Summary
Translocation into the endoplasmic reticulum (ER) is the first step in the biogenesis of thousands of eukaryotic endomembrane proteins. While functional ER translocation has been avidly studied, little is known about the quality control mechanisms that resolve faulty translocational states. One such faulty state is translocon clogging, in which the substrate fails to properly translocate and obstructs the translocon pore. To shed light on the machinery required to resolve clogging, we carried out a systematic screen in Saccharomyces cerevisiae that highlighted a role for the ER metalloprotease Ste24. We could demonstrate that Ste24 approaches the translocon upon clogging, interacts with and generates cleavage fragments of the clogged protein. Importantly, these functions are conserved in the human homologue, ZMPSTE24, while disease-associated mutant forms of ZMPSTE24 fail to clear the translocon. These results shed light on a new and critical task of Ste24, which safe-guards the essential process of translocation.
Introduction
Up to 30% of proteins in any eukaryotic proteome reside within the endomembrane system (Chen et al., 2005). The first step in the biogenesis of these numerous proteins involves their translocation, either partially or fully, into the endoplasmic reticulum (ER) through the translocon channel (Park and Rapoport, 2012). Endomembrane proteins, which are translated on cytosolic ribosomes, can be targeted and inserted into the ER through several pathways (Ast and Schuldiner, 2013; Aviram and Schuldiner, 2014). Based on its biophysical attributes, an endomembrane protein might translocate into the ER with the help of the signal recognition particle (SRP) targeting pathway, which tightly couples translation and translocation (Grudnik et al., 2009). Other substrates target and translocate into the ER through pathways that are not as tightly coupled to their translation, using SRP independent mechanisms. These SRP independent pathways use alternate means to thread their substrates into the ER, such as the Sec62/Sec63 machinery, which utilizes the luminal Hsp70 Kar2/BiP to ratchet proteins into the ER lumen (Park and Rapoport, 2012).
While the process of effective translocation has been well outlined, less is known regarding the safeguards in place to deal with faulty translocation in eukaryotes. Specifically, SRP independent translocation, which caters to a sizable fraction of endomembrane proteins (Ast et al., 2013; Jan et al., 2014), is more prone to difficulties than its SRP dependent counterpart. For example, these substrates can undergo premature cytosolic folding, precluding them from being linearly threaded through the translocon pore (Ast et al., 2014; Hamman et al., 1997; van den Berg et al., 2004). Although SRP independent substrates are bound by cytosolic chaperones prior to translocation (Ngosuwan et al., 2003), most likely to minimize instances of cytosolic folding, these chaperones disengage from the substrate once translocation has started (Plath and Rapoport, 2000). Thus an error-prone window of time exists in which a translocating SRP independent substrate, bound by Kar2/BiP in the ER lumen, may fold domains that are still in the cytosol. This protein can neither continue to translocate into the ER nor retrotranslocate into the cytosol and will become “clogged” in the translocon. Such a clogged protein would obstruct the translocon channel and preclude it from translocating other substrates, requiring external quality control measures to identify and resolve this debilitating stalemate. Such clogging events have been shown to occur in bacteria and require the actions of the protease FtsH to cleave the clogged translocon, inducing its turnover (van Stelten et al., 2009). However, a homologous or analogous pathway has yet to be described in eukaryotes.
To uncover potential quality control machineries that take part in relieving clogging events, we created an inducible clogging-prone construct (i.e. clogger). We crossed this construct into the yeast deletion and hypomorphic allele collection, querying the yeast genome for machineries that resolve the burden of clogging. This screen indicated that the ER metalloprotease Ste24 might have a role in this process. We could show that the clogged form of the construct indeed accumulated and translocation was slowed down in Δste24 cells expressing the clogger or in clogging-enhancing genetic backgrounds. Upon clogging, Ste24 approaches the SRP independent translocon, engages the clogged form of the construct and mediates its degradation. Notably, we could show that this declogging function of Ste24 is conserved for human ZMPSTE24. Together, these findings reveal a novel quality control mechanism overseeing translocation, at the heart of which lies the crucial and conserved metalloprotease Ste24/ZMPSTE24.
Results
A rapidly folding SRP independent protein generates translocon clogging
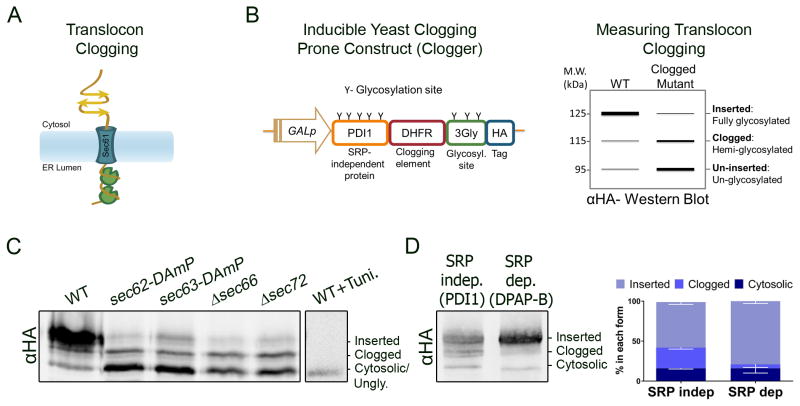
The protein-conducting channel of the endoplasmic reticulum (ER), the translocon, creates a hydrophilic channel ~15Å in diameter (Hamman et al., 1997; van den Berg et al., 2004). We hypothesized that this physical boundary might pose a problem for translocating substrates with domains that have been afforded a chance to fold in the cytosol. Preinsertional cytosolic folding of endomembrane proteins may become more abundant when translation and translocation are not coupled, as in the case of SRP independent proteins (Ast et al., 2013; Jan et al., 2014). Once an SRP independent substrate is engaged by the translocation machinery, but cannot transverse the translocon pore, translocon blocking (i.e. clogging) would occur. How the cell solves this debilitating problem that would disrupt ER homeostasis is unknown (Fig 1A).
Figure 1. A folding prone SRP independent protein can cause translocon clogging.
A. When translation and translocation are not tightly coupled, substrates can fold in the cytosol during translocation, stopping further insertion and clogging the translocon. B. A folding prone “clogger” construct can both induce and quantitate clogging. Under regulation of the galactose inducible promoter we fused the SRP independent substrate Pdi1 that bears 5 glycosylation sites, to the stably folding dihydrofolate reductase (DHFR) enzyme followed by 3 glycosylation sites and a hemagglutinin (HA) tag (left). When clogged this fusion protein can be easily recognized on a western blot as it runs as an intermediate hemi-glycosylated form (right). C. In WT cells, most of the clogger is fully glycosylated, while in mutants of the SRP independent translocon (sec62-DAmP, sec63-DAmP, Δsec66, Δsec72) the cytosolic unglycosylated band is prevalent. Treatment of WT cells with tunicamycin, blocking N-linked glycosylation, generates only the fastest migrating form of the clogger. Split panes represent different lanes on one gel, and are shown as such hereafter. D. The clogger construct was routed to the SRP dependent pathway by appending the hydrophobic core of the DPAP B signal anchor. 26% the SRP independent clogging construct becomes clogged, while only 5% of the SRP dependent counterpart does (n=3, Mean±SEM, p-value<0.001). See also Figure S1.
We set out to study how eukaryotic cells manages translocon clogging in the well-established endomembrane system of the yeast Saccharomyces cerevisiae. We built an inducible clogging prone construct (Fig 1B left), based on the SRP independent glycoprotein Pdi1 (Ng et al., 1996) under the control of a galactose-induced promoter. To tip the scales in favor of this construct folding at its C terminus (C′) and inducing clogging, we fused the yeast PDI1 to the Escherichia coli dehydrofolate reductase (DHFR) enzyme, which generates a rapid and stable fold (Lee et al., 2001). Importantly, E. coli DHFR folds stably even in the absence of methotrexate, precluding the need to add this toxic drug to induce clogging (Bhamidipati et al., 2005). Finally, 3 N-linked glycosylation (3Gly) sites and a hemagglutinin (HA) tag were placed at the C′ of the construct. Since N-linked glycosylation takes place in the ER lumen, and generates a size shift that can be assayed by western blot, this “clogger” construct would serve not only to induce translocon clogging, but also as a reporter for 3 translocational states by anti-HA western blot (Fig 1B right): 1) When fully inserted into the ER, all sites would be glycosylated to generate a slowly migrating form; 2) When clogged (i.e. Pdi1 has translocated and undergone glycosylation but the rapid folding of the DHFR in the cytosol prevented the rest of the protein from inserting), a hemi-glycosylated form would be apparent. This would result in an intermediate size band; 3) When uninserted, all of the construct would be un-glycosylated, producing a fast migrating form.
To verify that the clogger accurately reports on its translocational status the construct was inserted into either WT or mutant cells depleted for the various subunits of the SRP independent translocon (Fig 1C). Indeed, three migrational forms were present in all strains, indicating that all three states of insertion are detectable. As expected, the predominant fraction in WT cells was the inserted form, while the unglycosylated form was prevalent in cells with reduced translocation. Moreover, when glycosylation was chemically attenuated with the N-linked glycosylation inhibitor tunicamycin, only the fastest migrating form of the clogger was present. To further verify that the intermediate ‘clogged’ fraction of the construct is engaging the translocon, we performed a crosslinking experiment, exposing cells expressing the clogger to dithiobis succinimidyl propionate (DSP) and precipitating the Sec61 translocon (Fig S1A). Indeed, following crosslinking with DSP, the Sec61 precipitate is enriched for the clogged form of the construct. Importantly, there is no enrichment for binding with the abundant SRP independent protein Gas1, excluding the possibility that all proteins undergoing translocation are captured by this crosslinking. This intermediate clogged band is not the result of retrotranslocation, as cells lacking the cytosolic peptide-N-glycanase, Png1, (Suzuki et al., 2000) also contain this form of the clogger (Fig S1B). Finally, as predicted, the clogger disrupts ER homeostasis (Fig S1C), as significant activation of the unfolded protein response (UPR) was measured following expression of the clogger (p-value<0.01).
To verify that the clogged form that we see is a result of cytosolic folding, we set out to create a clogger in which translation was better coupled to translocation. To this end, we altered the clogger’s signal sequence to that of the established SRP dependent substrate DPAP-B (Ng et al., 1996). The SRP independent or dependent forms of the clogger were induced for an extensive period, to ensure that maximal clogging would take place. An anti-HA western blot revealed that at steady state 26% of the construct (SRP- indep.) was clogged, while for only 5% of the SRP dependent counterpart did clogging occur (Fig 1D). Therefore, it appears that clogging reflects the inherent complexity of targeting through the SRP independent pathway, which is intrinsically more prone to substrate folding in the cytosol.
A systematic genetic screen uncovers a role for Ste24 in translocon unclogging
As translocation is an essential process, we assumed that the absence of quality control machinery involved in relieving clogging (exacerbated by the expression of the clogger) would generate a significant growth delay. To find such strains we inserted the clogger construct into a systematic library of mutants in all yeast genes (Breslow et al., 2008; Giaever et al., 2002) using automated mating approaches (Cohen and Schuldiner, 2010). As a control, we expressed a cytosolic mCherry in the same collections. We then measured the growth rate (ie colony size) of each of these ~6000 haploid strains that contained a single genomic mutation as well as either the clogger or mCherry constructs (Fig 2A).
Figure 2. A systematic screen highlights a role for Ste24 in resolving translocon clogging.
A. The clogging construct was inserted into the yeast deletion and hypomorphic allele collections via SGA and its effect on growth was measured relative to a control protein (mCherry). B. ~110 mutants showed differential growth in the presence of the control protein or the clogger (FDR corrected p-value<0.05). Mutants attenuated for the SRP independent translocon, the unfolded protein response (UPR) and the ER protease Ste24 showed notably slower growth with the clogger (for a list of all strains see Table S1). C. Liquid growth was measured for WT and Δste24 cells either expressing the clogger or not (n=42, Mean±SD). D. Clogging in WT or Δste24 cells expressing the clogger was quantified by western blot, probing for the clogging construct with anti-HA. The clogged form of the construct accumulates in Δste24 cells. As a control, WT cells expressing the clogger were treated with tunicamycin. E. The localization of the endogenous SRP independent substrates Gas1, Kar2 and Pdi1 were assayed by western blot. Less translocation takes place when expressing the clogger in Δste24 cells. F. WT and mutant cells were analyzed by western blot for the localizations of Gas1, Kar2 and Pdi1. Mutant cells were either depleted of Ste24 (using a repressible Gal promoter on glucose), deleted for the auxillary translocon component Sec66 or both. SRP independent substrates accumulate in the cytosol when translocation becomes inefficient and the quality control actions of Ste24 are missing. See also Figure S2 and Tables S1–S4.
More than one hundred strains displayed a significantly altered growth pattern when expressing the clogger (FDR corrected p-value<0.05, Fig 2B, Table S1). One strain that grew better than expected upon clogger expression was deleted for the co-chaperone for Kar2, Scj1 (Schlenstedt et al., 1995). This suggests that when Kar2 is less active, potentially ratcheting SRP independent substrates into the ER at a slower rate, that less clogging is occurring or that such cases can be more easily resolved. We focused on mutations that lead to slower growth with the clogger (Fig 2B). First, strains mutated in known SRP independent translocon subunits displayed attenuated growth, serving as a validating internal control for the screen. Moreover, this slow growth was not a result of the simple overexpression of the SRP independent substrate, Pdi1 (Fig S2A). Second, strains deleted for Ire1 and Hac1 (Walter and Ron, 2011), the two main signaling components of the Unfolded Protein Response (UPR), also grew poorly upon clogger expression, in line with our observation that the clogger induces ER stress (Fig S1C). An unexpected yet intriguing observation was that cells lacking the ER localized metalloprotease Ste24 displayed significantly smaller colony size when expressing the clogger, but not when expressing Pdi1 alone (Fig S2B).
Ste24 is a highly conserved zinc metalloprotease embedded in the ER membrane whose active site is cytosolic (Fujimura-Kamada et al., 1997; Schmidt et al., 1998). We hypothesized that Ste24 could be an interesting candidate for the quality control machinery that resolves translocon clogging. To date, Ste24 has been characterized to take part in the pathway that processes proteins bearing a C′ CAAX motif (C -cysteine, A- aliphatic residue, X- any residue) (Michaelis and Barrowman, 2013), such as the yeast mating pheromone, a-factor, and mammalian Lamin A (Michaelis and Barrowman, 2012). However, hints at an additional role for Ste24 have accumulated in the literature. For example the deletion of Ste24, even in cells that do not express a-factor, generates notable ER stress (Jonikas et al., 2009), and interferes with membrane protein topology (Tipper and Harley, 2002), both phenotypes not shared by other a-factor processing enzymes.
To ascertain that loss of Ste24 was indeed detrimental to cells expressing the clogger, we first repeated the growth assay with a more precise liquid-growth measurement (Fig 2C). Indeed, while the WT grew more slowly with the clogger than without it, this growth delay was far more pronounced in a strain deleted for Ste24. Next, we directly assayed clogging (Fig 2D). Indeed, Δste24 cells contain highly elevated amounts of the clogged fraction as well as a significant amount of the cytosolic form of the clogger, further attesting to a blockage in translocation. Furthermore, inducing the clogger in the absence of Ste24 attenuated the ER entry of the endogenous SRP independent substrates Gas1 and Pdi1, as well as Kar2, which can utilize both SRP dependent and independent pathways for ER entry (Fig 2E) (Ast et al., 2013; Ng et al., 1996). It should be noted that these steady state measures likely under-represent the fraction of proteins that have failed to translocate, as untranslocated proteins are rapidly cleared from the cytosol by prERAD (Ast et al., 2014). On the other hand, SRP dependent translocation was not affected by the absence of Ste24 (Fig S2C), although expression of the clogger itself did attenuate the translocation of an SRP dependent substrate, possibly due to the activation of the UPR (Fig S1C).
To test that the role of Ste24 in clearing clogged translocons is not specific to our clogger construct, we analyzed a different clogging prone SRP independent substrate: Gas1 fused to the stably folding Red Fluorescent Protein (RFP-Gas1) (Fig S2D). While RFP-Gas1 is normally localized to the cell periphery, in Δste24 cells it was additionally found on the ER, indicating clogging or attenuated ER entry. This effect was not seen for the SRP dependent substrate, Hxt2-GFP, in the absence of Ste24 (Fig S2E).
Next, we set out to test whether endogenous proteins could clog the translocon. To this end, we mutated Sec66- which is required for early engagement and funneling of SRP independent substrates into the translocon (Harada et al., 2011; Plath et al., 1998). Indeed, in the double mutant lacking Sec66 and Ste24, SRP independent translocation is notably perturbed (Fig 2F). In these cells, Gas1, Kar2 and Pdi1 accumulate in the unglycosylated cytosolic forms, similar to what we had observed for cells lacking Ste24 and expressing the clogger (Fig 2E). This accumulation demonstrates that endogenous SRP-independent proteins can undergo clogging under physiological conditions, requiring the quality control actions of Ste24. These findings would explain the strong negative genetic interaction previously described between Ste24 and Sec66 (Costanzo et al., 2010).
The metalloprotease activity of Ste24is required for the new unclogging function
We next wondered whether translocon clogging seen in the absence of Ste24 is simply an indirect effect related to its previously described cellular roles, such as loss of CAAX processing, inducing ER stress or altered membrane protein topology. We first assessed clogging in the absence of other CAAX processing factors by testing proteins both upstream and downstream to Ste24 in the pathway, such as Ram1 or Axl1 (Fig 3A) (Michaelis and Barrowman, 2012). The absence of either of these proteins did not affect clogging. Furthermore, the CAAX processing mutants present in our initial screen (Δrce1 and Δaxl1) did not exhibit any growth defects with the clogger, although they were verified to be deleted (Fig S3A).
Figure 3. The role of Ste24 in relieving clogging is not an indirect effect of its previously described functions.
A. Western blotting with anti-HA to quantify clogging in CAAX processing mutants- Δste24, Δram1 or Δaxl1 demonstrates that only Δste24, but not other CAAX mutants, generates clogging B. WT cells expressing the clogger were treated with the ER stress inducing agent, DTT, and subsequent clogging was assessed by anti-HA western blot. (n=3, Mean±SEM) showing that clogging is not exacerbated by ER stress. C. A serial dilution growth assay was carried out for WT or Δste24 cells, bearing either an empty plasmid, a plasmid encoding for functional STE24 or for the proteolytically dead mutant, STE24E296G. These strains were examined for growth when the clogger is either repressed or expressed, showing that the metalloprotease activity of Ste24 is required to restore growth with the clogger. D. Clogging was assayed by western blot with anti-HA in Δste24 cells expressing the clogger and a plasmid encoding for either WT STE24 or STE24E296G. While Δste24 bearing the STE24 plasmid demonstrates complete rescue of the phenotype, the STE24E296G plasmid fails to rectify clogging and may even act as a dominant negative. See also Figure S3.
We then tested whether the ER stress known to occur in the Δste24 strain was indirectly causing clogging. We generated ER stress in WT cells expressing the clogger by exposing them to the reducing agent, DTT (Fig 3B). The induction of ER stress reduced the amount of uninserted protein, but did not alter the fraction of clogged protein. Furthermore, a verified deletion of Spf1, which induces more extensive ER stress than Δste24 (Jonikas et al., 2009), did not display a diminished colony size with the clogger (Fig S3B).
Finally, Δste24 has been shown to affect transmembrane protein topology. We therefore tested whether the SRP-independent translocon, which is itself made up of multiple transmembrane proteins, was still functional. To this end, we analyzed SRP independent translocation in Δste24 cells using an in-vitro insertion assay with the SRP independent substrate ppαF. ER membranes (microsomes) derived from either WT or Δste24 cells were able to translocate similar amounts of ppαF (Fig S3C). These results suggest that the increased clogging is not an indirect effect of a reduction in translocon availability or fidelity, ER stress or CAAX processing and indicate a more direct role for Ste24 in this process.
With this direct link in mind, we sought to test whether the metalloprotease activity of Ste24 is involved in clogging. While functional STE24 expressed from a plasmid rescued the growth delay of Δste24 cells expressing the clogger(Fig 3C) as well as the accumulation of clogged isoforms (Fig 3D), rescue was ablated when the conserved HExxH protease motif was mutated (STE24E296G) (Fujimura-Kamada et al., 1997). Together these results indicate that the metalloprotease activity of Ste24 is essential for its role in resolving clogging in a direct fashion.
Ste24 approaches, but does not cleave, the clogged translocon
If Ste24 clears clogged translocons by direct cleavage, either the translocon itself (as in bacteria (van Stelten et al., 2009)) or the clogger might be the processed substrate. In either case, Ste24 would have to be in close proximity to the translocation apparatus during clogging. To examine the proximity between Ste24 and the clogged translocon, we utilized the split Venus approach (Sung and Huh, 2007). We genomically fused the VC fragment to Ste24, while tagging the subunits of the SRP independent (Sec63, Sec66 and Sec72) or dependent (Srp102) translocon with the VN fragment (Fig 4A) so that all Venus fragments would face the cytosol (Kim et al., 2006). Notably, when the clogger protein was expressed in these cells, a dramatic increase in ER fluorescence could be seen for Ste24-VC paired with the VN tagged SRP independent translocon subunits (Fig 4B). Such an increase was not seen with Srp102-VN, the beta subunit of the SRP receptor or with the negative control (Erp2, an abundant ER protein not involved in translocation (Fig S4)). To rule out that this increase in Venus signal was an indirect readout of elevated protein levels due to UPR activation, we quantified this interaction in the absence of Hac1 (Walter and Ron, 2011). Under these conditions, we observed an even further elevation in interaction, which might be the result of more extensive clogging in Δhac1 cells as indicated by our initial screen (Fig 2B). Thus, it seems that Ste24 approaches the SRP independent translocon upon clogging.
Figure 4. Clogging recruits Ste24 to the SRP independent translocon.
A. The proximity of Ste24 to translocon subunits was visualized with a split Venus assay- fusing the C terminus of the fluorophore Venus (VC) to Ste24 and the N terminus (VN) to the auxiliary translocon subunits Sec63, Sec66, Sec72 or the SRP receptor subunit Srp102. Upon expression of the clogger, a ring-like ER signal is seen with Ste24 and subunits of the SRP independent translocon. Scale bar- 5μm. B. Fluorescence levels were measured for all split-Venus pairs in the presence or absence of the clogger by flow cytometry vs an untagged strain (n=3, Mean±SEM). Fluorescence in the presence of the clogger was increased when UPR was compromised (Δhac1), indicating that this is not an indirect increase due to this stress pathway. C. The amounts of the non essential subunits of the translocon, Sec66-GFP and Sec72-GFP, were examined with anti-GFP western blot in the presence or absence of the clogger in either WT or Δste24 strains and were unaltered. Pgk1 was used as a loading control. D. Translation was blocked by cycloheximide (CHX) in a WT strain lacking or expressing the clogger and samples were taken at the indicated time points. The samples were probed by western blot for endogenous Sec61, Sec62 and Sec63, showing that the half-life of these essential SRP independent translocon subunits is not altered upon clogger expression. See also Figure S4.
To test whether Ste24 was cleaving the translocon we first assayed the steady state amounts of the nonessential subunits of the SRP independent translocon, Sec66 and Sec72, in the absence or presence of the clogger, as well as Δste24 cells expressing the clogger (Fig 4C). In all of these conditions, the amounts of Sec66 and Sec72 remained the same. We then tested whether either the steady-state or the half-life of the essential SRP independent translocon subunits, Sec61, Sec62 and Sec63 (Belle et al., 2006), were altered upon expression of the clogger. (Belle et al., 2006)We found that the core translocon subunits were degraded at comparable rates under all conditions, suggesting that, unlike bacteria, they are not cleaved during clogging in eukaryotes (Fig 4D). It should be noted, however, that only a fraction of the translocon pool is clogged, and effects on the half-life of this pool might be masked by the general translocon population.
Ste24 cleaves the clogged protein
If Ste24 is not cleaving the translocon then it may be directly cleaving the clogged protein, in which case it would have to physically bind to it. To test for such an interaction, we generated a FLAG tagged form of the proteolytically dead STE24E296G, and carried out an immunoprecipitation experiment in the presence of the clogger (Fig 5A). We found that the clogged and unglycosylated forms did indeed coprecipitate with FLAG-STE24E296G. This unglycosylated fraction could represent a cytosolic protein that has not yet started to translocate or else a clogged form that has not undergone glycosylation. As a control, cells expressing the clogger but lacking FLAG-STE24E296G did not precipitate any form of the clogger.
Figure 5. Ste24 directly interacts with the clogged protein and cleaves it.
A. Cells expressing the clogger and an empty vector or a FLAG tagged version of the proteolytically dead STE24E296G were subjected to anti-flag immunoprecipitation. A 1/200 fraction of the sample input and the precipitate were subjected to WB, probing for both the clogger construct (Anti-HA) as well as STE24E296G (Anti-Flag). Ste24 binds to the clogged and unglycosylated forms of the construct. B. Cycloheximide (CHX) treatment of WT or Δste24 cells expressing the clogger was analyzed by anti-HA western blot (n=3, Mean±SEM), showed that only the clogged fraction is stabilized in Δste24 cells (p-value<0.05). Pgk1 was used as a loading control. C. Anti-HA western blot was carried out to examine fragments generated from the clogger in WT or Δste24 cells, without or with a genetic attenuation of the proteasome (scl1-DAmP). Clogger fragments not present in the absence of Ste24 are marked with an arrowhead. See also Figures S5 and S6.
We next tested whether Ste24 affects the degradation of the clogged protein (Fig 5B). Both in WT and Δste24 cells, the inserted form of the clogger was stable following cycloheximide treatment. In contrast, only 65% of the initial clogged fraction remained after 15 minutes in WT cells, whereas this form was completely stable in cells lacking Ste24 (p-value<0.05). This degradation is specific, as Ste24 did not affect the half-life of the poorly folding membrane protein, CFTR (Fig S5A).
If Ste24, whose active site faces the cytosol, directly cleaves the clogger then presumably it would create cytosolic cleavage fragments that would then be cleared by the proteasome (Fig 5C). To identify such fragments we genetically attenuated the proteasome with the hypomorphic allele scl1-DAmP, mutated for the alpha1 subunit of the 20S proteasome core (Lee et al., 1991). Deletion of Ste24 alone caused several fast migrating clogger fragments to accumulate, possibly indicating the presence of another, Ste24 independent, degradation pathway. However, when the proteasome was attenuated, several additional protein fragments could readily be seen. Some of these bands disappear in the double mutant scl1-DAmP Δste24 (Fig 5C, marked by an arrow head) although similar levels of proteins were present in the two samples (Fig S5B) pointing to Ste24 dependent cleavage intermediates. Notably, the sizes of these bands suggest that Ste24 is cleaving within Pdi1, and not at the interface between Pdi1 and DHFR.
The fact that that Ste24 cleaves inside a domain that should not be exposed to the cytosol during clogging indicates that there might be some force pulling the clogged protein out of the translocon as Ste24 scans for a cleavage site. One well-characterized molecular machine that generates a pulling force and extracts proteins from the ER membrane is the AAA ATPase Cdc48 (Rabinovich et al., 2002). Indeed, our original screen also revealed that cells deleted for Dfm1, a Cdc48 recruitment factor (Sato and Hampton, 2006) grew slowly when expressing the clogger (Fig S6A). Moreover, clogged proteins accumulated in Δdfm1 cells (Fig S6B). Indeed, we could visualize recruitment of Cdc48 to ER membrane foci upon clogger expression (Fig S6C). Dfm1 has recently been characterized to take part in the regulatory arm of ER associated degradation (ERAD-R) (Avci et al., 2014). However, the absence of another ERAD-R subunit, the protease Ypf1, does not affect clogging (Fig S6A and B), ruling out a general involvement of this pathway in unclogging. Thus, Dfm1 may have an additional, ERAD-R independent role, in recruiting Cdc48 to ratchet clogged proteins out of the translocon. This concerted action could potentially allow Ste24 to find a cleavage site within the broad spectrum of clogged proteins.
The function of Ste24 in unclogging is conserved to human ZMPSTE24
Ste24 is highly conserved from yeast to humans where the homologue, ZMPSTE24, has been characterized mainly for its role in CAAX processing of Lamin A (Fujimura-Kamada et al., 1997; Michaelis and Hrycyna, 2013). We wished to examine whether ZMPSTE24 can also function in translocon unclogging (Fig 6A and S7A). Indeed, while clogging doubles in the absence of Ste24 (p-value<0.05), the expression of ZMPSTE24 in yeast reduced clogging back to WT levels. We then tested whether 7 disease-associated forms of ZMPSTE24, which have been previously analyzed in yeast (Barrowman et al., 2012), could resolve clogging. While some mutations, such as L438F and W340R could reduce the amount of clogging, others, such as H335A or W450X were just as compromised for clogging, if not worse, as the deletion of Ste24. These defects are also apparent when measuring growth in the presence of the clogger (Fig S7B).
Figure 6. The unclogging activity of Ste24 is conserved in the human homologue, ZMPSTE24.
A. The fraction of the clogger found in each translocational form was quantified in Δste24 cells bearing plasmids encoding for functional ZMPSTE24 or disease-associated mutations (n=3, Mean±SEM). Lack of Ste24 doubles clogging (p-value<0.05). Mammalian ZMPSTE24, but not all disease-associated mutants, can restore clogging to WT levels. B. The fraction of clogged protein found in yeast expressing the WT or mutant forms of ZMPSTE24 (Fig 6A) was plotted against the known residual proteolitic activity of each ZMPSTE24 protease (Barrowman et al., 2012), showing a correlation (R2- 0.98). The outlier- mutation L438F is the only mutation located in the peptide-binding groove of ZMPSTE24 (Quigley et al., 2013). C. A human folding prone construct, made up of the luminal co-chaperone ERdj3, C terminally fused to GFP and 3 glycosylation sites (3Gly) was constructed. D. HEK293 cells were transfected with the human clogging prone construct and treated with the proteasome inhibitors MG132 or Bortezomib to stabilize cytosolic forms of the clogger. Additionally, cells were exposed to lopinavir (LPV) or tipranavir (TPV) to inhibit ZMPSTE24 function. Protein samples were extracted and analyzed by an anti-GFP western blot, showing an the accumulation of the hemiglycosylated clogged protein when ZMPSTE24 is inhibited. As a control, cells were treated with tunicamycin. See also Figure S7.
Each of the mutant forms of ZMPSTE24 has previously been assayed in-vitro for its processing activity on a CAAX motif (Barrowman et al., 2012). When we plotted the fraction of clogged protein present in each mutant relative to their residual processing ability (Fig 6B), we observed a strong correlation (R2= 0.98) supporting the requirement for the protease activity in clearance of the clogged protein. One interesting exception to this correlation was the L438F mutation. A recent structural study has revealed that L438 is found adjacent the peptide binding site of ZMPSTE24 (Quigley et al., 2013), suggesting that CAAX and clogged protein binding are not identical.
We then asked whether clogging could occur in human cells, and whether ZMPSTE24 would be required to resolve such cases. To this end, we constructed a human clogger (Fig 6C), based on the human ER Hsp40 ERdj3, which has been shown to require human Sec62 and Sec63 for efficient translocation (Lang et al., 2012). To the C′ of ERdj3, which contains one N-linked glycosylation site, we fused a tightly folding GFP as well as 3 additional sites for N-linked glycosylation. The human clogger was transfected into HEK293 cells (Fig 6D), which were subsequently treated with the HIV-protease inhibitors Lopinavir (LPV) or Tipranavir (TPV), both of which inhibit ZMPSTE24 in-vivo (Coffinier et al., 2007). To stabilize cytosolic forms of the mammalian clogger, we performed additional treatment with the proteasome inhibitors MG132 or Bortezomib. We hypothesized that under these conditions, clogging would be more predominant, as proteins are afforded a longer window of time in which they can remain in the cytosol. Indeed, we observe that the hemi-glycosylated, clogged form appears when the cells are treated with both a proteasome and ZMPSTE24 inhibitor. Thus, it seems that the function of Ste24 in clearing clogged proteins from the ER is conserved from yeast to humans and becomes important under conditions where cytosolic quality control is dysfunctional.
In summary, it appears that the very nature of SRP independent translocation makes it susceptible to unique challenges such as clogging (Fig 7 left). Upon clogging, Ste24 is recruited to the translocon, most likely with other factors involved in quality control. There, Ste24 proceeds to cleave the clogged protein, thereby resolving the stalemate scenario and salvaging the translocon, which can resume its essential task.
Figure 7. Ste24 approaches the translocon and cleaves clogged substrates during faulty translocation.
The SRP independent pathway is intrinsically more prone to translocon clogging, as translation and translocation are not tightly coupled. Thus partial folding of a cytosolic domain while the luminal domain has already engaged chaperones, can generate a scenario whereby the protein can neither proceed to insert nor be retrotranslocated. To resolve this stalemate, Ste24 engages with the SRP independent translocon, and cleaves the clogged substrate, whose fragments can then be degraded by the proteasome. Thus Ste24 function is essential for freeing clogged translocons for further rounds of insertion.
Discussion
The processes of ER targeting and translocation are vital to create a robustly functioning secretory pathway in cells. In bacteria, clogged SecY translocons are digested by the metalloprotease FtsH (van Stelten et al., 2009). Recent work in mammals has highlighted a role for the ribosomal quality control pathway and proteasomal degradation in clearing stalled translocation intermediates for SRP-dependent proteins (von der Malsburg et al., 2015). Similar studies in yeast analyzing SRP dependent substrates that faultily engage the translocon have implicated the ER associated degradation machinery in clearing this stalemate (Rubenstein et al., 2012). Thus, while it is clear that in all scenarios degradation is involved, each case seems to require quality control machinery tailored to the specific malfunction.
We set out to identify and understand the factors that resolve faulty SRP independent translocation, as this pathway contains a time window in which translocating substrates may fold in the cytosol. To this end, we generated a folding prone SRP independent construct that could serve to both induce translocon clogging and quantitate the amount of clogging taking place. In a genetic screen for reduced growth in the presence of this clogger, we identified the ER localized zinc metalloprotease Ste24 as essential for growth in the presence of increased clogging.
A growing body of work has indicated that Ste24, originally described for its function in CAAX processing, might have an additional role in maintaining ER homeostasis, and more specifically translocation. First, transcriptional analysis of the UPR response uncovered Ste24 to be significantly upregulated during ER stress (Travers et al., 2000). Inversely, the absence of Ste24 was shown to elicit marked ER stress (Jonikas et al., 2009). In addition, a systematic genetic analysis of the Kar2R217A mutant, in which the ER chaperone Kar2 exhibits a specific defect in SRP independent insertion, found a strong alleviating interaction with the deletion of Ste24 (Vembar et al., 2010). Possibly, under these conditions, the absence of Ste24 prevents SRP independent substrates that are slowly being ratcheted into the ER lumen by the Kar2R217A mutant from being prematurely degraded. Furthermore, a lack of Ste24 was shown to affect the topology of a model transmembrane protein in yeast (Tipper and Harley, 2002). Finally, the bacterial homologue of Ste24, HtpX, becomes essential in the absence of the established declogging protease FtsH, suggesting a conserved quality control link (Sakoh et al., 2005).
Our studies show that in the absence of Ste24, clogged proteins accumulate, impairing SRP independent translocation. Ste24 moves towards the SRP independent translocon upon clogging and, together with a pulling force (possibly exerted by Dfm1-recruited Cdc48), cleaves the clogged protein within Pdi1 itself. Interestingly, the analogous bacterial declogging system, FtsH couples an AAA ATPase module with a membrane metalloprotease to resolve clogging. Thus, it is possible that the function of FtsH was uncoupled in eukaryotes to consist of an AAA ATPase module provided by Cdc48 and a metalloprotease component provided by Ste24. In this manner, Ste24 can perhaps scan its substrates for segments that it can accommodate within its catalytic site, thus allowing it to cleave various clogged proteins. This mechanism of action would in fact be most economical, as it would sacrifice only the clogged translocating protein and rescue the large, multi-subunit complex of the Sec61 translocon.
Ste24/ZMPSTE24 appears to be a promiscuous protease, as even in its established substrates, the mating pheromone a-factor and Lamin A, two processing steps occur, on two internal sites that share little sequence similarity (Fujimura-Kamada et al., 1997; Michaelis and Barrowman, 2012, 2013; Schmidt et al., 1998). Indeed, all of the folding prone substrates that we tested: Pdi1, Gas1 and Erdj3, were affected in the absence of Ste24, although they contain no sequence or functional similarities. It should be noted that in cells lacking Ste24, we could not identify hemi-glycosylated clogging forms of endogenous proteins (Fig 2E and F). However, this is to be expected, as clogging most likely only takes place for a very small fraction of each endogenous protein undergoing translocation at a given time, making it difficult to identify a specific clogged form. So, how prevalent is clogging? The sizable number of SRP independent substrates (Ast et al., 2013) and the massive ER stress and UPR activation observed in the absence of Ste24 (Jonikas et al., 2009) would argue that clogging is not a rare event.
The promiscuity of Ste24 raises questions as to how this metalloprotease is regulated so it does not cleave every SRP independent substrate undergoing translocation. One layer of regulation appears to be the recruitment of Ste24 to the SRP independent translocon, as this proximity was notably enhanced upon clogger induction. How this regulation takes place remains to be seen. Interestingly, our initial clogging screen uncovered several post-translational modifiers required to maintain growth in the presence of the clogger. It would be interesting to investigate whether any of these additional factors serve to regulate Ste24.
In light of its key cellular roles, it is not surprising that Ste24 is a highly conserved protein, whose human homologue, ZMPSTE24, has been shown to perform all functions tested to date (Barrowman et al., 2012; Fujimura-Kamada et al., 1997; Young et al., 2005). We could show that ZMPSTE24 can completely compensate for the loss of yeast Ste24 and alleviate clogging, indicating that this cellular role has also been conserved. Indeed, recent works have highlighted that SRP independent translocation might be more prevalent in higher eukaryotes than was previously appreciated (Jan et al., 2014; Johnson et al., 2012; Shao and Hegde, 2011). These findings indicate that translocon unclogging would be as important in higher eukaryotes as it is in yeast, maintaining the need for dedicated quality control machinery. Indeed, treatment with lopinavir, one ZMPSTE24 inhibitor that generated clogging, has been previously shown to induce ER stress in multiple cell types. However the cause of this stress has not been uncovered (Taura et al., 2013). Our results here suggest this may be due, at least in part, to translocon clogging.
Humans mutations in ZMPSTE24 have been linked to a wide range of diseases, spanning from metabolic disorders to progeroid diseases of differential severity (Barrowman et al., 2012; Michaelis and Barrowman, 2013). When studying these disease-associated mutations, we could demonstrate that mutants in the metalloprotease domain accumulated clogged proteins in direct relation to their residual cleavage activity. This raises the possibility that one aspect of the disease mechanism, in addition to loss of Lamin A function, is a progressive slow down of the secretion apparatus, due to unresolved clogging and prolonged ER stress. One notable exception was the L438F mutation, which is found adjacent to the peptide binding site (Pryor et al., 2013; Quigley et al., 2013), suggesting that the substrate binding site might be more flexible for clogged proteins or even found in a different position. These findings highlight that we can differentiate between two actions of ZMPSTE24: binding to the substrate, which is different for CAAX motifs and clogged proteins; vs. cleavage, which appears to be similar for these two substrate groups.
In summary, our results shed light on a novel function of Ste24, a central and conserved metalloprotease long suspected to have a pivotal task in maintaining ER homeostasis. This ubiquitous protease orchestrates the process of translocon unclogging, thus ensuring that the essential flux of proteins entering the ER is maintained. More generally, translocation that is uncoupled from translation is not unique to the ER, and is found in other essential organelles such as mitochondria, chloroplasts and peroxisomes. The approaches set forth in this work can be utilized to study how translocon clogging is resolved in these organelles, shedding further light on the quality control machineries and mechanisms utilized to resolve this basic cellular obstacle.
Experimental Procedures
Plasmid construction
A complete list of plasmids and primers used in this study can be found in Sup. Tables S3 and S4. All cloning procedures were carried out with the restriction free method (van den Ent and Lowe, 2006). In order to generate the clogger construct, 3 N-linked glycosylation sites (3Gly) (AVAVNNTSAVAVAVNNTSAVAVAVNNTS) were cloned directly upstream to the HA tag in pYM-N24 GAL1p-3HA::NatR plasmid using primers 3Gly-pYM24clone-F and 3Gly-pYM24clone-R (primers were designed using Primers-4-Yeast (Yofe and Schuldiner, 2014)). Following this step, PDI1 was PCR amplified from the yeast genome, with the primers PDI-pYM24clone-F and PDI-pYM24clone-R and inserted upstream to the 3Gly-HA sequence. Finally, the E. coli DHFR gene was PCR-amplified from the CPY*-DHFR-HA plasmid ((Bhamidipati et al., 2005), and inserted between PDI1 and the 3Gly elements. Following its verification by sequencing, the clogger construct was inserted into the HO locus of all strains of interest. Further information is available in the extended experimental procedures.
Library creation and analysis
Introduction of the clogger or mCherry constructs into the yeast libraries was performed using Synthetic Genetic Array (SGA) techniques (Cohen and Schuldiner, 2010). Further information is available in the extended experimental procedures.
To analyze the growth of each deletion strain with the clogger or mCherry constructs, all custom libraries were replicated to rich media containing 2% galactose, and grown for 3 days at 30°C. Colony size was quantified for each strain using the Balony software (Young and Loewen, 2013) and normalized relative to other strains in the same row and column. Outliers from the linear regression pattern, ie strains bearing significantly different growth upon clogger induction, with a p-value<0.05, were identified, correcting for false discovery rate (Benjamini and Hochberg, 1995). Colony size of all strains with the two constructs can be found in Sup. Table S1.
Supplementary Material
Acknowledgments
We thank Michal Eisenberg and Idan Frumkin for their insightful comments on this manuscript. We are indebted to Marius Lemberg, Richard Zimmermann, Shachar Dagan, Oren Schuldiner and the Schuldiner lab-members for many fruitful discussions. We are grateful to Randy Schekman, Matthias Seedorf, Marius Lemberg, Peter Walter, Yves Bourbonnais, Richard Zimmermann, Stephen High, Naama Barkai, Howard Riezman, Won-Ki Huh, Jeffrey Brodsky, Hans Merzendorfer and Michal Sharon for plasmids and reagents. We thank the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH for supplying Lopinavir and Tipranavir. We are very grateful to Idan Frumkin for his help with the liquid growth assay. TA is supported by the Adams Fellowship Program of the Israel Academy of Sciences and Humanities. This work was supported by the generous support of the Adelis Foundation and the Berlin Family Foundation New Scientist Fund, a grant from the National Institutes of Health (R01GM041223) for SM as well as the Minerva foundation and the Israeli Science Foundation for MS.
Footnotes
Author Contributions
T.A, S.M and M.S. conceived and designed the experiments. T.A. performed the experiments. T.A and M.S. wrote the manuscript with input from S.M.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ast T, Aviram N, Chuartzman SG, Schuldiner M. A cytosolic degradation pathway, prERAD, monitors pre-inserted secretory pathway proteins. J Cell Sci. 2014;127:3017–3023. doi: 10.1242/jcs.144386. [DOI] [PubMed] [Google Scholar]
- Ast T, Cohen G, Schuldiner M. A Network of Cytosolic Factors Targets SRP-Indepenent Proteins to the Endoplasmic Reticulum. Cell. 2013;152:1134–1145. doi: 10.1016/j.cell.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Ast T, Schuldiner M. All roads lead to Rome (but some may be harder to travel): SRP-independent translocation into the endoplasmic reticulum. Crit Rev Biochem Mol Biol. 2013;48:273–288. doi: 10.3109/10409238.2013.782999. [DOI] [PubMed] [Google Scholar]
- Avci D, Fuchs S, Schrul B, Fukumori A, Breker M, Frumkin I, Chen CY, Biniossek ML, Kremmer E, Schilling O, et al. The yeast ER-intramembrane protease Ypf1 refines nutrient sensing by regulating transporter abundance. Mol Cell. 2014;56:630–640. doi: 10.1016/j.molcel.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Aviram N, Schuldiner M. Embracing the void--how much do we really know about targeting and translocation to the endoplasmic reticulum? Curr Opin Cell Biol. 2014;29:8–17. doi: 10.1016/j.ceb.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Barrowman J, Wiley PA, Hudon-Miller SE, Hrycyna CA, Michaelis S. Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity. Hum Mol Genet. 2012;21:4084–4093. doi: 10.1093/hmg/dds233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belle A, Tanay A, Bitincka L, Shamir R, O’Shea EK. Quantification of protein half-lives in the budding yeast proteome. Proc Natl Acad Sci U S A. 2006;103:13004–13009. doi: 10.1073/pnas.0605420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995:289–300. [Google Scholar]
- Bhamidipati A, Denic V, Quan EM, Weissman JS. Exploration of the topological requirements of ERAD identifies Yos9p as a lectin sensor of misfolded glycoproteins in the ER lumen. Mol Cell. 2005;19:741–751. doi: 10.1016/j.molcel.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Breslow DK, Cameron DM, Collins SR, Schuldiner M, Stewart-Ornstein J, Newman HW, Braun S, Madhani HD, Krogan NJ, Weissman JS. A comprehensive strategy enabling high-resolution functional analysis of the yeast genome. Nat Methods. 2008;5:711–718. doi: 10.1038/nmeth.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Yin Y, Gao G, Li S, Jiang Y, Gu X, Luo J. SPD--a web-based secreted protein database. Nucleic Acids Res. 2005;33:D169–173. doi: 10.1093/nar/gki093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C, Hudon SE, Farber EA, Chang SY, Hrycyna CA, Young SG, Fong LG. HIV protease inhibitors block the zinc metalloproteinase ZMPSTE24 and lead to an accumulation of prelamin A in cells. Proc Natl Acad Sci U S A. 2007;104:13432–13437. doi: 10.1073/pnas.0704212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y, Schuldiner M. Advanced methods for high-throughput microscopy screening of genetically modified yeast libraries. Methods Mol Biol. 2010;781:127–159. doi: 10.1007/978-1-61779-276-2_8. [DOI] [PubMed] [Google Scholar]
- Costanzo M, Baryshnikova A, Bellay J, Kim Y, Spear ED, Sevier CS, Ding H, Koh JL, Toufighi K, Mostafavi S, et al. The genetic landscape of a cell. Science. 2010;327:425–431. doi: 10.1126/science.1180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura-Kamada K, Nouvet FJ, Michaelis S. A novel membrane-associated metalloprotease, Ste24p, is required for the first step of NH2-terminal processing of the yeast a-factor precursor. J Cell Biol. 1997;136:271–285. doi: 10.1083/jcb.136.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- Grudnik P, Bange G, Sinning I. Protein targeting by the signal recognition particle. Biol Chem. 2009;390:775–782. doi: 10.1515/BC.2009.102. [DOI] [PubMed] [Google Scholar]
- Hamman BD, Chen JC, Johnson EE, Johnson AE. The aqueous pore through the translocon has a diameter of 40–60 A during cotranslational protein translocation at the ER membrane. Cell. 1997;89:535–544. doi: 10.1016/s0092-8674(00)80235-4. [DOI] [PubMed] [Google Scholar]
- Harada Y, Li H, Wall JS, Li H, Lennarz WJ. Structural studies and the assembly of the heptameric post-translational translocon complex. J Biol Chem. 2011;286:2956–2965. doi: 10.1074/jbc.M110.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan CH, Williams CC, Weissman JS. Principles of ER cotranslational translocation revealed by proximity-specific ribosome profiling. Science. 2014;346:1257521. doi: 10.1126/science.1257521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N, Vilardi F, Lang S, Leznicki P, Zimmermann R, High S. TRC-40 can deliver short secretory proteins to the Sec61 translocon. J Cell Sci. 2012 doi: 10.1242/jcs.102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonikas MC, Collins SR, Denic V, Oh E, Quan EM, Schmid V, Weibezahn J, Schwappach B, Walter P, Weissman JS, et al. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science. 2009;323:1693–1697. doi: 10.1126/science.1167983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Melen K, Osterberg M, von Heijne G. A global topology map of the Saccharomyces cerevisiae membrane proteome. Proc Natl Acad Sci U S A. 2006;103:11142–11147. doi: 10.1073/pnas.0604075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Benedix J, Fedeles SV, Schorr S, Schirra C, Schauble N, Jalal C, Greiner M, Hassdenteufel S, Tatzelt J, et al. Different effects of Sec61alpha, Sec62 and Sec63 depletion on transport of polypeptides into the endoplasmic reticulum of mammalian cells. J Cell Sci. 2012;125:1958–1969. doi: 10.1242/jcs.096727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Schwartz MP, Prakash S, Iwakura M, Matouschek A. ATP-dependent proteases degrade their substrates by processively unraveling them from the degradation signal. Mol Cell. 2001;7:627–637. doi: 10.1016/s1097-2765(01)00209-x. [DOI] [PubMed] [Google Scholar]
- Lee DH, Tamura T, Chung CH, Tanaka K, Ichihara A. Molecular cloning of the yeast proteasome PRS2 gene identical to the suppressor gene scl1+ Biochem Int. 1991;23:689–696. [PubMed] [Google Scholar]
- Michaelis S, Barrowman J. Biogenesis of the Saccharomyces cerevisiae pheromone a-factor, from yeast mating to human disease. Microbiol Mol Biol Rev. 2012;76:626–651. doi: 10.1128/MMBR.00010-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S, Barrowman J. Biogenesis of the Saccharomyces cerevisiae pheromone a-factor, from yeast mating to human disease. Microbiol Mol Biol Rev. 2013;76:626–651. doi: 10.1128/MMBR.00010-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S, Hrycyna CA. Biochemistry. A protease for the ages. Science. 2013;339:1529–1530. doi: 10.1126/science.1236764. [DOI] [PubMed] [Google Scholar]
- Ng DT, Brown JD, Walter P. Signal sequences specify the targeting route to the endoplasmic reticulum membrane. J Cell Biol. 1996;134:269–278. doi: 10.1083/jcb.134.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngosuwan J, Wang NM, Fung KL, Chirico WJ. Roles of cytosolic Hsp70 and Hsp40 molecular chaperones in post-translational translocation of presecretory proteins into the endoplasmic reticulum. J Biol Chem. 2003;278:7034–7042. doi: 10.1074/jbc.M210544200. [DOI] [PubMed] [Google Scholar]
- Park E, Rapoport TA. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu Rev Biophys. 2012;41:21–40. doi: 10.1146/annurev-biophys-050511-102312. [DOI] [PubMed] [Google Scholar]
- Plath K, Mothes W, Wilkinson BM, Stirling CJ, Rapoport TA. Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell. 1998;94:795–807. doi: 10.1016/s0092-8674(00)81738-9. [DOI] [PubMed] [Google Scholar]
- Plath K, Rapoport TA. Spontaneous release of cytosolic proteins from posttranslational substrates before their transport into the endoplasmic reticulum. J Cell Biol. 2000;151:167–178. doi: 10.1083/jcb.151.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor EE, Jr, Horanyi PS, Clark KM, Fedoriw N, Connelly SM, Koszelak-Rosenblum M, Zhu G, Malkowski MG, Wiener MC, Dumont ME. Structure of the integral membrane protein CAAX protease Ste24p. Science. 2013;339:1600–1604. doi: 10.1126/science.1232048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley A, Dong YY, Pike AC, Dong L, Shrestha L, Berridge G, Stansfeld PJ, Sansom MS, Edwards AM, Bountra C, et al. The structural basis of ZMPSTE24-dependent laminopathies. Science. 2013;339:1604–1607. doi: 10.1126/science.1231513. [DOI] [PubMed] [Google Scholar]
- Rabinovich E, Kerem A, Frohlich KU, Diamant N, Bar-Nun S. AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol Cell Biol. 2002;22:626–634. doi: 10.1128/MCB.22.2.626-634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein EM, Kreft SG, Greenblatt W, Swanson R, Hochstrasser M. Aberrant substrate engagement of the ER translocon triggers degradation by the Hrd1 ubiquitin ligase. J Cell Biol. 2012;197:761–773. doi: 10.1083/jcb.201203061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoh M, Ito K, Akiyama Y. Proteolytic activity of HtpX, a membrane-bound and stress-controlled protease from Escherichia coli. J Biol Chem. 2005;280:33305–33310. doi: 10.1074/jbc.M506180200. [DOI] [PubMed] [Google Scholar]
- Sato BK, Hampton RY. Yeast Derlin Dfm1 interacts with Cdc48 and functions in ER homeostasis. Yeast. 2006;23:1053–1064. doi: 10.1002/yea.1407. [DOI] [PubMed] [Google Scholar]
- Schlenstedt G, Harris S, Risse B, Lill R, Silver PA. A yeast DnaJ homologue, Scj1p, can function in the endoplasmic reticulum with BiP/Kar2p via a conserved domain that specifies interactions with Hsp70s. J Cell Biol. 1995;129:979–988. doi: 10.1083/jcb.129.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt WK, Tam A, Fujimura-Kamada K, Michaelis S. Endoplasmic reticulum membrane localization of Rce1p and Ste24p, yeast proteases involved in carboxyl-terminal CAAX protein processing and amino-terminal a-factor cleavage. Proc Natl Acad Sci U S A. 1998;95:11175–11180. doi: 10.1073/pnas.95.19.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Hegde RS. A Calmodulin-Dependent Translocation Pathway for Small Secretory Proteins. Cell. 2011;147:1576–1588. doi: 10.1016/j.cell.2011.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung MK, Huh WK. Bimolecular fluorescence complementation analysis system for in vivo detection of protein-protein interaction in Saccharomyces cerevisiae. Yeast. 2007;24:767–775. doi: 10.1002/yea.1504. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Park H, Hollingsworth NM, Sternglanz R, Lennarz WJ. PNG1, a yeast gene encoding a highly conserved peptide:N-glycanase. J Cell Biol. 2000;149:1039–1052. doi: 10.1083/jcb.149.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura M, Kariya R, Kudo E, Goto H, Iwawaki T, Amano M, Suico MA, Kai H, Mitsuya H, Okada S. Comparative analysis of ER stress response into HIV protease inhibitors: lopinavir but not darunavir induces potent ER stress response via ROS/JNK pathway. Free Radic Biol Med. 2013;65:778–788. doi: 10.1016/j.freeradbiomed.2013.08.161. [DOI] [PubMed] [Google Scholar]
- Tipper DJ, Harley CA. Yeast genes controlling responses to topogenic signals in a model transmembrane protein. Mol Biol Cell. 2002;13:1158–1174. doi: 10.1091/mbc.01-10-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- van den Berg B, Clemons WM, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- van den Ent F, Lowe J. RF cloning: a restriction-free method for inserting target genes into plasmids. J Biochem Biophys Methods. 2006;67:67–74. doi: 10.1016/j.jbbm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- van Stelten J, Silva F, Belin D, Silhavy TJ. Effects of antibiotics and a proto-oncogene homolog on destruction of protein translocator SecY. Science. 2009;325:753–756. doi: 10.1126/science.1172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vembar SS, Jonikas MC, Hendershot LM, Weissman JS, Brodsky JL. J domain co-chaperone specificity defines the role of BiP during protein translocation. J Biol Chem. 2010;285:22484–22494. doi: 10.1074/jbc.M110.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Malsburg K, Shao S, Hegde RS. The ribosome quality control pathway can access nascent polypeptides stalled at the Sec61 translocon. Mol Biol Cell. 2015;26:2168–2180. doi: 10.1091/mbc.E15-01-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Yofe I, Schuldiner M. Primers-4-Yeast: a comprehensive web tool for planning primers for Saccharomyces cerevisiae. Yeast. 2014;31:77–80. doi: 10.1002/yea.2998. [DOI] [PubMed] [Google Scholar]
- Young BP, Loewen CJ. Balony: a software package for analysis of data generated by synthetic genetic array experiments. BMC Bioinformatics. 2013;14:354. doi: 10.1186/1471-2105-14-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SG, Fong LG, Michaelis S. Prelamin A, Zmpste24, misshapen cell nuclei, and progeria--new evidence suggesting that protein farnesylation could be important for disease pathogenesis. J Lipid Res. 2005;46:2531–2558. doi: 10.1194/jlr.R500011-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.