Abstract
Although nicotine has been shown to improve attention deficits in schizophrenia, the neurobiological mechanisms underlying this effect are poorly understood. We hypothesized that nicotine would modulate attention‐associated neuronal response in schizophrenia patients in the ventral parietal cortex (VPC), hippocampus, and anterior cingulate based on previous findings in control subjects. To test this hypothesis, the present study examined response in these regions in a cohort of nonsmoking patients and healthy control subjects using an auditory selective attention task with environmental noise distractors during placebo and nicotine administration. In agreement with our hypothesis, significant diagnosis (Control vs. Patient) X drug (Placebo vs. Nicotine) interactions were observed in the VPC and hippocampus. The interaction was driven by task‐associated hyperactivity in patients (relative to healthy controls) during placebo administration, and decreased hyperactivity in patients after nicotine administration (relative to placebo). No significant interaction was observed in the anterior cingulate. Task‐associated hyperactivity of the VPC predicted poor task performance in patients during placebo. Poor task performance also predicted symptoms in patients as measured by the Brief Psychiatric Rating Scale. These results are the first to suggest that nicotine may modulate brain activity in a selective attention‐dependent manner in schizophrenia. Hum Brain Mapp 37:410–421, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: attention, fMRI, hippocampus, nicotine, schizophrenia, ventral parietal cortex
Abbreviations
- ANOVA
Analysis of variance
- BOLD
Blood oxygenation level dependent
- BP
blood pressure
- BPRS
Brief Psychiatric Rating Scale
- fMRI
Functional magnetic resonance imaging
- IR‐EPI
inversion recovery echo planar image
- MRI
Magnetic resonance imaging
- m
minutes
- NOS
not otherwise specified
- PET
positron emission tomography
- ROI
region‐of‐interest
- SPEM
smooth pursuit eye movement
- SART
sustained attention to response task
- SANS
Scale for the Assessment of Negative Symptoms
- VPC
Ventral parietal cortex
INTRODUCTION
Although pervasive positive symptoms (such as hallucinations) are necessary and sufficient to meet DSM‐5 criteria for schizophrenia [American Psychiatric Association, 2013], cognitive symptoms of the illness confer the heaviest burden on quality of life [Green, 1996]. Due in large part to significant cognitive impairment, patients suffer high rates of unemployment, homelessness, and poor everyday functioning [Torrey, 2006]. Unfortunately, no treatment has yet earned a federal indication for cognitive symptoms in schizophrenia.
One of the more striking cognitive deficits in schizophrenia is poor attention, particularly in the presence of distraction. As documented by McGhie and Chapman [1961] and later Venables [1964], patients commonly report being unable to ignore distracting noises in the environment, such as a fan whirring, clocking ticking, or traffic on a busy street. Although the neurobiological mechanisms are unclear, one hypothesis postulates that a reduction in inhibitory neuronal tone driven by the loss of nicotinic receptors on interneurons in schizophrenia patients impairs the ability of the brain to attenuate (or “gate”) response to repeated stimuli, thereby increasing distractibility [Miwa et al., 2011]. Evidence for a key role for the loss of these receptors in gating deficits in schizophrenia includes reduced expression of the α7 receptor subtype in the hippocampus and thalamus postmortem in patients [Court et al., 1999; Freedman et al., 1995], associations between the gating deficit, schizophrenia, and polymorphisms in the α7 receptor gene [Leonard et al., 2002], and high rates of smoking in the illness as a potential form of “self‐medication” [Winterer, 2010]. Mechanisms by which to restore normal function of the nicotinic system (e.g. α7 agonists) is a primary motivating factor behind the development of nicotinic potentiating agents designed to restore normal levels of nicotinic signaling and improve cognition in schizophrenia [Freedman, 2014].
Human functional magnetic resonance imaging (fMRI) studies have supported the hypothesis that hyperactivity (relative to healthy subjects), particularly of the hippocampus, may contribute to sensory flooding in schizophrenia. Hippocampal hyperactivity is often observed during simple sensory processing tasks, such as smooth pursuit eye movement (SPEM) [Tregellas et al., 2004], fixating on a point [Malaspina et al., 1999], watching faces [Holt et al., 2005], listening to repeated clicks [Tregellas et al., 2007], and listening to environmental noise [Tregellas et al., 2009]. Resting hippocampal hyperactivity also predicts cognitive deficits in schizophrenia, including attention deficits [Tregellas et al., 2014]. Interestingly, both nicotine and a nicotinic α7 receptor partial agonist reduce hippocampal hyperactivity in schizophrenia patients during SPEM [Tanabe et al., 2006; Tregellas et al., 2010; Tregellas et al., 2005] well as improve performance during sustained attention [Freedman et al., 2008; Harris et al., 2004]. The hippocampus is also hypothesized to be involved in attentional selection of memory encoding, and attention‐dependent engagement of the region may explain why important (novel, salient, and/or arousing) stimuli are better remembered than unimportant (redundant, distracting, and/or not arousing) stimuli [Chun and Turk‐Browne, 2007; Santangelo, 2015; Uncapher and Rugg, 2009].
Although nicotine has demonstrated efficacy as an attention enhancer in schizophrenia, the underlying neurobiological mechanisms remain poorly understood. Indeed, to our knowledge only one previous functional imaging study has examined the effects of nicotine during attention in schizophrenia patients. In that study, Hong et al. [2011] observed no significant task‐specific functional effects after acute administration of 21–35 mg of nicotine in smoking patients during a sustained attention task. No study, however, has yet examined the neuronal effects of nicotine during an attention task that uses distracting stimuli in schizophrenia. Filling in this knowledge gap is particularly important given that distractibility is a characteristic feature of attentional dysfunction in schizophrenia.
Our laboratory has previously developed an auditory attention task that requires subjects to respond (button press) to target auditory stimuli in the presence of distracting environmental noise [Smucny et al., 2013a, 2015]. We have examined the task‐associated effects of nicotine on this task in nonsmoking healthy subjects [Smucny et al., 2015], and found task‐dependent effects of nicotine in the ventral parietal cortex (VPC), hippocampus, and ventral anterior cingulate. Attention task‐associated nicotinic modulation of the VPC and ventral anterior cingulate is consistent with previous studies suggesting these regions are important for stimulus‐driven, “bottom up” processing and ignoring distraction, respectively [Corbetta and Shulman, 2002; Weissman et al., 2005; Weissman et al., 2004]. Functional modulation of the hippocampus were also not surprising based on its responsiveness to auditory stimuli [Kumar et al., 2014), role in auditory gating/filtering [Grunwald et al., 2003], and ability to undergo adapt different functional states in an attention‐dependent manner [Aly and Turk‐Browne, in press; Colgin et al., 2009; Klyachko and Stevens, 2006].
Based on these findings, we hypothesized that nicotine would alter response in these regions during our attention task in schizophrenia. We were particularly interested in nicotinic modulation of the hippocampus due to recent studies and reviews suggesting that pathology and hyperactivity of the area may be a key biological target for therapeutic intervention in schizophrenia [Heckers and Konradi, 2015; Schobel et al., 2013; Schobel et al., 2009; Tamminga et al., 2012; Tamminga et al., 2010; Tregellas, 2014; Tregellas et al., 2014]. The goal of this study, therefore, was to compare nicotine (vs. placebo) effects during an attention task in control subjects and schizophrenia patients. Only nonsmoking patients were recruited in order to maximize non‐psychopathological between‐group homogeneity. We also examined behavioral and clinical correlates as exploratory outcome measures.
MATERIALS AND METHODS
Subjects
Thirty‐seven subjects participated in this study—17 stable outpatients who had a primary diagnosis of schizophrenia and 20 healthy comparison subjects. Demographic and clinical information for participants was assessed by interview and is shown in Table 1. No significant group differences in age, gender, handedness, or ratio of never smokers/former smokers (> 3 months from last cigarette) were observed. No subjects were taking smoking cessation medication (e.g. varenicline) at the time of the study. Patients were recruited by referral from a University of Colorado psychiatrist. Patients were excluded for a diagnosis of neurological illness, head trauma, current smoking (< 3 months from last cigarette) or substance abuse, poor (inability to hear 60 dB sound pressure level 1000 and 1500 Hz tones in either ear) or unbalanced (> 10 dB threshold difference between each ear) hearing, failure to pass a physical examination, and magnetic resonance imaging (MRI) exclusion criteria (claustrophobia, weight > 250 lbs, metal in the body). Control subjects were excluded for all of the above as well as a diagnosis of Axis I mental illness or first‐degree family history of Axis I mental illness. Patients were medication stable (> 3 mo. with no change in medication). Schizophrenia patient comorbidities included six subjects with depression not otherwise specified (NOS), three with bipolar NOS, one with posttraumatic stress disorder, four with panic disorder, one with social phobia, two with generalized anxiety disorder, and one with obsessive compulsive disorder. Three patients had a history of alcohol abuse, one of cannabis dependence, and one of cocaine dependence. None of these patients, however, were substance dependent during or for at least 6 months prior to beginning the study (confirmed by urinalysis). All subjects were required to pass a nicotine tolerance test, in which the nicotine dose used for the experiment (7 mg) was administered > 3 d prior to the first fMRI scan. Criteria for passing the tolerance test were 1) less than a 20% change in heart rate or blood pressure (BP) for up to 90 minutes (m) post patch‐application, 2) no side effects other than mild/minor nausea, headache, lightheadness, buzz, clouded thinking, anxiety, or mouth tingling. All participants provided written informed consent in accordance with the principles of the Declaration of Helsinki and could withdraw from the study at any time. Subjects were compensated for participation. The Colorado Multiple Institutional Review Board approved the study.
Table 1.
Demographic and clinical data of participants
| Controls | Schizophrenia | |
|---|---|---|
| Age | 38.4 (12) | 44 (12) |
| Gender (M/F) | 11/9 | 12/5 |
| Smoking (never/former smokers) | 15/5 | 10/7 |
| Average total BPRS | 36.6 (7.7) | |
| Average total SANS | 4.2 (2.9) | |
| Meds: Typ/ATyp/Both/None | 1/15/0/1 |
Parentheses contain the standard deviation.
Abbreviations: BPRS, brief psychiatric rating scale; SANS; scale for the assessment of negative symptoms; Typ, # treated with typical antipsychotic medications; ATyp, # treated with atypical antipsychotic medications.
Study Design
This was a single‐blind, pseudo‐randomized, placebo‐controlled, crossover study. On each of two study visits, subjects were administered a 7 mg nicotine patch (Nicoderm) or a placebo patch (made in‐house) 70 m prior to MRI scanning. The order of study visits (placebo or nicotine) was counterbalanced across subjects. Subjects wore patches throughout scanning. Total time of patch application was approximately 120 m (70 m before scanning, 60 m during scanning). The attention task was performed approximately 10 m after the subject was placed in the scanner (∼80 m after patch application); the delay was because of localizer, high‐order shimming, and anatomical scans that preceded the functional scan. The 80 m latent period was used such that the attention task occurred during a time window corresponding to the peak plasma concentration of nicotine [Dempsey et al., 2013]. Based on previous work, the expected nicotine concentration during this period is expected to be approximately 4 ng/ml [Dempsey et al., 2013]. The placebo patch was tactilely similar to the nicotine patch and was affixed to the skin (upper arm) in the same manner as the nicotine patch. Subjects were asked to refrain from examining either patch during or after application as the placebo and drug patches were not visually identical. Furthermore, clothing covered patches such that they could not be readily observed after affixation. Patches were removed immediately after scanning. Visits were scheduled > 3 d apart. Heart rate and BP were monitored immediately prior to patch application, 30 and 60 m after patch application, and up to 60 m after patch removal. Physiological effects of nicotine were analyzed using a mixed‐effects model analysis of variance (ANOVA) in SPSS22, with time (pretreatment vs. posttreatment) and drug (placebo vs. nicotine) as within‐subjects factors and diagnosis (control vs. patient) as a between‐subjects factor.
Auditory Stimuli
For the attention task (see “Task Description”), synthetic audio recordings for the numbers 1–9 were downloaded from http://www.modeltalker.com. Number stimuli were adjusted to have the same onset with Adobe Audition.
For task‐overlaid noise distraction, environmental, “urban” noise stimuli were mixed as described previously [Tregellas et al., 2009]. Briefly, clips included segments from two talk radio shows, two classical musical pieces, sounds from a neighborhood block party, which included multiple background conversations and sounds from children playing, traffic sounds, a refrigerator motor cycling on and off, and frequent knocking sounds from glasses being set on countertops. Volumes of all of these elements were mixed so that no one element was readily identifiable. The subjective experience of the sound mixture was that of standing in a busy crowd of people, in which multiple conversations were occurring, with a low level of indistinguishable background music and other sounds. Urban noise distraction was presented at 80dB in the ear opposite the task‐relevant stimuli with MR‐compatible headphones (Resonance Technologies, Inc.).
Task Description
Subjects performed an auditory version of the Sustained Attention to Response Task (SART) [Seli et al., 2012]. For the SART, single‐digit numbers were aurally presented one at a time, and the subject was asked to respond (with a button press) (Lumina Response Pad, Cedrus Corp.) after each auditory stimulus (70 dB, presented in either the right or left ear), except for the number ‘3,’ in which case the subject was asked to withhold from responding. Subjects used their dominant hand for motor responses. The ear (right or left) in which the numbers were presented was pseudo‐randomized between subjects. Stimulus duration was 250 ms and interstimulus interval was 900 ms. Subjects performed two variations of the SART, the Ordered SART, and the Random SART. In the Ordered SART, the numbers were presented in order; in the Random SART, the numbers were presented pseudo‐randomly. Due to the predictability of Ordered SART, subjects may be able to correctly respond or withhold responding reflexively to the presence of any auditory stimulus. The unpredictability of Random SART, however, requires subjects to focus on specific stimulus features before making the appropriate response, increasing attentional demands [Smucny et al., 2013b]. The current SART variation (Ordered or Random) was highlighted and visually presented through MR‐compatible goggles (Resonance Technologies, Inc.) throughout the experiment. The identifier cue was presented 2.3 s before the first set of stimuli, as well 2.3 s before each time the condition switched from Ordered to Random (or vice‐versa). The subject was asked to respond as quickly and accurately as possible to help induce attentiveness.
The SART was presented as a block design, with four pseudo‐randomly dispersed conditions: Ordered‐Silent (ordered numbers with no noise distraction), Ordered‐Noisy (ordered numbers with noise distraction), Random‐Silent (random numbers with no noise distraction), and Random‐Noisy (random numbers with noise distraction). Seventy‐two blocks of 12.65 s each were administered, with 18 blocks per condition. Each block consisted of 9‐11 trials. Baseline data was collected from six 37.95 s fixation periods interspersed at regular intervals throughout the experiment. Total task duration was 18 m.
Recorded performance measures on the SART were 1) errors of commission, or incorrect button presses on ‘3’, 2) errors of omission, or failure to button press on the numbers 1, 2, and 4–9, and 3) reaction time. Percent correct responses were calculated as 100 – (percent errors commission + percent errors of omission). As a combination of all these measures provides a more accurate assessment of performance than each individual measure [Seli et al., 2013], they were combined into a single measure, “efficiency,” based on a previous SART study in schizophrenia [Chan et al., 2009]. Specifically, efficiency was defined as arcsin (√ (Percent Correct Responses/Reaction Time for Correct Responses)). Efficiency data were analyzed by mixed‐effects ANOVA in SPSS22 with drug (placebo vs. nicotine), SART difficulty (Ordered vs. Random) and distraction level (Silent vs. Noisy) as within‐subjects factors and diagnosis (Control vs. Patient) as a between‐subjects factor.
fMRI Scanning Parameters
Functional scans were collected using a clustered volume approach as described previously [Smucny et al., 2013b, 2013c]. Use of the clustered volume approach allowed stimuli to be presented while minimizing scanner noise. This technique has been shown to substantially improve signal detection in fMRI experiments using auditory stimuli, despite reducing the overall number of scans collected per experimental condition [Edmister et al., 1999]. We have previously used clustered volume acquisition in a number of auditory tasks in schizophrenia, including the SART [Smucny et al., 2013b,c,2015; Tregellas et al., 2007; Tregellas et al., 2009; Tregellas et al., 2012].
Studies were performed with at 3T GE Signa MR system using a standard quadrature head coil. Functional images were acquired with a gradient‐echo T2* Blood Oxygenation Level Dependent (BOLD) contrast technique, with TR = 12650 ms (as a clustered volume acquisition of 2000 ms, plus an additional 10650 ms silence interval), TE = 30 ms, FOV = 220 mm2, 642 matrix, 38 slices, 3.5 mm thick, 0.5 mm gap, angled parallel to the planum sphenoidale. Additionally, one inversion recovery echo planar image (IR‐EPI) (TI = 505 ms) volume was acquired to improve spatial normalization (see “fMRI Preprocessing”).
fMRI Preprocessing
Data were preprocessed using SPM8 (Wellcome Dept. of Imaging Neuroscience, London). Data from each subject were realigned to the first volume, normalized to the Montreal Neurological Institute template using the IR‐EPI as an intermediate to improve coregistration between images, and smoothed with an 8 mm FWHM Gaussian kernel.
Region‐of‐Interest (ROI) Analysis of BOLD Response
To account for both within‐group and within‐subject variance, a mixed effects analysis was implemented. Parameter estimates were generated for each individual in a first‐level analysis. First‐level effects were modeled with a double‐gamma function, without temporal derivatives, using the general linear model in SPM8. A 196 s high pass filter was applied to remove low‐frequency fluctuation in the BOLD signal. Rigid‐body movement parameters (up/down, left/right, forward/back, roll, pitch, yaw) were entered into the SPM8 design matrix as covariates of no interest. No significant effect of diagnosis, drug treatment, or drug X diagnosis interaction was observed for overall movement. “Task‐associated” contrast images were generated for each drug treatment condition (placebo and nicotine). “Task‐associated” response was defined as ((Random Noisy > Random Silent) > (Ordered Noisy > Ordered Silent)). Fixation periods were used as an implicit baseline.
A priori hypotheses were tested for response in three ROIs, the VPC, hippocampus, and anterior cingulate. The VPC, hippocampal, and anterior cingulate ROIs consisted of the supramarginal gyrus, hippocampus, and anterior cingulate AAL delineations in WFU Pickatlas [Maldjian et al., 2003] respectively. Mean task‐associated signal within each ROI was extracted for each subject using the Marsbar toolbox [Brett et al., 2002] and entered into SPS22 for ANOVA analysis. The primary contrast of interest, the drug (placebo vs. nicotine) X diagnosis (patient vs. control) interaction, was evaluated separately for each ROI, with drug as a within‐subjects factor and diagnosis as a between‐subjects factor. Significant interaction effects (omnibus F contrasts) were followed up by post‐hoc one‐tailed t‐tests in order to describe the directionality of effects.
RESULTS
Physiological Effects of Nicotine
Physiological effects of placebo vs. nicotine treatment are presented in Table 2. Physiological data were not available from one control subject due to an equipment malfunction. No significant time × drug × diagnosis interactions were observed on systolic BP (F(1,34) = 0.60, P = 0.44), diastolic BP (F(1,34) = 1.58, P = 0.22), or heart rate (F(1,34) = 0.063, P = 0.80). Across all subjects, no significant time (pretreatment vs. 60 m post‐treatment) × drug interactions were observed for systolic BP (F(1,34) = 2.84, P = 0.10), diastolic BP (F(1,34) = 0.070, P = 0.79), or heart rate F(1,34) = 4.07, P = 0.052).
Table 2.
Physiological effects of nicotine and placebo patch
| Group | Tx | Placebo | Nicotine | ||||
|---|---|---|---|---|---|---|---|
| Measure | Pretreatment | 60 m posttreatment | Δ | Pretreatment | 60 m posttreatment | Δ | |
| Controls | Systolic BP (mmHg) | 128 (4) | 121 (3) | −7 (3) | 127 (3) | 125 (2) | −2 (2) |
| Diastolic BP (mmHg) | 79 (2) | 77 (2) | −2 (2) | 79 (2) | 79 (2) | 0 (2) | |
| Heart Rate (bpm) | 75 (3) | 73 (3) | −2 (2) | 76 (3) | 77 (3) | 1 (2) | |
| Schizophrenia patients | Systolic BP (mmHg) | 135 (4) | 130 (4) | −5 (5) | 128 (4) | 125 (3) | −3 (2) |
| Diastolic BP (mmHg) | 79 (2) | 79 (3) | 0 (2) | 80 (2) | 78 (2) | −2 (2) | |
| Heart Rate (bpm) | 81 (4) | 81 (4) | 0 (2) | 84 (4) | 87 (4) | 3 (2) | |
Parentheses contain the standard error.
Abbreviations: BP, blood pressure; mmHg, mm of mercury; bpm, beats per minute
Behavioral Data
The primary behavioral measure of interest in this study was performance efficiency, a single metric that combines accuracy and reaction time (see Methods). Efficiency data for each SART condition (Ordered Silent, Ordered Noisy, Random Silent, Random Noisy) is presented in Table 3. Using this measure, significant main effects of difficulty (Ordered vs. Random; F(1,35) = 46.4, P < 0.001) and distraction level (Silent vs. Noisy; F(1,35) = 17.2, P < 0.001) were observed, indicative of decreased efficiency during the Random condition and Noisy condition relative to the Ordered and Silent conditions, respectively. No significant interactions were observed between SART condition and diagnosis (Control vs. Patient) or drug (Placebo vs. Nicotine).
Table 3.
Performance efficiency for each SART condition
| Condition | Controls | Schizophrenia patients | ||
|---|---|---|---|---|
| Placebo | Nicotine | Placebo | Nicotine | |
| Ordered Silent | 0.51 (0.018) | 0.52 (0.020) | 0.51 (0.025) | 0.51 (0.027) |
| Ordered Noisy | 0.50 (0.017) | 0.50 (0.021) | 0.48 (0.033) | 0.50 (0.026) |
| Random Silent | 0.44 (0.0076) | 0.44 (0.0080) | 0.43 (0.012) | 0.44 (0.015) |
| Random Noisy | 0.43 (0.0089) | 0.42 (0.010) | 0.39 (0.022) | 0.42 (0.016) |
Parentheses contain the standard error. Significant main effects of noise (Noisy > Silent) and difficulty (Random > Ordered) were observed (see Results), suggesting that these manipulations decrease behavioral performance.
Base behavioral measures (errors of commission, omission, and reaction times) are presented in Supporting Information Table I.
ROI Analysis of BOLD Response
The observed behavioral results suggest that attentional load is greater when number stimuli are random (relative to ordered) as well as during distracting noise (relative to silence). Therefore, we defined task‐associated BOLD signal effects as the signal resulting from the contrast ((Random Noisy > Random Silent) > (Ordered Noisy > Ordered Silent)).
Using mean task‐associated BOLD signal within anatomically defined ROIs as the primary measures of interest (see Methods), significant diagnosis × drug interactions were observed in the left VPC (F(1,35) = 6.98, P = 0.012) (Fig. 1) and left hippocampus (F(1,35) = 4.70, P = 0.037) (Fig. 2) but not the right VPC (F(1,35) = 2.17, P = 0.15), right hippocampus (F(1,35) = 0.31, P = 0.58), or anterior cingulate (F(1,35) = 0.050, P = 0.82).
Figure 1.
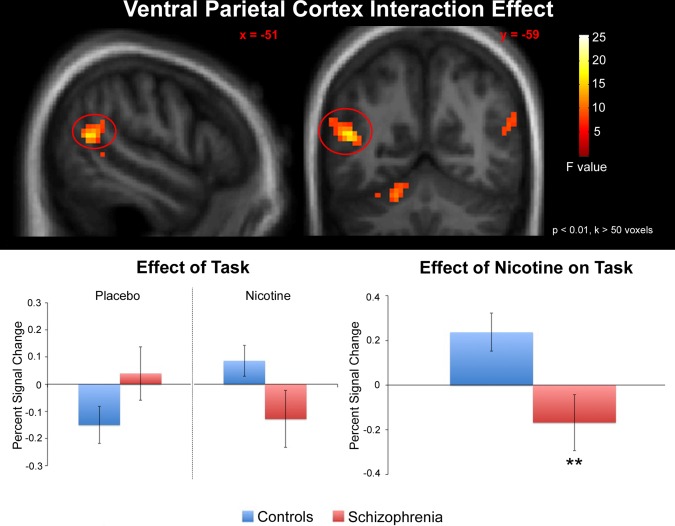
Effects of nicotine on task‐associated (see Methods for task definition) neuronal response in the left VPC. A significant drug X diagnosis interaction was observed. For visualization, statistical parametric maps are displayed in the neurologic convention (R on R) and thresholded at P < 0.01, cluster extent (k) > 50 voxels. Error bars represent the standard error. **Significant diagnosis X treatment interaction. Hum Brain Mapp 00:000–000, 2014. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 2.
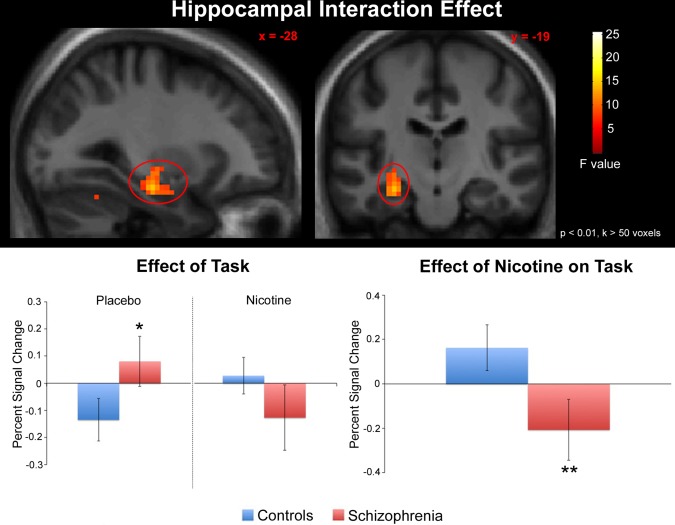
Effects of nicotine on task‐associated (see Methods for task definition) neuronal response in the left hippocampus. A significant drug X diagnosis interaction was observed. For visualization, statistical parametric maps are displayed in the neurologic convention (R on R) and thresholded at P < 0.01, cluster extent (k) > 50 voxels. Error bars represent the standard error. *Significantly increased response in patients relative to controls under placebo conditions. **Significant diagnosis X treatment interaction. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In the left VPC (Fig. 1), post‐hoc tests revealed increased response in controls after nicotine administration (relative to placebo) (P = 0.015) and a trend towards decreased response in patients after nicotine administration (relative to placebo) (P = 0.073). Between‐group comparisons also identified a trend towards greater response in patients (relative to controls) under placebo (P = 0.058) and reduced response in patients (relative to controls) under nicotine (P = 0.036).
In the left hippocampus (Fig. 2), post‐hoc tests revealed a trend towards increased response in controls after nicotine administration (relative to placebo) (P = 0.084) and a trend towards decreased response in patients after nicotine administration (relative to placebo) (P = 0.053). Between‐group comparisons showed greater response in patients (relative to controls) under placebo (P = 0.044) and no difference in response in patients (relative to controls) under nicotine (P = 0.12).
No significant correlations were observed between nicotinic effects on blood pressure or heart rate and effects on response in any ROI. Task associated‐response during placebo did not predict the magnitude of nicotinic effects for either group.
Behavioral Correlates
We examined correlations between task‐associated change in efficiency (ΔEff) and BOLD response in each ROI. ΔEff was calculated based on the efficiencies for each SART condition using the contrast ((Random > Ordered) > (Noisy > Silent)), consistent with the previously defined measure for task‐associated BOLD response. ΔEff therefore represents the change in performance due to increasing the task difficulty (from Ordered to Random) and distraction level (from Silent to Noisy).
In patients, ΔEff was negatively correlated with task‐associated left VPC response (r = −0.54, P = 0.026) (Fig. 3) as well as task‐associated anterior cingulate response (r = −0.59, P = 0.013).
Figure 3.
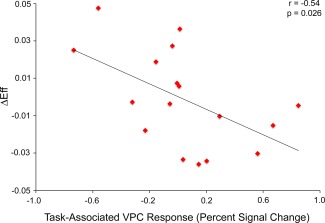
Negative correlation between task‐associated VPC response and task‐associated change in performance efficiency (see Results) in patients. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Clinical Correlates
In patients, ΔEff during placebo administration was significantly associated with total Brief Psychiatric Rating Scale (BPRS) score (r = 0.54, P = 0.027) (Fig. 4).
Figure 4.
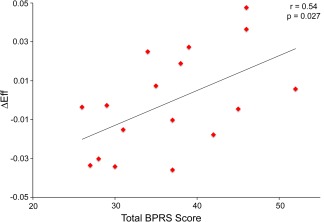
Positive correlation between total BPRS score and task‐associated change in performance efficiency (see Results) in patients. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The primary finding of this study was that significant diagnosis × drug interactions were observed on task‐associated response in the VPC and hippocampus. These effects were driven by (1) relative hyperactivity of these regions in patients during the placebo condition, and (2) decreased response in patients after nicotine administration. Poor task‐associated performance was also associated with VPC hyperactivity and overall symptomatology in the patient group.
Attention task‐associated nicotinic modulation of the VPC is in agreement with its demonstrated role in stimulus‐driven attention (“bottom‐up” attention) [Corbetta and Shulman, 2002]. The region is prominently recruited during attention tasks in which stimuli occur unexpectedly, i.e. interpreted as novel [Vossel et al., 2014]. In the context of the present study, hyperactivity in this region in patients under placebo conditions may represent increased noise‐associated processing and/or attentional load due to the noise being neurobiologically “interpreted” as “novel” throughout the task. Indeed, we have previously reported that patients who listen to the noise may find the voices within the recordings continuously bothersome [Tregellas et al., 2009]. A second possibility is that VPC activity is a reflection of task difficulty. The finding that hyperactivity predicted poor task‐associated performance in patients supports this hypothesis, although it is incongruent with the observation that nicotine increased VPC activity in controls without affecting performance. The observed correlation between VPC activity and performance also suggests that patients that show VPC hyperactivity may be particularly behaviorally sensitive to distraction.
The second region that showed significant task‐associated effects of nicotine was the hippocampus. Although classically defined as a region important for learning and memory, previous neuroimaging studies have demonstrated attention‐dependent modulation of the region [Aly and Turk‐Browne, in press; Uncapher and Rugg, 2009]. Patterns of hippocampal activity may thereby be a reflection of attention level [Aly and Turk‐Browne, in press], and help explain why attended stimuli are better remembered than unattended stimuli [Chun and Turk‐Browne, 2007]. In the context of the present study, one interpretation of the present findings is that in schizophrenia, the hippocampus is less able to alter its overall pattern of response (e.g. response amplitude) in order to filter out the distracting noise, inducing hyperactivity. Whether hippocampal hyperactivity is the result of poor stimulus filtering within the region itself and/or the consequence of an abnormal attentional process occurring elsewhere in the brain (e.g. in the VPC) is unclear. Regardless of the underlying causes of hippocampal hyperactivity, the present findings also suggest that nicotine may be able to normalize this phenotype, restoring task‐associated activity to levels in controls during placebo administration (Fig. 2). Nicotinic reduction of hippocampal hyperactivity may be due in part to the ability of the drug to activate nicotinic receptors on inhibitory interneurons in the region [Frazier et al., 1998].
One of the more striking features of schizophrenia is the high rate of smoking (70% or more) in the illness [Winterer, 2010]. Patients also consume more nicotine per cigarette and smoke more cigarettes per day than healthy smokers [Olincy et al., 1997; Williams et al., 2010]. Although patients smoke for many possible reasons [Winterer, 2010], an important contributing factor may be the ability of nicotine and other nicotinic agonists to restore sensory gating (e.g. P50 gating) deficits [Adler et al., 1993; Adler et al., 1992; Olincy et al., 2006; Zhang et al., 2012]. P50 gating is measured electrophysiologically as the difference (or ratio) of the 50 ms post‐stimulus neuronal response to the second of a pair of repeated identical click sounds, and is postulated to be an index of neuronal sensory inhibition [Miwa et al., 2011]. Loss of inhibition may in turn cause patients to be flooded with sensory information, leading to distractibility and predicting poor performance on attention tasks [Cullum et al., 1993; Smucny et al., 2013a]. Importantly, these deficits have been mapped to the hippocampus (among other areas) [Bak et al., 2014; Grunwald et al., 2003]. It is possible that the observed reduction in task‐associated response is related to the ability of nicotine to improve P50 gating. The nature of this association may be examined in future studies.
Interestingly, nicotine induced largely opposite task‐associated neuronal effects in control subjects compared to patients. Specifically, nicotine increased response of the VPC and hippocampus in controls, while decreasing response in patients. These results are similar to the direction of findings in P50 gating (paired‐click) studies, which show that nicotine decreases response to the second stimulus and improves gating in patients [Adler et al., 1993], and increases overall response to stimuli in healthy subjects that gate normally [Knott et al., 2010]. Pharmacologically, nicotine may be expected to have both excitatory and inhibitory effects. For example, in the hippocampus nicotine binds to both high‐affinity α4β2 receptors on excitatory pyramidal neurons as well as low‐affinity α7 receptors on inhibitory interneurons [Frazier et al., 1998; Papke, 2014]. The result that nicotine primarily has an inhibitory task‐associated effect in schizophrenia could be because of several factors, including (1) high activity at baseline in patients, (2) antipsychotic blockade of dopaminergic signaling occluding [Baskys et al., 1993] or otherwise reducing the excitatory effects [Medoff et al., 2001] of nicotine, and (3) differential expression of nicotinic receptor subtypes in nonsmoking patients vs. control subjects [Freedman et al., 1995; Mexal et al., 2010]. The first possibility is less likely as no correlations were observed between hyperactivity at baseline and the magnitude of nicotinic effects on task‐associated response. The second possibility may be examined in future studies that compare the effects of nicotine in unmedicated patients or at‐risk populations. Finally, positron emission tomography (PET) studies that examine nicotinic receptor availability may be used to determine the relationship between expression level and nicotinic effects on attention‐related processing.
Surprisingly, no drug × diagnosis interaction was observed in the anterior cingulate. As a highly significant correlation was observed between task performance and anterior cingulate response, however, this negative result may be because of the fact that patients and controls performed similarly during all task conditions (Table 3).
Our laboratory has recently published results from a study in which we examined neuronal response in schizophrenia during the SART using visual number stimuli with auditory noise distractors [Smucny et al., 2013b]. Interestingly, the nature response differences between patients and controls differed between the 2013 study and the present experiment. Specifically, in the previous study patients showed task‐associated hypoactivity of the VPC and hippocampus compared with hyperactivity in the present study. The discrepancy between these findings is not unexpected considering the fact that both decreased response [Carter et al., 2010; Tregellas et al., 2012; Weiss et al., 2007] and increased response [Carter et al., 2010; Weiss et al., 2003] have been previously observed during attention tasks in schizophrenia. One possible explanation for the observed discrepancy is that healthy subjects and schizophrenia patients may differ in how the brain adjusts its activity in response to attentional load. Specifically, a linear, positive relationship has been observed between load and activity in controls, whereas an inverted U‐shaped relationship has been observed in patients [Blasi et al., 2010]. It is possible that the use of cross‐modal vs. unimodal distractors represents a difference in task load that is reflected as relative hypoactivation and hyperactivation (respectively) in schizophrenia.
Some limitations of the present study should be noted. A single‐blind design was used as the nicotine and placebo patches were not visually identical and therefore it was impractical to blind the experimenter to the treatment. For this reason, subjects were instructed to refrain from examining the patches during the study. Furthermore, nicotine has known physiological effects (including those not measured in this study) that may reduce the effectiveness of the blind [Benowitz, 1998]. Patients were frequently comorbid for other psychiatric disorders, although high rates of comorbidity in schizophrenia [Buckley et al., 2009] suggests that our study remains “representative” of the nonsmoking patient population. Finally, the sample size was relatively small (n = 20 controls, 17 patients). Future studies may also examine the differential effects of nicotine in smokers vs. nonsmokers and/or former smokers.
Although the nicotinic receptor is one of the most promising therapeutic targets in schizophrenia, clinical trials using nicotinic agonists have shown mixed results in the illness [Deutsch et al., 2013; Freedman, 2014]. A potential method for improving the probability of success of these and other agents is to examine their effects on brain function using techniques such as fMRI in order to verify that a drug is having its intended biological effect [Tregellas, 2014]. Furthermore, fMRI‐based measures are often more sensitive than behavioral performance in measuring drug effects [Newhouse et al., 2011]. Therefore, fMRI validation of drug targeting is unlikely to require the large sample sizes necessary in late‐phase clinical trials. Our lab has previously reported that an α7 nicotinic receptor partial agonist may target localized functional abnormalities related to tasks such as SPEM (Tregellas et al., 2010) as well as in task‐nonspecific resting networks in schizophrenia [Tregellas et al., 2011]. Other nicotinic receptor signal‐promoting agents have also been shown to modulate brain activity on a network level [Smucny and Tregellas, 2013]. This study expands upon these findings by showing that regions involved in attention may be pharmacologically targeted in a task‐dependent manner and supports further investigation into the functional neurocognitive effects of other nicotinic‐based compounds in schizophrenia.
Supporting information
Supporting Information Table 1.
ACKNOWLEDGMENTS
The authors thank Debra Singel for assistance with data acquisition. The authors declare no competing financial interests in relation to this work.
REFERENCES
- Adler LE, Hoffer LD, Wiser A, Freedman R (1993): Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 150:1856–1861. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R (1992): Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry 32:607–616. [DOI] [PubMed] [Google Scholar]
- Aly, M , Turk‐Browne, NB : Attention Stabilizes Representations in the Human Hippocampus. Cereb Cortex (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013): Diagnostic and statistical manual of mental disorders. Washington, D.C.
- Bak N, Rostrup E, Larsson HB, Glenthoj BY, Oranje B (2014): Concurrent functional magnetic resonance imaging and electroencephalography assessment of sensory gating in schizophrenia. Hum Brain Mapp 35:3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskys A, Wang S, Remington G, Wojtowicz JM (1993): Haloperidol and loxapine but not clozapine increase synaptic responses in the hippocampus. Eur J Pharmacol 235:305–307. [DOI] [PubMed] [Google Scholar]
- Benowitz NL (1998): Nicotine Safety and Toxicity. New York, NY: Oxford University Press. [Google Scholar]
- Blasi G, Taurisano P, Papazacharias A, Caforio G, Romano R, Lobianco L, Fazio L, Di Giorgio A, Latorre V, Sambataro F, Popolizio T, Nardini M, Mattay VS, Weinberger DR, Bertolino A (2010): Nonlinear response of the anterior cingulate and prefrontal cortex in schizophrenia as a function of variable attentional control. Cereb Cortex 20:837–845. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB (2002): Region‐of‐interest analysis using an SPM toolbox. Neuroimage, 8th International Conference on Functional Mapping of the Human Brain, Aurora, Colorado. [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ (2009): Psychiatric comorbidities and schizophrenia. Schizophr Bull 35:383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JD, Bizzell J, Kim C, Bellion C, Carpenter KL, Dichter G, Belger A (2010): Attention deficits in schizophrenia–preliminary evidence of dissociable transient and sustained deficits. Schizophr Res 122:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RC, Wang Y, Cheung EF, Cui J, Deng Y, Yuan Y, Ma Z, Yu X, Li Z, Gong Q (2009): Sustained attention deficit along the psychosis proneness continuum: a study on the Sustained Attention to Response Task (SART). Cogn Behav Neurol 22:180–185. [DOI] [PubMed] [Google Scholar]
- Chun MM, Turk‐Browne NB (2007): Interactions between attention and memory. Curr Opin Neurobiol 17:177–184. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI (2009): Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462:353–357. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, Kerwin R, Perry R, Perry E (1999): Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha‐bungarotoxin and nicotine binding in the thalamus. J Neurochem 73:1590–1597. [DOI] [PubMed] [Google Scholar]
- Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, Griffith J, Adler LE, Freedman R (1993): Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr Res 10:131–141. [DOI] [PubMed] [Google Scholar]
- Dempsey DA, St Helen G, Jacob P, 3rd , Tyndale RF Benowitz NL (2013): Genetic and pharmacokinetic determinants of response to transdermal nicotine in white, black, and Asian nonsmokers. Clin Pharmacol Ther 94:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch SI, Schwartz BL, Schooler NR, Brown CH, Rosse RB, Rosse SM (2013): Targeting alpha‐7 nicotinic neurotransmission in schizophrenia: a novel agonist strategy. Schizophr Res 148:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM (1999): Improved auditory cortex imaging using clustered volume acquisitions. Hum Brain Mapp 7:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV (1998): Acetylcholine activates an alpha‐bungarotoxin‐sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci 18:1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R (2014): alpha7‐nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu Rev Med 65:245–261. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S (1995): Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 38:22–33. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman‐Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR (2008): Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry 165:1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF (1996): What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 153:321–330. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Boutros NN, Pezer N, von Oertzen J, Fernandez G, Schaller C, Elger CE (2003): Neuronal substrates of sensory gating within the human brain. Biol Psychiatry 53:511–519. [DOI] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R (2004): Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology 29:1378–1385. [DOI] [PubMed] [Google Scholar]
- Heckers, S , Konradi, C (2015): GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res 167:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Weiss AP, Rauch SL, Wright CI, Zalesak M, Goff DC, Ditman T, Welsh RC, Heckers S (2005): Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry 57:1011–1019. [DOI] [PubMed] [Google Scholar]
- Hong LE, Schroeder M, Ross TJ, Buchholz B, Salmeron BJ, Wonodi I, Thaker GK, Stein EA (2011): Nicotine enhances but does not normalize visual sustained attention and the associated brain network in schizophrenia. Schizophr Bull 37:416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko VA, Stevens CF (2006): Excitatory and feed‐forward inhibitory hippocampal synapses work synergistically as an adaptive filter of natural spike trains. PLoS Biol 4:e207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott VJ, Fisher DJ, Millar AM (2010): Differential effects of nicotine on P50 amplitude, its gating, and their neural sources in low and high suppressors. Neuroscience 170:816–826. [DOI] [PubMed] [Google Scholar]
- Kumar S, Bonnici HM, Teki S, Agus TR, Pressnitzer D, Maguire EA, Griffiths TD (2014): Representations of specific acoustic patterns in the auditory cortex and hippocampus. Proc Biol Sci 281:20141000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, Zerbe G, Olincy A, Ross RG, Adler LE, Freedman R (2002): Association of promoter variants in the alpha7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry 59:1085–1096. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Storer S, Furman V, Esser P, Printz D, Berman A, Lignelli A, Gorman J, Van Heertum R (1999): SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry 46:89–93. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J (1961): Disorders of attention and perception in early schizophrenia. Br J Med Psychol 34:103–116. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA (2001): Probing the human hippocampus using rCBF: Contrasts in schizophrenia. Hippocampus 11:543–550. [DOI] [PubMed] [Google Scholar]
- Mexal S, Berger R, Logel J, Ross RG, Freedman R, Leonard S (2010): Differential regulation of alpha7 nicotinic receptor gene (CHRNA7) expression in schizophrenic smokers. J Mol Neurosci 40:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa JM, Freedman R, Lester HA (2011): Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron 70:20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Potter AS, Dumas JA, Thiel CM (2011): Functional brain imaging of nicotinic effects on higher cognitive processes. Biochem Pharmacol 82:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R (2006): Proof‐of‐concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry 63:630–638. [DOI] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R (1997): Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry 42:1–5. [DOI] [PubMed] [Google Scholar]
- Papke RL (2014): Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem Pharmacol 89:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo V (2015): Forced to remember: when memory is biased by salient information. Behav Brain Res 283:1–10. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA (2013): Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA (2009): Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry 66:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli P, Cheyne JA, Barton KR, Smilek D (2012): Consistency of sustained attention across modalities: comparing visual and auditory versions of the SART. Can J Exp Psychol 66:44–50. [DOI] [PubMed] [Google Scholar]
- Seli P, Jonker TR, Solman GJ, Cheyne JA, Smilek D (2013): A methodological note on evaluating performance in a sustained‐attention‐to‐response task. Behav Res Methods 45:355–363. [DOI] [PubMed] [Google Scholar]
- Smucny J, Tregellas J (2013): Nicotinic modulation of intrinsic brain networks in schizophrenia. Biochem Pharmacol, 86:1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Eichman LC, Lyons E, Tregellas JR (2013a): Early sensory processing deficits predict sensitivity to distraction in schizophrenia. Schizophr Res 147:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Rojas DC, Eichman LC, Tregellas JR (2013b): Neural effects of auditory distraction on visual attention in schizophrenia. PLoS One 8:e60606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny J, Rojas DC, Eichman LC, Tregellas JR (2013c): Neuronal effects of auditory distraction on visual attention. Brain Cogn, 81:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucny, J , Olincy, A , Eichman, LS , Tregellas, JR (2015): Neuronal effects of nicotine during auditory selective attention. Psychopharmacology (Berl) 232:2017–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Southcott S, Sacco C, Wagner AD, Ghose S (2012): Glutamate dysfunction in hippocampus: relevance of dentate gyrus and CA3 signaling. Schizophr Bull 38:927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD (2010): The hippocampal formation in schizophrenia. Am J Psychiatry 167:1178–1193. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Martin LF, Freedman R (2006): Effects of nicotine on hippocampal and cingulate activity during smooth pursuit eye movement in schizophrenia. Biol Psychiatry 59:754–761. [DOI] [PubMed] [Google Scholar]
- Torrey, E.F. (2006) Surviving Schizophrenia: A Manual for Families, Patients, and Providers. New York, NY: Harper Collins. [Google Scholar]
- Tregellas JR (2014): Neuroimaging biomarkers for early drug development in schizophrenia. Biol Psychiatry 76:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC, Waldo MC, Gibson L, Wylie K, Du YP, Freedman R (2007): Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res 92:262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Ellis J, Shatti S, Du YP, Rojas DC (2009): Increased hippocampal, thalamic, and prefrontal hemodynamic response to an urban noise stimulus in schizophrenia. Am J Psychiatry 166:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Olincy A, Johnson L, Tanabe J, Shatti S, Martin LF, Singel D, Du YP, Soti F, Kem WR, Freedman R (2010): Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology 35:938–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Smucny J, Eichman L, Rojas DC (2012): The effect of distracting noise on the neuronal mechanisms of attention in schizophrenia. Schizophr Res 142:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Smucny J, Harris JG, Olincy A, Maharajh K, Kronberg E, Eichman LC, Lyons E, Freedman R (2014): Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am J Psychiatry 171:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe J, Rojas DC, Shatti S, Olincy A, Johnson L, Martin LF, Soti F, Kem WR, Leonard S, Freedman R (2011): Effects of an alpha 7‐nicotinic agonist on default network activity in schizophrenia. Biol Psychiatry 69:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Martin LF, Freedman R (2005): FMRI of response to nicotine during a smooth pursuit eye movement task in schizophrenia. Am J Psychiatry 162:391–393. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R (2004): Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry 161:315–321. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Rugg MD (2009): Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. J Neurosci 29:8270–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables PH (1964): Input dysfunction in schizophrenia. Prog Exp Pers Res 72:1–47. [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR (2014): Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 20:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EM, Golaszewski S, Mottaghy FM, Hofer A, Hausmann A, Kemmler G, Kremser C, Brinkhoff C, Felber SR, Fleischhacker WW (2003): Brain activation patterns during a selective attention test‐a functional MRI study in healthy volunteers and patients with schizophrenia. Psychiatry Res 123:1–15. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Siedentopf C, Golaszewski S, Mottaghy FM, Hofer A, Kremser C, Felber S, Fleischhacker WW (2007): Brain activation patterns during a selective attention test–a functional MRI study in healthy volunteers and unmedicated patients during an acute episode of schizophrenia. Psychiatry Res 154:31–40. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG (2005): Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex 15:229–237. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG (2004): The neural mechanisms for minimizing cross‐modal distraction. J Neurosci 24:10941–10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Lu SE, Kumar S, Shen J, Foulds J, Kipen H, Benowitz NL (2010): Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine Tob Res 12:855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G (2010): Why do patients with schizophrenia smoke? Curr Opin Psychiatry 23:112–119. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Liu L, Liu S, Hong X, Chen da C, Xiu MH, Yang FD, Zhang Z, Zhang X, Kosten TA, Kosten TR (2012): Short‐term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry 169:974–981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1.


