Abstract
Human pluripotent stem cells (hPSCs) offer unique opportunities for studying human biology, modeling diseases and for therapeutic applications. The simplest approach so far to generate human PSCs lines is through reprogramming of somatic cells from an individual by defined factors, referred to simply as reprogramming. Reprogramming circumvents the ethical issues associated with human embryonic stem cells (hESCs) and nuclear transfer hESCs (nt-hESCs), and the resulting induced pluripotent stem cells (hiPSCs) retain the same basic genetic makeup as the somatic cell used for reprogramming. Since the first report of iPSCs by Takahashi and Yamanaka, the molecular mechanisms of reprogramming have been extensively investigated. A better mechanistic understanding of reprogramming is fundamental not only to iPSC biology and improving the quality of iPSCs for therapeutic use, but also to our understanding of the molecular basis of cell identity, pluripotency and plasticity. Here we summarize the genetic, epigenetic and cellular events during reprogramming, and the roles of various factors identified thus far in the reprogramming process.
Introduction
Pluripotent stem cells (PSCs) can self-renew indefinitely in culture while maintaining the potential to differentiate into all cell lineages of an adult organism. Human PSCs (hPSCs) are relevant to a wide range of applications from basic biology to regenerative medicine. Aside from the promise of using hPSC-derived cells for cell replacement therapies, there is great potential of using hPSCs for modeling lineage decisions during differentiation and studying disease-relevant phenotypes that are manifested at the cellular level. Moreover, hPSCs also offer an attractive platform for drug efficacy and toxicity screening. Therefore, great efforts have been made to identify ways to generate PSCs, especially hPSCs. One approach is to derive PSCs through culturing various embryonic, adult or malignant cells with stem cell properties (Sidebar 1 and Figure 1). Among them, embryonic stem cells (ESCs) are the classic example of a PSC 1–3 and they remain the gold standard to which newly derived PSC lines are typically compared molecularly, through expression and epigenetic profiling and functionally, by assessing their differentiation potential in vitro and in vivo (Table 1). Another approach is to reset a somatic cell to a pluripotent state by exposing its nucleus to exogenous transacting factors. This is currently achieved by three methods: somatic cell nuclear transfer (SCNT), cell fusion, and direct reprogramming by defined transcription factors. SCNT allows generating ESCs (ntESC) from cloned embryos obtained through injection of a somatic nucleus into an enucleated oocyte. NtESCs have been derived from different species, including mouse 4 and more recently human somatic nuclei 5, 6 (Figure 1 (f)). SCNT and experiments involving fusions between PSCs and somatic cells (Figure 1 (g)) demonstrate that factors present in the egg and in PSCs have the ability to reset somatic nuclei to a pluripotent state 7. Based on these observations, Yamanaka and colleagues screened 24 pluripotency transcription factors and demonstrated that over-expression of the reprogramming factors Oct4, Sox2, Klf4 and c-Myc (referred to as OSKM) is sufficient to create induced pluripotent stem cells (iPSCs) from mouse fibroblasts (Figure 1 (h)) 8. Soon after this groundbreaking discovery, iPSCs were generated from human fibroblasts using the same 9–11 or a slightly different combination of reprogramming factors (OCT4, SOX2, NANOG and LIN28)12. Use of hiPSCs circumvents the ethical controversies associated with hESCs or nt-hESCs, and as one can easily generate hiPSCs that match the genetic background of any individual, this offers an ideal platform for cell replacement therapy and disease modeling.
Sidebar. Culture-derived Pluripotent Stem Cell lines.
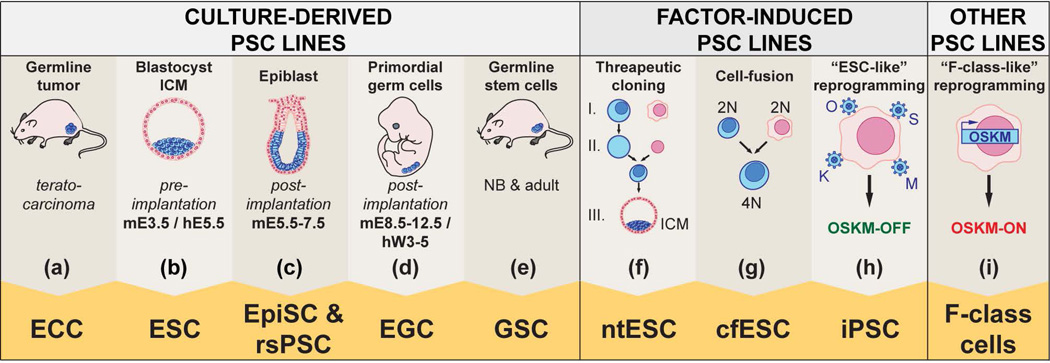
Embryonal carcinoma cells (ECCs): derived from mouse teratocarcinomas, these are the first PSC lines generated. ECCs self-renew and differentiate into multiple embryonic lineages in vitro and to some extent in vivo. Human ECCs show limited differentiation potential (Figure 1 (a)).
Embryonic stem cells (ESCs): derived from the inner cell mass (ICM) of pre-implantation mouse blastocysts, they differentiate into a wide range of cell types in vitro. Unlike ECCs, they also contribute to all lineages at a high frequency in chimera experiments. The first hESC lines were generated in 1998 (Figure 1 (b)).
Epiblast stem cells (EpiSCs) and region-selective pluripotent stem cells (rsPSC): derived from post-implantation mouse epiblasts using different culture conditions, they self-renew and differentiate into various cell types in vitro (Figure 1 (c)). Conventional hESCs are considered primed, as they share common features with primed mouse EpiSCs and are distinct from naïve mESCs. rsPSCs can be derived from primed hPSCs and contribute to post-implantation interspecies chimeric embryos 221.
Embryonic germ cells (EGCs) and germ line stem cells (GSCs): derived respectively from mouse primordial germ cells (Figure 1 (d)) or germ line stem cells (GSCs) from neonatal and adult testis (Figure 1 (e)), they resemble ESCs but retain some epigenetic features of their cell of origin. Human EGCs show limited self-renewal capacity and the pluripotency of human GSCs has been seriously questioned 222, 223.
Figure 1. Sources of pluripotent Stem Cells.
Culture-derived pluripotent stem cells (PSC) are generated from different in vivo cell types. (a) Embryonal carcinoma cells (ECCs), derived from germline tumors (teratocarcinomas); (b) embryonic stem cells (ESCs), derived from the inner cell mass (ICM) of pre-implantation mouse and human embryos at mouse embryonic day 3.5 (mE3.5) or human embryonic day 5.5 (hE5.5); (c) epiblast stem cells (EpiSCs) and region-selective pluripotent stem cells (rsPSCs), obtained from early post-implantation mouse embryos at mE5.5–7.5; (d) embryonic germ cells (EGCs) retrieved from mouse and human primordial germ cells (PGCs) respectively at mE8.5–12.5 or between weeks 3 and 5 of human development (hW3-5); (e) and germline-derived PSCs (GSCs), derived from spermatogonial stem cells of mouse neonatal and adult testes. In each of the above columns, the cell of origin of the different pluripotent stem cell lines is labeled in blue. Alternatively, exposing the nuclei of somatic cells to exogenous reprogramming factors can induce PSCs. (f) Nuclear transfer embryonic stem cells (ntESC) are obtained by reprogramming somatic nuclei (pink) with factors contained in an enucleated oocyte (blue), and cultured to the blastocyst stage to derive ntESCs from the ICM; (g) Through a similar approach, fusion between a somatic cell (pink) and a PSC (blue) gives rise to cell-fusion-derived tetraploid (4N) hybrid ESC (cfESC) lines; (h) Alternatively, over-expression of the reprogramming transcription factors, Oct4 (O), Sox2 (S), Klf4 (K) and cMyc (M) in a somatic cell (pink) using viral delivery (blue) allows generating induced pluripotent stem cells (iPSC); (i) F-class cells are generated through high and constitutive expression of OSKM in a somatic cell (pink) using an inducible integrated transgene (blue). F-Class cells share molecular and phenotypic features with iPSCs and ESCs.
Table 1.
Molecular and functional assays to assess the developmental potential of PSCs.
| Pluripotency assays | Methods | Limitations | Mouse | Human |
|---|---|---|---|---|
| Expression profiling | Immunostaining, qPCR, RNAseq to assess expression profile of pluripotency genes. |
Gene expression is not a functional test. |
YES | YES |
| Epigenetic profiling |
Chromatin: ChIP qRT-PCR, ChIPseq of common chromatin marks, PolI I, ... DNA: Targeted or genomewide DNA methylation patterns through bisulfite sequencing. |
Epigentic signature is not a functional test. |
YES | YES |
| In vitrodifferentiation | Directed differentia ion in culture towards specific linages or embrioid body formation and assayed for the expression of specific lineage markers. |
Marker expression is not a functional test. |
YES | YES |
| Teratoma | Injection of PSCs in immunocompromised mice and fomation of teratocarcinomas to test the di- fferentiation potencial of cells to various linages. |
Does not test the con- tribution of cells to nor- mal development. |
YES | YES |
| 2N Blatocyst complementation | Injection of PSCs into a diploid blastocyst to test the contribution of cells to normal deve- lopment trough formation of a chimeric mouse. |
Host cells may comple- ment non-autonomous cell defects of PSCs. |
YES | YES/NO* |
| Germline contribution | Injection of PSCs into a diploid blastocyst and breeding of the resulting chimera to test the contribution of cells to the germline. |
Complemetation by host cells. Epigenetic defects of PSCs not revealed. |
YES | NO |
| 4N Blatocyst complementation | Injection of PSCs into a tetraploid blastocyst. Since 4N cells cannot contribute to somatic linages, the embryo is 100% PSC-derived. |
Most stringent assay for pluripotency. Does not test trophectoderm potential. |
YES | NO |
This assay may be used to test the contribution of hPSCs at early stages of development but is limited at later stages for ethical reasons related with the formation of human-mouse chimeras.
This review aims to provide a summary of current understanding of the molecular mechanisms of iPSC generation. We first describe the current models of reprogramming and experiments attempting to dissect the stochastic vs deterministic nature of this process. Next, we delineate the different phases of reprogramming focusing on the major transcriptional and epigenetic changes observed upon expression of the reprogramming factors OSKM. We then functionally dissect the reprogramming process by first describing the roles of OSKM as well as various pluripotency-associated or lineage-restricted transcription factors during reprogramming. Next, we provide an overview of the roles of epigenetic remodeling factors involved in reprogramming including histone post-translational modifiers, histone variants, nucleosome remodelers, chromatin topology and DNA methylation/demethylation enzymes. Finally we cover the roles of non-coding RNAs, including microRNAs and long non-coding RNAs (lncRNAs), and the role of signaling pathways, including developmental, stress-induced and metabolic pathways during iPSC formation. Because the reprogramming field has been extremely prolific during these years, it is beyond the scope of this review to give an exhaustive account of all mechanistic studies completed on reprogramming. We apologize in advance to those whose work couldn’t be cited here.
I. Conceptual models of reprogramming
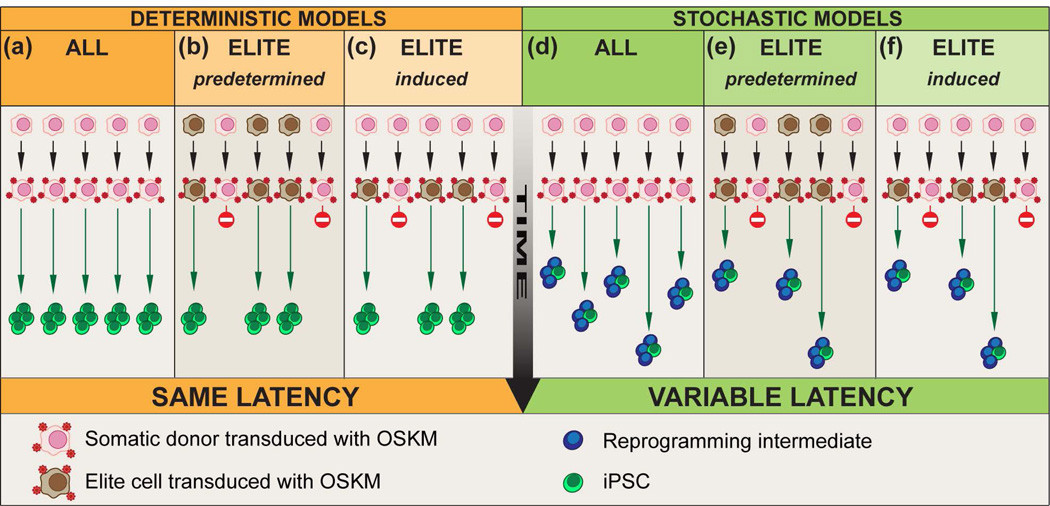
Reprogramming was initially described as an inefficient process requiring ectopic expression of OSKM for a period of time varying significantly between species. For instance using retroviral delivery, only ~ 0.5% of mouse embryonic fibroblasts (MEFs) 13 and ~ 0.0002% of human adult dermal fibroblasts (HDFs) 11 form iPSC lines in ~ 12 and ~ 25 days respectively. Why reprogramming is only achieved in such a small fraction of donor cells and what controls the kinetics of reprogramming? The models describing reprogramming fall into two categories: deterministic and stochastic (Figure 2). Deterministic models predict that somatic donor cells give rise to iPSCs with a fixed latency, while stochastic models suggest variable latencies. Both models are further divided into sub-categories where “all” or only a subset of “elite” cells are permissive to reprogramming.
Figure 2. Models of reprogramming.
Two types of models have been proposed to describe reprogramming: deterministic and stochastic. (a–c) Deterministic models predict that somatic donor cells give rise to iPSCs with a fixed latency (green arrows of same length). (d–f) Stochastic models predict that somatic donor cells give rise to iPSCs with variable latencies (green arrows of different lengths). In both models, either all (a, d) or only a subset of elite cells (brown) (b–c, e–f) is permissive to reprogramming and elite cells can be present in the donor population before reprogramming (predetermined) (b, e) or induced upon viral delivery (red dots) (c, f). The latency (green arrows) reflects the absolute time or the number of cell divisions required to produce an iPSC from the donor population.
I. I. All versus elite
It is important to determine whether the low efficiency of reprogramming is due to the limitation of the reprogramming techniques or the intrinsic inability of a terminally differentiated cell to be reprogrammed. Most initial reprogramming studies were performed in MEFs through the use of integrative viral vectors. Thus it has been proposed that reprogramming may require additional genetic events triggered by random transgene insertional mutagenesis in rare cells (Figure 2 (c, f)). However, systematic analysis of transgene insertions in mouse fibroblasts does not support this hypothesis 14. Random transgene integration is also known to cause heterogeneous transgene expression, and it is possible that optimal expression and stoichiometry of the reprogramming factors is only achieved in rare cells (Figure 2 (c, f)). Alternatively, it is possible that only a small fraction of elite somatic stem cells among the heterogeneous MEF culture can undergo reprogramming (Figure 2 (b, e)). Through the development of homogenous OSKM-delivery systems, up to 10% of MEFs can be reprogrammed. These systems include secondary reprogrammable MEFs 15 or transgenic reprogrammable MEFs, in which a doxycycline (dox)-inducible polycistronic OSKM expression cassette is integrated into the Collagen locus 16, 17. While the efficiency achieved with these systems argues against reprogramming occurring only in a very rare stem cell population, the majority of MEFs still fail to be reprogrammed, raising questions as to what is preventing the remaining cells from being reprogrammed.
This question has also been addressed in more defined cell types. Terminally differentiated B and T cells can both form iPSCs, arguing against the idea that only “less” differentiated cells can be reprogrammed 18. However, the developmental stage seems to affect the efficiency and kinetics of reprogramming, as hematopoietic stem and progenitor cells generate up to 300 times more iPSC colonies than terminally differentiated B and T cells 18. It has also been shown, in pre-B cells at least, that almost all cells (>92%) can give rise to reprogrammed colonies if the reprogramming factors are expressed for enough time 19. These observations argue that all cells, including terminally differentiated cells, can form iPSCs. They also highlight that reprogramming efficiency is influenced by the differentiation status, suggesting that the epigenetic and transcriptional state of the donor cell may affect its plasticity to become pluripotent.
I. II. Deterministic versus stochastic models
Reprogramming appears stochastic in many experimental settings. Most notably, single-cell cloning experiments show that the reprogramming kinetics varies even between individual clones of an apparently homogenous, lineage-committed cell population 19. This stochasticity may reflect differences in cell cycle stage, cell-intrinsic fluctuation in gene expression and epigenetic status. Supporting this notion, p53 knockdown increases the kinetics of iPSC generation in a cell-division-rate-dependent manner 19. Similarly, granulocyte monocyte progenitor (GMP) cells, which display an ultrafast cell cycle of 8 hours give rise to iPSC colonies almost synchronously in only four to five cell divisions upon OSKM expression 20. This study also highlights that one can achieve almost deterministic reprogramming using a privileged somatic cell population suggesting reprogramming models may be context-dependent. Along this line, a recent study suggests that chromatin de-repression through Mbd3 depletion allows rapid deterministic reprogramming of MEFs 21, though this effect also depends on the reprogramming context 22. In the next sections, we will discuss some of the mechanistic studies in more details, and we will also see that the identification of distinct reprogramming phases and the markers associated with them allows capturing subsets of cells in which reprogramming is much more likely to occur, if not absolutely deterministic. As such, at least part of the apparent stochastic nature of reprogramming may be attributed to our temporary partial understanding of the underlying mechanisms.
II. A molecular roadmap of reprogramming
Successful reprogramming requires the erasure of the somatic transcriptional and epigenetic signature in donor cells and the establishment and maintenance of the PSC transcriptome and epigenome in iPSCs. These two processes rely on the proper repression, activation and maintenance of different sets of transcription factors, non-coding RNAs, chromatin/DNA-modifying enzymes and signal-transduction pathways 23, which will be discussed in more details below. We would like to point out that most studies addressing the mechanism of reprogramming have been performed in MEFs using dox-inducible expression of OSKM. While this experimental setup has been extremely powerful to reveal the molecular events and the functions of many genes and pathways involved in reprogramming, the reprogramming process can be influenced by the species, the cell type, the reprogramming factors used and how the factors are introduced into donor cells. Therefore, much remains to be learned regarding divergent routes to pluripotency and alternative pluripotent states that share overlapping molecular and phenotypic features with ESCs.
II. I. Reprogramming phases
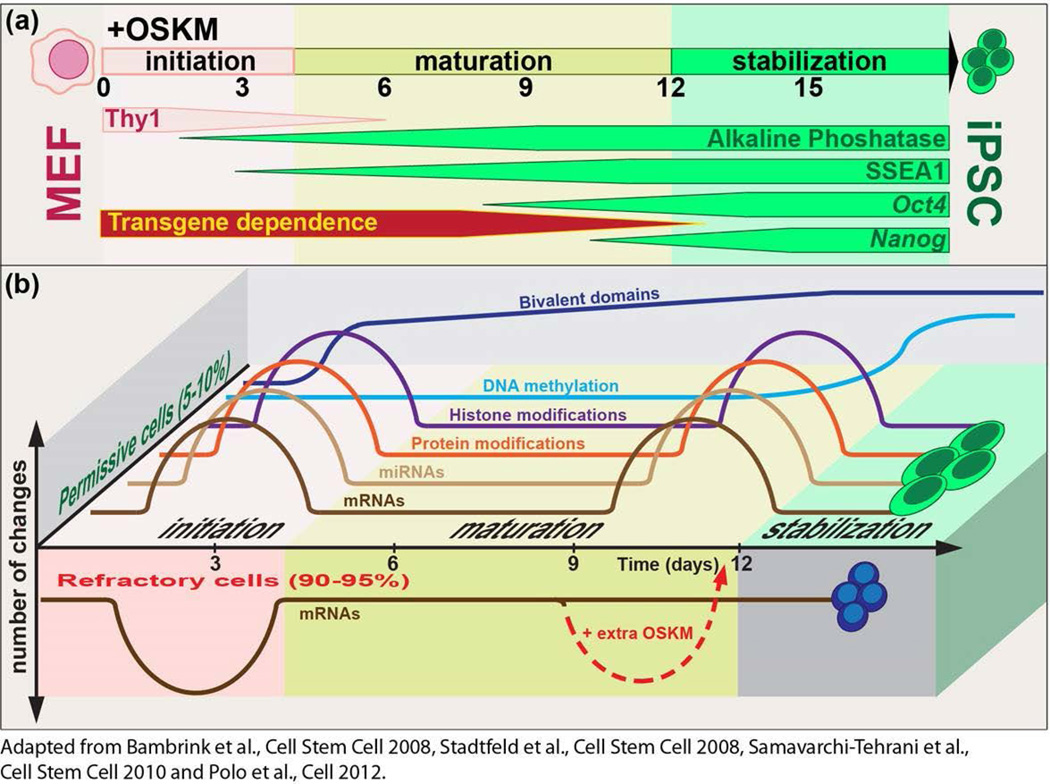
Experiments performed in Oct4-GFP or Nanog-GFP reporter MEFs using dox-inducible lentiviral vectors have shown that donor cells transition through a number of defined intermediates before reaching the pluripotent state. 1–2 days upon dox-induction of OSKM, the fibroblast-associated surface marker Thy1 is down-regulated coinciding with the up-regulation of the early pluripotency marker Alkaline phosphatase (Alpl) followed by embryonic antigen SSEA1, between day 3 and 5. Around day 8, reprogramming cells up-regulate the core pluripotency transcription factor Oct4 followed by Nanog 24, 25. In this setup, exogenous factor expression is required for at least 1.5 weeks to generate bona fide iPSC colonies that are transgene-independent 24, 25 (Figure 3 (a)). Global transcript and protein profiling during reprogramming of bulk MEF cultures and analysis of single cells have identified three phases in reprogramming: initiation, maturation and stabilization 26–29 (Figure 3(b) ).
Figure 3. Phases of Reprogramming.
(a) Kinetics of pluripotency-marker appearance and definition of the reprogramming phases. Alkaline phosphatase and Stage Specific Embryonic Antigen 1 (SSEA1) positive cells appear around day 2 and 3, respectively after OSKM transduction, coinciding with the down-regulation of the mesenchymal marker Thy1. GFP expressed from the endogenous Oct4 or Nanog loci is detected later, around day 9 and 10 respectively. The virally transduced factors need to be expressed for approximately 12 days to generate stable, transgene-independent iPSCs. (b) A schematic drawing illustrating the kinetics and the magnitude of the molecular changes observed during reprogramming. Permissive reprogramming cells (positive y axis) show a biphasic pattern of Protein, mRNA and microRNA (miRNA) expression changes following the kinetics of individual histone modifications. Bivalent domains are generated gradually after an initial burst and DNA methylation changes occur predominantly at the end of reprogramming. Refractory cells (negative y axis), undergo a similar wave of expression changes during the initiation phase but remain relatively stable afterwards. Forced expression of OKSM in refractory cells can rescue their ability to form iPSCs (Adapted from Polo et al., 2012).
II. I. I. The initiation phase
During the initiation phase MEFs undergo a coordinated mesenchymal-epithelial transition (MET), change their proliferation rate and up-regulate early PSC markers 26, 30. Single cell time-lapse microscopy has shown that the majority of cells undergo a MET, increase proliferation during this period 31–33 and single cell expression profiling of genes involved in reprogramming has uncovered stochastic variation in gene expression among cells during this phase 29. Transcriptional profiling of sorted Thy1−/SSEA1+ reprogramming intermediates between days 0 and 6 uncovers a first wave of global transcriptional changes involving mRNAs, miRNAs and lncRNAs, starting after OSKM expression and peaking around day 3. These changes affect cell proliferation, metabolism, cytoskeleton organization and developmental processes 27, 34, 35. Consistent with the hallmark MET and increased cell proliferation during the initiation phase, mesenchymal transcription factors are repressed (Snai1/2, Zeb1/2, Slug) 25, 36, 37, epithelial (Cdh1, Epcam, Cldn3, −4, −7, −11, Ocln, Crb3) and early pluripotency genes (Alpl, Fbxo15) are up-regulated 26, 27, 30, 38 together with DNA replication (Poli, Rfc4 and Mcm5), cell cycle progression (Ccnd1 and Ccnd2) and stress-induced genes (Cdkn1a and Cdkn2a) 36. Similar changes in protein expression are observed through proteomic analysis, identifying proteins involved in the regulation of pluripotency (Jarid2, Rif1, Tcf3, Eed) as well as general transcription regulation (Mediator, TAF, RNA Pol II, Nurd) 28.
Although SSEA1 identifies MEFs primed for reprogramming, only a small subset of sorted SSEA1+ cells progress successfully towards pluripotency at early reprogramming stages. Through a functional surface marker screen, a recent study identified an earlier set of surface markers (CD73, CD49d, CD200) transiently expressed in MEFs poised for reprogramming. CD73+, CD49d+, CD200+ cells express the transcriptional regulators Nr0b1 and Etv5 prior to up-regulation of other pluripotency regulators such as Rex1, Dppa2, Nanog and Sox2 39.
II. I. II. The maturation phase
The maturation phase is characterized by an initial period of stasis followed by a second wave of extensive transcriptional 26, 27, 38 and proteomic 28, 38 changes in MEFs between days 9 and 12. These changes affect development/stem cell genes (Oct4, Sall4, Nanog, Esrrb, Tcl1, Nr5a2) 27, 29, 38, 40 and hypoxia-responsive genes (ALDOC, ENO3, PKM2, Pfkl, Gpi, Pgk1) 28, 41, most of which are also up-reguated during the reprogramming of human fibroblasts. Moreover, proteins involved in vesicle-mediated transport, extracellular matrix, cell adhesion and EMT that were silenced during the initiation phase, are reactivated in reprogramming of MEFs between days 9 and 12 28.
The end of the maturation phase is marked by the up-regulation of Sox2 and other pluripotency genes in both mouse and human fibroblasts, leading to the establishment of the endogenous transcriptional network that sustains pluripotency independent of transgene expression 25–27, 29, 38. Transgene independence is a critical event marking the completion of the maturation phase. Interestingly, rather than classic pluripotency regulators (Oct4, Nanog, Sall4), a different set of genes seem to play a prominent role in this transition in MEFs, including pluripotency (Esrrb, Utf1, Lin28 and Dppa2), germline (Mnd1, Mutyh, Rad54b), cytoskeletal (Tuba3a, Pdzk1, Itgb7, Kirrel2) and RET-signalling (Nrtn, Kif26a) genes 29, 40. All together, these observations point towards an early stochastic and late deterministic model of reprogramming using the OSKM factors 42, 43.
II. I. III. The stabilization phase
The stabilization phase starts when iPSCs become transgene independent. At this stage, telomeres are actively elongated soon reaching embryonic levels 25, 44, DNA undergoes important changes, including reactivation of both copies of the X chromosome in a fraction of female mouse iPSCs 25, and 5-methylcytosine (5mC) marks are remodeled correlating with the up-regulation of genes involved in the regulation of DNA methylation (Aid, Tet and Dnmt family members) 27 (Table 2 (a)).
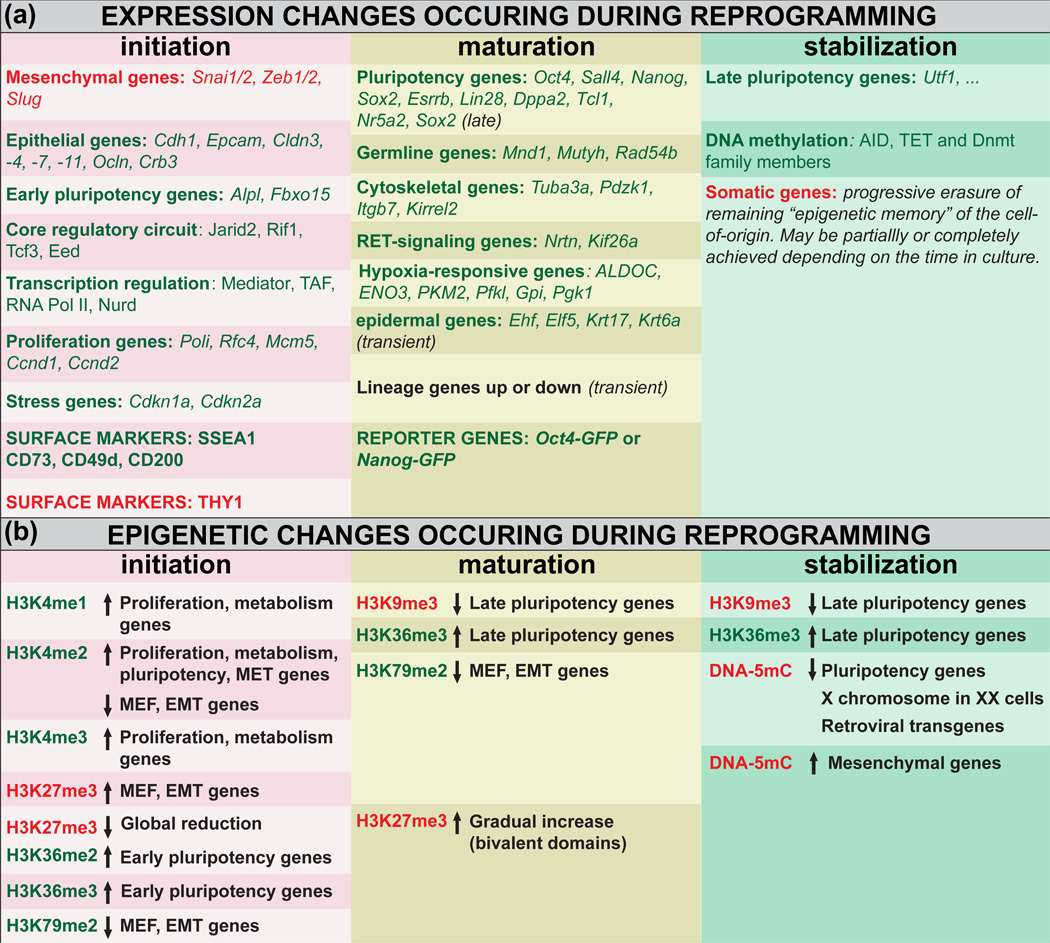
Table 2. Transcriptional and epigenetic changes during reprogramming.
(a) Expression changes (mRNA or protein). Genes in green are up-regulated and genes in red are down-regulated. (b) Epigenetic changes. Marks in green are generally associated with active loci and marks in red are generally associated with repressed loci. Arrows pointing up or down indicate respectively an increase or a decrease in the corresponding epigenetic mark. EMT, epithelial-to-mesenchymal transition; MEF, mouse embryonic fibroblast; MET, mesenchymal-to-epithelial transition. H3, histone H3; K4, K9, K27, K36, K79, Lysines 4, 9, 27, 36 and 79 of histone H3; me1, me2, me3, mono-, di- or tri-methylated respectively; DNA-5mC, 5-methyl cytosine DNA methylation.
II. II. Epigenetic changes during reprogramming
Bona fide iPSCs maintain pluripotency independent of transgene expression, suggesting the cells have acquired a different epigenetic state compared to the donor somatic cells and partially reprogrammed intermediates. The epigenetic modifications underlying the transcriptional changes observed during the initiation, maturation and stabilization phases of reprogramming involve genome-wide resetting of DNA methylation and histone post-translational modifications 45. Below we first provide a brief overview of commonly studied histone modifications.
Among the more than 100 known histone modifications, lysine methylation and acetylation are the most studied. In general, transcription start sites of actively transcribed genes are marked by trimethylated H3K4 (H3K4me3) and acetylated H3K27 (H3K27ac), and active enhancers are enriched with monomethylated H3K4 (H3K4me1) and H3K27ac. Gene bodies of actively transcribed genes are associated with trimethylated H3K36 (H3K36me3). Transcriptionally silenced heterochromatin is associated with trimethylated H3K9 (H3K9me3), whereas regulated gene repression is often associated with trimethylated H3K27 (H3K27me3). Notably, although H3K4 methylation marks active promoters in yeast, the presence of this mark at promoters and enhancers in mammalian cells does not necessarily correlate with gene expression. This difference results from the specific regulation of RNA polymerase II (Pol II) through pause and release at the promoter of mammalian cells and the presence of “bivalent promoters” containing both active (H3K4me3) and repressive (H3K27me3) marks in both PSC and terminally differentiated cells 23, 46.
II. II. I. Changes in histone marks
Unlike lineage-committed cells, which contain extensive regions of highly condensed heterochromatin, the chromatin of PSCs is more accessible, contains fewer heterochromatin foci, and correlates with a higher abundance of active- and a lower amount of repressive-histone post-translational modifications 47. Global reduction of H3K27me3 during the initiation phase of reprogramming is associated with the acquisition of a transient open/primed chromatin state and coincides with changes in RNA expression, loss of heterochromatin up to day 8 of reprogramming, followed by re-establishment of novel H3K27me3 marks 38. In contrast, H3K4me3/H3K27me3 bivalent domains are established gradually, starting around day 3 and increasing progressively until stable iPSCs are formed 27, 38. Unlike the H3K27me3 marks, the global H3K4me levels do not change significantly 38, but there are significant increases of monomethylated H3K4 (H3K4me1), H3K4me2 and H3K4me3 marks at proliferation and metabolism genes immediately upon OSKM induction, and H3K4me2 increases at MET and pluripotency genes and decreases at MEF-specific and EMT genes 37, 48–50.
Reprogramming also involves additional histone modifications at enhancers and promoters of key epithelial, mesenchymal and pluripotency genes. H3K36me2, whose depletion at CpG islands (CGIs: clusters of CpG dinucleotides, in which a cytosine precedes a guanosine) is thought to contribute to a transcriptionally permissive state 51, is removed in early responsive genes (Cdh1, Epcam) during the initiation phase 52. Sequential loss of H3K36me2/3 at the Ink4/Arf locus and the promoter of the microRNA cluster 302/367 are later events occurring during the maturation phase 53. H3K79 methylation is associated with active transcription and is usually enriched at gene bodies 54. Mesenchymal gene down-regulation during the initiation and maturation phase correlates with a reduction of H3K79me2 marks. As such, H3K36 and H3K79 methylation is believed to constitute a barrier for the acquisition of an epithelial fate during reprogramming 47. Another barrier to reprogramming is H3K9 methylation, and its removal during the late maturation phase marks the transition from pre-iPSCs to iPSCs 55.
II. II. II. Changes in DNA methylation
DNA methylation is considered the most stable epigenetic modification associated with gene silencing in lineage-committed cells. In mammals, DNA methylation occurs exclusively at the C5 position of cytosine (5mC), predominantly in the context of CpG dinucleotides. CpG clusters, called CGIs are predominantly found in gene promoters and represent major targets of DNA methylation in mammalian somatic cells. Generally speaking, promoter methylation is inversely correlated with gene expression 56.
PSCs possess a unique DNA methylation signature compared to lineage-committed cells. In particular, Oct4, Nanog and other pluripotency promoters are largely unmethylated and a significant number of tissue-specific promoters are hypermethylated 57. Genome-wide methylation studies reveal that in contrast to gene expression and histone modifications, global changes in DNA methylation occur predominantly at the end of the reprogramming process, coinciding with the up-regulation of different enzymes regulating DNA methylation. Hence, genome-wide demethylation of a large set of pluripotency-associated promoters including Nanog and Oct4 or de novo methylation of mesenchymal promoters such as Hoxa10, Gja8 occur in the late maturation phase 27, 38. Similarly, demethylation of the inactive X chromosome in female donor cells and down-regulation of the large noncoding RNA Xist (responsible for triggering X inactivation) occurs late after pluripotency genes are up-regulated and only in a fraction of iPSC lines 58–60 (Table 2 (b)).
II.III. Reprogramming intermediates, partially reprogrammed cells and alternative pluripotent states
Using reprogrammable MEFs, cells undergoing successful reprogramming transit in a linear fashion from Thy1+/SSEA1− to Thy1−/SSEA1− and to Thy1−/SSEA1+ in the first 6 days of reprogramming and eventually to a SSEA1+/Oct4+ state by days 9–12 27. Unlike Thy1−/SSEA1+ permissive cells, Thy1−/SSEA1− or refractory Thy1+/SSEA1− cells fail to properly activate PSC genes and repress mesenchymal genes, though this refractory state can be reversed in Thy1+/SSEA1− cells with an additional dose of OSKM. In addition, approximately 200 genes are aberrantly activated in refractory Thy1+/SSEA1− cells compared to progressing Thy1−/SSEA1+ cells at day 6 of reprogramming, including extracellular space/matrix, plasma membrane, retinoic acid binding and immune response transcripts, which may prevent reprogramming.
Analysis of Thy1−/SSEA1+ cells also reveals transient up- or down-regulation of gene expression specific to these reprogramming intermediates 26–28, 38. For instance, a large subset of transiently up-regulated genes are epidermal genes (Ehf, Elf5, Krt17, Krt6a) not expressed in either fibroblasts or iPSCs 61. A similar analysis used the expression of the surface antigen TRA-1–60 to identify intermediates during the reprogramming of human fibroblasts. This study has revealed transient up-regulation of primitive streak-like mesendoderm transcripts (T, EOMES, FOXH1) during reprogramming 62.
Transient activation/repression of developmental regulators during the maturation phase may reflect a permissive stage, as expression or silencing of certain lineage genes not directly involved in the acquisition of pluripotency may not be under selective pressure. Alternatively, transient activation or repression of some developmental regulators may facilitate the transition towards iPSCs. For example, over-expression of the FOXH1 gene mentioned above promotes OSKM-mediated HDF reprogramming. In either scenario, reprogramming intermediates may represent a transiently “plastic” state more amenable to cell-fate manipulation. This hypothesis has been supported by the successful derivation of epiblast stem cells 63, neural progenitors 64, endothelial cells 65 and cardiomyocytes 66 from fibroblasts transiently expressing OSKM and exposed to lineage-specific culture conditions. However, applying the same cardiac or neural transdifferentiation approach using a pluripotency-lineage tracing system suggests that the cells successfully transdifferentiating towards the cardiac or neural linages acquire transiently a pluripotency state 67, 68. Although, one cannot exclude that some transiently “plastic” cells may be able to directly convert into an alternative lineage, these experiments strongly suggest that OSKM transdifferentiation (OSKM-TD) requires passage through a transient iPSC state.
The success and apparent ease of reprogramming also raises interesting conceptual questions such as whether there could be alternative pluripotent states, such as the F-class cells recently generated using a PiggyBac transposon-mediated dox-inducible secondary reprogramming system 69 .These cells do not form typical ESC-like colonies and exhibit higher proliferative rates. Functionally, they differ from iPSC and ESCs in that although they can form teratomas containing tissue derivatives of the three germ layers, F-class cells don’t contribute to embryonic development when injected into mouse blastocysts 38, 69–72. Notably, this pluripotent state depends on high and constitutive OSKM expression. Sustained and high levels of OSKM result in rapid up-regulation and maintenance of Nanog and Sall4 expression in F-class cells, suggesting similarities in pluripotency network with iPSCs and ESCs. However, there are also notable differences in global methylation and histone marks among others, indicating that F-class cells represent an alternative pluripotent state 73. From a conceptual point of view, the transgene-dependency of F-class cells also challenges the traditional criterion that a fully reprogrammed cell (including cells created through lineage reprogramming not discussed in this review) must be able to maintain the cellular phenotype independent of continuous transgene expression.
III. A functional dissection of the reprogramming process
The acquisition and the maintenance of pluripotency depend on the activity of a large set of transcription factors, chromatin- and DNA-modifying enzymes, non-coding RNAs and signal-transduction pathways as outlined below.
III. I. Roles of transcription factors
III. I. I. OSKM
The transcription factors Oct4, Nanog and Sox2 (OSN) play a central role in maintaining the molecular and phenotypic features of PSCs, and their inactivation leads to loss of pluripotency 23. OSN form an auto-regulatory loop in PSCs by binding to their own and each other’s promoters. In addition, they co-occupy the promoters of hundreds of genes, half of them encoding genes actively expressed in PSCs, while the other half encoding developmental genes associated with lineage commitment that are silenced in PSCs 23.
cMyc is a facultative reprogramming factor that facilitates the emergence of reprogrammed cells 74, 75. Intersection of the transcriptional profiles identified at different stages of reprogramming with cMyc binding sites in PSCs reveals that the majority of cMyc targets are bound during the initiation phase. This is consistent with the finding that the main activity of cMyc is restricted to the initiation phase and its activity doesn’t seem to be mediated by the activation of pluripotency genes 37. One probable role of cMyc in reprogramming is through regulating cell proliferation: cMyc can repress cell cycle checkpoint genes, inhibit Cyclin-dependent kinase (CDK) inhibitors and associate with pre-replication complexes to promote DNA synthesis 76. Increased proliferation, in turn, may increase the probability for epigenetic resetting in the presence of OSK.
Overall, the binding sites of OSK evolve considerably during reprogramming but coincide at the onset of the stabilization phase with a PSC-like binding profile. More dramatic expression changes are usually observed in genes co-occupied by OSK in PSCs where they act as transcriptional activators, suggesting OSK play similar roles in reprogramming 36, 37, 77, 78. During the initiation phase, OSK bind to promoters and enhancers of active and repressed genes prior to significant histone modifications 37, 49, 50 including GLIS family zinc finger 1 (Glis1) 79, miR-302–367 cluster 80–82, Fbxo15, Fgf4, Sall4, Lin28 and MET genes 29, 30, 50. Interestingly, only half these enhancers are PSC-specific 49, the other half contains atypical distal elements suggesting OSK could be pioneer factors opening chromatin at critical pluripotency loci 50. Klf4 exerts a dual role during reprogramming, early in somatic gene repression (Tgfb1, Pdgfra, Col6a1), and late in pluripotency gene activation (Oct4, Tdgf1, Klf5) 27.
Widespread and promiscuous binding of OSK may result from the ectopic and non-physiological expression levels of the reprogramming transgenes. It has been proposed that, OSK interaction with Mediator, Cohesin complexes or RNA Pol II elongation factor ELL3 initially recruits these factors to non-canonical enhancers and promote chromatin opening 45, 83, 84. Supporting this idea transient expression of OSKM allows transdifferentiation of fibroblasts to other somatic lineages 63–66. Recent studies also show that cMyc is a global amplifier of gene expression, increasing transcription at all active promoters 85, 86. When OSK are over-expressed with cMyc, OSK factors may act as pioneer factors to enable cMyc binding to previously inaccessible distal elements on chromatin, and cMyc in turn may cooperatively enhance OSK binding and transcriptional activation capacity 50.
III. I. II. Other pluripotency-associated transcription factors
A number of additional totipotency- and pluripotency-associated transcription factors have been shown to replace part of the OSKM set and/or increase reprogramming efficiency. For instance iPSCs can be generated with OCT4, SOX2, NANOG and LIN28 (referred to as OSNL) from HDFs 12, or Sall4, Nanog, Esrrb and Lin28 (SNEL) from MEFs 42. Thus the pluripotent state can be achieved through different though likely convergent paths. On the other hand, differences have been observed in reprogramming using different factor combinations. For example, SNEL leads to significantly fewer iPSC colonies compared to OSKM, but the majority of iPSC lines established are of high quality based on the tetraploid complementation assay. In contrast, a combination of Oct4, Sox2, Sall4, Nanog and Esrrb (OSSNE) gives a much higher number of iPSC colonies but most lines exhibit poor developmental potential. These results suggest that the interplay between the reprogramming factors plays a critical role in fine-tuning the reprogramming process 42. Below we describe the roles of a number of pluripotency-associated reprogramming transcription factors other than the classic OSKM.
Nanog and Lin28 are perhaps the best studied reprogramming factors other than the OSKM factors. Together with Oct4 and Sox2, Nanog homeodomain transcription factor holds a central position in the pluripotency transcriptional network and is required for the maintenance of pluripotency in the epiblast and in ESCs 87. In OSNL-mediated reprogramming of human mesenchymal cells, NANOG is not required for the initial appearance of OCT4+ colonies, but improves the recovery of iPSC clones suggesting a role in the stabilization phase 12. Similarly, Nanog over-expression facilitates the transition from the maturation towards the stabilization phase during OKM-mediated reprogramming of mouse neural stem (NS) cells 88. The inclusion of Nanog in OSKM-mediated reprogramming of mouse B cells results in the formation of a similar numbers of iPSC colonies but with a significantly accelerated kinetics in a cell-division-rate-independent manner 19.
Lin28 is not absolutely required in the OSNL set, though removal of Lin28 leads to an approximately 5-fold reduction in the number of ESC-like colonies obtained from human mesenchymal cells 12. Lin28 may promote reprogramming through both miRNA-dependent and independent mechanisms 89. In ESCs, Lin28 binds and blocks the maturation of let-7 miRNA family members, which normally counteract the activity of cell-cycle-regulating miRNAs involved in ESC self-renewal. Lin28 can also regulate protein levels by controlling mRNA stability and has been shown to mediate post-transcriptional regulation of OCT4 in hESCs and cell-cycle regulators in mESCs 90. Thus LIN28’s function in the OSNL set may be analogous to that of cMYC (which is a let-7 target) in OSKM 91
Additional pluripotency-associated transcription factors have been shown to play a role in reprogramming, including Glis1, Sall4, and the orphan nuclear receptor Esrrb. Glis1 is enriched in unfertilized oocytes and one-cell stage embryos, and it promotes OSKM or OSK-mediated reprogramming in mouse and human fibroblasts 79. Glis1 has been shown to physically interact with OSK during reprogramming, and it contributes to the activation of multiple pro-reprogramming genes (Foxa2, Esrrb, Lin28a, Nanog). Glis1 over-expression also increases the expression of Mycn and Mycl1, but suppresses the expression of cMyc, and this altered balance between Myc family genes may also contribute to the effect of Glis1 in reprogramming. Sall4 is expressed in PSCs and its binding sites overlap with those of OSN 92, 93. When knocked down, ESCs differentiate toward a trophectoderm fate suggesting Sall4 contributes to the maintenance of the pluripotent state 94, 95. Sall4 over-expression with OSK in MEFs leads to a 2-fold increase in Nanog-GFP+ iPSC colonies compared to OSK alone, whereas Sall4 knockdown leads to a 2-fold reduction 96. Esrrb was identified through screening for factors to replace Klf4 in the OSKM cocktail, though OSEM exhibit a 2-fold decrease in efficiency compared to OSKM in reprogramming of MEFs. In ESCs, Esrrb targets many genes involved in self-renewal and pluripotency 97, 98 and its binding sites co-localize with Klf4, consistent with its capability to replace Klf4 in reprogramming 99.
III. I. III. Lineage transcription factors
A well-accepted model for the maintenance of pluripotency is that the core pluripotency factors prevent differentiation through binding and repression of key lineage specifiers in PSCs 100. In an interesting and not mutually-exclusive model, the pluripotency factors may act as early lineage specifiers and the balance of their antagonistic activities results in the maintenance of the undifferentiated state 101. Supporting the second model, Gata3 (a mesendoderm-lineage specifier) and Gmnn (an ectoderm-lineage specifier) can replace Oct4 and Sox2 respectively in OSKM-mediated reprogramming, and over-expression of Gata3 and Gmnn together with KM but without OS is sufficient to reprogram MEFs into iPSCs 102. A number of additional lineage transcription factors have been shown to work in similar ways as Gata3 and Gmnn in both mouse and human cells 102, 103. These results suggest a more dynamic and complex regulation of the induction, maintenance and exit of pluripotency than previously thought, though the exact mechanisms remain to be elucidated. By extension, one might also directly convert one somatic cell type into another (lineage reprogramming) through perturbing the balance between differentiation cues rather than over-expressing master transcription factors 104.
III. II. Roles of epigenetic remodeling factors
III. II. I. Histone post-translational modifiers
In PSCs, the promoters of most developmental genes regulated by Oct4, Sox2, and Nanog are co-occupied by Polycomb group (PcG) proteins, a family of epigenetic regulators responsible for the maintenance of transcriptional repression through H3K27 methylation 105. It is likely that reprogramming factors recruit, directly or indirectly, PcG proteins to silence developmental genes during the induction and maintenance of PSCs. Consistent with this notion, knockdown of H3K27 methyltransferases belonging to the PRC1 (BMI1, RING1) and PRC2 (EZH2, EED, SUZ12) PcG complexes substantially reduce the number of iPSC colonies obtained from human fibroblasts 106. Further studies have shown that Ezh2 negatively regulates TGFβ signaling components and pro-EMT miRNAs such as the miR-23a cluster through association with cMyc during the initiation phase of reprogramming in human fibroblasts 107.
Trithorax group (TrxG) complexes mediate H3K4 methylation, and H3K4me3 is enriched at actively transcribed promoters 108. During reprogramming, Wrd5, a core member of the mammalian TrxG complex, is up-regulated through direct binding of Oct4 to its promoter, and Wrd5 in turn promotes reprogramming by interacting with Oct4 109. Oct4 targets Wdr5 to pluripotency loci to re-establish H3K4me3 active marks leading to robust transcriptional activation. Consistent with this model, knockdown of Wdr5 results in decreased occupancy of Wdr5 on chromatin, global reduction of H3K4me3, locus-specific decrease of H3K4me3 at pluripotency-associated gene promoters including Oct4 and Nanog, and decreased efficiency of reprogramming in MEFs.
Inhibition of the H3K27 demethylase Utx (also known as Kdm6a) and the H3K36 demethylases Jhdm1a and Jhdm1b (also known as Kdm2a and Kdm2b) also decrease reprogramming efficiency. Utx has been shown to promote reprogramming through physically interacting with OSK and removing H3K27me3 from early pluripotency genes such as Fgf4, Sall4 or Sall1 110 Jhdm1b plays a prominent role during the initiation phase of reprogramming when it enhances the activation of early responsive genes through binding and demethylating their promoters 52. Moreover, Jhdm1a and Jhdm1b where also shown to mediate the enhancing effect of Vitamin C on reprogramming, by repressing the Ink4/Arf locus, increasing proliferation and suppressing senescence 53 (see chapter III. IV. III.).
H3K9 trimethylation (H3K9me3) is usually associated with repressed heterochromatin. During reprogramming, H3K9me3-containing regions have been shown to prevent OSKM binding in human fibroblasts 50 and their presence at pluripotency loci represents a major roadblock in the transition from pre- to fully reprogrammed mouse iPSCs 55, 111. Consistently, both the depletion of the heterochromatin protein-1γ (Cbx3), a protein known to recognize H3K9me3, or the H3K9 methyltransferases Ehmt1, Ehmt2 and Setdb1 increase mouse iPSC formation at early and late stages of reprogramming 111. H3K9 methyltransferases seem to act downstream of BMPs in combination with their corresponding demethylases. They constitute a switch controlling the transition from pre-iPSC to iPSC by regulating H3K9 methylation levels at core pluripotency loci 55.
Supporting an inhibitory role of H3K79me2-mediated gene expression in reprogramming, inhibition of the H3K79me2 methyltransferase DOT1L promotes reprogramming and can replace the reprogramming factors KLF4 and cMYC in OKSM 106. These effects are likely mediated through early up-regulation of NANOG and LIN28 and loss of H3K79me2 at EMT genes (SNAI1, SNAI2, ZEB1, TGFB2) 106.
III. II. II. Histone variants
Another important level of chromatin regulation is the incorporation of histone variants, which are often expressed at lower levels than major histone isoforms. For example, the histone variant H2A contributes to 1%–10% of the nucleosomes in the mammalian genome and gets phosphorylated by the ATM/ATR kinases upon DNA damage 112. We and others have detected significant increase in H2A.X phosphorylation (γ-H2A.X) during the initiation phase of reprogramming 44, 113, 114, suggesting ectopic expression of the reprogramming factors may trigger DNA damage. Supporting this hypothesis, homologous recombination genes including Brca1, Brca2 and Rad51 are required for efficient reprogramming 113, 115. More recent studies show that H2A.X depletion leads to decreased reprogramming efficiency 116 and that aberrant accumulation of H2A.X marks poor-quality iPSCs 42, 116.
MacroH2A histone variants are characterized by their large size and are generally associated with heterochromatin 117. MacroH2A variants are enriched in both differentiated mouse and human somatic cells 118–120. In mouse fibroblasts macroH2A1 and macroH2A2, together with H3K27me3, co-occupy repressed pluripotency genes 118, 119 and are highly enriched at Utx target genes, which are reactivated early during reprogramming 119. Similarly macroH2A1 occupies pluripotency and bivalent genes in human keratinocytes 120. More efficient reprogramming has been observed in macroH2A1 and macroH2A2-depleted mouse NSCs 118 and double knockout mouse fibroblasts 119. In human keratinocytes, macroH2A1 but not macroH2A2 knock-down leads to increased reprogramming efficiency, while macroH2A1 overexpression inhibits reprogramming 120. Thus macroH2A–mediated repression constitutes another barrier of reprogramming.
Unlike MacroH2A, other histones variants have been shown to promote reprogramming. TH2A and TH2B, which are highly expressed in mouse oocytes and contribute to activation of the paternal genome after fertilization, stimulate iPSC generation when over-expressed with OSKM in MEFs 121 . Moreover, knock-down and overexpression studies of SF1A (a histone-remodeling chaperone enriched in human oocytes) suggest this histone chaperone promotes reprogramming of HDFs 122.
III. II. III. Nucleosome remodelers
Chromatin structure is also regulated by ATP-dependent chromatin remodeling factors that influence DNA accessibility through insertion, replacement or removal of nucleosomes. Deletion of components of the BAF (mammalian SWI/SNF) chromatin remodeling complex leads to defects in ESC self-renewal and differentiation 123. Through a combined functional and quantitative proteomics screen, BRG1 and BAF155, two components of BAF, were found enriched in reprogramming-competent cell extracts. They increase OSKM-mediated reprogramming of MEFS by promoting demethylation of the promoters of pluripotency genes such as Oct4, Nanog and Rex1 124.
Other chromatin-remodeling proteins including Chd1 and INO80 have been shown to be required for pluripotency and reprogramming. Chd1 associates with euchromatin in ESCs and preferentially targets genes involved in chromatin organization and transcription 125. INO80 co-occupies pluripotency gene promoters in an OCT4- and WDR5-dependent manner, maintaining open chromatin and facilitating the recruitment of Mediator and RNA Pol II for gene activation 126. Both Chd1 and INO80 are required for efficient reprogramming 125, 126.
Mbd3 is a core member of the nucleosome remodeling and deacetylation (NuRD) repressor complex, and its exact roles in reprogramming are still under debate. In MEFs, Mbd3 overexpression significantly reduces reprogramming and its knockdown using shRNAs leads to a 10-fold increase in reprogramming efficiency 127. Using optimized Mbd3 genetic deletion, OSKM transgene delivery and 2i/LIF naive pluripotency culture conditions Mbd3−/− MEFs can be reprogrammed with close to 95% efficiency 21. During OSKM-mediated reprogramming of MEFs, the genes bound by Mbd3 and Chd4 (another NuRD component) are enriched for Klf4, Oct4, Sox2 and Esrrb targets. OSKM-mediated recruitment of Mbd3/NuRD repressor complex seems to act as a potent epigenetic barrier to reprogramming 21. In contrast to these observations, Mbd3 ablation in neural stem cells reduces iPSC formation 22, and its knockdown in human fibroblasts differentiated from hESCs has a negative effect on iPSC induction 106. These discrepancies may result from differences in experimental setups, and the roles of Mbd3 may be sensitive to the reprogramming context.
III. II. IV. Chromatin topology
Beside transcriptional and epigenetic regulation, chromatin topology constitutes an additional layer of gene regulation in vertebrate genomes. Differentiation of PSCs leads to relocation of pluripotency loci towards the nuclear lamina where they get silenced. It has also been shown that differentiation disrupts the physical interactions (looping) between promoters and enhancers at highly transcribed pluripotency loci. Such interactions rely on the proper binding of mediator and cohesin complexes to these regulatory sequences. These observations suggest that proper reorganization of chromatin architecture may also be required for successful reprogramming 128.
Different subunits of mediator and cohesin bind to the promoters and enhancers of Oct4 and Nanog in ESCs 83, 129. During reprogramming, they have been shown to be recruited by the reprogramming factor Klf4 at the Oct4 locus 130 and their knockdown inhibits reprogramming 129. The re-establishment of proper long-range interactions characterizes pre-iPSCs, precedes transcriptional activation of both Nanog and Oct4 loci and is required for successful reprogramming 129–131. These results strongly support a causal relationship between the acquisition of a pluripotency-specific 3D chromatin structure and the transcriptional activation of key pluripotency loci.
III. II. V. DNA methylation/demethylation enzymes
DNA methylation marks gene inactivation in general and is considered the most stable epigenetic modification. It is established by the de novo methyltransferases Dnmt3a and Dnmt3b and preserved through cell division by the maintenance methyltransferase Dnmt1 132. Silencing of somatic genes and activation of pluripotent genes during reprogramming involves DNA methylation and demethylation respectively. DNMT3a and Dnmt3a/3b knockdown in human and mouse fibroblasts have mild effects on reprogramming 133, suggesting that somatic gene silencing is mainly triggered by chromatin remodeling during reprogramming.
On the other hand, DNA demethylation of pluripotency loci is a critical event in reprogramming, and it involves both passive and active mechanisms 134. Down-regulation of Dnmt1 promotes passive, replication-dependent DNA demethylation and facilitates the transition from the maturation to the stabilization phase during reprogramming 36. Alternatively, active DNA demethylation can be achieved through TET–mediated hydroxylation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) and the subsequent replacement of 5hmC with an unmethylated cytosine through TDG-mediated base excision repair 134. 5hmC levels increase and correlate with TET1 activation in human fibroblasts undergoing reprogramming 135. Tet1 over-expression stimulates reprogramming and can replace exogenous Oct4 expression 136, 137, Tet1 or Tet2 over-expression with Nanog enhance reprogramming, whereas inhibition of Nanog or Tet2 compromises iPSC formation 88, 138, 139. Triple Tet1, 2, 3-null and TDG-null MEFs cannot be reprogrammed demonstrating an absolute requirement of oxidative demethylation for reprogramming 140. However, this requirement appears specific to mesenchymal cells, as keratinocytes and neural progenitor cells can be successfully reprogrammed without any Tet genes. At day 4 of reprogramming, two proteins, poly(ADP-ribose) polymerase-1 (Parp1, also known as Artd1) and Tet2, are recruited to pluripotency promoters 139. Nanog has been shown to target both Tet1 and Tet2 to many pluripotency loci 138, and Tet2 induces hydroxymethylation of key pluripotency loci (Nanog, Oct4, Esrrb) 139. Parp1 has a complementary role in the establishment of early epigenetic marks during somatic cell reprogramming by regulating 5mC modification 139. There is also evidence suggesting that Parp1-mediated poly(ADP-ribosyl)ation of Sox2 leads to the dissociation of Sox2 from the Fgf4 enhancer and relieves the inhibitory effect of excessive Sox2 on Ffg4 expression in PSCs and during reprogramming 141, 142.
In addition to the TET proteins, activation-induced cytidine deaminase (AID) is also suggested to mediate DNA demethylation during development through base excision repair 143. However, the exact roles of AID during reprogramming have been debated. AID has been shown to be required for active demethylation of somatic cell nuclei in somatic-pluripotent cell fusion experiments 144, but this finding has been challenged by a following report 145. In OSKM-mediated reprogramming, AID over-expression leads to a 2-fold increase in iPSC generation 146, but no effects have been observed in subsequent studies 147–149. Transient shRNA knockdown between day 0–2 of reprogramming has been shown to result in a 4–5 fold reduction in iPSC colony formation 146. However, a later study reports that AID null MEFs are initially hyper-responsive to OSKM-mediated reprogramming and give rise to six-fold more Nanog+ colonies compared to control MEFs 147. This study also shows that by 4 weeks, most AID null iPSC colonies have differentiated, suggesting a requirement of AID for the stabilization of the pluripotent phenotype 147, but this finding has not been replicated in recent OSK-reprogramming studies 148, 149. Therefore all studies so far agree that AID is not absolutely required for reprogramming, but there is disagreement about whether there is a subtle requirement, suggesting the roles of AID is sensitive to the reprogramming context.
III. III. Role of non-coding RNAs
III. III. I. microRNAs
MicroRNAs (miRNAs) represent a class of non-coding RNAs that are ~22 nucleotides long. In mammals, miRNAs act as post-transcriptional repressors through mediating mRNA degradation or translation blockage. A subset of miRNAs is specifically expressed in PSCs and progressively silenced during differentiation. During reprogramming, miRNAs change their expression profile in a biphasic manner like mRNAs and proteins. One wave occurs during the initiation phase and the second happens at the end of the maturation phase. miRNAs up-regulation often correlates with the down-regulation of their target genes such as Lats2, TgfbR2, and Akt1 targeted by miR-294, and Lin28, N-Myc, and Sall4 targeted by let-7c 27. Mouse PSCs deficient for Dicer or DGCR8, two enzymes essential for miRNA biogenesis, are viable but show defects in both proliferation and differentiation. In MEFs, shRNA knockdown of key miRNA processing enzymes including Dicer, Drosha or Ago2 results in a dramatic decrease in the number of iPSC colonies generated with OSKM 150 suggesting miRNAs are also required in the acquisition of pluripotency.
Inhibition of the MEF-enriched miRNAs miR-21, miR-29a, let-7 151, 152 or deletion of miR-34 153 has a positive effect during the initiation phase of reprogramming. These miRNAs act as barriers to iPSC generation through modulating the activity of different pathways antagonizing reprogramming (TGFβ, MAP kinase, ERK1/2 and p53) 151–154. Binding of the EMT factor Snai1 to let-7 promoter inhibits let-7 expression and releases Lin28 repression 155. Moreover, Snai1 and Nanog have been shown to co-occupy the regulatory region and activate the transcription of several pluripotency-associated genes including Lin28 and miR-290–295. Interestingly, Snai1 and its homolog Snai2 exert opposite effects on reprogramming: ectopic expression of Snai1 or conversely Snai2 depletion promotes this process 156. On the other hand, over-expression of miR-93, miR-106b 150, miR-130/301/721 157, miR-29b 158 or miR-135b 159 enhance iPSC generation. miR-93, miR-130/301/721 and miR-29b modulate MET through targeting TGF-G receptor II (miR-93, miR-106b ), Meox2 (miR-130/301/721) and Dnmt3a and Dnmt3b. This results in the up-regulation of epithelial genes such as E-cadherin, Epcam or Cldn3. Moreover, miR-135b modulates Wisp1, a key regulator of several extra cellular matrix proteins, 150, 157, 159.
A family of ESC cell cycle promoting miRNAs, known as the ESCC family, targets multiple inhibitors of the CyclinE-Cdk2 pathway and is highly expressed in ESCs promoting their unique cell cycle program 160. Over-expression of miR-290 cluster (miR-291-3p, miR-294 and miR-295) 161 or the closely related miR-106 family 81, 150 enhances reprogramming of MEFs. Similarly, ESCC miR-302 and miR-372 promote reprogramming of human fibroblasts through regulating cell cycle and blocking TGFβ-induced EMT 82. Furthermore, the ESCC miR-302/367 cluster is a direct target of Oct4 and Sox2 in PSCs 162, and its over-expression increases OSK-mediated iPSC generation through targeting TGFβ receptor II 81. Notably, over-expression of miR302/367 is sufficient to reprogram both MEFs and human fibroblasts, yielding respectively 100- and 2-fold more iPSC colonies than OSKM reprogramming with a faster kinetics. Hdac inhibition through valproic acid (VPA) treatment 163 is however required to achieve miRNA-mediated reprogramming in MEFs 80.
These results highlight the importance of miRNAs in reprogramming, and miRNAs may offer an easier alternative to OSKM reprogramming for rapid generation of therapeutically relevant human iPSCs.
III. III. II. Long non-coding RNAs (lncRNAs)
Long non-coding RNAs (lncRNAs) represent a class of non-coding RNAs of >200 nucleotides in length. LncRNAs can activate or repress transcription in cis or in trans. Several studies indicate that lncRNAs are members of the PSC self-renewal regulatory circuit and are required for proper differentiation 164. A subset of lncRNAs binds one or more chromatin modification complexes, including readers, writers, or erasers of repressive histone modifications including polycomb complexes 165.
Relatively few specific lncRNAs have been studied in details for their roles in reprogramming. The lncRNA “regulator of reprogramming” (lncRNA-RoR) was identified through comparing lncRNA expression profiles between human fibroblasts, iPSCs and ESCs. Among a group of 28 “iPSC-enriched” lncRNAs, lncRNA-RoR inhibition or over-expression either decreases or increases the respective efficiency of iPSC formation, providing the first demonstration for functional contribution of a lncRNA during reprogramming 166. However, loss-of-function of most lncRNAs up-regulated during reprogramming has no significant effect on reprogramming, although transcriptional analysis suggests some of them may play a role in suppressing lineage genes (Ladr49, Ladr83, Ladr317) or activating metabolic genes (Ladr86, Ladr91) 167.
III. IV. Roles of signal transduction pathways
III. IV. I. Developmental signaling pathways
Several signaling pathways central to the regulation of pluripotency in PSCs also play important roles in reprogramming likely because they regulate components of the pluripotency transcriptional network. Activation of the Jak-Stat3 pathway by the cytokine leukemia inhibitory factor (LIF) and BMP-SMAD signaling is essential for the maintenance of mESCs. Other secreted factors, including Wnt, activin/nodal, and bFGF contribute to pluripotency in various culture conditions of mESCs and hESCs 168. Dual chemical inhibition of MEK (Erk1/Erk2) and canonical Wnt/β-catenin pathways (GSK-3) also known as the 2i condition is sufficient to sustain self-renewal of mouse ESCs in the absence of any extrinsic signal, a condition referred to as the ground state pluripotency 169. When applied at 7–9 days of reprogramming, MEK inhibition limits the growth of non-iPSC colonies and promotes the growth of reprogrammed iPSCs from neural progenitor cells (NPCs) 170. Moreover, Wnt3a–conditioned media increase MEF reprogramming with OSK 171, and GSK-3 inhibition allows reprogramming MEFs with only two factors OS 172.
Wnt seems to mediate its effect on reprogramming through repression of Tcf3, which binds and inhibit Oct4, Nanog and Sox2 in mouse ESCs 173. Moreover, β-catenin is indirectly regulated by Nanog and is necessary for conversion of pre-iPSCs into iPSCs 174. Combining 2i and LIF promotes the transition of partially reprogrammed cells toward bona fide iPSCs 175. Similarly, activation of the Jak-Stat3 pathway by LIF promotes reprogramming by facilitating the transition from partially reprogrammed cells toward fully reprogrammed iPSCs 176. The conventional hPSCs bear more similarities to primed mouse EpiSCs than the naïve mESCs. For a long time, it was unclear whether hPSCs could adopt a naïve pluripotent state. Recently, several studies have reported the generation of naïve hPSCs using different combinations of chemical compounds and cytokines 177–182. Naive hPSCs, including naïve hESCs and hiPSCs, share numerous morphological and molecular similarities with naive mESCs, suggesting conservation of a naïve pluripotency program in vitro.
Other developmental or disease-related pathways, including cyclic AMP 183, PI3K 184, Hippo/Yap 185, Src 186, Fgf 141 and Notch pathways 187 have also been shown to modulate reprogramming efficiency or functionally replace some of the reprogramming factors. Notably, inhibition of TGFβ using small molecule inhibitors allows reprogramming MEFs with only OK 188, 189 likely through promoting MET 30. Building upon these and other findings on chemicals that enhance reprogramming 190, Hou and colleagues performed an extensive small-molecules screen and identified a combination of seven chemical compounds sufficient to reprogram mouse somatic cells with up to 0.2% efficiency. Thus rather than imposing the pluripotency program through transcription factor over-expression, reprogramming can be achieved through simply modulating molecular pathways not strictly related to pluripotency 191.
Importantly, the effects of signaling molecules may depend on the donor cell type and the reprogramming stage. While more than 80% of MEFs reprogram through OSKM expression, TGFβ inhibition, Wnt activation and ascorbic acid treatment, hepatoblast or blood progenitors only require TGFβ inhibition or Wnt activation alone respectively to achieve similar reprogramming efficiencies with OSKM 192. In reprogramming MEFs, BMP promotes MET in the early initiation phase 26 but blocks the late transition of pre-iPSC to iPSCs 55; whereas Wnt signaling has inhibitory effects early and a stimulatory role late in reprogramming 193.
III. IV. II. Stress-induced signaling pathways
The p53 tumor-suppressor processes a variety of extra- and intracellular stress signals (e.g. DNA damage, oxidative stress, …) into different cellular responses including DNA repair, cell cycle arrest, senescence, and programmed cell death. An important module regulating p53 activity is the p53-p19 (ARF)-Mdm2 network: p53 transcriptionally represses p19 and activates Mdm2, Mdm2 blocks p53 transcriptional activation and promotes its polyubiquitination and proteasome-mediated degradation, p19 binds to Mdm2 and inhibits its ubiquitin ligase activity 194. Elevated p53 in response to stress signals or elevated p19 as a consequence of oncogenic signals or release of E2F, results in p53 stabilization and up-regulation of its targets genes including pro-apoptotic Bcl2 family members and p21 (CIP1), a CDK (cyclin-dependent kinase) inhibitor, leading to cell-cycle arrest in the G1 and G2 phases 195.
Several studies have shown that p53 deletion or depletion in MEFs leads to the formation of significantly higher numbers of iPSC colonies 44, 196–198. Contrary to Nanog over-expression however, p53 inhibition affects reprogramming kinetics and efficiency in a proliferation-dependent manner 19. In wild-type fibroblasts, over-expression of the reprogramming factors triggers DNA damage and oxidative stress, leading to a significant increase in the number of apoptotic and senescent cells 44, 113, 197, 199. These effects correlate with an increase in p53 level and activity, and up-regulation of p19, p21 and Mdm2 196, 197, 199. This p53-mediated response is particularly pronounced in cells harboring pre-existing DNA alterations, such as shorter telomeres, suggesting that p53 limits reprogramming through elimination of genetically abnormal cells 200. Consistently, as observed for p53, down-regulation of p19, Mdm2 and p21 also increases reprogramming efficiency 196–198
The Ink4/Arf locus encodes two important tumor suppressors: p16 (Ink4a) and p15 (Ink4b) in addition to p19. P16 and p15 bind and inhibit the cyclin-D-dependent kinases Cdk4 and Cdk6, which in turn are important to relieve the cell-cycle inhibitory activity of the Retinoblastoma (Rb) tumour suppressor, and Rb controls multiple cellular functions, including proliferation, survival, differentiation, metabolism, and genomic stability 201. In wild-type fibroblasts silencing of the Ink4/Arf locus accompanies the establishment of iPSCs, and single or combined deletion or depletion of the genes encoded by this locus promotes reprogramming 198, 199, 202. Rb null MEFs also reprogram with a higher efficiency. Considering the regulation of Rb by p15/p16, Rb may act through limiting proliferation during reprogramming as shown for p53. However, contrary to this prediction, Rb does not significantly affect cell proliferation, and instead acts predominantly through globally repressing the pluripotency network in somatic cells 203.
Ataxia-telangiectasia mutated (Atm) has a critical role in the cellular response to DNA double-strand breaks (DSBs). Upon DNA DSBs, Atm is activated and phosphorylates p53 and H2AX. H2AX recruits Mbc1 at the site of the break and triggers DNA repair by recruiting the homologous recombination (HR) repair machinery including Brca1, Brca2, Rad51, 53BP. Supporting the central role of p53 in eliminating DNA-damaged cells during reprogramming, MEFs deficient in either Atm or 53BP1 show a decrease in reprogramming efficiency 44, 204, 205. Moreover, HR DNA DSB repair genes such as Brca1, Brca2, Rad51 113, 115, 206, Fanconi anemia genes involved in both DNA DSB repair and interstrand cross-link (ICLs) processing 114, 207–210 and non-homologous end joining (NHEJ) DSB repair genes 211, 212 have all been shown to be required for efficient reprogramming to a pluripotent state.
These observations suggest that reprogramming and malignant transformation shares common features. Similar to cancer, reprogramming occurs in a small fraction of cells and proceeds through a multi-step process involving global transcriptional, epigenetic, metabolic and cellular changes. Unlike somatic donor cells, iPSC are immortal in culture, and these cells can form benign tumors (teratomas) when transplanted into immunocompromised mice. Molecularly, OSKM have each been shown to act as oncogenes in different contexts, and many chromatin and DNA methylation regulators cooperating with OSKM during reprogramming are associated with tumorigenesis. These similarities suggest that cancer-initiating cells may need to overcome similar barriers as iPSCs during tumor development. This is exemplified by the silencing of the Ink/Arf locus, which is required for iPSC formation and also observed in a large number of tumors. A major difference however exists: bona fide iPSCs display a normal karyotype and support full development in tetraploid complementation experiments, whereas most cancer cells are aneuploid and characterized by limited differentiation potential 128, 213.
III. IV. III. Metabolic signaling pathways
Normal somatic cells usually generate energy from mitochondrial oxidative phosphorylation (OXPHOS), whereas PSCs and cancer cells rely mainly on glycolysis for energy production. In both PSCs and cancer cells, glycolytic genes are up-regulated, mitochondrial activity is reduced, and lactate production is significantly increased. While ambient air has a concentration of ~21% O2, the concentration is much lower in tissues, between ~0.7% – 7%, and the O2 concentration experienced by blastocysts and many cancer cells in vivo is close to the lower end of this range. The dependency of PSCs on glycolysis to produce ATP may therefore reflect an adaptation of ICM cells to the low-oxygen niche in the blastocyst 214.
Lowering oxygen concentration in culture from normoxic (~21%) to hypoxic (~5%) conditions has been shown to enhance the generation of iPSCs from mouse and human somatic cells 215. Additionally, reprogramming efficiency can be increased through activation of glycolysis or blockade of mitochondrial OXPHOS using fructose 2,6-bisphosphate (PFK1 activator), 2,4-dinitrophenol (mitochondria decoupler), quercetin (HIF activator), or PS48 (PDK1 activator) 216. The cellular adaptation to hypoxic conditions is mainly mediated through the activation of the oxygen-sensitive transcription factors, hypoxia-inducible factors (HIFs). Interestingly, HIF1α has been shown to activate Notch signaling and maintain the undifferentiated state of various stem and progenitor cell populations 217, and HIF2α binds to Oct4 promoter and regulates its expression in PSCs 218. During reprogramming of human fibroblasts, knockdown of either HIF1α or HIF2α reduces the number of iPSC colonies formed, and over-expression of non-degradable forms of either proteins during early phases of reprogramming significantly improves reprogramming efficiency and accelerates the switch toward glycolytic metabolism 41.
Treatment with Ascorbic Acid (Vitamin C, or Vc), a potent antioxidant, significantly improves the efficiencies of both OSK- and OSKM-mediated MEF reprogramming. This effect is mediated at least in part through a reduction of cell senescence as suggested by a significant reduction in p53 and p21 levels in Vc-treated reprogramming cells 219. Vc also improves iPSC quality through preventing aberrant silencing of the imprinted Dlk1-Dio3 locus, suggesting a direct role of Vc in epigenetic remodeling 220. Supporting this notion, Vc promotes H3K36me2/3 demethylation during reprogramming, an activity mediated by Vc-dependent demethylating dioxygenases Jhdm1a/1b. In addition, Jhdm1b accelerates cell cycle progression and suppresses cell senescence by repressing the Ink4/Arf locus, and cooperates with Oct4 to activate the miR-302/367 cluster 53. In addition to Jhdm1a/1b, Vc also modulates TET1 function and 5hmC formation at loci critical for MET during reprogramming 137.
Conclusion
Since Takahashi and Yamanaka’s demonstration that reprogramming can be achieved through ectopic expression of a surprisingly small number of pluripotency-associated transcription factors, a large body of work has contributed to the improvement of the reprogramming methods and a better understanding of the mechanisms underlying this process. Several recent studies have achieved almost deterministic reprogramming using privileged donor cells or through epigenetic manipulation, suggesting that the initial stochasticity attributed to the process is context-dependent. These advances are not only fundamental to our understanding of pluripotency and the nature of cell identity in general, but are also of critical importance to the therapeutic application of reprogramming.
One important consideration is the safety of the cells for therapy. For instance, initial studies have identified transgene integration as a major limitation of hiPSC in a therapeutic setup due to risks of tumorigenesis associated with random transgene insertional mutagenesis and potential reactivation of the reprogramming transgenes. This issue has been mitigated through the development of non-integrating reprogramming methods 224. Independent of transgene insertion, several studies have identified different types of genetic alterations in hiPSCs. Some of these alterations may result from passaging, other mutations have been identified in rare donor cells, and some are acquired de novo during the reprogramming process. While these mutations highlight the importance of screening multiple patient-derived iPSC lines in order to select the safer line(s) for therapeutic purposes, the extent of these alterations compared to hESCs is not dramatic 225.
Another area of intensive investigation is regarding the similarity between hiPSCs and hESCs, which remains an important question relevant to both basic stem cell biology and the therapeutic use of hPSCs. While initial reprogramming studies highlighted the strong similarity between hiPSCs and hESCs, further analysis revealed significant differences in their transcriptomes, epigenomes, in vitro and in vivo differentiation potentials. Some of these differences may reflect intrinsic variability in the molecular and functional properties of PSCs. Some may be inherent to hiPSC as a consequence of the reprogramming itself 226. For instance, incomplete epigenetic remodeling, referred to as a memory of the cell-of-origin, has been described in hiPSC lines, and epigenetic memory has been suggested to be responsible for lineage-specific bias of hiPSCs during differentiation, though this bias diminishes through passaging and could be prevented by optimizing the reprogramming conditions 227. Recently, nt-hESC lines have been established from human cells 5, 6. Two studies comparing isogenic nt-hESCs and hiPSC lines reveal that the two reprogramming methods induce comparable numbers of de novo coding mutations 228, 229. However, while one study suggests that the transcriptional and epigenetic profiles of nt-hESCs are closer to hESCs than hiPSCs 229, the other study identified equal numbers of epigenetic alterations in nt-hESCs and hiPSCs, suggesting these differences are inherent to reprogramming regardless of the exact reprogramming approach 228. It remains to be seen whether and how any differences among hiPSCs, nt-hESCs and hESCs affect the usage of the cells for disease modeling and cell replacement therapy. The answers to these questions may become clearer as we gain a better understanding of reprogramming.
Footnotes
Related Articles
| Subtopic | Article title |
|---|---|
| e.g., Fertilization to Gastrulation | Direct cellular reprogramming in Caenorhabditis elegans: facts, models, and promises for regenerative medicine |
| The microRNA regulation of stem cells |
Contributor Information
Federico González, Developmental Biology Program, Sloan-Kettering Institute, 1275 York Avenue, New York, New York 10065, USA; gonzalf1@mskcc.org.
Danwei Huangfu, Developmental Biology Program, Sloan-Kettering Institute, 1275 York Avenue, New York, New York 10065, USA; HuangfuD@mskcc.org.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Wakayama T, Tabar V, Rodriguez I, Perry AC, Studer L, Mombaerts P. Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science. 2001;292:740–743. doi: 10.1126/science.1059399. [DOI] [PubMed] [Google Scholar]
- 5.Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada M, Johannesson B, Sagi I, Burnett LC, Kort DH, Prosser RW, Paull D, Nestor MW, Freeby M, Greenberg E, et al. Human oocytes reprogram adult somatic nuclei of a type 1 diabetic to diploid pluripotent stem cells. Nature. 2014;510:533–536. doi: 10.1038/nature13287. [DOI] [PubMed] [Google Scholar]
- 7.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 13.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–1181. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 14.Varas F, Stadtfeld M, de Andres-Aguayo L, Maherali N, di Tullio A, Pantano L, Notredame C, Hochedlinger K, Graf T. Fibroblast-derived induced pluripotent stem cells show no common retroviral vector insertions. Stem Cells. 2009;27:300–306. doi: 10.1634/stemcells.2008-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R, Staerk J, Markoulaki S, Jaenisch R. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat Biotechnol. 2008;26:916–924. doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey BW, Markoulaki S, Beard C, Hanna J, Jaenisch R. Single-gene transgenic mouse strains for reprogramming adult somatic cells. Nat Methods. 2010;7:56–59. doi: 10.1038/nmeth.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stadtfeld M, Maherali N, Borkent M, Hochedlinger K. A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nat Methods. 2010;7:53–55. doi: 10.1038/nmeth.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eminli S, Foudi A, Stadtfeld M, Maherali N, Ahfeldt T, Mostoslavsky G, Hock H, Hochedlinger K. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41:968–976. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP, van Oudenaarden A, Jaenisch R. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo S, Zi X, Schulz VP, Cheng J, Zhong M, Koochaki SH, Megyola CM, Pan X, Heydari K, Weissman SM, et al. Nonstochastic reprogramming from a privileged somatic cell state. Cell. 2014;156:649–662. doi: 10.1016/j.cell.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rais Y, Zviran A, Geula S, Gafni O, Chomsky E, Viukov S, Mansour AA, Caspi I, Krupalnik V, Zerbib M, et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502:65–70. doi: 10.1038/nature12587. [DOI] [PubMed] [Google Scholar]
- 22.dos Santos RL, Tosti L, Radzisheuskaya A, Caballero IM, Kaji K, Hendrich B, Silva JC. MBD3/NuRD facilitates induction of pluripotency in a context-dependent manner. Cell Stem Cell. 2014;15:102–110. doi: 10.1016/j.stem.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–159. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, Woltjen K, Nagy A, Wrana JL. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Polo JM, Anderssen E, Walsh RM, Schwarz BA, Nefzger CM, Lim SM, Borkent M, Apostolou E, Alaei S, Cloutier J, et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansson J, Rafiee MR, Reiland S, Polo JM, Gehring J, Okawa S, Huber W, Hochedlinger K, Krijgsveld J. Highly coordinated proteome dynamics during reprogramming of somatic cells to pluripotency. Cell Rep. 2012;2:1579–1592. doi: 10.1016/j.celrep.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buganim Y, Faddah DA, Cheng AW, Itskovich E, Markoulaki S, Ganz K, Klemm SL, van Oudenaarden A, Jaenisch R. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. 2012;150:1209–1222. doi: 10.1016/j.cell.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F, Zhuang Q, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Araki R, Jincho Y, Hoki Y, Nakamura M, Tamura C, Ando S, Kasama Y, Abe M. Conversion of ancestral fibroblasts to induced pluripotent stem cells. Stem Cells. 2010;28:213–220. doi: 10.1002/stem.282. [DOI] [PubMed] [Google Scholar]
- 32.Megyola CM, Gao Y, Teixeira AM, Cheng J, Heydari K, Cheng EC, Nottoli T, Krause DS, Lu J, Guo S. Dynamic migration and cell-cell interactions of early reprogramming revealed by high-resolution time-lapse imaging. Stem Cells. 2013;31:895–905. doi: 10.1002/stem.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith ZD, Nachman I, Regev A, Meissner A. Dynamic single-cell imaging of direct reprogramming reveals an early specifying event. Nat Biotechnol. 2010;28:521–526. doi: 10.1038/nbt.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein BE, van Oudenaarden A, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, Bernstein BE, Jaenisch R, Lander ES, Meissner A. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sridharan R, Tchieu J, Mason MJ, Yachechko R, Kuoy E, Horvath S, Zhou Q, Plath K. Role of the murine reprogramming factors in the induction of pluripotency. Cell. 2009;136:364–377. doi: 10.1016/j.cell.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussein SM, Puri MC, Tonge PD, Benevento M, Corso AJ, Clancy JL, Mosbergen R, Li M, Lee DS, Cloonan N, et al. Genome-wide characterization of the routes to pluripotency. Nature. 2014;516:198–206. doi: 10.1038/nature14046. [DOI] [PubMed] [Google Scholar]
- 39.Lujan E, Zunder ER, Ng YH, Goronzy IN, Nolan GP, Wernig M. Early reprogramming regulators identified by prospective isolation and mass cytometry. Nature. 2015 doi: 10.1038/nature14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golipour A, David L, Liu Y, Jayakumaran G, Hirsch CL, Trcka D, Wrana JL. A late transition in somatic cell reprogramming requires regulators distinct from the pluripotency network. Cell Stem Cell. 2012;11:769–782. doi: 10.1016/j.stem.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT, Ruohola-Baker H. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. 2014;14:592–605. doi: 10.1016/j.stem.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buganim Y, Markoulaki S, van Wietmarschen N, Hoke H, Wu T, Ganz K, Akhtar-Zaidi B, He Y, Abraham BJ, Porubsky D, et al. The developmental potential of iPSCs is greatly influenced by reprogramming factor selection. Cell Stem Cell. 2014;15:295–309. doi: 10.1016/j.stem.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David L, Polo JM. Phases of reprogramming. Stem Cell Res. 2014;12:754–761. doi: 10.1016/j.scr.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460:1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buganim Y, Faddah DA, Jaenisch R. Mechanisms and models of somatic cell reprogramming. Nat Rev Genet. 2013;14:427–439. doi: 10.1038/nrg3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 47.Liang G, Zhang Y. Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res. 2013;23:49–69. doi: 10.1038/cr.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt R, Plath K. The roles of the reprogramming factors Oct4, Sox2 and Klf4 in resetting the somatic cell epigenome during induced pluripotent stem cell generation. Genome Biol. 2012;13:251. doi: 10.1186/gb-2012-13-10-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koche RP, Smith ZD, Adli M, Gu H, Ku M, Gnirke A, Bernstein BE, Meissner A. Reprogramming factor expression initiates widespread targeted chromatin remodeling. Cell Stem Cell. 2011;8:96–105. doi: 10.1016/j.stem.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soufi A, Donahue G, Zaret KS. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blackledge NP, Zhou JC, Tolstorukov MY, Farcas AM, Park PJ, Klose RJ. CpG islands recruit a histone H3 lysine 36 demethylase. Mol Cell. 2010;38:179–190. doi: 10.1016/j.molcel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang G, He J, Zhang Y. Kdm2b promotes induced pluripotent stem cell generation by facilitating gene activation early in reprogramming. Nat Cell Biol. 2012;14:457–466. doi: 10.1038/ncb2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang T, Chen K, Zeng X, Yang J, Wu Y, Shi X, Qin B, Zeng L, Esteban MA, Pan G, et al. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell. 2011;9:575–587. doi: 10.1016/j.stem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen AT, Zhang Y. The diverse functions of Dot1 and H3K79 methylation. Genes Dev. 2011;25:1345–1358. doi: 10.1101/gad.2057811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen J, Liu H, Liu J, Qi J, Wei B, Yang J, Liang H, Chen Y, Chen J, Wu Y, et al. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. Nat Genet. 2013;45:34–42. doi: 10.1038/ng.2491. [DOI] [PubMed] [Google Scholar]
- 56.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 57.Berdasco M, Esteller M. DNA methylation in stem cell renewal and multipotency. Stem Cell Res Ther. 2011;2:42. doi: 10.1186/scrt83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pasque V, Tchieu J, Karnik R, Uyeda M, Sadhu Dimashkie A, Case D, Papp B, Bonora G, Patel S, Ho R, et al. X chromosome reactivation dynamics reveal stages of reprogramming to pluripotency. Cell. 2014;159:1681–1697. doi: 10.1016/j.cell.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim JS, Choi HW, Arauzo-Bravo MJ, Scholer HR, Do JT. Reactivation of the inactive X chromosome and post-transcriptional reprogramming of Xist in iPSCs. J Cell Sci. 2015;128:81–87. doi: 10.1242/jcs.154294. [DOI] [PubMed] [Google Scholar]
- 60.Kim KY, Hysolli E, Tanaka Y, Wang B, Jung YW, Pan X, Weissman SM, Park IH. X Chromosome of female cells shows dynamic changes in status during human somatic cell reprogramming. Stem Cell Reports. 2014;2:896–909. doi: 10.1016/j.stemcr.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Malley J, Skylaki S, Iwabuchi KA, Chantzoura E, Ruetz T, Johnsson A, Tomlinson SR, Linnarsson S, Kaji K. High-resolution analysis with novel cell-surface markers identifies routes to iPS cells. Nature. 2013;499:88–91. doi: 10.1038/nature12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takahashi K, Tanabe K, Ohnuki M, Narita M, Sasaki A, Yamamoto M, Nakamura M, Sutou K, Osafune K, Yamanaka S. Induction of pluripotency in human somatic cells via a transient state resembling primitive streak-like mesendoderm. Nat Commun. 2014;5:3678. doi: 10.1038/ncomms4678. [DOI] [PubMed] [Google Scholar]
- 63.Han DW, Greber B, Wu G, Tapia N, Arauzo-Bravo MJ, Ko K, Bernemann C, Stehling M, Scholer HR. Direct reprogramming of fibroblasts into epiblast stem cells. Nat Cell Biol. 2011;13:66–71. doi: 10.1038/ncb2136. [DOI] [PubMed] [Google Scholar]
- 64.Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, Ragoussis J, Huang Y, Han JD, Zeng L, et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci U S A. 2012;109:13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 67.Bar-Nur O, Verheul C, Sommer AG, Brumbaugh J, Schwarz BA, Lipchina I, Huebner AJ, Mostoslavsky G, Hochedlinger K. Lineage conversion induced by pluripotency factors involves transient passage through an iPSC stage. Nat Biotechnol. 2015;33:761–768. doi: 10.1038/nbt.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maza I, Caspi I, Zviran A, Chomsky E, Rais Y, Viukov S, Geula S, Buenrostro JD, Weinberger L, Krupalnik V, et al. Transient acquisition of pluripotency during somatic cell transdifferentiation with iPSC reprogramming factors. Nat Biotechnol. 2015;33:769–774. doi: 10.1038/nbt.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tonge PD, Corso AJ, Monetti C, Hussein SM, Puri MC, Michael IP, Li M, Lee DS, Mar JC, Cloonan N, et al. Divergent reprogramming routes lead to alternative stem-cell states. Nature. 2014;516:192–197. doi: 10.1038/nature14047. [DOI] [PubMed] [Google Scholar]
- 70.Benevento M, Tonge PD, Puri MC, Hussein SM, Cloonan N, Wood DL, Grimmond SM, Nagy A, Munoz J, Heck AJ. Proteome adaptation in cell reprogramming proceeds via distinct transcriptional networks. Nat Commun. 2014;5:5613. doi: 10.1038/ncomms6613. [DOI] [PubMed] [Google Scholar]
- 71.Clancy JL, Patel HR, Hussein SM, Tonge PD, Cloonan N, Corso AJ, Li M, Lee DS, Shin JY, Wong JJ, et al. Small RNA changes en route to distinct cellular states of induced pluripotency. Nat Commun. 2014;5:5522. doi: 10.1038/ncomms6522. [DOI] [PubMed] [Google Scholar]
- 72.Lee DS, Shin JY, Tonge PD, Puri MC, Lee S, Park H, Lee WC, Hussein SM, Bleazard T, Yun JY, et al. An epigenomic roadmap to induced pluripotency reveals DNA methylation as a reprogramming modulator. Nat Commun. 2014;5:5619. doi: 10.1038/ncomms6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu J, Izpisua Belmonte JC. Stem cells: A designer’s guide to pluripotency. Nature. 2014;516:172–173. doi: 10.1038/516172a. [DOI] [PubMed] [Google Scholar]
- 74.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 75.Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 77.Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 78.Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maekawa M, Yamaguchi K, Nakamura T, Shibukawa R, Kodanaka I, Ichisaka T, Kawamura Y, Mochizuki H, Goshima N, Yamanaka S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature. 2011;474:225–229. doi: 10.1038/nature10106. [DOI] [PubMed] [Google Scholar]
- 80.Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liao B, Bao X, Liu L, Feng S, Zovoilis A, Liu W, Xue Y, Cai J, Guo X, Qin B, et al. MicroRNA cluster 302–367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J Biol Chem. 2011;286:17359–17364. doi: 10.1074/jbc.C111.235960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Subramanyam D, Lamouille S, Judson RL, Liu JY, Bucay N, Derynck R, Blelloch R. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol. 2011;29:443–448. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, Ebmeier CC, Goossens J, Rahl PB, Levine SS, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin C, Garruss AS, Luo Z, Guo F, Shilatifard A. The RNA Pol II elongation factor Ell3 marks enhancers in ES cells and primes future gene activation. Cell. 2013;152:144–156. doi: 10.1016/j.cell.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin CY, Loven J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42–49. doi: 10.1038/sj.cr.7310125. [DOI] [PubMed] [Google Scholar]
- 88.Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Viswanathan SR, Daley GQ. Lin28: A microRNA regulator with a macro role. Cell. 2010;140:445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 90.Shyh-Chang N, Daley GQ. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell. 2013;12:395–406. doi: 10.1016/j.stem.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 92.Lim CY, Tam WL, Zhang J, Ang HS, Jia H, Lipovich L, Ng HH, Wei CL, Sung WK, Robson P, et al. Sall4 regulates distinct transcription circuitries in different blastocyst-derived stem cell lineages. Cell Stem Cell. 2008;3:543–554. doi: 10.1016/j.stem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 93.Yang J, Chai L, Fowles TC, Alipio Z, Xu D, Fink LM, Ward DC, Ma Y. Genome-wide analysis reveals Sall4 to be a major regulator of pluripotency in murine-embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:19756–19761. doi: 10.1073/pnas.0809321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Q, Chen X, Zhang J, Loh YH, Low TY, Zhang W, Zhang W, Sze SK, Lim B, Ng HH. Sall4 interacts with Nanog and co-occupies Nanog genomic sites in embryonic stem cells. J Biol Chem. 2006;281:24090–24094. doi: 10.1074/jbc.C600122200. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J, Tam WL, Tong GQ, Wu Q, Chan HY, Soh BS, Lou Y, Yang J, Ma Y, Chai L, et al. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol. 2006;8:1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- 96.Tsubooka N, Ichisaka T, Okita K, Takahashi K, Nakagawa M, Yamanaka S. Roles of Sall4 in the generation of pluripotent stem cells from blastocysts and fibroblasts. Genes Cells. 2009;14:683–694. doi: 10.1111/j.1365-2443.2009.01301.x. [DOI] [PubMed] [Google Scholar]
- 97.Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, DeCoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- 98.Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 99.Feng B, Jiang J, Kraus P, Ng JH, Heng JC, Chan YS, Yaw LP, Zhang W, Loh YH, Han J, et al. Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nat Cell Biol. 2009;11:197–203. doi: 10.1038/ncb1827. [DOI] [PubMed] [Google Scholar]
- 100.Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Loh KM, Lim B. A precarious balance: pluripotency factors as lineage specifiers. Cell Stem Cell. 2011;8:363–369. doi: 10.1016/j.stem.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 102.Shu J, Wu C, Wu Y, Li Z, Shao S, Zhao W, Tang X, Yang H, Shen L, Zuo X, et al. Induction of pluripotency in mouse somatic cells with lineage specifiers. Cell. 2013;153:963–975. doi: 10.1016/j.cell.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Montserrat N, Nivet E, Sancho-Martinez I, Hishida T, Kumar S, Miquel L, Cortina C, Hishida Y, Xia Y, Esteban CR, et al. Reprogramming of human fibroblasts to pluripotency with lineage specifiers. Cell Stem Cell. 2013;13:341–350. doi: 10.1016/j.stem.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 104.Ben-David U, Nissenbaum J, Benvenisty N. New balance in pluripotency: reprogramming with lineage specifiers. Cell. 2013;153:939–940. doi: 10.1016/j.cell.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 105.Aloia L, Di Stefano B, Di Croce L. Polycomb complexes in stem cells and embryonic development. Development. 2013;140:2525–2534. doi: 10.1242/dev.091553. [DOI] [PubMed] [Google Scholar]
- 106.Onder TT, Kara N, Cherry A, Sinha AU, Zhu N, Bernt KM, Cahan P, Marcarci BO, Unternaehrer J, Gupta PB, et al. Chromatin-modifying enzymes as modulators of reprogramming. Nature. 2012;483:598–602. doi: 10.1038/nature10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rao RA, Dhele N, Cheemadan S, Ketkar A, Jayandharan GR, Palakodeti D, Rampalli S. Ezh2 mediated H3K27me3 activity facilitates somatic transition during human pluripotent reprogramming. Sci Rep. 2015;5:8229. doi: 10.1038/srep08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 109.Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, Ding J, Ge Y, Darr H, Chang B, et al. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145:183–197. doi: 10.1016/j.cell.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mansour AA, Gafni O, Weinberger L, Zviran A, Ayyash M, Rais Y, Krupalnik V, Zerbib M, Amann-Zalcenstein D, Maza I, et al. The H3K27 demethylase Utx regulates somatic and germ cell epigenetic reprogramming. Nature. 2012;488:409–413. doi: 10.1038/nature11272. [DOI] [PubMed] [Google Scholar]
- 111.Sridharan R, Gonzales-Cope M, Chronis C, Bonora G, McKee R, Huang C, Patel S, Lopez D, Mishra N, Pellegrini M, et al. Proteomic and genomic approaches reveal critical functions of H3K9 methylation and heterochromatin protein-1gamma in reprogramming to pluripotency. Nat Cell Biol. 2013;15:872–882. doi: 10.1038/ncb2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Banaszynski LA, Allis CD, Lewis PW. Histone variants in metazoan development. Dev Cell. 2010;19:662–674. doi: 10.1016/j.devcel.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gonzalez F, Georgieva D, Vanoli F, Shi ZD, Stadtfeld M, Ludwig T, Jasin M, Huangfu D. Homologous recombination DNA repair genes play a critical role in reprogramming to a pluripotent state. Cell Rep. 2013;3:651–660. doi: 10.1016/j.celrep.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muller LU, Milsom MD, Harris CE, Vyas R, Brumme KM, Parmar K, Moreau LA, Schambach A, Park IH, London WB, et al. Overcoming reprogramming resistance of Fanconi anemia cells. Blood. 2012;119:5449–5457. doi: 10.1182/blood-2012-02-408674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Navarro S, Moleiro V, Molina-Estevez FJ, Lozano ML, Chinchon R, Almarza E, Quintana-Bustamante O, Mostoslavsky G, Maetzig T, Galla M, et al. Generation of iPSCs from genetically corrected Brca2 hypomorphic cells: implications in cell reprogramming and stem cell therapy. Stem Cells. 2014;32:436–446. doi: 10.1002/stem.1586. [DOI] [PubMed] [Google Scholar]
- 116.Wu T, Liu Y, Wen D, Tseng Z, Tahmasian M, Zhong M, Rafii S, Stadtfeld M, Hochedlinger K, Xiao A. Histone variant H2A.X deposition pattern serves as a functional epigenetic mark for distinguishing the developmental potentials of iPSCs. Cell Stem Cell. 2014;15:281–294. doi: 10.1016/j.stem.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 117.Buschbeck M, Di Croce L. Approaching the molecular and physiological function of macroH2A variants. Epigenetics. 2010;5:118–123. doi: 10.4161/epi.5.2.11076. [DOI] [PubMed] [Google Scholar]
- 118.Pasque V, Radzisheuskaya A, Gillich A, Halley-Stott RP, Panamarova M, Zernicka-Goetz M, Surani MA, Silva JC. Histone variant macroH2A marks embryonic differentiation in vivo and acts as an epigenetic barrier to induced pluripotency. J Cell Sci. 2012;125:6094–6104. doi: 10.1242/jcs.113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gaspar-Maia A, Qadeer ZA, Hasson D, Ratnakumar K, Leu NA, Leroy G, Liu S, Costanzi C, Valle-Garcia D, Schaniel C, et al. MacroH2A histone variants act as a barrier upon reprogramming towards pluripotency. Nat Commun. 2013;4:1565. doi: 10.1038/ncomms2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barrero MJ, Sese B, Kuebler B, Bilic J, Boue S, Marti M, Izpisua Belmonte JC. Macrohistone variants preserve cell identity by preventing the gain of H3K4me2 during reprogramming to pluripotency. Cell Rep. 2013;3:1005–1011. doi: 10.1016/j.celrep.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 121.Shinagawa T, Takagi T, Tsukamoto D, Tomaru C, Huynh LM, Sivaraman P, Kumarevel T, Inoue K, Nakato R, Katou Y, et al. Histone variants enriched in oocytes enhance reprogramming to induced pluripotent stem cells. Cell Stem Cell. 2014;14:217–227. doi: 10.1016/j.stem.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 122.Gonzalez-Munoz E, Arboleda-Estudillo Y, Otu HH, Cibelli JB. Cell reprogramming Histone chaperone ASF1A is required for maintenance of pluripotency and cellular reprogramming. Science. 2014;345:822–825. doi: 10.1126/science.1254745. [DOI] [PubMed] [Google Scholar]
- 123.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singhal N, Graumann J, Wu G, Arauzo-Bravo MJ, Han DW, Greber B, Gentile L, Mann M, Scholer HR. Chromatin-Remodeling Components of the BAF Complex Facilitate Reprogramming. Cell. 2010;141:943–955. doi: 10.1016/j.cell.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 125.Gaspar-Maia A, Alajem A, Polesso F, Sridharan R, Mason MJ, Heidersbach A, Ramalho-Santos J, McManus MT, Plath K, Meshorer E, et al. Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature. 2009;460:863–868. doi: 10.1038/nature08212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang L, Du Y, Ward JM, Shimbo T, Lackford B, Zheng X, Miao YL, Zhou B, Han L, Fargo DC, et al. INO80 facilitates pluripotency gene activation in embryonic stem cell self-renewal, reprogramming, and blastocyst development. Cell Stem Cell. 2014;14:575–591. doi: 10.1016/j.stem.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luo M, Ling T, Xie W, Sun H, Zhou Y, Zhu Q, Shen M, Zong L, Lyu G, Zhao Y, et al. NuRD blocks reprogramming of mouse somatic cells into pluripotent stem cells. Stem Cells. 2013;31:1278–1286. doi: 10.1002/stem.1374. [DOI] [PubMed] [Google Scholar]
- 128.Apostolou E, Hochedlinger K. Chromatin dynamics during cellular reprogramming. Nature. 2013;502:462–471. doi: 10.1038/nature12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Apostolou E, Ferrari F, Walsh RM, Bar-Nur O, Stadtfeld M, Cheloufi S, Stuart HT, Polo JM, Ohsumi TK, Borowsky ML, et al. Genome-wide chromatin interactions of the Nanog locus in pluripotency, differentiation, and reprogramming. Cell Stem Cell. 2013;12:699–712. doi: 10.1016/j.stem.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wei Z, Gao F, Kim S, Yang H, Lyu J, An W, Wang K, Lu W. Klf4 organizes long-range chromosomal interactions with the oct4 locus in reprogramming and pluripotency. Cell Stem Cell. 2013;13:36–47. doi: 10.1016/j.stem.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 131.Zhang H, Jiao W, Sun L, Fan J, Chen M, Wang H, Xu X, Shen A, Li T, Niu B, et al. Intrachromosomal looping is required for activation of endogenous pluripotency genes during reprogramming. Cell Stem Cell. 2013;13:30–35. doi: 10.1016/j.stem.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 132.Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- 133.Pawlak M, Jaenisch R. De novo DNA methylation by Dnmt3a and Dnmt3b is dispensable for nuclear reprogramming of somatic cells to a pluripotent state. Genes Dev. 2011;25:1035–1040. doi: 10.1101/gad.2039011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502:472–479. doi: 10.1038/nature12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang T, Wu H, Li Y, Szulwach KE, Lin L, Li X, Chen IP, Goldlust IS, Chamberlain SJ, Dodd A, et al. Subtelomeric hotspots of aberrant 5-hydroxymethylcytosine-mediated epigenetic modifications during reprogramming to pluripotency. Nat Cell Biol. 2013;15:700–711. doi: 10.1038/ncb2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao Y, Chen J, Li K, Wu T, Huang B, Liu W, Kou X, Zhang Y, Huang H, Jiang Y, et al. Replacement of Oct4 by Tet1 during iPSC induction reveals an important role of DNA methylation and hydroxymethylation in reprogramming. Cell Stem Cell. 2013;12:453–469. doi: 10.1016/j.stem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 137.Chen J, Guo L, Zhang L, Wu H, Yang J, Liu H, Wang X, Hu X, Gu T, Zhou Z, et al. Vitamin C modulates TET1 function during somatic cell reprogramming. Nat Genet. 2013;45:1504–1509. doi: 10.1038/ng.2807. [DOI] [PubMed] [Google Scholar]
- 138.Costa Y, Ding J, Theunissen TW, Faiola F, Hore TA, Shliaha PV, Fidalgo M, Saunders A, Lawrence M, Dietmann S, et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature. 2013;495:370–374. doi: 10.1038/nature11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Doege CA, Inoue K, Yamashita T, Rhee DB, Travis S, Fujita R, Guarnieri P, Bhagat G, Vanti WB, Shih A, et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488:652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hu X, Zhang L, Mao SQ, Li Z, Chen J, Zhang RR, Wu HP, Gao J, Guo F, Liu W, et al. Tet and TDG mediate DNA demethylation essential for mesenchymal-to-epithelial transition in somatic cell reprogramming. Cell Stem Cell. 2014;14:512–522. doi: 10.1016/j.stem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 141.Weber FA, Bartolomei G, Hottiger MO, Cinelli P. Artd1/Parp1 regulates reprogramming by transcriptional regulation of Fgf4 via Sox2 ADP-ribosylation. Stem Cells. 2013;31:2364–2373. doi: 10.1002/stem.1507. [DOI] [PubMed] [Google Scholar]
- 142.Gao F, Kwon SW, Zhao Y, Jin Y. PARP1 poly(ADP-ribosyl)ates Sox2 to control Sox2 protein levels and FGF4 expression during embryonic stem cell differentiation. J Biol Chem. 2009;284:22263–22273. doi: 10.1074/jbc.M109.033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu SC, Zhang Y. Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol. 2010;11:607–620. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bhutani N, Brady JJ, Damian M, Sacco A, Corbel SY, Blau HM. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature. 2010;463:1042–1047. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Foshay KM, Looney TJ, Chari S, Mao FF, Lee JH, Zhang L, Fernandes CJ, Baker SW, Clift KL, Gaetz J, et al. Embryonic stem cells induce pluripotency in somatic cell fusion through biphasic reprogramming. Mol Cell. 2012;46:159–170. doi: 10.1016/j.molcel.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bhutani N, Decker MN, Brady JJ, Bussat RT, Burns DM, Corbel SY, Blau HM. A critical role for AID in the initiation of reprogramming to induced pluripotent stem cells. FASEB J. 2013;27:1107–1113. doi: 10.1096/fj.12-222125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kumar R, DiMenna L, Schrode N, Liu TC, Franck P, Munoz-Descalzo S, Hadjantonakis AK, Zarrin AA, Chaudhuri J, Elemento O, et al. AID stabilizes stem-cell phenotype by removing epigenetic memory of pluripotency genes. Nature. 2013;500:89–92. doi: 10.1038/nature12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Habib O, Habib G, Do JT, Moon SH, Chung HM. Activation-induced deaminase-coupled DNA demethylation is not crucial for the generation of induced pluripotent stem cells. Stem Cells Dev. 2014;23:209–218. doi: 10.1089/scd.2013.0337. [DOI] [PubMed] [Google Scholar]
- 149.Shimamoto R, Amano N, Ichisaka T, Watanabe A, Yamanaka S, Okita K. Generation and characterization of induced pluripotent stem cells from Aid-deficient mice. PLoS One. 2014;9:e94735. doi: 10.1371/journal.pone.0094735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li Z, Yang CS, Nakashima K, Rana TM. Small RNA-mediated regulation of iPS cell generation. EMBO J. 2011;30:823–834. doi: 10.1038/emboj.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang CS, Li Z, Rana TM. microRNAs modulate iPS cell generation. RNA. 2011;17:1451–1460. doi: 10.1261/rna.2664111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–626. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Choi YJ, Lin CP, Ho JJ, He X, Okada N, Bu P, Zhong Y, Kim SY, Bennett MJ, Chen C, et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat Cell Biol. 2011;13:1353–1360. doi: 10.1038/ncb2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang CS, Rana TM. Learning the molecular mechanisms of the reprogramming factors: let’s start from microRNAs. Mol Biosyst. 2013;9:10–17. doi: 10.1039/c2mb25088h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Unternaehrer JJ, Zhao R, Kim K, Cesana M, Powers JT, Ratanasirintrawoot S, Onder T, Shibue T, Weinberg RA, Daley GQ. The Epithelial-Mesenchymal Transition Factor SNAIL Paradoxically Enhances Reprogramming. Stem Cell Reports. 2014 doi: 10.1016/j.stemcr.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gingold JA, Fidalgo M, Guallar D, Lau Z, Sun Z, Zhou H, Faiola F, Huang X, Lee DF, Waghray A, et al. A genome-wide RNAi screen identifies opposing functions of Snai1 and Snai2 on the Nanog dependency in reprogramming. Mol Cell. 2014;56:140–152. doi: 10.1016/j.molcel.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pfaff N, Fiedler J, Holzmann A, Schambach A, Moritz T, Cantz T, Thum T. miRNA screening reveals a new miRNA family stimulating iPS cell generation via regulation of Meox2. EMBO Rep. 2011;12:1153–1159. doi: 10.1038/embor.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guo X, Liu Q, Wang G, Zhu S, Gao L, Hong W, Chen Y, Wu M, Liu H, Jiang C, et al. microRNA-29b is a novel mediator of Sox2 function in the regulation of somatic cell reprogramming. Cell Res. 2013;23:142–156. doi: 10.1038/cr.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li Z, Dang J, Chang KY, Rana TM. MicroRNA-mediated regulation of extracellular matrix formation modulates somatic cell reprogramming. RNA. 2014 doi: 10.1261/rna.043745.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27:459–461. doi: 10.1038/nbt.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Card DA, Hebbar PB, Li L, Trotter KW, Komatsu Y, Mishina Y, Archer TK. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol. 2008;28:6426–6438. doi: 10.1128/MCB.00359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ng SY, Johnson R, Stanton LW. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J. 2012;31:522–533. doi: 10.1038/emboj.2011.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Flynn RA, Chang HY. Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell. 2014;14:752–761. doi: 10.1016/j.stem.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim DH, Marinov GK, Pepke S, Singer ZS, He P, Williams B, Schroth GP, Elowitz MB, Wold BJ. Single-Cell Transcriptome Analysis Reveals Dynamic Changes in lncRNA Expression during Reprogramming. Cell Stem Cell. 2015;16:88–101. doi: 10.1016/j.stem.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007;3:7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- 169.Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shi Y, Do JT, Desponts C, Hahm HS, Scholer HR, Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 171.Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young RA, Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li W, Zhou H, Abujarour R, Zhu S, Young Joo J, Lin T, Hao E, Scholer HR, Hayek A, Ding S. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells. 2009;27:2992–3000. doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Marucci L, Pedone E, Di Vicino U, Sanuy-Escribano B, Isalan M, Cosma MP. beta-catenin fluctuates in mouse ESCs and is essential for Nanog-mediated reprogramming of somatic cells to pluripotency. Cell Rep. 2014;8:1686–1696. doi: 10.1016/j.celrep.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 175.Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang J, van Oosten AL, Theunissen TW, Guo G, Silva JC, Smith A. Stat3 activation is limiting for reprogramming to ground state pluripotency. Cell Stem Cell. 2010;7:319–328. doi: 10.1016/j.stem.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chan YS, Goke J, Ng JH, Lu X, Gonzales KA, Tan CP, Tng WQ, Hong ZZ, Lim YS, Ng HH. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell. 2013;13:663–675. doi: 10.1016/j.stem.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 178.Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 179.Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci U S A. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D, Santos F, Clarke J, Mansfield W, et al. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell. 2014;158:1254–1269. doi: 10.1016/j.cell.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L, et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 2014;15:471–487. doi: 10.1016/j.stem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S, et al. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111:4484–4489. doi: 10.1073/pnas.1319738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang Y, Adjaye J. A cyclic AMP analog, 8-Br-cAMP, enhances the induction of pluripotency in human fibroblast cells. Stem Cell Rev. 2011;7:331–341. doi: 10.1007/s12015-010-9209-3. [DOI] [PubMed] [Google Scholar]
- 184.Liao J, Marumoto T, Yamaguchi S, Okano S, Takeda N, Sakamoto C, Kawano H, Nii T, Miyamato S, Nagai Y, et al. Inhibition of PTEN tumor suppressor promotes the generation of induced pluripotent stem cells. Mol Ther. 2013;21:1242–1250. doi: 10.1038/mt.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Staerk J, Lyssiotis CA, Medeiro LA, Bollong M, Foreman RK, Zhu S, Garcia M, Gao Q, Bouchez LC, Lairson LL, et al. Pan-Src family kinase inhibitors replace Sox2 during the direct reprogramming of somatic cells. Angew Chem Int Ed Engl. 2011;50:5734–5736. doi: 10.1002/anie.201101042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ichida JK, Tcw J, Williams LA, Carter AC, Shi Y, Moura MT, Ziller M, Singh S, Amabile G, Bock C, et al. Notch inhibition allows oncogene-independent generation of iPS cells. Nat Chem Biol. 2014;10:632–639. doi: 10.1038/nchembio.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Maherali N, Hochedlinger K. Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Curr Biol. 2009;19:1718–1723. doi: 10.1016/j.cub.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K, et al. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang Y, Li W, Laurent T, Ding S. Small molecules, big roles -- the chemical manipulation of stem cell fate and somatic cell reprogramming. J Cell Sci. 2012;125:5609–5620. doi: 10.1242/jcs.096032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 192.Vidal SE, Amlani B, Chen T, Tsirigos A, Stadtfeld M. Combinatorial Modulation of Signaling Pathways Reveals Cell-Type-Specific Requirements for Highly Efficient and Synchronous iPSC Reprogramming. Stem Cell Reports. 2014;3:574–584. doi: 10.1016/j.stemcr.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ho R, Papp B, Hoffman JA, Merrill BJ, Plath K. Stage-specific regulation of reprogramming to induced pluripotent stem cells by Wnt signaling and T cell factor proteins. Cell Rep. 2013;3:2113–2126. doi: 10.1016/j.celrep.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Michael D, Oren M. The p53-Mdm2 module and the ubiquitin system. Semin Cancer Biol. 2003;13:49–58. doi: 10.1016/s1044-579x(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 195.Spike BT, Wahl GM. p53, Stem Cells, and Reprogramming: Tumor Suppression beyond Guarding the Genome. Genes Cancer. 2011;2:404–419. doi: 10.1177/1947601911410224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Izpisua Belmonte JC. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, Khalil A, Rheinwald JG, Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Banito A, Rashid ST, Acosta JC, Li S, Pereira CF, Geti I, Pinho S, Silva JC, Azuara V, Walsh M, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009;23:2134–2139. doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Marion RM, Strati K, Li H, Tejera A, Schoeftner S, Ortega S, Serrano M, Blasco MA. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell. 2009;4:141–154. doi: 10.1016/j.stem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 201.Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 202.Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M, Blasco MA, Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460:1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kareta MS, Gorges LL, Hafeez S, Benayoun BA, Marro S, Zmoos AF, Cecchini MJ, Spacek D, Batista LF, O’Brien M, et al. Inhibition of pluripotency networks by the rb tumor suppressor restricts reprogramming and tumorigenesis. Cell Stem Cell. 2015;16:39–50. doi: 10.1016/j.stem.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fukawatase Y, Toyoda M, Okamura K, Nakamura K, Nakabayashi K, Takada S, Yamazaki-Inoue M, Masuda A, Nasu M, Hata K, et al. Ataxia telangiectasia derived iPS cells show preserved x-ray sensitivity and decreased chromosomal instability. Sci Rep. 2014;4:5421. doi: 10.1038/srep05421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kinoshita T, Nagamatsu G, Kosaka T, Takubo K, Hotta A, Ellis J, Suda T. Ataxia-telangiectasia mutated (ATM) deficiency decreases reprogramming efficiency and leads to genomic instability in iPS cells. Biochem Biophys Res Commun. 2011;407:321–326. doi: 10.1016/j.bbrc.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 206.Soyombo AA, Wu Y, Kolski L, Rios JJ, Rakheja D, Chen A, Kehler J, Hampel H, Coughran A, Ross TS. Analysis of induced pluripotent stem cells from a BRCA1 mutant family. Stem Cell Reports. 2013;1:336–349. doi: 10.1016/j.stemcr.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Raya A, Rodriguez-Piza I, Guenechea G, Vassena R, Navarro S, Barrero MJ, Consiglio A, Castella M, Rio P, Sleep E, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460:53–59. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rio P, Banos R, Lombardo A, Quintana-Bustamante O, Alvarez L, Garate Z, Genovese P, Almarza E, Valeri A, Diez B, et al. Targeted gene therapy and cell reprogramming in Fanconi anemia. EMBO Mol Med. 2014;6:835–848. doi: 10.15252/emmm.201303374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yung SK, Tilgner K, Ledran MH, Habibollah S, Neganova I, Singhapol C, Saretzki G, Stojkovic M, Armstrong L, Przyborski S, et al. Brief report: human pluripotent stem cell models of fanconi anemia deficiency reveal an important role for fanconi anemia proteins in cellular reprogramming and survival of hematopoietic progenitors. Stem Cells. 2013;31:1022–1029. doi: 10.1002/stem.1308. [DOI] [PubMed] [Google Scholar]
- 210.Liu GH, Suzuki K, Li M, Qu J, Montserrat N, Tarantino C, Gu Y, Yi F, Xu X, Zhang W, et al. Modelling Fanconi anemia pathogenesis and therapeutics using integration-free patient-derived iPSCs. Nat Commun. 2014;5:4330. doi: 10.1038/ncomms5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tilgner K, Neganova I, Moreno-Gimeno I, Al-Aama JY, Burks D, Yung S, Singhapol C, Saretzki G, Evans J, Gorbunova V, et al. A human iPSC model of Ligase IV deficiency reveals an important role for NHEJ-mediated-DSB repair in the survival and genomic stability of induced pluripotent stem cells and emerging haematopoietic progenitors. Cell Death Differ. 2013;20:1089–1100. doi: 10.1038/cdd.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Molina-Estevez FJ, Lozano ML, Navarro S, Torres Y, Grabundzija I, Ivics Z, Samper E, Bueren JA, Guenechea G. Brief report: impaired cell reprogramming in nonhomologous end joining deficient cells. Stem Cells. 2013;31:1726–1730. doi: 10.1002/stem.1406. [DOI] [PubMed] [Google Scholar]
- 213.Suva ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339:1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 215.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 216.Zhu S, Li W, Zhou H, Wei W, Ambasudhan R, Lin T, Kim J, Zhang K, Ding S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7:651–655. doi: 10.1016/j.stem.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 218.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6:71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 220.Stadtfeld M, Apostolou E, Ferrari F, Choi J, Walsh RM, Chen T, Ooi SS, Kim SY, Bestor TH, Shioda T, et al. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nat Genet. 2012;44:398–405. S391–S392. doi: 10.1038/ng.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu J, Okamura D, Li M, Suzuki K, Luo C, Ma L, He Y, Li Z, Benner C, Tamura I, et al. An alternative pluripotent state confers interspecies chimaeric competency. Nature. 2015;521:316–321. doi: 10.1038/nature14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Surani MA. Cellular reprogramming in pursuit of immortality. Cell Stem Cell. 2012;11:748–750. doi: 10.1016/j.stem.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 223.Gaspar-Maia A, Alajem A, Meshorer E, Ramalho-Santos M. Open chromatin in pluripotency and reprogramming. Nat Rev Mol Cell Biol. 2011;12:36–47. doi: 10.1038/nrm3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gonzalez F, Boue S, Izpisua Belmonte JC. Methods for making induced pluripotent stem cells: reprogramming a la carte. Nat Rev Genet. 2011;12:231–242. doi: 10.1038/nrg2937. [DOI] [PubMed] [Google Scholar]
- 225.Liang G, Zhang Y. Genetic and epigenetic variations in iPSCs: potential causes and implications for application. Cell Stem Cell. 2013;13:149–159. doi: 10.1016/j.stem.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sullivan GJ, Bai Y, Fletcher J, Wilmut I. Induced pluripotent stem cells: epigenetic memories and practical implications. Mol Hum Reprod. 2010;16:880–885. doi: 10.1093/molehr/gaq091. [DOI] [PubMed] [Google Scholar]
- 228.Johannesson B, Sagi I, Gore A, Paull D, Yamada M, Golan-Lev T, Li Z, LeDuc C, Shen Y, Stern S, et al. Comparable frequencies of coding mutations and loss of imprinting in human pluripotent cells derived by nuclear transfer and defined factors. Cell Stem Cell. 2014;15:634–642. doi: 10.1016/j.stem.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 229.Ma H, Morey R, O’Neil RC, He Y, Daughtry B, Schultz MD, Hariharan M, Nery JR, Castanon R, Sabatini K, et al. Abnormalities in human pluripotent cells due to reprogramming mechanisms. Nature. 2014;511:177–183. doi: 10.1038/nature13551. [DOI] [PMC free article] [PubMed] [Google Scholar]