Abstract
An increase in faunivory is a consistent component of human evolutionary models. Animal matter is energy- and nutrient-dense and can provide macronutrients, minerals, and vitamins that are limited or absent in plant foods. For female humans and other omnivorous primates, faunivory may be of particular importance during the costly periods of pregnancy and early lactation. Yet, because animal prey is often monopolizable, access to fauna among group-living primates may be mediated by social factors such as rank. Wild chimpanzees (Pan troglodytes) across Africa habitually consume insects and/or vertebrates. However, no published studies have examined patterns of female chimpanzee faunivory during pregnancy and early lactation relative to non-reproductive periods, or by females of different rank. In this study, we assessed the influence of reproductive state and dominance rank on the consumption of fauna (meat and insects) by female chimpanzees of Gombe National Park, Tanzania. Using observational data collected over 38 years, we tested (a) whether faunivory varied by reproductive state, and (b) if high-ranking females spent more time consuming fauna than lower-ranking females. In single-factor models, pregnant females consumed more meat than lactating and baseline (meaning not pregnant and not in early lactation) females, and high-ranking females consumed more meat than lower-ranking females. A two-factor analysis of a subset of well-sampled females identified an interaction between rank and reproductive state: lower-ranking females consumed more meat during pregnancy than lower-ranking lactating and baseline females did. High-ranking females did not significantly differ in meat consumption between reproductive states. We found no relationships between rank or reproductive state with insectivory. We conclude that, unlike insectivory, meat consumption by female chimpanzees is mediated by both reproductive state and social rank. We outline several possible mechanisms for these patterns, relate our findings to meat-eating patterns in women from well-studied hunter-gatherer societies, and discuss potential avenues for future research.
Keywords: Dominance Rank, Reproduction, Faunivory, Meat, Insectivory, Diet
Introduction
A greater reliance on faunivory relative to other hominoids is a consistent component of human evolutionary models (e.g., Dart, 1953; Lee and de Vore, 1968; Bunn, 1981, 2006). There is considerable interest and debate among evolutionary anthropologists over the relationships between life history, social organization, encephalization, and diet (including faunivory) in the hominin lineage (e.g., Kaplan et al., 2000; Kennedy, 2005; Barrickman et al., 2008). However, most researchers agree that more frequent consumption of animal prey, and particularly meat, is likely to have been a key dietary shift for the genus Homo (Bunn, 2009). For omnivorous primates, including humans, the nutrients acquired from consuming animals may be of particular importance to females and young offspring (Allen 2000, 2005; Murphy and Allen 2003; Herrera and Heymann 2004). Understanding the relationships between sociality, faunivory and reproduction in living nonhuman primates is therefore critical for clarifying how humans are (or are not) different from other hominoids and mammals more generally. Faunivory patterns among living apes can provide critical insights into the relationship between female reproduction and diet during early hominin evolution.
Mammalian pregnancy and lactation are energetically and nutritionally costly (Clutton-Brock and Harvey 1978; Lee, 1987; Gittleman and Thompson, 1988; Rogowitz, 1996; Butte et al., 1999; Dufour and Sauther, 2002; Emery Thompson et al., 2012). Maternal deficiencies in macronutrients, vitamins, and minerals during pregnancy and lactation have measurable impacts on infant development, health and survival across diverse mammalian species, including humans (McKenzie et al., 1975; Halas et al., 1983; Neuringer et al., 1984; Allen, 2000, 2005; Markham et al., 2011). Beyond the direct and substantial costs of producing milk, mothers may experience additional costs from associating with dependent offspring, including increased energetic expenditure due to carrying their young, reduced travel speeds, and/or reduced foraging efficiency (Hunt 1989; Altmann and Samuels, 1992; Sanchez et al., 1999; Wrangham, 2000; Shradin and Anzenberger, 2001; Pontzer and Wrangham, 2006). Mammalian mothers adopt several strategies to respond to the increased energetic and nutritional demands of pregnancy and lactation. They may draw from their own bodily reserves for fat, protein, and minerals (Adair and Popkin, 1992; Bell et al., 2000, Oftedal, 2000; Kovacs, 2001; Sarli et al., 2004; Emery Thompson, 2013) and/or increase their metabolic efficiency (Poppitt et al., 1994). They may also shift their feeding and foraging patterns to increase food quality, amount consumed, and/or food intake rate (Silk 1986; Berger 1991; Muruthi et al., 1991; Rydell 1993; Sauther 1994; Kunz et al., 1995; McCabe and Fedigan, 2007; Dias et al., 2011). These changes may include shifts in dietary composition. For example, East African chimpanzees (Pan troglodytes schweinfurthii) are known to increase the proportion of fruit in their diet (and by inference, improve dietary quality) during pregnancy and lactation (Murray et al., 2009). In at least some omnivorous primates, dietary shifts in these reproductive periods include changes in faunivory (meaning the consumption of animals, vertebrate or otherwise). For example, lactating titi monkeys (Callicebus cupreus) increase the proportion of fauna in their diet (Herrera and Heymann, 2004), although white-faced capuchins (Cebus capucinus) do not (McCabe and Fedigan, 2007).
Fauna (including both vertebrate and invertebrate prey) are consumed by many extant primates (Lambert, 2007) and were likely an important part of the diet for the earliest primates (Sussman, 1991; Cartmill, 1992). Compared to most plant foods, animal-source foods are generally energy and nutrient-dense (Lieberman, 1987; Milton, 2003). Animal tissues contain high-quality protein and lipids, and often include specific macronutrients (e.g., omega-3 fatty acids such as DHA), vitamins (e.g, vitamin B12), and minerals that are limited or absent in plant foods (Ramos-Elorduy et al., 1997; Crawford et al., 1999; Cordain et al. 2001; Murphy and Allen, 2003; Neumann et al., 2003; Ramos-Elorduy, 2008). Given the nutritional value of faunivory, it is unsurprising that populations of all great ape clades habitually consume vertebrates (chimpanzees: Newton-Fisher, 2014; bonobos [Pan paniscus]: Surbeck and Hohmann, 2008, 2009; orangutans [Pongo spp.]: Hardus et al., 2012) and/or invertebrates (chimpanzees: Goodall, 1968; McGrew, 1992; bonobos: Badrian et al., 1981; Badrian and Melenky, 1984; orangutans: van Schaik et al., 1996; Fox et al., 2004; gorillas [Gorilla spp.]: Tutin and Fernandez, 1992; Ganas and Robbins, 2004). Vertebrate and invertebrate prey can differ in body size by orders of magnitude, yet can have comparable macronutrient composition when compared on a gram-to-gram basis (DeFoliart, 1989, 1992; O’Malley and Power, 2012). The consumption of animals is likely to yield important nutritional benefits for great apes, yet comprehensive nutritional data of the most common chimpanzee prey are limited to analyses of insects (e.g., Deblauwe and Janssens 2008; O’Malley and Power 2012, 2014). Nutrient deficiencies are likely to have fitness consequences during periods of high reproductive investment such as pregnancy and early lactation, but to our knowledge no published studies have examined how faunivory patterns vary during pregnancy and lactation in chimpanzees or any other great ape species.
Due to their varied and seasonal diet, members of the genus Pan are intriguing subjects in which to examine variation in female faunivory patterns across reproductive states. While chimpanzees are ripe-fruit specialists, all long-term study populations are known to consume some fauna (reviewed in Newton-Fisher, 2014). Pruetz (2006) reported that the proportion of animal foods in the diet of chimpanzees varies from 3–13% among long-term study sites (although dietary measures varied across study sites). Notably, the consumption of vertebrates (carnivory) comprised only 0.33 – 6% of these diets based on faecal analyses. Insects (predominantly eusocial insects such as termites [Isoptera], and ants and honeybees [Hymenoptera]) make up the remainder (McGrew, 1992), and so arguably make a greater contribution to chimpanzee diets than do vertebrate prey. Most meat is acquired and consumed by adult male chimpanzees but is shared with other group members, including females (Stanford et al., 1994; Mitani and Watts, 2001; Gilby, 2006). While the degree to which meat sharing serves strategic social functions is debated (Stanford et al., 1994; Mitani and Watts, 2001; Gomes and Boesch, 2009; Gilby 2006, Gilby et al., 2010), there is consensus that meat affords consumers with important macronutrients, vitamins and minerals (Stanford, 1996; Tennie et al., 2014), and that chimpanzees behave in ways consistent with it being a highly valued food resource (Gilby, 2006).
Given the energetic and nutritional costs of reproduction for female chimpanzees (Emery Thompson et al., 2012), dietary variation by reproductive state is expected. However, there are also theoretical reasons to expect faunivory to vary with female social status. Wright et al. (2014) found that higher-ranking female gorillas had higher foraging efficiency compared to lower-ranking females. In wild bonobos, where females are dominant over males, prey carcasses are controlled more often by females- particularly high-ranking females (Fruth and Hohmann, 2002). Female bonobos are frequent and active participants in hunts, although it is not yet clear whether they hunt as often as males do (Surbeck and Hohmann, 2006). Despite relatively low levels of overt female aggression (reviewed in Murray et al., 2007; Miller et al., 2014), high female dominance rank affords myriad benefits to female chimpanzees, including higher-quality core ranging areas (Murray et al., 2006; Emery Thompson et al., 2008), shorter inter-birth intervals (Jones et al., 2010), reduced offspring mortality, and more rapidly maturing daughters (Pusey et al., 1997). These disparities may stem, at least in part, from a high-ranking female’s ability to maintain greater access to high quality foods or greater feeding efficiency even in the presence of conspecific competitors (Wittig and Boesch, 2003; Wright et al., 2014). As they are discrete, high-energy, nutrient-dense packages, we hypothesize that vertebrate and invertebrate prey are useful dietary components with which to examine female contest competition (in this case, meaning competition for spatially or temporally clumped and therefore monopolizable resources; Nicholson, 1954; van Schaik and van Noordwijk, 1986, 1988). McGrew (1992; Table 5.6) presented tantalizing evidence that chimpanzee females’ meat consumption may correlate positively with reproductive success, but did not account for possible confounds such as rank. Mackworth-Young and McGrew (2014) presented evidence of a positive correlation in termite-fishing patterns over a five-year period with lifetime reproductive success of female chimpanzees, but noted that social rank was a likely confounding variable.
In this study, we analyzed 38 years of demographic and feeding data on chimpanzees of the Kasekela community of Gombe National Park, Tanzania, to assess the influence of reproductive state and rank on female faunivory. First, we tested the hypothesis that faunivory varies by reproductive state, with the prediction that females who are pregnant or in early lactation (meaning with an infant of 0–24 months of age) spend more of their feeding time on animals compared to females not heavily investing in reproduction. We focused on mothers with infants in the first two years of life since in this period the offspring are predominantly dependent upon the mother for nutrition, and the demands of lactation are greatest (Murray et al., 2009; Emery Thompson et al., 2012). Notably, at Gombe, no chimpanzee infants orphaned at this stage have survived their mother’s death (Goodall, 1986). Second, we tested the hypothesis that consumption patterns of animal foods are consistent with intra-community contest competition, with the prediction that high-ranking females spend a greater percentage of their foraging time consuming fauna than lower-ranking females. High rank correlates with higher quality core areas and higher quality diets in chimpanzee females (e.g., Murray et al., 2006), but a clear relationship between female rank and faunivory remains untested. Finally, we tested for an interaction between these factors on patterns of faunivory for a subset of females for whom sufficient data were available in a given reproductive state and rank (e.g., pregnant and high-ranking). Variation in patterns of faunal consumption by reproductive and social status would support our hypothesis that, despite its relatively small fraction of the chimpanzee diet, animal source foods are a high-value food resource, and that faunivory patterns have consequences for maternal and fetal health (and by extension, for female reproductive success).
Materials and methods
Study site
We investigated faunivory patterns among adult female chimpanzees (≥12 years old; following Murray et al., 2006, 2009) in the Kasekela community of Gombe National Park, Tanzania. This well-habituated population has been the subject of continuous behavioral observation since 1960 (Goodall, 1986). During our study period, the community varied in size from 38–62 individuals with 12–24 adult females.
Datasets
In order to examine how faunivory varies by reproductive state and dominance rank, we analyzed focal follow data from two long-term datasets, using data collected from 1974 through 2012. The first ‘B-record’ dataset focuses on adult members of the community. Each day, research staff followed one adult chimpanzee from night nest-to-night nest (~12 hours) and collected continuous data on its feeding behavior (including food species and part), party composition, and social interactions with others, including aggression and vocalizations. Research staff rotated through adult members of the community, with the goal of collecting at least one follow on each individual per month (for further details see Goodall, 1986; Wilson, 2012). The second ‘family follows’ dataset focuses on a subset of community mothers and their offspring. During family follows, data were collected on the target mother, youngest dependent offspring, and next oldest offspring (if present). Behaviors such as traveling, resting, feeding, and grooming were collected at 1-minute point samples, while behavioral events such as aggression and vocalizations recorded ad libitum. Maximum family follow duration varied over the years of the study from 6 hours to ~12 hours. We categorized each follow of an adult female in both datasets based on the target’s reproductive state and dominance rank determined as described below. We removed duplicate/overlapping follows between the two datasets (keeping the longer of overlapping follows).
Reproductive state
We assigned female follows to one of three reproductive states: pregnancy, early lactation, or non-pregnant, non-early-lactation (henceforth called ‘baseline’). We defined pregnancy as 226 days prior to the date of birth (the average gestation length for the Kasekela population [Boehm and Pusey, 2013]). We defined early lactation as the first two years of an infant’s life when infants are most dependent on their mothers for nutrition and cannot survive on their own. To consider how patterns during these costly reproductive states compared to faunivory by females that are not heavily investing in reproduction, the baseline category included only follows on females who were neither pregnant nor in early lactation. Female chimpanzees exhibit exaggerated sexual swellings and become sexually receptive around ovulation, and some studies have reported that males are more likely to share meat with sexually receptive females (Stanford, 1998; but see Gilby et al., 2010, Mitani and Watts, 2001). Accordingly, we further restricted the baseline dataset to females of estrous state ‘0’ (meaning the female had no indications of a developing, shrinking, or full sexual swelling) in analyses of reproductive states, to avoid this possible confounding factor and address our primary interest in variance during periods of reproductive investment.
Dominance rank
We determined female dominance rank by the direction and frequency of dyadic pant-grunts, vocalizations that serve as formal indicators of subordinance (Bygott, 1979). Pant-grunts between all individuals were recorded ad libitum in both datasets. We calculated modified David’s scores (de Vries et al., 2006) for females over two-year periods (e.g., 1974 – 1975, 1976 – 1977, and so on). We classified females as high-ranking for a two-year period if their score was greater than 0.5 S.D. above the mean. We characterized all other females as lower-ranking. If no pant-grunts involving a particular female were recorded for a given period, we assigned her last known rank category to her. While prior studies of female relationships have shown that relative ranks among specific females tended to be stable over time, they have also found that an individual’s rank increased with age (Pusey et al., 1997; Murray et al., 2006). Therefore, we assigned follows that occurred during pregnancy and early lactation (see above) a rank status based on the female’s rank on the infant’s birthdate to ensure a consistent rank on all follows linked to a particular infant. For baseline female follows, we assigned rank status based on the female’s rank on the date of the follow.
Faunivory metrics
We analyzed two types of faunivory. Vertebrate prey were primarily colobus monkeys (Procolobus tephrosceles), bush piglets (Potamochoerus larvatus), and forest antelope (Tragelaphus scriptus) fawns. Henceforth we refer to vertebrates as “meat” although this category includes all vertebrate material, such as skin, bones, and eggs. Invertebrates consisted primarily of termites (Macrotermes, Pseudacanthotermes) and ants (Dorylus, Camponotus, Crematogaster, and Oecophylla). Henceforth we refer to invertebrates as “insects”, though this category also includes insect-induced galls in Chlorophora excelsa leaves as well as honey and honeycomb (of Apis mellifera, Trigona spp. and Meliponini). Honey is included as part of insect consumption in our analyses, but it is a relatively small component of female insectivory (on average, 1.60% of insectivory time, and 0.05% of total feeding time) and the results for insectivory we report here are consistent whether or not honey is included. The most frequent form of insectivory at Gombe is termite-fishing, in which chimpanzees use flexible probes of vegetation inserted into holes in termite mounds to capture Macrotermes subhyalinus soldiers (for a full description see Goodall, 1986; McGrew, 1992).
Our faunivory metrics were percent of focal feeding time devoted to faunivory, calculated as:
The percent of feeding time devoted to faunivory was calculated for each female, binned according to reproductive state, social rank, or both, depending on the analyses. For example, to calculate the percent of feeding time on meat during pregnancy, the number of minutes Female X was observed feeding on meat while pregnant was divided by the number of minutes Female X was observed feeding on any identified food while pregnant, multiplied by 100. Likewise, to calculate the percent of meat in the diet during pregnancy for a high ranking female, the number of minutes Female X was observed feeding on meat while pregnant and high-ranking was divided by the total number of minutes Female X was observed feeding on all identified foods while pregnant and high-ranking, then multiplied by 100.
Strictly speaking, both foraging time and feeding time are measures of feeding effort, rather than measures of actual food intake (Schülke et al., 2006; Nakagawa, 2009). However, in our datasets meat consumption was not recorded until a capture had actually been made and meat was being eaten. Also, in an analysis of Gombe insectivory, O’Malley and Power (2014) found that with the exception of termite-fishing, all tool-assisted insectivory bouts of ≥1min in duration in their dataset yielded at least some insects, For termite fishing, only 16% (n = 10/61) of the observed bouts of ≥1min were completely unsuccessful. While our measure of insectivory therefore includes some time spent foraging as well as actual feeding time, we are nevertheless confident that feeding time as described above is a reasonable proxy for consumption of animals in these analyses.
Analyses
In order to examine differences in faunivory based on rank or reproductive state, we used a series of linear mixed models (SAS 9.3, PROC MIXED) with female ID included as a random factor to account for repeated and uneven sampling of individual females. For each of our two faunivory categories (meat and insects) we ran three models using percent of focal feeding time devoted to faunivory as the response variable: Model 1) with reproductive state (Pregnant/Early Lactation/Baseline) as a fixed main effect, Model 2) with categorical rank (High/Lower) as a fixed main effect, and Model 3) with rank, reproductive state, and the 2-way interaction term as fixed effects (n=6 analyses on three different models in total). This approach maximized the number of females included in the two models focused on main effects, while allowing us to examine the interaction between reproductive state and rank in the third model for those females for whom there were enough data to do so. Because consumption of vertebrate prey is a relatively small fraction of feeding time (Goodall 1986) and less likely to be captured than other feeding behaviors, we imposed an inclusion criterion of at least 200 hours of observation on a given female per rank and/or reproductive state. Specifically, a female had to be observed for at least 200 hours in a given reproductive state to be included in model 1(npregnant = 8, nearly lactation = 20, nbaseline= 22), at least 200 hours in a given rank to be included in model 2 (nhigh = 12, nlower = 28), and at least 200 hours in a given reproductive state and rank category to be included in model 3 (nhigh, pregnant = 5, nhigh, lactatation= 7, nhigh, baseline = 11, nlower, pregnant = 4, nlower, early lactation= 17, nlower, baseline= 17). At the 200 hour threshold some vertebrate consumption was captured for the majority of females included in the model, and this allowed us to maximize the number of females included in each analysis. In all analyses the percent fauna in the diet was arcsine square root transformed to meet the assumptions of normality and homogeneity of variance. Model assumptions were evaluated using diagnostic residuals plots.
Results
Our initial dataset included n=33 females that met the inclusion criterion of 200 hours of observation, regardless of rank or reproductive state (X̄ = 1621.4±393.4 S.E. observation hours). Consistent with prior studies on this and other wild chimpanzee populations, insectivory comprised a greater percent of females’ feeding time than meat-eating did (meat: X̄ =1.77 ± 0.22% S.E.; insects: 5.66 ± 0.68% S.E., Wilcoxon signed ranks: V=52, p < 0.001). Termite fishing made up the greatest percent of insectivory by females (X̄ =57.20 ± 4.13% S.E.). We found the same patterns described below for total insectivory in an analysis of termite fishing alone, and so do not discuss those results here. To ensure that there were not significant longitudinal changes in faunivory patterns over the course of the study, we examined summary data binned across decades (1974–1983, 1984–1993, and so on; following the same 200h exclusion criteria per female) to confirm that the broad patterns described below for rank and reproductive state were consistent over time.
Faunivory by Reproductive State
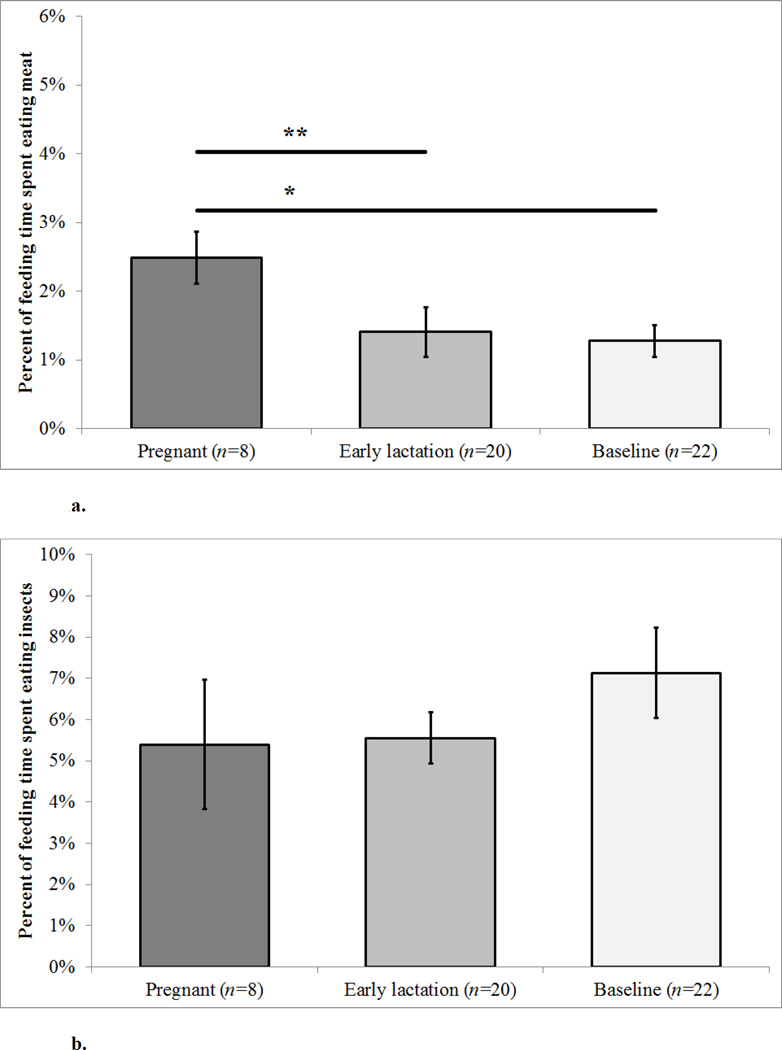
Reproductive state was a significant predictor of the percent of feeding time on meat (F2,24= 3.87, p = 0.035; [Figure 1a; Table 1]). Pregnant females spent a greater percent of their feeding time eating meat than females in early lactation (X̄pregnant = 2.49 ± 0.37% S.E., X̄early lactation = 1.41 ± 0.36% S.E., X̄baseline = 1.27 ± 0.23% S.E.; Tukey’s pairwise test; p = 0.030) and tended to eat more meat than baseline females (Tukey’s; p = 0.068). We found no differences between reproductive states in the percent of feeding time spent eating insects (X̄pregnant = 5.40 ± 1.57% S.E., X̄early lactation = 5.56 ± 0.62% S.E., X̄baseline = 7.14 ± 1.09% S.E., F2,24= 1.41, p = 0.263) [Figure 1b].
Figure 1. a–b. Mean Faunivory by Reproductive State.
Error bars represent the standard error (S.E.) The n value gives the number of females in each reproductive state. Individual females may appear in more than one reproductive state category. ** indicates a pairwise difference (p<0.05) and * indicates a tendency (p<0.10) as determined by Tukey’s test.
Table 1.
Summary of Linear Mixed Model Results (Model 1).
| Variable | Estimate | S.E. | Numerator df |
Denominator df |
t value | F | p1 |
|---|---|---|---|---|---|---|---|
| Model 1: Reproductive State | |||||||
| Dependent variable: % feeding time on meat2 | |||||||
| Intercept | 0.102 | 0.013 | 2 | 23 | 7.70 | <0.001 | |
| Reproductive State | 2 | 24 | 3.87 | 0.035** | |||
| Pregnant3 | 0.055 | 0.023 | 2.35 | 0.028 | |||
| Early Lactation3 | −0.010 | 0.017 | −0.57 | 0.573 | |||
| Dependent variable: % feeding time on insects2 | |||||||
| Intercept | 0.259 | 0.017 | 2 | 23 | 15.40 | <.001 | |
| Reproductive State | 2 | 24 | 1.41 | 0.263 | |||
| Pregnant2 | −0.029 | 0.027 | −1.06 | 0.300 | |||
| Early Lactation2 | −0.031 | 0.019 | −1.59 | 0.124 | |||
p values denoted with ** indicate an effect (p<0.05).
after arcsine square-root transformation,
compared to baseline.
Faunivory by Rank
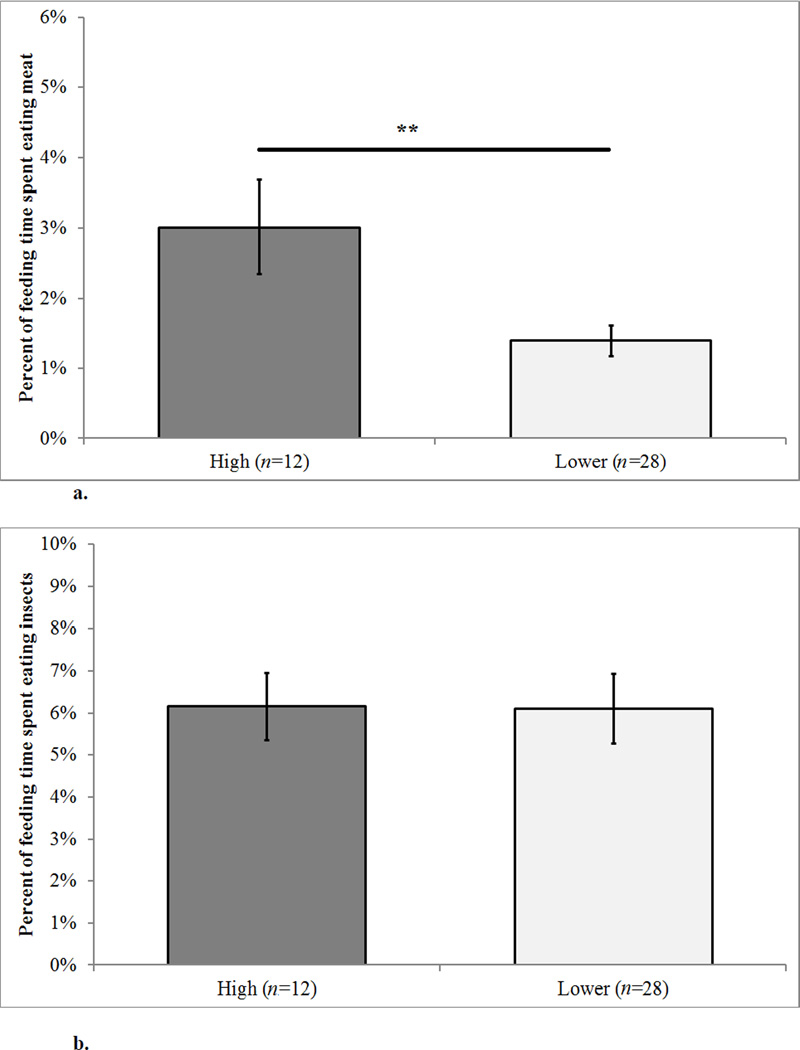
High-ranking females spent more of their feeding time eating meat than lower-ranking females did (X̄high= 3.01 ± 0.67% S.E., X̄lower = 1.39 ± 0.22% S.E., F1,8= 7.32, p = 0.027 [Figure 2a; Table 2]). We found no rank differences in time spent consuming invertebrates (X̄high = 6.16 ± 0.80% S.E., X̄lower = 6.10 ± 0.83% S.E., F1,8= 0.02, p = 0.891[Figure 2b]).
Figure 2. a–b. Mean Faunivory by Dominance Rank.
Error bars represent the S.E. The n value gives the number of females in each rank category. Individual females may appear in more than one rank category. ** indicates a difference (p<0.05).
Table 2.
Summary of Linear Mixed Model Results (Model 2).
| Variable | Estimate | S.E. | Numerator df |
Denominator df |
t value | F | p1 |
|---|---|---|---|---|---|---|---|
| Model 2 (Rank only) | |||||||
| Dependent variable: % feeding time on meat2 | |||||||
| Intercept | 0.104 | 0.012 | 1 | 30 | 8.95 | <0.001 | |
| Rank | 1 | 8 | 2.71 | 7.32 | 0.027** | ||
| High3 | 0.056 | 0.021 | |||||
| Dependent variable: % feeding time on insects2 | |||||||
| Intercept | 0.236 | 0.015 | 1 | 30 | 15.89 | <0.001 | |
| Rank | 1 | 8 | −0.14 | 0.02 | 0.891 | ||
| High3 | 0.004 | 0.025 | |||||
p values denoted with ** indicate an effect (p<0.05).
after arcsine square-root transformation,
compared to Lower.
Interaction of Reproductive State and Rank
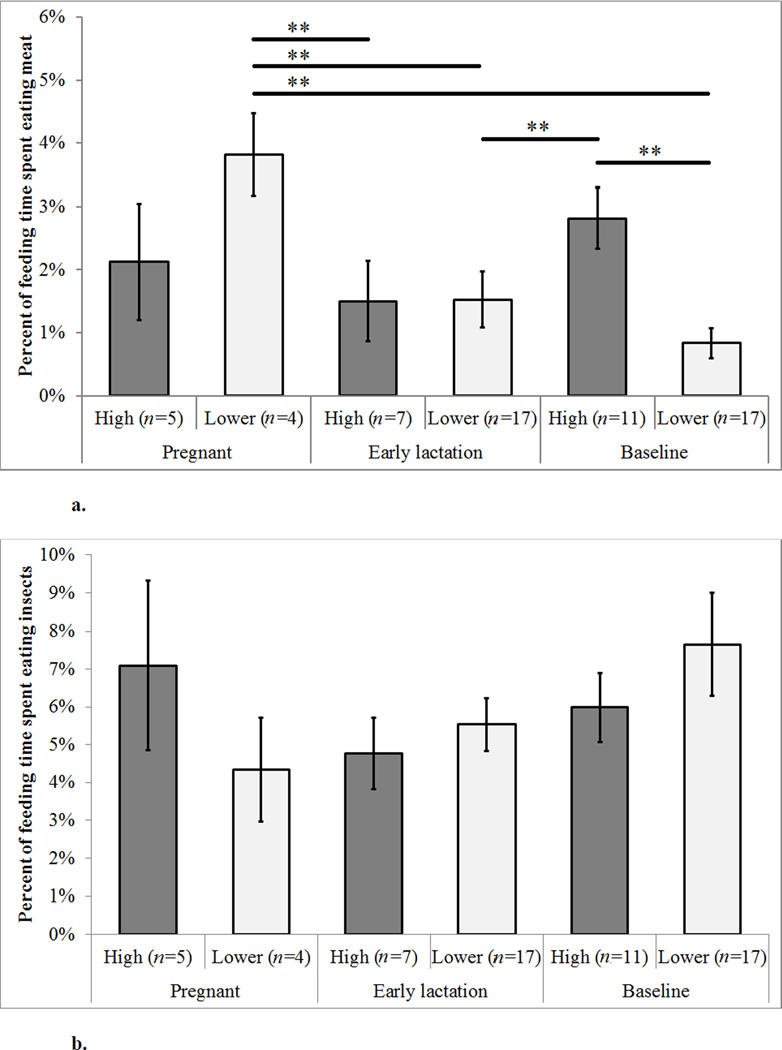
Using the set of well-sampled females (n=23) that met our inclusion criteria of 200h for at least one reproductive state by rank category, we found that the interaction predicted consumption of meat [Table 3]. High-ranking, baseline females spent a greater percent of feeding time eating meat than lower-ranking, baseline females (X̄high, baseline= 2.81 ± 0.48% S.E., X̄low, baseline = 0.83 ± 0.23% S.E.; Tukey’s pairwise test; p = 0.009). Meat eating by females of high rank did not differ across reproductive states(X̄high, pregnant = 2.12 ± 0.92% S.E., X̄high, early lactation = 1.50 ± 0.64% S.E., X̄high, baseline = 2.81 ± 0.48% S.E.)[Figure 3a]. However, among lower-ranking females, those that were pregnant spent a greater percent of feeding time eating meat than lactating females (X̄lower, pregnant = 3.82 ± 0.65% S.E., X̄lower, early lactation = 1.52 ± 0.44% S.E., Tukey’s; p= 0.002) or baseline females did (X̄lower, baseline = 0.83 ± 0.23% S.E.; Tukey’s; p<0.001). Lower-ranking, pregnant females also consumed more meat than high-ranking, lactating females (Tukey’s; p = 0.013), and high-ranking, baseline females consumed more meat than lower-ranking, lactating females (Tukey’s; p = 0.043). We found no evidence of an interaction of reproductive state and rank with percent of feeding time devoted to insectivory (X̄high, pregnant = 7.09 ± 2.24% S.E., X̄high, early lactation = 4.77 ± 0.94% S.E., X̄high, baseline = 5.99 ± 0.92% S.E., X̄lower, pregnant = 4.34 ± 1.37% S.E., X̄lower, early lactation = 5.54± 0.70% S.E., X̄lower, baseline = 7.65± 1.35% S.E.)[Figure 3b].
Table 3.
Summary of Linear Mixed Model Results (Model 3).
| Variable | Estimate | S.E. | Numerator df |
Denominator df |
t value | F | p1 |
|---|---|---|---|---|---|---|---|
| Model 3: Reproductive State * Rank | |||||||
| Dependent variable: % feeding time on meat2 | |||||||
| Intercept | 0.079 | 0.016 | 2 | 22 | 5.01 | <0.001 | |
| Reproductive State | 2 | 33 | 6.71 | <0.004** | |||
| Pregnant3 | 0.146 | 0.031 | 4.68 | <0.001 | |||
| Early Lactation3 | 0.015 | 0.019 | 0.80 | 0.432 | |||
| Rank | 1 | 33 | 0.04 | 0.844 | |||
| High3 | 0.082 | 0.022 | 3.73 | <0.001 | |||
| Reproductive State * Rank | 2 | 33 | 9.27 | 0.001** | |||
| Pregnant3, High4 | −0.179 | 0.043 | −4.17 | <0.001 | |||
| Early Lactation3, High4 | −0.078 | 0.032 | −2.40 | 0.022 | |||
| Dependent variable: % feeding time on insects2 | |||||||
| Intercept | 0.264 | 0.018 | 2 | 22 | 14.40 | <0.001 | |
| Reproductive State | 2 | 33 | 1.66 | 0.207 | |||
| Pregnant3 | −0.063 | 0.035 | −1.82 | 0.078 | |||
| Early Lactation3 | −0.032 | 0.021 | −1.53 | 0.136 | |||
| Rank | 1 | 33 | 0.14 | 0.715 | |||
| High4 | −0.021 | 0.025 | −0.86 | 0.394 | |||
| Reproductive State * Rank | 2 | 33 | 1.94 | 0.159 | |||
| Pregnant3, High4 | 0.088 | 0.048 | 1.84 | 0.075 | |||
| Early Lactation3, High4 | −0.002 | 0.036 | −0.05 | 0.963 | |||
p values denoted with ** indicate an effect (p<0.05).
after arcsine square root transformation,
compared to baseline,
compared to low.
Figure 3. a–b: Mean Faunivory by Reproductive State and Rank.
Errors bar represent the S.E. The n value gives the number of females in each reproductive state by rank bin. Individual females may appear in more than one reproductive state by rank category. ** indicates a pairwise difference (p<0.05) as determined by Tukey’s test.
Discussion
Despite considerable research interest in faunivory and its role in human evolution, little is known about how animal consumption varies by reproductive state or social status in hominoid females. We found that pregnant chimpanzees spent a greater percentage of feeding time on meat than females in other reproductive states. Consistent with prior studies regarding the benefits of high rank (e.g., Wittig and Boesch, 2003; Murray et al., 2006), we also found that high-ranking females spent a greater percentage of their feeding time consuming meat than lower-ranking females. Closer inspection of these broad patterns on a subset of well-sampled females revealed that differences were driven by the feeding patterns of lower-ranking females. High-ranking females maintained relatively consistent meat consumption regardless of reproductive state, while lower-ranking females increased their meat consumption in pregnancy. Thus, while high-ranking baseline females consumed more meat than lower-ranking baseline females, among lower-ranking individuals, pregnant females consumed more meat than lactating or baseline females. Moreover, the percent of feeding time on meat by pregnant, lower-ranking females was significantly higher than that of high-ranking, lactating females.
As noted above, feeding time is a measure of foraging effort, not a direct measure of food intake or the significance of that food in the diet. Nevertheless, a slight shift in overall feeding time on foods that are both energy- and nutrient-dense likely represents major differences in macronutrient and micronutrient intake for females on short- and long-term timescales. Only limited macronutrient estimates are available for vertebrate prey consumed by chimpanzees (Ajayi, 1979), although energy, macronutrient and mineral values for all major Kasekela insect prey have been determined by O’Malley and Power (2012, 2014). In general, relative to plant foods, both vertebrate tissues and insects are energy- and nutrient-dense. Animal tissues contain highly digestible (and therefore high quality) protein, variable (but often high) fatty acid content, and high levels of zinc (Zn), selenium (Se), phosphorus (P), potassium (K), sodium (Na) and iron (Fe) (the latter in the easily absorbed haem form) (Lieberman, 1987; Milton, 2003; Pereira and Vicente, 2013). Animal foods also provide consistently high levels of vitamins, including vitamin B12. B12 plays critical roles in the development and functioning of the brain and nervous system, yet is absent from virtually all wild plant tissues and must be acquired either as a product of gastrointestinal microbial activity or through the consumption of animal tissues (Watanabe, 2007). While plant foods can and do contain high levels of protein and fats, and can be high in both mineral and vitamin content, few plant foods are comparable to animal-sourced foods in providing such a broad spectrum of both macro- and micronutrients.
For female chimpanzees, the benefits of faunivory are more likely to be more nutritional than energetic (Tennie et al., 2009; 2014) as the energetic yields from frugivory can be quite high (e.g., up to 30kcal/min from figs; Wrangham et al., 1993). While analyses of hunting patterns at one study site suggest that hunting is calorically-motivated (Taï: Boesch, 1994; Boesch and Boesch-Achermann, 2000), the likelihood of hunting increases during periods of relative plant food abundance at others (Gombe: Gilby et al., 2006; Kanyawara: Gilby and Wrangham, 2007). Hunting is estimated to be energetically costly at these sites (Tennie et al., 2009), particularly given that many hunts are unsuccessful and that meat is not equally shared among participants. As females do not hunt as often as males, they are not necessarily incurring hunting-related energetic costs, although they also consume meat less often and in smaller amounts than males (Goodall, 1986, Stanford et al., 1994). We therefore argue that the available data for Gombe support the meat-scrap hypothesis (Gilby et al., 2008, Tennie et al., 2009, 2014), in which the consumption of even small amounts of animal prey serves as a source of macronutrients such as fats, as well as vitamins and minerals that are limited or absent in most plant foods. Given the well-established effects of prenatal (e.g., Brown and Susser, 2003) or infant (e.g., Lozoff et al., 2000, Walker et al., 2007) nutrient deficiencies on health outcomes in humans and in other animals (e.g., Hurley and Mutch, 1973; Rajalakshmi and Nakhasi, 1974; Chmurzynska, 2010), we hypothesize that pregnancy may be a critical period for dietary intake of nutrients such as omega-3 fatty acids, vitamin B12, and minerals such as haem iron, sodium and zinc. Although faunivory represents, on average, a small percentage of female feeding time (7.43%; see above), the difference in average feeding time on meat between, for example, high-versus lower-ranking baseline females (2.81 versus 0.83%) or lower-ranking pregnant versus baseline females (3.82 versus 0.83%) nevertheless represents roughly a three and a half to five-fold increase in feeding effort on these energy- and nutrient-dense foods between rank and reproductive state, respectively. To the extent that these differences in feeding time represent differences in actual meat intake, we hypothesize that they have a real biological impact on females’ nutritional status.
Dominant females’ higher overall meat consumption and consistency across reproductive states may reflect that they experience a greater degree of tolerance by males. In the Kasekela community of Gombe, dominant females had lower levels of faecal glucocorticoid metabolite (FGM; a measure of stress) in larger and male-dominated parties compared to lower-ranking females (Markham et al., 2014). We hypothesize that if males tend to be more tolerant of high-ranking females in social groups, and high-ranking females experience less social stress, dominant females may be able to beg more persistently or successfully from males. Greater meat consumption by high-ranking females may also reflect stronger kinship ties with male hunters, at least at Gombe. In the Kasekela community (unlike most other long-term study communities across Africa), up to 50% of females remain in their natal community (Pusey et al., 1997). Since dominance rank increases with age (Pusey et al., 1997; Murray et al., 2006), dominant females are more likely to have one or more adult sons or brothers in the community compared to subordinate females. Food-sharing between adult primates, although rare in the wild, is often biased towards maternal relatives (Feistner and McGrew, 1989; Silk et al., 2013). Chimpanzee males may gain inclusive fitness benefits by preferentially sharing meat with (or tolerating more scrounging from) their mothers or sisters. Interestingly, Fruth and Hohmann (2002) found no kin bias in meat sharing among adult bonobos. Boesch et al. (2006) found that mutualism, rather than kinship, best explained hunting behavior among male chimpanzees of the Taï Forest (Côte d’Ivoire). However, kin-biased meat-sharing among adults of different sexes has, to our knowledge, not been systematically examined at any long-term chimpanzee research site.
The observed pattern of increased meat-eating by lower-ranking females during pregnancy is particularly compelling when we consider that successful hunts by chimpanzees typically occur in relatively large, male-biased groups (Stanford et al., 1994; Mitani and Watts, 2001; Gilby et al., 2006). As noted above, lower-ranking females experience more stress in such groups compared to dominant females (Markham et al., 2014). Future work will therefore be required to identify the mechanisms through which low-ranking, pregnant females increase the percent of meat in their diet. Hypotheses include (a) that males may share meat more often, or in greater quantities, specifically with low-ranking, pregnant females, (b) that lower-ranking, pregnant females may increase the duration, intensity, or persistence of begging for meat, and (c) that social rank and reproductive state mediate hunting behavior by female chimpanzees themselves. One possible contributing factor for hypotheses (a) and (b) is that female chimpanzees sometimes continue to produce estrous swellings for several months into a pregnancy (Wallis and Goodall, 1983). While debate over the evolutionary function of estrous swellings continues (Nunn, 1999; Domb and Pagel, 2001, Deschner et al., 2004), one proximate effect of these swellings is to increase male interest in the females bearing them (Girolami and Bielert, 1987; Deschner et al., 2004). Although excluded from our analyses of reproductive state for the reasons noted above, the proportion of feeding time on meat by fully swollen estrous females was, on average, more than one and a half times that of pregnant females, and roughly three times that of early lactation or baseline females (see Supplementary Online Material [SOM] Figures 1a–b). However, prior work has shown that the presence of estrous females does not increase the likelihood of hunts, and that estrous females are neither more likely to receive meat from males, nor receive larger quantities of meat from males compared to other (non-estrous) females (Gilby, 2006; Gilby et al., 2010). Studies of the Gombe population have yielded conflicting but largely negative evidence for a direct, short-term exchange of “meat for sex” between adult males and estrous females (Stanford et al., 1994; Stanford, 1996; Gilby, 2006; Gilby et al., 2010). Gilby (2006) found no evidence that male chimpanzees at Gombe share meat based on female sexual receptivity, although that study did not consider female relatedness to meat possessors, female social rank, or aspects of reproductive state (e.g., pregnancy) beyond the presence or absence of an estrous swelling. As discussed above, kin-biased meat sharing among adults, which has not been examined at Gombe, may also be important in understanding the interaction of rank and reproductive state on female meat consumption. Additionally, as they typically have a lower-quality diet than high-ranking females (Murray et al., 2006), low-ranking females might be motivated to beg for meat more persistently while pregnant (or in the weeks immediately prior to pregnancy) regardless of whether or not they exhibit estrous swellings, in order to avoid the negative consequences of prenatal nutrient deficiencies. These factors could result in greater meat consumption by these females without necessarily reflecting socially-strategic sharing by males as predicted by the original “meat-for-sex” hypothesis, which has some parallels to indirect effects of male hunting success on female reproduction in humans.
The lower meat consumption among lactating females (of both high- and lower- rank) may simply reflect that females with young infants are less likely to join large parties (where hunts are more likely to be successful), or reflect an aversion to hunting parties specifically, which might be dangerous for infants (Tennie et al., 2014). While female chimpanzees do not hunt as often as males (Stanford et al. 1994), Goodall (1986) noted that the frequency of female hunts while they are alone or in family groups is probably underestimated.
In contrast to carnivory, insectivory did not vary with dominance rank or reproductive state. Some insects consumed at Gombe have comparable macronutritional value to meat on a gram-for-gram basis (O’Malley and Power, 2012). Estimated yields from consumption of the most common insect prey (Macrotermes termites and Dorylus ants) can meet or substantially contribute to chimpanzees’ estimated daily requirements for several dietary minerals (O’Malley and Power, 2014). However, the small mass of insect prey on a per-unit basis means that termite fishing and other common forms of insectivory among Gombe chimpanzees have low energetic and nutritional yields on a per-minute basis. While in theory, a female chimpanzee seeking to increase the percent of fauna in her diet might more easily “up-regulate” insectivory, in practice, the returns for common forms of insectivory may only be nutritionally significant for bouts of mean to maximum duration (e.g., in termite-fishing), or the duration of bouts may be curtailed by prey countermeasures (e.g., in dipping for Dorylus ants) (O’Malley and Power 2014). Time spent consuming insects may reduce the time spent consuming other foods in which energetic yields can be much higher than for insectivory (e.g., figs; Wrangham et al., 1993). Feeding sites for invertebrates (e.g., termite mounds), are scattered throughout the community range; in some cases prey may be readily available at predictable locations, but most profitably obtained only during certain times of year (e.g., Macrotermes [Goodall, 1986]). Other insect prey may be available year round but encountered only opportunistically (e.g., Dorylus ants [Pascual-Garrido et al., 2013]). While conflicts over mound access do occur between females (O’Malley, personal observation), the existence of dozens of productive termite mounds across the community range (O’Malley, 2011) and multiple fishing holes at most mounds suggests that these represent less monopolizable animal resources than vertebrate carcasses. In contrast to hunting, insectivory (with the exception of honeybee nest raiding) poses little or no physical risk to a chimpanzee predator (Tennie et al., 2014). Our findings do not mean that invertebrate consumption is unimportant for females in this community (indeed, the consistently high fraction of insects in the diet across female reproductive states and rank suggests otherwise), but do suggest that patterns of insect consumption are not strongly influenced by intra-community female competition.
Comparing our results to the literature on meat consumption in well-studied modern hunter-gatherer populations highlights intriguing similarities and contrasts, as well as potential avenues for future research (Table 4). Well-studied hunter-gatherers typically consume significantly more meat per annum than chimpanzees (Cordain et al., 2000; Stanford, 2001), and fauna (including meat, insects, and honeycomb) are usually among the most valued food for both men and women (Hill et al., 1984; Berbesque and Marlowe, 2007). There are clear prestige benefits for men to be known as a good hunter (reviewed in Smith 2004). Being a good hunter is positively associated with reproductive success (Hill and Hurtado 1996, Marlowe 1999, Weissner 2002). The families of good hunters often consume more meat (Hawkes et al., 2013; Wood and Marlowe 2013; but also see Kaplan et. al., 1984), and have greater seasonal weight gains (Hawkes, 1993). As noted by Marlowe (2001), the primary effects of male provisioning, including of meat, are earlier weaning (Hawkes et al.,1998) and reduced inter-birth intervals (Kaplan et al., 2000), two metrics with clear implications for female reproductive success. Despite these benefits, there are surprisingly few quantitative data on the relationships between faunivory patterns of women with reproductive state. The best evidence for faunivory shifts relating to reproductive state among foragers demonstrates that young children negatively impact foraging returns for women (but not men, who increase their foraging returns [Marlowe, 2003; Hurtado et al., 1992]). We have found no evidence for broad dietary shifts per se in pregnancy among well-studied hunter-gatherer populations. However, food taboos (which can include meat and eggs [reviewed by Spielmann 1989]) and aversions (which vary cross-culturally, but also can include meat and eggs [Dickens and Trethowan, 1978; Hook 1978]) in pregnancy and lactation are documented in many cultures, including those of hunter-gatherers. Such taboos and aversions may cause a decrease in faunivory among women, particularly in the first trimester when aversions are strongest. A possible functional explanation for such patterns in pregnancy is the avoidance of pathogens or levels of certain nutrients (e.g., Vitamin A) that can be toxic to a fetus at high levels. On the other hand, such taboos are linked to nutritional deficiencies in pregnancy and negative birth outcomes (Spielmann, 1989). More study on this topic is needed, particularly among hunter-gatherer groups.
Table 4.
Comparison of meat-eating and dietary quality patterns in two well-studied hunter gatherer groups with chimpanzees of Gombe.
| Homo sapiens | Pan troglodytes | |||
|---|---|---|---|---|
| Hadza [Tanzania]1 | Ache [Paraguay]2 | Gombe [Tanzania]3 | ||
| Meat is a highly valued or highly ranked food resource | Yes | Yes | Yes | |
| Meat acquisition is male-biased | Yes | Yes | Yes | |
| Access to, or consumption of, meat correlates with one or more metrics of reproductive success for females | Yes | Yes | Unclear (but rank does correlate with metrics of reproductive success) | |
| Males share meat preferentially with: | ||||
| mates and/or offspring | Yes | No (on multi-day hunting trips) | No | |
| adult relatives | Unclear | Unclear | Unclear | |
| Females exhibit foraging changes or dietary shifts in pregnancy (vs. baseline) | ||||
| Change in foraging efficiency | Unclear | Unclear | Unclear | |
| Change in dietary quality | Unclear | Unclear | Increases (more fruit/meat) | |
| Change in meat consumption | Unclear (Food taboos?) | Unclear (Food taboos?) | Increases (if lower-ranking) | |
| Females exhibit foraging changes or dietary shifts with dependent infants (vs. baseline) | ||||
| Change in foraging efficiency | Decrease | Decrease | Unclear(increase travel distances) | |
| Change in dietary quality | Unclear (husband’s returns increase) | Unclear | Increases (more fruit) | |
| Change in meat consumption | Unclear | Unclear | No | |
Sources:
(Hadza)1 Hawkes et al. 1998; Marlowe 2003; Berbesque and Marlowe 2007; Wood and Marlowe 2013;
(Ache)2 Hill et al., 1984; Kaplan et al., 1984; Kaplan and Hill, 1985; Hurtado et al., 1992;
(Gombe)3 Stanford, 1996; Gilby, 2006; Murray et al., 2006; 2009; Pontzer and Wrangham, 2006; Gilby et al.,2010; this study.
The energetic and nutritional contribution of meat to hunter-gatherer diets varies widely (Crawford et al., 1999, Cordain et al., 2001). However, myriad human clinical trials and animal model studies have documented the close relationship between human maternal condition and diet with fetal and postnatal health (reviewed by Godfrey and Barker, 2000). Among modern hunter-gatherers, women may be more consistently energetically and nutritionally constrained than men (Spielmann, 1989). In industrialized countries, macro- and micro-nutrient deficiencies in pregnant women are a health concern (reviewed in Blumfield et al., 2013). In the developing world, supplementing the diets of pregnant women and young children with animal-source foods is a successful strategy for avoiding or alleviating a variety of health problems (Allen, 2003, 2005; Murphy and Allen, 2003; Neumann et al., 2003). At the same time, excessive consumption of protein on a regular basis – roughly 50% of total calories – leads to liver and kidney impairment and potentially other serious health consequences (Speth 1989, 1990, 1991). Speth (1991) noted that the ceiling for safe levels of protein consumption may actually be much lower in pregnant women. A diet high in protein is linked to negative birth outcomes, particularly under conditions of energy restriction. Whether a protein intake ceiling may also apply to chimpanzees, and more importantly, whether even the most faunivorous wild chimpanzee females ever approach it, remains unclear. Testing this hypothesis would require more comprehensive data on nutritional composition and long-term food intake patterns on chimpanzees than are currently available in the literature.
Given the variability above and the gap between patterns in modern humans and those of our ancestors, our results are important for understanding the role of faunivory in hominin evolution. Broadly speaking, a higher quality diet, including greater animal consumption, is hypothesized to have relaxed constraints on the size and structure of energetically expensive brain tissue in later hominins (Aiello and Wheeler, 1995; Milton, 2003; Navarrete et al., 2011). The human pattern of low infant mortality and longer life expectancy partially reflects the adoption of broad and flexible diets, including consumption of diverse animal prey (Hockett and Haws, 2003). Increased faunivory has been linked to distinct human life-history patterns in Homo relative to other apes (Finch and Stanford, 2004; Schuppli et al., 2012) and to the social organization of human ancestors, including provisioning, sexual division of labor, and a greater reliance on home bases (Isaac, 1978; Rose and Marshall, 1996; Kaplan et al., 2000; Bunn, 2009). Though there is growing evidence for butchery of large animals using stone tools prior to the emergence of the genus Homo (McPherron et al., 2010; Harmond et al. 2015; Thompson et al. 2015), this is likely to have been preceded by predation on smaller game at frequencies or in ways that may not be discernable in the fossil and archaeological records, and so resembles in some ways the hunting patterns of chimpanzees (Stanford, 1996; Pruetz et al., 2015). Extinct hominins’ access to high-quality, monopolizable food resources such as meat is likely to have been socially mediated, as is the case for chimpanzees, bonobos and human foragers. However, there remain some important differences between human foragers and chimpanzees. At minimum, these include differences in how often, and in what quantity, meat is consumed and how it is shared (Stanford, 1996, 2001). It is unclear whether faunivory patterns by modern humans and chimpanzees shift across reproductive states in similar ways (as reviewed above). However, the dietary shifts documented in this study suggest that even in a social hominoid species for whom meat is a small fraction of the diet, high-ranking adult females maintain relatively consistent meat consumption patterns, while lower-ranking females increase meat consumption specifically during pregnancy – a period when maternal nutrient deficiencies are known to be particularly costly in terms of offspring health and development (e.g., Hurley and Mutch, 1973; Allen, 2000, 2005). To further explore this relationship, more data are needed on meat intake rates and nutritional composition, as well as analyses of faunivory patterns as they relate to female reproductive metrics such as inter-birth intervals, infant survivorship, and infant maturation rates. While neither chimpanzees nor bonobos are stand-ins for any extinct hominin species, examining the significance of faunivory in the female diet in living Pan remains a useful line of inquiry in reconstructing the role of animal foods in human evolution.
Conclusions
Using observational data collected over nearly four decades, we have provided evidence that consumption of meat by female chimpanzees varies with dominance rank and reproductive state, consistent with meat being a highly-valued and monopolizable food. High- ranking females spent relatively more of their feeding time consuming meat and were consistent in their carnivorous feeding time across reproductive states, while lower-ranking females spent a relatively greater proportion of their feeding time in carnivory during pregnancy compared to other reproductive states. Among baseline females, high-ranking chimpanzees spent a greater proportion of their feeding time on meat than lower-ranking chimpanzees. Interestingly, patterns of insectivory did not vary with either rank or reproductive state in any of our three models. Our results suggest that patterns of meat consumption (but not insect consumption) are mediated by social rank and reproductive state. However, a clear relationship between faunivory and maternal health, infant gestation, and infant survival in chimpanzees remains to be established.
Supplementary Material
Acknowledgements
For permission to conduct research in Gombe National Park, we thank Tanzania National Parks (TANAPA), the Tanzania Wildlife Research Institute (TAWIRI), and the Tanzanian Commission for Science and Technology (COSTECH). We are grateful to the Jane Goodall Institute for their financial support of the long-term research effort, and to the Gombe Stream Research Centre staff for maintaining data collection. Data digitization was funded by the National Institutes of Health (R00HD057992, R01 AI058715), the National Science Foundation (DBS-9021946, SBR-9319909, BCS-0452315, LTREB-1052693), the Harris Steel Group, the Windibrow Foundation, the Carnegie Corporation, the University of Minnesota, Duke University, the Leo S. Guthman Foundation, and the National Geographic Society. Behavioral analyses were supported by the National Institutes of Health (R00HD057992) and by a George Washington University Selective Excellence Grant. We are grateful to the numerous interns and research assistants who have helped digitize both behavioral datasets. Finally, we thank the entire George Washington University Primate Behavioral Ecology lab for invaluable feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robert C. O’Malley, Email: omalleyrc@gmail.com.
Margaret A. Stanton, Email: maggiestanton@gmail.com.
Ian C. Gilby, Email: ian.gilby@asu.edu.
Elizabeth V. Lonsdorf, Email: elonsdor@fandm.edu.
Anne Pusey, Email: anne.pusey@duke.edu.
A. Catherine Markham, Email: markham.catherine@gmail.com.
Carson M. Murray, Email: cmmurray@gwu.edu.
REFERENCES
- Adair LS, Popkin BM. Prolonged lactation contributes to depletion of maternal energy reserves in Filipino women. J. Nutr. 1992;122:1643. doi: 10.1093/jn/122.8.1643. [DOI] [PubMed] [Google Scholar]
- Aiello LC, Wheeler P. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 1995;36:199–221. [Google Scholar]
- Ajayi SS. Utilization of forest wildlife in West Africa. Paper prepared for the FAO Forestry Department. 1979 FO:Misc./79/26 (unpublished). [Google Scholar]
- Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am. J. Clin. Nutr. 2000;71:1280s–1284s. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- Allen LH. Interventions for micronutrient deficiency control in developing countries: past, present and future. J. Nutr. 2003;133:3875S–3878S. doi: 10.1093/jn/133.11.3875S. [DOI] [PubMed] [Google Scholar]
- Allen LH. Multiple micronutrients in pregnancy and lactation: an overview. Am. J. Clin. Nutr. 2005;81:1206S–1212S. doi: 10.1093/ajcn/81.5.1206. [DOI] [PubMed] [Google Scholar]
- Altmann J, Samuels A. Costs of maternal care: infant-carrying in baboons. Behav. Ecol. Sociobiol. 1992;29:391–398. [Google Scholar]
- Badrian N, Malenky RK. Feeding ecology of Pan paniscus in the Lomako forest, Zaire. In: Susman RL, editor. The Pygmy Chimpanzee: Evolutionary Biology and Behavior. New York: Plenum Press; 1984. pp. 275–299. [Google Scholar]
- Badrian N, Badrian A, Susman RL. Preliminary observations on the feeding behaviour of Pan paniscus in the Lomako forest of central Zaire. Primates. 1981;22:173–181. [Google Scholar]
- Barrickman NL, Bastian ML, Isler K, van Schaik CP. Life history costs and benefits of encephalization: a comparative test using data from long-term studies of primates in the wild. J. Hum. Evol. 2008;54:568–590. doi: 10.1016/j.jhevol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Bell AW, Burhans WS, Overton TR. Protein nutrition in late pregnancy, maternal protein reserves and lactation performance in dairy cows. Proc. Nutr. Soc. 2000;59:119–126. doi: 10.1017/s0029665100000148. [DOI] [PubMed] [Google Scholar]
- Berbesque JC, Marlowe FW. Sex differences in food preferences of Hadza hunter-gatherers. Evol. Psychol. 2009;7:601–616. [Google Scholar]
- Berger J. Pregnancy incentives, predation constraints and habitat shifts: experimental and field evidence for wild bighorn sheep. Anim. Behav. 1991;41:61–77. [Google Scholar]
- Blumfield ML, Hure AJ, Macdonald-Wicks L, Smith R, Collins CE. A systematic review and meta-analysis of micronutrient intakes during pregnancy in developed countries. Nutr. Rev. 2013;71(2):118–132. doi: 10.1111/nure.12003. [DOI] [PubMed] [Google Scholar]
- Boehm EE, Pusey AE. Measuring gestation length in the chimpanzees of Gombe National Park [Abstract]. Annual Meeting of the American Journal of Physical Anthropologists; Wiley-Blackwell. 2013. p. 84. [Google Scholar]
- Boesch C. Hunting strategies of Gombe and Tai chimpanzees. In: Wrangham RW, McGrew WC, deWaal FBM, Heltne PG, editors. Chimpanzee Cultures. Cambridge: Harvard University Press; 1994. pp. 77–91. [Google Scholar]
- Boesch C, Boesch-Achermann H. The Chimpanzees of the Taï Forest: Behavioural Ecology and Evolution. Oxford: Oxford University Press; 2000. [Google Scholar]
- Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr. Bull. 2008;34:1054–1063. doi: 10.1093/schbul/sbn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesch C, Boesch H, Vigilant L. Cooperative hunting in chimpanzees: kinship or mutualism? In: Kappeler P, van Schaik C, editors. Cooperation in Primates and Humans. Berlin Heidelberg: Springer; 2006. pp. 139–150. [Google Scholar]
- Bunn HT. Archaeological evidence for meat-eating by Plio-Pleistocene hominids from Koobi Fora and Olduvai Gorge. Nature. 1981;291:574–577. [Google Scholar]
- Bunn HT. Evolution of the Human Diet: the Known, the Unknown, and the Unknowable. New York: Oxford University Press; 2006. Meat made us human; pp. 191–211. [Google Scholar]
- Butte NF, Hopkinson JM, Mehta N, Moon JK, Smith EOB. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am. J. Clin. Nutr. 1999;69:299–307. doi: 10.1093/ajcn/69.2.299. [DOI] [PubMed] [Google Scholar]
- Bygott D. Agonistic behavior and dominance among wild chimpanzees. In: Hamburg DA, McCown ER, editors. The Great Apes. Menlo Park: Benjamin Cummings; 1979. pp. 405–427. [Google Scholar]
- Cartmill M. New views on primate origins. Evol. Anthropol. 1992;1:105–111. [Google Scholar]
- Chmurzynska A. Fetal programming: link between early nutrition, DNA methylation, and complex diseases. Nutr. Rev. 2010;68:87–98. doi: 10.1111/j.1753-4887.2009.00265.x. [DOI] [PubMed] [Google Scholar]
- Clark C. A preliminary report on weaning among chimpanzees of the Gombe National Park, Tanzania. In: Chevalier-Skolnikoff S, Poirier F, editors. Primate Bio-Social Development. New York: Garland Press; 1977. pp. 235–260. [Google Scholar]
- Clutton-Brock TH, Harvey PH. Mammals, resources and reproductive strategies. Nature. 1978;273(5659):191–195. doi: 10.1038/273191a0. [DOI] [PubMed] [Google Scholar]
- Cordain L, Miller JB, Eaton SB, Mann N, Holt SH, Speth JD. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am. J. Clin. Nutr. 2000;71(3):682–692. doi: 10.1093/ajcn/71.3.682. [DOI] [PubMed] [Google Scholar]
- Cordain L, Watkins BA, Mann NJ. Fatty acid composition and energy density of foods available to African hominids. World Rev. Nutr. Diet. 2001;90:144–161. doi: 10.1159/000059813. [DOI] [PubMed] [Google Scholar]
- Crawford MA, Bloom M, Broadhurst CL, Schmidt WF, Cunnane SC, Galli C, Gehbremeskel K, Linseisen F, Lloyd-Smith J, Parkington J. Evidence for unique function of docosahexaenoic acid during the evolution of the modern human brain. Lipids. 1999;34:S39–S47. doi: 10.1007/BF02562227. [DOI] [PubMed] [Google Scholar]
- Dart R. The predatory transition from ape to man. Int. Anthropol. Linguist. Rev. 1953;1:493–502. [Google Scholar]
- de Vries H, Stevens JM, Vervaecke H. Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 2006;71:585–592. [Google Scholar]
- Deblauwe I, Janssens GPJ. New insights in insect prey choice by chimpanzees and gorillas in southeast Cameroon: the role of nutritional value. Am. J. Phys. Anthropol. 2008;135:42–55. doi: 10.1002/ajpa.20703. [DOI] [PubMed] [Google Scholar]
- DeFoliart GR. The human use of insects as food and as animal feed. Bull. Entomol. Soc. Am. 1989;35:22–35. [Google Scholar]
- DeFoliart GR. Insects as human food. Crop Protect. 1992;11:395–399. [Google Scholar]
- Deschner T, Heistermann M, Hodges K, Boesch C. Female sexual swelling size, timing of ovulation, and male behavior in wild West African chimpanzees. Horm. Behav. 2004;46:204–215. doi: 10.1016/j.yhbeh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Dias PAD, Rangel-Negrín A, Canales-Espinosa D. Effects of lactation on the time-budgets and foraging patterns of female black howlers (Alouatta pigra) Am. J. Phys. Anthropol. 2011;145:137–146. doi: 10.1002/ajpa.21481. [DOI] [PubMed] [Google Scholar]
- Dickens G, Trethowan H. Cravings and a versions during pregnancy. J. Psychosomatic Res. 1971;15:259–268. doi: 10.1016/0022-3999(71)90037-7. [DOI] [PubMed] [Google Scholar]
- Domb LG, Pagel M. Sexual swellings advertise female quality in wild baboons. Nature. 2001;410:204–206. doi: 10.1038/35065597. [DOI] [PubMed] [Google Scholar]
- Dufour DL, Sauther ML. Comparative and evolutionary dimensions of human pregnancy and lactation. Am. J. Hum. Biol. 2002;14:584–602. doi: 10.1002/ajhb.10071. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M. Reproductive ecology of female chimpanzees. Am. J. Primatol. 2013;75(3):222–237. doi: 10.1002/ajp.22084. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Wrangham RW. Diet and reproductive function in wild female chimpanzees (Pan troglodytes schweinfurthii) at Kibale National Park, Uganda. Am. J. Phys. Anthropol. 2008;135:171–181. doi: 10.1002/ajpa.20718. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Wrangham RW. The energetics of lactation and the return to fecundity in wild chimpanzees. Behav. Ecol. 2012;23:1234–1241. [Google Scholar]
- Feistner AT, McGrew WC. Food-sharing in primates: a critical review. Persp. Primate Biol. 1989;3:21–36. [Google Scholar]
- Finch CE, Stanford CB. Meat-adaptive genes and the evolution of slower aging in humans. Q. Rev. Biol. 2004;79:3–50. doi: 10.1086/381662. [DOI] [PubMed] [Google Scholar]
- Fox EA, van Schaik CP, Sitompul A, Wright DN. Intra-and interpopulational differences in orangutan (Pongo pygmaeus) activity and diet: implications for the invention of tool use. Am. J. Phys. Anthropol. 2004;125:162–174. doi: 10.1002/ajpa.10386. [DOI] [PubMed] [Google Scholar]
- Fruth B, Hohmann G. How bonobos handle hunts and harvests: why share food? In: Boesch B, Hohmann G, Marchant LF, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge: Cambridge University Press; 2002. pp. 231–243. [Google Scholar]
- Galdikas B, Wood J. Birth spacing patterns in humans and apes. Am. J. Phys. Anthropol. 1990;83:185–191. doi: 10.1002/ajpa.1330830207. [DOI] [PubMed] [Google Scholar]
- Ganas J, Robbins MM. Intrapopulation differences in ant eating in the mountain gorillas of Bwindi Impenetrable National Park, Uganda. Primates. 2004;45:275–278. doi: 10.1007/s10329-004-0089-5. [DOI] [PubMed] [Google Scholar]
- Gilby IC. Meat sharing among the Gombe chimpanzees: harassment and reciprocal exchange. Anim. Behav. 2006;71:953–963. [Google Scholar]
- Gilby IC, Wrangham RW. Risk-prone hunting by chimpanzees (Pan troglodytes schweinfurthii) increases during periods of high diet quality. Behav. Ecol. Sociobiol. 2007;61:1771–1779. [Google Scholar]
- Gilby IC, Eberly LE, Pintea L, Pusey A. Ecological and social influences on the hunting behaviour of wild chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 2006;72:169–180. [Google Scholar]
- Gilby IC, Eberly LE, Wrangham RW. Economic profitability of social predation among wild chimpanzees: individual variation promotes cooperation. Anim. Behav. 2008;75:351–360. [Google Scholar]
- Gilby IC, Emery-Thompson M, Ruane JD, Wrangham R. No evidence of short-term exchange of meat for sex among chimpanzees. J. Hum. Evol. 2010;59:44–53. doi: 10.1016/j.jhevol.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Gittleman JL, Thompson SD. Energy allocation in mammalian reproduction. Am. Zool. 1988;28:863–875. [Google Scholar]
- Girolami L, Bielert C. Female perineal swelling and its effects on male sexual arousal: an apparent sexual releaser in the chacma baboon (Papio ursinus) Int. J. Primatol. 1987;8:651–661. [Google Scholar]
- Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000;71(5):1344s–1352s. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- Gomes CM, Boesch C. Wild chimpanzees exchange meat for sex on a long-term basis. PLoS ONE. 2009;4:e5116. doi: 10.1371/journal.pone.0005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J. The Behaviour of Free-living Chimpanzees in the Gombe Stream Reserve. London: Baillière, Tindall & Cassell; 1968. [Google Scholar]
- Goodall J. The Chimpanzees of Gombe : Patterns of Behavior. Cambridge: Harvard University Press; 1986. [Google Scholar]
- Halas E, Eberhardt M, Diers M, Sandstead H. Learning and memory impairment in adult rats due to severe zinc deficiency during lactation. Physiol. Behav. 1983;30:371–381. doi: 10.1016/0031-9384(83)90140-3. [DOI] [PubMed] [Google Scholar]
- Hardus ME, Lameira AR, Zulfa A, Atmoko SSU, de Vries H, Wich SA. Behavioral, ecological, and evolutionary aspects of meat-eating by Sumatran orangutans (Pongo abelii) Int. J. Primatol. 2012;33:287–304. doi: 10.1007/s10764-011-9574-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmand S, Lewis JE, Feibel CS, Lepre CJ, Prat S, Lenoble A, Boes X, Quinn RL, Brenet M, Arroyo A, Taylor N, Clement S, Daver G, Brugal J-P, Leakey L, Mortlock RA, Wright JD, Lokorodi S, Kirwa C, Kent DV, Roche H. 3.3-million-year-old stone tools from Lomekwi, West Turkana, Kenya. Nature. 2015;521:310–315. doi: 10.1038/nature14464. [DOI] [PubMed] [Google Scholar]
- Hawkes K. Why hunter-gatherers work. Curr. Anthropol. 1993;34:341–362. [Google Scholar]
- Hawkes K, O’Connell JF, Jones NB, Alvarez H, Charnov EL. Grandmothering, menopause, and the evolution of human life histories. Proc. Nat. Acad. Sci. 1998;95(3):1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K, O'Connell JF, Jones NB. Hadza meat sharing. Evol. Hum. Behav. 2001;22:113–142. doi: 10.1016/s1090-5138(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Hawkes K, O’Connell JF, Jones NGB. More lessons from the Hadza about men’s work. Hum. Nat. 2014;25(4):596–619. doi: 10.1007/s12110-014-9212-5. [DOI] [PubMed] [Google Scholar]
- Herrera E, Heymann EW. Does mom need more protein? Preliminary observations on differences in diet composition in a pair of red titi monkeys (Callicebus cupreus) Folia Primatol. 2004;75:150–153. doi: 10.1159/000078304. [DOI] [PubMed] [Google Scholar]
- Hill KR, Hurtado AM. Ache Life History: The Ecology and Demography of Foraging People. New York: Aldene de Gruyter; 1996. [Google Scholar]
- Hill K, Kaplan H, Hawkes K, Hurtado AM. Foraging decisions among Ache hunter-gatherers: new data and implications for optimal foraging models. Ethol. Sociobiol. 1987;8:1–36. [Google Scholar]
- Hockett B, Haws J. Nutritional ecology and diachronic trends in Paleolithic diet and health. Evol. Anthropol. 2003;12:211–216. [Google Scholar]
- Hook EB. Dietary cravings and aversions during pregnancy. Am. J. Clin. Nutr. 1978;31:1355–1362. doi: 10.1093/ajcn/31.8.1355. [DOI] [PubMed] [Google Scholar]
- Hunt KD. Ph.D. Dissertation. University of Michigan; 1989. Positional behavior in Pan troglodytes at the Mahale Mountains and Gombe Stream National Parks, Tanzania. [DOI] [PubMed] [Google Scholar]
- Hurley LS, Mutch PB. Prenatal and postnatal development after transitory gestational zinc deficiency in rats. J. Nutr. 1973;103:649–656. doi: 10.1093/jn/103.5.649. [DOI] [PubMed] [Google Scholar]
- Hurtado AM, Hill K, Hurtado I, Kaplan H. Trade-offs between female food acquisition and child care among Hiwi and Ache foragers. Hum. Nat. 1992;3:185–216. doi: 10.1007/BF02692239. [DOI] [PubMed] [Google Scholar]
- Isaac GL. The food sharing behavior of protohuman hominids. Sci. Am. 1978;238:90–109. doi: 10.1038/scientificamerican0478-90. [DOI] [PubMed] [Google Scholar]
- Jones JH, Wilson ML, Murray CM, Pusey A. Phenotypic quality influences fertility in Gombe chimpanzees. J. Anim. Ecol. 2010;79:1262–1269. doi: 10.1111/j.1365-2656.2010.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlenberg SM, Emery-Thompson M, Wrangham RW. Female competition over core areas in Pan troglodytes schweinfurthii Kibale National Park, Uganda. Int. J. Primatol. 2008;29:931–947. [Google Scholar]
- Kaplan H, Hill K, Hawkes K, Hurtado A. Food sharing among Ache hunter-gatherers of eastern Paraguay. Curr. Anthropol. 1984:113–115. [Google Scholar]
- Kaplan H, Hill K. Hunting ability and reproductive success among male Ache foragers: Preliminary results. Curr. Anthropol. 1985a;26:131–133. [Google Scholar]
- Kaplan H, Hill K. Food sharing among Ache foragers: Tests of explanatory hypotheses. Curr. Anthropol. 1985b;26:223–246. [Google Scholar]
- Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 2000;9:156–185. [Google Scholar]
- Kennedy GE. From the ape's dilemma to the weanling's dilemma: early weaning and its evolutionary context. J. Hum. Evol. 2005;48:123–145. doi: 10.1016/j.jhevol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kovacs CS. Calcium and bone metabolism in pregnancy and lactation. J. Clin. Endocrinol. Metab. 2001;86:2344–2348. doi: 10.1210/jcem.86.6.7575. [DOI] [PubMed] [Google Scholar]
- Kunz T, Whitaker J, Wadanoli M. Dietary energetics of the insectivorous Mexican free-tailed bat (Tadarida brasiliensis) during pregnancy and lactation. Oecologia. 1995;101:407–415. doi: 10.1007/BF00329419. [DOI] [PubMed] [Google Scholar]
- Lambert JE. Primate nutritional ecology: feeding biology and diet at ecological and evolutionary scales. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK, editors. Primates in Perspective. New York: Oxford University Press; 2007. pp. 482–495. [Google Scholar]
- Lee PC. Nutrition, fertility and maternal investment in primates. J. Zool. 1987;213:409–422. [Google Scholar]
- Lee PC. The meanings of weaning: growth, lactation, and life history. Evol Anthropol. 1996;5:87–98. [Google Scholar]
- Lee RB, DeVore I. Man the Hunter. New York: Aldine de Gruyter; 1968. [Google Scholar]
- Lieberman LS. Biocultural consequences of animals versus plants as sources of fats, proteins, and other nutrients. In: Harris M, Ross EB, editors. Food and Evolution: Towards a Theory of Human Food Habits. Philadelphia: Temple University Press; 1987. pp. 225–258. [Google Scholar]
- Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:e51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- Mackworth-Young C, McGrew WC. Elementary technology correlates with lifetime reproductive success in wild chimpanzees, but why? Pan Africa News. 2014;21:12–15. [Google Scholar]
- Markham AC, Gesquiere LR, Bellenger J-P, Alberts SC, Altmann J. White monkey syndrome and presumptive copper deficiency in wild savannah baboons. Am. J. Primatol. 2011;73:1160–1168. doi: 10.1002/ajp.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham AC, Santymire RM, Lonsdorf EV, Heintz MR, Lipende I, Murray CM. Rank effects on social stress in lactating chimpanzees. Anim. Behav. 2014;87:195–202. doi: 10.1016/j.anbehav.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlowe F. Showoffs or providers? The parenting effort of Hadzamen. Evol. Hum. Behav. 1999;20:391–404. [Google Scholar]
- Marlowe F. Male contribution to diet and female reproductive success among foragers. Curr. Anthropol. 2001;42(5):755–759. [Google Scholar]
- Marlowe FW. A critical period for provisioning by Hadza men: Implications for pair bonding. Evol. Hum. Behav. 2003;24:217–229. [Google Scholar]
- McCabe G, Fedigan L. Effects of reproductive status on energy intake, ingestion rates, and dietary composition of female Cebus capucinus at Santa Rosa, Costa Rica. Int. J. Primatol. 2007;28:837–851. [Google Scholar]
- McGrew WC. Chimpanzee Material Culture: Implications for Human Evolution. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- McKenzie JM, Fosmire GJ, Sandstead HH. Zinc deficiency during the latter third of pregnancy: effects on fetal rat brain, liver, and placenta. J. Nutr. 1975;105:1466–1475. doi: 10.1093/jn/105.11.1466. [DOI] [PubMed] [Google Scholar]
- McPherron SP, Zeresenay Alemseged Z, Marean CW, Wynn JG, Reed D, Geraads D, Bobe R, Béarat HA. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature. 466:857–860. doi: 10.1038/nature09248. [DOI] [PubMed] [Google Scholar]
- Miller JA, Pusey AE, Gilby IC, Schroepfer-Walker K, Markham AC, Murray CM. Competing for space: female chimpanzees are more aggressive inside than outside their core areas. Anim. Behav. 2014;87:147–152. doi: 10.1016/j.anbehav.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton K. The critical role played by animal source foods in human (Homo) evolution. J. Nutr. 2003;133:3886S–3892S. doi: 10.1093/jn/133.11.3886S. [DOI] [PubMed] [Google Scholar]
- Mitani JC, Watts DP. Why do chimpanzees hunt and share meat? Anim. Behav. 2001;61:915–924. [Google Scholar]
- Murphy SP, Allen LH. Nutritional importance of animal source foods. J. Nutr. 2003;133:3932S–3935S. doi: 10.1093/jn/133.11.3932S. [DOI] [PubMed] [Google Scholar]
- Murray CM, Eberly LE, Pusey AE. Foraging strategies as a function of season and rank among wild female chimpanzees (Pan troglodytes) Behav. Ecol. 2006;17:1020–1028. [Google Scholar]
- Murray CM, Mane SV, Pusey AE. Dominance rank influences female space use in wild chimpanzees, Pan troglodytes : towards an ideal despotic distribution. Anim. Behav. 2007;74:1795–1804. [Google Scholar]
- Murray CM, Lonsdorf EV, Eberly LE, Pusey AE. Reproductive energetics in free-living female chimpanzees (Pan troglodytes schweinfurthii) Behav. Ecol. 2009;20:1211–1216. [Google Scholar]
- Muruthi P, Altmann J, Altmann S. Resource base, parity, and reproductive condition affect females' feeding time and nutrient intake within and between groups of a baboon population. Oecologia. 1991;87:467–472. doi: 10.1007/BF00320408. [DOI] [PubMed] [Google Scholar]
- Nakagawa N. Feeding rate as valuable information in primate feeding ecology. Primates. 2009;50:131–141. doi: 10.1007/s10329-009-0129-2. [DOI] [PubMed] [Google Scholar]
- Navarrete A, van Schaik CP, Isler K. Energetics and the evolution of human brain size. Nature. 2011;480:91–93. doi: 10.1038/nature10629. [DOI] [PubMed] [Google Scholar]
- Neumann CG, Bwibo NO, Murphy SP, Sigman M, Whaley S, Allen LH, Guthrie D, Weiss RE, Demment MW. Animal source foods improve dietary quality, micronutrient status, growth and cognitive function in Kenyan school children: background, study design and baseline findings. J. Nutr. 2003;133:3941S–3949S. doi: 10.1093/jn/133.11.3941S. [DOI] [PubMed] [Google Scholar]
- Neuringer M, Connor WE, Van Petten C, Barstad L. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J. Clin. Invest. 1984;73:272–276. doi: 10.1172/JCI111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton-Fisher NE. The Hunting Behavior and Carnivory of Wild Chimpanzees. 2nd ed. Berlin: Springer-Verlag; 2014. [Google Scholar]
- Nicholson AJ. An outline of the dynamics of animal populations. Aust. J. Zool. 1954;2:9–65. [Google Scholar]
- Nunn CL. The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim. Behav. 1999;58:229–246. doi: 10.1006/anbe.1999.1159. [DOI] [PubMed] [Google Scholar]
- O'Malley RC. Ph.D. Dissertation. University of Southern California; 2011. Environmental, nutritional and social aspects of insectivory by Gombe chimpanzees. [Google Scholar]
- O'Malley RC, Power ML. Nutritional composition of actual and potential insect prey for the Kasekela chimpanzees of Gombe National Park, Tanzania. Am. J. Phys. Anthropol. 2012;149:493–503. doi: 10.1002/ajpa.22151. [DOI] [PubMed] [Google Scholar]
- O'Malley RC, Power ML. The energetic and nutritional yields from insectivory for Kasekela chimpanzees. J. Hum. Evol. 2014:46–58. doi: 10.1016/j.jhevol.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Oftedal OT. Use of maternal reserves as a lactation strategy in large mammals. Proc. Nutr. Soc. 2000;59:99–106. doi: 10.1017/s0029665100000124. [DOI] [PubMed] [Google Scholar]
- Pascual-Garrido A, Umaru B, Allon O, Sommer V. Apes finding ants: predator–prey dynamics in a chimpanzee habitat in Nigeria. Am. J. Primatol. 2013;75(12):1231–1244. doi: 10.1002/ajp.22187. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Wrangham RW. Ontogeny of ranging in wild chimpanzees. Int. J. Primatol. 2006;27:295–309. [Google Scholar]
- Poppitt SD, Prentice AM, Goldberg GR, Whitehead RG. Energy-sparing strategies to protect human fetal growth. Am. J. Obstet. Gynecol. 1994;171:118–125. doi: 10.1016/s0002-9378(94)70087-7. [DOI] [PubMed] [Google Scholar]
- Pruetz JD, Bertolani P, Ontl KB, Lindshield S, Shelley M, Wessling EG. New evidence on the tool-assisted hunting exhibited by chimpanzees (Pan troglodytes verus) in a savannah habitat at Fongoli, Sénégal. Royal Soc. Op. Sci. 2015;2(4):140507. doi: 10.1098/rsos.140507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey A, Williams J, Goodall J. The influence of dominance rank on the reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- Rajalakshmi R, Nakhasi H. Effects of prenatal and postnatal nutritional deficiency on brain lipid composition in rats. Exp. Neurol. 1974;44:103–112. doi: 10.1016/0014-4886(74)90050-8. [DOI] [PubMed] [Google Scholar]
- Ramos-Elorduy J. Energy supplied by edible insects from Mexico and their nutritional and ecological importance. Ecol. Food. Nutr. 2008;47:280–297. [Google Scholar]
- Ramos-Elorduy J, Moreno JMP, Prado EE, Perez MA, Otero JL, De Guevara OL. Nutritional value of edible insects from the state of Oaxaca, Mexico. J. Food Compos. Anal. 1997;10:142–157. [Google Scholar]
- Rogowitz GL. Trade-offs in energy allocation during lactation. Am. Zool. 1996;36:197–204. [Google Scholar]
- Rose L, Marshall F. Meat eating, hominid sociality, and home bases revisited. Curr. Anthropol. 1996;37:307–338. [Google Scholar]
- Rydell J. Variation in foraging activity of an aerial insectivorous bat during reproduction. J. Mammal. 1993:503–509. [Google Scholar]
- Sanchez S, Pelaez F, Gil-Bürmann C, Kaumanns W. Costs of infant-carrying in the cotton-top tamarin (Saguinus oedipus) Am. J. Primatol. 1999;48:99–111. doi: 10.1002/(SICI)1098-2345(1999)48:2<99::AID-AJP2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Sarli M, Hakim C, Rey P, Zanchetta J. Osteoporosis during pregnancy and lactation. Medicina (Mex) 2004;65:533–540. [PubMed] [Google Scholar]
- Schradin C, Anzenberger G. Costs of infant carrying in common marmosets, Callithrix jacchus: an experimental analysis. Anim. Behav. 2001;62:289–295. [Google Scholar]
- Schülke O, Chalise MK, Koenig A. The importance of ingestion rates for estimating food quality and energy intake. Am. J. Primatol. 2006;68:951–965. doi: 10.1002/ajp.20300. [DOI] [PubMed] [Google Scholar]
- Schuppli C, Isler K, van Schaik CP. How to explain the unusually late age at skill competence among humans. J. Hum. Evol. 2012;63:843–850. doi: 10.1016/j.jhevol.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Silk JB, Brosnan SF, Henrich J, Lambeth SP, Shapiro S. Chimpanzees share food for many reasons: the role of kinship, reciprocity, social bonds and harassment on food transfers. Anim. Behav. 2013;85:941–947. doi: 10.1016/j.anbehav.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EA. Why do good hunters have higher reproductive success? Hum. Nat. 2004;15:343–364. doi: 10.1007/s12110-004-1013-9. [DOI] [PubMed] [Google Scholar]
- Spielmann KA. A review: dietary restrictions on hunter-gatherer women and the implications for fertility and infant mortality. Hum. Ecol. 1989;17:321–345. doi: 10.1007/BF00889022. [DOI] [PubMed] [Google Scholar]
- Speth JD. Early hominid subsistence strategies in seasonal habitats. J. Archaeol. Sci. 1987;14(1):13–29. [Google Scholar]
- Speth JD. Early hominid hunting and scavenging: the role of meat as an energy source. J. Hum. Evol. 1989;18:329–343. [Google Scholar]
- Speth JD, Widdowson EM, Oftedal OT, Foley RA, Van Soest P. Protein selection and avoidance strategies of contemporary and ancestral foragers: Unresolved issues [and discussion] Philos. T. Roy. Soc. 1991;B334:265–270. doi: 10.1098/rstb.1991.0115. [DOI] [PubMed] [Google Scholar]
- Stanford CB. The hunting ecology of wild chimpanzees: implications for the evolutionary ecology of Pliocene hominids. Amer. Anthropol. 1996;98:96–113. [Google Scholar]
- Stanford CB. Chimpanzee and Red Colobus: The Ecology of Predator and Prey. Cambridge: Harvard University Press; 1998. [Google Scholar]
- Stanford CB. The Hunting Ape: Meat Eating and the Origins of Human Behavior. Princeton: Princeton University Press; 1999. [Google Scholar]
- Stanford CB. A comparison of social meat-foraging by chimpanzees and human foragers. In: Stanford CB, Bunn HT, editors. Meat Eating and Human Evolution. Oxford: Oxford University Press; 2001. pp. 122–140. [Google Scholar]
- Stanford CB, Wallis J, Mpongo E, Goodall J. Hunting decisions in wild chimpanzees. Behaviour. 1994a:1–18. [Google Scholar]
- Stanford CB, Wallis J, Matama H, Goodall J. Patterns of predation by chimpanzees on red colobus monkeys in Gombe National Park, 1982–1991. Am. J. Phys. Anthropol. 1994b;94(2):213–228. doi: 10.1002/ajpa.1330940206. [DOI] [PubMed] [Google Scholar]
- Surbeck M, Hohmann G. Primate hunting by bonobos at Lui Kotale, Salonga National Park. Curr. Biol. 2008;18:R906–R907. doi: 10.1016/j.cub.2008.08.040. [DOI] [PubMed] [Google Scholar]
- Surbeck M, Fowler A, Deimel C, Hohmann G. Evidence for the consumption of arboreal, diurnal primates by bonobos (Pan paniscus) Am. J. Primatol. 2009;71:171–174. doi: 10.1002/ajp.20634. [DOI] [PubMed] [Google Scholar]
- Sussman RW. Primate origins and the evolution of angiosperms. Am. J. Primatol. 1991;21:209–223. doi: 10.1002/ajp.1350230402. [DOI] [PubMed] [Google Scholar]
- Tennie C, Gilby IC, Mundry R. The meat-scrap hypothesis: small quantities of meat may promote cooperative hunting in wild chimpanzees (Pan troglodytes) Behav. Ecol. Sociobiol. 2009;63:421–431. [Google Scholar]
- Tennie C, O'Malley RC, Gilby I. Why do chimpanzees hunt? A cost-benefit analysis of acquiring and consuming vertebrate versus invertebrate prey. J. Hum. Evol. 2014;71:38–45. doi: 10.1016/j.jhevol.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Thompson JC, McPherron SP, Bobe R, Reed D, Barr WA, Wynn JG, Marean CW, Geraads D, Alemseged Z. Taphonomy of fossils from the hominin-bearing deposits at Dikika, Ethiopia. J. Hum. Evol. 2015;86:112–135. doi: 10.1016/j.jhevol.2015.06.013. [DOI] [PubMed] [Google Scholar]
- Tutin CEG, Fernandez M. Insect eating by sympatric lowland gorillas (Gorilla g. gorilla) and chimpanzees (Pan t. troglodytes) in the Lopé Reserve, Gabon. Am. J. Primatol. 1992;28:29–40. doi: 10.1002/ajp.1350280103. [DOI] [PubMed] [Google Scholar]
- van Schaik CP, van Noordwijk MA. The hidden costs of sociality: intra-group variation in feeding strategies in Sumatran long-tailed macaques (Macaca fascicularis) Behaviour. 1986;99:296–315. [Google Scholar]
- van Schaik CP, van Noordwijk MA. Scramble and contest in feeding competition among female long-tailed macaques (Macaca fascicularis) Behaviour. 1988;105:77–98. [Google Scholar]
- van Schaik C, Fox E, Sitompul A. Manufacture and use of tools in wild Sumatran orangutans. Naturwissenschaften. 1996;83:186–188. doi: 10.1007/BF01143062. [DOI] [PubMed] [Google Scholar]
- Walker SP, Wachs TD, Meeks Gardner J, Lozoff B, Wasserman GA, Pollitt E, Carter JA. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- Wallis J, Goodall J. Anogenital swelling in pregnant chimpanzees of Gombe National Park. Am. J. Primatol. 1993;31:89–98. doi: 10.1002/ajp.1350310202. [DOI] [PubMed] [Google Scholar]
- Watanabe F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007;232(10):1266–1274. doi: 10.3181/0703-MR-67. [DOI] [PubMed] [Google Scholar]
- Wiessner P. Hunting, healing, and Hxaroexchange: A long-term perspective on !Kung (Ju/’hoansi) large-game hunting. Evol. Hum. Behav. 2002;23:407–436. [Google Scholar]
- Wilson ML. Long-term studies of the chimpanzees of Gombe National Park, Tanzania. In: Kappeler PM, Watts DP, editors. Long-term Field Studies of Primates. Berlin Heidelberg: Springer; 2012. pp. 357–384. [Google Scholar]
- Wittig RM, Boesch C. Food competition and linear dominance hierarchy among female chimpanzees of the Tai National Park. Int. J. Primatol. 2003;24:847–867. [Google Scholar]
- Wood BM, Marlowe FW. Household and kin provisioning by Hadza men. Hum. Nature. 2013;24(3):280–317. doi: 10.1007/s12110-013-9173-0. [DOI] [PubMed] [Google Scholar]
- Wrangham RW. Why are male chimpanzees more gregarious than mothers? A scramble competition hypothesis. In: Kappeler PM, editor. Primate Males: Causes and Consequences of Variation in Group Composition. Cambridge: Cambridge University Press; 2000. pp. 248–258. [Google Scholar]
- Wrangham R, Conklin N, Etot G, Obua J, Hunt K, Hauser M, Clark A. The value of figs to chimpanzees. Int. J. Primatol. 1993;14:243–256. [Google Scholar]
- Wright E, Robbins AM, Robbins MM. Dominance rank differences in the energy intake and expenditure of female Bwindi mountain gorillas. Behav. Ecol. Sociobiol. 2014;68:957–970. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.