Abstract
Numerous studies have linked air pollution with adverse birth outcomes, but relatively few have examined differential associations across the socioeconomic gradient. To evaluate interaction effects of gestational nitrogen dioxide (NO2) and area-level socioeconomic deprivation on fetal growth, we used: 1) highly spatially-resolved air pollution data from the New York City Community Air Survey (NYCCAS); and 2) spatially-stratified principle component analysis of census variables previously associated with birth outcomes to define area-level deprivation. New York City (NYC) hospital birth records for years 2008–2010 were restricted to full-term, singleton births to non-smoking mothers (n = 243,853). We used generalized additive mixed models to examine the potentially non-linear interaction of nitrogen dioxide (NO2) and deprivation categories on birth weight (and estimated linear associations, for comparison), adjusting for individual-level socio-demographic characteristics and sensitivity testing adjustment for co-pollutant exposures. Estimated NO2 exposures were highest, and most varying, among mothers residing in the most-affluent census tracts, and lowest among mothers in residing in mid-range deprivation tracts. In non-linear models, we found an inverse association between NO2 and birth weight in the least-deprived and most-deprived areas (p-values < 0.001 and 0.05, respectively) but no association in the mid-range of deprivation (p=0.8). Likewise, in linear models, a 10 ppb increase in NO2 was associated with a decrease in birth weight among mothers in the least-deprived and most-deprived areas of −16.2 g (95% CI: −21.9 to −10.5) and −11.0 g (95% CI: −22.8 to 0.9), respectively, and a non-significant change in the mid-range areas [β = 0.5 g (95% CI: −7.7 to 8.7)]. Linear slopes in the most- and least-deprived quartiles differed from the mid-range (reference group) (p-values < 0.001 and 0.09, respectively). The complex patterning in air pollution exposure and deprivation in NYC, however, precludes simple interpretation of interactive effects on birth weight, and highlights the importance of considering differential distributions of air pollution concentrations, and potential differences in susceptibility, across deprivation levels.
Keywords: nitrogen dioxide, socioeconomic deprivation, term birth weight
1. Introduction
There is considerable attention on the role of prenatal air pollution exposure on adverse birth outcomes (Shah et al. 2011; Stieb et al. 2012). Despite a growing understanding of the biological mechanisms underlying this association, including systemic oxidative stress (Kannan et al. 2006; Burton and Jauniaux 2011) and inflammation (Munoz-Suano et al. 2011), epidemiological evidence remains inconclusive. This mixed evidence may be attributable to differing exposure assignment methods and measurement error (Dadvand et al. 2013), or to varying co-pollutant exposures and adjustment methods (Woodruff et al. 2009). Alternatively, inconsistencies may arise from incomplete adjustment for confounding, or from differential exposure-response relationships across populations. Of particular concern is sufficiently accounting for socioeconomic deprivation, which may be spatially correlated with air pollution (Clark et al. 2014; Tian et al. 2013), and thus may confound measures of association, or may operate synergistically through common biological pathways [e.g., chronic stress-induced inflammation, or dysregulation of immune and endocrine systems (Clougherty and Kubzansky 2009; Schwartz et al. 2011)].
The need to integrate socioeconomic context and environmental pollution exposures into health research has long been recognized (IOM 1999; Gee and Payne-Sturges 2004; Morello-Frosch and Shenassa 2006), and there is growing attention to the role of multiple exposures and heightened physiologic susceptibility [i.e., allostatic load (McEwen and Seeman 1999)] in driving health disparities (Nweke et al. 2011; Sexton and Linder 2011). There is substantial evidence for adverse impacts of area-level deprivation on pregnancy outcomes, even after accounting for individual socioeconomic position (SEP) (Picket et al. 2002; O’Campo et al. 2008; Blumenshine et al. 2010). However, only a few studies have examined differential associations between exposure to air pollution (or traffic-related proxy variables) and fetal growth outcomes across the socioeconomic gradient. Among these studies, results range from no interaction with fine particulate matter (particles with aerodynamic diameter < 2.5 μm3, PM2.5) or ozone (Gray et al. 2014), to heightened associations with carbon monoxide (CO) and nitrogen dioxide (NO2) (Morello-Frosch et al. 2010) or distance-weighted traffic density in low-SEP areas (Wilhelm and Ritz 2003), to heightened associations with residential proximity to highway in high-SEP areas (Généreux et al. 2008). These mixed results may arise from real differences in exposure and/ or susceptibility across populations, or from methodological differences (e.g., socioeconomic measures, or pollution exposure assignment). Disentangling the complex relationships between social and environmental exposures requires large and diverse samples, detailed exposure and outcome information, and innovative analytic strategies to address spatial confounding (Ness et al. 2013).
This is the first study, to our knowledge, to consider potential non-linear associations and effect modification between NO2 and area-level deprivation on term birth weight. Specifically, we used vital records and hospital data covering in New York City (NYC) births 2008–2010 to examine: (1) mutually-adjusted NO2 and area-level deprivation associations with birth weight and (2) differential associations between NO2 and birth weight by deprivation levels, adjusted for individual-level SEP and co-occurring PM2.5. We focus on fetal growth among term births, which has important lifecourse and population health implications (Barker et al. 2002). To quantify area-level deprivation, we developed a composite index of area-level deprivation, which reflects the spatial heterogeneity of socioeconomic factors across NYC. We build on a study of air pollution and birth outcomes in NYC which was designed to minimize spatial and temporal uncertainty in air pollution exposure estimates in a densely populated city (Ross et al. 2013; Savitz et al. 2014). We previously reported significant associations between fine-scale NO2 and PM2.5 and term birth weight, and observed that variance in exposure estimates were primarily spatial for NO2 vs. temporal for PM2.5 (Savitz et al. 2014). Because our deprivation index does not vary temporally over the study period, we focus here on spatial variation in NO2 exposures over the entire pregnancy.
2. Methods
2.1 Study population
Vital records for 348,585 live births to mothers residing within the five boroughs of NYC during 2008–2010 were merged with patient-level data from the New York State Department of Health Statewide Planning and Research Cooperative System (SPARCS), covering all licensed NYC healthcare facilities. We restricted the study population to full-term (37 to 42 weeks gestation), singleton births with no congenital anomalies, born to (self-reported) non-smoking mothers with complete residential address and covariate data, leaving 243,853 births. Exclusion criteria for implausible clinical values and fixed cohort bias (Strand et al. 2011) in this population are detailed elsewhere (Savitz et al. 2014).
2.2 Term birth weight outcome and covariates
We examined changes in term birth weight as a continuous variable. We adjusted for individual-level covariates previously associated with fetal growth, including: maternal age, pre-pregnancy body mass index (BMI), receipt of prenatal care (yes/ no), number of previous lives births, and gestational age (in weeks). We included three measures of maternal SEP, including: Medicaid status (yes/ no), years of education (< 9, 9 – 11, 12, 13–15, 16, or > 16), and race/ ethnicity (White, Black, Hispanic, or Asian) cross-classified by United States (US)- or foreign-born status. To account for temporal trends in pollution we adjusted for year and season of conception, as in our prior analysis of this data (Savitz et al. 2014).
2.3 Composite index of area-level socioeconomic deprivation
We adapted Messer et al.’s (2006) area-level deprivation index originally developed to reflect between-city differences in prevalence in, and combinations of, SEP indicators using spatially-stratified principle component analysis (PCA). This effort to capture distinct SEP typologies using cities as spatial regimes, or strata, represented an important methodological innovation, as traditional application of data reduction techniques can obscure heterogeneity in spatial patterns in SEP (Pickett and Pearl 2001). Here, we adapted this approach to describe intra-urban SEP heterogeneity across NYC census tracts, and propose a geostatistical technique for identifying optimal spatial strata for PCA. Based on Messer et al.’s (2006) literature review of census SEP variables previously associated with birth outcomes, we selected 20 indicators covering multiple domains of deprivation – educational attainment, employment, occupation, housing, poverty, and racial/ ethnic composition – from the American Communities Survey (ACS) 2005–09 five-year estimates, to best match years of air pollution and outcome data (Supplemental Table 1). We used census tracts as our unit of analysis to maximize comparability with other studies of area-level SEP and birth outcomes (Krieger et al. 2003; Janevic et al. 2010), excluding tracts with total residential population fewer than 20 persons (n = 62 of 2,216).
To identify spatial strata which maximized autocorrelation in each tract-level SEP indicator, and minimized correlations between strata, we used Local Indicators of Spatial Association (LISA) statistics to quantify between-tract clustering (Anselin 1995). More information on the LISA statistic, and our process for identifying boroughs (n = 5) as the optimal spatial strata, can be found in supplemental materials (Supplemental Figure 1).
We followed a standard PCA process to reduce the number of highly-correlated variables to the minimum number of uncorrelated components. Specifically, following initial extraction of components and corresponding eigenvalues, we selected the number of components based on eigenvalues > 1, Scree plots, and proportion of variance > 5%. We then used the rotated (varimax) solution to identify SEP variables that loaded strongly (> ±0.40) on more than one component, suggesting that the variable captured more than one underlying construct, and could be omitted to increase between-factor differences. After generating a city-wide PCA solution, we repeated the above steps within each borough, to ensure that locally-important variables and relationships, possibly obscured in the city-wide PCA, could be retained and contribute to the final deprivation index. We tallied variables that loaded > ±0.40 in two or more borough-level PCA solutions, which were then included with those retained by the initial city-wide solution in a second city-wide PCA process.
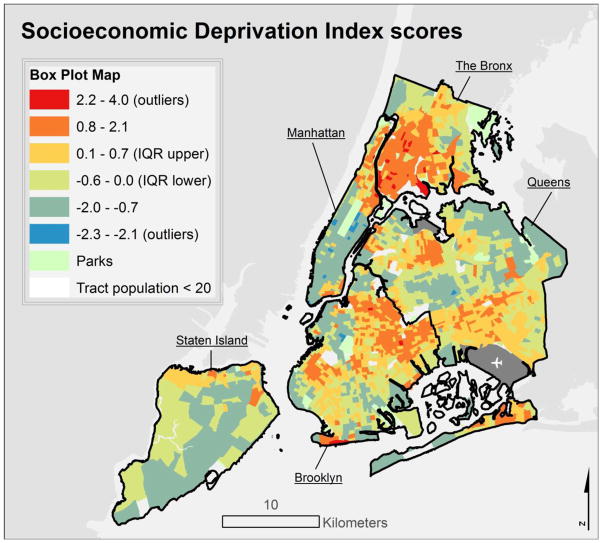
The final socioeconomic deprivation index (SDI) solution based on census tracts retained seven ACS variables: population rates of residents with a college degree, unemployed, residential crowding, management or professional occupation, below 200% of the Federal Poverty Level (FPL), households receiving public assistance, and non-White racial composition. The first component factor explained 56% of overall variance in retained variables. The initial city-wide solution, in contrast, retained fewer, slightly different variables, and the first component explained only 41% of overall variance (Supplemental Table 1). We operationalized the SDI as tract-level factor scores for the first component of the PCA solution, in keeping with Messer et al. (2006), such that higher scores indicated greater tract-level socioeconomic deprivation (Figure 1); tract SDI mean score = 0, standard deviation (SD) = 1, range −2.33 – 4.01. PCA was implemented in SAS v9 (Cary, NC).
Figure 1.
Socioeconomic deprivation index scores, with higher scores indicating greater census tract-level deprivation.
2.4 Air pollution exposure assessment
Fine-scale ambient pollution data from the New York City Community Air Survey (NYCCAS) was used to derive near-residence maternal NO2 and PM2.5 exposure estimates. NYCCAS methods and results are detailed elsewhere (Matte et al. 2013; Clougherty et al. 2013). Briefly, NYCCAS utilized a spatial saturation design to measure multiple air pollutants across 150 locations, repeated across four seasons and over two years. Monitors were positioned at street-level (10–12 feet), and collected integrated two-week samples in each season from December 2008 through December 2010. Prior analyses reported greater spatial variability in NO2 and greater temporal variability in PM2.5 (Clougherty et al. 2013; Savitz et al. 2014). Because our SDI measure used multi-year census variables to maximize precision in spatial variability in SEP (which is not time-varying over the course of study), we focus here on the full-gestation period for NO2 exposure assessment, and consider co-pollutant adjustment for full-gestation PM2.5 in sensitivity analyses.
Births were geocoded to mother’s residential address at delivery, and NYCCAS pollution concentration surfaces were used to estimate near-residence exposure as the mean concentration within a 300m radial buffer. Exposure estimates were then temporally adjusted using regulatory monitoring data to match individual-level gestation periods, as detailed in Ross et al. 2013.
2.5 Statistical analyses
We used generalized additive mixed models to estimate associations between area-level deprivation, maternal air pollution exposure, and term birth weight, allowing for flexible estimation of non-linear exposure-response relationships using penalized splines (Wood 2003). A random intercept accounted for the clustering of mothers within census tracts. We first considered mutually-adjusted non-linear effects of NO2 and area-level deprivation (i.e., SDI) on term birth weight, with adjustment for maternal SEP and covariates (Model 1). We then examined differential NO2-birth weight associations by SDI levels by allowing the smooth relationship between NO2 and birth weight to differ by quartile of census tract-level SDI (Model 2). Cut-points for three-level SDI categories were set at the 25th and 75th percentiles of factor scores across mothers (−0.46 and 1.03, respectively). We also examined the linear interaction between NO2 with SDI quartiles (Model 3), to quantitatively compare the estimated slopes across the SDI levels. For interaction models, we combined middle-range SDI quartiles (Q2 and Q3) due to similar observed relationships between pollutant exposures and birth weight in these quartiles. Regression models were implemented R statistical software v3.1.0.
2.6 Sensitivity analyses
First, to investigate whether the observed interaction between NO2 and tract-level SDI was driven by clustering of similar-SEP mothers within a tract, we examined modification of the NO2-birth weight association by maternal SEP characteristics, adjusted for area-level deprivation. Second, because NO2 and PM2.5 have some common sources, and thus may be spatially confounded, we re-fit all models with adjustment for maternal PM2.5 exposure estimates.
This research protocol was approved by Institutional Review Boards at the NYC Department of Health and Mental Hygiene, Brown University and University of Pittsburgh.
3. Results
Mothers in the study population reflected the socio-demographic diversity of NYC (Table 1). Overall, 71.5% of mothers reported fewer than 16 years of education [roughly the equivalent of a college degree (BA)] and 61.1% of deliveries were eligible for Medicaid coverage. Mothers living in least-deprived (high-SEP, SDI Q1) tracts had higher mean educational attainment (33.5% < BA) and lower mean Medicaid eligibility rates (23.8%), compared to mothers living in the most-deprived (low-SEP, SDI Q4) tracts (92.7% < BA, 83.6% Medicaid eligibility). Overall, 55% of mothers were foreign-born, with the highest proportion of non-native mothers reporting Hispanic and Asian ethnicities. Ethnicity varied across SDI levels; more foreign- and US-born White and foreign-born Asian mothers lived in high-SEP tracts (20.3, 44.3, and 13.5%, respectively), versus higher proportions of foreign- and US-born Black and Hispanic mothers in low-SEP tracts (10.3, 17.7, 36.9, and 20.6, respectively). Mothers in high-SEP tracts were generally older, with lower parity, and lower pre-pregnancy BMI, compared to mothers in low-SEP tracts. The majority of mothers across SDI levels received prenatal care (overall 99.5%). Few births were less than 2,500 g (2.64%), which were slightly less common (2.24%, p < 0.001) among mothers in high-SEP tracts.
Table 1.
Study population characteristics, by SDI levels.
| Study Population | High-SEP tracts (SDI Q1) | Mid-range SEP tracts (SDI Q2 + Q3) | Low-SEP tracts (SDI Q4) | |
|---|---|---|---|---|
|
| ||||
| n = 243,853 | n = 60,963 | n = 121,809 | n = 61,081 | |
|
| ||||
| Term birth weight (g) | % (n) | % (n) | % (n) | % (n) |
|
| ||||
| < 1,500 | 0.04 (88) | 0.04 (26) | 0.03 (32) | 0.05 (30) |
| 1,500 – 2,499 | 2.6 (6,402) | 2.2 (1,361) | 2.7 (3,291) | 2.9 (1,750) |
| 2500 – 3,999 | 90.3 (220,156) | 90.2 (54,978) | 90.3 (110,017) | 90.3 (55,161) |
| ≥ 4,000 | 7.1 (17,207) | 7.5 (4,598) | 7.0 (8,469) | 6.8 (4,140) |
|
| ||||
| Maternal SEP | % (n) | % (n) | % (n) | % (n) |
|
| ||||
| Education | ||||
| < 9 yrs. | 8.1 (19,731) | 2.1 (1,300) | 8.8 (10,700) | 12.7 (7,731) |
| 9 – 11 yrs. | 17.6 (42,819) | 4.3 (2,622) | 17.8 (21,719) | 30.3 (18,487) |
| 12 yrs. (High school) | 23.9 (58,286) | 10.3 (6,266) | 28.4 (35,544) | 28.7 (17,476) |
| 13 – 15 yrs. | 21.9 (53,376) | 16.8 (10,249) | 24.9 (30,293) | 21.0 (12,825) |
| 16 yrs. (BA) | 16.3 (39,793) | 33.2 (20,213) | 13.2 (16,129) | 5.7 (3,451) |
| > 16 yrs. | 12.2 (29,857) | 33.3 (20,213) | 6.9 (8,424) | 1.8 (1,120) |
|
| ||||
| Medicaid status | ||||
| Yes | 61.1 (149,106) | 23.8 (14,485) | 68.6 (83,582) | 83.6 (51,039) |
| No | 38.9 (94,747) | 86.2 (46,478) | 31.4 (38,227) | 16.4 (10.042) |
|
| ||||
| Ethnicity | ||||
| US-born White | 19.4 (47,233) | 44.3 (27,021) | 14.6 (17,725) | 4.1 (2,496) |
| Foreign-born White | 9.4 (22,912) | 20.3 (12,387) | 8.0 (9,763) | 1.3 (762) |
| US-born Black | 12.0 (29,339) | 2.8 (1,732) | 13.8 (16,779) | 17.7 (10,828) |
| Foreign-born Black | 9.8 (23,856) | 2.1 (1,295) | 13.4 (16,299) | 10.3 (6,262) |
| US-born Hispanic | 12.4 (30,346) | 6.5 (3,974) | 11.3 (13,794) | 20.6 (12,578) |
| Foreign-born Hispanic | 21.8 (53,248) | 7.4 (4,529) | 21.5 (26,161) | 36.9 (22,558) |
| US-born Asian | 1.2 (2,899) | 2.9 (1,783) | 0.8 (981) | 0.2 (135) |
| Foreign-born Asian | 14.0 (34,020) | 13.5 (8,251) | 16.7 (20,307) | 8.9 (5,462) |
|
| ||||
| Adjustment covariates | % (n) | % (n) | % (n) | % (n) |
|
| ||||
| Maternal age (years) | ||||
| < 20 | 6.6 (16,108) | 1.7 (1,024) | 6.6 (8,056) | 11.5 (7.028) |
| 20 – < 25 | 20.8 (50,608) | 8.1 (4,964) | 23.4 (28,504) | 28.1 (17,140) |
| 25 – < 30 | 26.6 (64,814) | 20.0 (12,178) | 28.9 (35,145) | 28.6 (17,491) |
| 30 – < 35 | 26.4 (64,481) | 37.8 (23,062) | 24.3 (29,556) | 19.4 (11,863) |
| 35 – < 40 | 15.3 (37,246) | 25.1 (15,324) | 13.2 (16,025) | 9.7 (5,897) |
| ≥ 40 | 4.4 (10,596) | 7.2 (4,411) | 3.7 (4,523) | 2.7 (1,662) |
|
| ||||
| Pre-pregnancy BMI | ||||
| < 18.5 (Underweight) | 5.5 (13,445) | 6.4 (4,108) | 5.3 (6,456) | 4.7 (2,881) |
| 18.5 – < 25 (Normal) | 54.3 (132,442) | 68.7 (41,851) | 51.6 (62,810) | 45.5 (27,781) |
| 25 – < 30 (Overweight) | 23.7 (57,842) | 16.3 (9,929) | 25.5 (31,082) | 27.6 (16,831) |
| ≥ 30 (Obese) | 16.5 (40,124) | 8.3 (5,075) | 17.6 (21,461) | 22.3 (13,588) |
|
| ||||
| Prenatal care received | ||||
| Yes | 99.5 (242,570) | 99.6 (60,746) | 99.5 (121,156) | 99.3 (60,668) |
| No | 0.5 (1,283) | 0.4 (217) | 0.5 (653) | 0.7 (413) |
|
| ||||
| Previous live births | ||||
| 0 | 46.6 (113,644) | 56.3 (34,314) | 44.0 (53,582) | 42.2 (25,748) |
| 1 | 29.5 (71,990) | 29.3 (17,884) | 29.9 (36,356) | 29.1 (17,741) |
| 2 | 13.5 (33,011) | 9.4 (5,727) | 14.3 (17,433) | 16.1 (9,851) |
| ≥ 3 | 10.3 (25,208) | 5.0 (3,038) | 11.9 (14,429) | 12.7 (7,741) |
|
| ||||
| Gestational age (weeks) | ||||
| 37 | 8.1 (19,654) | 7.0 (4,284) | 8.6 (10,147) | 8.6 (5,223) |
| 38 | 18.5 (44,994) | 17.6 (10,727) | 18.7 (22,876) | 18.7 (11,391) |
| 39 | 34.5 (84,237) | 35.0 (21,319) | 34.7 (41,742) | 34.7 (21,176) |
| 40 | 29.6 (72,284) | 31.7 (19,288) | 28.7 (35,454) | 28.7 (17,542) |
| 41 | 8.6 (21,002) | 8.2 (4,975) | 8.8 (10,569) | 8.8 (5,368) |
| 42 | 0.7 (1,682) | 0.6 (370) | 0.8 (931) | 0.6 (381) |
|
| ||||
| Conception season | ||||
| Dec – Feb | 28.8 (70,242) | 28.4 (17,305) | 29.0 (35,326) | 28.8 (17,611) |
| Mar – May | 20.4 (49,686) | 20.0 (12,200) | 20.4 (24,839) | 20.7 (12,647) |
| Jun – Aug | 22.0 (53,670) | 22.4 (13,654) | 22.0 (26,787) | 21.7 (13,229) |
| Sep – Nov | 28.8 (70,255) | 29.2 (17,804) | 28.6 (34,857) | 28.8 (17,594) |
|
| ||||
| Conception year | ||||
| 2007 | 16.7 (40,812) | 16.8 (10,212) | 16.7 (20,292) | 16.9 (10,308) |
| 2008 | 38.7 (94,238) | 38.7 (23,562) | 38.6 (47,042) | 38.7 (23,634) |
| 2009 | 37.2 (90,615) | 37.2 (22,709) | 37.2 (45,301) | 37.0 (22,605) |
| 2010 | 7.5 (18,188) | 7.4 (4,480) | 7.5 (9,174) | 7.4 (4,534) |
|
| ||||
| Full-gestation air pollution exposure estimate | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| NO2 near-residence mean concentration (ppb) | 26.8 (5.3) | 28.1 (8.0) | 25.7 (3.9) | 27.8 (3.6) |
| PM2.5 near-residence mean concentration (μg/m3) | 11.8 (1.9) | 12.3 (2.4) | 11.3 (1.5) | 12.2 (1.7) |
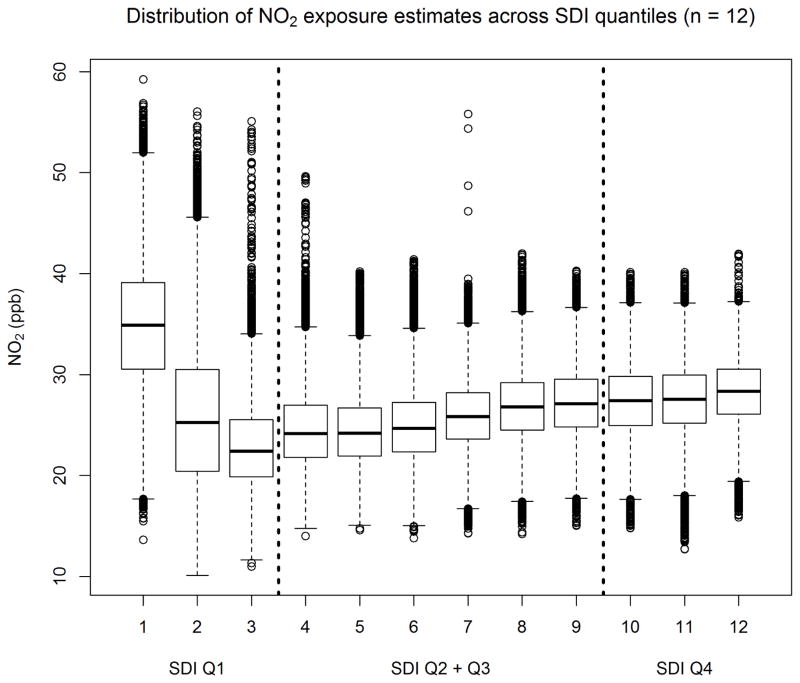
Maternal air pollution exposures varied spatially, and by the SDI; the distribution of NO2 across SDI levels exhibited an inverted J-shaped relationship, with highest, and most variable, exposures in high-SEP tracts forming a negative relationship within SDI Q1, while, in the middle- and lower-SEP tracts (SDI Q2 – Q4), NO2 and SDI showed a weak but positive correlation (Figure 2). The interquartile range for full-gestation maternal NO2 exposure was 6.25 ppb. NO2 and PM2.5 exposure estimates were correlated [Pearson rho = 0.81 (p < 0.001)], and both were weakly inversely correlated with SDI [NO2 rho = −0.12 (p < 0.05), PM2.5 rho = −0.11 (p < 0.05)].
Figure 2.
Maternal NO2 exposure estimates, by SDI quantiles. The most affluent quartile of tracts (SDI Q1) contains the highest and most varying NO2 levels.
3.1 Mutually-adjusted associations of SDI and NO2 with term birth weight
In Model 1, SDI showed a negative linear association with term birth weight, while NO2 exhibited negative non-linear associations with birth weight (Supplemental Figure 2), with strongest associations below approximately 20 ppb, flat between 20 to 30 ppb, and a shallow slope above 30 ppb. Gestational age, receipt of prenatal care, pre-pregnancy BMI, maternal age, and maternal education were positively associated with birth weight (Table 2). Offspring of US- and foreign-born Black, US-born Hispanic, and US- and foreign-born Asian mothers had lower average birth weights, as did births in later study years. Medicaid status and conception season did not significantly predict birth weight.
Table 2.
Linear coefficient estimates for change in term birth weight (g) for covariates from Models 1 –3.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Covariates | Effect estimate (g) | 95% CIs | Effect estimate (g) | 95% CIs | Effect estimate (g) | 95% CIs |
| Intercept | 2773.4 | 2746.2, 2800.6 | 2773.2 | 2746.0, 2800.4 | 2774.0 | 2739.2, 2808.7 |
|
| ||||||
| Ethnicity | ||||||
| US-born White [REF] | -- | -- | -- | -- | -- | -- |
| Foreign-born White | 5.7 | −1.2, 12.6 | 5.8 | −1.1, 12.7 | 5.2 | −1.5, 12.0 |
| US-born Black | −113.8 | −121.2, −106.3 | −113.3 | −120.7, −105.8 | −113.8 | −121.1, −106.4 |
| Foreign-born Black | −78.5 | −86.3, −70.8 | −77.9 | −85.6, −70.1 | −78.6 | −86.2, −70.9 |
| US-born Hispanic | −38.2 | −45.4, −30.9 | −37.9 | −45.1, −30.6 | −38.3 | −45.4, −31.1 |
| Foreign-born Hispanic | −1.4 | −8.1, 5.3 | −1.0 | −7.7, 5.7 | −1.6 | −8.1, 5.0 |
| US-born Asian | −104.5 | −120.3, −88.6 | −104.3 | −120.2, −88.4 | −104.8 | −120.4, −89.2 |
| Foreign-born Asian | −87.7 | −94.5, −80.8 | −87.5 | −94.4, −80.7 | −88.4 | −95.2, −81.7 |
|
| ||||||
| Maternal education | ||||||
| < 9 yrs. [REF] | -- | -- | -- | -- | -- | -- |
| 9 – 11 yrs. | 12.2 | 10.1, 25.6 | 12.2 | 4.9, 19.5 | 12.2 | 5.0, 19.4 |
| 12 yrs. (High school) | 17.5 | 41.0, 57.1 | 17.5 | 10.5, 24.6 | 17.5 | 10.7, 24.4 |
| 13 – 15 yrs. | 34.7 | 57.2, 74.2 | 34.8 | 27.3, 42.2 | 34.7 | 27.4, 41.9 |
| 16 yrs. (BA) | 36.9 | 66.1, 84.4 | 37.1 | 28.8, 45.4 | 37.1 | 28.9, 45.2 |
| > 16 yrs. | 36.1 | 50.8, 73.6 | 36.2 | 27.1, 45.4 | 36.3 | 27.3, 45.2 |
|
| ||||||
| Medicaid status | ||||||
| No [REF] | -- | -- | -- | -- | -- | -- |
| Yes | 1.5 | −3.0, 5.9 | 1.5 | −3.0, 5.9 | 1.3 | −3.1, 5.0 |
|
| ||||||
| Maternal age (years) | ||||||
| < 20 [REF] | -- | -- | -- | -- | -- | -- |
| 20 – < 25 | 17.8 | 10.4, 24.6 | 17.8 | 10.0, 25.5 | 17.7 | 10.0, 25.3 |
| 25 – < 30 | 49.0 | 27.3, 42.1 | 48.9 | 40.8, 56.9 | 48.9 | 40.8, 56.9 |
| 30 – < 35 | 65.7 | 28.6, 45.3 | 65.5 | 57.1, 74.0 | 65.7 | 57.3, 74.2 |
| 35 – < 40 | 75.2 | 27.0, 45.2 | 75.1 | 65.9, 84.2 | 75.2 | 65.9, 84.2 |
| ≥ 40 | 62.2 | −3.0, 5.9 | 62.1 | 50.6, 73.5 | 62.2 | 51.0, 73.4 |
|
| ||||||
| Pre-pregnancy BMI | ||||||
| < 18.5 (Underweight) [REF] | -- | -- | -- | -- | -- | -- |
| 18.5 – < 25 (Normal) | 95.3 | 87.8, 102.8 | 95.3 | 87.8, 102.8 | 95.3 | 87.9, 102.6 |
| 25 – < 30 (Overweight) | 159.7 | 151.6, 167.8 | 159.7 | 151.6, 167.8 | 159.7 | 151.8, 167.6 |
| ≥ 30 (Obese) | 215.5 | 207.0, 224.0 | 215.5 | 207.0, 224.0 | 215.6 | 207.2, 223.9 |
|
| ||||||
| Prenatal care received | ||||||
| No [REF] | -- | -- | -- | -- | -- | -- |
| Yes | 32.2 | 9.1, 55.2 | 32.2 | 9.2, 55.3 | 32.1 | 9.5, 54.7 |
|
| ||||||
| Previous live births | ||||||
| 0 [REF] | -- | -- | -- | -- | -- | -- |
| 1 | 68.4 | 64.3, 72.5 | 68.4 | 64.3, 72.5 | 68.3 | 64.3, 72.4 |
| 2 | 77.2 | 71.6, 82.8 | 77.3 | 71.7, 82.8 | 77.2 | 71.7, 82.7 |
| ≥ 3 | 76.9 | 70.3, 83.5 | 77.0 | 70.3, 83.6 | 76.7 | 70.2, 83.2 |
|
| ||||||
| Gestational age (weeks) | ||||||
| 37 [REF] | -- | -- | -- | -- | -- | -- |
| 38 | 198.8 | 191.7, 205.8 | 198.8 | 191.8, 205.8 | 198.8 | 191.9, 205.7 |
| 39 | 347.5 | 341.0, 354.0 | 347.5 | 341.0, 354.1 | 347.6 | 341.2, 354.0 |
| 40 | 454.8 | 448.2, 461.5 | 454.9 | 448.3, 461.6 | 454.9 | 448.1, 461.4 |
| 41 | 585.9 | 577.7, 594.1 | 585.9 | 577.7, 594.1 | 585.9 | 577.7, 594.1 |
| 42 | 648.5 | 627.6, 669.4 | 648.7 | 627.8, 669.7 | 648.5 | 628.0, 669.0 |
|
| ||||||
| Conception season | ||||||
| Dec – Feb [REF] | -- | -- | -- | -- | -- | -- |
| Mar – May | 1.4 | −3.6, 6.4 | 1.4 | −3.6, 6.3 | 1.5 | −3.4, 6.4 |
| Jun – Aug | 4.4 | −0.6, 9.3 | 4.2 | −0.7, 9.2 | 4.1 | −0.7, 9.0 |
| Sep – Nov | −2.3 | −7.1, 2.4 | −2.5 | −7.2, 2.3 | −2.5 | −7.2, 2.1 |
|
| ||||||
| Conception year | ||||||
| 2007 [REF] | -- | -- | -- | -- | -- | -- |
| 2008 | −11.7 | −16.9, −6.5 | −11.4 | −16.6, −6.2 | −11.3 | −16.4, −6.3 |
| 2009 | −17.8 | −23.4, −12.2 | −17.3 | −23.0, −11.6 | −16.8 | −22.4, −11.3 |
| 2010 | −30.4 | −38.9, −22.0 | −29.8 | −38.4, −21.2 | −28.7 | −37.1, −20.3 |
3.2 Modification of the NO2-birth weight association by SDI levels
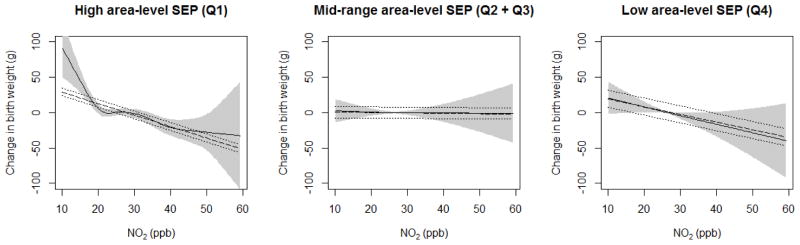
In Model 2, we observed decreasing term birth weight with increasing pollution exposures in the highest- and lowest-SDI quartiles, and a flat association in the middle-range SDI level (Q2 + Q3) (Figure 3). Among high-SEP tracts (SDI Q1), increasing NO2 below approximately 20 ppb, and above approximately 30 ppb, was associated with lower birth weights (p-value < 0.001). Among lower-SEP tracts (SDI Q4), there was a near-linear negative relationship between NO2 and birth weights (p = 0.05), and no association in the mid-range SDI group (p-value = 0.8).
Figure 3.
Exposure-response functions of NO2 with birth weight, at different levels of area-level SDI, adjusted for maternal SEP and covariates (Models 2 and 3). Shaded areas indicate 95% confidence intervals for non-linear association (Model 2). For comparison, linear slope and 95% confidence intervals in dashed lines (Model 3). Linear NO2-birth weight slopes (i.e., birth weight reduction) were −16.2 g (95% CI: −21.9, −10.5), 0.5 g (95% CI: −7.7, 8.7), and −11.0 g (95% CI: −22.8, 0.9) per 10 ppb increase in NO2, for SDI Q1, SDI Q2 + Q3, and SDI Q4 groups, respectively.
In Model 3, linear NO2-birth weight slopes (i.e., birth weight reductions) were −16.2 g (95% CI: −21.9, −10.5), 0.5 g (95% CI: −7.7, 8.7), and −11.0 g (95% CI: −22.7, 0.9) per 10 ppb increase in NO2, for the lowest, middle, and highest SDI groups (SDI Q1, SDI Q2 + Q3, and SDI Q4), respectively. Compared to the mid-range SDI group (reference), p-values for interaction for SDI Q1 and Q4 were < 0.001 and 0.09, respectively. Covariate estimates in Models 2 and 3 were unchanged from Models 1 (Table 2).
3.3 Sensitivity analyses
Tests for modification of the NO2-birth weight association by individual-level SEP indicators with adjustment for area-level deprivation were null or weak (Supplemental Table 3). We observed no evidence for modification by educational attainment, and modest non-significant modification by Medicaid status; among Medicaid-eligible mothers, each 10 ppb increase in NO2 conferred a 6.6 g decrement (95% CI: −13.1, −0.1) in birth weight, versus −14.0 g (95% CI: −19.3, −8.7) among non-eligible mothers. Similarly, we observed attenuated NO2-birth weight associations among foreign-born White and Asian mothers – a 10 ppb increase in NO2 was associated with birth weight decrements of 4.9 g (95% CI: −14.1, 4.3) and 0.3 g (95% CI: −9.6, 9.0), respectively – versus greater decrements among US-born White and US- and foreign-born Black and Hispanic mothers [−15.8 g (95% CI −22.4, −9.2); −15.4 g (95% CI: −28.5, −2.3); −16.5 g (−30.1, −2.9); −14.8 g (95% CI: −25.8, −3.8); and −11.6 g (95% CI: −21.2, −2.0), respectively].
Adjusting for PM2.5 co-exposures did not change covariate coefficient estimates across all models (Supplemental Table 4). A smooth term for PM2.5 added to Model 1 appeared slightly protective above approximately 20 μg/m3, but was not statistically significant (Supplemental Figure 3). In Model 2, adding a smooth term for PM2.5 was not statistically significant (p-value = 0.6), and did not substantively alter the NO2-SDI interaction result (see Supplemental Figure 4); non-linear inverse association between NO2 and birth weight, which was most pronounced in the least-deprived areas, null in the mid-range of deprivation, and inverse (near-significant) among the most-deprived areas (p-values < 0.001, 0.9, and 0.08, respectively). Likewise in Model 3, adding a linear term for PM2.5 produced similar NO2-birth weight slopes (i.e., birth weight reductions): −16.8 g (95% CI: −24.1, −8.0), −0.8 g (95% CI: −10.5, 9.0), and −11.7 g (95% CI: −24.3, 2.4) per 10 ppb increase in NO2, for the lowest, middle, and highest SDI groups, respectively. Compared to the mid-range SDI group (reference), p-values for interaction for SDI Q1 and Q4 were < 0.001 and 0.08, respectively.
4. Discussion
Our findings indicate complex spatial patterning of air pollution and deprivation in NYC. The non-linear relationship between gestational air pollution exposure and area-level deprivation we observed are consistent with the one other NYC analysis of their joint spatial distribution (Hajat et al. 2013), and echo other studies reporting higher air pollution concentrations in more affluent urban areas of Los Angeles County (Molitor et al. 2011) and Rome, Italy (Forastiere et al. 2007). While the spatial heterogeneity in deprivation and air pollution vary across cities and regions, they appear to be positively correlated in many US cities (Bell and Ebisu 2012; Miranda et al. 2011; Gray et al. 2013); better understanding their joint distributions may be important for discerning mixed evidence for deprivation as a modifier of the air pollution-birth weight association.
Our interaction results indicate differences in birth weight decrements along different parts of the exposure-response curve, in linear and non-linear models. The relatively steep exposure-response function describing mothers in the most-affluent quartile of census tracts (SDI Q1) may be due to higher average and more variable near-residence pollution exposures among this group. By comparison, the NO2-birthweight slope was somewhat lower (though not significantly so) in the most-deprived quartile (SDI Q4), where pollution exposures were also lower. Alternately, this differential association by SDI may be due to unmeasured deprivation-related behavioral (e.g., time-activity patterns) or structural factors, potentially associated with both air pollution and birth outcomes. However, the varying distribution of the estimated NO2 exposures across the SDI gradient raises challenges for interpreting the differences in NO2-birth weight slopes as “modification,” because the observed differences in the slopes may also be due to the difference in NO2’s variance and concentration ranges.
The magnitude of our findings for linear effects of NO2 on birth weight, across deprivation levels, are comparable to some US studies (Bell at al. 2007; Darrow et al. 2011). Though few studies have examined modification of air pollution effects on birth outcomes by area-level SEP, the majority have found heightened associations in lower-SEP areas (Wilhelm and Ritz 2003; Morello-Frosch et al. 2010; Grey et al. 2014). Specifically, Morello-Frosch et al. (2010) found approximately 13 g decrements, on average, in birth weight per 10 ppb increase in NO2 among mothers living in census tracts with ≥ 22% of residents living in households with income under the FPL, vs. lesser, but statistically significant, negative associations in areas with 0 to 22% of residents living in poverty (approximately 6 to 9 g decrements per 10 ppb NO2). In comparison to this step-wise exposure-response relationship, we observed similar magnitude decrements in higher-SEP tracts [−16.2 g (95% CI: −21.9, −10.5) per 10 ppb increase in NO2], but null and weaker effects in mid-range and lower-SEP tracts. Gray et al. (2014) found increased odds of adverse birth outcomes among mothers residing in census tracts with lower mean household income, but found no significant interaction with PM2.5 or O3, potentially due to low variability in modeled air pollution exposures by area-level SEP across North Carolina. In contrast, Généreux et al. (2008) found that closer residential proximity to a highway conferred greater odds of low birth weight only among mothers in the wealthiest areas of Montréal, Canada, though mothers in the poorest areas were more likely to live within 200m of a highway. Further studies are needed to understand whether these mixed results are a function of locally-specific differences in exposure and/ or susceptibility patterns, or to different deprivation metrics and/ or air pollution exposure assignment methods.
4.1 Limitations
Though we sought to minimize uncertainty in exposure assignment, our air pollution exposure assessment was limited because near-residence estimates (a) do not encompass daily activities, and (b) assume that the mother maintained the same residential location recorded at the time of birth for the full gestation. Though we tested adjustment for co-pollutant PM2.5 exposures, our use of the total mass concentration, instead of specific constituents, may have obscured impacts of key elevated PM2.5 constituents in NYC, the spatial distributions of which may not be accurately captured by the total mass distribution [e.g., nickel (NYC DOHMH 2010)]. Likewise, our area-level deprivation assessment was conducted using census tract units, which may be poor proxies for lived neighborhood spaces (Diez Roux 2001).
4.2 Strengths
The primary strength of this analysis is our fine-scale, spatially-informed exposure assignment for both air pollution and contextual deprivation. Adapting Messer et al.’s (2006) method for calculating the socioeconomic deprivation index bolsters comparability with other investigations of area-level deprivation and birth outcomes. Here, we employ spatial regimes for improving accuracy and local-specificity in estimating contextual deprivation, potentially of particular interest in studies of joint effects of social and environmental exposures. Importantly, spatial regimes can be identified and evaluated empirically using geostatistical techniques (e.g., LISA) commonly used in econometrics (Paelinck and Klaassen 1979; Anselin 2009), and more recently in air pollution modeling (Sampson et al. 2013). These methods offer promising approaches for environmental health research, especially where exposure-outcome relationships may be heterogeneous across space. Another strength is our consideration of non-linear exposure-response relationships and non-linear interactions, and our comparison to linear models. We adjusted for multiple maternal SEP indicators, and tested whether our observed area-level deprivation modification was driven by compositional, rather than contextual, factors. In keeping with the “ethnic framework” for birth outcomes research (Janevic et al. 2010), we included both maternal ethnicity and nativity (i.e., US- vs. foreign-born).
5. Conclusion
Our findings suggest possible differential associations between air pollution and fetal growth by area-level socioeconomic deprivation, but also illustrate the complexity in determining the “interaction” of these risk factors because of their uneven joint distribution. Spatially-refined exposure assessment and a flexible modeling approach suggest where adverse birth outcomes may arise from disproportionate exposure burdens, or from differential susceptibility to exposures. The apparent role of contextual deprivation impacts, as distinct from individual-level and compositional impacts, reinforces the need to design studies to disentangle which components of area-level deprivation may be driving differential susceptibility, and to elucidate their physiological and/ or behavioral mechanisms (Clougherty et al. 2014).
Supplementary Material
Highlights.
We examined prenatal NO2 exposure, socioeconomic context and term birth weight.
We observed highest air pollution levels in least-deprived areas of NYC.
NO2 was associated with lower birth weight in the least- and most-deprived areas.
Complex pattern of exposure complicates interpretation of interaction models.
Acknowledgments
Funding: This research was supported by the National Institute of Environmental Health Sciences (grant # 1R01ES019955-01 to Brown University) and by the U.S. Environmental Protection Agency (grant # R834576 to the University of Pittsburgh).
ABBREVIATIONS
- ACS
US Census American Community Survey
- BMI
body mass index
- FPL
Federal Poverty Level
- IQR
inter-quartile range
- LISA
Local Indicators of Spatial Association
- NO2
nitrogen dioxide
- NYCCAS
New York City Community Air Survey
- PCA
principle component analysis
- PM2.5
fine particulate matter with aerodynamic diameter < 2.5 microns
- SD
standard deviation
- SDI
socioeconomic deprivation index
- SEP
socioeconomic position
- SPARCS
New York State Department of Health Statewide Planning and Research Cooperative System
- US
United States
Footnotes
Conflict of interest: The authors declare no competing financial or non-financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anselin L. Local Indicators of Spatial Association – LISA. Geographical Analysis. 1995;27:93–115. [Google Scholar]
- Anselin L. Thirty Years of Spatial Econometrics. Tempe, AZ: GeoDa Center for Geospatial Analysis and Computation; 2009. [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1253–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115(7):1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ Health Perspect. 2012;120(12):1699–1704. doi: 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsley DA, Kuh E, Welsch RE. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. Hoboken, NJ: Wiley-Interscience; 2005. [Google Scholar]
- Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman P. Socioeconomic Disparities in Adverse Birth Outcomes: A Systematic Review. Am J Prev Med. 2010;39(3):263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Oxidative Stress. Best Pract Res Clin Obstet Gynaecol. 2011;25:287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LP, Millet DB, Marshall JD. National patterns in environmental injustice and inequality: outdoor NO2 air pollution in the United States. PLoS One. 2014;9(4):e94431. doi: 10.1371/journal.pone.0094431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty JE, Kubzansky LD. A Framework for Examining Social Stress and Susceptibility to Air Pollution in Respiratory Health. Environ Health Perspect. 2009;117(9):1351–1358. doi: 10.1289/ehp.0900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clougherty JE, Kheirbek I, Eisl HM, Ross Z, Pezeshki G, Gorzynski JE, et al. Intra-urban spatial variability in wintertime street-level concentrations of multiple combustion-related air pollutants: The New York City Community Air Survey (NYCCAS) J Expo Sci Environ Epidemiol. 2013;23(3):232–240. doi: 10.1038/jes.2012.125. [DOI] [PubMed] [Google Scholar]
- Clougherty JE, Shmool JLC, Kubzansky LD. The Role of Non-Chemical Stressors in Mediating the Socioeconomic Susceptibility to Environmental Chemicals. Curr Envir Health Rpt. 2014 doi: 10.1007/s40572-014-0031-y. [DOI] [Google Scholar]
- Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121(3):267–373. doi: 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow LA, Klein M, Strickland MJ, et al. Ambient air pollution and birth weight in full-term infants in Atlanta, 1994–2004. Environ Health Perspect. 2011;119(5):731–737. doi: 10.1289/ehp.1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV. Investigating Neighborhood and Area Effects on Health. Am J Public Health. 2001;91(11):1783–89. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forastiere F, Stafoggia M, Tasco C, Picciotto S, Agabiti N, Cesaroni G, et al. Socioeconomic Status, Particulate Air Pollution and Daily Mortality: Differential Exposure or Differential Susceptibility. Am J Ind Med. 2007;50(3):208–216. doi: 10.1002/ajim.20368. [DOI] [PubMed] [Google Scholar]
- Gee GC, Payne-Sturges DC. Environmental health disparities: a framework for integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112(17):1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Généreux M, Auger N, Goneau M, Daniel M. Neighborhood socioeconomic status, maternal education and adverse birth outcomes among mothers living near highways. J Epidemiol Community Health. 2008;62(8):695–700. doi: 10.1136/jech.2007.066167. [DOI] [PubMed] [Google Scholar]
- Gray SC, Edwards SE, Schultz BD, Miranda ML. Assessing the impact of race, social factors and air pollution on birth outcomes: a population-based study. Environ Health. 2014;13(4) doi: 10.1186/1476-069X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux AV, Adar SD, Auchincloss AH, Lovasi GS, O’Neill MS, Sheppard L, Kaufman JD. Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2013;121(11–12):1325–1333. doi: 10.1289/ehp.1206337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (IOM) Toward Environmental Justice: Research, Education, and Health Policy Needs. Washington DC: The National Academies Press; 1999. [PubMed] [Google Scholar]
- Janevic T, Stein CR, Savitz DA, Kaufman JS, Mason SM, Herring AH. Neighborhood Deprivation and Adverse Birth Outcomes among Diverse Ethnic Groups. Ann Epidemiol. 2010;20(6):445–451. doi: 10.1016/j.annepidem.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ Health Perspect. 2006;114(11):1636–1641. doi: 10.1289/ehp.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures – the public health disparities geocoding project. Am J Public Health. 2003;93(10):1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matte TD, Ross Z, Kheirbek I, Eisl H, Johnson S, Gorczynski JE, et al. Monitoring intraurban spatial patterns of multiple combustion air pollutants in New York City: design and implementation. J Expo Sci Environ Epidemiol. 2013;23(3):223–231. doi: 10.1038/jes.2012.126. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann NY Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J, et al. The Development of a Standardized Neighborhood Deprivation Index. J Urban Health. 2006;83(6):1041–1062. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda ML, Edwards SE, Keating MH, Paul CJ. Making the environmental justice grade: the relative burden of air pollution exposure in the United States. Int J Res Public Health. 2011;8(6):1755–1771. doi: 10.3390/ijerph8061755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitor J, Su JG, Molitor NT, Rubio VT, Richardson S, Hastie D, et al. Identifying vulnerable populations through an examination of the association between multipollutant profiles and poverty. Environ Sci Technol. 2011;45(18):7754–7760. doi: 10.1021/es104017x. [DOI] [PubMed] [Google Scholar]
- Morello-Frosch R, Shenassa ED. The environmental “riskscape” and social inequality: implications for explaining maternal and child health disparities. Environ Health Perspect. 2006;114(8):1150–1153. doi: 10.1289/ehp.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Suano A, Hamilton AB, Betz Gimme shelter: the immune system during pregnancy. Immunol Rev. 2011;241(1):20–38. doi: 10.1111/j.1600-065X.2011.01002.x. [DOI] [PubMed] [Google Scholar]
- Ness RB, Bodnar L, Holzman C, Platt RW, Savitz DA, Shaw GM, et al. Thoughts on the Future of Reproductive and Perinatal Epidemiology. Pediatr Perinat Epidemiol. 2012;27(1):11–19. doi: 10.1111/ppe.12017. [DOI] [PubMed] [Google Scholar]
- New York City Department of Health and Mental Hygiene (NYC DOHMH) Nickel Concentrations in Ambient Fine Particles: Winter Monitoring, 2008–2009. New York, NY: 2010. New York City Community Air Survey. [Google Scholar]
- Nweke OC, Payne-Sturges D, Garcia L, Lee C, Zenick H, Grevatt P, et al. Symposium on Integrating the Science of Environmental Justice into Decision-Making at the Environmental Protection Agency. Am J Public Health. 2011;101(S1):S19–S29. doi: 10.2105/AJPH.2011.300368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Campo P, Burke JG, Culhane J, Elo IT, Eyster J, Holzman C, et al. Neighborhood deprivation and preterm birth among non-Hispanic Black and White women in eight geographic areas in the United States. Am J Epidemiol. 2008;167(2):166–163. doi: 10.1093/aje/kwm277. [DOI] [PubMed] [Google Scholar]
- Paelinck J, Klaassen L. Spatial Econometrics. Farnborough, England: Saxon House; 1979. [Google Scholar]
- Ponce NA, Hoggart KJ, Wilhelm M, Ritz B. Preterm Birth: The interaction of Traffic-related Air Pollution with Economic Hardship in Los Angeles Neighborhoods. Am J Epidemiol. 2005;162(2):140–148. doi: 10.1093/aje/kwi173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KE, Pearl M. Multilevel analyses of neighborhood socioeconomic context and health outcomes: a critical review. J Epidemiol Community Health. 2001;55(2):111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross Z, Ito K, Johnson S, Yee M, Pezeshki G, Clougherty JE, et al. Spatial and temporal estimation of air pollutants in New York City: exposure assignment for use in a birth outcomes study. Environ Health. 2013;12(51) doi: 10.1186/1476-069X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Richards M, Szpiro AA, Bergen S, Sheppard L, Larson TV, Kaufman JD. A regionalized national universal kriging model using Partial Least Squares regression for estimating annual PM2.5 concentrations in epidemiology. Atmos Environ. 2013;75:383–392. doi: 10.1016/j.atmosenv.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz DA, Bobb JF, Carr JL, Clougherty JE, Dominici F, Elston B, et al. Ambient Fine Particulate Matter, Nitrogen Dioxide, and Term Birth Weight in New York, New York. Am J Epidemiol. 2014;179(4):457–466. doi: 10.1093/aje/kwt268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton K, Linder SH. Cumulative risk assessment for combined health effects from chemical and nonchemical stressors. Am J Public Health. 2011;101S(1):S81–S88. doi: 10.2105/AJPH.2011.300118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Bellinger D, Glass T. Exploring potential sources of differential vulnerability and susceptibility in risk from environmental hazards to expand the scope of risk assessment. Am J Public Health. 2011;101(Supp 1):S94–S101. doi: 10.2105/AJPH.2011.300272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS, Balkhair T Knowledge Synthesis Group on Determinants of Preterm/LBW births. Air pollution and birth outcomes: a systematic review. Environ Int. 2011;37(2):498–516. doi: 10.1016/j.envint.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environ Res. 2012;117(2012):100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Strand LD, Barnett AG, Tong S. Methodological challenges when estimating the effects of season and seasonal exposures on birth outcomes. BMC Med Res Methodol. 2011;11:49–57. doi: 10.1186/1471-2288-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian N, Xue J, Barzyk TM. Evaluating socioeconomic and racial differences in traffic-related metrics in the United States using a GIS approach. J Expos Sci Environ Epidemiol. 2013;23(2):215–222. doi: 10.1038/jes.2012.83. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ritz B. Residential proximity to traffic and adverse birth outcomes in Los Angeles county, California, 1994–1996. Environ Health Perspect. 2003;111(2):207–216. doi: 10.1289/ehp.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN. Thin plate regression splines. J R Stat Soc B. 2003;65(1):95–114. [Google Scholar]
- Woodruff TJ, Parker JD, Darrow LA, Slama R, Bell M, Choi H, et al. Methodological issues in studies of air pollution and reproductive health. Environ Res. 2009;109(3):311–320. doi: 10.1016/j.envres.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.