Abstract
Background: Alzheimer disease is the main cause of dementia in middle-aged and elderly people. Considering the improving effects of creatine supplementation on cognitive performance, this study aimed to determine the effects of creatine supplementation on learning, memory, and apoptosis in an experimental model of Alzheimer’s disease.
Methods: Thirty-two male Wistar rats each weighing 250±50 grams were divided into four groups. The AdCr+ (Aβ injection, creatine supplementation) and AdCr- groups (Aβ injection, no creatine supplementation) were injected bilaterally with amyloid beta (Aβ) (0.2μg in each CA1 area), and the sham group was injected with normal saline in the same area. After the injection, the AdCr+ group received a diet of 2% creatine for six weeks. The control group underwent no surgical or dietary intervention. After six weeks the Morris Water Maze (MWM) test was administered, to measure learning and memory retrieval. After sacrificing the animals, TUNEL staining for an anti-apoptosis assay was performed for the sham, AdCr+, and AdCr- groups. All groups were compared by independent ttest using SPSS software.
Results: Results of MWM show that rats in sham and control groups performed better than those in the AdCr- and AdCr+ groups. Compared to sham group, AdCr+ and AdCr- groups had more TUNEL positive neurons count. Results indicated no differences between the AdCr+ and AdCrgroups in learning, memory retrieval, and percentage of TUNEL positive neurons.
Conclusion: After Aβ injection, creatine supplementation had no effect on learning, memory retrieval, or neuron apoptosis in male Wistar rats.
Keywords: Creatine supplementation, Amyloid beta, Alzheimer disease, Memory, Learning, Apoptosis
Introduction
Alzheimer disease is the main cause of dementia in middle-aged and elderly people (1). Impairedshort-term memory and environmental disorientation are early hallmarks of the disease (2). There aremedications to treat thisdisease but efforts to control Alzheimer have been disappointing so far. Social and medical supports, however, are useful for increasing the quality of life for patients (1).
Studieshave shown thatbrain function is improvedwhen the fuel supplies for neurons, are increased. Considering the crucial role of creatine in neuron energy homeostasis, it seems that creatine could improve brain function by improving the energy status of neurons (3). One study showed that mental training increased creatine levels in neurons (4). Increased creatine levels in the brain following creatine supplementation have also been reported in humans and other rodents (5-9). Acute oral creatine supplementation has been shown to reduce mental fatigue and to decrease task-responsive oxygen demand to activated areas on performance of a serial calculation task (10). Five grams per day of creatine supplementation in healthy subjects appears to enhance performance on validated tests related to intelligence and working memory. Although a dose of 0.03 gr/kg creatine supplementationin young adults had no effect on short-term memory, 20gr/day ofcreatine supplementation for a weekinhealthyelderly was effectiveinimprovingshort-termmemoryand spatial memory (3, 11, 12).
The effects of creatine supplementation on short-term memory in people suffering from Alzheimer disease has not yet been studied; however, there is some evidence that neuronal creatine kinase activity declines in neurons under the toxic effects of amyloid beta (13) and another study showed that creatine content of brain decreases during conversion from Mild Cognitive Impairment (MCI) to Manifest Dementia (MD) (14). One in vitro study showed the neuroprotective effects of creatine against amyloid beta, while some other studies have found creatine deposits in the neurons of humans with Alzheimer disease and in the neurons of transgenic mice models of Alzheimer (15-17).
Studies on healthy individuals that have shown the positive effects of creatine supplementation on short-term memory and spatial memory along with studies that have reported the neuroprotective effects of creatine give rise to a curiosity about how creatine supplementation can help people suffering from memory impairment, progression of dementia, and neuron loss related to Alzheimer disease (3, 11, 12, 15). Since inflammation is reportedly caused by creatine deposition in the neurons of both animal models of Alzheimer’s disease and people afflicted with it, a big risk is attached to prescribing creatine in a clinical trial (16); thus, the current study investigates the effects of creatine supplementation on spatial memory and neuronal apoptosis in an experimental model of Alzheimer disease.
Methods
Animals
Adult male Wistar rats (200-250g) were obtained from the Pastor Institute (Tehran, Iran).
They were caged at a constant temperature of 21 ±1°C with controlled 12h/12h light-dark cycles, and ad libitum access to food and water. The rats were given one week to habituate to the facilities, and then experimental procedures were begun. The guidelines of the Committee of Care in the Use of Experimental Animals were followed, and study procedures were approved by the Ethics Committee of Tehran University of Medical Sciences. Efforts were made to minimize animal suffering and reduce the number of animals used.
Study design
The animals were divided into four groups (n=8 per group): control, sham, AdCr-, and AdCr+. Animals in the sham, AdCr-, and AdCr+ groups underwent stereotaxic surgery. Those in the AdCr- and AdCr+ groups were given a bilateral amyloid beta injection (4 µl) in the CA1 hippocampus (0.5µg/µl), while the sham group was given a normal saline injection in the same area of the brain. After surgery, the AdCr+ group received creatine monohydrate powder mixed in their CHO (2% creatine/diet) while the other groups continued receiving normal CHO. The control group had no surgical or dietary intervention during the study period. After six weeks of dietary intervention, behavioral tests carried on and also histological tests performed after sacrificing the animals.
Surgery
Human β-amyoid 1-42 (Sigma-Aldrich USA) was solved in 0.1M phosphate buffer saline (PBS; PH=7.4) and then aliquoted and stored at -70°C until use. Every aliquot was incubated at room temperature for 48 hours before injection. The animals were anesthetized with intraperitoneal ketamine (100mg/kg) and Xylazine (10 mg/kg) and then injected bilaterally under stereotaxic conditions with Aβ or normal saline into the CA1 hippocampus (AP=3.9mm, LR=2.2mm, D=2.7mm). Injections were performed at a rate of 0.5µl/min using a Hamilton syringe attached to the stereotax apparatus. Four micro-liters of Aβ (0.5µg/µl) solution injected in every hippocampus, and the needle was kept in place for one minute after injection before being slowly retracted.
Supplementation
Two percent creatine(Sigma-Aldrich USA) was mixed into the normal CHO using an electric mill. After adding some water and cutting the paste, the creatine and CHO mixture were dried in a 30-45 minute exposure to a continuous warm air stream. After injection, animals in the AdCr+ group had free access to this creatine-CHO mixture for six weeks.
Morris Water Maze
The Morris water maze (MWM) procedure was performed 6 weeks after the Aβ injection.
Apparatus
The water maze consisted of a pool (155 cm in diameter) filled with water (21±1◦C) to a level 10 cm from the edge of the tank. A transparent Plexiglas platform (10cm diameter) was located 1.5 cm below the surface in the eastern quadrant of the tank (target quadrant). Climbing onto the platform was the only means of escape from the water. The walls surrounding the pool were decorated with distinct extra maze spatial cues which were kept in fixed positions during the entire experiment to allow the animals to find the hidden platform. The animals’ movements were recorded by a CCD camera (Panasonic Inc., Japan) hanging from the ceiling above the MWM apparatus, and locomotion tracking was measured using Ethovision software (version XT7, the Netherlands), a video tracking system for automated analysis of animal behavior.
Habituation
Twenty-four hours before starting the hidden platform training, rats were given 60 seconds to swim in the tank without the platform in order to adapt to their environment.
Procedure
The reference spatial learning and memory tests were performed based on the procedure previously conducted in our laboratory with some modifications for training; the platform was submerged in the eastern quadrant of the pool. Its place remained unchanged throughout training, but animals were released into the water (while facing the tank wall) from different locations chosen randomly from the south, north, west, northwest and southwest between the trials. Rats received one training session per day for 3 consecutive days. Each session consisted of four trials with 1-min inter-trial intervals. The animals were allowed to swim for 60 sec to localize the platform’s position in the tank. Rats that did not find the platform within 60 sec were directed to it and allowed to rest on it for 10 sec. Twenty-four hours after the third session, the spatial probe test was given. In it, the platform was removed, and rats were allowed to swim for 60 sec before they were removed. Animals were released into the water at a location near the platform. Behavior was recorded with a video tracking system. Escape latencies, time spent in target quadrant, and swim speed were recorded for subsequent analysis.
Preparation for histological tests
The rats were anesthetized and their brains were fixed through a transcardial fixation surgery (normal saline and 4% paraformaldehyde solution) within 24 hours after the spatial probe test. After that, the brains were kept in a 4% paraformaldehyde solution. In preparation for the histological staining, the rat brains were embedded with paraffin, cut into consecutive 5 µm transverse sections by a microtome, and placed on the poly-D-lysine-coated glass slides. Alternate sections were stained with hematoxylin and eosin (H&E) to observe general morphologic alterations, and neighboring sections were used for thioflavin t and TUNEL staining.
Thioflavin
In order to detect Aβ plaques in brain sections thioflavin-t (Sigma-Aldrich USA)was used for staining. Brain sections were deparaffinized in xylene, rehydrated, covered with thioflavin-t solution (0.5% thioflavin-t in 0.1N HCl), and incubated at room temperature and away from light for 10 min. Slides were immersed in distilled water twice before being observed through a fluorescent microscope.
TUNEL
In order to detect apoptotic neurons in brain sections the TUNEL staining protocol was performed by use of Kit (TUNEL Apoptosis Detection Kit,Millipour, and UK). Brain sections were deparaffinized in xylene, rehydrated, immersed in PBS, and then incubated at 37°C for 30 min. Of PBS, sections were covered with diluted protein kinase and incubated at 37°C for 20 min. To halt protein kinase activity, sections were washed in PBS solution (4×2 minutes). Every brain section was covered with 50µl TdT buffer for 10 min. The TdT buffer was removed using a sampler, and then each section was covered with 50µl TdT mixture solution (90% TdT buffer, 5% dUTP-biotin, 5% TdT) and incubated for 60 min at 37°C. Slides were washed for 5 min by immersing them in TB buffer at room temperature and then immersed in PBS (4×2 min). After the washing process, sections were covered with 50µl of blocking solution and incubated for 20 min at room temperature. After removing the blocking buffer with a sampler, 50µl Avidin-FTC mixture (1 part Avidin FTC, 9 parts blocking solution) was used for every section, and slides were incubated at 37°C for 30 min away from light. To obtain the mean percentage of apoptotic neurons in the hippocampus area, the number of TUNEL positive cells were counted in three adjacent 40X microscopic fields.
Statistical analysis
The data were presented as mean±SD using SPSS v.16. Mean escape time, distance traveled to the platform, time spent in target quadrant, swimming speed, and percentage of apoptotic cells were analyzed using the independent samples t-test, to compare sham and control groups, sham and AdCr- groups and also AdCr- and AdCr+.
Results
Thioflavin
Thioflavin staining was performed to confirm the induction of Alzheimer’s in animals. Beta amyoid (Aβ) plaques were observed in the brain sections derived from animals in the AdCr+ and AdCr- groups, but not in the brain sections of those from the sham group or the control group (Fig. 1).
Fig. 1 .
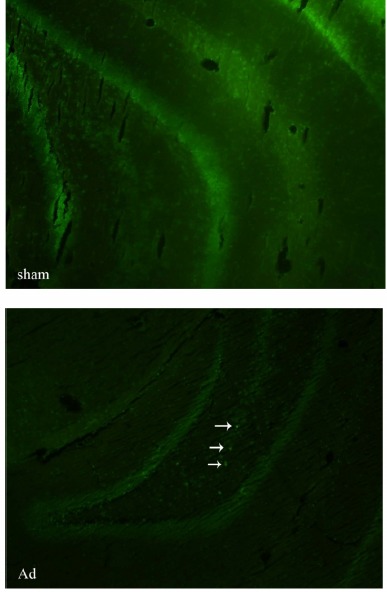
Thioflavin-T staining of amyloid beta plaques. Arrows show the amyloid beta plaques in the brain section derived from an Alzheimer’s induced (Ad). No amyloid beta plaque is visible in brain section of the rat from sham group (sham). Obiective × 10
Table 1. Descriptive statistics of escape time (in 3 days of trainnig), distance travel (in 3 days of trainnig), time spent (in target quadrant in probe test) and percentage of TUNEL positive neurons in brain section of rats,in different groups
Table 1 . Descriptive statistics of escape time (in 3 days of trainnig), distance travel (in 3 days of trainnig), time spent (in target quadrant in probe test) and percentage of TUNEL positive neurons in brain section of rats,in different groups .
| Group | Escape timea (Mean±SD) | Distance Travel(Cm)b (Mean±SD | Time spent(Cm)c (Mean±SD) | TUNELd (Mean±SD) |
| Control | 17.2±1.19 | 374.0±19.76 | 33.57±2.58 | - |
| Sham | 20.9±0.99 | 425.8±20.48 | 35.91±0.91 | 4.83±1.32 |
| AdCr- | 27.9±0.53 | 645.1±35.12 | 31.99±0.78 | 24.00±4.45 |
| AdCr+ | 29.5±1.07 | 650.0±24.65 | 30.37±2.00 | 21.25±3.47 |
a. Sham & Control, Independent sample t-test (p=0.098), Sham & AdCr-, Independent sample t-test (p=0.001), AdCr- & AdCr+, Independent sample t-test (P=0.102)
b. Sham & Control, Independent sample t-test (p=0.119), Sham & AdCr-, Independent sample t-test (p=0.002), AdCr- & AdCr+, Independent sample t-test (P=0.455)
c. Sham & Control, Independent sample t-test (p=0.222), Sham & AdCr-, Independent sample t-test (p=0.017), AdCr- & AdCr+, Independent sample t-test (P=0.235)
d. Sham & AdCr-, Independent sample t-test (=0.001), AdCr- & AdCr+, Independent sample t-test (p=0.322)
Learning and memory retrieval
Rats in the AdCr- group had a higher mean escape time than rats in the sham (p<0.001) and control groups (p=0.001), but no significant difference was shown between the AdCr+ and AdCr- groups (p=0.102)(Table 1 & Fig. 2). These data showed the learning impairment in AdCr+ and AdCr- groups and that creatine supplementation had no effect on the learning ability of rats after Aβ injection. These results were confirmed by comparison of the mean distance that rats traveled to find the platform in training trials (Table 1 & Fig. 3).
Fig. 2 .
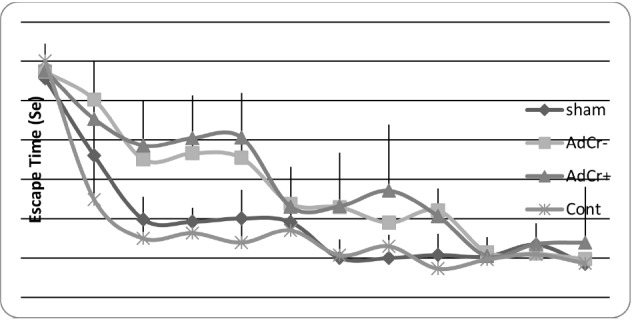
Mean escape time in every single
Fig. 3 .
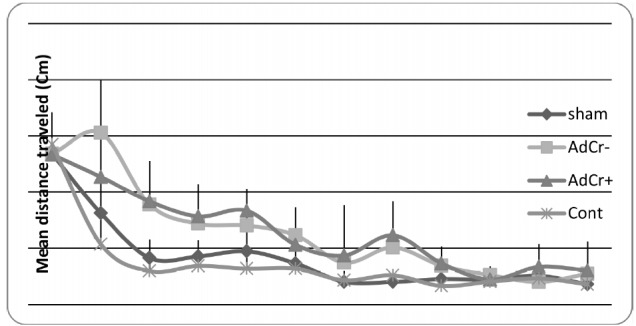
Mean distance traveled to the platform in every single training
Rats in the AdCr- group spent less time in the target quadrant than rats in the sham and control groups (p=0.017). There was no significant difference between rats in the AdCr+ and AdCr- groups (p=0.235), which indicates that after Aβ injection creatine supplementation had no effect on the ability to retrieve memories (Table 1).
Apoptosis
The TUNEL staining protocol was performed to detect apoptotic neurons in brain sections (Fig. 4). As is obvious in Fig. 4 the brain sections of rats in the AdCr+ and AdCr- groups contained more TUNEL positive neurons than those of the sham group.
Fig. 4 .
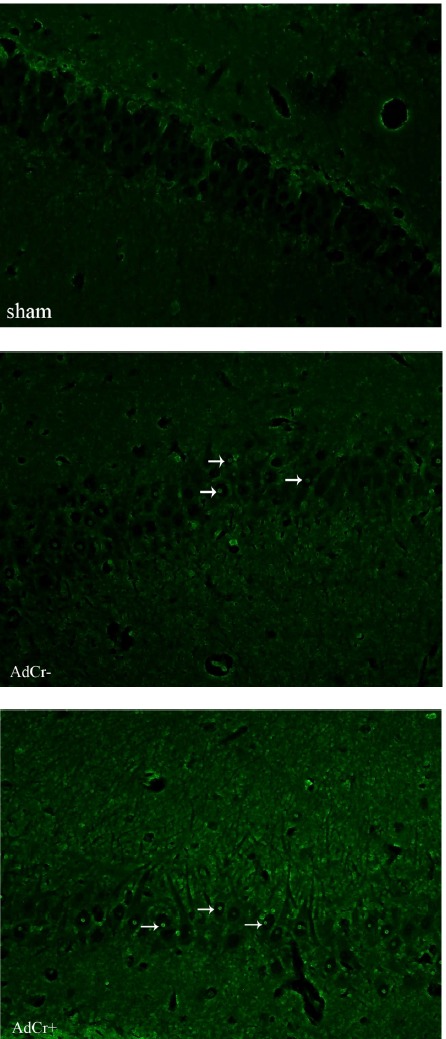
TUNEL staining. Arrows show the TUNEL positive neurons in brain section picture. Alzheimer induced rats (AdCr- & AdCr+, groups) have more TUNEL positive neurons compare to rats in sham group. Objective × 40
The mean percentage of TUNEL positive neurons in the study group (minus the control group) is presented in Table 1. The percentage of TUNEL positive neurons found in the brain sections of rats in the AdCr+ and AdCr-groups was significantly higher than those found in the sham group (p=0.010, p=0.001), but no significant difference was shown in the percentage of TUNEL positive neurons of the AdCr+ and AdCr-groups (P=0.322), which means that after Aβ injection creatine supplementation did not affect the apoptotic effect of Aβ in neurons.
Discussion
The results of our study showed that memory impairments and apoptosis induction caused by a single injection of Aβ could not be affected by six weeks of creatine supplementation started right after the injection while there are evidence that suggest improving effects of creatine supplementation on learning, memory and mental performance (3, 10, 11). The reason that we could not see any improving effects of creatine supplementation on learning and memory may be due to a possible down regulation of creatine kinase activity caused by Aβinjection.
A reduction in creatine kinase activity caused by Aβ has been reported in Alzheimer’s disease and in experimental and cell culture model studies (16, 18). One study reported 86% reduction in creatine kinase activity (19). This decrease is important in the pathogenesis of Alzheimer’s disease, and it is claimed to be the main cause of cognition impairment in elderly individuals and in Alzheimer’s disease (18). Thus the metabolism of creatine in brain is quite different in situations like Alzheimer’s disease and elderly. McMorris et al reported possitive effects of creatine supplementation in healthy elderly indivituals (11) suggesting the creatine supplementation could have improving effects in Alzheimer’s disease as well. Nonetheless our result showed no improvement in learning or memory retrieval among rats in the AdCr+ group (compared with rats in the AdCr- group), however creatine kinase activity was not measured, and this is one limitation of this study. Based on the results of previous studies, the researchers assumed that the reduction in creatine kinase activity was too severe to attenuate the cognitive side effects by supplying more substrate for the enzyme through creatine supplementation (18, 19). It is also possible that the role of decreased creatine kinase activity in cognitive impairment in the elderly and people with Alzheimer’s disease is somewhat exaggerated. Furtherer studies are warranted to investigate effects of creatine supplementation on mental functions in different experimental models of Alzheimer’s disease and to clarify the mechanisms by measuring the creatine kinase activity during a similar study.
Present study also showed no difference in mean percentages of TUNEL positive neurons among rats in the AdCr+ and AdCr- groups which is in line with our behavioral tests. Thisresults indicated that after Aβ injection, creatine supplementation did not show any neuroprotective effects against Aβ toxicity. Country to the results of Brewer‘s study which showed neuroprotective effects of creatine against Aβ in vitro (15) due to slow rate of increase in brain creatine following oral creatine supplementation (18). On the other hand, knowing that supplementation started after Aβ injection, the increase in the brain creatine supply was probably too slow to show the neuroprotective effects of creatine against Aβ toxicity. Further research is needed to evaluate the neuroprotective effects of creatine supplementation when it starts before the Aβ injection.
Conclusion
After Aβ injection creatine supplementation had no effect on learning, memory retrieval, or neuron apoptosis in male Wistar rats. More studies are needed in the field.
Acknowledgements
The authorsextend their sincere thanks to ProfessorFereshtehMotamedi for her helpful cooperation in this study.We would like to thank the Research Council of Tehran University of Medical Sciences for their financial support (18530).
Conflict of interest
The authors have no conflict of interest.
Cite this article as: AliMohammadi M, Eshraghian M, Zarindast M.R, Aliaghaei A, Pishva H. Effects of creatine supplementation on learning, memory retrieval, and apoptosis in an experimental animal model of Alzheimer disease. Med J Islam Repub Iran 2015 (4 October). Vol. 29:273.
References
- 1.Akhondzadeh S, Noroozian M, Mohammadi M, Ohadinia S, Jamshidi AH, Khani M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: a double blind, randomised, placebo controlled trial. Journal of Neurology, Neurosurgery & Psychiatry 2003 July 1, 2003;74(7):863–6. doi: 10.1136/jnnp.74.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER. et al. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64(12):2063–8. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 3.Rae C, Digney AL, McEwan SR, Bates TC. Oral creatine monohydrate supplementation improves brain performance: a double–blind, placebo–controlled, cross–over trial. Proceedings of the Royal Society of London Series B: Biological Sciences. 2003;270(1529):2147–50. doi: 10.1098/rspb.2003.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valenzuela MJ, Jones M, Caroline Rae WW, Graham S, Shnier R, Sachdev P. Memory training alters hippocampal neurochemistry in healthy elderly. NeuroReport. 2003;14(10):1333–7. doi: 10.1097/01.wnr.0000077548.91466.05. [DOI] [PubMed] [Google Scholar]
- 5.Dechent P, Pouwels PJW, Wilken B, Hanefeld F, Frahm J. Increase of total creatine in human brain after oral supplementation of creatine-monohydrate. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1999;277(3):698–704. doi: 10.1152/ajpregu.1999.277.3.R698. [DOI] [PubMed] [Google Scholar]
- 6.Dedeoglu A, Kubilus JK, Yang L, Ferrante KL, Hersch SM, Beal MF. et al. Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington's disease transgenic mice. J Neurochem. 2003;85(6):1359–67. doi: 10.1046/j.1471-4159.2003.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK. et al. Neuroprotective Effects of Creatine in a Transgenic Mouse Model of Huntington's Disease. The Journal of Neuroscience. 2000;20(12):4389–97. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R. et al. Neuroprotective Effects of Creatine and Cyclocreatine in Animal Models of Huntington’s Disease. The Journal of Neuroscience. 1998;18(1):156–63. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan JW, Takahashi K. Cerebral energetic effects of creatine supplementation in humans. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2007;292(4):R1745–R50. doi: 10.1152/ajpregu.00717.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe A, Kato N, Kato T. Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neuroscience Research. 2002;42(4):279–85. doi: 10.1016/s0168-0102(02)00007-x. [DOI] [PubMed] [Google Scholar]
- 11.McMorris T, Mielcarz G, Harris RC, Swain JP, Howard A. Creatine supplementation and cognitive performance in elderly individuals. Neuropsychology, development, and cognition Section B, Aging, Neuropsychology and Cognition. 2007;14(5):517–28. doi: 10.1080/13825580600788100. [DOI] [PubMed] [Google Scholar]
- 12.Rawson ES, Lieberman HR, Walsh TM, Zuber SM, Harhart JM, Matthews TC. Creatine supplementation does not improve cognitive function in young adults. Physiology & Behavior. 2008;95(1-2):130–4. doi: 10.1016/j.physbeh.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Aksenov M, Aksenova M, Butterfield DA, Markesbery WR. Oxidative modification of creatine kinase BB in Alzheimer's disease brain. Journal of neurochemistry. 2000;74(6):2520–7. doi: 10.1046/j.1471-4159.2000.0742520.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuzyk A, Kastyak M, Agrawal V, Gallant M, Sivakumar G, Rak M. et al. Association among amyloid plaque, lipid, and creatine in hippocampus of TgCRND8 mouse model for Alzheimer disease. Journal of Biological Chemistry. 2010;285(41):31202–7. doi: 10.1074/jbc.M110.142174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brewer GJ, Wallimann TW. Protective Effect of the Energy Precursor Creatine Against Toxicity of Glutamate and β-Amyloid in Rat Hippocampal Neurons. Journal of neurochemistry. 2000;74(5):1968–78. doi: 10.1046/j.1471-4159.2000.0741968.x. [DOI] [PubMed] [Google Scholar]
- 16.Gallant M, Rak M, Szeghalmi A, Del Bigio M, Westaway D, Yang J. et al. Focally Elevated Creatine Detected in Amyloid Precursor Protein (APP) Transgenic Mice and Alzheimer Disease Brain Tissue. Journal Name: Journal of Biological Chemistry. 2006;281(1):39576. doi: 10.1074/jbc.C500244200. [DOI] [PubMed] [Google Scholar]
- 17.Pilatus U, Lais C, de Rochmont AdM, Kratzsch T, Frölich L, Maurer K. et al. Conversion to dementia in mild cognitive impairment is associated with decline of N-actylaspartate and creatine as revealed by magnetic resonance spectroscopy. Neuroimaging. 2009;173(1):Neuroimaging 2009;173(1). doi: 10.1016/j.pscychresns.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Wyss M, Kaddurah-Daouk R. Creatine and Creatinine Metabolism. Physiological Reviews. 2000;80(3):1107–213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 19. David S, Shoemaker M, Haley BE. Abnormal properties of creatine kinase in Alzheimer's disease brain: Correlation of reduced enzyme activity and active site photolabeling with aberrant cytosol-membrane partitioning. Molecular Brain. [DOI] [PubMed]


