Abstract
Background: Alterations in the neuromuscular control of the spine were found in patients with chronic low back pain (CLBP). Sudden loading of the spine is assumed to be the cause of approximately 12% of lower back injuries. However, some aspects of this problem, such as alterations in the sensory–motor control of the spine, remain questionable. This study investigated postural and neuro– motor changes in trunk muscles during sudden upper limb loading in patients with CLBP.
Methods: Electromyography of the erector spinae (ES) and transverses abdominis/internal oblique (TrA/IO) and external oblique (EOA) muscles were recorded in 20 patients with CLBP and 20 asymptomatic individuals with eyes open (EO) and eyes closed (EC) conditions. Moreover, measurements of the center of pressure (COP) and vertical ground reaction force (GRF) or Fz were recorded using a force plate. Data were analyzed using paired t-test and independent t-test at the significance level of 0.05.
Results: In patients with CLBP, decreased electrical activity of the ES muscle was observed under both the EO and EC conditions and that of the TrA/IO muscle was observed under the EO condition (p< 0.05). Other findings included a shorter peak latency of the ES muscle in the EO condition and a greater increase in the peak latency of the ES muscle following the EC condition (p< 0.05). No significant differences were observed in COP and GRF measurements between the groups.
Conclusion: Electrical muscle activity may indicate less stiffening or preparatory muscle activity in the trunk muscle of patients with CLBP. Altered latency of the muscle may lead to microtrauma of lumbar structures and CLBP.
Keywords: Low back pain, Abdominal muscles, Electromyography, Posture
Introduction
Low back pain (LBP) is one of the most common conditions leading to disability worldwide (1). Scientists often suggest that the occurrence of LBP is a fact of life and that researchers and clinicians should attempt to prevent LBP progression to chronicity. LBP cases that progress to a chronic state lead to a heavy cost burden (2,3)
Changes in neuromuscular control or trunk muscle activity in patients with LBP have been examined in several investigations. Patients with chronic low back pain (CLBP) have been observed to experience delayed or absent trunk muscle activation following limb movement or loading. These changes may lead to microtrauma in spinal structures (4). Inefficient erector spinae (ES) and abdominal muscle responses can increase the flexion moment of the lumbar spine and lead to damage to lumbar tissues (5). The results of previous studies suggest that in order to diminish kinematic displacement during sudden flexion loading, muscles should act in a preparatory coactivation manner (6).
In addition, an essential requirement for performing daily activities is appropriate postural control (7). Impaired postural control has been reported in patients with CLBP. These alterations in these patients’ postural control present as increased postural sway and a difficulty controlling posture during unstable conditions. Moreover, these patients have indicated less recovery of their postural balance following perturbation (8).
Sudden loading of the spine frequently occurs during slips, falls, hits, and adding an extra load to an object held in one’s hands. In addition, unexpectedly moving the contents of a container can suddenly load the spine (9-12). These conditions can situate the spine in an instable condition and are thought to be the cause of about 12% of lower back injuries. In these conditions, the neuromuscular system must respond quickly to maintain postural balance and produce efficient muscular activity (12). Moreover, during sudden neuromuscular system responses, increased trunk muscle activation and mechanical loading on the spine will occur. Under unexpected loading, the central nervous system (CNS) attempts to compensate for deviations between the desired and actual kinematics, which may increase the possibility of excessive spinal loads. Such excessive forces acting on the spine are thought to be a major cause of damage to this region (10-12).
Earlier onset and less electrical activity of the paraspinal muscles following visual prediction have been observed in asymptomatic subjects. In addition, increased electrical muscle activity before load onset has been observed in those subjects (13). On the other hand, limited effects of prediction and impaired postural control were observed in CLBP patients. In addition to above findings, changes in the motor control of the trunk muscles have been found following experimentally induced pain (14) . On the contrary, similarities between paraspinal muscle responses have been found in some studies using healthy controls and CLBP patients (15).
It is thought that pain intensity should be a 3 score or below it on the visual analog scale (VAS≤ 3) to detect alterations in the sensory-motor control of the spine. Pain intensities above this level can confound findings further because researchers cannot determine whether these alterations are related to increased pain or altered sensory-motor control (16).
Based on earlier studies, it can be assumed that the impaired motor control of the spine may result in LBP chronicity (17). Furthermore, neuromuscular and postural responses and the effects of visual prediction during sudden upper limb loading in CLBP patients are still unclear. To our knowledge, no study has synchronously investigated neuromuscular and postural responses during sudden upper limb loading using postural and electromyographic variables in this subgroup of CLBP patients. These variables were as follows: muscle electrical activity, onset and peak latencies of the trunk muscles, ground reaction force (GRF) and center of pressure (COP) measures.The first aim of the present study was to determine whether motor and postural control responses in eye-open (EO) and eye-closed (EC) conditions would be different during sudden upper limb loading in asymptomatic and CLBP subjects. The second aim of the present study was to determine if visual prediction could change trunk muscle response latencies and decrease its magnitude. The third aim of the present study was to determine whether postural control parameters were changed following visual prediction. The fourth aim of the present study was to determine if any differences exist between CLBP and asymptomatic subjects in terms of motor and postural control because of visual prediction.
Methods
Study Population
Twenty patients with CLBP (7 male and 13 female) and 20 asymptomatic subjects (10 male and 10 female) aged between 18 and 45 years were participated in the present study (18).Two groups were matched by age, height, and weight. In the present study, LBP is defined as reporting any pain, burning sensation, tenderness, or discomfort between the edge of the twelfth ribs and the gluteal folds. The inclusion criteria for the LBP subjects were as follows: persistent or intermittent mechanical LBP (movement exacerbates or begins the pain) for three months or further (19-21); VAS of equal or less than three (16); absence of any lumbar radicular signs or any respiratory, neurological, or other orthopedic conditions aside from LBP (22). The inclusion criteria for the asymptomatic subjects were the absence of any respiratory, neurological, or orthopedic conditions and experiencing LBP in the previous two years required to rest at home or a job-related disability (23). The exclusion criteria for both groups were unwillingness to continue the test and the generation or exacerbation of pain during the test.
Flowchart 1 .
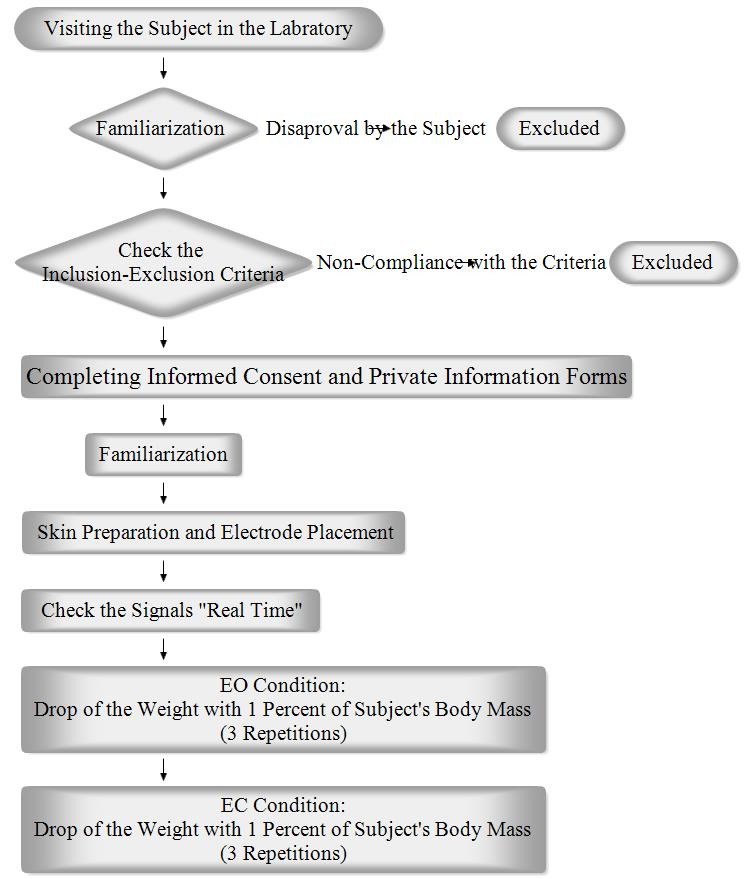
Illustrates the study procedure
Methodology of the Test
At the beginning of this quasi-experimental study, each subject was given an explanation of how the test would be performed. If the subject gave his or her consent to continue, the inclusion criteria were checked. Each subject then gave informed consent before participating in the study. After a subject completed the personal information form, he or she became familiarized with the test. Next, the electrodes were attached to the relevant muscles. The electromyography (EMG) signals were checked “Real Time” and the subject was instructed on how to stand and position his or her upper limbs. The subject then stood over the force plate with his or her knees completely extended and elbows flexed at 90°. The subject held a basket in his or her hands with his or her ears covered and eyes were either closed or open. A box weighing about 1% of the subject’s body mass was fixed by an electromagnet to a bracket situated approximately at eye level, directly above the basket. At random times and without warning, the weight was released into the basket (23). A total of six consecutive measurements were taken in a sequence of three trials with the subject’s eyes open and three with his or her eyes closed. The basket was equipped with a marker switch that indicated the impact time. Flowchart 1 illustrates the study procedure. This study was approved by the Tehran University of Medical Sciences Ethics Board (Ethical code: 130/3428; date: 18/3/2013), and it was performed according to the Declaration of Helsinki.
Electromyography and Force Plate Recordings
For this study, EMG recording was conducted using the ME 6000 (Mega Electronics Ltd. Kuopio, Finland). To facilitate the EMG recording of the muscles studied on the right and the left sides, the subjects’ skin was prepared by shaving, rubbing, and cleaning the skin with alcohol prep pads. The medial points of each pair of surface EMG electrodes (Ag–AgCl discs) were then placed as described in previous studies with the transversusabdominus/internal oblique (TrA/IO) approximately 2 cm medial and inferior to the anterior superior iliac spine (24), the external oblique (EOA) 10 cm lateral to the umbilicus with a vertical orientation of 45° (6), and the ES muscle at the L3–L4 level approximately 4 cm lateral from the midline. The center-to-center electrode distance was 2.5 cm, and the electrodes were longitudinally oriented along the fibers of the muscle (25). EMG data were band-pass filtered between 20 Hz and 500 Hz (2) and sampled at 1 kHz using Qualisys Track Manager software version 2.7 (QTM, Gothenburg, Sweden). Thereafter, data were exported for analysis to MATLAB software version 7 (MathWorks, USA). A time window of 200 ms before and 250 ms after perturbation was selected for the analysis (26). The root-mean-square (RMS) of the EMG signal was then normalized according to the previous method used by Kanekar et al and Silfies et al (27,28). Consequently, the integral of EMG signal (IEMG), representing muscle activity, and the muscle onset latencies were calculated using the MATLAB software. A muscle was considered to respond with an onset when the RMS signal crossed the threshold of the mean plus three standard deviations of the pre-perturbation baseline signals. To avoid considering any EMG spike activity as a muscle onset during the signal processing, it was necessary to cross the threshold for at least 20 ms (26). The time to peak of the muscle electrical activity (peak latency) was also calculated (29). A force plate device (Kistler Company, Switzerland) was used to measure the Fz (vertical component of ground reaction force) peak latency; the total center of pressure (COP) excursion; and COP excursion along the Y axis (YCOP).The data from the marker switch, the force plate, and the EMG systems were collected synchronously (30).
Statistical Analysis
The data were analyzed using SPSS software version 18 (SPSS Inc, Chicago, USA). The normal distribution of the data was confirmed through the Kolmogorov-Smirnov test. Paired t-tests were used to calculate within-group differences after the EC condition. Independent t-tests were used to calculate between-group differences for the EO and EC conditions. For the force and COP data, the mean value of three measurements was calculated. For the EMG data, the mean value of three measurements for each side was calculated. Then the values for the left and right sides were pooled for subsequent analysis (15) . P value< 0.05 was considered as significant.
Results
Descriptive Statistics and Analysis of Differences between CLBP and Asymptomatic Groups
The demographic characteristics of the subjects were not significantly different between groups. During this study, electromyography and force data for 46 subjects were recorded. The data from the six subjects were excluded due to noise in the force plate signals. Tables 1 and 2 illustrate the means and standard deviations of the studied variables for both EO and EC conditions. Furthermore, this table lists the P values calculated using the independent t-test to compare the asymptomatic and CLBP group differences. As the Table 1 shows, in the LBP patients, shorter peak latency in the ES muscle in the EO condition (P=0.026), less electrical activity of TrA/IO in EO condition (p=0.044) and ES in the EO and EC conditions were seen (p=0.045), (p=0.028).
Table 1 . The descriptive statistics of the EMG variables for both the EO and EC conditions .
| Item | Muscle | Condition |
Asymptomatic (Mean±SD) |
LBP (Mean±SD) |
p | ||
| Onset Latency(ms) | TrA/IO | EO | 20±60 | 55±219 | 0.087 | ||
| EC | 86±46 | 88±52 | 0.799 | ||||
| EOA | EO | 36±77 | 36±118 | 0.995 | |||
| EC | 111±75 | 69±39 | 0.082 | ||||
| ES | EO | -9±100 | -36±334 | 0.828 | |||
|
Peak Latency
(ms) IEMG |
TrA/IO EOA ES TrA/IO EOA ES |
EC EO EC EO EC EO EC EO EC EO EC EO EC |
56±41 102±44 118±45 99±56 128±63 92±45 114±52 0.95±0.28 1.07±0.39 1.01±0.34 1.13±0.45 1.37±0.33 1.46±0.57 |
59±32 88±61 115±45 75±46 104±54 61±39 115±35 0.78±0.22 0.86±0.30 0.93±0.32 0.98±0.33 1.18±0.24 1.16±0.24 |
0.311 0.416 0.819 0.170 0.210 0.026 0.944 0.044 0.068 0.453 0.082 0.045 0.038 |
||
TrA/IO: TransversusAbdominus/ Internal Oblique, EOA: External Oblique, ES: Erector Spinae
EO: eye open, EC: eye closed
Significant P values are underlined.
Table 2 . The descriptive statistics of the force plate variables for both the EO and EC conditions .
| Item | Muscle | Condition |
Asymptomatic (Mean±SD) |
LBP (Mean±SD) |
p |
|
Fz peak latency (ms) COP excursion (mm) YCOP excursion(mm) |
EO EC EO EC EO EC |
106±16 121±18 190±41 192±33 143±33 144±25 |
106±23 127±30 190±48 194±42 142±36 145±30 |
0.992 0.427 0.886 0.829 0.732 0.940 |
Within- and Between-group Analysis of Response Changes Following EC
Table 3 provides the P values calculated using a paired t-test to compare the dependent variables for EO and EC conditions in each group. In addition, this table lists the p-values calculated using the independent t-test to investigate any difference between the CLBP and asymptomatic subjects in terms of motor and postural strategies as a result of visual prediction. As the Tables 3 and 4 show, in the asymptomatic subjects, shorter onset latencies of TrA/IO and EOA muscles (p=0.002), (p=0.026), shorter peak latency of EOA muscle (p=0.001) and shorter Fz peak latency (p=0.003) were found following EO condition. Furthermore, in the LBP patients shorter peak latencies of EOA and ES muscles (p=0.084), (p<0.001) were observed in EO condition compared to EC condition. Moreover, in these patients shorter Fz peak latency (p=0.004) were found in EO condition compared to EC condition. EC condition produced more increase in ES muscle’s peak latency in the LBP subjects compared to the asymptomatic subjects (p=0.037).
Table 3 . P values calculated using a paired t-test to compare EMG parameters in EO and EC conditions in each group .
| Item | Asymptomatic | LBP | |
|
Onset latency Peak latency IEMG |
TrA/IO EOA ES TrA/IO EOA ES TrA/IO EOA ES |
0.002 0.026 0.818 0.192 0.001 0.054 0.137 0.205 0.261 |
0.781 0.395 0.580 0.159 0.084 <0.001 0.114 0.443 0.669 |
TrA/IO: TransversusAbdominus/ Internal Oblique, EOA: External Oblique, ES: Erector Spinae
Significant p-values are underlined.
Table 4 . P values calculated using a paired t-test to compare force plate parameters in EO and EC conditions in each group .
| Item | Asymptomatic | LBP |
|
Fz peak latency COP excursion YCOP excursion |
0.003 0.814 0.711 |
0.004 0.174 0.166 |
Significant P values are underlined.
Discussion
This study investigated altered neuromuscular control and possible changes in visual information processing in CLBP patients. The subjects in the present study had little to no pain at the time of testing (VAS ≤ 3). Therefore, any changes in neuromuscular responses seen in the present study are mainly considered to be long-term alterations in sensory-motor control in CLBP patients with minimal or no pain (31). As described earlier in the result section, less electrical activity and shorter peak latency of the trunk muscles were observed in the CLBP patients. Furthermore, in the CLBP patients, shorter peak latencies of ES muscle in EO condition and more increase in ES muscle’s peak latency after eye closing were found.
As Table 1 illustrates, the electrical activity of the TrA/IO and ES muscles in EO conditions and the ES muscle in EC condition in the LBP group were significantly less than that of the asymptomatic group. This decrease in electrical muscle activity may indicate that subjects with CLBP have less stiffening or preparatory muscle activity in the trunk muscles, especially in TrA/IO and ES muscles during sudden upper limb loading. The decrease may also be seen as an attempt by the CNS to minimize stresses on lumbar tissues through muscle inhibition in a compensatory mechanism (32,33). These changes in trunk muscle activity may lead to lower back injuries and pain, due to microtrauma. The observed decrease in electrical muscle activity in the present study is consistent with Sihvonen et alstudy which showed decreased electrical muscle activity during functional movements in LBP patients (34). On the other hand, this decrease in electrical trunk muscle activity is in contrast with D’hooge et alstudy which showed increased electrical activity of the erector spinae muscles with and without load (35). In a study of motor behaviors based on task types, Richardson et alconcluded that slow arm movements could not reveal the differences in CLBP and asymptomatic groups; but they found these differences with rapid arm movements. In view of the present and above studies and the recent theory of “adaptation to pain”, which describes chronic pain and motor control, controversial results may be due to different groups studied and the diversity of tasks (36). Unlike no between groups differences in the TrA/IO electrical muscle activity in the EC condition, the electrical activity of this muscle in CLBP patients was significantly less than that of the asymptomatic subjects in the EO condition. The less electrical muscle activity observed in the patients, may suggest that they cannot increase activity in the TrA/IO muscle as much as asymptomatic subjects following visual prediction of loading.
Older theories of chronic pain and motor control are questionable. Changes in motor control are sometimes interpreted in the context of the pain–spasm–pain model, which states that pain results in increased muscle activity, thus causing increased pain. On the other hand, the pain adaptation model postulates that pain decreases muscle activation when the muscle is activated as agonists and increases muscle activation when the muscle is activated as antagonists (32). Given the flexion moment production in the spine during sudden upper limb loading, only the decreased electrical activity of the TrA/IO in the LBP group is consistent with the pain adaptation model (32).
As demonstrated in Table 3, no significance between-group differences were observed in response changes following visual predictions in muscle onset latencies. In contrast, Leinonenetalreported that impairment of the neuro-motor system shortens ES muscle latency following visual prediction during upper limb loading in CLBP patients. CLBP patients participated in their study had a considerable pain and disability. It may then be supposed that changes in those subjects’ motor control were possibly due to pain rather than altered sensory-motor control (15). In subjects with significant pain, pain itself and its related features (e.g., its attention-demanding requirements and stress or fear) may disrupt motor output because of the increased demand placed on information-processing resources (31). Furthermore, some motor responses examined in the present study were not significantly different in CLBP subjects in comparison to asymptomatic subjects. This may be due to the fact that the CLBP patients participating in the present study had little to no pain during testing.
The COP excursion has been used as a measure of balance performance in patients with non-specific LBP in previous studies (37). In the present study, Fz peak latency was significantly decreased by visual prediction in both groups. It is clear that a visual prediction of loading can cause earlier changes in the vertical ground reaction force (Fz). The remaining Fz and COP measures studied were not significantly different between and within groups. Leinonen et al postulated that postural control in patients with CLBP is diminished because these patients demonstrated a larger sway in comparison to asymptomatic controls in EO or EC conditions (38). The considerable pain and disability experienced by subjects in their study may have caused neuro-motor changes. On the other hand, Brumagne et al found no significant differences in postural balance between asymptomatic and CLBP subjects while the subjects stood quietly on a firm support surface. However, they observed significant differences between the groups when the subjects stood on an unstable support surface (foam). In that study, CLBP subjects showed significantly larger sways compared to the asymptomatic subjects during the EC condition (7). Because detecting postural instability is task-dependent, some of the force and COP measures studied did not demonstrate significant between-group differences.
In the CLBP patients, the ES muscle in the EO condition reached a peak of electrical activity more rapidly. This may be considered as a fear of movement and poor response modulation on the part of these patients. In addition, EC causes a higher increase in the peak latency of the ES muscle in CLBP patients compared to asymptomatic subjects. This may be due to the fact that CLBP patients rely more on visual inputs to modulate their motor responses than do asymptomatic individuals. Some studies considered CLBP patients’ higher reliance on visual inputs as a dysfunction in these patients’ proprioceptive systems (39). Muscle peak latencies that occur too early or too late may cause inappropriate loading on the spinal musculoskeletal system, micro trauma, inflammation, and, consequently, LBP chronicity (31,40).
Conclusion
decreased electrical activity of the ES muscle during both EO and EC conditions and of the TrA/IO muscle in the EO condition were observed in the patients with CLBP. These findings may suggest the lower stiffening effect of the TrA/IO and ES muscles on the spinal structures in CLBP patients. Also, a shorter peak latency of the ES muscle in an EO condition and a higher increase in the peak latency of the ES muscle following the EC condition may provide microtrauma and consequently LBP chronicity.
The present study limitation was absence of the kinematic data of the subjects during the test that could be interpreted with kinetic and electromyographic data globally.
For the future studies, the authors suggest setting a study with an additional athletes group with CLBP for further discussing the neuromuscular control changes in these patients.
Acknowledgements
This study was performed in Gait & Motion Analysis Laboratory, Department of Physiotherapy, School of Rehabilitation Sciences, Iran University of Medical Sciences. Financial support for this study was provided by the Iran University of Medical Sciences; grant number 1391/د/ 26/1024, date 1391/12/23.
Conflict of interest
The authors declare no conflict of interest.
Cite this article as: Akbari M, Sarrafzadeh J, Maroufi N, Haghani H. Changes in postural and trunk muscles responses in patients with chronic nonspecific low back pain during sudden upper limb loading. Med J Islam Repub Iran 2015 (21 September). Vol. 29:265.
References
- 1.Salekzamani Y, Mirzaee S, Shakouri S, Nezami N. Pain Relieving Effect of Thermoplastic Lumbosacral Orthosis with Adjustable Posterior Pad in Chronic Non-Specific Low Back Pain. IRCMJ. 2011;13(12):903. [PMC free article] [PubMed] [Google Scholar]
- 2.Reeves N, Cholewicki J, Milner T. Muscle reflex classification of low-back pain J ELECTROMUOGR. KINES. 2005;15(1):53–60. doi: 10.1016/j.jelekin.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Hong J, Reed C, Novick D, Happich M. Costs associated with treatment of chronic low back pain: an analysis of the UK General Practice Research Database. Spine. 2013;38(1):75–82. doi: 10.1097/BRS.0b013e318276450f. [DOI] [PubMed] [Google Scholar]
- 4.Massé-Alarie H, Flamand VH, Moffet H, Schneider C. Corticomotor control of deep abdominal muscles in chronic low back pain and anticipatory postural adjustments EXP. BRAIN RES. 2012;218(1):99–109. doi: 10.1007/s00221-012-3008-9. [DOI] [PubMed] [Google Scholar]
- 5.Hwang JH, Lee YT, Park DS, Kwon TK. Age affects the latency of the erector spinae response to sudden loading. CLIN BIOMECH. 2008;23(1):23–9. doi: 10.1016/j.clinbiomech.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Granata KP, Orishimo KF, Sanford AH. Trunk muscle coactivation in preparation for sudden loadJ. ELECTROMUOGR KINES. 2001;11(4):247–54. doi: 10.1016/s1050-6411(01)00003-7. [DOI] [PubMed] [Google Scholar]
- 7.Brumagne S, Janssens L, Knapen S, Claeys K, Suuden-Johanson E. Persons with recurrent low back pain exhibit a rigid postural control strategy. EUR SPINE J. 2008;17(9):1177–84. doi: 10.1007/s00586-008-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brumagne S, Janssens L, Janssens E, Goddyn L. Altered postural control in anticipation of postural instability in persons with recurrent low back pain. Gait & posture. 2008;28(4):657–62. doi: 10.1016/j.gaitpost.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Zwambag DP, Freeman NE, Brown SH. The effect of elbow flexor fatigue on spine kinematics and muscle activation in response to sudden loading at the hands. J ELECTROMUOGR KINES. 2015 doi: 10.1016/j.jelekin.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Bazrgari B, Shirazi-Adl A, Larivière C. Trunk response analysis under sudden forward perturbations using a kinematics-driven model. J BIOMECH. 2009;42(9):1193–200. doi: 10.1016/j.jbiomech.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Vera-Garcia FJ, Elvira JL, Brown SH, McGill SM. Effects of abdominal stabilization maneuvers on the control of spine motion and stability against sudden trunk perturbationsJ. ELECTROMUOGR KINES. 2007;17(5):556–67. doi: 10.1016/j.jelekin.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Mawston GA, McNair PJ, Boocock MG. The effects of prior exposure, warning, and initial standing posture on muscular and kinematic responses to sudden loading of a hand-held box. CLIN BIOMECH. 2007;22(3):275–81. doi: 10.1016/j.clinbiomech.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Leinonen V, Kankaanpää M, Hänninen O, Airaksinen O, Taimela S. Paraspinal muscle responses during sudden upper limb loading. EUR J APPL PHYSIOL. 2002;88(1-2):42–9. doi: 10.1007/s00421-002-0664-7. [DOI] [PubMed] [Google Scholar]
- 14.Kiesel KB, Uhl T, Underwood FB, Nitz AJ. Rehabilitative ultrasound measurement of select trunk muscle activation during induced pain. Manual therapy. 2008;13(2):132–8. doi: 10.1016/j.math.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Leinonen V, Kankaanpää M, Luukkonen M, Hänninen O, Airaksinen O, Taimela S. Disc herniation-related back pain impairs feed-forward control of paraspinal muscles. Spine. 2001;26(16):E367–E72. doi: 10.1097/00007632-200108150-00014. [DOI] [PubMed] [Google Scholar]
- 16.Henry SM, Hitt JR, Jones SL, Bunn JY. Decreased limits of stability in response to postural perturbations in subjects with low back painCLIN. BIOMECH. 2006;21(9):881–92. doi: 10.1016/j.clinbiomech.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Geisser ME, Ranavaya M, Haig AJ, Roth RS, Zucker R, Ambroz C. et al. A meta-analytic review of surface electromyography among persons with low back pain and normal, healthy controls. The journal of pain. 2005;6(11):711–26. doi: 10.1016/j.jpain.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25(8):947–54. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Wälti P, Kool J, Luomajoki H. Short-term effect on pain and function of neurophysiological education and sensorimotor retraining compared to usual physiotherapy in patients with chronic or recurrent non-specific low back pain, a pilot randomized controlled trial BMC MUSCULOSKEL. DIS. 2015;16(1):83. doi: 10.1186/s12891-015-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dankaerts W, O’Sullivan P. The validity of O’Sullivan’s classification system (CS) for a sub-group of NS-CLBP with motor control impairment (MCI): overview of a series of studies and review of the literature. Manual Therapy. 2011;16(1):9–14. doi: 10.1016/j.math.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Slaboda JC, Boston JR, Rudy TE, Lieber SJ. Classifying subgroups of chronic low back pain patients based on lifting patternsARCH PHYS MED. REHAB. 2008;89(8):1542–9. doi: 10.1016/j.apmr.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 22. Magee DJ. Orthopedic physical assessment: Elsevier Health Sciences; 2013.
- 23.Moseley GL, Hodges PW, Gandevia SC. External perturbation of the trunk in standing humans differentially activates components of the medial back muscles. The Journal of physiology. 2003;547(2):581–7. doi: 10.1113/jphysiol.2002.024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall PW, Murphy BA. Muscle activation changes after exercise rehabilitation for chronic low back pain. ARCH PHYS MED REHAB. 2008;89(7):1305–13. doi: 10.1016/j.apmr.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 25.Hashemirad F, Talebian S, Hatef B, Kahlaee AH. The relationship between flexibility and EMG activity pattern of the erector spinae muscles during trunk flexion–extension. Journal of Electromyography and Kinesiology. 2009;19(5):746–53. doi: 10.1016/j.jelekin.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Brown SH, McGill SM. The intrinsic stiffness of the in vivo lumbar spine in response to quick releases: Implications for reflexive requirementsJ ELECTROMUOGR. KINES. 2009;19(5):727–36. doi: 10.1016/j.jelekin.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Kanekar N, Santos MJ, Aruin AS. Anticipatory postural control following fatigue of postural and focal muscles. Clinical Neurophysiology. 2008;119(10):2304–13. doi: 10.1016/j.clinph.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Silfies SP, Squillante D, Maurer P, Westcott S, Karduna AR. Trunk muscle recruitment patterns in specific chronic low back pain populationsCLIN. BIOMECH. 2005;20(5):465–73. doi: 10.1016/j.clinbiomech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Bressel E, Willardson JM, Thompson B, Fontana FE. Effect of instruction, surface stability, and load intensity on trunk muscle activityJ ELECTROMUOGR. KINES. 2009;19(6):e500–e4. doi: 10.1016/j.jelekin.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 30. Fashkhami AN, Rahimi A, Kalantari KK. The Voluntary Response Index in Electromyographic Study During Landing Test of the Patients With ACL Deficiency: A New Study Protocol. 2014. [DOI] [PMC free article] [PubMed]
- 31.Hodges PW, Moseley GL. Pain and motor control of the lumbopelvic region: effect and possible mechanismsJ ELECTROMUOGR. KINES. 2003;13(4):361–70. doi: 10.1016/s1050-6411(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 32.van Dieën JH, Cholewicki J, Radebold A. Trunk muscle recruitment patterns in patients with low back pain enhance the stability of the lumbar spine. Spine. 2003;28(8):834–41. [PubMed] [Google Scholar]
- 33. Boyling JD, Jull G. Grieve's modern manual therapy: Elsevier; 2004.
- 34.Sihvonen T, Lindgren KA, Airaksinen O, Manninen H. Movement disturbances of the lumbar spine and abnormal back muscle electromyographic findings in recurrent low back pain. Spine. 1997;22(3):289–95. doi: 10.1097/00007632-199702010-00012. [DOI] [PubMed] [Google Scholar]
- 35.D’hooge R, Hodges P, Tsao H, Hall L, MacDonald D, Danneels L. Altered trunk muscle coordination during rapid trunk flexion in people in remission of recurrent low back painJ ELECTROMUOGR. KINES. 2013;23(1):173–81. doi: 10.1016/j.jelekin.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Hodges PW. Pain and motor control: from the laboratory to rehabilitationJ ELECTROMUOGR. KINES. 2011;21(2):220–8. doi: 10.1016/j.jelekin.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Ruhe A, Fejer R, Walker B. Center of pressure excursion as a measure of balance performance in patients with non-specific low back pain compared to healthy controls: a systematic review of the literatureEUR. SPINE J. 2011;20(3):358–68. doi: 10.1007/s00586-010-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leinonen V, Kankaanpää M, Luukkonen M, Kansanen M, Hänninen O, Airaksinen O. et al. Lumbar paraspinal muscle function, perception of lumbar position, and postural control in disc herniation-related back pain. Spine. 2003;28(8):842–8. [PubMed] [Google Scholar]
- 39.Tsay A, Allen T, Proske U, Giummarra M. Sensing the body in chronic pain: A review of psychophysical studies implicating altered body representationNEUROSCI. BIOBEHAVE R. 2015;52:221–32. doi: 10.1016/j.neubiorev.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 40. Richardson C, Hodges P, Hides J. Therapeutic exercise for lumbopelvic stabilization: a motor control approach for the treatment and prevention of low back pain: Churchill Livingstone; 2004.


