Abstract
Regulatory T cells (Tregs) play an important role in the regulation of T cell-mediated immune responses through suppression of T cell proliferation and secretion of inhibitory cytokines such as IL-10 and TGF-β. Impaired Treg numbers and function have been associated with numerous diseases and an imbalance between pro-inflammatory/pro-atherogenic cells and Tregs promotes atherosclerotic disease. Restoration of this balance by inducing Tregs has great therapeutic potential to prevent cardiovascular disease. In addition to suppressing differentiation and function of effector T cells, Tregs have been shown to induce anti-inflammatory macrophages, inhibit foam cell formation and to influence cholesterol metabolism. Furthermore, Tregs suppress immune responses of endothelial cells and innate lymphoid cells. In this review we focus on the recent knowledge on Treg subsets, their activity and function in atherosclerosis and discuss promising strategies to use Tregs as a therapeutic tool to prevent cardiovascular disease.
Introduction
Tregs form an important T cell subclass that provides protection against autoimmunity and may be used for treatment of autoimmune-like disorders such as atherosclerosis.1 Various subsets of Tregs exist, but the best-characterized are CD4+FoxP3+CD25hiCD127lo cells that comprise 5-10% of the CD4+ T cells in human blood, lymphoid tissue, and epithelial barrier tissues.2 A significant fraction of these CD4+ Tregs develops in the thymus and are called natural or thymic Tregs. The transcription factor Helios is reported to be exclusively expressed in thymic Tregs, although this has been disputed.3 Tregs exert their immunosuppressive function mainly through secretion of the inhibitory cytokines IL-10 and TGF-β, and cell-cell contact, mediated by membrane-bound TGF-β, cytotoxic T lymphocyte-associated antigen (CTLA-4) and/or glucocorticoid-induced TNF receptor family related protein (GITR).4, 5 In addition to the natural Tregs, CD4+ Tregs differentiate from naïve CD4+ T cells in secondary lymphoid organs, and are called adaptive or peripheral Tregs, which include CD4+FoxP3+CD25hiCD127lo cells with a similar phenotype to natural Treg, as well as IL-10 producing T regulatory type 1 cells (Tr1), TGF-β producing T helper-3 cells (Th3), and CD8+Foxp3+ Tregs.6-11
The importance of Tregs in modulation of immune responses in atherosclerosis has been demonstrated in several studies in mice where Tregs were partially or entirely depleted. LDLr−/− mice lacking CD28 or CD80/CD86, costimulatory molecules that are essential for Treg development and homeostasis, show decreased Treg numbers associated with an increase in atherosclerosis12, and treatment of ApoE−/− mice with a Treg depleting CD25-specific antibody (PC61) aggravates lesion development.12 The contribution of Foxp3+ Tregs to atherosclerosis development was first elucidated by a partial depletion of Foxp3+ Tregs using a dendritic cell-based vaccination that provoked cytotoxic T cell responses against Foxp3-expressing cells leading to enhanced atherosclerosis.13 Recently, Klingenberg et al. showed that a specific depletion of Foxp3+ Tregs using DEREG/LDLr−/− mice increases atherosclerosis development 2.1-fold.14
The focus of this review will be on the development of experimental therapies to increase the frequency of Tregs to reduce atherosclerosis and on their potency as a new immune-therapy to treat cardiovascular disease.
Frequency and characterization of regulatory T cells in atherosclerosis
Tregs have been found in both mouse and human atherosclerotic lesions15, 16 and most studies show that Treg numbers are reduced in hypercholesterolemic mice and cardiovascular patients compared to healthy controls (Table 1).
Table 1.
Frequencies of Tregs in experimental atherosclerosis and cardiovascular patients.
| Treg phenotype | Frequency in disease | References | |
|---|---|---|---|
| Mice | CD4+CD25+ or CD25+Foxp3+ | ↓ In lymphoid organs ApoE−/− mice compared to C57Bl6 mice or young ApoE−/− mice | Mor 200717 |
| CD4+Foxp3+ | ↑ in spleen of hypercholesterolemia LDLr−/− mice | Maganto-Garcia 201119 | |
| ↓ in circulation and aorta of hypercholesterolemic LDLr−/− mice | |||
| CD4+CD25+Foxp3+ | ↓ in hypercholesterolemic ApoE−/− mice | Wang 201418 |
| Treg phenotype | Frequency in disease | References | |
|---|---|---|---|
| Humans | CD4+CD25high | ↓ numbers and suppressive capacity In ACS patients compared with stable angina patients and healthy individuals, also reduced Foxp3 and CTLA-4 expression | Mor 200621 |
| ↔ no correlation between circulating Tregs and the thickness of the carotid artery | Ammirati 20 1022 | ||
| ↓ in non ST-elevated ACS patients compared to controls | |||
| ↑ in ST-elevated acute MI patients compared to controls | |||
| CD3+Foxp3+ | ↓ low numbers present in all stages of atherosclerotic lesions | De Boer 200715 | |
| CD4+Foxp3+ | ↓ numbers associated with higher release of pro-inflammatory cytokines and increased risk for acute coronary events but not stroke | Wigren 201223 | |
| ↓ in MI patients compared to healthy individuals | Ghourbani Gazar 201224 | ||
| ↓ in vulnerable lesions | Dietel 201320 | ||
| CD4+CD25+Foxp3+ | ↓ in ACS patients (stable angina, unstable angina and acute MI) compared with healthy individuals, associated with expansion of Th1 cells in unstable angina and acute MI patients | Han 200725 | |
| ↓ numbers and serum IL-10 in patients with recurrent cardiac events compared with stable patients | George 20 1 249 | ||
| ↓ numbers and enhanced DNA methylation of the Treg-specific demethylated region in Foxp3 in ACS patients compared with controls | Jia 201326 | ||
| CD4+CD25+CD127low | ↔ no correlation between Treg levels and intima-media thicknes (also no correlation with Foxp3 and IL-10 mRNA, or IL-10 serum levels) | Ammirati 201022 | |
| ↓ in non-ST elevation ACS patients | Zhang 201228 | ||
| ↑ in thrombi | Klingenberg 2014 | ||
| CD4+ICOS+ | ↓ in MI and stable angina patients compared to healthy individuals | Ghourbani Ganor 201224 | |
| CD4+LAP+ | ↓ in MI (ST-elevation and non-ST-elevation patients) compared with stable angina patients and healthy controls | Lin 201333 | |
| ↓ numbers and suppressive capacity in unstable angina and acute MI patients compared with chronic stable angina and chest pain syndrome patients | Zhu 201434 | ||
| CD4+GARP+ | ↓ numbers unstable angina and acute MI patients compared with stable angina and chest pain syndrome patients | Zhu 201434 | |
| CD4+CD25+GARP+ | ↓ numbers, suppressive capacity and TGF-β in ACS patients compared with stable angina and control patients | Meng 201437 |
Low numbers of Tregs in atherosclerosis
ApoE−/− mice have reduced numbers of Tregs, identified as either CD4+CD25+ cells or CD25+Foxp3+ cells, in lymphoid organs compared with C57BL/6 mice and younger ApoE−/− mice that have no evident atherosclerotic lesions.17 When fed a high fat diet, ApoE−/− mice showed a reduction in CD4+CD25+Foxp3+ cells compared with mice fed a regular diet.18 In LDLr−/− mice, circulating and lesional CD4+Foxp3+ Tregs peak 4 weeks after initiation of a high-fat diet but these numbers subsequently decline, resulting in an accumulation of effector T cells that contribute to disease progression.19 Similarly, low levels of circulating human Tregs are associated with an increased risk to develop acute coronary syndrome (ACS) (Table 1) and decreased lesional Tregs are associated with increased lesion vulnerability.20 Interpretation of these studies may be complicated because the term ‘ACS’ often comprises different patient groups, including those suffering from unstable angina, non ST-elevation myocardial infarction (MI) and ST-elevation MI. Moreover, control groups vary in different studies, including either healthy individuals with angiographically confirmed normal coronary arteries or stable angina patients and chest pain syndrome patients. Overall, in most of these studies, patients with unstable angina and non ST-elevation MI show reduced peripheral Tregs in the blood compared with healthy individuals or stable angina patients.
Originally, Tregs were characterized as CD4+CD25high cells that express Foxp3 to maintain their suppressive capacity and as shown in Table 1 numerous studies validate their association with the development of ACS.21-26 Foxp3 is however also transiently upregulated in activated effector human T cells.27 Therefore, additional markers for the characterization of Tregs are required for accurate identification, including CD127, ICOS (inducible T cell costimulator), LAP (latency-associated peptide), and GARP (glycoprotein A repetitions predominant). CD127 (IL-7 receptor) is down-regulated on Tregs and inversely correlated with Foxp3 expression. Although Ammirati et al. found no correlation between circulating CD4+CD25+CD127low cells and intima-media thickness of the common carotid artery22, Zhang et al. found less CD4+CD25+CD127low cells in patients suffering from non-ST elevated ACS.28 ICOS is involved in IL-10-mediated effector T cell suppression and in mice ICOS deficiency accelerates atherosclerosis via decreased numbers and suppressive function of Foxp3+ Tregs.29 Importantly, ICOS+ Treg levels were decreased in MI and stable angina patients compared to healthy individuals.24 LAP forms a complex with TGF-β, maintaining its latency/inactive state and exerts regulatory activity independent of Foxp3 by releasing mature TGF-β. CD4+LAP+ cells and TGF-β levels were elevated in mice treated with thymic stromal lymphopoietin (TSLP)/TSLP-loaded DCs30, oral FTY72031 or nasal oxLDL32, leading to reduced atherosclerosis. Recently, reduced circulating CD4+LAP+ Tregs have been found in ACS patients compared with stable angina and control patients.33, 34 Moreover, CD4+LAP+ Tregs from ACS patients show a defect in their suppressive capacity compared with those from control groups.34 GARP, associates with latent TGF-β/LAP on Tregs and regulates the bioavailability and activation of TGF-β.35 In humans GARP is only expressed on activated Tregs, while in mice GARP has also been found on some resting Tregs.36 Reduced circulating CD4+GARP+ Tregs and CD4+CD25+GARP+ Tregs are seen in ACS patients compared with those with stable angina/chest pressure syndrome and healthy individuals.34, 37 In addition, CD4+CD25+GARP+ Tregs isolated from ACS patients showed a reduced ability to suppress effector T cells.
Possible mechanisms underlying the low frequency of Tregs in atherosclerosis
Several mechanisms underlying the inverse correlation between Tregs and atherosclerosis progression have been explored. Possibly, survival of Tregs is impaired since Zhang et al. observed increased apoptosis in Tregs of non-ST elevated ACS patients compared with Tregs of chronic stable angina/chest pain syndrome patients.28 Tregs from non-ST elevated ACS patients contain lower mRNA levels of the anti-apoptotic gene Bcl-2 and higher levels of the pro-apoptotic gene Bak. Moreover, they showed that oxLDL induced apoptosis of Tregs and in light of elevated oxLDL levels in non-ST elevation ACS patients, which may suggest that oxLDL is involved in the Treg defect in cardiovascular disease patients. Previously, Mor et al. already observed that oxLDL can reduce numbers of CD4+CD25+ Tregs in vitro, partially because of apoptosis induction.21 It was also found that oxLDL dose-dependently increased methylation of the Treg-specific demethylated region within the Foxp3 gene, thereby reducing Foxp3 expression in PBMCs isolated from healthy individuals.26 Most interestingly, reduced Treg levels defined as demethylation at the Foxp3 demethylated region are observed in ACS patients and this was associated with the severity of ACS. Another possibility that might explain reduced Treg numbers in cardiovascular patients was proposed by Zhang et al. who found that non-ST elevation ACS patients have impaired thymic Treg output determined by lower circulating CD45RO−CD45RA+CD31+ Tregs compared with chronic stable angina/chest pain syndrome patients.28
Function of regulatory T cells in atherosclerosis
In addition to lower numbers of Tregs in the circulation and atherosclerotic lesions, multiple studies report a dysfunction in their suppressive capacity during disease. Tregs from ApoE−/− mice show hampered inhibition of effector T cells compared with Tregs isolated from C57BL/6 mice17 and the suppressive function of human Tregs isolated from peripheral blood of patients with ACS is strongly decreased as compared with patients with stable angina and normal coronary artery subjects.21, 34, 37 These studies strongly suggest that patients suffering from cardiovascular disease would benefit from increased numbers of athero-protective Tregs. Most research is therefore focused on expanding the potency of Tregs as a new immune-therapy to inhibit pro-inflammatory immune responses in atherosclerosis (see Figure 1).
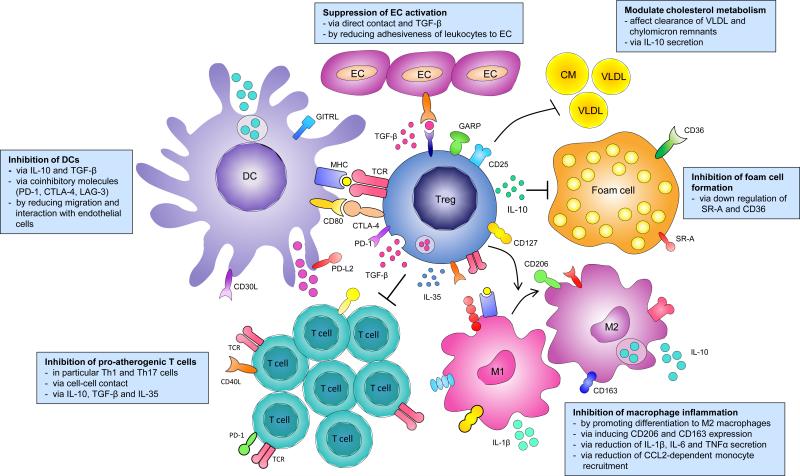
Figure 1.
Regulatory T cells; mechanism of action in atherosclerosis. Regulatory T cells can modulate several processes involved in the development of atherosclerosis. Tregs can inhibit pro-atherogenic T cells, dendritic cell (DC) activation and migration, macrophage inflammation, foam cell formation, endothelial cell (EC) activation and can affect cholesterol metabolism.
Inhibition of effector T cells
Tregs show the ability to suppress pro-atherogenic effector T cells in atherosclerosis. The majority of the pathogenic CD4+ T cells in atherosclerosis are effector Th1 cells which via secretion of IFN-γ stimulate the recruitment of monocytes and T cells into the plaque, increase lipid uptake by macrophages and activate lesional APCs.38, 39 Correspondingly, deficiency in T-bet40 or IFN-γ41 attenuates atherosclerosis. Multiple studies in mice have shown that inducing Tregs in atherosclerosis affects Th1 cells31, 42 and in cardiovascular patients an inverse correlation between Th1 cells and Tregs exists. Whereas circulating Th1 cells are expanded in patients with stable angina, unstable angina and acute MI compared with healthy individuals, Tregs are reduced.25 IL-17 producing Th17 cells form another pro-inflammatory subset of effector CD4+ T cells43, 44, although mouse studies have shown both Th17 cells or IL-17 may have either pro- or anti-atherogenic effects. Nonetheless, a significant negative correlation between Th17 and Treg cell frequencies has been found in the circulation of patients with unstable carotid artery lesions.45 This imbalance between Th17 cells and Tregs has been confirmed46, suggesting one of the mechanisms through which Tregs regulate atherosclerosis is the inhibition of Th17 cells. Interestingly, the ability of Treg to suppress Th1 or Th17 responses may require differential stimulation of the Treg by IFNγ and IL-17 vs. IL-10, respectively.47
The inhibitory cytokines IL-10 and TGF-β strongly contribute to Treg-mediated suppression of effector T cells in atherosclerosis. In mice, IL-10-producing Tr1 cells reduce immune responses in ApoE−/− mice resulting in a decreased plaque size and inflammation as shown by lower levels of IFN-γ.48 In addition, Tregs and serum IL-10 are decreased in vulnerable patients that have had recurrent cardiac events in comparison with stable patients.49 Deficiencies in total or T cell specific TGF-β signaling accelerate atherosclerosis and induce an unstable plaque phenotype in hypercholesterolemic mice.50, 51 A relatively new cytokine associated with Treg-mediated effector T cell suppression is IL-35.52 IL-35 consists of Epstein-Barr virus-induced gene 3 (EBI3) and IL-12 p35 (IL-12A) and both of these subunits are strongly coexpressed in human atherosclerotic lesions.53 Recently it was shown that serum IL-35 is decreased in patients with acute MI, unstable angina and stable angina compared with patients with chest pain syndrome54, suggesting Treg-associated IL-35 can be a novel target to prevent atherosclerosis.
Inhibition of DCs
Dendritic cells (DCs) are major contributors to the pathogenesis of atherosclerosis, in part because of their essential role in activating T cell activation. An inverse correlation between mature DCs expressing fascin and Tregs exists in atherosclerotic lesions; in vulnerable lesions fascin-expressing DCs are increased whereas Tregs are decreased compared to patients with stable lesions.20 Tregs can inhibit DCs via their immunosuppressive cytokines, IL-10 and TGF-β, but also via cell surface molecules such as CTLA-4, programmed death-1 and their ligands PD-L1/2, and lymphocyte activation gene-3 (LAG-3). CTLA-4 expressed on Tregs binds to CD80/CD86 on DCs thereby blocking the ability of DC to activate naïve T cells. Increased mRNA levels of CTLA-4 have been associated with increased Tregs and reduced atherosclerosis in several studies.55-57 Moreover, treatment with a recombinant CTLA-4 Ig fusion protein, abatacept, reduced intimal thickening in a femoral artery cuff model in ApoE3*Leiden mice through reduced activation of IFN-γ producing Th1 cells and elevated IL-10 producing Tregs.58 Signaling via coinhibitory PD-1 expressed on Tregs and PD-L1/2 on DCs also inhibits their activation. Mice deficient in either PD-1 or PD-L1/2 showed aggravated atherosclerosis, mediated by increased effector T cell responses.59, 60 LAG-3 is upregulated on activated Tregs and can bind with higher affinity than CD4 to MHC-II on the DC,61 which suppresses maturation and the immune-stimulatory capacity of DCs62, however, its importance in atherosclerosis remains to be determined.
Inhibition of macrophage inflammation and foam cell formation
Tregs can also exert atheroprotective effects by promoting the differentiation of mouse M1 macrophages towards an anti-inflammatory M2 macrophage.63 Co-culture of Tregs with monocytes from healthy individuals induced an M2 phenotype as illustrated by the surface expression of CD206 (mannose receptor), CD163 (haemoglobin scavenger receptor), elevated CCL18 production and phagocytic activity.64 Moreover, in response to LPS these Treg-treated monocytes strongly reduced secretion of pro-inflammatory cytokines.
The transition of macrophages into foam cells is a hallmark of atherosclerosis. It has been shown that Tregs can impede this process by inhibiting lipid accumulation in peritoneal macrophages via the down-regulation of scavenger receptor class A (SR-A) and CD36, but Tregs do not affect reverse cholesterol transport.63 In addition, lesional Tregs may suppress MCP-1 expression and monocyte recruitment into plaques, thereby reducing foam cell macrophage accumulation.65
Enhancing lesion stability
Since M2 macrophages promote collagen synthesis Tregs contribute to lesion stability by inducing M2 macrophages. In line with this finding, expansion of Tregs enhanced lesion stabilization in a regression model of atherosclerosis.42 Additionally, Tregs dose-dependently increase lesion stability, as measured by decreased macrophage and lipid content and increased smooth muscle cell and collagen content, and lower the incidence of lesion disruption in ApoE−/− mice.66 Moreover, Tregs inhibited expression of inflammatory cytokines and the matrix metalloproteinases MMP-2 and MMP-9, and enhanced P4Hα1 expression in atherosclerotic lesions.66
Treg effects on cholesterol metabolism
Overexpression of IL-10, a hallmark cytokine of Tregs, reduces VLDL en LDL levels in serum of LDLr−/− mice.67 Recent studies have revealed a direct role for Tregs in cholesterol metabolism since depletion of Tregs using DEREG mice significantly increases atherosclerosis associated with a 1.7-fold increase in plasma cholesterol levels.14 More specifically, VLDL levels were increased because the clearance of VLDL and chylomicron remnants was inhibited in the absence of Tregs. They found reduced expression of sortilin-1 in the liver and increased plasma enzyme activity of lipoprotein lipase, hepatic lipase and phospholipid transfer protein in Treg-depleted mice. In addition, Treg expansion in a regression model of atherosclerosis significantly reduced cholesterol levels compared with control mice.42
Suppression of endothelial activation
Interestingly, adaptive Foxp3+ Tregs have also been shown to suppress TNFα and IL-1β-mediated endothelial selectin expression and subsequently reduced leukocyte adhesiveness.68 In vivo, these adaptive Tregs inhibit acute inflammation in a peritonitis model via adherence to inflamed endothelium and secretion of inhibitory cytokines.
Treg-cell based therapy
The usage of Tregs as a therapeutic agent shows great potential in the treatment of atherosclerosis. An early study showed that an adoptive transfer of CD4+CD25+ T cells in mice causes a reduction in atherosclerotic lesion development12 and research is nowadays focused on the development of Foxp3+ T cells, either ex vivo or via expansion in vivo.
Adoptive transfer of Tregs
A possible strategy to use Tregs for therapy is an adoptive transfer of ex vivo expanded Tregs. This procedure will require substantial numbers of Tregs, which can be achieved by in vivo isolation of naive or Foxp3+CD4+ T cells from peripheral blood and subsequent ex vivo Treg induction and/or expansion to obtain large numbers for therapy using appropriate cytokine mixtures.69, 70 Trials on adoptive Treg therapy for GVHD, transplantation, and autoimmunity have used varying numbers of Tregs, ranging from 5×106 to 2.6×109, injected once or twice.71-74 Treg based therapy has proven to be effective and no significant adverse effects have been reported. Most studies used polyclonal autologous Treg preparations, although alloantigen-stimulated preparations have been used for allograft tolerance. There are insufficient data to assess how long the effects of transferred Tregs last. More data from these ongoing trials will be needed to inform the design of trials for atherosclerotic disease.
Induction of Tregs in vivo
Alternatively, Tregs can be expanded in vivo. This can be achieved by targeting Treg via administration of an IL-2/anti-IL-2 immune complex. Administration of IL-2/anti-IL-2 complex to Western-type diet fed LDLr−/− mice significantly expanded IL-10 producing Tregs up to 10-fold in the circulation and several (lymphoid) organs. This expansion of Tregs potently suppressed effector T cells and reduced initial atherosclerotic lesion formation, whereas, in combination with a vigorous lowering of blood lipid levels, it enhanced lesion stability in LDLr−/− mice with pre-existing lesions.42 Future research should reveal whether administration of this IL-2 complex would also be beneficial in patients with cardiovascular disease. Another frequently used method to induce Tregs is administration of antigens via a tolerogenic route. Administration of atherosclerosis relevant antigens such as oxLDL56, HSP6075, β2-glycoprotein I76 and ApoB100 peptide77, 78 via oral, nasal and subcutaneous routes has been shown to suppress atherosclerosis in mice by increasing antigen specific Tregs through the induction of tolerogenic DCs. Recent approaches also combine several peptide antigens derived from ApoB100 and HSP60 to induce a variety of antigen-specific Tregs.79 The induction of regulatory T cells may be further improved by combining a relevant antigen with the cholera toxin B80, as shown by the atheroprotective effect of a construct consisting of an apoB100 peptide combined with CTB.81 One may speculate that these approaches will in the near future form the basis for First-In-Humans clinical trials.
Induction of tolerogenic DCs
A final approach to enhance the function and induction of Tregs may be to enhance the tolerogenic function of DCs. Oral and nasal routes of administration of antigens rely on the interaction of these antigens with tolerogenic DCs and subsequent induction of Tregs, This approach can be mimicked in vitro by incubating DCs with atherosclerosis related antigens in the presence of IL-10 to induce a tolerogenic DC phenotype. Subsequent adoptive transfer of these antigen loaded DCs induces an atheroprotective affect via the induction of Tregs.82 Various other methods for inducing a tolerogenic DC phenotype have been described and are being applied to atherosclerosis studies in mice.83
Polyclonal Tregs vs. antigen-specific Tregs
For potential Treg therapies based on adoptive transfers of Tregs three kinds of Tregs can be used; general Tregs expanded ex vivo, antigen-specific Tregs expanded ex vivo, or induced Tregs differentiated from naïve CD4+ T cells in the presence of IL-2, αCD3/CD28, TGF-β both with or without retinoic acid (possibly antigen-specific). The obvious advantage of induced Tregs is that a large number of cells can be produced with relative ease. However, maintaining stability of induced Tregs is a major complicating factor and Blazar argued that induced Tregs have disappeared 14 days after infusion, which limits long-term effects. Non-specific Tregs can be isolated from peripheral blood of patients and ex vivo expanded for maximally three rounds before they also lose their phenotype. The use of antigen-specific Tregs could be extremely effective in treating atherosclerosis. In addition to studies using oral or nasal administration of HSP60, Yang et al. showed that adoptive transfer of HSP60-specific CD4+CD25high cells via in vitro induction through HSP60-loaded DCs inhibits atherosclerosis formation.84 Although several candidates of atherosclerosis specific antigens such as oxLDL, HSP60 and ApoB100, have been investigated, to date the dominant relevant antigens that are recognized by pro-atherogenic T cells are not known. This complicates the approach of using athero-antigen-specific Tregs. Moreover, antigen-specific Tregs studied in autoimmune diseases have a very low frequency and therefore treatment with antigen-specific Tregs would also require repeated rounds of in vitro expansion to achieve a sufficient amount of cells and this often results in phenotype loss. Possibly Treg antigen-specificity can be achieved in vivo by for example combining oral tolerance induction against e.g. oxLDL with administration of low dose IL-2 or an IL-2/anti-IL-2 complex that potently expands Tregs.
Summary and Outlook
Regulatory T cells are important regulators of immune responses and may hold great potential to be used as a therapeutic in atherosclerosis since enhanced Treg numbers are associated in experimental models for atherosclerosis and in clinical studies with a positive outcome of cardiovascular disease.
Various experimental approaches suggest a differential role for Tregs in different stages of atherosclerosis, since Tregs inhibit initial stages of atherosclerosis but are also important in the stabilization of well-established lesions during progression of disease.
Experimental therapies are based on the expansion of Tregs and by inducing tolerance induction against atherosclerosis specific antigens leading to Tregs that inhibit atherosclerosis. It can be anticipated that improvement of these experimental therapies by enhancing the tolerogenic capacity of the antigen and enhancing the tolerogenic function of the dendritic cells during tolerance induction will lead to a clinical application and a new therapy for atherosclerosis.
Significance.
Regulatory T cells (Tregs) play an important role in atherosclerosis and impaired Treg numbers promote atherosclerosis, whereas their induction lowers the burden of atherosclerosis. Tregs are atheroprotective by inhibiting effector T cells, by inducing an anti-inflammatory phenotype in macrophages, by lowering foam cell formation and inducing a tolerogenic phenotype in dendritic cells. Tregs mainly induce these effects via the secretion of the inhibitory cytokines IL-10 and TGF-β and via co-inhibitory pathways. The induction of Tregs in experimental models for disease via the intranasal, oral or subcutaneous administration of atherosclerosis-related antigens such as oxidized LDL, apoB100 peptides and HSP60 leads to the induction of antigen specific Tregs that inhibit the initiation and progression of atherosclerosis, which may be superior to the induction of polyclonal Tregs. It can be anticipated that these experimental therapies will lead to a clinical application and the development of a tolerogenic vaccine for atherosclerosis.
Acknowledgments
We acknowledge the support from the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation, Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development and the Royal Netherlands Academy of Sciences, for the GENIUS project “Generating the best evidence-based pharmaceutical targets for atherosclerosis” (CVON2011-19). AHL is supported by NIH R01HL087282.
NONSTANDARD ABBREVIATIONS AND ACRONYMS
- Tregs
Regulatory T cells
- DCs
Dendritic cells
- CTLA4
Cytotoxic T Lymphocyte-associated Antigen-4
- GITR
glucocorticoid-induced TNF receptor family related protein
- Tr1
T regulatory type 1 cells
- Th3
T helper-3 cells
- ACS
Acute Coronary Syndrome
- ICOS
Inducible T cell COStimulator
- LAP
Latency-Associated Peptide
- PD-L
Programmed Death-Ligand
- LAG-3
Lymphocyte Activation Gene-3
- MI
Myocardial Infarction
Footnotes
Disclosures
None
References
- 1.von Boehmer H, Daniel C. Therapeutic opportunities for manipulating t(reg) cells in autoimmunity and cancer. Nature reviews. Drug discovery. 2013;12:51–63. doi: 10.1038/nrd3683. [DOI] [PubMed] [Google Scholar]
- 2.Yu N, Li X, Song W, Li D, Yu D, Zeng X, Li M, Leng X, Li X. Cd4(+)cd25 (+)cd127 (low/−) t cells: A more specific treg population in human peripheral blood. Inflammation. 2012;35:1773–1780. doi: 10.1007/s10753-012-9496-8. [DOI] [PubMed] [Google Scholar]
- 3.Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and helios− cells coexist within the natural foxp3+ t regulatory cell subset in humans. J Immunol. 2013;190:2001–2008. doi: 10.4049/jimmunol.1201379. [DOI] [PubMed] [Google Scholar]
- 4.von Boehmer H. Mechanisms of suppression by suppressor t cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by cd4(+)cd25(+) regulatory t cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacchetta R, Gregori S, Roncarolo MG. Cd4+ regulatory t cells: Mechanisms of induction and effector function. Autoimmun Rev. 2005;4:491–496. doi: 10.1016/j.autrev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A cd4+ t-cell subset inhibits antigen-specific t-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 8.O'Garra A, Vieira P. Regulatory t cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 9.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O'Garra A. Il-10-secreting regulatory t cells do not express foxp3 but have comparable regulatory function to naturally occurring cd4+cd25+ regulatory t cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory t cell clones induced by oral tolerance: Suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 11.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 12.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory t cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 13.van Es T, van Puijvelde GH, Foks AC, Habets KL, Bot I, Gilboa E, Van Berkel TJ, Kuiper J. Vaccination against foxp3(+) regulatory t cells aggravates atherosclerosis. Atherosclerosis. 2010;209:74–80. doi: 10.1016/j.atherosclerosis.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 14.Klingenberg R, Gerdes N, Badeau RM, Gistera A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Luscher TF, Jauhiainen M, Sparwasser T, Hansson GK. Depletion of foxp3+ regulatory t cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC. Low numbers of foxp3 positive regulatory t cells are present in all developmental stages of human atherosclerotic lesions. PLoS ONE. 2007;2:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heller EA, Liu E, Tager AM, Yuan Q, Lin AY, Ahluwalia N, Jones K, Koehn SL, Lok VM, Aikawa E, Moore KJ, Luster AD, Gerszten RE. Chemokine cxcl10 promotes atherogenesis by modulating the local balance of effector and regulatory t cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 17.Mor A, Planer D, Luboshits G, Afek A, Metzger S, Chajek-Shaul T, Keren G, George J. Role of naturally occurring cd4+ cd25+ regulatory t cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:893–900. doi: 10.1161/01.ATV.0000259365.31469.89. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Mao S, Zhan Z, Yu K, He C, Wang C. Effect of hyperlipidemia on foxp3 expression in apolipoprotein e-knockout mice. Journal of cardiovascular medicine. 2014;15:273–279. doi: 10.2459/JCM.0b013e3283641b9c. [DOI] [PubMed] [Google Scholar]
- 19.Maganto-Garcia E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic changes in regulatory t cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124:185–195. doi: 10.1161/CIRCULATIONAHA.110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietel B, Cicha I, Voskens CJ, Verhoeven E, Achenbach S, Garlichs CD. Decreased numbers of regulatory t cells are associated with human atherosclerotic lesion vulnerability and inversely correlate with infiltrated mature dendritic cells. Atherosclerosis. 2013;230:92–99. doi: 10.1016/j.atherosclerosis.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of cd4(+)cd25(+) regulatory t cells in patients with acute coronary syndromes. European heart journal. 2006;27:2530–2537. doi: 10.1093/eurheartj/ehl222. [DOI] [PubMed] [Google Scholar]
- 22.Ammirati E, Cianflone D, Banfi M, Vecchio V, Palini A, De Metrio M, Marenzi G, Panciroli C, Tumminello G, Anzuini A, Palloshi A, Grigore L, Garlaschelli K, Tramontana S, Tavano D, Airoldi F, Manfredi AA, Catapano AL, Norata GD. Circulating cd4+cd25hicd127lo regulatory t-cell levels do not reflect the extent or severity of carotid and coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1832–1841. doi: 10.1161/ATVBAHA.110.206813. [DOI] [PubMed] [Google Scholar]
- 23.Wigren M, Bjorkbacka H, Andersson L, Ljungcrantz I, Fredrikson GN, Persson M, Bryngelsson C, Hedblad B, Nilsson J. Low levels of circulating cd4+foxp3+ t cells are associated with an increased risk for development of myocardial infarction but not for stroke. Arterioscler Thromb Vasc Biol. 2012;32:2000–2004. doi: 10.1161/ATVBAHA.112.251579. [DOI] [PubMed] [Google Scholar]
- 24.Ghourbani Gazar S, Andalib A, Hashemi M, Rezaei A. Cd4(+)foxp3(+) treg and its icos(+) subsets in patients with myocardial infarction. Iranian journal of immunology : IJI. 2012;9:53–60. [PubMed] [Google Scholar]
- 25.Han SF, Liu P, Zhang W, Bu L, Shen M, Li H, Fan YH, Cheng K, Cheng HX, Li CX, Jia GL. The opposite-direction modulation of cd4+cd25+ tregs and t helper 1 cells in acute coronary syndromes. Clin Immunol. 2007;124:90–97. doi: 10.1016/j.clim.2007.03.546. [DOI] [PubMed] [Google Scholar]
- 26.Jia L, Zhu L, Wang JZ, Wang XJ, Chen JZ, Song L, Wu YJ, Sun K, Yuan ZY, Hui R. Methylation of foxp3 in regulatory t cells is related to the severity of coronary artery disease. Atherosclerosis. 2013;228:346–352. doi: 10.1016/j.atherosclerosis.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J, Hori S. Plasticity of foxp3(+) t cells reflects promiscuous foxp3 expression in conventional t cells but not reprogramming of regulatory t cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Zhang WC, Wang J, Shu YW, Tang TT, Zhu ZF, Xia N, Nie SF, Liu J, Zhou SF, Li JJ, Xiao H, Yuan J, Liao MY, Cheng LX, Liao YH, Cheng X. Impaired thymic export and increased apoptosis account for regulatory t cell defects in patients with non-st segment elevation acute coronary syndrome. The Journal of biological chemistry. 2012;287:34157–34166. doi: 10.1074/jbc.M112.382978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH. Impaired regulatory t-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 30.Yu K, Zhu P, Dong Q, Zhong Y, Zhu Z, Lin Y, Huang Y, Meng K, Ji Q, Yi G, Zhang W, Wu B, Mao Y, Cheng P, Zhao X, Mao X, Zeng Q. Thymic stromal lymphopoietin attenuates the development of atherosclerosis in apoe−/− mice. Journal of the American Heart Association. 2013;2:e000391. doi: 10.1161/JAHA.113.000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang K, Li SQ, Wang WJ, Liu LS, Jiang YG, Feng PN, Wang YQ, Wang SM. Oral fty720 administration induces immune tolerance and inhibits early development of atherosclerosis in apolipoprotein e-deficient mice. International journal of immunopathology and pharmacology. 2012;25:397–406. doi: 10.1177/039463201202500209. [DOI] [PubMed] [Google Scholar]
- 32.Zhong Y, Wang X, Ji Q, Mao X, Tang H, Yi G, Meng K, Yang X, Zeng Q. Cd4+lap + and cd4 +cd25 +foxp3 + regulatory t cells induced by nasal oxidized low-density lipoprotein suppress effector t cells response and attenuate atherosclerosis in apoe−/− mice. Journal of clinical immunology. 2012;32:1104–1117. doi: 10.1007/s10875-012-9699-7. [DOI] [PubMed] [Google Scholar]
- 33.Lin YZ, Lu SH, Lu ZD, Huang Y, Shi Y, Liu L, Wang XY, Ji QW. Downregulation of cd4+lap+ and cd4+cd25+ regulatory t cells in acute coronary syndromes. Mediators of inflammation. 2013;2013:764082. doi: 10.1155/2013/764082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu ZF, Meng K, Zhong YC, Qi L, Mao XB, Yu KW, Zhang W, Zhu PF, Ren ZP, Wu BW, Ji QW, Wang X, Zeng QT. Impaired circulating cd4+ lap+ regulatory t cells in patients with acute coronary syndrome and its mechanistic study. PLoS One. 2014;9:e88775. doi: 10.1371/journal.pone.0088775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang R, Zhu J, Dong X, Shi M, Lu C, Springer TA. Garp regulates the bioavailability and activation of tgfbeta. Molecular biology of the cell. 2012;23:1129–1139. doi: 10.1091/mbc.E11-12-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards JP, Fujii H, Zhou AX, Creemers J, Unutmaz D, Shevach EM. Regulation of the expression of garp/latent tgf-beta1 complexes on mouse t cells and their role in regulatory t cell and th17 differentiation. J Immunol. 2013;190:5506–5515. doi: 10.4049/jimmunol.1300199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng K, Zhang W, Zhong Y, Mao X, Lin Y, Huang Y, Lang M, Peng Y, Zhu Z, Liu Y, Zhao X, Yu K, Wu B, Ji Q, Zeng Q. Impairment of circulating cd4cd25garp regulatory t cells in patients with acute coronary syndrome. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2014;33:621–632. doi: 10.1159/000358639. [DOI] [PubMed] [Google Scholar]
- 38.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein e−/− mice. The American journal of pathology. 2000;157:1819–1824. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. Ifn-gamma potentiates atherosclerosis in apoe knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitman SC, Ravisankar P, Daugherty A. Ifn-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein e−/− mice. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 42.Foks AC, Frodermann V, ter Borg M, Habets KL, Bot I, Zhao Y, van Eck M, van Berkel TJ, Kuiper J, van Puijvelde GH. Differential effects of regulatory t cells on the initiation and regression of atherosclerosis. Atherosclerosis. 2011;218:53–60. doi: 10.1016/j.atherosclerosis.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 43.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector th17 and regulatory t cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 44.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. Tgfbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of il-17-producing t cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Liu ZD, Wang L, Lu FH, Pan H, Zhao YX, Wang SJ, Sun SW, Li CL, Hu XL. Increased th17 cell frequency concomitant with decreased foxp3+ treg cell frequency in the peripheral circulation of patients with carotid artery plaques. Inflammation research : official journal of the European Histamine Research Society ... [et al.] 2012;61:1155–1165. doi: 10.1007/s00011-012-0510-2. [DOI] [PubMed] [Google Scholar]
- 46.Zheng Y, Wang Z, Deng L, Yuan X, Ma Y, Zhang G, Gantier MP, Liu JP, Shen L, Xu D. Osteopontin promotes inflammation in patients with acute coronary syndrome through its activity on il-17 producing cells. Eur J Immunol. 2012;42:2803–2814. doi: 10.1002/eji.201242475. [DOI] [PubMed] [Google Scholar]
- 47.Chaudhry A, Rudensky AY. Control of inflammation by integration of environmental cues by regulatory t cells. J Clin Invest. 2013;123:939–944. doi: 10.1172/JCI57175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mallat Z, Gojova A, Brun V, Esposito B, Fournier N, Cottrez F, Tedgui A, Groux H. Induction of a regulatory t cell type 1 response reduces the development of atherosclerosis in apolipoprotein e-knockout mice. Circulation. 2003;108:1232–1237. doi: 10.1161/01.CIR.0000089083.61317.A1. [DOI] [PubMed] [Google Scholar]
- 49.George J, Schwartzenberg S, Medvedovsky D, Jonas M, Charach G, Afek A, Shamiss A. Regulatory t cells and il-10 levels are reduced in patients with vulnerable coronary plaques. Atherosclerosis. 2012;222:519–523. doi: 10.1016/j.atherosclerosis.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Mallat Z, Gojova A, Marchiol-Fournigault C, Esposito B, Kamate C, Merval R, Fradelizi D, Tedgui A. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89:930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- 51.Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of tgf-beta signaling in t cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine il-35 contributes to regulatory t-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 53.Kempe S, Heinz P, Kokai E, Devergne O, Marx N, Wirth T. Epstein-barr virus-induced gene-3 is expressed in human atheroma plaques. The American journal of pathology. 2009;175:440–447. doi: 10.2353/ajpath.2009.080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin Y, Huang Y, Lu Z, Luo C, shi Y, Zeng Q, Cao Y, Liu L, Wang X, Ji Q. Decreased plasma il-35 levels are related to the left ventricular ejection fraction in coronary artery diseases. PLoS One. 2012;7:e52490. doi: 10.1371/journal.pone.0052490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Puijvelde GH, van Es T, van Wanrooij EJ, Habets KL, de Vos P, van der Zee R, van Eden W, van Berkel TJ, Kuiper J. Induction of oral tolerance to hsp60 or an hsp60-peptide activates t cell regulation and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2677–2683. doi: 10.1161/ATVBAHA.107.151274. [DOI] [PubMed] [Google Scholar]
- 56.van Puijvelde GH, Hauer AD, de Vos P, van den Heuvel R, van Herwijnen MJ, van der Zee R, van Eden W, van Berkel TJ, Kuiper J. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114:1968–1976. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]
- 57.Dietrich T, Hucko T, Schneemann C, Neumann M, Menrad A, Willuda J, Atrott K, Stibenz D, Fleck E, Graf K, Menssen HD. Local delivery of il-2 reduces atherosclerosis via expansion of regulatory t cells. Atherosclerosis. 2012;220:329–336. doi: 10.1016/j.atherosclerosis.2011.09.050. [DOI] [PubMed] [Google Scholar]
- 58.Ewing MM, Karper JC, Abdul S, de Jong RC, Peters HA, de Vries MR, Redeker A, Kuiper J, Toes RE, Arens R, Jukema JW, Quax PH. T-cell co-stimulation by cd28-cd80/86 and its negative regulator ctla-4 strongly influence accelerated atherosclerosis development. International journal of cardiology. 2013;168:1965–1974. doi: 10.1016/j.ijcard.2012.12.085. [DOI] [PubMed] [Google Scholar]
- 59.Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the pd-1/pd-l pathway in mice. J Clin Invest. 2007;117:2974–2982. doi: 10.1172/JCI31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. Pd-1 protects against inflammation and myocyte damage in t cell-mediated myocarditis. J Immunol. 2012;188:4876–4884. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA. Role of lag-3 in regulatory t cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 62.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S, Tybulewicz V, Vignali D, Clynes R. Regulatory t cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of mhc class ii. J Immunol. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 63.Lin J, Li M, Wang Z, He S, Ma X, Li D. The role of cd4+cd25+ regulatory t cells in macrophage-derived foam-cell formation. Journal of lipid research. 2010;51:1208–1217. doi: 10.1194/jlr.D000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. Cd4+cd25+foxp3+ regulatory t cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires myd88 signaling in dcs. J Clin Invest. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meng X, Li W, Yang J, Zhang K, Qin W, An G, Gao F, Wang Y, Zhang C, Zhang Y. Regulatory t cells prevent plaque disruption in apolipoprotein e-knockout mice. International journal of cardiology. 2013;168:2684–2692. doi: 10.1016/j.ijcard.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 67.Von Der Thusen JH, Kuiper J, Fekkes ML, De Vos P, Van Berkel TJ, Biessen EA. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in ldlr−/− mice. FASEB J. 2001;15:2730–2732. doi: 10.1096/fj.01-0483fje. [DOI] [PubMed] [Google Scholar]
- 68.Maganto-Garcia E, Bu DX, Tarrio ML, Alcaide P, Newton G, Griffin GK, Croce KJ, Luscinskas FW, Lichtman AH, Grabie N. Foxp3+-inducible regulatory t cells suppress endothelial activation and leukocyte recruitment. J Immunol. 2011;187:3521–3529. doi: 10.4049/jimmunol.1003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trenado A, Fisson S, Braunberger E, Klatzmann D, Salomon BL, Cohen JL. Ex vivo selection of recipient-type alloantigen-specific cd4(+)cd25(+) immunoregulatory t cells for the control of graft-versus-host disease after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2004;77:S32–34. doi: 10.1097/01.TP.0000106470.07410.CA. [DOI] [PubMed] [Google Scholar]
- 70.Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, Porter SB. In vitro-expanded human cd4(+)cd25(+) t-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated mlr cultures. Blood. 2004;104:453–461. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 71.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, McGlave PB, Blazar BR, Wagner JE. Infusion of ex vivo expanded t regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, Mysliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded cd4+cd25+cd127- t regulatory cells. Clin Immunol. 2009;133:22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, Aloisi T, Perruccio K, Ruggeri L, Balucani C, Pierini A, Sportoletti P, Aristei C, Falini B, Reisner Y, Velardi A, Aversa F, Martelli MF. Tregs prevent gvhd and promote immune reconstitution in hla-haploidentical transplantation. Blood. 2011;117:3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 74.Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Derkowska I, Juscinska J, Owczuk R, Szadkowska A, Witkowski P, Mlynarski W, Jarosz-Chobot P, Bossowski A, Siebert J, Trzonkowski P. Therapy of type 1 diabetes with cd4(+)cd25(high)cd127-regulatory t cells prolongs survival of pancreatic islets - results of one year follow-up. Clin Immunol. 2014;153:23–30. doi: 10.1016/j.clim.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 75.van Puijvelde GH, van Es T, van Wanrooij EJ, Habets KL, de Vos P, van der Zee R, van Eden W, van Berkel TJ, Kuiper J. Induction of oral tolerance to hsp60 or an hsp60-peptide activates t cell regulation and reduces atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2677–2683. doi: 10.1161/ATVBAHA.107.151274. [DOI] [PubMed] [Google Scholar]
- 76.George J, Yacov N, Breitbart E, Bangio L, Shaish A, Gilburd B, Shoenfeld Y, Harats D. Suppression of early atherosclerosis in ldl-receptor deficient mice by oral tolerance with beta 2-glycoprotein i. Cardiovasc Res. 2004;62:603–609. doi: 10.1016/j.cardiores.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 77.Fredrikson GN, Soderberg I, Lindholm M, Dimayuga P, Chyu KY, Shah PK, Nilsson J. Inhibition of atherosclerosis in apoe-null mice by immunization with apob-100 peptide sequences. Arterioscler Thromb Vasc Biol. 2003;23:879–884. doi: 10.1161/01.ATV.0000067937.93716.DB. [DOI] [PubMed] [Google Scholar]
- 78.Herbin O, Ait-Oufella H, Yu W, Fredrikson GN, Aubier B, Perez N, Barateau V, Nilsson J, Tedgui A, Mallat Z. Regulatory t-cell response to apolipoprotein b100-derived peptides reduces the development and progression of atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32:605–612. doi: 10.1161/ATVBAHA.111.242800. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Zhao X, Zhang S, Wang S, Du P, Qi G. Apob-100 and hsp60 peptides exert a synergetic role in inhibiting early atherosclerosis in immunized apoe-null mice. Protein and peptide letters. 2011;18:733–740. doi: 10.2174/092986611795445987. [DOI] [PubMed] [Google Scholar]
- 80.Sun JB, Xiang Z, Smith KG, Holmgren J. Important role for fcgammariib on b lymphocytes for mucosal antigen-induced tolerance and foxp3+ regulatory t cells. J Immunol. 2013;191:4412–4422. doi: 10.4049/jimmunol.1301324. [DOI] [PubMed] [Google Scholar]
- 81.Klingenberg R, Lebens M, Hermansson A, Fredrikson GN, Strodthoff D, Rudling M, Ketelhuth DF, Gerdes N, Holmgren J, Nilsson J, Hansson GK. Intranasal immunization with an apolipoprotein b-100 fusion protein induces antigen-specific regulatory t cells and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:946–952. doi: 10.1161/ATVBAHA.109.202671. [DOI] [PubMed] [Google Scholar]
- 82.Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein b-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123:1083–1091. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]
- 83.Van Brussel I, Schrijvers DM, Van Vre EA, Bult H. Potential use of dendritic cells for anti-atherosclerotic therapy. Current pharmaceutical design. 2013;19:5873–5882. doi: 10.2174/1381612811319330006. [DOI] [PubMed] [Google Scholar]
- 84.Yang K, Li D, Luo M, Hu Y. Generation of hsp60-specific regulatory t cell and effect on atherosclerosis. Cellular immunology. 2006;243:90–95. doi: 10.1016/j.cellimm.2007.01.002. [DOI] [PubMed] [Google Scholar]