Abstract
We previously reported the rapid and robust clinical effects of ketamine versus saline infusions in a proof-of-concept crossover trial in unmedicated adults with obsessive-compulsive disorder (OCD). This study examined the concurrent neurochemical effects of ketamine versus saline infusions using proton magnetic resonance spectroscopy (1H MRS) during the clinical proof-of-concept crossover trial. Levels of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) and the excitatory neurochemicals glutamate+glutamine (Glx) were acquired in the medial prefrontal cortex (MPFC), a region implicated in OCD pathology. Seventeen unmedicated OCD adults received two intravenous infusions at least 1 week apart, one of saline and one of ketamine, while lying supine in a 3.0 T GE MR scanner. The order of each infusion pair was randomized. Levels of GABA and Glx were measured in the MPFC before, during, and after each infusion and normalized to water (W). A mixed effects model found that MPFC GABA/W significantly increased over time in the ketamine compared with the saline infusion. In contrast, there were no significant differences in Glx/W between the ketamine and saline infusions. Together with earlier evidence of low cortical GABA in OCD, our findings suggest that models of OCD pathology should consider the role of GABAergic abnormalities in OCD symptomatology.
Keywords: Obsessive-compulsive disorder (OCD), Ketamine, Glutamate-glutamine, Gamma-aminobutyric acid, Magnetic resonance spectroscopy, Medial prefrontal cortex
1. Introduction
Obsessive-compulsive disorder (OCD) is associated with dysfunction in frontostriatal circuits (Maia et al., 2008; Ahmari et al., 2013; Burguiere et al., 2013), and is characterized by repetitive thoughts (obsessions) and behaviors (compulsions) (Koran et al., 2007). Treatment of OCD with serotonin reuptake inhibitors (SRIs) results in a long lag time (2-3 months) before clinical benefit that is typically only partially effective (Pigott and Seay, 1999; Doughterty et al., 2004). Moreover, the only evidence-based medication strategy for augmenting SRIs in OCD is the addition of antipsychotics; however, this is effective in only one third of patients (Bloch et al., 2006). Identifying pharmacological treatments beyond SRIs may not only advance therapeutic options, but also open new hypotheses regarding the mechanisms underlying OCD psychopathology.
Converging lines of evidence from in vivo brain-imaging, genetic, and pharmacological studies implicate abnormalities of glutamate, the most abundant excitatory neurotransmitter, in the pathogenesis of OCD (Pittenger et al., 2011). Recently, we showed that a single subanesthetic dose of the glutamatergic N-methyl-D-aspartate receptor antagonist ketamine rapidly and robustly reduces OCD symptoms in the absence of an SRI (Rodriguez et al., 2013), supporting the “glutamate hypothesis of OCD” originally proposed by Rosenberg et al. (Rosenberg et al., 2000; for review, see Pittenger et al., 2011). In addition, abnormalities in the inhibitory neurotransmitter system of gamma-aminobutyric acid (GABA) have been reported in several mental disorders (Gonzalez-Burgos and Lewis, 2012; Han et al., 2014), including OCD (Greenberg et al., 2000; Zai et al., 2005a; Zai et al., 2005b; Richter et al., 2012). As a result, therapeutic interventions targeting glutamatergic and GABAergic dysregulations are attracting considerable interest.
Currently, proton magnetic resonance spectroscopy (1H MRS) is the only noninvasive neuroimaging technique that allows investigation of glutamatergic and GABAergic abnormalities within brain regions of interest in vivo. MRS studies of the effects of ketamine in the medial prefrontal cortex (MPFC) and other brain regions in healthy (Rowland et al., 2005; Stone et al., 2012; Taylor et al., 2012) and depressed (Valentine et al., 2011) human subjects have been reported. These studies examined one or two time points, with some (Rowland et al., 2005; Stone et al., 2012) but not all (Valentine et al., 2011; Taylor et al., 2012) documenting increases in glutamatergic compounds in response to ketamine. These findings are relevant to OCD, given the clinical findings of ketamine described above (Rodriguez et al., 2013) and the fact that earlier MRS studies in OCD reported Glx and GABA abnormalities in the MPFC (Rosenberg et al., 2004; Starck et al., 2008; Yucel et al., 2008; Simpson et al., 2012).
We and others have previously reported the clinical effects of ketamine in OCD (Rodriguez et al., 2011; Bloch et al., 2012; Rodriguez et al., 2013). This study is the first to investigate the neurochemical effects of ketamine in OCD. Specifically, we used 1H MRS to dynamically monitor the changes in the levels of GABA and Glx in the MPFC of 17 medication-free adults with OCD during administration of ketamine and saline. Consonant with current OCD models of glutamatergic abnormalities (Pittenger et al., 2011) and reports of ketamine increasing glutamatergic compounds in some MRS studies (Rowland et al., 2005; Stone et al., 2012), we hypothesized that ketamine, compared with saline, would increase Glx in the MPFC in OCD. Given recent evidence of GABA abnormalities in OCD (Simpson et al., 2012), exploratory analyses examined whether ketamine would also increase MPFC GABA levels.
2. Methods
2.1. Participants
The Institutional Review Board of the New York State Psychiatric Institute (NYSPI)/Columbia University approved the study. Subjects were recruited by clinical referral and advertisements and provided written informed consent before participation.
Eligible outpatients were between the ages of 18 and 55 with a principal diagnosis of OCD for at least 1 year and were at least moderately symptomatic (Yale-Brown Obsessive-Compulsive Scale [YBOCS (Goodman et al., 1989a; Goodman et al., 1989b)] score ≥ 16). Subjects were required to be off all psychotropic medications, to have failed at least one earlier trial of SRI and/or cognitive behavioral therapy with exposure and response prevention (EX/RP), or to have refused these treatments for individual reasons.
Subjects were excluded for comorbid major depression (MDD) as documented by a Hamilton Depression Rating Scale (HDRS-17) score ≥ 25 (Hamilton, 1960), as well as for bipolar, psychotic, or eating disorders, substance dependence (including nicotine), substance abuse within the past year, or prominent suicidal ideation. Other comorbidity was permitted (e.g., social anxiety, specific phobia) if the OCD symptoms were the most severe and impairing. Subjects with a first degree relative with schizophrenia were also excluded. In addition, potential participants were excluded if they had an unstable medical or neurological condition that increased the risk of participation (e.g,. dementia), if they had ferromagnetic material in the body that may have presented a risk to the subject or interfered with MRS, if they were pregnant or nursing, or if they were having concurrent EX/RP treatment.
Trained clinicians determined eligibility during a clinical interview; diagnoses were confirmed by trained raters using the Structured Clinical Interview for DSM-IV (SCID-IV) (First et al., 1996). Treatment history was confirmed with the referring clinician, chart review, and/or pharmacy records.
2.2. Procedures
2.2.1. Infusion during MR acquisition
Seventeen participants with OCD were administered two 40-min intravenous infusions, one of saline and one of ketamine (0.5 mg/kg), at least 1 week apart, while lying supine in a General Electric 3.0-T EXCITE MRI scanner at the New York State Psychiatric Institute. The order of each infusion pair was randomized. Participants were instructed to fast before each scan. Vital signs were monitored continuously during the infusion with electrocardiogram, blood oxygen saturation, and blood pressure cuff. Each scanning session lasted 90 min and included a structural MRI and six 13-min MRS acquisitions (one at baseline [0-13 min], three during ketamine infusion [15-28, 30-43, 45-58 min], and two post-infusion scans [60-73, 75-88 min]).
2.2.2. Clinical assessments
For a full description of the clinical assessments, we previously reported in a nearly identical sample examining the clinical effects of ketamine in OCD (see Rodriguez et al., 2013). Below we describe assessments directly relevant to our current study of the neurochemical effects of ketamine in OCD. To assess rapid changes in OCD symptoms during and after the infusion, patients self-rated the severity of their obsessions using the OCD-visual analog scale (VAS) (Rodriguez et al., 2011; Rodriguez et al., 2013), a modified self-rating scale used previously to detect acute changes in OCD symptoms (Greenberg et al., 1998; Murphy et al., 1989), at baseline, during the infusion, at 90, 110, 230 min post-infusion, and daily for 1 week post-infusion. An independent evaluator, blind to treatment, evaluated participants at baseline using the Y-BOCS, which appraises obsessive and compulsive symptoms over the prior week. The Y-BOCS checklist provided scores for each OCD patient along five symptom dimensions (contamination and cleaning, taboo thoughts, doubt and checking, symmetry and ordering, and hoarding), using the approach recommended by Pinto et al. (Pinto et al., 2008; Pinto et al., 2009). The HDRS-17 was used to evaluate depression severity at baseline.
2.2.3. Structural MRI
A three-plane, low-resolution, high-speed localizer imaging series was obtained, followed by a volumetric T1-weighted spoiled gradient-recalled echo (SPGR) scan (repetition time/echo time=7.12/2.86, flip angle=9°, field of view 256×256mm2, image matrix size = 256×256, slice thickness 1 mm; voxel size 1×1×1mm3). A neuroradiologist confirmed that all MRI scans were free of gross structural abnormalities.
2.2.4. In vivo 1H MRS time course
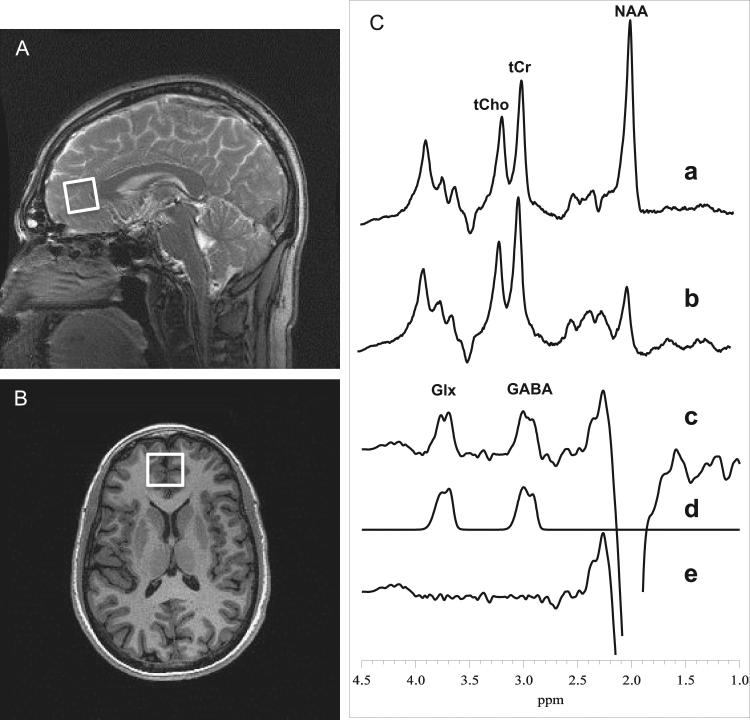
Brain spectra containing GABA and Glx resonances were acquired with an eight-channel phased-array head coil and the volume-selective PRESS J-editing difference method (Rothman et al., 1993), as fully described previously (Geramita et al., 2011; Simpson et al., 2012; Mullins et al., 2014) and demonstrated in Fig. 1. While optimized for GABA detection, this pulse sequence also achieves reliable detection of the combined resonances of Glx at 3.7 p.p.m. (Kegeles et al., 2006; Geramita et al., 2011) due to their high structural, chemical and magnetic similarities with GABA. Spectra were acquired from a 2.5 × 3.0 × 2.5-cm3 (18.8-cm3) MPFC voxel that was positioned anterior to the genu of the corpus callosum, and included portions of Brodmann areas 24, 32, and 10, including a portion of the pregenual anterior cingulate cortex (ACC) (Fig. 1A and 1B). Each of six MRS data frames were recorded in 13 min with the J-editing technique using TE/TR 68/1500 ms and 256 interleaved excitations (512 total), and included a 2-min gap between the data frames for automatic readjustment of the static field homogeneity (shims) by the host computer. The achieved static field homogeneity, as assessed from the full width at half-maximum (FWHM) of the unsuppressed voxel tissue water resonance, was typically 12-13 Hz, with 20 Hz being the upper limit that was considered acceptable. The strong unsuppressed voxel tissue water signal, necessary for eight-channel coil data reconstruction and intensity normalization, was automatically recorded in two excitations with the water suppression and J-editing pulses turned off, and then stored in separate memory blocks within the same raw data file as the neurotransmitter signals, which were recorded immediately thereafter and stored in subsequent memory blocks. The metabolite levels were expressed semi-quantitatively as ratios to the unsuppressed voxel tissue water, with no correction for relaxation effects.
Fig. 1.
Medial prefrontal cortex (MPFC) voxel and sample 1H spectral data. (A) Sagittal and (B) axial localizer images showing MPFC voxel size and location. (C) J-edited PRESS 1H MR spectra (Geramita et al., 2011; Mullins et al., 2014) acquired in 13 min from the MPFC voxel using TE/TR 68/1500 ms, and 256 interleaved excitations (total 512) with editing radiofrequency pulse off (a) or on (b). With the editing pulse off (a), a standard PRESS spectrum is obtained and yields high-quality spectra for deriving NAA, tCr, and tCho. (c) The difference of the subspectra in (a) and (b) showing the detected GABA and Glx peaks. (d) The best-fit model spectrum. (e) The residual difference between the experimental and fitted spectra. The fitting procedure yields areas under the GABA and Glx peaks, which are proportional to concentrations.
The alternative of fitting Glx in the unedited spectrum was considered, but not pursued for two reasons. First, the Glx resonance in the unedited spectrum greatly overlaps with other resonances, which are mostly eliminated in the difference spectrum to simplify spectral fitting, and sits atop a difficult to model baseline; and, second, we found that our test-retest reliability data for Glx values obtained from our analysis of the difference spectra were as reliable as for similarly derived GABA values (Kegeles et al., 2006). In our view, the similarity in test-reliability estimates for GABA and Glx makes a compelling case for fitting the Glx resonance in the much simpler difference spectra because the two resonances being fitted are unobstructed and fully visible and the baseline is relatively flat.
2.2.5. MRS data processing
MRS data quality assessment, processing and quantification procedures have been described (Simpson et al., 2012; Chen et al., 2014). Briefly, eight-channel phased-array coil data that met our quality assessment criteria with respect to spectral resolution and subject head motion during the scans (Simpson et al., 2012; Chen et al., 2014) were combined into single regular time-domain free-induction decay signals, using a C-language computer program that implemented a previously published algorithm (Brown, 2004). The unsuppressed voxel tissue water signal acquired with each receiver coil element provided the required relative array coil sensitivities. The two resulting free-induction decay signals for the editing pulse on/off scans were subtracted in the time domain to yield the difference signal, which was filtered with an exponential multiplication function corresponding to a 5-Hz line broadening and then Fourier-transformed to obtain the GABA and Glx spectra (Fig. 1C). The area under each neurotransmitter peak was obtained using a robust and highly optimized public-domain Levenberg–Marquardt nonlinear least-squares IDL minimization routine (Markwardt, 2009) to model the resonances in the frequency domain as a linear combination of pseudo-Voigt functions (Fig. 1C); this enables more precise analysis of line shapes that consist of mixtures of Lorentzian and Gaussian functions (Marshall et al., 2000), as is often the case for in vivo spectra. The IDL-fitting procedure automatically reports the covariance matrix for the estimated set of MRS parameters, from which the parameter correlational matrix, statistical errors at one SD, and uncertainties are computed as measures of goodness of fit (Markwardt, 2009), which, along with visual examination of the fits, were used for quality assessment of the data as previously described (see Supplementary material in Simpson et al. (2012). To enable groupwise comparisons, the GABA and Glx peak areas were expressed as ratios relative to the area of the unsuppressed voxel tissue water (W). Voxel segmentation and metabolite correction were not conducted, given the focus of the study is on metabolite changes over time within subjects.
2.3. Statistical analyses
Glx/W levels were fitted using mixed effects linear models with infusion type (ketamine/saline), infusion order, MRS data frame, and responder status as fixed effects, and subject and scan (nested within subject) as random effects. GABA/W levels were explored using similar models. Where the overall F-test was significant (p <0.05, two-tailed), post hoc analyses were conducted to identify potential effects of ketamine at each of the six 13-min acquisitions to give a more detailed picture of which time points are driving the trend; correction for multiple comparisons was not conducted for pos thoc analyses, given their exploratory nature. We first tested for neurochemical carryover effects using a paired t-test between baseline Glx/W and GABA/W levels between the first and second infusions (GABA/W: p=0.383; Glx/W: p = 0.673); finding no evidence of carryover effects, we collapsed the neurochemical data across phases in the final analyses.
3. Results
3.1. Sample characteristics
In Table 1, the demographic and clinical characteristics of the 16 OCD subjects with viable MRS data are provided, after the exclusion of one subject who was an extreme outlier (z-scores>3 for Glx/W in the MPFC). These participants were a nearly identical sample to those whose clinical findings we previously reported (Rodriguez et al., 2013), except for the participant excluded due to non-viable MRS data and two new participants. The final MRS sample (n=16) had clinically significant symptoms, with a mean YBOCS score of 26 (SD=4.2) and a range from 20 (moderate) to 37 (extreme) OCD, with mean illness duration of 17.3 years (SD=9.7). As shown in Table 1, 11 of the 16 OCD subjects (69%) had no other psychiatric comorbidity. Two met criteria for co-morbid depression, with baseline HDRS-17 scores of 13 and 16. No participants were receiving medication or psychotherapy at the time of MRS scanning, with the last SRI dose a mean of 3 (SD=2) years earlier. All five OCD symptom dimensions were represented, with most participants exhibiting symptoms in more than one dimension (cleaning and contamination symptoms, n=12; symmetry and ordering, n=11; hoarding, n=7; doubt and checking, n=9; taboo thoughts, n=8). Comparison of the demographics of the two groups randomized to different infusion order showed no statistically significant differences between groups in sex, age, race, or number of earlier SRI trials (p-values ranged from 0.13 to 0.39).
Table 1.
Demographic and clinical characteristics
| Demographic and clinical characteristics (n = 16) | |
|---|---|
| Demographics | |
| Mean age in years (SD) | 32.9 (7.5) |
| Number of males/females | 9/7 |
| Race-ethnicity | 1A/7AA/5C/3H |
| Mean age of OCD onset in years (SD) | 16.4 (8.9) |
| Baseline clinical scores | |
| Mean baseline YBOCS (SD) | 26.0 (4.2) |
| Mean baseline HDRS-17 (SD) | 3.9 (4.6) |
| Number of subjects with current DSM-IV Axis I comorbidity | |
| OCD only | 11 |
| OCD with MDD only | 2 |
| OCD with social anxiety only | 2 |
| OCD with social anxiety and specific phobia | 1 |
| Treatment history | |
| Mean number of prior SRI trials (SD) | 1.8 (2.2) |
| Number of subjects who had at least one EX/RP trial | 7 |
Abbreviations: A=Asian; AA=African American; C=Caucasian; EX/RP=cognitive behavioral therapy with exposure and response prevention; F=female; H=Hispanic; HDRS-17=17-Item Hamilton Depression Rating Scale; M=male; MDD=major depressive disorder; OCD=obsessive-compulsive disorder; SRI=serotonin reuptake inhibitor; SD=standard deviation; Y-BOCS=Yale-Brown Obsessive-Compulsive Scale.
3.2. Effects of ketamine on MPFC Glx and GABA levels in vivo
In the MPFC voxel, OCD subjects did not show significant differences between ketamine and saline conditions over time in Glx/W (F=0.65; df=6, 141; p=0.689), but they did show modest differences in GABA/W (F=2.16; df=6, 146; p=0.048) over six successive 13-min acquisitions (Table 2). Repeating the analyses with the first treatment phase only gives similar conclusions (i.e., OCD subjects did not show significant differences between the ketamine and saline conditions over time in Glx/W [F=1.45; df=6, 64; p=0.210], but they did show modest differences in GABA/W [F=1.90; df=6, 66; p=0.094] over six successive acquisitions, although not meeting our a priori standard for significance).
Table 2.
Medial prefrontal cortex (MPFC) metabolite levels by infusion type
| Scan 1 | Scan 2 | Scan 3 | Scan 4 | Scan 5 | Scan 6 | ||
|---|---|---|---|---|---|---|---|
| MRS metabolite | Infusion | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Glx/Wa | Ketamine | 1.69 (0.31) | 1.61 (0.36) | 1.64 (0.42) | 1.60 (0.27) | 1.67 (0.36) | 1.59 (0.38) |
| Glx/Wa | Saline | 1.58 (0.36) | 1.70 (0.25) | 1.64 (0.34) | 1.65 (0.32) | 1.68 (0.22) | 1.64 (0.26) |
| GABA/Wa | Ketamine | 2.32 (0.44)* | 2.44 (0.29) | 2.33 (0.31) | 2.42 (0.45) | 2.56 (0.54)*† | 2.33 (0.34) |
| GABA/Wa | Saline | 2.44 (0.45) | 2.33 (0.32) | 2.52 (0.48) | 2.41 (0.59) | 2.26 (0.31)† | 2.40 (0.44) |
The gray shaded box visually illustrates the approximate duration of 40-min infusion (ketamine or saline); the exact end of the infusion is 3 min before the end of fourth scan. Post hoc tests within the framework of the mixed effect model showed GABA/W significantly increased during scan 5 (60-73 min post-infusion) in the ketamine compared with the saline condition (t(146)=2.38, p=0.02)† and compared with baseline (t(72)=2.22, p=0.03)*(see Results section).
Abbreviations: Glx/W= Glutamate-glutamine level normalized to water; GABA/W=Gamma-aminobutyric acid level normalized to water; SD=standard deviation. Symbols (†and *) both denote significant differences (p<0.05).
10−3 institutional units
3.3. Clinical correlations
Post hoc tests within the framework of the mixed effect model showed GABA/W significantly increased 60-73 min post-infusion in the ketamine compared with the saline condition (t(146)=2.38, p=0.02) and compared with baseline (t(72)=2.22, p=0.03). The change in GABA/W from baseline to 60-73 min post-infusion was positively correlated with changes in OCD symptoms as measured by the OCD-VAS both during and after the infusion at all time points up to day 7 (r-values ranged from 0.46 to 0.63, p-values ranged from 0.01 to 0.06), but the change was not significantly correlated with dissociative symptoms or psychotic symptoms using the Clinician Administered Dissociative Symptoms Scale and the Brief Psychiatric Rating Scale.
4. Discussion
This is the first report to examine the time course of neurochemical effects of a single intravenous infusion of ketamine in OCD. Contrary to our hypothesis, in this proof-of-concept study, ketamine did not significantly increase MPFC Glx/W levels in unmedicated adult OCD participants over time. However, ketamine did significantly increase MPFC GABA/W levels over time. Post hoc analyses showed that a single time point, approximately 1 hour post-ketamine infusion, was driving this effect.
Our Glx/W finding is in agreement with a study that found no differences in cortical Glx in healthy volunteers between ketamine and saline groups (Taylor et al., 2012), and a study that did not detect differences in cortical glutamate+glutamine in healthy volunteers between pre- and post-ketamine infusion (in a region partially overlapping with and slightly dorsal to the voxel in the present study) (Stone et al., 2012).
That GABA/W increased in our sample is interesting given recent findings indicating GABA abnormalities in OCD. Specifically, abnormalities in cortical inhibitory processes in OCD have been reported using transcranial magnetic stimulation paradigms (Greenberg et al., 2000; Richter et al., 2012). In addition, a study of the association between markers in the GABAB receptor 1 (GABBR1) gene and OCD found evidence for GABBR1 as a susceptibility factor in OCD (Zai et al., 2005). Finally, we previously reported that unmedicated patients with OCD show baseline GABA deficits in MPFC compared with matched healthy controls (Simpson et al., 2012); low baseline MPFC GABA levels may be part of the brain's attempt to regulate or compensate for OCD symptoms.
Exploratory post hoc analyses revealed increases in GABA/W at approximately 1 hour post-infusion during the ketamine compared with the saline infusion and compared with baseline. The change in GABA/W levels was positively correlated with changes in OCD symptoms, but not correlated with (and thus unlikely to be manifestations of) psychotic or dissociative symptoms. This is also consistent with lack of significant correlation between psychotic or dissociative symptoms and ketamine blood-level findings or clinical OCD symptoms, which we previously reported in a nearly identical sample examining the clinical effects of ketamine in OCD (Rodriguez et al., 2013). The post-ketamine increase in GABA/W is consistent with recent studies suggesting that ketamine concurrently activates a subpopulation of GABAergic interneurons and projection neurons in the cortex (Whittington et al., 2000; Hong et al., 2010; McNally et al., 2011; Kocsis et al., 2012; Vinck et al., 2013). The finding is interesting given that ketamine also impacts oscillatory activity in a manner consistent with evolving models of cortical microcircuitry, suggesting that rhythmic physiological signals (thought to modulate or “gate” processing of inputs) are mediated by precisely timed activation of GABA interneurons and projection neurons (Whittington et al., 2000; Cardin et al., 2009; Gonzalez-Burgos and Lewis, 2012). That change in GABA/W was positively correlated with change in OCD symptoms is counterintuitive and warrants further study in a larger sample: we speculate that greater GABA/W changes during ketamine infusion may reflect greater underlying GABAergic abnormalities in OCD.
Understanding the underlying brain basis of GABA and how it relates to obsessions and compulsions across disorders as traditionally defined (i.e., exploring OCD-related disorders, disorders often comorbid with OCD [e.g. tic disorder] and OCD symptom dimensions) in a larger sample in future studies is needed given emerging findings of GABA abnormalities in other disorders (Kalanithi et al., 2005; Bronfeld et al., 2013; Pogorelov et al., 2015). Although participants in the present study did not endorse tics, and dimensions were not correlated with clinical effect, larger samples may prove fruitful for this type of analysis.
Our findings need to be tempered in light of several limitations. First, the sample size was small. As a result, we may have insufficient statistical power to rule out type I error (e.g., distinguishing baseline variability from ketamine's effects at specific time points) or addressing important potential confounds like gender. Second, although the J-editing method can separate GABA from Glx, it cannot separate the individual components that form Glx (i.e., glutamate from glutamine). Given that one study has shown glutamine (thought to be a marker of neurotransmitter glutamate release) rather than glutamate increases in healthy volunteers administered ketamine (Rowland et al., 2005), while another has shown glutamate increases (Stone et al., 2012), future studies investigating the response of the glutamatergic system to drug effects should aim to derive less ambiguous data by using MRS methods (e.g. 2D J-resolved at 7 T) that are capable of reliably measuring glutamate and glutamine separately. At the same time, Glx obtained with J-editing is predominantly glutamate and shows excellent test-retest reliability (Shungu, 2013; Shungu et al., 2013). In addition, MRS measures whole-tissue levels; thus, an increase in glutamate release without an elevation in overall tissue levels might not be detected by MRS. Furthermore, the GABA MRS peak is an admixture of signals from both mobile macromolecules and GABA (Behar, 1994); thus, if ketamine affects macromolecules, the observed changes in the study may not be exclusively due to changes in GABA. Also, low tissue concentration of GABA necessitates relatively large voxels (e.g., 10-20 cm3) to attain adequate signal-to-noise ratio for reliable quantification in a clinically tolerable scan time. Given Glx levels may be sensitive to voxel white-matter content, it is possible that putative changes in Glx levels are masked by the white matter that is unavoidably present in the large voxels required by GABA J-editing. Nevertheless, these methods have found Glx differences in the same brain region following ketamine in healthy subjects (Kegeles et al., 2013) and in depressed subjects (Milak et al., in press), as well as in schizophrenia compared with controls (Kegeles et al., 2012), indicating that Glx changes in OCD are smaller than in these conditions. Third, although we did not find evidence of a neurochemical carryover effect, we cannot rule out that clinical carryover effects (Rodriguez et al., 2013) may have non-specific effects on neurochemical events. Finally, because of the psychoactive effects of ketamine, blinding patients to the dissociative side effects was difficult (Rodriguez et al., 2013). Thus, the design of future studies of ketamine's effects in OCD should use a parallel design (with an active control that can mimic dissociative side effects) and incorporate MRS assessments within 1-3 days following the infusion.
In conclusion, we found that a single ketamine infusion increased GABA/W levels in adults with OCD in a MPFC voxel that included the pregenual ACC, but we did not find that ketamine increased Glx/W levels. Further research is warranted to examine how GABA and glutamate abnormalities in the fronto-striatal brain circuits contribute to the pathophysiology of OCD.
Highlights.
The neurochemical effects of ketamine versus saline infusions were examined in 17 unmedicated patients with obsessive-compulsive disorder (OCD) using proton magnetic resonance spectroscopy (1H MRS).
Levels of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) and the excitatory neurochemicals glutamate+glutamine (Glx) were acquired in the medial prefrontal cortex (MPFC).
A mixed effects model found that MPFC GABA/W significantly increased over time in the ketamine compared with the saline infusion.
Findings suggest that models of OCD pathology should consider the role of GABAergic abnormalities in OCD symptomatology.
Acknowledgements
This investigation was supported by grants from National Institute of Mental Health, K23MH092434 (Dr. Rodriguez), K24MH09155 (Dr. Simpson), Gray Matters and Sidney R. Baer, Jr. Foundation Fellowships (Dr. Rodriguez and Dr. Moore), Pisetsky Young Investigator Award (Dr. Rodriguez), Molberger Scholar Award (Dr. Rodriguez), and the New York State Psychiatric Institute. Dr. Rodriguez also utilized the Clinical Research Resource (CRR) within the Irving Institute for Clinical and Translational Research (Columbia's CTSA), which is funded through NCATS/NIH CTSA UL1 TR000040. We thank those individuals with OCD who generously donated their time to participate in this research study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding and disclosures
Dr. Carolyn I. Rodriguez, Amanda Levinson, Dr. R. Todd Ogden, Xiangling Mao, Dr. Matthew S. Milak, Donna Vermes, Ms. Shan Xie, Liane Hunter, Dr. Holly Moore, and Dr. Dikoma C. Shungu report no financial relationships with commercial interests or potential conflicts of interest. Dr. Lawrence S. Kegeles has received research funds from Amgen and Pfizer. Dr. Pamela Flood reports an equity interest in Signature Pharmaceuticals. Dr. Helen Blair Simpson has received research funds from Janssen Pharmaceuticals (2006-2012) and Transcept Pharmaceuticals (2011-2013), royalties from Cambridge University Press and UpToDate, Inc., and provided a 1-hour consultation for Quintiles, Inc. (September, 2012).
References
- Abrantes AM, Strong DR, Cohn A, Cameron AY, Greenberg BD, Mancebo MC, Brown RA. Acute changes in obsessions and compulsions following moderate-intensity aerobic exercise among patients with obsessive-compulsive disorder. J Anxiety Disord. 2009;23:923–927. doi: 10.1016/j.janxdis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, Gordon JA, Hen R. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Spencer DD, Petroff OA. Analysis of macromolecule resonances in 1H NMR spectra of human brain. Magnetic Resonance in Medicine. 1994;32:294–302. doi: 10.1002/mrm.1910320304. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry. 2006;11:622–632. doi: 10.1038/sj.mp.4001823. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Wasylink S, Landeros-Weisenberger A, Panza KE, Billingslea E, Leckman JF, Krystal JH, Bhagwagar Z, Sanacora G, Pittenger C. Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry. 2012;72:964–970. doi: 10.1016/j.biopsych.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfeld M, Yael D, Belelovsky K, Bar-Gad I. Motor tics evoked by striatal disinhibition in the rat. Frontiers in Systems Neuroscience. 2013;7:50. doi: 10.3389/fnsys.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MA. Time-domain combination of MR spectroscopy data acquired using phased-array coils. Magnetic Resonance in Medicine. 2004;52:1207–1213. doi: 10.1002/mrm.20244. [DOI] [PubMed] [Google Scholar]
- Burguiere E, Monteiro P, Feng G, Graybiel AM. Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science. 2013;340:1243–1246. doi: 10.1126/science.1232380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Stanford AD, Mao X, Abi-Dargham A, Shungu DC, Lisanby SH, Schroeder CE, Kegeles LS. GABA level, gamma oscillation, and working memory performance in schizophrenia. NeuroImage. Clinical. 2014;4:531–539. doi: 10.1016/j.nicl.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Rauch SL, Jenike MA. Pharmacotherapy for obsessive-compulsive disorder. J. Clin. Psychol. 2004;60:1195–1202. doi: 10.1002/jclp.20083. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York, NY.: 1996. [Google Scholar]
- Geramita M, van der Veen JW, Barnett AS, Savostyanova AA, Shen J, D.R., W., Marenco S. Reproducibility of prefrontal gamma-aminobutyric acid measuremetns with J-edited spectroscopy. NMR Biomed. 2011;24:1089–1098. doi: 10.1002/nbm.1662. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38:950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch. Gen. Psychiatry. 1989a;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch. Gen. Psychiatry. 1989b;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Benjamin J, Martin JD, Keuler D, Huang SJ, Altemus M, Murphy DL. Delayed obsessive-compulsive disorder symptom exacerbation after a single dose of a serotonin antagonist in fluoxetine-treated but not untreated patients. Psychopharmacology (Berl) 1998;140:434–444. doi: 10.1007/s002130050787. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Ziemann U, Cora-Locatelli G, Harmon A, Murphy DL, Keel JC, Wassermann EM. Altered cortical excitability in obsessive-compulsive disorder. Neurology. 2000;54:142–147. doi: 10.1212/wnl.54.1.142. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Jones CJ, Scheuer T, Catterall WA. Enhancement of inhibitory neurotransmission by GABAA receptors having alpha2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron. 2014;81:1282–1289. doi: 10.1016/j.neuron.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Buchanan RW, O'Donnell P, Thaker GK, Weiler MA, Lahti AC. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 2010;35:632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles L, Mao X, Ojeil N, Massuda R, Pedrini M, Chen M, Slifstein M, Abi-Dargham A, Milak M, Rodriguez C, Shungu D. Time course of ketamine effects on brain glutamate and GABA levels measured with 1H MRS. Schizophrenia Bulletin. 2013;39:S140. [Google Scholar]
- Kegeles LS, Mao X, Dyke J, Gonzalez J, Soones T, Shungu DC. Test-retest reliability of dorsolateral prefrontal cortical GABA measuremnt using an 8-channel phased-array head cil with the J-editing technique at 3T. Proc Intl Soc Magn Reson Med. 2006;14:489. [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, Gil R, Slifstein M, Abi-Dargham A, Lisanby SH, Shungu DC. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- Kocsis B. Differential role of NR2A and NR2B subunits in N-methyl-D-aspartate receptor antagonist-induced aberrant cortical gamma oscillations. Biol Psychiatry. 2012;71:987–995. doi: 10.1016/j.biopsych.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koran LM, Hanna GL, Hollander E, Nestadt G, Simpson HB. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164:5–53. [PubMed] [Google Scholar]
- Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Development and psychopathology. 2008;20:1251–1283. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt C. Non-linear least squares fitting in IDL with MPFIT. Proceedings of Astronomical Data Analysis Softward and Systems. 2009;411:251–254. [Google Scholar]
- Marshall I, Bruce SD, Higinbotham J, MacLullich A, Wardlaw JM, Ferguson KJ, Seckl J. Choice of spectroscopic lineshape model affects metabolite peak areas and area ratios. Magnetic Resonance in Medicine. 2000;44:646–649. doi: 10.1002/1522-2594(200010)44:4<646::aid-mrm20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- McNally JM, McCarley RW, McKenna JT, Yanagawa Y, Brown RE. Complex receptor mediation of acute ketamine application on in vitro gamma oscillations in mouse prefrontal cortex: modeling gamma band oscillation abnormalities in schizophrenia. Neuroscience. 2011;199:51–63. doi: 10.1016/j.neuroscience.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak M, Proper C, Mulhern S, Parter A, Kegeles L, Ogden R, Mao X, Rodriguez CI, Oquendo M, Suckow R, Cooper T, Shungu D, Mann J. Amino acid neurotransmitter response to a single subanesthetic dose of ketamine monitored in major depressive disorder in vivo by proton magnetic resonance spectroscopy. Molecular Psychiatry. doi: 10.1038/mp.2015.83. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O'Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Cardiff Symposium on M.R.S.o.G., Edden RA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Mueller EA, Hill JL, Tolliver TJ, Jacobsen FM. Comparative anxiogenic, neuroendocrine, and other physiologic effects of m-chlorophenylpiperazine given intravenously or orally to healthy volunteers. Psychopharmacology (Berl) 1989;98:275–282. doi: 10.1007/BF00444705. [DOI] [PubMed] [Google Scholar]
- Pigott TA, Seay SM. A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorder. J Clin Psychiatry. 1999;60:101–106. doi: 10.4088/jcp.v60n0206. [DOI] [PubMed] [Google Scholar]
- Pinto A, Greenberg BD, Grados MA, Bienvenu OJ, Samuels JF, Murphy DL, Hasler G, Stout RL, Rauch SL, Shugart YY, Pauls DL, Knowles JA, Fyer AJ, McCracken JT, Piacentini J, Wang Y, Willour VL, Cullen B, Liang KY, Hoehn-Saric R, Riddle MA, Rasmussen SA, Nestadt G. Further development of YBOCS dimensions in the OCD Collaborative Genetics study: symptoms vs. categories. Psychiatry Research. 2008;160:83–93. doi: 10.1016/j.psychres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Greenberg BD, Murphy DL, Nestadt G, Rasmussen SA. Using individual items to clarify OCD symptom structure: the case for five factors. Am J Psychiatry. 2009;166:728–729. doi: 10.1176/appi.ajp.2009.09020287. author reply 729-731. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol Ther. 2011;132:314–332. doi: 10.1016/j.pharmthera.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorelov V, Xu M, Smith HR, Buchanan GF, Pittenger C. Corticostriatal interactions in the generation of tic-like behaviors after local striatal disinhibition. Exp Neurol. 2015;265:122–128. doi: 10.1016/j.expneurol.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter MA, de Jesus DR, Hoppenbrouwers S, Daigle M, Deluce J, Ravindran LN, Fitzgerald PB, Daskalakis ZJ. Evidence for cortical inhibitory and excitatory dysfunction in obsessive compulsive disorder. Neuropsychopharmacology. 2012;37:1144–1151. doi: 10.1038/npp.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Flood P, Simpson HB. Rapid resolution of obsessions after an infusion of intravenous ketamine in a patient with treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011;72:567–569. doi: 10.4088/JCP.10l06653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, Flood P, Simpson HB. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38:2475–2483. doi: 10.1038/npp.2013.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000;39:1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, Bhandari R, Rose M, Ivey J, Boyd C, Moore GJ. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry. 2004;43:1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, Barrow R, Yeo R, Lauriello J, Brooks WM. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- Shungu DC. Demystifying the “Glx” peak that co-edits with GABA by J-editing. 2nd Cardiff International Symposium on MRS of GABA; Cardiff, Wales, UK.. 2013. [Google Scholar]
- Shungu DC, Mao X, Gu M, Milak MS, Weiduschat N, Mayer D, Spielman D, Mann JJ, Kegeles LS. ‘Glx’ measured by J-editing/MEGA-PRESS is primarily ‘pure’ glutamate…or is it? . Proc Intl Soc Mag Reson Med. 2013;21:3985. [Google Scholar]
- Simpson HB, Shungu DC, Bender J, Jr., Mao X, Xu X, Slifstein M, Kegeles LS. Investigation of cortical glutamate-glutamine and gamma-aminobutyric acid in obsessive-compulsive disorder by proton magnetic resonance spectroscopy. Neuropsychopharmacology. 2012;37:2684–2692. doi: 10.1038/npp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck G, Ljungberg M, Nilsson M, Jonsson L, Lundberg S, Ivarsson T, Ribbelin S, Ekholm S, Carlsson A, E., F.-A., Carlsson ML. A 1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: relationship between metabolite concentrations and symptom severity. J Neural Transm. 2008;115:1051–1062. doi: 10.1007/s00702-008-0045-4. [DOI] [PubMed] [Google Scholar]
- Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, Krystal JH, Nutt D, Barker GJ. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17:664–665. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Tiangga ER, Mhuircheartaigh RN, Cowen PJ. Lack of effect of ketamine on cortical glutamate and glutamine in healthy volunteers: a proton magnetic resonance spectroscopy study. J Psychopharmacol. 2012;26:733–737. doi: 10.1177/0269881111405359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. Neuroimaging. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Womelsdorf T, Buffalo EA, Desimone R, Fries P. Attentional modulation of cell-class-specific gamma-band synchronization in awake monkey area v4. Neuron. 2013;80:1077–1089. doi: 10.1016/j.neuron.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. International Journal of Psychophysiology. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Yucel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, Velakoulis D, Pantelis C. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust N Z J Psychiatry. 2008;42:467–477. doi: 10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
- Zai G, Arnold P, Burroughs E, Barr CL, Richter MA, Kennedy JL. Evidence for the gamma-amino-butyric acid type B receptor 1 (GABBR1) gene as a susceptibility factor in obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2005a;134B:25–29. doi: 10.1002/ajmg.b.30152. [DOI] [PubMed] [Google Scholar]
- Zai G, King N, Wong GW, Barr CL, Kennedy JL. Possible association between the gamma-aminobutyric acid type B receptor 1 (GABBR1) gene and schizophrenia. Eur Neuropsychopharmacol. 2005b;15:347–352. doi: 10.1016/j.euroneuro.2004.12.006. [DOI] [PubMed] [Google Scholar]