Abstract
Objective
The objective of this study is to evaluate whether trophoblast yield obtained by trophoblast retrieval and isolation from the cervix (TRIC) is affected by pregnancy outcome, gestational age (GA) at retrieval, maternal body mass index (BMI), parity, or maternal age.
Methods
TRIC was performed on 224 ongoing pregnancies between 5–20 weeks GA. Trophoblast cells were isolated from cervical cells using anti-human leukocyte antigen (HLA)-G antibody coupled to magnetic nanoparticles. Purity was assessed by the percentage of isolated cells that express β-hCG. Patient records were monitored until delivery, and pregnancy outcomes were determined. Trophoblast yield was compared to GA at time of collection, maternal BMI, parity, maternal age, and outcome of pregnancy, using linear regression.
Results
There was no effect of GA, maternal BMI, parity, and maternal age on trophoblast yield. Trophoblast yield decreased significantly with early pregnancy loss (EPL) compared to uncomplicated pregnancies that delivered at term. Trophoblast yield with preeclampsia or intrauterine growth restriction were decreased compared to healthy term outcomes, however, they did not reach statistical significance.
Conclusions
If TRIC becomes available as a method for non-invasive prenatal testing, our data demonstrate that it is unaffected by BMI and is useful as early as 5 weeks GA.
INTRODUCTION
Trophoblast cells are shed from the placenta into the lower uterine segment, and can be retrieved from the cervix as early as 5 weeks gestational age (GA).1,2 Multiple investigators have obtained trophoblast cells from the cervix of ongoing pregnancies and evaluated their use for non-invasive prenatal testing, including detection of trisomies 18 and 21, Rh fetal status in Rh negative mothers, and hemoglobinopathies.3–7 Several approaches to identify trophoblast cells in endocervical specimens have included morphological identification and micromanipulation to isolate cell clumps that resemble trophoblast,4,8–10 or the use of trophoblast markers, including various cytokeratins and antigens expressed by villous and extravillous trophoblast cells.11–13 Monoclonal antibodies specific for trophoblast cells have been used in combination with laser capture microdissection to obtain fetal DNA from cervical specimens, with confirmation of fetal origin by quantitative PCR.14,15 We identified trophoblast cells in endocervical specimens by labeling with a monoclonal antibody against human leukocyte antigen (HLA)-G,16 a protein present on trophoblast cells and not on maternal cervical cells.17 Trophoblast cells account for approximately one in 2000 cells from endocervical samples,16 which is more favorable by about three orders of magnitude compared to the estimated proportion of fetal cells in maternal blood.18 A significant decrease in the proportion of trophoblast/cervical cells was found in samples collected between 6–20 weeks GA from patients with an ectopic pregnancy or blighted ovum, as compared to healthy term pregnancies. It was speculated that trophoblast yield could predict abnormal pregnancies, although the sample number was small.
A limiting feature of using trophoblast cells obtained from the cervix for non-invasive prenatal testing was the presence of an excess of maternal cells that had to be distinguished from fetal cells, and the inability to efficiently isolate the fetal cells. We recently developed trophoblast retrieval and isolation from the cervix (TRIC), a safe procedure to obtain trophoblast cells from the cervix with a high degree of purity in ongoing pregnancies, using HLA-G for immunomagnetic isolation.19 TRIC can reliably identify fetal gender as early as 5 weeks GA by fluorescence in situ hybridization or the polymerase chain reaction.19 The isolated cells are intact as evidenced by the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay. Additionally, immunocytochemical characterization indicates that the cells obtained by TRIC are of the extravillous trophoblast phenotype.
Current clinical practice utilizes circulating cell-free fetal DNA obtained from maternal plasma, which is adversely affected by early GA and maternal obesity.20 Additionally, cell-free fetal DNA cannot ensure the presence of an intact fetal genome, particularly at early GA, limiting its diagnostic value.21 Initial studies suggest that early GA does not negatively impact TRIC;16,19 however, its limitations are not well understood. Development of a small placenta or pathologies that reduce trophoblast invasion could negatively impact the ability to obtain trophoblast cells by TRIC. Therefore, a goal of this study was to determine if fewer cells are isolated from patients that later have a pregnancy loss, a small for gestational age fetus, or preeclampsia. In the present study, we evaluated whether early GA, maternal obesity, parity, maternal age, and poor placentation adversely affect trophoblast yield during TRIC.
MATERIALS AND METHODS
Patients Selection
Trophoblast cells are being collected by TRIC in an ongoing study, from which 224 patients were enrolled between 2011–2014. Medical records were evaluated to obtain maternal age, GA at time of retrieval, maternal body mass index (BMI), and parity, as well as uncomplicated pregnancies with full term deliveries, and pregnancies with complications, including preeclampsia (PE), intrauterine growth restriction (IUGR), or early pregnancy loss (EPL). PE was defined as new onset proteinuria and elevated blood pressure after 20 weeks GA,22 and IUGR was defined as birth weight below the 10th percentile for GA.23 The Institutional Review Board of Wayne State University approved the study, and each subject provided written informed consent prior to participation. The inclusion criteria included pregnant women between the ages of 18 and 45, and a GA from 5 to 20 weeks. Exclusion criteria included women who were experiencing active vaginal bleeding and pregnancies with multiple gestation. GA was determined by the date of the last menstrual period and first ultrasound.
Endocervical Sampling
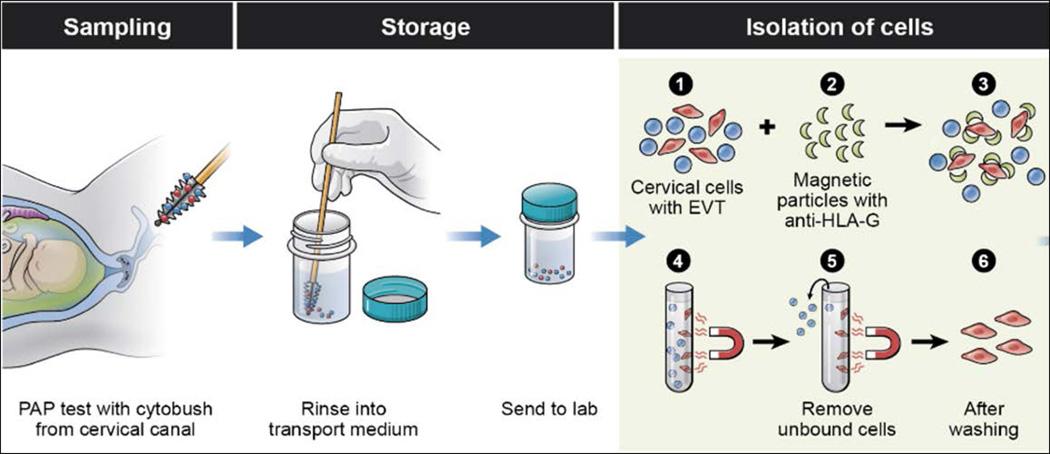
Endocervical samples were obtained as described previously (Fig. 1).19 Briefly, a speculum was inserted into the vagina and endocervical samples were collected using a cytobrush and a ThinPrep Kit (Hologic, Inc., Marlborough, MA) containing 20 ml of PreservCyt fixative solution. The specimens were transferred to the laboratory, where they were acidified with 500 µl of glacial acetic acid for 5 minutes to dissolve the mucous. Samples were centrifuged at 400 × g for 5 minutes at 4°C. The supernatant was removed and the cell pellet was re-suspended in 20 ml of ice-cold sterile phosphate buffered saline (PBS). Specimens were then washed by centrifugation and re-suspended two more times with 20 mL of PBS, and on the final wash, the volume was brought to 10 mL with PBS at 4°C.
Figure 1.
Trophoblast retrieval and isolation from the cervix (TRIC) isolates trophoblast cells from the cervix of ongoing pregnancies. Collection of cervical specimens is performed in an identical fashion as a pap smear, using a cytobrush and placing the specimen into a ThinPrep fixative. At the laboratory, isolation of the trophoblast cells (red) from the cervical cells (blue) is accomplished by incubating the cervical sample with nanoparticles bound to anti-HLA-G. The specimen is placed in a magnetic separator while the non-bound cervical cells are aspirated from the bound trophoblast cells.
Isolation of Trophoblast Cells
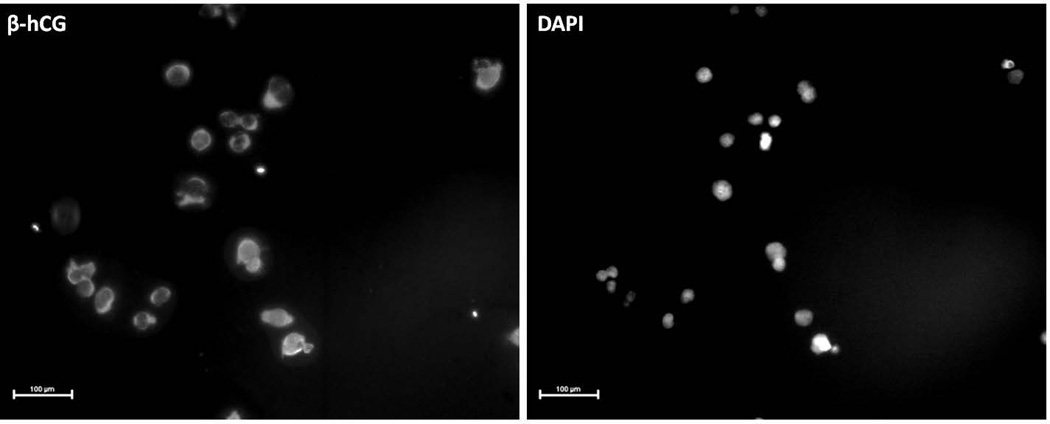
The endocervical specimens were centrifuged and re-suspended in 1 ml PBS, combined with mouse anti-HLA-G antibody conjugated to 250 nm magnetic nanoparticles (Clemente Associates, Madison, CT), and incubated overnight at 4°C with mixing. The EVT cells bound to magnetic nanoparticles were then immobilized on a DynaMag-Spin magnet (Life Technologies) for 10 minutes. The non-bound cells were collected, followed with three washings in 1 ml of PBS. The bound trophoblast cells were counted and checked for purity by immunocytochemical labeling of the trophoblast marker, human chorionic gonadotropin β- subunit (β-hCG). Purity is reported as percentage of cells labeled with β-hCG (trophoblast cells) versus total cells (DAPI), as described previously.19
Statistical Analysis
The trophoblast yield data were subjected to a natural log transformation (ln) prior to subsequent analysis, because the data were not normally distributed. The non-parametric Mann-Whitney-Wilcoxon Test (binomial) or the Kruskal Wallis Test (ordinal values) was employed for comparison. Linear regression was used to examine the association of log-transformed trophoblast yield and other variables. p values <0.05 were considered statistically significant. All statistical computations were performed using the open-source R software (http://www.r-project.org/).
RESULTS
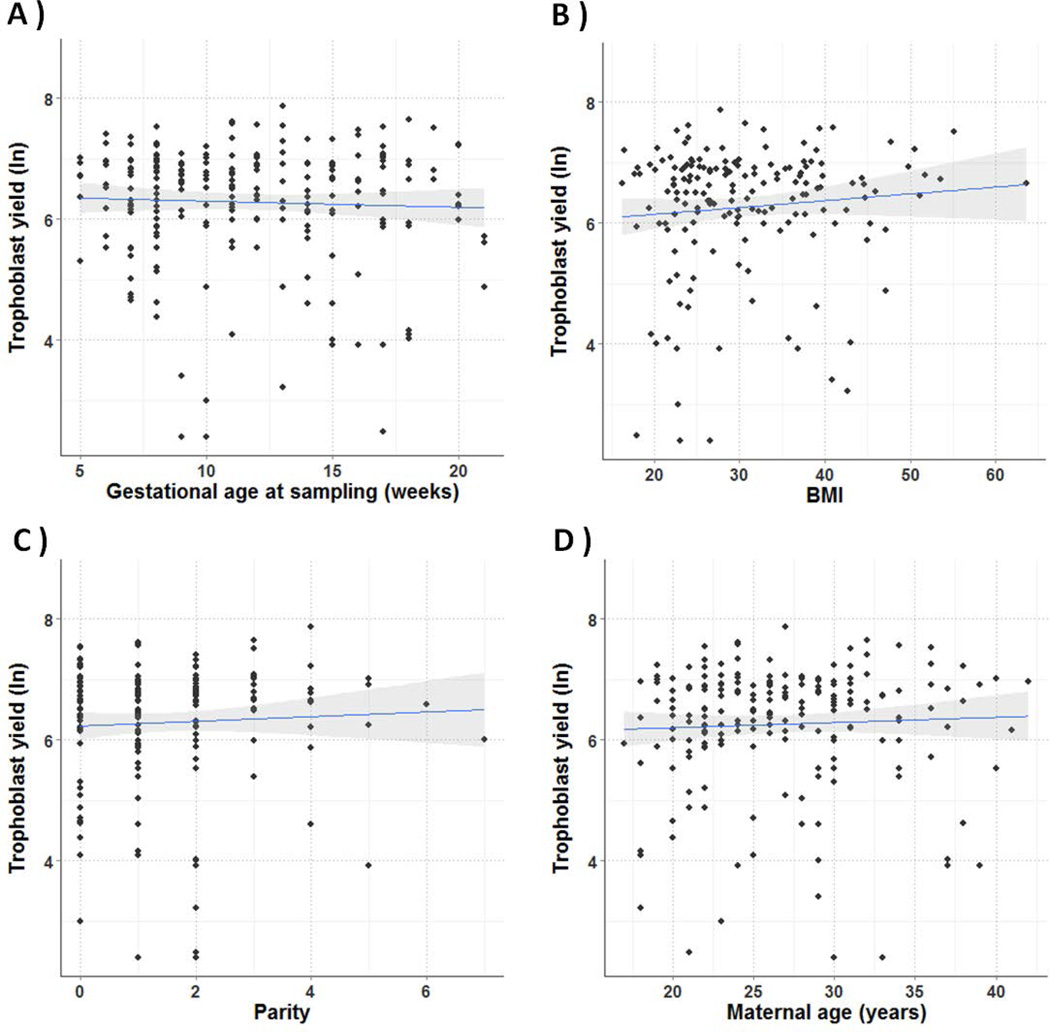
The median trophoblast yield from all 224 specimens evaluated was 675 (IQR, 399-1010) cells, and the median purity based on β-hCG staining was 97.9% (IQR 93.3-100) (Fig. 2). Trophoblast cells were isolated between 5 and 20 weeks GA, with 42.9% prior to 10 weeks GA. Median GA, BMI, parity, and maternal age were 11 weeks (IQR 8-15), 28.6 (IQR 23.7-37.1), 1 (IQR 0-2), and 26 years of age (IQR 22-30), respectively. There was no correlation between GA, BMI, parity, or maternal age and trophoblast yield (Fig. 3).
Figure 2.
Assessment of trophoblast purity by β-hCG expression. Isolated trophoblast cells labeled with anti-β-hCG were examined by immunofluorescence microscopy. Image on the left represents β-hCG staining, and the image on the right represents DAPI staining of the same field of cells. Scale bar represents 100 µm.
Figure 3.
Correlation between trophoblast yield (log transformed) and GA, maternal BMI, parity, and maternal age. The number of isolated trophoblast cells was correlated to GA (A), maternal BMI (B), parity (C), and maternal age (D), using linear regression. No significant correlations were found.
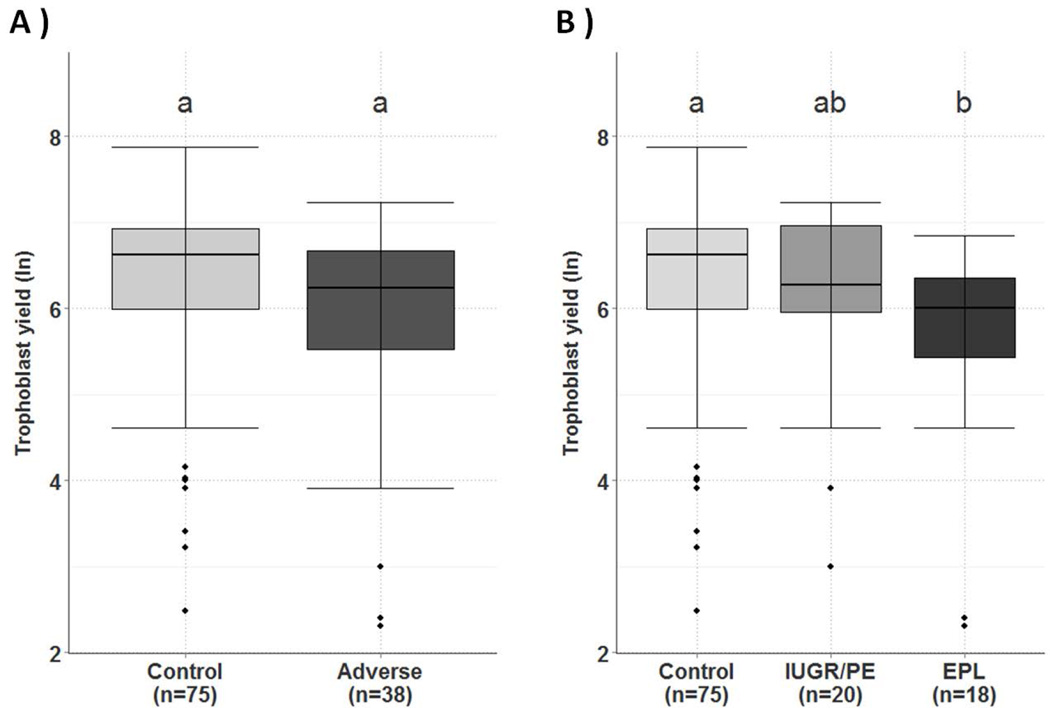
Among the 224 patients, 113 were either uncomplicated term outcomes (n=75), or pregnancies with adverse outcomes (n=38), including PE and/or IUGR (n=20), and EPL (n=18). Although the trophoblast yield in adverse outcomes compared to the healthy control cohort was decreased (median= 510 (IQR 250-786) vs. 750 (IQR 400-1020), it did not reach statistical significance (p=0.052; Fig. 4A). Sub-analysis of the adverse cohort revealed a significant decrease (p <0.05) in trophoblast yield in the EPL cohort (median = 407 (IQR 227-572)) compared to the healthy cohort (Fig. 4B), but in the PE/IUGR cohort (median= 530 (IQR 387-1050) no significance was found (p= 0.674). Median purity of the EVT cells obtained in the healthy cohort group, EPL, and IUGR/PE groups were 98% (IQR 93.2-100), 95.7% (IQR 92.2-100) and 97.7% (IQR 95.1-100), respectively.
Figure 4.
Trophoblast yield (log transformed) was compared to pregnancy outcome. Trophoblast yield obtained by TRIC was compared in pregnancies with adverse outcomes (N= 38), including PE, IUGR, and EPL to pregnancies with uncomplicated term deliveries (N= 75). Statistical analysis was performed with the Kruskal Wallis test. A. There was no significant difference in trophoblast yield in pregnancies with adverse outcomes (PE, IUGR, and EPL) compared to uncomplicated term controls. B. Further subdividing the adverse group into PE/IUGR (N=20) and EPL (N=18) revealed a significant decrease (P<0.05) in the EPL cohort compared to the control cohort (N=75). Boxes represent 25th to 75th percentiles, horizontal lines within boxes represent the median, bars are 1.5× IQR. The diamonds represent outliers. Samples labeled above with different letters are significantly different.
DISCUSSION
Currently, prenatal genetic testing relies on invasive methods such as chorionic villous sampling or amniocentesis to acquire fetal tissue. Although these methods are reliable for genetic diagnosis, the earliest they can be performed is 10 weeks GA with a fetal loss risk of approximately 0.5%, or less with an experienced physician.24,25 Cell-free fetal DNA is widely available for prenatal genetic screening after 10 weeks GA, but is negatively impacted by GA and obesity.21,26 Currently, cell-free fetal DNA cannot be used diagnostically, and results should be confirmed by invasive testing.27 TRIC can isolate intact fetal cells,19 and could provide a minimally-invasive, alternative approach for prenatal genetic testing, if validated. The present study suggests that trophoblast yield obtained by TRIC is as robust at 5 weeks as 20 weeks GA, although fewer samples were available at 5 weeks than later GAs. Furthermore, TRIC is unaffected by maternal obesity, parity or age.
We found a significant decrease in trophoblast cells isolated from pregnancies with EPL compared to pregnancies with healthy term deliveries. In a previous report, we found a significant decrease in trophoblast cells from crude cervical smears in pregnancies with a blighted ovum, compared to successful intrauterine pregnancies.16 We are aware that the total number of cells per specimen varies, which would influence trophoblast yield. Nevertheless, our data confirm previously published results analyzing both adverse outcome and gestational age in which trophoblast yield was normalized to total cell number.16
Abnormal placentation, as observed in EPL, PE and IUGR, is characterized by increased trophoblast apoptosis and shedding,28 releasing fetal DNA fragments into the maternal circulation.29–31 Here, we show decreased numbers of trophoblast cells migrating to the cervix in pregnancies with adverse outcomes, reflecting the abnormal placentation associated with these pathologies.
TRIC provides for the first time intact fetal cells in adequate numbers for downstream molecular and genetic testing without a negative impact of GA or BMI. Additional studies will be required to establish this approach as a diagnostic tool for prenatal testing. The current study provides the foundation for future validation of TRIC.
What’s already known about this topic?
Trophoblast retrieval and isolation from the cervix (TRIC) safely provides trophoblast cells as early as 5 weeks gestation with a high degree of purity.
TRIC isolates extravillous trophoblast cells by immunomagnetic isolation, using anti-HLA-G antibody.
What does this study add?
Gestational age at time of collection, maternal body mass index, parity and maternal age do not affect trophoblast yield following TRIC.
Early pregnancy loss is associated with a significant decrease in trophoblast yield compared to uncomplicated term outcomes.
Acknowledgments
Study funding/competing interest(s): This research was supported in part by the Intramural Research Program of the NICHD, grants from the NIH (HD071408 and HL128628), the W.K. Kellogg Foundation, the March of Dimes Foundation, and Perkin Elmer, Inc.
MPD, SD and DRA have a pending patent and receive payment for intellectual property that has been licensed on their behalf by Wayne State University to Perkin Elmer, Inc.
REFERENCES
- 1.Imudia AN, Kumar S, Diamond MP, et al. Transcervical retrieval of fetal cells in the practice of modern medicine: a review of the current literature and future direction. Fertil Steril. 2010;93(6):1725–1730. doi: 10.1016/j.fertnstert.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cioni R, Bussani C, Bucciantini S, Scarselli G. Fetal cells in a transcervical cell sample collected at 5 weeks of gestation. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2005;18(4):271–273. doi: 10.1080/14767050500246391. [DOI] [PubMed] [Google Scholar]
- 3.Adinolfi M, Davies A, Sharif S, et al. Detection of trisomy 18 and Y-derived sequences in fetal nucleated cells obtained by transcervical flushing. Lancet. 1993;342(8868):403–404. doi: 10.1016/0140-6736(93)92816-c. [DOI] [PubMed] [Google Scholar]
- 4.Sherlock J, Halder A, Tutschek B, et al. Prenatal detection of fetal aneuploidies using transcervical cell samples. J Med Genet. 1997;34(4):302–305. doi: 10.1136/jmg.34.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sifakis S, Ghatpande S, Seppo A, et al. Prenatal diagnosis of trisomy 21 through detection of trophoblasts in cervical smears. Early human development. 2010;86(5):311–313. doi: 10.1016/j.earlhumdev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Adinolfi M, Sherlock J, Kemp T, et al. Prenatal detection of fetal RhD DNA sequences in transcervical samples. Lancet. 1995;345(8945):318–319. doi: 10.1016/s0140-6736(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 7.Adinolfi M, El-Hashemite N, Sherlock J, et al. Prenatal detection of Hb mutations using transcervical cells. Prenatal diagnosis. 1997;17(6):539–543. doi: 10.1002/(sici)1097-0223(199706)17:6<539::aid-pd106>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Bussani C, Cioni R, Scarselli B, et al. Strategies for the isolation and detection of fetal cells in transcervical samples. Prenat Diagn. 2002;22(12):1098–1101. doi: 10.1002/pd.469. [DOI] [PubMed] [Google Scholar]
- 9.Bussani C, Cioni R, Mattei A, et al. Prenatal diagnosis of common aneuploidies in transcervical samples using quantitative fluorescent-PCR analysis. Mol Diagn Ther. 2007;11(2):117–121. doi: 10.1007/BF03256231. [DOI] [PubMed] [Google Scholar]
- 10.Tutschek B, Sherlock J, Halder A, et al. Isolation of fetal cells from transcervical samples by micromanipulation: molecular confirmation of their fetal origin and diagnosis of fetal aneuploidy. Prenat Diagn. 1995;15(10):951–960. doi: 10.1002/pd.1970151010. [DOI] [PubMed] [Google Scholar]
- 11.Bulmer JN, Rodeck C, Adinolfi M. Immunohistochemical characterization of cells retrieved by transcervical sampling in early pregnancy. Prenat Diagn. 1995;15(12):1143–1153. doi: 10.1002/pd.1970151211. [DOI] [PubMed] [Google Scholar]
- 12.Katz-Jaffe MG, Mantzaris D, Cram DS. DNA identification of fetal cells isolated from cervical mucus: potential for early non-invasive prenatal diagnosis. BJOG. 2005;112(5):595–600. doi: 10.1111/j.1471-0528.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- 13.Miller D, Briggs J, Rahman MS, et al. Transcervical recovery of fetal cells from the lower uterine pole: reliability of recovery and histological/immunocytochemical analysis of recovered cell populations. Hum Reprod. 1999;14(2):521–531. doi: 10.1093/humrep/14.2.521. [DOI] [PubMed] [Google Scholar]
- 14.Bulmer JN, Cioni R, Bussani C, et al. HLA-G positive trophoblastic cells in transcervical samples and their isolation and analysis by laser microdissection and QF-PCR. Prenat Diagn. 2003;23(1):34–39. doi: 10.1002/pd.511. [DOI] [PubMed] [Google Scholar]
- 15.Mantzaris D, Cram DS. Potential of syncytiotrophoblasts isolated from the cervical mucus for early non-invasive prenatal diagnosis: evidence of a vanishing twin. Clin Chim Acta. 2015;438(1):309–315. doi: 10.1016/j.cca.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Imudia AN, Suzuki Y, Kilburn BA, et al. Retrieval of trophoblast cells from the cervical canal for prediction of abnormal pregnancy: a pilot study. Hum Reprod. 2009;24(9):2086–2092. doi: 10.1093/humrep/dep206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mcmaster MT, Librach CL, Zhou Y, et al. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. Journal of immunology. 1995;154(8):3771–3778. [PubMed] [Google Scholar]
- 18.Mouawia H, Saker A, Jais JP, et al. Circulating trophoblastic cells provide genetic diagnosis in 63 fetuses at risk for cystic fibrosis or spinal muscular atrophy. Reprod Biomed Online. 2012;25(5):508–520. doi: 10.1016/j.rbmo.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Bolnick JM, Kilburn BA, Bajpayee S, et al. Trophoblast retrieval and isolation from the cervix (TRIC) for noninvasive prenatal screening at 5 to 20 weeks of gestation. Fertil Steril. 2014;102(1):135–142. e6. doi: 10.1016/j.fertnstert.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang E, Batey A, Struble C, et al. Gestational age and maternal weight effects on fetal cell-free DNA in maternal plasma. Prenat Diagn. 2013;33(7):662–666. doi: 10.1002/pd.4119. [DOI] [PubMed] [Google Scholar]
- 21.Norwitz ER, Levy B. Noninvasive Prenatal Testing: The Future Is Now. Reviews in obstetrics and gynecology. 2013;6(2):48–62. [PMC free article] [PubMed] [Google Scholar]
- 22.Milne F, Redman C, Walker J, et al. The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community. BMJ. 2005;330(7491):576–580. doi: 10.1136/bmj.330.7491.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American College Of O, Gynecologists. ACOG Practice bulletin no. 134: fetal growth restriction. Obstetrics and gynecology. 2013;121(5):1122–1133. doi: 10.1097/01.AOG.0000429658.85846.f9. [DOI] [PubMed] [Google Scholar]
- 24.American College Of O, Gynecologists. ACOG Practice Bulletin No. 88, December 2007. Invasive prenatal testing for aneuploidy. Obstetrics and gynecology. 2007;110(6):1459–1467. doi: 10.1097/01.AOG.0000291570.63450.44. [DOI] [PubMed] [Google Scholar]
- 25.Wapner RJ. Invasive prenatal diagnostic techniques. Semin Perinatol. 2005;29(6):401–404. doi: 10.1053/j.semperi.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Vora NL, Johnson KL, Basu S, et al. A multifactorial relationship exists between total circulating cell-free DNA levels and maternal BMI. Prenatal diagnosis. 2012;32(9):912–914. doi: 10.1002/pd.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American College Of O, Gynecologists Committee On G. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstetrics and gynecology. 2012;120(6):1532–1534. doi: 10.1097/01.AOG.0000423819.85283.f4. [DOI] [PubMed] [Google Scholar]
- 28.Huppertz B, Kingdom JC. Apoptosis in the trophoblast--role of apoptosis in placental morphogenesis. Journal of the Society for Gynecologic Investigation. 2004;11(6):353–362. doi: 10.1016/j.jsgi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Lau TW, Leung TN, Chan LY, et al. Fetal DNA clearance from maternal plasma is impaired in preeclampsia. Clinical chemistry. 2002;48(12):2141–2146. [PubMed] [Google Scholar]
- 30.Tjoa ML, Cindrova-Davies T, Spasic-Boskovic O, et al. Trophoblastic oxidative stress and the release of cell-free feto-placental DNA. The American journal of pathology. 2006;169(2):400–404. doi: 10.2353/ajpath.2006.060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Shen Y, Ge Q, et al. Quantification of maternal serum cell-free fetal DNA in early-onset preeclampsia. International journal of molecular sciences. 2013;14(4):7571–7582. doi: 10.3390/ijms14047571. [DOI] [PMC free article] [PubMed] [Google Scholar]