al., 2014). The same study and others also identified NRXN1, NLGN1, GRIN1 (NR1) and GRIN2B (NR2B) as autism risk genes. Thus our findings, showing that these proteins work in concert to establish proper thalamocortical connectivity, provide an important mechanistic insight into how mutations in these genes may lead to synaptic dysfunction in autism. In fact, abnormal thalamocortical connectivity has been reported in autism patients (Nair et al., 2013). Future studies investigating the role of hevin in the pathophysiology of neurological disorders could determine if the modulation of synaptic adhesions by hevin, and its receptors can provide a molecular mechanism that could be exploited for therapeutic strategies.
EXPERIMENTAL PROCEDURES
(Detailed Experimental Procedures can be found in the Supplemental Data section.)
RGC culture system to study synapse formation in vitro
RGCs were purified and cultured as previously described (Kucukdereli et al., 2011). On DIV4 purified recombinant hevin (WT or mutants) were added for an additional 6 days. Synapse quantification in RGC cultures were performed as described in (Ippolito and Eroglu, 2010).
DNA constructs and protein purification
Hevin, hevin truncation, and deletion mutants were all cloned into pAPtag5 vector. Recombinant proteins were purified from conditioned media of transfected HEK cells by using Ni-NTA resin (Qiagen). Plasmid constructs for NLs and Nrxs were described in (Chih et al., 2005; Chih et al., 2006; Graf et al., 2004; Siddiqui et al., 2010). TM fusion constructs: cDNA encoding the transmembrane region of LDL-TM and the cytoplasmic tail of CD46 was cloned into AP or hevin fragment containing pAPtag5 plasmids. Fc-tagged fusion constructs: cDNA encoding Fc protein was cloned into the pAPtag5 vector at N or C termini of hevin fragments or Nrx1-ECDs. Recombinant Fc-tagged proteins were purified from HEK293-conditioned media using HiTRAP protein G affinity columns (GE Healthcare).
RGC Transfections
RGCs were transfected using the Lipofectamine LTX (Invitrogen) reagent on DIV5. Hevin treatments started 2 days post-shRNA transfection. The number of synapses on EGFP positive cells was quantified as in (Ippolito and Eroglu, 2010).
Presynaptic “Hemisynapse” Assay
HA-tagged TM constructs or NL1-expressing HEK293 cells were co-cultured with RGCs (DIV6). 3 days later, cells were fixed and stained for synapsin-1 and imaged using a Zeiss 710 inverted confocal microscope.
Postsynaptic “Bead Clustering” Assay
Fc-tagged proteins on protein G-coated magnetic beads were added onto cultured RGCs. After 3 days, RGCs were fixed and stained for homer-1 and Fc. Confocal images of cells were taken at 40× magnification for quantification.
Nrx-NL co-clustering assay
CFP-tagged α or β Nrx1-expressing COS7 cells were co-cultured with HA-NL1B-expressing RGCs (DIV6) in the presence or absence of purified hevin or hevin mutants (100nM each). After 3 days of co-culture, cells were fixed and stained. Confocal images of CFP-expressing COS7 cells were analyzed for clustering of NL1B.
Hevin Injections and Synapse Analyses
Hevin KO (Kucukdereli et al., 2011) and NL1 KO (Varoqueaux et al., 2006) mice were previously described. The Nrx1α KOs (Grayton et al., 2013) were received from Catherine Fernandes (King’s College London) and crossed into a mixed background.
Hevin or Hevin-ΔDE were injected into the V1 cortices of P13 Hevin KO, and NL1 HET or KO mice and the brains were harvested and analyzed for synapse numbers at P16 using methods described in (Risher et al., 2014).
AAV2/5 Preparation and Administration
Full-length hevin cDNA was cloned into the pZac2.1 gfaABC1D-Cyto-GCaMP3 viral vector (Addgene plasmid #44331) replacing the GCaMP3 sequence. Human GFAP promoter (gfaABC1D) is highly selective for astrocytes (Shigetomi et al., 2013). 50–100nl of AAV2/5-Hevin (1.82×1013GC/ml) virus was injected into layer IV of V1B of Hevin KOs at P3.
Ocular Dominance Plasticity Recordings
At P23, littermate Hevin WT, HET, KO or virus-injected Hevin KO mice were anesthetized with isoflurane. Electrodes were implanted at stereotaxic coordinates corresponding to layer 4 of V1B in each cortical hemisphere. At P26, mice were presented with a visual stimulus to each eye independently and VEPs were recorded. Recordings were made of >100 stimulus presentations/animal, and peak to trough measurements of the largest amplitude VEP response from each eye were measured and averaged. After the baseline recordings, mice were anesthetized with isoflurane, and one eyelid was stitched and glued shut. Mice remained monocularly deprived for a period of 7 days and VEP recording procedure was repeated. Contralateral Bias Indices (CBI) was calculated as the ratio of the contralateral VEP over the ipsilateral VEP response for each mouse.
Statistics
Statistical analyses were performed using Statistica Software. Individual statistical tests can be found in their respective figure legends.
Supplementary Material
Acknowledgments
We thank Drs Anne-Marie Craig and Tabreez Siddique for providing Nrx constructs, Dr Helene Sage for sharing many hevin reagents, and Drs Anne West and Nicola Allen for critical reading of the manuscript. This research was funded by a NIH/NIDA R01 (DA031833) and Brumley Neonatal Perinatal Research Institute Grant to CE, a NIH/NIDCR R01 (DE22743 and DE17794) to RRJ, by a R01 NIH/NINDS (NS083897) to DLS and by Swiss National Science Foundation to PS. SKS and HD are Ruth K. Broad International Postdoctoral Fellows. JAS is funded by F31 NIH/NINDS (NS092419). AP was funded by an HHMI VIP grant. CE is a Holland-Trice Brain Research Scholar, Esther and Joseph Klingenstein Fund Fellow and Alfred P. Sloan Fellow.
Footnotes
AUTHOR CONTRIBUTIONS
SKS, JAS, NP, HD, YHK, L-JP, AP, DLS, RRJ, AEM, and CE designed research; SKS, JAS, NP, HD, YHK, L-JP, IHK, ACM, WSR-J, AP, ZE, and CE performed research; PS contributed reagents and data analysis tools; SKS, JAS, NP, HD, YHK, AP, EE, AEM, and CE analyzed data; SKS, JAS, AEM, and CE wrote the paper.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baudouin S, Scheiffele P. SnapShot: Neuroligin-neurexin complexes. Cell. 2010;141:908, 908 e901. doi: 10.1016/j.cell.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Bear MF, Kleinschmidt A, Gu QA, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Nicoll R. Molecular machines integrate coincident synaptic signals. Cell. 1993;72(Suppl):65–75. doi: 10.1016/s0092-8674(05)80029-7. [DOI] [PubMed] [Google Scholar]
- Budreck EC, Kwon OB, Jung JH, Baudouin S, Thommen A, Kim HS, Fukazawa Y, Harada H, Tabuchi K, Shigemoto R, et al. Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:725–730. doi: 10.1073/pnas.1214718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Allen NJ, Eroglu C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harbor perspectives in biology. 2015;7 doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JE, Law K, Bear MF. Anatomical origins of ocular dominance in mouse primary visual cortex. Neuroscience. 2009;161:561–571. doi: 10.1016/j.neuroscience.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Current opinion in neurobiology. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L, Fromer M, Walker S, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayton HM, Missler M, Collier DA, Fernandes C. Altered social behaviours in neurexin 1alpha knockout mice resemble core symptoms in neurodevelopmental disorders. PloS one. 2013;8:e67114. doi: 10.1371/journal.pone.0067114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Shi SH, Ule J, Ruggiu M, Barker LA, Darnell RB, Jan YN, Jan LY. Common molecular pathways mediate long-term potentiation of synaptic excitation and slow synaptic inhibition. Cell. 2005;123:105–118. doi: 10.1016/j.cell.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Ippolito DM, Eroglu C. Quantifying synapses: an immunocytochemistry-based assay to quantify synapse number. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont ML, Sanlaville D, Redon R, Raoul O, Cormier-Daire V, Lyonnet S, Amiel J, Le Merrer M, Heron D, de Blois MC, et al. Array-based comparative genomic hybridisation identifies high frequency of cryptic chromosomal rearrangements in patients with syndromic autism spectrum disorders. Journal of medical genetics. 2006;43:843–849. doi: 10.1136/jmg.2006.043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IG, Paladino T, Gurd JW, Brown IR. Molecular cloning of SC1: a putative brain extracellular matrix glycoprotein showing partial similarity to osteonectin/BM40/SPARC. Neuron. 1990;4:165–176. doi: 10.1016/0896-6273(90)90452-l. [DOI] [PubMed] [Google Scholar]
- Kahler AK, Djurovic S, Kulle B, Jonsson EG, Agartz I, Hall H, Opjordsmoen S, Jakobsen KD, Hansen T, Melle I, et al. Association analysis of schizophrenia on 18 genes involved in neuronal migration: MDGA1 as a new susceptibility gene. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2008;147B:1089–1100. doi: 10.1002/ajmg.b.30726. [DOI] [PubMed] [Google Scholar]
- Krahe TE, Medina AE. Activation of NMDA receptors is necessary for the recovery of cortical binocularity. Journal of neurophysiology. 2010;103:2700–2706. doi: 10.1152/jn.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E440–449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Dean C, Isacoff E. Alternative splicing of neuroligin regulates the rate of presynaptic differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:11435–11446. doi: 10.1523/JNEUROSCI.2946-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Muller RA. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain : a journal of neurology. 2013;136:1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V, Pizzorusso T, Maffei L. The visual physiology of the wild type mouse determined with pattern VEPs. Vision research. 1999;39:3071–3081. doi: 10.1016/s0042-6989(99)00022-x. [DOI] [PubMed] [Google Scholar]
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- Risher WC, Patel S, Kim IH, Uezu A, Bhagat S, Wilton DK, Pilaz LJ, Singh Alvarado J, Calhan OY, Silver DL, et al. Astrocytes refine cortical connectivity at dendritic spines. eLife. 2014;3 doi: 10.7554/eLife.04047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EB, Meredith MA, Ramoa AS. Suppression of NMDA receptor function using antisense DNA block ocular dominance plasticity while preserving visual responses. Journal of neurophysiology. 1998;80:1021–1032. doi: 10.1152/jn.1998.80.3.1021. [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Schreiner D, Simicevic J, Ahrne E, Schmidt A, Scheiffele P. Quantitative isoform-profiling of highly diversified recognition molecules. eLife. 2015;4:e07794. doi: 10.7554/eLife.07794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bushong EA, Haustein MD, Tong X, Jackson-Weaver O, Kracun S, Xu J, Sofroniew MV, Ellisman MH, Khakh BS. Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. The Journal of general physiology. 2013;141:633–647. doi: 10.1085/jgp.201210949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7495–7506. doi: 10.1523/JNEUROSCI.0470-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SL, Trachtenberg JT. Experience-dependent binocular competition in the visual cortex begins at eye opening. Nature neuroscience. 2007;10:370–375. doi: 10.1038/nn1844. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Kreiman G, Lyckman A, Mukherjee S, Yu H, Horng S, Sur M. Gene expression changes and molecular pathways mediating activity-dependent plasticity in visual cortex. Nature neuroscience. 2006;9:660–668. doi: 10.1038/nn1689. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nature neuroscience. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver MS, Workman G, Cardo-Vila M, Arap W, Pasqualini R, Sage EH. Processing of the matricellular protein hevin in mouse brain is dependent on ADAMTS4. The Journal of biological chemistry. 2010;285:5868–5877. doi: 10.1074/jbc.M109.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurov V, Stead JD, Merali Z, Palkovits M, Faludi G, Schild-Poulter C, Anisman H, Poulter MO. Molecular pathway reconstruction and analysis of disturbed gene expression in depressed individuals who died by suicide. PloS one. 2012;7:e47581. doi: 10.1371/journal.pone.0047581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.