Abstract
Tumorigenesis is dependent on the reprogramming of cellular metabolism as both direct and indirect consequence of oncogenic mutations. A common feature of cancer cell metabolism is the ability to acquire necessary nutrients from a frequently nutrient-poor environment and utilize these nutrients to both maintain viability and build new biomass. The alterations in intracellular and extracellular metabolites that can accompany cancer-associated metabolic reprogramming have profound effects on gene expression, cellular differentiation and the tumor microenvironment. In this Review, we have organized known cancer-associated metabolic changes into six hallmarks: (1) deregulated uptake of glucose and amino acids, (2) use of opportunistic modes of nutrient acquisition, (3) use of glycolysis/TCA cycle intermediates for biosynthesis and NADPH production, (4) increased demand for nitrogen, (5) alterations in metabolite-driven gene regulation, and (6) metabolic interactions with the microenvironment. While few tumors display all six hallmarks, most display several. The specific hallmarks exhibited by an individual tumor may ultimately contribute to better tumor classification and aid in directing treatment.
INTRODUCTION
While the first observations on metabolic alterations that are characteristic for tumors were first made nearly a century ago, the field of cancer metabolism has become a topic of a renewed interest in the past decade. Aided by new biochemical and molecular biological tools, studies in cancer cell metabolism have expanded our understanding of the mechanisms and functional consequences of tumor-associated metabolic alterations at various stages of tumorigenesis. In particular, it has become evident that tumorigenesis-associated metabolic alterations encompass all stages of cell-metabolite interaction, (a) affecting the metabolite influx through conferring an increased ability to acquire the necessary nutrients; (b) shaping the way the nutrients are preferentially assigned to metabolic pathways that contribute to cellular tumorigenic properties, as well as (c) exerting long-ranging effects on cellular fate, among which are alterations in differentiation of cancer cells themselves as well as of the components of the tumor microenvironment (Figure 1). In this Perspective, we take a detailed look at distinct hallmarks of tumorigenesis-associated metabolic reprogramming, and examine the functional contribution of these hallmarks to the establishment and maintenance of the tumorigenic state.
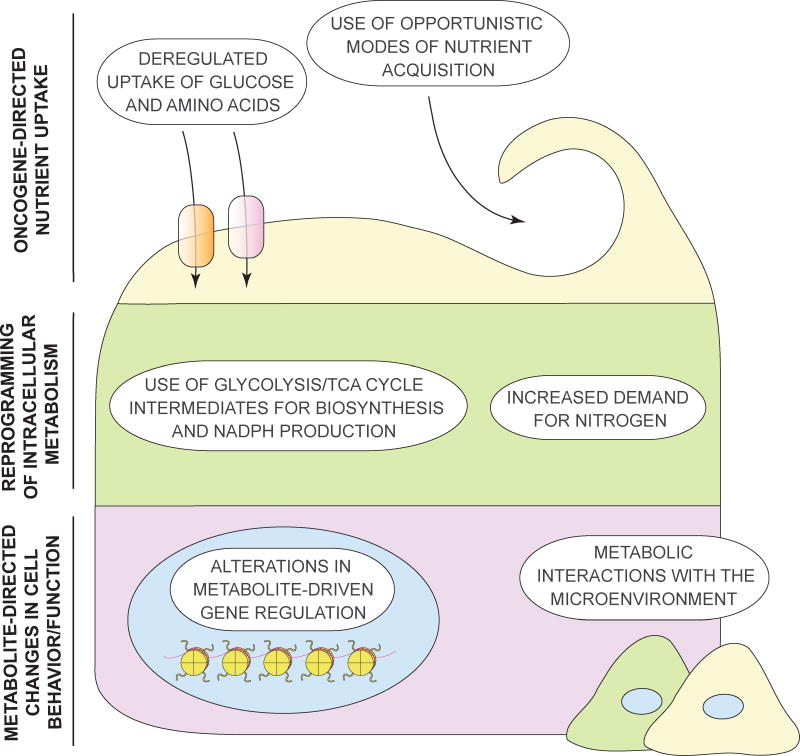
Figure 1. The emerging hallmarks of cancer metabolism.
Cancer cells accumulate metabolic alterations that allow them to gain access to conventional nutrient sources as well as to unconventional nutrient sources, utilize these nutrients towards the creation of new biomass to sustain deregulated proliferation, and take advantage of the ability of select metabolites to affect the fate of cancer cells themselves as well as a variety of normal cell types within the tumor microenvironment. Three layers of cell-metabolite interaction are depicted, all of which become reprogrammed in cancer. On top are the adaptations that involve nutrient uptake (Hallmarks 1 and 2), followed by alterations to intracellular metabolic pathways (Hallmarks 3 and 4) in the middle. Finally, long-ranging effects of metabolic reprogramming on the cancer cell itself (Hallmark 5), as well as on other cells within its microenvironment (Hallmark 6) are depicted at the bottom.
DEREGULATED UPTAKE OF GLUCOSE AND AMINO ACIDS
In order to fulfill the biosynthetic demands associated with proliferation, a cell must increase the import of nutrients from the environment. Two principal nutrients that support survival and biosynthesis in mammalian cells are glucose and glutamine. Through the catabolism of glucose and glutamine, a cell maintains pools of diverse carbon intermediates, which are utilized as building blocks for the assembly of various macromolecules. In addition, controlled oxidation of carbon skeletons of glucose and glutamine allows a cell to capture their reducing power either in the form of NADH and FADH2, which mediate the transfer of electrons to the electron transport chain to fuel ATP generation, or in the form of a related cofactor NADPH, which provides reducing power for a wide variety of biosynthetic reactions, as well as helps maintain cellular redox capacity.
A markedly increased consumption of glucose by tumors in comparison to the non-proliferating normal tissues was first described more than 90 years ago by the German physiologist Otto Warburg (Warburg O, 1924; Warburg et al., 1927). This observation has been confirmed in a variety of tumor contexts and shown to correlate with poor tumor prognosis (Som et al., 1980). Positron emission tomography (PET)-based imaging of the uptake of a radioactive fluorine-labeled glucose analog, 18F-fluorodeoxyglucose (18F-FDG) has been successfully used in the clinic for tumor diagnosis and staging, as well as for monitoring responsiveness to treatment (Almuhaideb et al., 2011).
Glutamine, a second principal growth-supporting substrate contributes not only carbon, but also reduced nitrogen for the de novo biosynthesis of a number of diverse nitrogen-containing compounds. Thus, glutamine provides the nitrogen required for the biosynthesis of purine and pyrimidine nucleotides, glucosamine-6-phosphate, as well as non-essential amino acids. Glutamine also has been reported to play a role in the uptake of essential amino acids. While non-essential amino acids can be produced by mammalian cells de novo, essential amino acids must be acquired from external sources. Interestingly, the import of an essential amino acid leucine through the plasma membrane-localized neutral amino acid antiporter LAT1 was shown to be coupled to a simultaneous efflux of glutamine (Nicklin et al., 2009). In such a manner, intracellular glutamine may facilitate the import of a broad range of LAT1 substrates, including leucine, isoleucine, valine, methionine, tyrosine, tryptophan and phenylalanine (Yanagida et al., 2001).
The high demand of proliferating tumor cells for glutamine was first described by the American physiologist Harry Eagle in 1950s, who demonstrated that the optimal growth of cultured HeLa cells requires a 10- to a 100-fold molar excess of glutamine in culture medium relative to other amino acids (Eagle, 1955). Furthermore, glutamine was found to be the most rapidly consumed amino acid by Ehrlich ascites carcinomas as well as by a number of hepatomas and carcinosarcomas proliferating in vivo (Marquez et al., 1989; Sauer et al., 1982). In fact, numerous tumorigenic contexts are associated with the depletion of glutamine from the tumor environment as compared to the corresponding normal tissue (Marquez et al., 1989; Rivera et al., 1988; Roberts and Frankel, 1949; Yuneva et al., 2012). Following the success of the 18F -FDG imaging paradigm, 18F-labeled glutamine tracers have recently shown promise in preclinical and early clinical studies (Lieberman et al., 2011; Venneti et al., 2015). Use of 18F-labeled glutamine as a tracer appears to provide potentially useful tumor information where the use of 18F-fluorodeoxyglucose is not feasible – for instance, in imaging of tumors that are localized to sites of heavy glucose utilization, such as the brain.
What causes tumor cells to internalize high quantities of glucose and glutamine? Normally, despite being surrounded by nutrient-rich plasma and the extracellular fluid, metazoan cells do not import nutrients in a constitutive manner. On the contrary, nutrient uptake is strictly regulated by growth factor signaling (Thompson, 2011) (Figure 2). For instance, when deprived of growth factors, hematopoietic and neuronal cells fail to consume glucose in quantities sufficient to even maintain cellular bioenergetics (Lindsten et al., 2003; Rathmell et al., 2000). This, in turn, negatively affects cell size, mitochondrial potential and ATP generation – all despite the abundance of glucose in the culture medium (Rathmell et al., 2000). However, survival of growth-factor deprived cells can be readily restored by a combined expression of a plasma membrane glucose transporter GLUT1 and the first enzyme of the glycolytic pathway hexokinase (HK) (Rathmell et al., 2003).
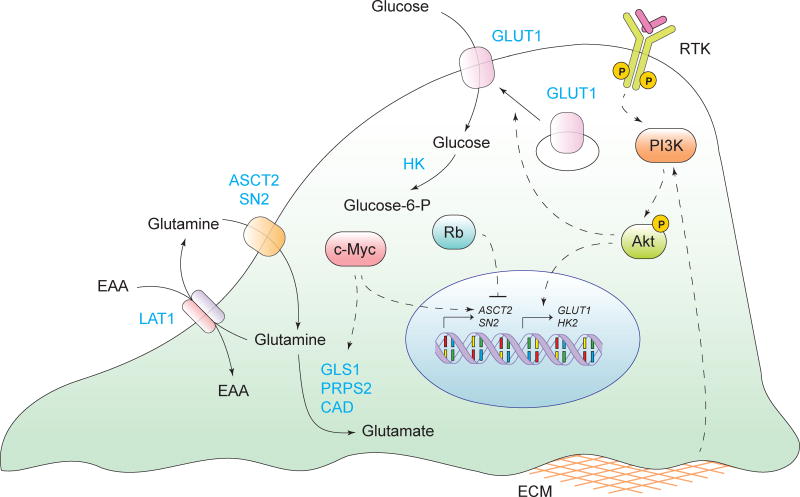
Figure 2. Deregulated uptake of glucose and amino acids.
Aberrantly activated oncogenes and loss of tumor suppressors deregulates the import of glucose and amino acids into cancer cells. Solid arrows depict the movement of metabolites or proteins and metabolic reactions. Dashed arrows depict positive and negative regulatory effects of signal transduction components. RTK, receptor tyrosine kinase; GLUT1, glucose transporter 1; ASCT2/SN2, glutamine transporter; LAT1, neutral amino acid transporter; EAA, essential amino acids; GLS1, glutaminase 1; PRPS2, phosphoribosyl pyrophosphate synthetase 2; CAD, carbamoyl-phosphate synthetase 2; HK, hexokinase; ECM, extracellular matrix.
In addition to soluble growth factors, interactions of cells with the extracellular matrix play a role in regulating glucose uptake. Culturing mammary epithelial cells under conditions where the cells are detached from the extracellular matrix compromises glucose uptake and results in a depressed mitochondrial potential and decreased ATP levels (Grassian et al., 2011; Schafer et al., 2009). Taken together, these observations suggest that the influx of glucose into cells is not driven by the immediate bioenergetic needs of a cell, but on the contrary, is rationed strictly as a result of extracellular stimuli.
In contrast to their normal counterparts, which require adhesion- and growth-factor-driven signaling inputs to maintain survival and proliferation, cancer cells accumulate oncogenic alterations that convey to them a significant degree of independence from these external requirements (Hanahan and Weinberg, 2000; Vogelstein and Kinzler, 2004). In particular, genetic alterations that target PI-3 kinase, its negative regulators PTEN and INPP4B, as well as activating mutations and gene amplifications in a variety of upstream receptor tyrosine kinases, result in constitutive glucose uptake and metabolism in diverse cancer types.
A point of convergence of signals from receptor tyrosine kinases as well as from the extracellular matrix, PI3K/Akt signaling acts as a master regulator of glucose uptake. PI3K/Akt signaling promotes both the expression of glucose transporter GLUT1 mRNA and the translocation of GLUT1 protein from the endomembranes to the cell surface (Barthel et al., 1999; Wieman et al., 2007). In addition, Akt potentiates the activity of the hexokinase (HK), which phosphorylates glucose molecules, thus preventing their efflux back into the extracellular space, as well as of the phosphofructokinase (PFK) enzyme, which catalyzes the key irreversible step of glycolysis (Deprez et al., 1997; Gottlob et al., 2001). In fact, the 18F-FDG-PET signal intensity in tumors correlates closely with the level of PI3K/Akt pathway activity, and is attenuated by PI-3 kinase and receptor tyrosine kinase inhibitors (Benz et al., 2011; Lheureux et al., 2013; Ma et al., 2009). Furthermore, exogenous expression of a constitutively active form of Akt alone can stimulate glycolysis and is sufficient to restore cell size, viability, mitochondrial potential and ATP levels in growth factor-deprived cells in a manner that is dependent upon the presence of glucose in the culture medium (Plas et al., 2001; Rathmell et al., 2003). Constitutively active form of Akt also prevents a decrease in ATP levels triggered by the loss of cellular attachment (Schafer et al., 2009). In addition, Akt signaling is central to facilitating glucose uptake in physiological settings of increased biosynthetic demand. For instance, targeted deletion of Akt1 in the mouse mammary gland abrogates a lactation-induced increase in glucose uptake, resulting in insufficient milk production (Boxer et al., 2006).
However, the PI3K/Akt signaling module is not the only oncogenic stimulus that plays a role in facilitating glucose uptake. Other oncogenic signaling proteins - notably, Ras, have been found to upregulate GLUT1 mRNA expression and increase cellular glucose consumption (Murakami et al., 1992). Taken together, multiple growth signaling nodes that become aberrantly activated in cancer share an ability to facilitate cellular access to glucose, a principal metabolic substrate.
The signaling pathways that regulate glutamine uptake are still being elucidated. The transcription factor c-myc, which is upregulated in proliferating cells and is frequently targeted by amplification in various tumor types, is a principal driver of glutamine utilization by proliferating cells (Wang et al., 2011b; Wise et al., 2008). To this end, c-myc induces the transcription of glutamine transporters ASCT2 and SN2, and in addition, promotes expression of glutamine-utilizing enzymes glutaminase (GLS1), phosphoribosyl pyrophosphate synthetase (PRPS2) and carbamoyl-phosphate synthetase 2 (CAD), which support transporter-facilitated glutamine uptake by converting glutamine to glutamate (Eberhardy and Farnham, 2001; Gao et al., 2009; Mannava et al., 2008). The resulting glutamate cannot exit the cell through glutamine transport and as it accumulates, promotes TCA cycle anaplerosis and stimulates uptake of cysteine by acting as an exchange substrate for the cysteine antiporter, xCT (Conrad and Sato, 2012).
In addition to a positive regulatory input from c-myc, glutamine uptake is also subject to negative regulation by the Rb tumor suppressor family of proteins. The deletion of Rb family proteins has been shown to upregulate the uptake and utilization of glutamine via the E2F-dependent upregulation of ASCT2 and GLS1 (Reynolds et al., 2014). Taken together, c-myc and E2F, two critical facilitators of cell division, exert their effects, in part, via enabling the access of cells to glutamine - a metabolic substrate critical for the biosynthetic demands that accompany DNA replication.
For any given cell, an attempt to proliferate in the absence of proper metabolic resources could end in a disaster. To avoid this, aberrantly activated oncogenes and/or loss of tumor suppressors can lock cancer cells in a state where they constitutively scavenge available glucose, glutamine and essential amino acids from the extracellular environment, which, in turn, enables their uncontrolled proliferation.
USE OF OPPORTUNISTIC MODES OF NUTRIENT ACQUISITION
Despite the avidity with which they take up glucose and amino acids, cancer cells in vivo often encounter conditions of nutrient scarcity as a result of the increased rates of nutrient consumption and the inadequacies of the tumor vascular supply (Vaupel et al., 1989). To deal with depleted supplies of the normal anabolic precursors, certain cancers acquire mutations that activate the cellular ability to utilize alternative ways of obtaining necessary nutrients. For instance, plasma and interstitial fluid of tissues is rich in soluble protein, yet extracellular protein is not generally utilized as a source of amino acids. Remarkably, expression of mutant Ras or c-Src alleles provides cells with a mechanism that allows them to recover free amino acids through the lysosomal degradation of extracellular proteins (Commisso et al., 2013) (Figure 3A). In contrast to the uptake of low molecular weight nutrients, which enter the cell through dedicated transporters, capture of extracellular macromolecules occurs through macropinocytosis, a process in which bulk extracellular fluid is taken up into giant vesicles (Kerr and Teasdale, 2009). Macropinocytosis is stimulated by Ras- and c-Src-driven actin cytoskeleton remodeling. Fluid-filled macropinosomes are trafficked into the interior of the cell, where they fuse with lysosomes and the engulfed proteins are subjected to proteolytic degradation, liberating free amino acids. Indeed, supplementation of culture medium with physiological levels (20–30 mg/mL) of serum albumin enables proliferation of KRASG12D-transformed MEFs in the absence of an essential amino acid leucine (Palm et al., 2015). Albumin supplementation has even been shown to restore the proliferation of a KRASG12D-driven mouse pancreatic cancer line in the culture medium that was devoid of all free amino acids (Kamphorst et al., 2015). In contrast to the effect of KRASG12D, PI3K/Akt signaling does not promote cellular utilization of extracellular protein, despite the ability of this pathway to stimulate the uptake of low molecular weight nutrients (Palm et al., 2015).
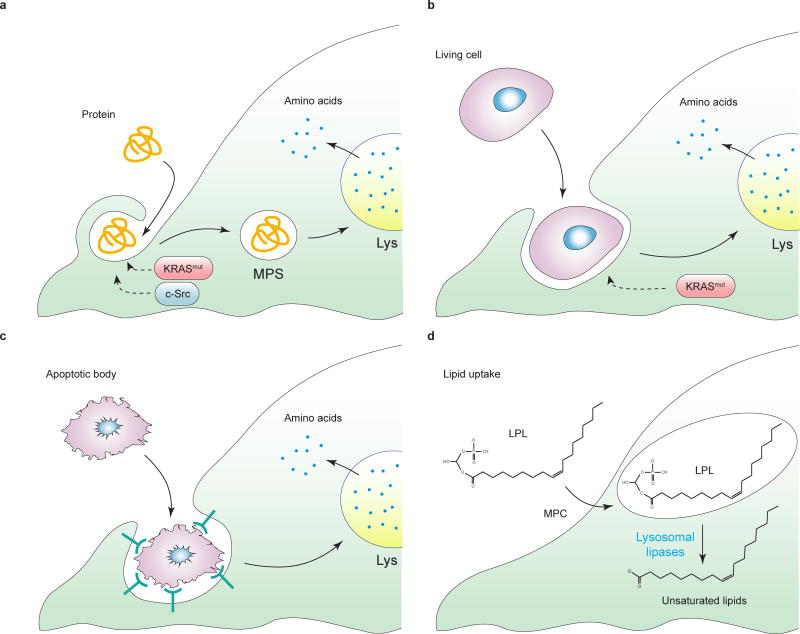
Figure 3. Use of opportunistic modes of nutrient acquisition.
When free amino acids are unavailable, cancer cells may recover amino acids from (a) extracellular proteins via macropinocytosis. MPS, macropinosome, Lys, lysosome, (b) entosis of living cells, or (c) phagocytosis of apoptotic bodies. d, in conditions of oxygen deficiency, which prevents the de novo desaturation of stearate (C18:0) into oleate (C18:1), monounsaturated fatty acids such as oleate (C18:1) can be recovered from extracellular lysophospholipids (LPL). MPC, macropinocytosis; SCD1, stearoyl-CoA desaturase 1. X represents a lipid head group.
Remarkably, mTORC1 inhibitors greatly increase the cellular ability to recover amino acids from captured extracellular protein and enhance their growth in the absence of essential amino acids (Palm et al., 2015). Thus, the utilization of extracellular protein as nutrients is repressed by mTORC1 under amino acid-replete conditions and is only utilized as an emergency source in conditions when free amino acids are insufficient. A spatial difference in the effect of rapamycin treatment in a KRASG12D-driven mouse model of pancreatic cancer provides a striking illustration of this paradigm. While rapamycin suppresses cancer cell proliferation at the tumor margin where the vascular delivery of nutrients is effective, rapamycin enhances cell proliferation within the poorly-vascularized tumor core by promoting enhanced lysosomal degradation of extracellular proteins (Palm et al., 2015).
In addition to soluble extracellular proteins, free amino acids can be recovered from the engulfment and digestion of entire living cells via a process known as entosis (Figure 3B), as well as from phagocytosis of apoptotic cellular corpses (Figure 3C) (Krajcovic et al., 2013; Stolzing and Grune, 2004). In a manner similar to macropinocytosis, cargo engulfed via entosis or phagocytosis becomes digested within lysosomes, providing a supply of amino acids to support cell survival and proliferation during conditions of amino acid deficit (Krajcovic et al., 2013). Interestingly, cells that harbor a mutant KRAS allele are more likely to perpetrate entosis than to be consumed in this process (Sun et al., 2014). Thus, KRAS-mutant cells may not only have a nutritional advantage over their non-mutant neighbors, but also actively eliminate the latter. Such a property may contribute to cell-cell competition within tumors, leading to the emergence of more aggressive cell populations.
A second consequence of a lack of vascular nutrient delivery in growing tumors is an emergence of hypoxic areas, which leads to a suppression of a number of biosynthetic reactions that require molecular oxygen as an electron acceptor. For example, hypoxia compromises stearoyl-CoA desaturase 1 (SCD1)-catalyzed introduction of double bonds into the de novo produced fatty acids, creating a deficit of unsaturated fatty acid species. To supplement for the missing fatty acids, hypoxic cells import “ready-made” unsaturated fatty acids from the surrounding environment in a form of single acyl chain-containing lysophospholipids (Kamphorst et al., 2013) (Figure 3D). Accordingly, removal of serum lipids triggers ER stress and apoptosis in certain cancer cells, when cultured under hypoxic conditions (Young et al., 2013).
In other cancer cells, the extracellular liberation of free fatty acids from more complex lipid species is increased. An elevated expression of lipoprotein lipase (LPL) and monoacylglycerol lipase (MAGL) across diverse cancer subtypes and correlates with invasiveness (Kuemmerle et al., 2011; Nomura et al., 2010). Finally, while some tumors develop adaptations that allow them to recover fatty acids directly from plasma, others induce the release of stored lipids from the neighboring normal cells. For instance, upregulation of a long chain fatty acid-binding protein FABP4 on the surface of metastatic ovarian cancer cells allows them to acquire fatty acids directly from the adipocytes of the omental fat (Nieman et al., 2011).
Taken together, tumor cells utilize opportunistic modes of nutrient acquisition, which allow them to survive and proliferate in metabolically unfavorable conditions. These adaptations involve an ability to access normally inaccessible nutrient sources, as well as to recover premade molecules when their synthesis within the cell has been compromised.
Even in the absence of available extracellular nutrients, cancer cells have been shown to utilize adaptive metabolic pathways to sustain their viability. In particular, cancer cells can withstand long periods of nutrient deprivation via the self-catabolic process of macroautophagy (Boya et al., 2013). During macroautophagy, intracellular macromolecules and whole organelles are enveloped by double membrane structures and fuse with lysosomes. Once inside a lysosome, the engulfed cargo is degraded by resident proteases and lipases to liberate free amino and fatty acids. Indeed, autophagy allows nutrient- or growth factor-deprived cells to maintain their viability in culture for weeks (Lum et al., 2005). In addition, deletion of a core component of the autophagy machinery, Atg7, was shown to dramatically change the nature of lung tumors driven by KrasG12D and BrafV600E oncogenes from malignant adenocarcinomas to benign oncocytomas (Guo et al., 2013; Strohecker et al., 2013). However, autophagy cannot supply cells with new biomass and thus cannot support proliferation in nutrient-poor conditions.
USE OF GLYCOLYSIS/TCA CYCLE INTERMEDIATES FOR BIOSYNTHESIS AND NADPH PRODUCTION
Cell proliferation not only increases the amount of nutrients a cell requires as outlined above, but also actively changes the way nutrients are used. Indeed, when a tumor cell is quiescent, glucose is preferentially utilized for mitochondrial acetyl-CoA generation, which is then subjected to oxidation in the tricarboxylic (TCA) cycle. The electrons extracted from the oxidative reactions of the TCA cycle are shuttled through NAD+/NADH and FAD/FADH2 to the electron transport chain, creating an electrochemical gradient that fuels ATP production.
The carbon economy of a proliferating cell differs dramatically from that of a cell that is quiescent (Vander Heiden et al., 2009) (Figure 4A). The major use of reduced carbon in proliferating cells is for the biosynthesis of a diverse array of biomolecules – among which are fatty acids and cholesterol, pentose and hexose sugar derivatives, glycerol, nucleotides and non-essential amino acids. To achieve this, a proliferating cell must first transform acquired nutrients into diverse pools of structural intermediates (Figure 4B). These molecules include cytosolic acetyl-CoA, one-carbon-carrying folate cycle units and S-adenosylmethionine (SAM), as well as a battery of glycolytic and TCA cycle intermediates. In addition, many biosynthetic reactions are reductive by nature and thus require a source of reducing power. For instance, generation of palmitic acid consumes 14 reducing equivalents, while building a cholesterol molecule requires 26 (Lunt and Vander Heiden, 2011). A designated donor of reducing equivalents that is used in cellular biosynthetic reactions is NADPH. Generation of NADPH from NADP+ is enabled by the controlled oxidation of carbon substrates in pathways distinct from those that generate NADH to support mitochondrial electron transport. Thus, a proliferating cell must allocate a portion of its carbon substrates to be used in NADPH production.
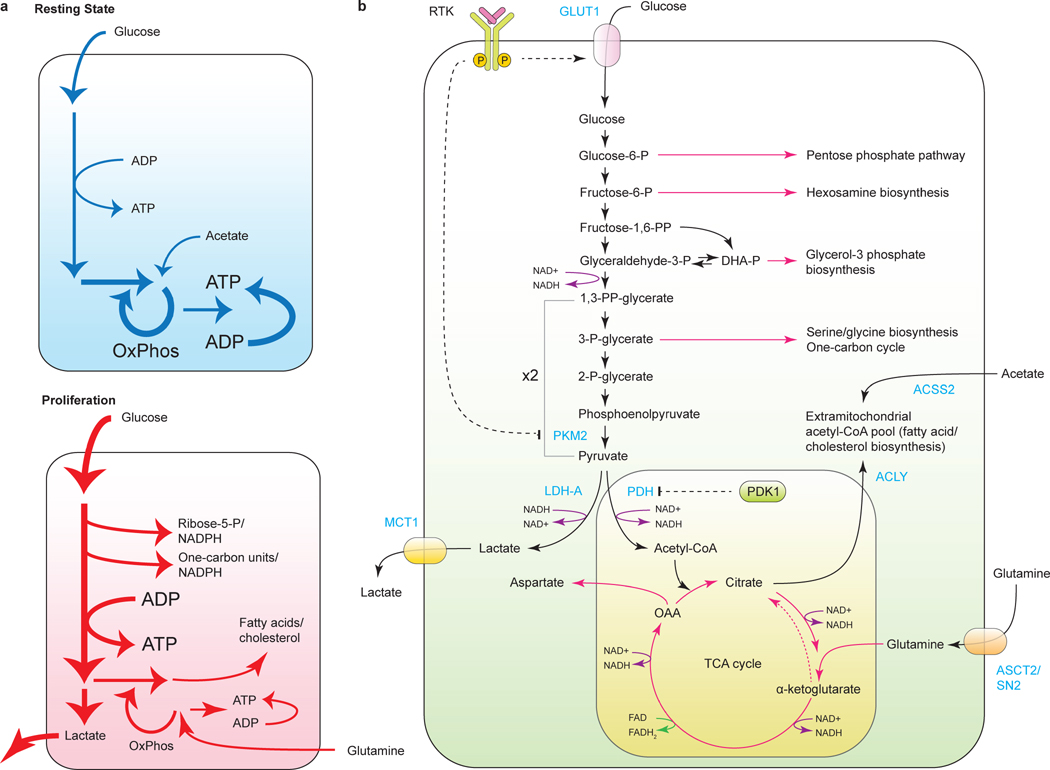
Figure 4. Use of glycolysis/TCA cycle intermediates for biosynthesis and NADH production.
a, differences in the central carbon metabolism of a cell in a quiescent state compared to a proliferating cell; b, diverse biosynthetic outputs of central carbon metabolism. RTK, receptor tyrosine kinase; GLUT1, glucose transporter 1; PKM2, pyruvate kinase M2; ACSS2, acetyl-CoA synthetase 2; LDH-A, lactate dehydrogenase A; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; ACLY, ATP-citrate lyase; MCT1, monocarboxylate transporter 1; ASCT2/SN2, glutamine transporter.
The reprogramming of carbon metabolism by proliferating cells provides part of the explanation for Warburg’s original observations concerning cancer metabolism. Experimenting with tumor slices incubated with glucose ex vivo, Warburg demonstrated that despite consuming massive amounts of glucose and being exposed to ambient oxygen, cancer cells did not take advantage of the bioenergetic benefits offered by the coupling of glycolysis to the TCA cycle. On the contrary, when grown in glucose-rich medium, any cells converted the surplus pyruvate to lactate, which, in turn, was secreted into the extracellular environment. Working under assumption that the major use of glucose in cells was to generate ATP, Warburg and many subsequent investigators misinterpreted this phenomenon, attributing it to the “irreversible injuring of respiration”, which leaves tumor cells with a considerably less efficient way of deriving ATP from substrate-level phosphorylation reactions of glycolysis (Warburg, 1956).
However, subsequent investigations into the mitochondrial status of tumor cells have shown that by and large, tumor cells possess functional mitochondria and retain the ability to conduct oxidative phosphorylation. In fact, targeted depletion of mitochondrial DNA reduces the tumorigenic potential of cancer cell lines in vitro and in vivo (Cavalli et al., 1997; Morais et al., 1994; Tan et al., 2015). Moreover, preferential conversion of glucose into lactate has been later demonstrated in genetically normal proliferating cells, as well as in cells infected by viruses (Brand et al., 1986; Chambers et al., 2010; Noch and Khalili, 2012). These observations suggest that, rather than being an adaptation to a defect in respiration, the Warburg effect is a regulated metabolic state and may, in fact, be beneficial during a time of increased biosynthetic demand.
Why then do proliferating cells convert excess pyruvate to lactate rather than transport the excess pyruvate into the mitochondria to maintain oxidative phosphorylation? The answer is just now coming into focus. Surprisingly, proliferating cells have only a modest increase in their consumption of ATP relative to their need for precursor molecules and reducing equivalents in the form of NADPH. Glucose catabolism is a robust provider of these precursors and reducing equivalents. In contrast, TCA cycle activity which generates NADH and ATP is the major negative regulator of glucose metabolism. By converting excess pyruvate to lactate, proliferating cells prevent accumulation of cytosolic NADH and reduce ATP production promoting continued cytosolic glucose metabolism free from feedback repression by excess mitochondrial ATP generation.
This realization has spurred further exploration of advantages that the decoupling of glycolysis from oxidative phosphorylation, might offer to a proliferating cell. Though glycolysis is classically depicted as a single chain of molecular events that leads to the generation of pyruvate, a number of glycolytic intermediates can be diverted into branching pathways, generating diverse biosynthetic precursors. First in the series of these branching pathways is the pentose phosphate pathway (PPP), in which glucose-6-phosphate becomes partially oxidized to generate NADPH and ribose-5-phosphate – a structural component of nucleotides. Utilization of PPP is frequently elevated in tumorigenesis. The key enzymes in the pathway, transketolase-like 1 (TKTL1) and transaldolase (TALDO) are frequently overexpressed in cancer (Wang et al., 2011a; Xu et al., 2009b). Both oncogenes and tumor suppressors have been shown to regulate PPP activity. For instance, Ras-driven transformation induces transcriptional upregulation of enzymes that mediate ribose-5-phosphate biosynthesis (Ying et al., 2012). Furthermore, wild-type, but not the mutant, p53 inhibits PPP by directly binding and inactivating the rate-limiting PPP enzyme glucose-6-phosphate dehydrogenase (G6PD) (Jiang et al., 2011).
Fructose-6-phosphate, which follows glucose-6-phosphate in the glycolytic pathway, can also leave glycolysis and become utilized as a substrate for hexosamine biosynthesis. A first committed step of hexosamine biosynthesis is a glutamine fructose-6-phospate aminotransferase 1 (GFPT1)-catalyzed production of glucosamine-6-phosphate from fructose-6-phosphate and glutamine. Through the generation of N-acetylglucosamine (GlcNAc), a substrate for N- and O-linked glycosylation, the hexosamine pathway provides substrates for cellular glycosylation reactions as well as heparan sulfate and hyaluronic acid biosynthesis, potentiates receptor-mediated signaling and regulates the stability of select proteins, including c-myc (Itkonen et al., 2013; Spiro, 2002; Wellen et al., 2010).
The next glycolytic intermediate that is utilized in macromolecular synthesis is dihydroxyacetone phosphate (DHAP). DHAP is converted by glycerol-3-phosphate dehydrogenase 1 (GPD1) into glycerol-3-phosphate, which is utilized in the biosynthesis of diverse phospholipids, a major structural component of cellular membranes.
Perhaps the most intensely studied growth-promoting mechanism that shunts metabolites out of the glycolytic pathway is the use of 3-phosphoglycerate as a precursor for the biosynthesis of serine, glycine, and as a means to generate methyl donor groups and NADPH. As metabolic flux studies reveal, cancer cells may utilize as much as 50% of glucose-derived carbon in serine biosynthesis and its subsequent catabolism (Locasale et al., 2011). A rate-limiting serine biosynthesis enzyme, 3-phosphoglycerate dehydrogenase (PHGDH), is frequently focally amplified in breast cancer and melanoma and is required for the growth of PHGDH-amplified cells in vitro as well as in vivo (Locasale et al., 2011; Possemato et al., 2011).
Serine has a unique metabolic role in the cell as a major substrate for the so-called one-carbon, or folate cycle. To this end, its γ-carbon can be transferred to a carrier molecule, tetrahydrofolate (THF), in a reaction catalyzed by serine hydroxylmethyltransferase 2 (SHMT2) in the mitochondria, and SHMT1 in the cytosol, generating 5, 10-methylene-THF and glycine. 5, 10-methylene-THF then undergoes a series of oxidative-reductive transformations, creating a battery of one-carbon-THF species (Tibbetts and Appling, 2010). One-carbon-THF species are utilized as substrates for the biosynthesis of purines, thymidine, as well as in the production of S-adenosylmethionine, a principal substrate for cellular methylation reactions. In addition, stepwise oxidation of one-carbon-THF species was recently shown to produce up to 50% of all cellular NADPH (Fan et al., 2014). Accordingly, hypoxia-driven induction of SHMT2 expression was shown to protect cells from hypoxia-associated oxidative stress (Ye et al., 2014). Thus, one-carbon pathway metabolites contribute to a number of cellular biosynthetic and regulatory processes. Finally, methylene tetrahydrofolate dehydrogenase 2 (MTHFD2), a component of the mitochondrial arm of the one-carbon pathway, was found to be among the top three most frequently overexpressed metabolic enzymes in cancer, suggesting that alterations in the one-carbon pathway may be universally selected for in tumorigenesis (Nilsson et al., 2014).
In conclusion, glycolytic intermediates leave glycolysis to take part in diverse biosynthetic reactions; accordingly, the rate-limiting enzymes within branching pathways of glycolysis are frequently upregulated in tumors. To balance biosynthetic outputs of glycolysis with its role in providing pyruvate that supports TCA cycle activity, proliferating cells have evolved a novel mechanism by which to regulate the last step of glycolysis. This step is regulated by pyruvate kinase (PK), an enzyme that converts phosphoenolpyruvate to the final product of glycolysis, pyruvate. With the exception of liver and kidney, which express tissue-specific PK isoforms PKL and PKR, most tissues express the PKM (muscle) form of PK. PKM exists in two splice variants (Noguchi et al., 1986). While PKM1 is more efficient at producing pyruvate, the majority of proliferating cells and essentially all cancer cells express primarily the PKM2 variant (Christofk et al., 2008a).
Unlike PKM1, PKM2’s activity is highly regulated. Thus, PKM2 is inhibited by tyrosine phosphorylation and activated by serine (Chaneton et al., 2012; Christofk et al., 2008b; Ye et al., 2012). In such a manner, growth factor-dependent signal transduction inhibits PKM2, leading to accumulation of glycolytic intermediates until the growing cell’s need for a catabolizable pool of free serine is saturated. In support of this paradigm, a reintroduction of the PKM1 isoform compromises cancer cell proliferation and tumorigenesis in vivo, in part by negatively affecting serine-dependent biosynthesis (Ye et al., 2012). In addition to serine, PKM2 can also be allosterically activated by excess accumulation of other byproducts of glucose metabolism including succinylaminoimidazolecarboxamide (SAICAR), an intermediate product of ribose-5-phosphate biosynthesis (Keller et al., 2012).
The emerging evidence favors the hypothesis that glycolysis is utilized by proliferating cells as a versatile production line that generates metabolic intermediates for numerous biosynthetic processes. Any excess glycolytic flux not utilized for biosynthesis is preferentially converted to lactate to help preserve a sufficient pool of NAD+ to sustain glycolysis and avoid flooding the mitochondria with a supply of NADH that would suppress the TCA cycle. Excess NADH generated by glycolysis is not imported into mitochondria of proliferating cells solely through chemical shuttles but is also utilized to convert the glycolytic intermediate DHAP into glycerol-3-phosphate that donates electrons directly into the electron transport chain utilizing glycerol-3-phosphate dehydrogenase (mGPDH) localized to the outer mitochondrial membrane. By using FAD as an electron acceptor, the mGPDH-catalyzed reaction reduces net mitochondrial electrical chemical potential from NADH generated via glycolysis. In fact, increased activity of GPDH has been detected in prostate cancer cells, insulinomas and carcinoid tumors (Chowdhury et al., 2005; MacDonald et al., 1990).
Mitochondria are protected further from excess glycolysis because any pyruvate that does enter the TCA cycle is channeled into citrate that is secreted into the cytosol through the mitochondrial tricarboxylate carrier and broken down into acetyl-CoA and oxaloacetate. Oxaloacetate is converted to malate and re-imported into mitochondria to maintain anaplerosis, while the acetyl-CoA serves as a precursor for lipid biosynthesis and protein acetylation. The electrons entering the mitochondrial electron transport chain through glycerol phosphate dehydrogenase activity and Complex I as a result of mitochondrial conversion of malate to oxaloacetate are sufficient to maintain both mitochondrial integrity and ATP production despite reduced catabolic TCA cycle activity.
A variety of oncogenes have been shown to contribute to the metabolic adaptations of proliferating cells outlined above. For instance, c-myc coordinately increases the expression of PDK1, lactate dehydrogenase A (LDH-A), an enzyme which catalyzes the reductive conversion of pyruvate to lactate, and monocarboxylate transporter (MCT1), which facilitates the efflux of lactate into the extracellular space (Wahlstrom and Arsenian Henriksson, 2015). In addition to c-myc, TCF/β-catenin signaling has also been shown to upregulate MCT1 and PDK1 transcription (Pate et al., 2014). Finally, stabilization of HIF1α by hypoxia or in various oncogenic contexts also triggers coordinated transcriptional upregulation of LDH-A and PDK1 (Kim et al., 2006; Papandreou et al., 2006).
Even with these adaptations, proliferating cells often accumulate electron transport flux that exceeds the capacity of the ATP synthase, resulting in the formation of excess reactive oxygen species (ROS). In fact, the damaging consequences of the overproduction of ROS from the overloaded electron transport chain may underlie the phenomenon of oncogene-induced cellular senescence (OIS). Triggered by the introduction of a mutant BRAF or RAS alleles, OIS is associated with a profound oxidative damage and irreversible growth arrest (Courtois-Cox et al., 2008). Interestingly, attenuation of the activity of pyruvate dehydrogenase (PDH), a gatekeeper enzyme that converts pyruvate into acetyl-CoA, via the ectopic expression of its negative regulator pyruvate dehydrogenase kinase 1 (PDK1) or an RNAi-mediated depletion of a PDH phosphatase PDP2 was found to bypass mutant BRAFV600E-induced OIS and potentiate tumorigenesis (Kaplon et al., 2013).
Whereas overproduction of ROS is detrimental to cell growth and survival, moderate levels of ROS, which are generated either during respiration or in a targeted manner by a class of NADPH oxidases, constitute an important signaling input that contributes to the maintenance of the tumorigenic state (Sullivan and Chandel, 2014). To this end, ROS act as inhibitors of protein phosphatases such as PTEN and PTP1B, as well as activators of Src family kinases and MAPK (Denu and Tanner, 1998; Kamata et al., 2005; Lee et al., 2002; Yan and Berton, 1996). Furthermore, increased ROS levels facilitate the activation of HIF1α and NRF2 transcription factors, thus promoting transcriptional programs that further contribute to tumorigenesis (Bertout et al., 2008; Sporn and Liby, 2012).
In contrast to the proliferating bulk of a tumor, accumulating evidence suggests that quiescent subpopulations of tumor cells are markedly less glycolytic and exhibit higher dependence on oxidative phosphorylation, as well as elevated expression of mitochondrial respiratory components. Among such populations are stem-like subclones that emerge following the oncogene ablation in vivo, as well as circulating tumor cells (CTCs) (LeBleu et al., 2014; Viale et al., 2014). Such apparent dichotomy in utilization of carbon for either predominantly biosynthetic or predominantly bioenergetic purposes may explain why tumors retain the capacity for oxidative phosphorylation, despite the original hypothesis put forth by Otto Warburg.
In addition to glycolytic intermediates, growth factor signaling promotes the use of select TCA cycle intermediates in the generation of biosynthetic precursors as well. In particular, PI3K/Akt activation allows a cell to expand its cytosolic pool of acetyl-CoA, a substrate for the de novo biosynthesis of fatty acids. To this end, Akt activates the ATP-citrate lyase (ACLY) enzyme, which catalyzes cytosol-localized cleavage of the TCA cycle-derived citrate into acetyl-CoA and oxaloacetate (Bauer et al., 2005; Berwick et al., 2002).
While the de novo biosynthesis of fatty acids is low in normal adult tissues – with the exception of lipogenic tissues such as liver, adipose tissue or mammary epithelium in the period of lactation, tumorigenesis is associated with a dramatic increase in lipid production (Li and Cheng, 2014; Menendez and Lupu, 2007). An increased capacity for the producing lipids de novo not only facilitates the formation of lipid bilayers, but also enables a cell to alter its membrane composition in favor of oxidative damage-resistant saturated fatty acids as means of adapting to oxidative stress (Rysman et al., 2010). The biosynthesis of fatty acid chains begins with the carboxylation of cytosolic acetyl-CoA by acetyl-CoA carboxylase (ACC) to produce malonyl-CoA, which is further assembled into long fatty acid chains by fatty acid synthase (FASN). In fact, all three major components involved in fatty acyl chain biosynthesis – ACLY, ACC and FASN – are frequently upregulated in transformed cells; furthermore, their inhibition reduces tumor cell growth in vitro and in vivo (Chajes et al., 2006; Flavin et al., 2010; Migita et al., 2008; Milgraum et al., 1997). In addition to fatty acid production, the de novo biosynthesis of cholesterol from malonyl-CoA plays a role in tumorigenesis as well. Thus, interference with cholesterol biosynthesis by inhibiting a rate-limiting enzyme, hydroxymethylglutaryl coenzyme A reductase (HMGCR) restores the normal acinar morphology of breast cancer cells, suggesting that changes in membrane composition and fluidity may have an effect on tissue architecture and anchorage-independent growth (Freed-Pastor et al., 2012).
In addition to supplying citrate, the TCA cycle also provides metabolic precursors for the biosynthesis of non-essential amino acids including aspartate and asparagine. In fact, aspartate biosynthesis has recently been shown to be critically dependent upon a cell’s ability to conduct oxidative phosphorylation (Birsoy et al., 2015; Sullivan et al., 2015). To maintain the ability to synthesize these amino acids, their production must be carefully balanced with an anaplerotic influx into the cycle. The major anaplerotic substrate in growing cells is glutamine. Glutamine can be captured in mitochondria by glutaminase and the resulting glutamate converted into α-ketoglutarate by either glutamate dehydrogenase or amino acid transaminases. Most proliferating cells depend on a continuous supply of glutamine to maintain the integrity of TCA cycle intermediates (DeBerardinis et al., 2007). In c-myc-transformed cells in particular, glutamine deprivation leads to the collapse of the TCA cycle and triggers cell death, which can be rescued by the addition of oxaloacetate or a membrane-permeable form of α-ketoglutarate (Wise et al., 2008; Yuneva et al., 2007). Oxidation of glutamine-derived α-ketoglutarate into oxaloacetate not only helps to maintain the ability of cells to synthesize citrate. Thus, the resulting oxaloacetate can be converted to malate, which, in turn, can be further oxidized by the malic enzyme (ME1) to pyruvate in a reaction that produces NADPH in a manner independent of glucose (Son et al., 2013). Finally, α-ketoglutarate may provide a source of citrate for the de novo lipogenesis in a setting in which hypoxia or certain oncogenic contexts create a deficit of glucose-derived acetyl-CoA. In a process referred to as reductive carboxylation, a section of the TCA cycle reverses itself, converting α-ketoglutarate into citrate, which can be then used to generate a source of cytosolic acetyl-CoA (Metallo et al., 2012; Wise et al., 2011).
Glutamine-derived α-ketoglutarate is not the only anaplerotic substrate that can be exploited by proliferating cells. In fact, tracing the fate of glucose-derived carbons in human patients and in mouse models of glioblastoma and non-small cell lung carcinoma (NSCLC) reveals that at least in these tumorigenic contexts, the principal source for the TCA cycle intermediates is not glutamine, but glucose (Maher et al., 2012; Marin-Valencia et al., 2012; Sellers et al., 2015). Anaplerotic entry of glucose-derived carbons into the TCA cycle takes place via carboxylation of pyruvate into the TCA cycle intermediate oxaloacetate, catalyzed by pyruvate carboxylase (PC). Indeed, survival of glutamine-deprivation-resistant derivatives of a glioblastoma cell line in culture was found to be dependent on pyruvate carboxylase (Cheng et al., 2011). Moreover, PC inhibition in NSCLC-derived cell lines suppresses fatty acid biosynthesis and dramatically reduces proliferation even in the presence of ample glutamine (Sellers et al., 2015). These reports highlight that the cell lineage context, as well as potential secondary effects associated with the adaptation of cells to tissue culture conditions may play an important role in the choice of an anaplerotic substrate.
Finally, extracellular acetate has recently been shown to be utilized by some cancers as a source of acetyl-CoA for biosynthesis. Specifically, a variety of tumor types localized to the brain, including primary glioblastomas as well as brain metastases that originated from other tissues, have been shown to assimilate exogenous acetate and incorporate acetate-derived carbons into de novo synthesized fatty acids (Comerford et al., 2014; Mashimo et al., 2014; Schug et al., 2015). Imported acetate is converted to acetyl-CoA in a reaction catalyzed by acetyl-CoA synthetase 2 (ACSS2), which is a target for amplification in breast cancer. Thus, hypoxia-associated deficit of acetyl-CoA can be augmented not only through the reductive carboxylation of α-ketoglutarate, but also by increasing the utilization of free acetate from plasma and interstitial fluid. Furthermore, upregulation of ACSS2 may potentially allow cells to recycle acetate released from deacetylation of histones and other cellular proteins as well.
INCREASED DEMAND FOR NITROGEN
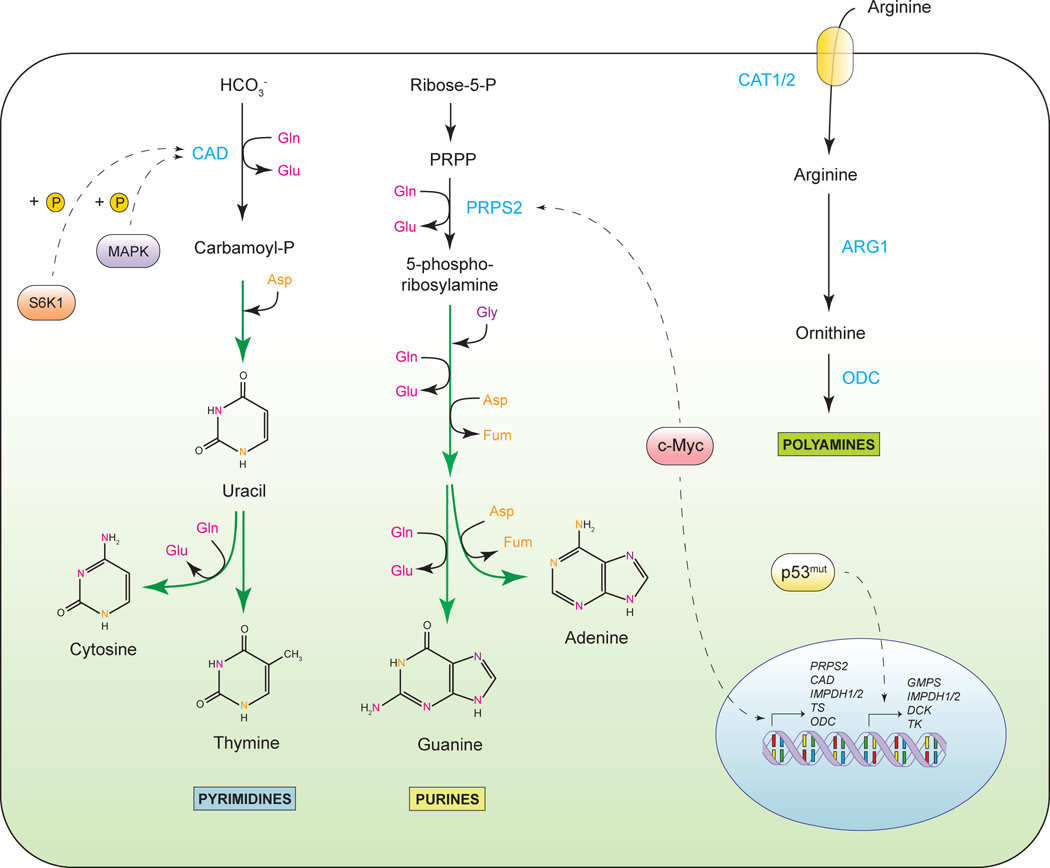
In addition to increasing the consumption of carbon in biosynthetic pathways, growth signaling concomitantly elevates the cellular demand for reduced nitrogen. Indeed, a proliferating cell must synthesize a number of nitrogen-containing molecules de novo, including nucleotides, non-essential amino acids and polyamines. As discussed above, the transcriptional effects of c-myc and E2F result in enhanced cellular uptake of glutamine. A non-essential amino acid containing two atoms of reduced nitrogen, glutamine serves as the principal way in which reduced nitrogen is transported between cells in metazoan organisms. The amide group of glutamine is an indispensable donor of nitrogen for the biosynthesis of purine and pyrimidine bases (Figure 5). Thus, one glutamine molecule is used in the production of uracil and thymine, cytosine and adenine each require two, and building a guanine base costs a cell three molecules of glutamine. In addition, the assembly of both pyrimidine and purine rings utilizes aspartate, which is derived from the transamination of the TCA cycle metabolite oxaloacetate and glutamic acid, both of which are catabolites of glutamine. Thus, glutamine is a key structural building block in the biosynthesis of nucleotides. Accordingly, glutamine levels have been shown to be rate-limiting for cell cycle progression, and glutamine deprivation leads to cell cycle arrest in the S-phase in certain cellular contexts (Fontenelle and Henderson, 1969; Gaglio et al., 2009).
Figure 5. Increased demand for nitrogen.
Sources of nitrogen atoms and regulation of nucleotide and polyamine biosynthesis. PRPP, phosphoribosyl pyrophosphate; PRPS2, phosphoribosyl pyrophosphate synthetase 2; CAD, carbamoyl-phosphate synthetase 2; Gln, glutamine, Glu, glutamate; Gly, glycine; Asp, aspartate; Fum, fumarate; IMPDH1/2, inosine-5-monophosphate dehydrogenase; TS, thymidylate synthase; GMPS, guanosine monophosphate synthetase; DCK, deoxycytidine kinase; TK, thymidine kinase, ARG1, arginase 1; ODC, ornithine decarboxylase; CAT1/2, cationic amino acid transporter.
The oncogene c-myc orchestrates nucleotide biosynthesis by upregulating the expression of a number of nucleotide biosynthesis enzymes. Among the c-myc-regulated targets are phosphoribosyl pyrophosphate synthetase 2 (PRPS2), which catalyzes a first step of purine biosynthesis, as well as carbamoyl phosphate synthetase II (CAD), which initiates the pyrimidine ring-building cascade (Cunningham et al., 2014; Eberhardy and Farnham, 2001; Mannava et al., 2008). Other targets of c-myc that are involved in nucleotide biosynthesis are thymidylate synthase (TS) and inosine monophosphate dehydrogenase 1 (IMPDH1) and 2 (IMPDH2) (Liu et al., 2008; Mannava et al., 2008). Thus, c-myc not only facilitates glutamine uptake, but also promotes its utilization in the biogenesis of purine and pyrimidine bases. Besides the regulatory inputs from c-myc, mutant p53 alleles have also been shown to facilitate expression of nucleotide biosynthesis genes, among which are IMPDH1 and 2, GMP synthetase (GMPS) and nucleoside salvage enzymes deoxycytidine kinase (DCK) and thymidine kinase (TK1) (Kollareddy et al., 2015). Furthermore, activity of CAD enzyme is regulated via phosphorylation by MAPK and by mTORC1-dependent S6 kinase (Ben-Sahra et al., 2013; Graves et al., 2000; Robitaille et al., 2013). In this manner, mTORC1-driven CAD activation may enable the cell to regulate pyrimidine biogenesis in response to the levels of intracellular glutamine, as the latter contributes to mTORC1 activation (Duran et al., 2012; Jewell et al., 2015).
In addition to being utilized for nucleotide biosynthesis, glutamine can be directly deamidated to glutamate by glutaminase which is frequently upregulated in cancer cells in a c-myc-dependent manner. Glutamine-derived glutamate serves as a donor of nitrogen for the production of a number of non-essential amino acids via transamination. In contrast, biosynthesis of asparagine from aspartate, catalyzed by asparagine synthetase (ASNS), utilizes the amide nitrogen of glutamine. Though structurally similar to glutamine, asparagine is the only amino acid that mammalian cells do not catabolize. Yet despite this, asparagine plays a crucial regulatory role in conditions of glutamine deprivation (Zhang et al., 2014). Exactly how asparagine supports cell survival and adaptation to glutamine depletion remains to be fully elucidated. ASNS is frequently upregulated in tumors and is associated with poor prognosis (Sircar et al., 2012; Zhang et al., 2014). In contrast, acute lymphoblastic leukemia cells lack ASNS expression, leading to asparagine auxotrophy. Depletion of plasma asparagine by a recombinant form of bacterial L-Asparaginase is an effective anticancer intervention for this cancer type (Hill et al., 1967; Pieters et al., 2011).
Despite the existence of a glutamine biosynthetic pathway in mammalian cells, the majority of proliferating cells in culture require an exogenous supply of glutamine. Interestingly, some cell types, such as embryonic stem cells and luminal breast cancer cells, are capable of proliferating in the absence of glutamine in the culture medium, indicating that in some cellular contexts, glutamine can be produced de novo (Carey et al., 2015; Kung et al., 2011). In addition, human glioblastoma samples implanted directly into the brains of immunocompromised mice were found to accumulate glutamine relative to the normal brain tissue (Marin-Valencia et al., 2012); furthermore, a similar accumulation of glucose-derived glutamine within the tumor tissue was observed in a mouse model of c-Met-driven liver cancer (Yuneva et al., 2012). Glutamine synthetase (GS) has been found to be overexpressed in some cancers (Osada et al., 2000); yet its role and its mode of activation remain to be fully understood.
Arginine, despite being a non-essential amino acid, also becomes conditionally essential in some tumorigenic contexts (Figure 5). A carrier of four nitrogen atoms, arginine serves as a precursor for a wide variety of nitrogenous compounds, among which are polyamines, creatine, agmatine and pyrroline-5-carboxylate, a precursor for the biosynthesis of proline. The de novo biosynthesis of arginine is an integral part of the urea cycle, where it is derived from argininosuccinate in a cleavage reaction catalyzed by argininosuccinate lyase (ASL). Argininosuccinate, in turn, is produced by the enzyme argininosuccinate synthase (ASS1) from citrulline and aspartate. Intriguingly, both ASS1 and ASL enzymes are frequently epigenetically silenced in melanoma, renal cell carcinoma and hepatocellular carcinoma (Bowles et al., 2008; Ensor et al., 2002; Yoon et al., 2007). Reduced expression of ASS1 and ASL are associated with poor prognosis and resistance to chemotherapy (McAlpine et al., 2014; Nicholson et al., 2009). Accordingly, the auxotrophy of tumors for arginine is being exploited in the development of anticancer therapies (Delage et al., 2010).
Why do tumors opt out of the de novo production of arginine and instead begin relying on its exogenous influx? One possibility is that the inactivation of ASL and ASS1 allows cancer cells to accumulate ornithine, which is then utilized in the production of polyamines, a class of nitrogen-containing polycationic aliphatic carbon molecules. Polyamine levels increase in proliferating cells; moreover, polyamines have been shown to inhibit apoptosis and promote tumor growth and invasion (Casero and Marton, 2007; Gerner and Meyskens, 2004). Another possible reason for arginine auxotrophy in cancer may be related to the fact that the suppression of ASS1-driven argininosuccinate production leads to an accumulation of its substrate aspartate, which can then be applied toward nucleotide biosynthesis.
In addition to its effects on glutamine metabolism, c-myc-orchestrated remodeling of non-essential amino acid metabolism involves the metabolism of proline (Phang et al., 2008). Proline can be produced either from glutamate or from arginine-derived ornithine via a common intermediate, pyrroline-5-carboxylate. The expression of the principal enzyme in proline biosynthesis, pyrroline-5-carboxylate reductase (PYCR1), is upregulated by c-myc (Liu et al., 2012). In fact, a meta-analysis of metabolic enzyme expression across diverse tumor types identified PYCR1 as one of the most commonly overexpressed genes in tumors (Nilsson et al., 2014). Reciprocally, proline oxidase (POX), which mediates proline degradation, is negatively controlled by c-myc via miR-23b, and is a p53 target as well (Donald et al., 2001; Liu et al., 2012). Expression of POX has been shown to inhibit tumor cell growth by triggering cell cycle arrest in the G2 phase (Liu et al., 2009). The contribution of altered proline metabolism to tumorigenesis remains to be elucidated. One explanation suggests that the elevated proline pool may facilitate production of collagen and new extracellular matrix deposition, facilitating tumor invasion. Taken together, while this area of cancer metabolism remains under active investigation, it is clear that similarly to carbon, the metabolism of nitrogen undergoes complex reprogramming in tumorigenesis.
ALTERATIONS IN METABOLITE-DRIVEN GENE REGULATION
Aberrantly activated growth and survival signals that drive tumorigenesis facilitate the reprogramming of cancer cell metabolism to enable increased nutrient acquisition and biosynthesis. However, metabolic networks themselves are not merely passive recipients of growth signals, but quite the contrary, directly transmit the information about the cellular metabolic state to a diverse array of regulatory enzymes, among which are those that mediate the deposition and removal of epigenetic marks from chromatin (Katada et al., 2012)(Figure 6).
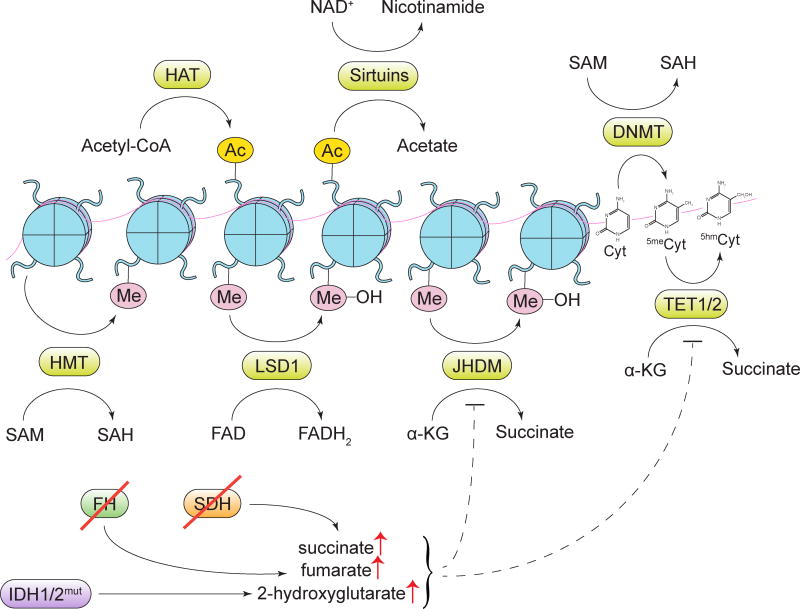
Figure 6. Alterations in metabolite-driven gene regulation.
Diverse metabolites serve as cofactors or substrates for enzymes involved in deposition and removal of epigenetic marks. HAT, histone acetyltransferase enzymes; Ac, an acetyl mark; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; DNMT, DNA methyltransferase enzymes; HMT, histone methyltransferase enzymes; Me, a methyl mark; LSD1, lysine-specific histone demethylase 1; JHDM, Jumonji domain-containing histone demethylase enzymes; Cyt, cytosine; 5meCyt, 5-methylcytosine; 5hmCyt, 5-hydroxymethylcytosine; TET1/2, ten-eleven translocation methylcytosine dioxygenase 1/2; α-KG, α-ketoglutarate; SDH, succinate dehydrogenase; FH, fumarate hydratase; IDH1/2, isocitrate dehydrogenase 1/2.
A key metabolite that builds up when cells metabolize more glucose than needed for bioenergetic support is cytosolic acetyl-CoA. Cytosolic acetyl-CoA is the obligate substrate for enzymes that acetylate histones and other proteins. The deposition of acetyl marks on histones is associated with the increased accessibility of the genomic DNA for the assembly of transcriptional complexes, and has a rapid turnover rate. Histone acetylation is exquisitely sensitive to alterations in the cellular nutritional and signaling status (Cai et al., 2011; Shi and Tu, 2013). Indeed, withdrawal and readdition of glucose, as well as activation of oncogenic signaling via introduction of an oncogenic KRAS mutant or a constitutively active form of Akt, increase total histone acetylation, which, in turn, promotes the enhanced and broader gene expression (Lee et al., 2014). Immunohistochemical analysis of glioblastoma and prostate tumor samples has shown that Akt activation levels closely correlate with global histone acetylation status (Lee et al., 2014). Activated Akt expands the extramitochondrial pool of acetyl-CoA by activating ACLY, which converts cytosolic citrate into acetyl-CoA and oxaloacetate. Furthermore, studies have shown that both the glucose- and oncogene-driven increases in global histone acetylation are blocked by ACLY inhibition (Lee et al., 2014; Wellen et al., 2009).
In addition to acetyl-CoA, a p300 histone acetyltransferase has recently been shown to utilize crotonyl-CoA as a substrate as well. Notably, deposition of crotonyl marks on select amino acid residues within histone tails was found to activate gene expression even more potently than acetyl marks (Sabari et al., 2015). Crotonyl-CoA can be produced from the catabolism of lysine and tryptophan, as well as of a short-chain fatty acid butyrate. In fact, a large variety of novel histone marks, such as formylation, propionylation, butyrylation, malonylation and succinylation have recently been identified by tandem mass spectrometry (Chen et al., 2007; Wisniewski et al., 2008; Xie et al., 2012). Further investigation of these marks has potential to dramatically expand the number of metabolic inputs into the regulation of global patterns of gene expression.
Numerous methylation reactions in the cell, including the deposition of methyl marks on histone tails, cytosine methylation on DNA and adenosine methylation on mRNA utilize S-adenosylmethionine (SAM) as a donor of methyl groups. SAM is a product of the one-carbon metabolic pathway and is fueled by serine catabolism as described above. A number of recent reports demonstrate that histone and DNA methylation is sensitive to alterations in SAM levels (Chiang et al., 2009; Shyh-Chang et al., 2013; Towbin et al., 2012).
The removal of acetyl and methyl marks is also guided by the cellular metabolic state. For instance, sirtuins, a class of deacetylases that catalyze the removal of acetyl marks from histone and non-histone proteins, utilize NAD+ as a cofactor, while FAD serves as a cofactor for a lysine-specific demethylase LSD1 (Imai et al., 2000; Shi et al., 2004). Sensitive to changes in NAD+ or FAD availability, these enzymes orchestrate global post-translational and epigenetic changes that promote energy conservation (Hino et al., 2012; Schwer and Verdin, 2008).
A wide variety of post-translational modifications in the cell are carried out by the members of a large class of α-ketoglutarate-dependent dioxygenases. Among α-ketoglutarate-dependent dioxygenases are a TET family of DNA demethylases, Jumonji C family of histone demethylases, mRNA demethylases FTO and ALKBH5, and a family of prolyl hydroxylase (PHD) enzymes, which, among other processes, regulate HIF1α levels in response to oxygen availability and oxidative stress. The reaction mechanism of α-ketoglutarate-dependent dioxygenases involves a concurrent oxidation of a co-substrate α-ketoglutarate into succinate. Accordingly, intracellular levels of α-ketoglutarate can directly influence the activity of these enzymes. In addition, α-ketoglutarate-dependent dioxygenases are prone to the inhibition by their reaction product, succinate, as well as by fumarate, the downstream product of succinate degradation in the TCA cycle. For instance, a biallelic loss of succinate dehydrogenase (SDH), an alteration found in familial paragangliomas and pheochromocytomas, as well as in a subset of sporadic gastrointestinal stromal tumors, creates a build-up of succinate (Astuti et al., 2001; Janeway et al., 2011). Similarly, loss of a fumarate-metabolizing enzyme fumarate hydratase (FH) and accumulation of fumarate is found in the familial cancer syndrome HLRCC (hereditary leiomyomatosis and renal cell cancer) and in a subset of paragangliomas and pheochromocytomas as well (Tomlinson et al., 2002). Tumors that exhibit SDH- and FH loss share a number of phenotypic features that are consistent with dioxygenase inhibition, among which is a characteristic global increase in DNA methylation (Killian et al., 2013; Letouze et al., 2013; Xiao et al., 2012), as well as elevated levels of HIF1α (Selak et al., 2005). In fact, the latter, at least in the context of SDH loss, can be reversed by the addition of a membrane-permeable form of α-ketoglutarate (MacKenzie et al., 2007).
Another prominent class of tumor-associated genetic alterations that modulate the activity of α-ketoglutarate-dependent dioxygenases are gain-of-function mutations of isocitrate dehydrogenase 1 (IDH1) and isocitrate dehydrogenase 2 (IDH2). IDH1 and IDH2 mutations have been identified in low-grade glioma, where they represent a likely initiator lesion, as well as in chondrosarcoma, cholangiocarcinoma, and acute myeloid leukemia (AML) (Balss et al., 2008; Borger et al., 2012; Cohen et al., 2013; Mardis et al., 2009; Parsons et al., 2008; Paschka et al., 2010). Mutant alleles of IDH1 and IDH2 exhibit an unusual neomorphic enzymatic function. In contrast to wild-type isocitrate dehydrogenases, which convert the TCA cycle metabolite isocitrate to α-ketoglutarate, a mutant form of IDH prefers to use α-ketoglutarate as a substrate, catalyzing its conversion to the D-enantiomer of 2-hydroxyglutarate (2-HG) (Dang et al., 2010; Ward et al., 2010). Owing to its structural similarity to α-ketoglutarate, 2-HG acts as a competitive inhibitor of α-ketoglutarate-dependent dioxygenases (Xu et al., 2011). Indeed, IDH-driven gliomas, leukemias and chondrosarcomas display a prominent CpG island hypermethylation, reminiscent of the hypermethylator phenotype seen in SDH- and FH-deficient cancers (Figueroa et al., 2010; Lu et al., 2013; Turcan et al., 2012). IDH mutations in AML are mutually exclusive with inactivating mutations in TET2 methylcytosine hydroxylases, further implicating mutant IDH as a bona fide driver of epigenetic remodeling (Figueroa et al., 2010). Furthermore, a knock-in of a mutant IDH1 allele into normal hematopoietic cells and into chondrocytes in vivo triggers an aberrant expansion of targeted cell lineages (Hirata et al., 2015; Sasaki et al., 2012). In various cellular settings, ectopic expression of a mutant IDH allele or treatment with exogenous D-2-HG is sufficient to induce DNA and histone hypermethylation and block cellular differentiation (Losman et al., 2013; Lu et al., 2012). Conversely, small molecule-mediated inhibition of mutant IDH1 in glioma and IDH2 in leukemia markedly promoted differentiation in these contexts (Rohle et al., 2013; Wang et al., 2013). The abundance of 2-HG has been utilized for 1H magnetic resonance spectroscopy-based imaging of IDH-mutant gliomas (Choi et al., 2012). Moreover, small molecule inhibitors of IDH1 and IDH2 have been reported to induce remissions in phase I clinical trials for treatment of AML patients with the respective mutations.
Notably, some tumor types display elevations in 2-HG even in the absence of IDH mutations. For instance, elevated 2-HG levels have been detected in triple-negative breast cancer (Terunuma et al., 2014). In addition, a specific increase in the L enantiomer of 2-HG, which was shown to exert similar, but not identical, inhibitory effects on α-ketoglutarate-dependent dioxygenases was found in clear cell renal cell carcinoma, where it correlates with increased methylcytosine levels (Shim et al., 2014). Furthermore, L-2-HG levels were found to increase in response to hypoxia as a result of promiscuous substrate use by LDH-A and malate dehydrogenase (MDH) (Intlekofer et al., 2015), and two recent studies implicate L-2-HG as a metabolic mediator of the cellular stress response to hypoxia (Intlekofer et al., 2015; Oldham et al., 2015).
METABOLIC INTERACTIONS WITH THE MICROENVIRONMENT
The information about a metabolic state of a cell affects not only its own long-term decision-making, but also has the potential to influence the fate of other cells in its vicinity. Indeed, a variety of genetically stable cell types, among which are tumor-associated fibroblasts, endothelial cells, as well as innate and adaptive immune system components, are known to undergo characteristic phenotypic changes as a consequence of residing in the vicinity of a growing tumor (Hanahan and Coussens, 2012). How cancer cells reprogram their microenvironment to assist tumor growth and dissemination is an area of intense investigation, but it is clear that such reprogramming encompasses multiple strategies, among which are secreted growth factors and alterations to the extracellular matrix and cell-cell interactions.
In addition to these inputs, proliferating cancer cells alter the metabolic composition of the extracellular milieu around them as well (Figure 7). The high utilization of extracellular glucose and glutamine by cancer cells results in the accumulation of extracellular lactate, which was shown to affect a number of cell types within the tumor microenvironment. Increased lactate levels promote the emergence of an immune-permissive microenvironment by attenuating dendritic and T cell activation and monocyte migration (Fischer et al., 2007; Goetze et al., 2011; Gottfried et al., 2006). In addition, lactate stimulates the polarization of resident macrophages to a so-called M2 state, which plays a role in immunosuppression and wound healing (Carmona-Fontaine et al., 2013; Colegio et al., 2014). Furthermore, lactate accumulation is instrumental for the promotion of angiogenesis. Thus, lactate promotes the stabilization of HIF1α and activates NF-κB and PI-3 kinase signaling in endothelial cells, as well as induces secretion of a pro-angiogenic factor VEGF from tumor-associated stromal cells (Constant et al., 2000; Schmid et al., 2007; Sonveaux et al., 2012; Vegran et al., 2011). Increased levels of lactate also stimulate hyaluronic acid production by fibroblasts, which may contribute to tumor invasiveness (Stern et al., 2002).
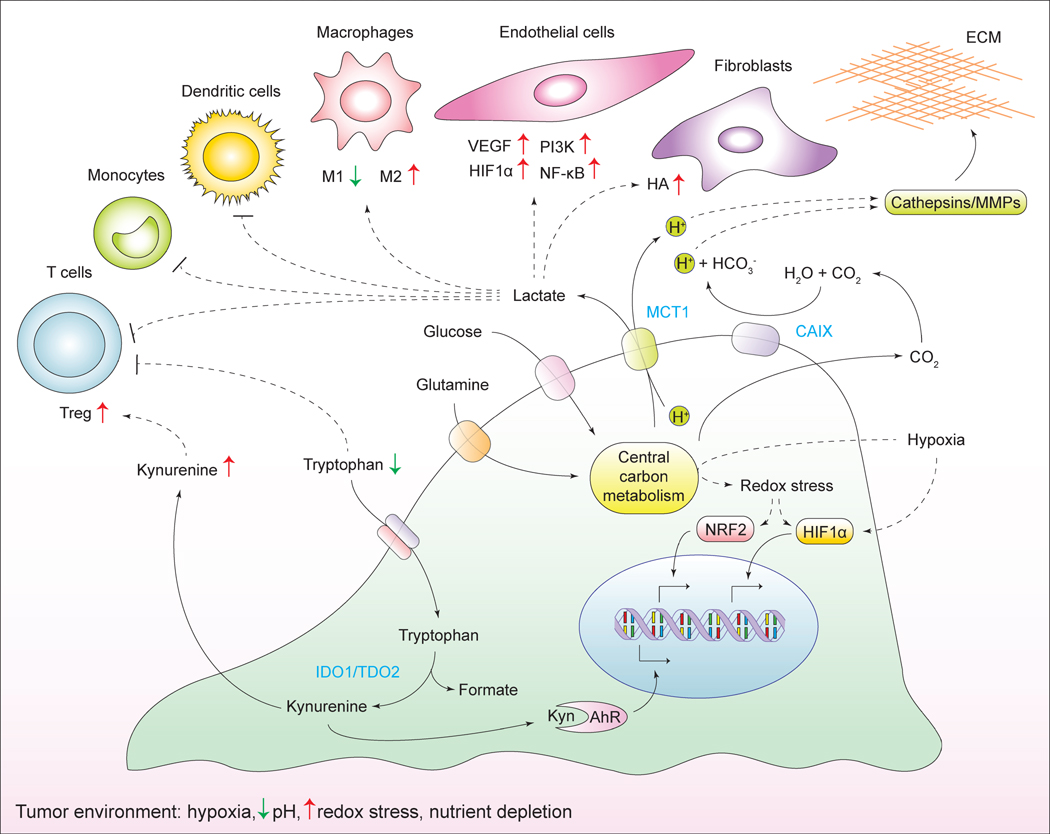
Figure 7. Metabolic interactions with the microenvironment.
Cancer cells alter the chemical composition of the extracellular milieu, which exerts pleiotropic effects on the phenotypes of normal cells that reside in the vicinity of the tumor, as well as the extracellular matrix. Reciprocally, the microenvironment affects the metabolism and signaling responses of cancer cells themselves. ECM, extracellular matrix; Treg, regulatory T cells; HA, hyaluronic acid; MMPs, matrix metalloproteinases; MCT1, monocarboxylate transporter 1; CAIX, carbonic anhydrase IX; IDO1, indoleamine-2, 3-dioxygenase 1; TDO2, tryptophan-2, 3-dioxygenase 2; Kyn, kynurenine; AhR, aryl -hydrocarbon receptor.
The secretion of lactate into the extracellular space via the monocarboxylate transporter MCT1 is coupled to the co-transport of H+, which causes acidification of the cellular microenvironment. In addition, the surplus of CO2 generated in mitochondrial decarboxylation reactions contributes to the extracellular acidification as well. To this end, CO2 diffuses into the extracellular space, where it becomes converted to H+ and HCO3− by a class of extracellular carbonic anhydrases (Swietach et al., 2007). Expression of carbonic anhydrases, in particular the CAIX isoform, is elevated during hypoxia (Svastova et al., 2004). Increased extracellular acidification stimulates the proteolytic activity of matrix metalloproteinases (MMPs) and cathepsins, promoting the degradation of the extracellular matrix components and enhancing tumor invasion (Martinez-Zaguilan et al., 1996; Rothberg et al., 2013).
Whereas lactate accumulation and concomitant acidification of the extracellular space may be seen as collateral effects of cancer-specific metabolic reprogramming, some tumors employ a distinct strategy as means of promoting the emergence of an immune-permissive microenvironment around them. Specifically, numerous solid tumor types overexpress tryptophan-degrading dioxygenases indoleamine-2, 3-dioxygenase (IDO1) and tryptophan-2, 3-dioxygenase (TDO2), which catalyze the conversion of an essential amino acid, tryptophan, into its derivative, kynurenine (Munn and Mellor, 2007). As a consequence, tryptophan depletion triggers amino acid deprivation-associated apoptosis of effector T cells (Fallarino et al., 2002). Furthermore, accumulated kynurenine acts as a ligand for aryl hydrocarbon receptor (AhR) (Opitz et al., 2011). In a manner that is dependent on AhR, kynurenine promotes regulatory T-cell phenotype, further contributing to the suppression of antitumor immune responses (Fallarino et al., 2006). Finally, kynurenine potentiates autocrine signaling through AhR on cancer cells themselves, promoting the degradation of the extracellular matrix and invasion (Opitz et al., 2011). Small molecule inhibitors of IDO1 are currently being tested in clinical trials (Vacchelli et al., 2014).
Reciprocally, the conditions within the tumor microenvironment have profound effects on the metabolism of a cancer cell. As discussed earlier, tumors are often faced with nutrient- and oxygen-poor surroundings and develop various nutrient-scavenging strategies to bypass these limitations. Furthermore, hypoxia impedes the ability of cells to carry out oxidative phosphorylation and other reactions that require oxygen and disrupts the redox balance, affecting cellular signaling and transcriptional programs. Taken together, reciprocal interactions between cancer cells and their microenvironment impose a selective pressure that further shapes cancer cell metabolism and actively contributes to the emergence of a more aggressive state.
REMAINING QUESTIONS
Oncogene-driven metabolic reprogramming allows cancer cells to maintain deregulated proliferation, withstand metabolic challenges that are associated with oxygen and nutrient limitations, maintain a dedifferentiated state through alterations in global patterns of gene expression, and corrupt the surrounding microenvironment into actively assisting tumor growth and dissemination. While most of the studies to this point have been focused on alterations in the metabolism of glucose and glutamine, cancer cells utilize a great variety of other nutrients, among which are sulfur–containing amino acids cysteine and methionine, essential fatty acids, choline, trace metals, and vitamins. We are only beginning to understand the extent to which these nutrients contribute to tumorigenesis.
For instance, multiple lines of evidence indicate that the metabolism of sulfur may undergo reprogramming in cancer. Thus, cancer cells upregulate the uptake of cysteine via the transcriptional upregulation of the xCT transporter, as well as secondary to the increased rate of glutamine utilization by cells (Lo et al., 2008). In fact, no less than 30% of imported glutamine was shown to leave the cell through the xCT transporter as glutamate, while being exchanged for cysteine (Collins et al., 1998). Consistent with its heavy utilization by cancer cells, cysteine was found to be the second most depleted amino acid in pancreatic tumors in comparison to normal pancreatic tissue (Kamphorst et al., 2015). Cysteine has several metabolic fates in proliferating cells, among which are the biosynthesis of glutathione and iron-sulfur clusters, as well as of hydrogen sulfide (H2S), a gasotransmitter with complex and not yet fully defined functions in cellular physiology, among which are protection from oxidative stress, increased mitochondrial respiration, protection from apoptosis and facilitation of angiogenesis (Wu et al., 2015).
Alterations in serine metabolism of tumors have received considerable attention in the past four years. Notably, in addition to the de novo-produced serine, the inputs from the exogenous one-carbon donors may be affecting one-carbon metabolism and SAM levels as well. Indeed, dietary serine and glycine deficiency has been shown to impair xenograft growth of colon cancer cells in mice (Maddocks et al., 2013). Two other dietary compounds that can be utilized as donors of methyl groups for SAM production are choline and its derivative betaine. Increased choline uptake is observed in breast and prostate cancer; in fact, radioactive 18F- and 11C-choline is used in the clinic for PET-based tumor imaging (Krause et al., 2013; Kwee et al., 2007). High dietary intake of choline has been linked to a significantly increased risk of lethal prostate cancer (Richman et al., 2012). In contrast, a diet high in choline and betaine was associated with a decreased breast and lung cancer risk (Xu et al., 2009a; Ying et al., 2013; Zhang et al., 2013). Furthermore, diets deficient in choline and betaine were shown to directly affect histone and DNA methylation in a number animal models (Du et al., 2009; Mehedint et al., 2010; Niculescu et al., 2006), suggesting that alterations in exogenous methyl donor availability may have strong effects on cellular epigenetic state and thus contribute to tumorigenesis.
The effect of various micronutrients – namely, vitamins and trace metals – on tumor growth is a promising area of research. Micronutrients are utilized as cofactors for various enzymes and play complex and not yet fully defined roles in regulating cellular metabolic circuits, as well as signal transduction. For instance, ascorbic acid acts as a cofactor for the α-ketoglutarate-dependent dioxygenases. Notably, ascorbic acid treatment was shown to affect DNA methylation in embryonic stem cells and mouse embryonic fibroblasts in a manner that is dependent on TET2 (Blaschke et al., 2013; Minor et al., 2013). As many cancers are characterized by increased methylation of CpG islands, alterations in ascorbic acid import may thus affect their epigenetic state as well. Furthermore, levels of select trace metals, such as zinc and copper, are altered in cancer cells compared to the normal tissue. Copper levels in particular can be elevated as much as 2–3 fold in breast and ovarian tumors, as well as in leukemia (Gupte and Mumper, 2009). Interestingly, copper was recently shown to be required for BRAFV600E-driven ERK activation in melanoma cells, and the effect of copper depletion could be rescued by the constitutive activation of ERK (Brady et al., 2014).
Finally, the contribution of a broad spectrum of metabolites produced by the body’s “forgotten organ”, i.e., microbiota, to tumor initiation and progression is only beginning to be elucidated (Garrett, 2015). Taken together, the concept of metabolic alterations of cancer cells, first called to attention nearly a century ago, continues to uncover new connections between nutrient utilization and tumorigenic state. Furthermore, the study of cancer metabolism is not only shedding light on carcinogenesis but is also revealing new principles of how the biochemistry of anabolic metabolism is orchestrated to support normal cell growth, proliferation, and differentiation.
Acknowledgments
We thank the members of the Thompson laboratory, particularly Dr. Tullia Lindsten, for critical reading of the manuscript, and Leeza Menon for help with manuscript preparation. C.B.T. is supported by grants from the NIH and NCI, and is a founder of Agios Pharmaceuticals and a member of its scientific advisory board. C.B.T. also serves on the board of directors of Merck and Charles River Laboratories. N.N.P. is supported by the Terri Brodeur Breast Cancer Foundation postdoctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almuhaideb A, Papathanasiou N, Bomanji J. 18F-FDG PET/CT imaging in oncology. Ann Saudi Med. 2011;31:3–13. doi: 10.4103/0256-4947.75771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti D, Latif F, Dallol A, Dahia PL, Douglas F, George E, Skoldberg F, Husebye ES, Eng C, Maher ER. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- Barthel A, Okino ST, Liao J, Nakatani K, Li J, Whitlock JP, Jr, Roth RA. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem. 1999;274:20281–20286. doi: 10.1074/jbc.274.29.20281. [DOI] [PubMed] [Google Scholar]
- Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–6322. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz MR, Herrmann K, Walter F, Garon EB, Reckamp KL, Figlin R, Phelps ME, Weber WA, Czernin J, Allen-Auerbach MS. (18)F-FDG PET/CT for monitoring treatment responses to the epidermal growth factor receptor inhibitor erlotinib. J Nucl Med. 2011;52:1684–1689. doi: 10.2967/jnumed.111.095257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DC, Hers I, Heesom KJ, Moule SK, Tavare JM. The identification of ATP-citrate lyase as a protein kinase B (Akt) substrate in primary adipocytes. J Biol Chem. 2002;277:33895–33900. doi: 10.1074/jbc.M204681200. [DOI] [PubMed] [Google Scholar]
- Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell. 2015;162:540–551. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke K, Ebata KT, Karimi MM, Zepeda-Martinez JA, Goyal P, Mahapatra S, Tam A, Laird DJ, Hirst M, Rao A, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500:222–226. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles TL, Kim R, Galante J, Parsons CM, Virudachalam S, Kung HJ, Bold RJ. Pancreatic cancer cell lines deficient in argininosuccinate synthetase are sensitive to arginine deprivation by arginine deiminase. Int J Cancer. 2008;123:1950–1955. doi: 10.1002/ijc.23723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer RB, Stairs DB, Dugan KD, Notarfrancesco KL, Portocarrero CP, Keister BA, Belka GK, Cho H, Rathmell JC, Thompson CB, et al. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell Metab. 2006;4:475–490. doi: 10.1016/j.cmet.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL, Thiele DJ, et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature. 2014;509:492–496. doi: 10.1038/nature13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand K, Leibold W, Luppa P, Schoerner C, Schulz A. Metabolic alterations associated with proliferation of mitogen-activated lymphocytes and of lymphoblastoid cell lines: evaluation of glucose and glutamine metabolism. Immunobiology. 1986;173:23–34. doi: 10.1016/S0171-2985(86)80086-9. [DOI] [PubMed] [Google Scholar]
- Cai L, Sutter BM, Li B, Tu BP. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol Cell. 2011;42:426–437. doi: 10.1016/j.molcel.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Bucci V, Akkari L, Deforet M, Joyce JA, Xavier JB. Emergence of spatial structure in the tumor microenvironment due to the Warburg effect. Proc Natl Acad Sci U S A. 2013;110:19402–19407. doi: 10.1073/pnas.1311939110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casero RA, Jr, Marton LJ. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov. 2007;6:373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- Cavalli LR, Varella-Garcia M, Liang BC. Diminished tumorigenic phenotype after depletion of mitochondrial DNA. Cell Growth Differ. 1997;8:1189–1198. [PubMed] [Google Scholar]
- Chajes V, Cambot M, Moreau K, Lenoir GM, Joulin V. Acetyl-CoA carboxylase alpha is essential to breast cancer cell survival. Cancer Res. 2006;66:5287–5294. doi: 10.1158/0008-5472.CAN-05-1489. [DOI] [PubMed] [Google Scholar]
- Chambers JW, Maguire TG, Alwine JC. Glutamine metabolism is essential for human cytomegalovirus infection. J Virol. 2010;84:1867–1873. doi: 10.1128/JVI.02123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaneton B, Hillmann P, Zheng L, Martin AC, Maddocks OD, Chokkathukalam A, Coyle JE, Jankevics A, Holding FP, Vousden KH, et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491:458–462. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sprung R, Tang Y, Ball H, Sangras B, Kim SC, Falck JR, Peng J, Gu W, Zhao Y. Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol Cell Proteomics. 2007;6:812–819. doi: 10.1074/mcp.M700021-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Mates JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci U S A. 2011;108:8674–8679. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang EP, Wang YC, Chen WW, Tang FY. Effects of insulin and glucose on cellular metabolic fluxes in homocysteine transsulfuration, remethylation, S-adenosylmethionine synthesis, and global deoxyribonucleic acid methylation. J Clin Endocrinol Metab. 2009;94:1017–1025. doi: 10.1210/jc.2008-2038. [DOI] [PubMed] [Google Scholar]
- Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, Yang XL, Mashimo T, Raisanen JM, Marin-Valencia I, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18:624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SK, Gemin A, Singh G. High activity of mitochondrial glycerophosphate dehydrogenase and glycerophosphate-dependent ROS production in prostate cancer cell lines. Biochem Biophys Res Commun. 2005;333:1139–1145. doi: 10.1016/j.bbrc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008a;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008b;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- Cohen AL, Holmen SL, Colman H. IDH1 and IDH2 mutations in gliomas. Curr Neurol Neurosci Rep. 2013;13:345. doi: 10.1007/s11910-013-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CL, Wasa M, Souba WW, Abcouwer SF. Determinants of glutamine dependence and utilization by normal and tumor-derived breast cell lines. J Cell Physiol. 1998;176:166–178. doi: 10.1002/(SICI)1097-4652(199807)176:1<166::AID-JCP18>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK, Walters H, Tantawy MN, Fu A, Manning HC, et al. Acetate dependence of tumors. Cell. 2014;159:1591–1602. doi: 10.1016/j.cell.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (−) : cystine supplier and beyond. Amino Acids. 2012;42:231–246. doi: 10.1007/s00726-011-0867-5. [DOI] [PubMed] [Google Scholar]
- Constant JS, Feng JJ, Zabel DD, Yuan H, Suh DY, Scheuenstuhl H, Hunt TK, Hussain MZ. Lactate elicits vascular endothelial growth factor from macrophages: a possible alternative to hypoxia. Wound Repair Regen. 2000;8:353–360. doi: 10.1111/j.1524-475x.2000.00353.x. [DOI] [PubMed] [Google Scholar]
- Courtois-Cox S, Jones SL, Cichowski K. Many roads lead to oncogene-induced senescence. Oncogene. 2008;27:2801–2809. doi: 10.1038/sj.onc.1210950. [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Moreno MV, Lodi A, Ronen SM, Ruggero D. Protein and nucleotide biosynthesis are coupled by a single rate-limiting enzyme, PRPS2, to drive cancer. Cell. 2014;157:1088–1103. doi: 10.1016/j.cell.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2010;465:966. doi: 10.1038/nature09132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T, Szlosarek PW. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2010;126:2762–2772. doi: 10.1002/ijc.25202. [DOI] [PubMed] [Google Scholar]
- Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- Deprez J, Vertommen D, Alessi DR, Hue L, Rider MH. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J Biol Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- Donald SP, Sun XY, Hu CA, Yu J, Mei JM, Valle D, Phang JM. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 2001;61:1810–1815. [PubMed] [Google Scholar]
- Du YP, Peng JS, Sun A, Tang ZH, Ling WH, Zhu HL. Assessment of the effect of betaine on p16 and c-myc DNA methylation and mRNA expression in a chemical induced rat liver cancer model. BMC Cancer. 2009;9:261. doi: 10.1186/1471-2407-9-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, Hall MN. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Eagle H. The minimum vitamin requirements of the L and HeLa cells in tissue culture, the production of specific vitamin deficiencies, and their cure. J Exp Med. 1955;102:595–600. doi: 10.1084/jem.102.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardy SR, Farnham PJ. c-Myc mediates activation of the cad promoter via a post-RNA polymerase II recruitment mechanism. J Biol Chem. 2001;276:48562–48571. doi: 10.1074/jbc.M109014200. [DOI] [PubMed] [Google Scholar]
- Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002;62:5443–5450. [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- Flavin R, Peluso S, Nguyen PL, Loda M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6:551–562. doi: 10.2217/fon.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenelle LJ, Henderson JF. Sources of nitrogen as rate-limiting factors for purine biosynthesis de novo in Ehrlich ascites tumor cells. Biochim Biophys Acta. 1969;177:88–93. doi: 10.1016/0304-4165(69)90067-1. [DOI] [PubMed] [Google Scholar]
- Freed-Pastor WA, Mizuno H, Zhao X, Langerod A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li W, Polotskaia A, et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148:244–258. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio D, Soldati C, Vanoni M, Alberghina L, Chiaradonna F. Glutamine deprivation induces abortive s-phase rescued by deoxyribonucleotides in k-ras transformed fibroblasts. PLoS One. 2009;4:e4715. doi: 10.1371/journal.pone.0004715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS. Cancer and the microbiota. Science. 2015;348:80–86. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner EW, Meyskens FL., Jr Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39:453–463. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- Gottfried E, Kunz-Schughart LA, Ebner S, Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A, Kreutz M. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107:2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassian AR, Coloff JL, Brugge JS. Extracellular matrix regulation of metabolism and implications for tumorigenesis. Cold Spring Harb Symp Quant Biol. 2011;76:313–324. doi: 10.1101/sqb.2011.76.010967. [DOI] [PubMed] [Google Scholar]
- Graves LM, Guy HI, Kozlowski P, Huang M, Lazarowski E, Pope RM, Collins MA, Dahlstrand EN, Earp HS, 3rd, Evans DR. Regulation of carbamoyl phosphate synthetase by MAP kinase. Nature. 2000;403:328–332. doi: 10.1038/35002111. [DOI] [PubMed] [Google Scholar]
- Guo JY, Karsli-Uzunbas G, Mathew R, Aisner SC, Kamphorst JJ, Strohecker AM, Chen G, Price S, Lu W, Teng X, et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 2013;27:1447–1461. doi: 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. 2009;35:32–46. doi: 10.1016/j.ctrv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hill JM, Roberts J, Loeb E, Khan A, MacLellan A, Hill RW. L-asparaginase therapy for leukemia and other malignant neoplasms. Remission in human leukemia. JAMA. 1967;202:882–888. [PubMed] [Google Scholar]
- Hino S, Sakamoto A, Nagaoka K, Anan K, Wang Y, Mimasu S, Umehara T, Yokoyama S, Kosai K, Nakao M. FAD-dependent lysine-specific demethylase-1 regulates cellular energy expenditure. Nat Commun. 2012;3:758. doi: 10.1038/ncomms1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M, Sasaki M, Cairns RA, Inoue S, Puviindran V, Li WY, Snow BE, Jones LD, Wei Q, Sato S, et al. Mutant IDH is sufficient to initiate enchondromatosis in mice. Proc Natl Acad Sci U S A. 2015;112:2829–2834. doi: 10.1073/pnas.1424400112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Dematteo RG, Venneti S, Finley LW, Lu C, Judkins AR, Rustenburg AS, Grinaway PB, Chodera JD, Cross JR, et al. Hypoxia Induces Production of L-2-Hydroxyglutarate. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V, Svindland A, Schlomm T, Mills IG. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res. 2013;73:5277–5287. doi: 10.1158/0008-5472.CAN-13-0549. [DOI] [PubMed] [Google Scholar]
- Janeway KA, Kim SY, Lodish M, Nose V, Rustin P, Gaal J, Dahia PL, Liegl B, Ball ER, Raygada M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JL, Kim YC, Russell RC, Yu FX, Park HW, Plouffe SW, Tagliabracci VS, Guan KL. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347:194–198. doi: 10.1126/science.1259472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, Yang X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol. 2011;13:310–316. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, Thompson CB, Rabinowitz JD. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A. 2013;110:8882–8887. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75:544–553. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, van der Burg SH, Verdegaal EM, Cascante M, Shlomi T, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- Katada S, Imhof A, Sassone-Corsi P. Connecting threads: epigenetics and metabolism. Cell. 2012;148:24–28. doi: 10.1016/j.cell.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Keller KE, Tan IS, Lee YS. SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions. Science. 2012;338:1069–1072. doi: 10.1126/science.1224409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- Killian JK, Kim SY, Miettinen M, Smith C, Merino M, Tsokos M, Quezado M, Smith WI, Jr, Jahromi MS, Xekouki P, et al. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3:648–657. doi: 10.1158/2159-8290.CD-13-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kollareddy M, Dimitrova E, Vallabhaneni KC, Chan A, Le T, Chauhan KM, Carrero ZI, Ramakrishnan G, Watabe K, Haupt Y, et al. Regulation of nucleotide metabolism by mutant p53 contributes to its gain-of-function activities. Nat Commun. 2015;6:7389. doi: 10.1038/ncomms8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajcovic M, Krishna S, Akkari L, Joyce JA, Overholtzer M. mTOR regulates phagosome and entotic vacuole fission. Mol Biol Cell. 2013;24:3736–3745. doi: 10.1091/mbc.E13-07-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause BJ, Souvatzoglou M, Treiber U. Imaging of prostate cancer with PET/CT and radioactively labeled choline derivates. Urol Oncol. 2013;31:427–435. doi: 10.1016/j.urolonc.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Kuemmerle NB, Rysman E, Lombardo PS, Flanagan AJ, Lipe BC, Wells WA, Pettus JR, Froehlich HM, Memoli VA, Morganelli PM, et al. Lipoprotein lipase links dietary fat to solid tumor cell proliferation. Mol Cancer Ther. 2011;10:427–436. doi: 10.1158/1535-7163.MCT-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung HN, Marks JR, Chi JT. Glutamine synthetase is a genetic determinant of cell type-specific glutamine independence in breast epithelia. PLoS Genet. 2011;7:e1002229. doi: 10.1371/journal.pgen.1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee SA, DeGrado TR, Talbot JN, Gutman F, Coel MN. Cancer imaging with fluorine-18-labeled choline derivatives. Semin Nucl Med. 2007;37:420–428. doi: 10.1053/j.semnuclmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, de Carvalho FM, Damascena A, Domingos Chinen LT, Rocha RM, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. 1001–1015. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JV, Carrer A, Shah S, Snyder NW, Wei S, Venneti S, Worth AJ, Yuan ZF, Lim HW, Liu S, et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 2014;20:306–319. doi: 10.1016/j.cmet.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Letouze E, Martinelli C, Loriot C, Burnichon N, Abermil N, Ottolenghi C, Janin M, Menara M, Nguyen AT, Benit P, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739–752. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Lheureux S, Lecerf C, Briand M, Louis MH, Dutoit S, Jebahi A, Giffard F, Fournier CB, Batalla A, Poulain L, et al. (18)F-FDG Is a Surrogate Marker of Therapy Response and Tumor Recovery after Drug Withdrawal during Treatment with a Dual PI3K/mTOR Inhibitor in a Preclinical Model of Cisplatin-Resistant Ovarian Cancer. Transl Oncol. 2013;6:586–595. doi: 10.1593/tlo.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Cheng JX. Direct visualization of de novo lipogenesis in single living cells. Sci Rep. 2014;4:6807. doi: 10.1038/srep06807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman BP, Ploessl K, Wang L, Qu W, Zha Z, Wise DR, Chodosh LA, Belka G, Thompson CB, Kung HF. PET imaging of glutaminolysis in tumors by 18F-(2S,4R)4-fluoroglutamine. J Nucl Med. 2011;52:1947–1955. doi: 10.2967/jnumed.111.093815. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Golden JA, Zong WX, Minarcik J, Harris MH, Thompson CB. The proapoptotic activities of Bax and Bak limit the size of the neural stem cell pool. J Neurosci. 2003;23:11112–11119. doi: 10.1523/JNEUROSCI.23-35-11112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW, Phang JM. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci U S A. 2012;109:8983–8988. doi: 10.1073/pnas.1203244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Borchert GL, Donald SP, Diwan BA, Anver M, Phang JM. Proline oxidase functions as a mitochondrial tumor suppressor in human cancers. Cancer Res. 2009;69:6414–6422. doi: 10.1158/0008-5472.CAN-09-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Li F, Handler J, Huang CR, Xiang Y, Neretti N, Sedivy JM, Zeller KI, Dang CV. Global regulation of nucleotide biosynthetic genes by c-Myc. PLoS One. 2008;3:e2722. doi: 10.1371/journal.pone.0002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M, Wang YZ, Gout PW. The x(c)- cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losman JA, Looper RE, Koivunen P, Lee S, Schneider RK, McMahon C, Cowley GS, Root DE, Ebert BL, Kaelin WG., Jr (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science. 2013;339:1621–1625. doi: 10.1126/science.1231677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Venneti S, Akalin A, Fang F, Ward PS, Dematteo RG, Intlekofer AM, Chen C, Ye J, Hameed M, et al. Induction of sarcomas by mutant IDH2. Genes Dev. 2013;27:1986–1998. doi: 10.1101/gad.226753.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- Ma WW, Jacene H, Song D, Vilardell F, Messersmith WA, Laheru D, Wahl R, Endres C, Jimeno A, Pomper MG, et al. [18F]fluorodeoxyglucose positron emission tomography correlates with Akt pathway activity but is not predictive of clinical outcome during mTOR inhibitor therapy. J Clin Oncol. 2009;27:2697–2704. doi: 10.1200/JCO.2008.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MJ, Warner TF, Mertz RJ. High activity of mitochondrial glycerol phosphate dehydrogenase in insulinomas and carcinoid and other tumors of the amine precursor uptake decarboxylation system. Cancer Res. 1990;50:7203–7205. [PubMed] [Google Scholar]
- MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, Watson DG, Gottlieb E. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–3289. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks OD, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E, Vousden KH. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–546. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher EA, Marin-Valencia I, Bachoo RM, Mashimo T, Raisanen J, Hatanpaa KJ, Jindal A, Jeffrey FM, Choi C, Madden C, et al. Metabolism of [U-13 C]glucose in human brain tumors in vivo. NMR Biomed. 2012;25:1234–1244. doi: 10.1002/nbm.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannava S, Grachtchouk V, Wheeler LJ, Im M, Zhuang D, Slavina EG, Mathews CK, Shewach DS, Nikiforov MA. Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells. Cell Cycle. 2008;7:2392–2400. doi: 10.4161/cc.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z, et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez J, Sanchez-Jimenez F, Medina MA, Quesada AR, Nunez de Castro I. Nitrogen metabolism in tumor bearing mice. Arch Biochem Biophys. 1989;268:667–675. doi: 10.1016/0003-9861(89)90335-4. [DOI] [PubMed] [Google Scholar]
- Martinez-Zaguilan R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996;14:176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagandla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–1614. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine JA, Lu HT, Wu KC, Knowles SK, Thomson JA. Down-regulation of argininosuccinate synthetase is associated with cisplatin resistance in hepatocellular carcinoma cell lines: implications for PEGylated arginine deiminase combination therapy. BMC Cancer. 2014;14:621. doi: 10.1186/1471-2407-14-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehedint MG, Niculescu MD, Craciunescu CN, Zeisel SH. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 2010;24:184–195. doi: 10.1096/fj.09-140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M, Ushijima M, Mashima T, Seimiya H, Satoh Y, et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008;68:8547–8554. doi: 10.1158/0008-5472.CAN-08-1235. [DOI] [PubMed] [Google Scholar]
- Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res. 1997;3:2115–2120. [PubMed] [Google Scholar]
- Minor EA, Court BL, Young JI, Wang G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem. 2013;288:13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais R, Zinkewich-Peotti K, Parent M, Wang H, Babai F, Zollinger M. Tumor-forming ability in athymic nude mice of human cell lines devoid of mitochondrial DNA. Cancer Res. 1994;54:3889–3896. [PubMed] [Google Scholar]
- Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Nishiyama T, Shirotani T, Shinohara Y, Kan M, Ishii K, Kanai F, Nakazuru S, Ebina Y. Identification of two enhancer elements in the gene encoding the type 1 glucose transporter from the mouse which are responsive to serum, growth factor, and oncogenes. J Biol Chem. 1992;267:9300–9306. [PubMed] [Google Scholar]
- Nicholson LJ, Smith PR, Hiller L, Szlosarek PW, Kimberley C, Sehouli J, Koensgen D, Mustea A, Schmid P, Crook T. Epigenetic silencing of argininosuccinate synthetase confers resistance to platinum-induced cell death but collateral sensitivity to arginine auxotrophy in ovarian cancer. Int J Cancer. 2009;125:1454–1463. doi: 10.1002/ijc.24546. [DOI] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006;20:43–49. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C, Huang J, Asplund A, Mootha VK. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun. 2014;5:3128. doi: 10.1038/ncomms4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noch E, Khalili K. Oncogenic viruses and tumor glucose metabolism: like kids in a candy store. Mol Cancer Ther. 2012;11:14–23. doi: 10.1158/1535-7163.MCT-11-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Inoue H, Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. 1986;261:13807–13812. [PubMed] [Google Scholar]
- Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140:49–61. doi: 10.1016/j.cell.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham WM, Clish CB, Yang Y, Loscalzo J. Hypoxia-Mediated Increases in l-2-hydroxyglutarate Coordinate the Metabolic Response to Reductive Stress. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- Osada T, Nagashima I, Tsuno NH, Kitayama J, Nagawa H. Prognostic significance of glutamine synthetase expression in unifocal advanced hepatocellular carcinoma. J Hepatol. 2000;33:247–253. doi: 10.1016/s0168-8278(00)80365-7. [DOI] [PubMed] [Google Scholar]
- Palm W, Park Y, Wright K, Pavlova NN, Tuveson DA, Thompson CB. The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell. 2015;162:259–270. doi: 10.1016/j.cell.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Kronke J, Bullinger L, Spath D, Kayser S, Zucknick M, Gotze K, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28:3636–3643. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- Pate KT, Stringari C, Sprowl-Tanio S, Wang K, TeSlaa T, Hoverter NP, McQuade MM, Garner C, Digman MA, Teitell MA, et al. Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 2014;33:1454–1473. doi: 10.15252/embj.201488598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang JM, Donald SP, Pandhare J, Liu Y. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids. 2008;35:681–690. doi: 10.1007/s00726-008-0063-4. [DOI] [PubMed] [Google Scholar]
- Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, Goekbuget N, Schrappe M, Pui CH. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117:238–249. doi: 10.1002/cncr.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23:7315–7328. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Vander Heiden MG, Harris MH, Frauwirth KA, Thompson CB. In the absence of extrinsic signals, nutrient utilization by lymphocytes is insufficient to maintain either cell size or viability. Mol Cell. 2000;6:683–692. doi: 10.1016/s1097-2765(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Lane AN, Robertson B, Kemp S, Liu Y, Hill BG, Dean DC, Clem BF. Control of glutamine metabolism by the tumor suppressor Rb. Oncogene. 2014;33:556–566. doi: 10.1038/onc.2012.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman EL, Kenfield SA, Stampfer MJ, Giovannucci EL, Zeisel SH, Willett WC, Chan JM. Choline intake and risk of lethal prostate cancer: incidence and survival. Am J Clin Nutr. 2012;96:855–863. doi: 10.3945/ajcn.112.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera S, Azcon-Bieto J, Lopez-Soriano FJ, Miralpeix M, Argiles JM. Amino acid metabolism in tumour-bearing mice. Biochem J. 1988;249:443–449. doi: 10.1042/bj2490443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E, Frankel S. Free amino acids in normal and neoplastic tissues of mice as studied by paper chromatography. Cancer Res. 1949;9:645–648. 643 pl. [PubMed] [Google Scholar]
- Robitaille AM, Christen S, Shimobayashi M, Cornu M, Fava LL, Moes S, Prescianotto-Baschong C, Sauer U, Jenoe P, Hall MN. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science. 2013;339:1320–1323. doi: 10.1126/science.1228771. [DOI] [PubMed] [Google Scholar]
- Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg JM, Bailey KM, Wojtkowiak JW, Ben-Nun Y, Bogyo M, Weber E, Moin K, Blum G, Mattingly RR, Gillies RJ, et al. Acid-mediated tumor proteolysis: contribution of cysteine cathepsins. Neoplasia. 2013;15:1125–1137. doi: 10.1593/neo.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniels VW, Machiels J, et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- Sabari BR, Tang Z, Huang H, Yong-Gonzalez V, Molina H, Kong HE, Dai L, Shimada M, Cross JR, Zhao Y, et al. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol Cell. 2015;58:203–215. doi: 10.1016/j.molcel.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brustle A, Harris IS, Holmes R, Wakeham A, Haight J, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488:656–659. doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer LA, Stayman JW, 3rd, Dauchy RT. Amino acid, glucose, and lactic acid utilization in vivo by rat tumors. Cancer Res. 1982;42:4090–4097. [PubMed] [Google Scholar]
- Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SA, Gaumann A, Wondrak M, Eckermann C, Schulte S, Mueller-Klieser W, Wheatley DN, Kunz-Schughart LA. Lactate adversely affects the in vitro formation of endothelial cell tubular structures through the action of TGF-beta1. Exp Cell Res. 2007;313:2531–2549. doi: 10.1016/j.yexcr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Schug ZT, Peck B, Jones DT, Zhang Q, Grosskurth S, Alam IS, Goodwin LM, Smethurst E, Mason S, Blyth K, et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell. 2015;27:57–71. doi: 10.1016/j.ccell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008;7:104–112. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Sellers K, Fox MP, Bousamra M, 2nd, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest. 2015;125:687–698. doi: 10.1172/JCI72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Tu BP. Acetyl-CoA induces transcription of the key G1 cyclin CLN3 to promote entry into the cell division cycle in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2013;110:7318–7323. doi: 10.1073/pnas.1302490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Shim EH, Livi CB, Rakheja D, Tan J, Benson D, Parekh V, Kho EY, Ghosh AP, Kirkman R, Velu S, et al. L-2-Hydroxyglutarate: an epigenetic modifier and putative oncometabolite in renal cancer. Cancer Discov. 2014;4:1290–1298. doi: 10.1158/2159-8290.CD-13-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyh-Chang N, Locasale JW, Lyssiotis CA, Zheng Y, Teo RY, Ratanasirintrawoot S, Zhang J, Onder T, Unternaehrer JJ, Zhu H, et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science. 2013;339:222–226. doi: 10.1126/science.1226603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar K, Huang H, Hu L, Cogdell D, Dhillon J, Tzelepi V, Efstathiou E, Koumakpayi IH, Saad F, Luo D, et al. Integrative molecular profiling reveals asparagine synthetase is a target in castration-resistant prostate cancer. Am J Pathol. 2012;180:895–903. doi: 10.1016/j.ajpath.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Som P, Atkins HL, Bandoypadhyay D, Fowler JS, MacGregor RR, Matsui K, Oster ZH, Sacker DF, Shiue CY, Turner H, et al. A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med. 1980;21:670–675. [PubMed] [Google Scholar]
- Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonveaux P, Copetti T, De Saedeleer CJ, Vegran F, Verrax J, Kennedy KM, Moon EJ, Dhup S, Danhier P, Frerart F, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One. 2012;7:e33418. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R, Shuster S, Neudecker BA, Formby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res. 2002;276:24–31. doi: 10.1006/excr.2002.5508. [DOI] [PubMed] [Google Scholar]
- Stolzing A, Grune T. Neuronal apoptotic bodies: phagocytosis and degradation by primary microglial cells. FASEB J. 2004;18:743–745. doi: 10.1096/fj.03-0374fje. [DOI] [PubMed] [Google Scholar]
- Strohecker AM, Guo JY, Karsli-Uzunbas G, Price SM, Chen GJ, Mathew R, McMahon M, White E. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discov. 2013;3:1272–1285. doi: 10.1158/2159-8290.CD-13-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell. 2015;162:552–563. doi: 10.1016/j.cell.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Luo T, Ren Y, Florey O, Shirasawa S, Sasazuki T, Robinson DN, Overholtzer M. Competition between human cells by entosis. Cell Res. 2014;24:1299–1310. doi: 10.1038/cr.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svastova E, Hulikova A, Rafajova M, Zat’ovicova M, Gibadulinova A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004;577:439–445. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26:299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J, Bajzikova M, Kovarova J, Peterka M, Yan B, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015;21:81–94. doi: 10.1016/j.cmet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Terunuma A, Putluri N, Mishra P, Mathe EA, Dorsey TH, Yi M, Wallace TA, Issaq HJ, Zhou M, Killian JK, et al. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J Clin Invest. 2014;124:398–412. doi: 10.1172/JCI71180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CB. Rethinking the regulation of cellular metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:23–29. doi: 10.1101/sqb.2012.76.010496. [DOI] [PubMed] [Google Scholar]
- Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- Towbin BD, Gonzalez-Aguilera C, Sack R, Gaidatzis D, Kalck V, Meister P, Askjaer P, Gasser SM. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell. 2012;150:934–947. doi: 10.1016/j.cell.2012.06.051. [DOI] [PubMed] [Google Scholar]
- Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchelli E, Aranda F, Eggermont A, Sautes-Fridman C, Tartour E, Kennedy EP, Platten M, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2014;3:e957994. doi: 10.4161/21624011.2014.957994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- Venneti S, Dunphy MP, Zhang H, Pitter KL, Zanzonico P, Campos C, Carlin SD, La Rocca G, Lyashchenko S, Ploessl K, et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med. 2015;7:274ra217. doi: 10.1126/scitranslmed.aaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sanchez N, Marchesini M, Carugo A, Green T, Seth S, Giuliani V, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Wahlstrom T, Arsenian Henriksson M. Impact of MYC in regulation of tumor cell metabolism. Biochim Biophys Acta. 2015;1849:563–569. doi: 10.1016/j.bbagrm.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Wang C, Guo K, Gao D, Kang X, Jiang K, Li Y, Sun L, Zhang S, Sun C, Liu X, et al. Identification of transaldolase as a novel serum biomarker for hepatocellular carcinoma metastasis using xenografted mouse model and clinic samples. Cancer Lett. 2011a;313:154–166. doi: 10.1016/j.canlet.2011.08.031. [DOI] [PubMed] [Google Scholar]
- Wang F, Travins J, DeLaBarre B, Penard-Lacronique V, Schalm S, Hansen E, Straley K, Kernytsky A, Liu W, Gliser C, et al. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–626. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011b;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Warburg OPK, Negelein E. Über den Stoffwechsel der Carcinomzelle. Biochem Zeitschr. 1924;152:309–344. [Google Scholar]
- Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell. 2010;17:225–234. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW, Rabinowitz JD, Coller HA, Thompson CB. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24:2784–2799. doi: 10.1101/gad.1985910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski JR, Zougman A, Mann M. Nepsilon-formylation of lysine is a widespread post-translational modification of nuclear proteins occurring at residues involved in regulation of chromatin function. Nucleic Acids Res. 2008;36:570–577. doi: 10.1093/nar/gkm1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Si W, Wang M, Lv S, Ji A, Li Y. Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide. 2015;50:38–45. doi: 10.1016/j.niox.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y, et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Dai J, Dai L, Tan M, Cheng Z, Wu Y, Boeke JD, Zhao Y. Lysine succinylation and lysine malonylation in histones. Mol Cell Proteomics. 2012;11:100–107. doi: 10.1074/mcp.M111.015875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Xiao MT, Liu LX, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Gammon MD, Zeisel SH, Bradshaw PT, Wetmur JG, Teitelbaum SL, Neugut AI, Santella RM, Chen J. High intakes of choline and betaine reduce breast cancer mortality in a population-based study. FASEB J. 2009a;23:4022–4028. doi: 10.1096/fj.09-136507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zur Hausen A, Coy JF, Lochelt M. Transketolase-like protein 1 (TKTL1) is required for rapid cell growth and full viability of human tumor cells. Int J Cancer. 2009b;124:1330–1337. doi: 10.1002/ijc.24078. [DOI] [PubMed] [Google Scholar]
- Yan SR, Berton G. Regulation of Src family tyrosine kinase activities in adherent human neutrophils. Evidence that reactive oxygen intermediates produced by adherent neutrophils increase the activity of the p58c-fgr and p53/56lyn tyrosine kinases. J Biol Chem. 1996;271:23464–23471. doi: 10.1074/jbc.271.38.23464. [DOI] [PubMed] [Google Scholar]
- Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima J, Fukasawa Y, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514:291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- Ye J, Fan J, Venneti S, Wan YW, Pawel BR, Zhang J, Finley LW, Lu C, Lindsten T, Cross JR, et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014;4:1406–1417. doi: 10.1158/2159-8290.CD-14-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Mancuso A, Tong X, Ward PS, Fan J, Rabinowitz JD, Thompson CB. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc Natl Acad Sci U S A. 2012;109:6904–6909. doi: 10.1073/pnas.1204176109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying J, Rahbar MH, Hallman DM, Hernandez LM, Spitz MR, Forman MR, Gorlova OY. Associations between dietary intake of choline and betaine and lung cancer risk. PLoS One. 2013;8:e54561. doi: 10.1371/journal.pone.0054561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon CY, Shim YJ, Kim EH, Lee JH, Won NH, Kim JH, Park IS, Yoon DK, Min BH. Renal cell carcinoma does not express argininosuccinate synthetase and is highly sensitive to arginine deprivation via arginine deiminase. Int J Cancer. 2007;120:897–905. doi: 10.1002/ijc.22322. [DOI] [PubMed] [Google Scholar]
- Young RM, Ackerman D, Quinn ZL, Mancuso A, Gruber M, Liu L, Giannoukos DN, Bobrovnikova-Marjon E, Diehl JA, Keith B, et al. Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress. Genes Dev. 2013;27:1115–1131. doi: 10.1101/gad.198630.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Mates JM, Alonso FJ, Wang C, Seo Y, et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15:157–170. doi: 10.1016/j.cmet.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CX, Pan MX, Li B, Wang L, Mo XF, Chen YM, Lin FY, Ho SC. Choline and betaine intake is inversely associated with breast cancer risk: a two-stage case-control study in China. Cancer Sci. 2013;104:250–258. doi: 10.1111/cas.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fan J, Venneti S, Cross JR, Takagi T, Bhinder B, Djaballah H, Kanai M, Cheng EH, Judkins AR, et al. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell. 2014;56:205–218. doi: 10.1016/j.molcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]