Abstract
Background: The present study aimed to investigate the Nailfold Capillaroscopy (NC) features of the patients with dermatomyositis (DM) and its correlation with their disease activity indices, physical findings, and laboratory results.
Methods: The present cross-sectional study was conducted on 27 DM patients above 16 years old who had referred to an(there are 3 clinics not one) outpatient rheumatology clinics from 2012 to 2013. Nailfold capillaroscopy and calculation of disease activity indices were performed separately for all the patients by two rheumatologists who were blinded to each other's results. Statistical analyses were performed using chi-square and Mann-Whitney U tests.
Results: The mean age of the patients was 39.2±14.1 years with the mean disease duration of 13.1±15.2 months (range: 1-72 months). Myopathic electromyography (EMG) findings showed a strong association with scleroderma pattern (p=0.015). However, disease activity in each organ system and global disease activity showed no significant association between scleroderma pattern and other NC findings. (Disease activity in each organ system and also global disease activity were both assessed to see if they are associated with scleroderma pattern and other NC findings so if we use between it means we are looking for an association between scleroderma pattern and other NC findings and this is not what we have done and is wrong.)
Conclusion: This study revealed no significant relationship between disease activity indices and NC features. Thus, it may be more precise to interpret the results of NC in conjunction with other physical and laboratory findings.
Keywords: Dermatomyositis, Nails, Capillaroscopy
Introduction
Dermatomyositis (DM) is a chronic and idiopathic inflammatory disorder involving the skin and muscles. However, comprehensive epidemiologic data are not available for determining the true incidence and prevalence of DM since most studies are different regarding sex, age, and location (1). Some studies have reported the annual incidence of DM to be fewer than 10 per million individuals (2-6). DM has a bimodal peak of incidence, one at 5-14 and the other at 45-64 years of age. Inaddition, women are affected two to three times more than men. Considering the immunohistopathology and response to immunosuppression, DM is an immune-mediated disorder (7).
Bohan and Peter proposed criteria for diagnosis and classification of DM. These criteria rely on symmetric proximal muscle weakness, typical rash of DM, elevated serum muscle enzymes, and myopathic changes on electromyography (8,9). Besides, the classic muscular manifestation is slowly progressive proximal muscle weakness. The patients suffering from DM complain about difficulties in climbing up the steps, arising from chair, or combing and washing their hair. Afterwards, it may involve the distal parts, as well (10). Some major dermatologic features of this disorder include Gottron papules (11,12), heliotrope rash, Gottron sign (12), shawl sign, V sign (13), Mechanic's hands (10), nailfold telangiectasia, and prominent periungual erythema (11). Other organs, such as lung, heart, and esophagus, are involved, as well. Moreover, the cardiac manifestations are pericarditis, myocarditis, arrhythmias, and conduction blocks (14-16). Furthermore, Interstitial Lung Disease (ILD) and hypoventilation are the common pulmonary complications which are evaluated by pulmonary function test and high resolution CT scanning (17-19). Serum level of Creatine Kinase (CK) is the most common enzyme used for evaluation of myositis. Laboratory test abnormalities are common in polymyositis and dermatomyositis and differ among clinical and demographic groups (20-22). Other enzymes found to be abnormally high in DM are aldolase, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and/or lactate dehydrogenase (LDH) (23). Overall, several myositis specific antibodies are found in myositis patients with anti-Jo-1 antibody being the most common one; however, it is not commonly seen in DM (24).
Microvascular involvement is a remarkable characteristic in DM (25-27). Activation and deposition of complement leads to reduction of capillary density and the subsequent compensatory dilation of the remaining capillaries (28). Small vessels could be easily visualized at nailfolds. Nailfold Capillaroscopy (NC) is a noninvasive, reproducible, and inexpensive imaging technique for evaluating microcirculation in vivo (29-33). It is accomplished by magnification of microvascular structures. Commonly, DM shows characteristic patterns in NC. Systemic sclerosis and its related disorders have also similar capillaroscopic features (34,35). These patterns may even be useful in diagnosis (36-38).
Nailfold capillaroscopy findings have been well described in DM, but their associations with the disease activity have rarely been investigated especially in adults (39-44). It is noteworthy that similar studies have never been conducted in the Iranian population. Direct visualization and assessment of nailfold capillaries may help us evaluate the disease activity (40,45-48). According to what was mentioned above, we hypothesized that NC might be a useful and reliable tool for assessing the disease activity. Thus, the present study aims to determine any possible correlation between NC changes and the disease activity.
Methods
Patients
This cross-sectional study was conducted at the rheumatology clinics affiliated to Shiraz University of Medical Sciences from March 2012 to March 2013.
The inclusion criteria of the study were: (1) being above 16 years old, (2) fulfilling the criteria of Bohan and Peter for definite and probable DM (8, 9), and (3) having at least six evaluable nailbeds for capillaroscopy.
On the other hand, the exclusion criteria of the study were diagnosis of overlap syndrome, having a history of diabetes mellitus, and smoking. The patients whose fingers where hard to look through including those with very thick skin and traumatized fingers were excluded from the study, as well.
The study was explained to all the participants and written informed consent was obtained from each one. After all, 27patients fulfilling all the inclusion criteria of the study were enrolled into the investigation. For each participant, a questionnaire was completed that included items on demographic characteristics, disease duration, a checklist of inclusion and exclusion criteria, the results of the disease activity evaluation, and NC features.
The eligible patients were evaluated by the principal investigator for their disease activity. Afterwards, they were referred to an experienced rheumatologist for NC. It should be mentioned that the principal investigator was blind to the NC results. The rheumatologist accomplishing NC was blind to the results of the clinical assessment, as well.
Clinical assessment
In order to evaluate the disease activity, Myositis Disease Activity Assessment Visual Analog Scale (VAS) (MYOACT) section of Myositis Disease Activity Assessment Tool (MDAAT) version 2-2005 was used. MYOACT is the physician's assessment of the disease activity in various organ systems using a VAS. In this study, constitutional symptoms along with cutaneous, skeletal, gastrointestinal, pulmonary, and cardiac involvement were assessed. There is a 10-cm VAS for each organ system in MYOACT. It is used to score the overall severity of the disease activity in each organ system and a global extramuscular VAS (49).
Nailfold capillaroscopy technique
Before NC, each patient spent at least twenty minutes at room temperature (20-25˚C). All the patients were asked to avoid caffeinated drinks at least four hours before NC. Nailfold capillaroscopy was carried out using a stereomicroscope (Euromax ST1740, Netherlands) with ×250 magnification. Immersion oil was applied on nailfold bed to improve resolution. In this study, eight fingers of the two hands excluding the thumbs were assessed. The fingers with thick nailfolds and ulcerated ones were not studied. We also took a photograph of each evaluated nailfold.
Interpretation of nailfold capillaroscopy findings
The following parameters were analyzed in each patient: (1) Dimension of the larger part of the capillary loop was measured and >0.020mm was reported as dilated loop; giant loops >0.050mm and their mean number were reported, too (Fig. 1) (50), (2) avascular areas defined as the absence of 2 or more successive capillaries (51), (3) capillary loop length considered as normal and elongated if the length of the visible part of the capillary relative to the venular plexus was <300 and ≥300 µm, respectively (Fig. 2) (36), (4) the patterns of microhemorrhages consisting of pericapillary and punctate, pericapillary, confluent, and no hemorrhages (Fig. 3), (5) blood flow patterns which were categorized as normal, stasis and slowing, and flocculation, (6) presence of disturbed distribution of capillaries to the nailbeds, (7) mean number of capillaries in one millimeter, and (8) morphology that was categorized as normal hairpin, subtle changes, and anomaly. Tortuosity and crossing were considered as subtle changes. Besides, anomalies included meandering, ectasia, ramification, bushy loops (Fig. 4), arborization, branching (neoangiogenesis) (Fig. 5), and bizarre shape capillaries (50,52).
Fig. 1 .
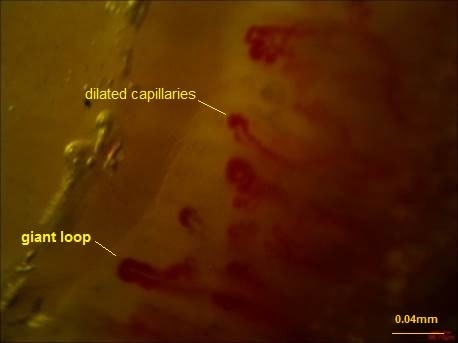
Dilated capillaries and giant loop (DrZareinejad)
Fig. 2 .
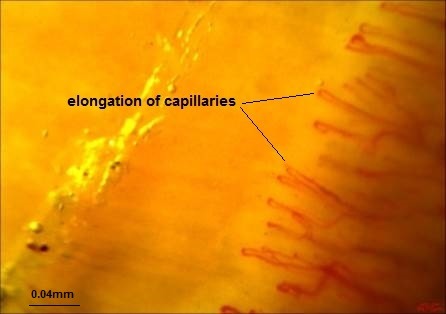
Elongation of capillaries (DrZareinejad)
Fig. 3 .
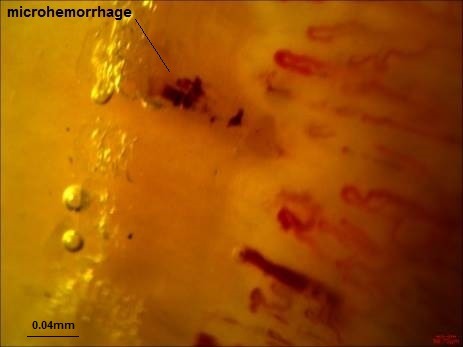
Microhemorrhage (Dr. Zareinejad)
Fig. 4 .
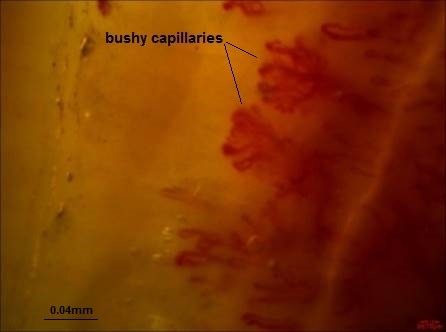
Bushy capillaries (Dr. Zareinejad)
Fig. 5 .
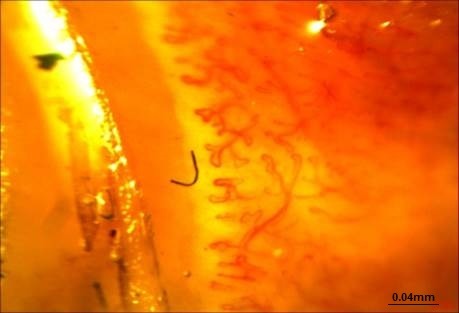
Neoangiogenesis (Dr. Zareinajd)
The early scleroderma pattern (Sclearly) is defined by a few capillary hemorrhages, a few (<4 altered capillaries/mm) giant capillaries, and relatively well-preserved capillary distribution without obvious loss of capillaries. On the other hand, the active scleroderma pattern (Scl- active) is defined by frequent capillary hemorrhages, frequent (>6 altered capillaries/mm) giant capillaries, a moderate loss (20-30%) of capillaries, mild disorganization of the capillary architecture, and absent or mildly ramified capillaries. Yet, the late scleroderma pattern (Scl-late) is defined by few or absent giant capillaries and hemorrhages, an irregular enlargement of the capillaries, severe loss (50-70%) of capillaries with extensive avascular areas, disorganization of the normal capillary array, and ramified/bushy capillaries (50).
Statistical analysis
All the statistical analyses were performed using SPSS software, v. 15 (SPSS, Chicago, IL, USA). The results were expressed as crude frequencies and percentages where appropriate. Descriptive results for numeric variables were presented as the mean value ± standard deviation (SD). Chi-square test and Mann-Whitney U test were used to compare the variables between the study groups. Besides, P value < 0.05 was considered as statistically significant.
Results
The present study was conducted on 27 patients, including 23 females (85.2%) and four males (14.8%). The mean age of the participants was 39.2±14.1 years with the mean disease duration of 13.1±15.2 months (range: 1-72 months). In addition, 25 patients (92.6%) were diagnosed as definite DM and two (7.4%) as probable DM.
Clinical and laboratory findings
The clinical and laboratory findings are summarized in Table 1.
Table 1 . Presence of different clinical and laboratory findings in patients with dermatomyositis .
| Present n(%) | Absence n(%) | |
| Pyrexia | 8(29.7%) | 19(70.3%) |
| Weight loss | 15(55.5%) | 12(45.5%) |
| Fatigue | 20(74.1%) | 7(25.9%) |
| Cutaneous ulceration | 12(45.5%) | 15(55.5%) |
| Erythroderma | 12(45.5%) | 15(55.5%) |
| Panniculitis | 1(3.7%) | 26(96.3%) |
| Erythematous rashes: withsecondary changes* withoutsecondary changes |
17(62.9%) 16(59.3%) |
10(37.1%) 11(40.7%) |
| Heliotrope rash | 12(45.5%) | 15(55.5%) |
| Gottron’s papules/sign | 24(88.9%) | 3(11.1%) |
| Periungual capillary changes | 25(92.6%) | 2(7.4%) |
| Alopecia Diffuse hair loss Focal, patchy with erythema |
9(33.3%) 1(3.7%) |
18(66.7%) 26(96.3%) |
| Mechanics hands | 12(45.5%) | 15(55.5%) |
| Arthritis Severe active polyarthritis Moderately active arthritis Mild arthritis |
4(14.8%) 5(18.5%) 6(22.2%) |
23(85.2%) 22(81.5%) 21(77.8%) |
| Arthralgia | 15(55.5%) | 12(45.5%) |
| Dysphagia: Moderate/severe dysphagia Mild dysphagia |
7(25.9%) 7(25.9%) |
20(74.1%) 20(74.1%) |
| Abdominal pain Severe Moderate Mild |
1(3.7%) 0 0 |
26(96.3%) 27(100%) 27(100%) |
| xRespiratory muscle weakness withoutILD†: Dyspnea at rest Dyspnea on exertion |
0 0 |
27(100%) 27(100%) |
| Active reversible ILD Dyspnea or cough due to ILD Parenchymal abnormalities on chest x-ray or HRCT‡ and/or ground glass shadowing on HRCT Pulmonary Function Tests: ≥ 10% change in FVC§ |
8(29.7%) 8(29.7%) 8(29.7%) |
19(70.3%) 19(70.3%) 19(70.3%) |
| Dysphonia: Moderate to severe Mild |
1(3.7%) 2(7.4%) |
26(96.3%) 25(92.6%) |
| Pericarditis | 1(3.7%) | 26(96.3%) |
| Myocarditis | 0 | 27(100%) |
| Arrhythmia: Severe arrhythmia Other arrhythmia, except sinus tachycardia |
0 0 |
27(100%) 27(100%) |
| Sinus tachycardia | 7(25.9%) | 20(74.1%) |
| Myositis: Severe muscle inflammation Moderate muscle inflammation Mild muscle inflammation |
17(62.9%) 19(70.3%) 19(70.3%) |
10(37.1%) 8(29.7%) 8(29.7%) |
| Myalgia | 4(14.8%) | 23(85.2%) |
*e.g. accompanied by erosions, vesiculobullous change or necrosis, †ILD: interstitial lung disease, ‡HRCT: high resolution CT scan, §FVC: Functional vital capacity
Nailfold capillaroscopy findings
1. Distribution: Distribution was normal in 2 patients (7.4%) and abnormal in 25 ones (92.6%).
2. Morphology: Overall, 8 patients (29.6%) had subtle changes among whom, five had tortuosity and four had crossing (One patient showed both changes). Besides, different patterns of anomalies were detected in 22 patients (81.4%). Anomaly patterns are summarized in Table 2.
Table 2 . Presence of different anomalous morphologies in our patients with dermatomyositis .
| Presence, n(%) | Absence, n(%) | |
|
Neoangiogenesis Meandering Ectasia Bizarre shape capillaries |
20(74%) 3(11.1%) 1(3.7%) 6(22.2%) |
7(26%) 24(89.9%) 26(96.3%) 21(77.8%) |
3. Dimension: In this study, twenty four patients (88.9%) had abnormal dimensions among whom, 16 showed giant loops.
4. Avascular area: Avascular area was detected in 15 patients (55.6%).
5. Elongated capillary loops: A total of five patients (18.5%) presented this finding.
6. Hemorrhage: In this study, eighteen patients (66.7%) had hemorrhagic patterns among whom, 15, 5, and 1 had punctuate, pericapillary, and large confluent hemorrhage, respectively. In addition, three patients had both punctuate and pericapillary patterns.
7. Blood flow: A total of six patients (22.2%) showed abnormal blood flow.
8. Scleroderma pattern: Scleroderma pattern was noted in 24 patients (88.9%) among whom, early, active, and late patterns were observed in 1, 9, and 14 patients, respectively. However, three patients had nonspecific patterns.
9. Mean capillary density: The mean capillary density was 5.8±1.8mm.
Association between clinical, laboratory findings and different scleroderma patterns (we here, wanted to know if clinical findings and laboratory findings were associated with different scleroderma patterns or not, if we omit “and” we consider them as 3 groups with the same value but here, we did not search for association between clinical findings and laboratory findings. As a matter of fact clinical and laboratory findings are one group and different scleroderma patterns are the other group.
The association between different scleroderma patterns and clinical and laboratory findings and their frequencies is summarized in Table 3. At the time of investigation, 19 patients (70.3%) had myopathic electromyography (EMG) findings which showed a strong association with the scleroderma pattern (p=0.015).
Table 3 . Association between different scleroderma patterns and clinical or laboratory features .
| Features | Scleroderma pattern | ||||
| Early pattern n (%) | Active pattern n (%) | Late pattern n (%) | Non-specific pattern n (%) | p | |
|
Symptoms and signs, % |
|||||
| Fever | 1(16.7%) | 3(50%) | 1(16.7%) | 1(16.7%) | 0.20 |
| Heliotrope rash | 0 | 6(50%) | 4(33.3%) | 2(16.7%) | 1 |
| Gottron papules | 1(4.3%) | 8(34.8%) | 11(47.8%) | 3(13%) | 1 |
| Gottron's sign | 0 | 8(42.1%) | 10(52.6%) | 1(5.3%) | 0.56 |
| V sign | 1(4.3%) | 2(28.6%) | 3(42.9%) | 1(14.3%) | 0.76 |
| Shawl sign | 0 | 2(66.7%) | 1(33.3%) | 0 | 0.41 |
| Preorbital edema | 1(16.7%) | 2(33.3%) | 2(33.3%) | 1(16.7%) | 0.53 |
| Calcification | 0 | 5(50%) | 4(40%) | 1(10%) | 0.60 |
| Mechanic hand | 1(8.3%) | 4(33.3%) | 6(50%) | 1(8.3%) | 0.60 |
| Facial erythema | 1(16.7%) | 7(46.7%) | 6(40%) | 1(6.7%) | 0.06 |
| Arthritis | 1(25%) | 1(25%) | 1(25%) | 1(25%) | 0.71 |
| Polyarthralgia | 1(16.7%) | 7(46.7%) | 6(40%) | 1(6.7%) | 0.06 |
| ILD* | 0 | 3(33.3%) | 4(44.4%) | 2(22.2%) | 0.41 |
| Dysphasia | 0 | 3(37.5%) | 4(50%) | 1(12.5%) | 1 |
| Muscle weakness | 0 | 9(45%) | 9(45%) | 2(10%) | 0.56 |
| Laboratory findings, % | |||||
| CK† | 25% | 17.9% | 7.1% | 50% | 0.29 |
| ESR‡ | 1(5.5%) | 8(44.5%) | 7(38.9%) | 2(11.1%) | 0.16 |
| CRP§ | 0 | 2(40%) | 2(40%) | 1(20%) | 1 |
| ANA|| | 1(7.7%) | 5(38.5%) | 7(53.8%) | 0 | 0.11 |
| Anti RO antibody | 0 | 0 | 4(100%) | 0 | 0.46 |
| Anti JO1 antibody | 0 | 2(50%) | 2(50%) | 0 | 0.71 |
| Myopathic EMG¶ | 1(5.3%) | 9(47.3%) | 8(42.1%) | 1(5.3%) | 0.015 |
*ILD: Interstitial lung disease, †CK: creatine kinase, ‡ESR: Erythrocyte sedimentation rate. A level of >12 in male and >18 in female was considered abnormal [52]. §CRP: C-Reactive Protein. A level of >5 mg/L was considered as abnormal.
||ANA: Antinuclear antibody, ¶EMG: electromyography
Association between myositis disease activity organ systems and scleroderma pattern
Table 4 summarizes the association between myositis disease activity organ systems and the scleroderma pattern in the patients with dermatomyositis.
Table 4 . Association between myositis disease activity organ systems and scleroderma pattern in patients with dermatomyositis .
|
Myositis disease activity organ systems |
Scleroderma pattern (Mean± SD*) |
Non-specific pattern (Mean± SD) |
p |
|
Constitutional disease activity Cutaneous disease activity Skeletal disease activity Gastrointestinal disease activity Pulmonary disease activity Cardiac disease activity Muscular disease activity Global disease activity |
2.92±2.50 3.50±2.16 1.79±1.97 1.21±2.20 1.79±2.78 0.96±1.70 3.96±3.11 5.25±2.55 |
4.67±3.05 3.00±2.64 2.67±4.62 2.67±4.62 3.33±3.05 0 5.00±3.00 5.67±2.08 |
0.30 0. 78 0.60 0.53 0.49 0.50 0.63 0.81 |
*SD: Standard deviation
Association between myositis disease activity organ systems as well as laboratory findings and NVC parameters
The association between myositis disease activity and NVC parameters is presented in Table 5.
Table 5 . Association between nailfoldcapillaroscopy features and myositis disease activity organ systems in our patients with dermatomyositis .
| Myositis disease activity organ systems, (Mean±SD*) |
Dilated capillary loops |
Avascular areas | Elongated capillary loops |
Microhem orrhage |
Distribution | Neoangiogenesis | Giant loop | p |
| Constitutional disease activity | 3±2.5( 0.69) | 2.7±2.2(0.39) | 5±3(0.068) | 2.8±2.3(0.39) | 3±2.6(0.87) | 2.8±2.6(0.40) | 3.5±2.6(0.50) | 3.7±2.3(0.60) |
| Cutaneous disease activity | 3.5±2.2(0.92) | 3.5±2.1(0.95) | 4.4±2.3(0.27) | 3.2±2.1(0.51) | 3.4±2.1(1) | 3.5±2.5(0.84) | 3.6±1.8(0.72) | 4±2.3(0.53) |
| Skeletal disease activity | 2±2.4(0.32) | 1.9±2(0.91) | 3.6±2.9(0.063) | 1.7±2(0.66) | 1.9±2.3(1) | 1.9±12.2(0.86) | 2.5±2.8(0.24) | 3±2.3(0.18) |
| Gastrointestinal disease activity | 1±2.8(0.52) | 1.3±2.2(0.93) | 1.6±3.6(0.82) | 0.9±1.9( 0.22) | 1.5±2.5(0.72) | 1.5±2.5(0,68) | 1.1±2.6(0.65) | 0(0.16) |
| Pulmonary disease activity | 2.1±2.9(0.39) | 1.7±2.8(0.63) | 3.2±3.3(0.27) | 1.6±2.7( 0.38) | 2±2.8(1) | 1.8±2.8(0.64) | 2.4±3(0.48) | 3.2±3.3(0.25) |
| Cardiac disease activity | 1±1.7(0.33) | 0.7±1.6(0.67) | 1.4±2(0.40) | 1±1.6( 0.56) | 0.9±1.7(0.76) | 0.9±1.6(0.92) | 1.1±1.8(0.57) | 1.7±2.1(0.24) |
| Muscular disease activity | 4.1±3.1(0.96) | 4.1±2.8(0.99) | 5.6±3.2(0.21) | 4±3(0.95) | 4.3±3(0.16) | 3.8±3.3(0.48) | 4.7±2.8(0.37) | 5.8±1.6(0.11) |
| Global disease activity |
5.4±2.4(0.64) |
5.1±2.5(0.70) |
6.4±3(0.27) |
5.5±2.3(0.57) | 5.4±2.5(0.47) | 5.2±2.6(0.79) | 5.5±2.6(0.70) | 6.7±1.7(0.13) |
*SD: Standard deviation
The patients with increased capillary loop dimension showed the following indices more frequently: periungual capillary changes (p=0.009), anti Ro antibody (p=0.049), and erythroderma rashes without secondary changes (p=0.097).
Moreover, avascular areas were more frequently observed with diffuse alopecia (p=0.09), mechanic hands (p=0.07), and high CRP values (p=0.056); however, none were statistically significant.
The patients with elongated capillaries had higher rates of arthralgia (p=0.041) and higher ALT levels (p=0.024).
Furthermore, microhemorrhages were significantly related to myalgia (p=0.041).
Discussion
The present study aimed to investigate the association between the clinical and laboratory findings and DM disease activity and the NC findings in the Iranian population. The patients with increased capillary dimension showed significant periungual capillary. Besides, the patients with elongated capillaries showed higher rates of arthralgia and ALT levels. The study results revealed a significant association between microhemorrhages and myalgia. In addition, myopathic EMG pattern was associated with the scleroderma pattern.
In the present study, no significant association was found between VAS of disease activity and the NVC patterns in the patients with DM. In a study conducted among the Japanese patients, the scleroderma pattern was associated with muscle disease activity and elevated serum CK levels. In addition, loss of capillaries was significantly associated with muscle and global disease activity. Also, a significant relationship was found between hemorrhage and cutaneous disease activity (27). However, these results were on the contrary to those of the present study. The results of two studies conducted on the patients with juvenile dermatomyositis demonstrated that the patients with normal end row loops had lower skin disease activity (54, 55).
The differences between those studies and the current one may be due to the possible genetic and endogenous host factors. The differences between the disease durations in these studies may have played a role, as well. Specific environmental and exogenous factors might have also interacted with the results. Furthermore, Rocella et al. concluded that the patients with normal end-row loop should not be treated less aggressively for their disease (54). This may indicate that normal or mild abnormal NC findings do not necessarily correlate with a less active and milder disease with better prognosis. Another study showed that the NC data at diagnosis could not be used to predict the course of the disease (41). Although some items of each organ system were independently associated with certain NC findings, the disease activity indices were not significantly associated with the NC findings. Similar to some previous studies (27), the majority of our patients (89%) showed the scleroderma pattern.
The present study had some limitations. We evaluated a limited number of patients with dermatomyositis. Besides, our patients did not have long term NC follow up. Few previous studies did NC by two specialists. However, similar to many other studies, NC was accomplished by one expert rheumatologist in the current study. Thus, we may not be able to extrapolate the results to a wider population.
Conclusion
The results of this study showed that myopathic EMG pattern was significantly associated with the scleroderma pattern. However, no significant association was found between VAS of disease activity and other NC patterns in the patients with DM, while some indices of certain clinical manifestations on presentation were significantly associated with specific NC features. Future studies are recommended to investigate more cases with long-term follow ups. Although NC may be a dynamic tool for exploring the microvascular system and be useful in monitoring the progression of microangiopathy, because of various results regarding the relationship between disease activity and NC features, we propose that until their precise evaluation in large and multicenter studies, they should be interpreted cautiously and in combination with other findings.
Acknowledgements
Research Improvement Center of Shiraz University of Medical Sciences and Ms. A. Keivanshekouh are appreciated for improving the use of English in the manuscript.
Conflict of Interest
The authors declare that they have no conflicts of interest in the research.
Cite this article as: Shenavandeh S, Zarei Nezhad M. Association of nailfold capillary changes with disease activity, clinical and laboratory findings in patients with dermatomyositis. Med J Islam Repub Iran 2015 (11 July). Vol. 29:233.
References
- 1.Bernatsky S, Joseph L, Pineau CA, Bélisle P, Boivin JF, Banerjee D. et al. Estimating the prevalence of polymyositis and dermatomyositis from administrative data: Age, sex and regional differences. Ann Rheum Dis. 2009;68:1192–6. doi: 10.1136/ard.2008.093161. [DOI] [PubMed] [Google Scholar]
- 2.Oddis C, Conte C, Steen V. Incidence of polymyositis-dermatomyositis: A 20 year study of hospital diagnosed cases in Allegheny County, PA 1963-1982. J Rheumatol. 1990;17:1329–1334. [PubMed] [Google Scholar]
- 3.Mastaglia FL, Phillips BA. Idiopathic inflammatory myopathies: Epidemiology, classification, and diagnostic criteria. Rheum Dis Clin North Am. 2002;28:723–741. doi: 10.1016/s0889-857x(02)00021-2. [DOI] [PubMed] [Google Scholar]
- 4.Medsger TA, Dawson WN, Masi AT. The epidemiology of polymyositis. Am J Med. 1970;48:715–723. doi: 10.1016/s0002-9343(70)80006-7. [DOI] [PubMed] [Google Scholar]
- 5.Patrick M, Buchbinder R, Jolley D. et al. Incidence of inflammatory myopathies in Victoria, Australia, and evidence of spatial clustering. J Rheumatol. 1999;26:1094–1100. [PubMed] [Google Scholar]
- 6.Weitoft T. Occurrence of polymyositis in the county of Gavleborg, Sweden. Scand J Rheumatol. 1997;26:104–106. doi: 10.3109/03009749709115827. [DOI] [PubMed] [Google Scholar]
- 7.Dalakas MC. Muscle biopsy findings in inflammatory myopathies. Rheum Dis Clin North Am. 2002;28:779–98. doi: 10.1016/s0889-857x(02)00030-3. [DOI] [PubMed] [Google Scholar]
- 8.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 9.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N Engl J Med. 1975;292:403–7. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 10.Dalakas M. Polymyositis, dermatomyositis, and inclusion-body myositis. N Engl J Med. 1991;325:1487–98. doi: 10.1056/NEJM199111213252107. [DOI] [PubMed] [Google Scholar]
- 11.Santmyire-Rosenberger B, Dugan EM. Skin involvement in dermatomyositis. Curr Opin Rheumatol. 2003;15(6):714–22. doi: 10.1097/00002281-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Sontheimer RD. Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. DermatolClin. 2002;20:387–408. doi: 10.1016/s0733-8635(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 13.Christopher-Stine L, Plotz P. Adult inflammatory myopathies. Best Pract Res ClinRheumatol. 2004;18:331–44. doi: 10.1016/j.berh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Danko K, Ponvi A, Constantin T, Borgulya G, Szegedi G. Long-term survival of patients with idiopathic inflammatory myopathies according to clinical features: a longitudinal study of 162 cases. Medicine. 2004;83(1):35–42. doi: 10.1097/01.md.0000109755.65914.5e. [DOI] [PubMed] [Google Scholar]
- 15.Gottdiener JS, Sherber HS, Hawley RJ, Engel WK. Cardiac manifestations in polymyositis. Am J Cardiol. 1978;41(7):1141–9. doi: 10.1016/0002-9149(78)90871-8. [DOI] [PubMed] [Google Scholar]
- 16.Marie J, Hachulla E, Cherin P, Hellot MF, Herson S, Levesque H. et al. Opportunistic infections in polymyositis and dermatomyositis. Arthritis Rheum. 2005;53:155–65. doi: 10.1002/art.21083. [DOI] [PubMed] [Google Scholar]
- 17.Hepper NG, Ferguson RH, Howard FM Jr. Three types of pulmonary involvement in polymyositis. Med Clin North Am. 1964;48:1031–42. doi: 10.1016/s0025-7125(16)33432-0. [DOI] [PubMed] [Google Scholar]
- 18.Dickey BF, Myers AR. Pulmonary disease in polymyositis/dermatomyositis. Semin Arthritis Rheum. 1984;14:60–76. doi: 10.1016/0049-0172(84)90010-6. [DOI] [PubMed] [Google Scholar]
- 19. W. Koopman and L. Moreland, editors. Arthritis and Allied Conditions. A Textbook of Rheumatology. Philadelphia: Lippincott, Williams, and Wilkins; 2005. pp. 1593-1620.
- 20.Munsat TL, Baloh R, Pearson CM, Fowler WJ. Serum enzyme alterations in neuromuscular disorders. J Am Med Assoc. 1973;226:1536–43. [PubMed] [Google Scholar]
- 21.Rider LG, Miller FW. Laboratory evaluation of the inflammatory myopathies. ClinDiagn Lab Immunol. 1995;2(1):1–9. doi: 10.1128/cdli.2.1.1-9.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briani C, Doria A, Sarzi-Puttini P, Dalakas MC. Update on idiopathic inflammatory myopathies. Autoimmunity. 2006;39:161–70. doi: 10.1080/08916930600622132. [DOI] [PubMed] [Google Scholar]
- 23.Mammen AL. Dermatomyositis and polymyositis: Clinical presentation, autoantibodies, and pathogenesis. Ann N Y Acad Sci. 2010;1184:134–53. doi: 10.1111/j.1749-6632.2009.05119.x. [DOI] [PubMed] [Google Scholar]
- 24.Mercer KL, Moore TL, Chinoy H, Murray AK, Vail A, Cooper RG. et al. Quantitative nailfold video capillaroscopy in patients with idiopathic inflammatory myopathy. Rheumatology. 2010;49:1699–705. doi: 10.1093/rheumatology/keq051. [DOI] [PubMed] [Google Scholar]
- 25.Selva-O'Callaghan A, Fonollosa-Pla V, Trallero-Araguás E, Martínez-Gómez X, Simeon-Aznar CP, Labrador-Horrillo M. et al. Nailfold capillary microscopy in adults with inflammatory miopathy. Semin Arthritis Rheum. 2010;39:398–404. doi: 10.1016/j.semarthrit.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Mugii N, Hasegawa M, Matsushita T, Hamaguchi Y, Horie S, Yahata T. et al. Association between nailfold capillary findings and disease activity in dermatomyositis. Rheumatology. 2011;50:1091–8. doi: 10.1093/rheumatology/keq430. [DOI] [PubMed] [Google Scholar]
- 27.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971–82. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 28.Ingegnoli F, Zeni S, Gerloni V, Fantini F. Capillaroscopic observations in childhood rheumatic diseases and healthy controls. Clin Exp Rheumatol. 2005;23:905–11. [PubMed] [Google Scholar]
- 29.Cutolo M, Sulli A, Secchi ME, Paolino S, Pizzorni C. Nailfold capillaroscopy is useful for the diagnosis and follow-up of autoimmune rheumatic diseases. A future tool for the analysis of microvascular heart involvement? Rheumatology. 2006;45 Suppl 4:43–6. doi: 10.1093/rheumatology/kel310. [DOI] [PubMed] [Google Scholar]
- 30.Cutolo M, Sulli A, Secchi ME, Pizzorni C. Capillaroscopy and rheumatic diseases: state of the art. Z Rheumatologie. 2006;65:290–6. doi: 10.1007/s00393-006-0071-2. [DOI] [PubMed] [Google Scholar]
- 31.Dolezalova P, Young SP, Bacon PA, Southwood TR. Nailfold capillary microscopy in healthy children and in childhood rheumatic diseases: a prospective single blind observational study. Ann Rheum Dis. 2003;62:444–9. doi: 10.1136/ard.62.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortes S, Cutolo M. Capillaroscopic patterns in rheumatic diseases. ActaRheumatol Port. 2007;32:29–36. [PubMed] [Google Scholar]
- 33.Blockmans D, Beyens G, Verhaeghe R. Predictive value of nailfoldcapillaroscopy in the diagnosis of connective tissue diseases. Clin Rheumatol. 1996;15:148–53. doi: 10.1007/BF02230332. [DOI] [PubMed] [Google Scholar]
- 34.Minkin W, Rabhan NB. Office nail fold capillary microscopy using ophthalmoscope. J Am AcadDermatol. 1982;7:190–3. doi: 10.1016/s0190-9622(82)70107-0. [DOI] [PubMed] [Google Scholar]
- 35.Kabasakal Y, Elvins DM, Ring EF, McHugh NJ. Quantitative nailfoldcapillaroscopy findings in a population with connective tissue disease and in normal healthy controls. Ann Rheum Dis. 1996;55:507–12. doi: 10.1136/ard.55.8.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarrat P. Periungualcapillaroscopy in children with scleroderma and dermatomyositis. J Mal Vasc. 1983;8:175–7. [PubMed] [Google Scholar]
- 37.Nagy Z, Czirjak L. Nailfold digital capillaroscopy in 447 patients with connective tissue disease and Raynaud's disease. J EurAcadDermatolVenereol. 2004;18:62–8. doi: 10.1111/j.1468-3083.2004.00853.x. [DOI] [PubMed] [Google Scholar]
- 38.Smith RL, Sundberg J, Shamiyah E, Dyer A, Pachman LM. Skin involvement in juvenile dermatomyositis is associated with loss of end row nailfold capillary loops. J Rheumatol. 2004;31:1644–9. [PubMed] [Google Scholar]
- 39.Nascif AK, Terreri MT, Len CA, Andrade LE, Hilario MO. Inflammatory myopathies in childhood: correlation between nailfoldcapillaroscopy findings and clinical and laboratory data. J Pediatr. 2006;82:40–5. doi: 10.2223/JPED.1435. [DOI] [PubMed] [Google Scholar]
- 40.Christen-Zaech S, Seshadri R, Sundberg J, Paller AS, Pachman LM. Persistent association of nailfoldcapillaroscopy changes and skin involvement over thirty-six months with duration of untreated diseases in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;58:571–6. doi: 10.1002/art.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selva-O’Callaghan A, Fonollosa-Pla V, Trallero-Araguas E, Martinez-Gomez X, Simeon-Aznar CP, Labrador-Horrillo M. et al. Nailfold capillary microscopy in adults with inflammatory myopathy. Semin Arthritis Rheum. 2010;39(5):398–404. doi: 10.1016/j.semarthrit.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Fujisawa T, Suda T, Nakamura Y, Enomoto N, Ide K, Toyoshima M. et al. Differences in clinical features and prognosis of interstitial lung diseases between polymyositis and dermatomyositis. J Rheumatol. 2005;32:58–64. [PubMed] [Google Scholar]
- 43.Aschwanden M, Daikeler T, Jaeger KA, Thalhammer C, Gratwohl A, Matucci-Cerinic M. et al. Rapid improvement of nailfoldcapillaroscopy after intense immunosuppression for systemic sclerosis and mixed connective tissue disease. Ann Rheum Dis. 2008;67:1057–9. doi: 10.1136/ard.2007.082008. [DOI] [PubMed] [Google Scholar]
- 44.Feldman BM, Rider LG, Dugan L, Miller FW, Schneider R. Nailfold capillaries as indicators of disease activity in juvenile idiopathic inflammatory myopathies. Arthritis Rheum. 1999;42 Suppl 9:181. [Google Scholar]
- 45.Pachman L, Mendez E, Lechman T, Sundberg J, Shamiyeh E, Dyer A. The rash of juvenile Dermatomyositis is associated with derangement of capillaries. Arthritis Rheum. 2000;43 Suppl 9:380. [Google Scholar]
- 46.Spencer-Green G, Crowe WE, Levinson JE. Nailfold capillary abnormalities and clinical outcome in childhood dermatomyositis. Arthritis Rheum. 1982;25:954_8. doi: 10.1002/art.1780250807. [DOI] [PubMed] [Google Scholar]
- 47.Spencer-Green G, Schlesinger M, Bove KE, Levinson JE, Schaller JG, Hanson V. et al. Nailfold capillary abnormalities in childhood rheumatic diseases. J Pediatrics. 1983;102:341–6. doi: 10.1016/s0022-3476(83)80645-3. [DOI] [PubMed] [Google Scholar]
- 48. Rider LG, Werth VP, Huber AM, Alexanderson H, Rao AP, Ruperto N, et al. (2011), Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), Physician Global Damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care Res, 63: S118–S157. doi: 10.1002/acr.20532 [DOI] [PMC free article] [PubMed]
- 49. Cutolo M, editor. Atlas of Capillaroscopy in Rheumatic Diseases. Milan: Elsevier; 2010. pp. 115-18.
- 50.Cutolo M, Sulli A, Pizzorni C, Accardo S. Nailfoldvideocapillaroscopy assessment of microvascular damage in systemic sclerosis. J Rheumatol. 2000;27:155–60. [PubMed] [Google Scholar]
- 51. Lambova, S. N. The role of capillaroscopy in rheumatology [Dissertation]. Gießen: Justus Liebig Univ. Giessen; 1995.
- 52.Wetteland P, Røger M, Solberg HE, Iversen OH. Population-based erythrocyte sedimentation rates in 3910 subjectively healthy Norwegian adults A statistical study based on men and women from the Oslo area. J Intern Med. 1996;240(3):125–31. doi: 10.1046/j.1365-2796.1996.30295851000.x. [DOI] [PubMed] [Google Scholar]
- 53.Ostrowski RA, Sullivan CL, Seshadri R, Morgan GA, Pachman LM. Association of normal nailfold end row loop numbers with a shorter duration of untreated disease in children with juvenile dermatomyositis. Arthritis & Rheumatism. 2010;62(5):1533–8. doi: 10.1002/art.27379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmeling H, Stephens S, Goia C, Manlhiot C, Schneider R, Luthra S. et al. Nailfold capillary density is importantly associated over time with muscle and skin disease activity in juvenile dermatomyositis. Rheumatology. 2011;50(5):885–93. doi: 10.1093/rheumatology/keq407. [DOI] [PubMed] [Google Scholar]
- 55.Pavlov-Dolijanovic S, Damjanov N, Stojanovic R, VujasinovicStupar N, Stanisavljevic D. Scleroderma pattern of nailfold capillary changes as predictive value for the development of a connective tissue disease: a follow-up study of 3,029 patients with primary Raynaud’s phenomenon. Rheumatol Int. 2012;32(10):3039–45. doi: 10.1007/s00296-011-2109-2. [DOI] [PubMed] [Google Scholar]


