Abstract
Aims
We examined whether unhealthy alcohol consumption, which negatively impacts HIV outcomes, changes after HIV care entry overall and by several factors. We also compared using phosphatidylethanol (PEth, an alcohol biomarker) to augment self-report to using self-report alone.
Design
Prospective one-year observational cohort study with quarterly visits.
Setting
Large rural HIV clinic in Mbarara, Uganda.
Participants
208 adults (89 women and 119 men) entering HIV care, reporting any prior year alcohol consumption.
Measurements
Unhealthy drinking was PEth+ (≥ 50 ng/ml) or Alcohol Use Disorders Test – Consumption+ (AUDIT-C+, over 3 months, women ≥ 3; men ≥ 4). We calculated adjusted odds ratios (AOR) for unhealthy drinking per month since baseline, and interactions of month since baseline with perceived health, number of HIV symptoms, anti-retroviral therapy (ART), gender, and self-reported prior unhealthy alcohol use.
Findings
The majority of participants (64%) were unhealthy drinkers (PEth+ or AUDIT-C+) at baseline. There was no significant trend in unhealthy drinking overall (per-month AOR: 1.01; 95% CI: 0.94–1.07), while the per-month AORs were 0.91 (95% CI: 0.83–0.99) and 1.11 (95% CI: 1.01–1.22) when participants were not yet on ART and on ART, respectively (interaction p-value <0.01). In contrast, 44% were AUDIT-C+; the per-month AORs for being AUDIT-C+ were 0.89 (95% CI: 0.85–0.95) overall, and 0.84 (95% CI: 0.78–0.91) and 0.97 (95% CI: 0.89–1.05) when participants were not on and on ART, respectively.
Conclusions
Unhealthy alcohol use among Ugandan adults entering HIV care declines prior to the start of anti-retroviral therapy but rebounds with time. Augmenting self-reported alcohol use with biomarkers increases the ability of current alcohol use measurements to detect unhealthy alcohol use.
Keywords: unhealthy alcohol use, phosphatidylethanol, biomarker, self-report, Africa, brief intervention, HIV, Uganda, Africa, trend
Introduction
Alcohol is currently the most commonly used recreational drug in sub-Saharan Africa (SSA) and in Uganda. While 40% of Ugandans are lifetime abstainers, consumption is very high among current drinkers (23.7 liters of pure alcohol per capita), which is on par with drinking levels in Russia (22.3 liters)1, a country known for high alcohol consumption.2 The HIV prevalence in Uganda is 7.4%,3 and alcohol is a risk factor for lower antiretroviral therapy (ART) adherence worldwide.4 In Southwest Uganda, unhealthy alcohol use among persons with HIV was a strong predictor of poor ART adherence, lower CD4 cell counts (measuring immune system health), and detectable HIV viral load (indicating treatment failure) in a cohort of persons initiating ART.5 Therefore, decreasing unhealthy alcohol consumption among those with HIV is vital to improving HIV treatment outcomes and reducing onward HIV transmission in SSA. However, little is known about the patterns of alcohol consumption after HIV diagnosis and initiation of ART.
Receiving a diagnosis of a serious chronic illness such as HIV provides an opportunity for a “teachable moment” that has been theorized to motivate health-related behavior change.6 Consistent with the Health Belief Model,7 individuals newly entering HIV care may perceive a number of health-related threats such as symptoms or functional declines that motivate engagement in behavior change to optimize health outcomes.8 Specifically, entry into HIV care represents an important “cue to action” where individuals may be especially driven to reduce alcohol use in order to reduce HIV symptoms.9 However, these efforts to decrease drinking may be short-lived after initiating ART, once cues to action such as HIV-related symptoms and ART initiation-related side effects resolve.10
We recently found substantial reductions in self-reported alcohol use after HIV diagnosis11 and after ART initiation in Uganda.12 In addition to HIV-related cues to action described above, a possible explanation for these declines is that new HIV patients in these clinics are screened for alcohol consumption using the Alcohol Use Disorders Identification Test – Consumption (AUDIT-C)13 and provided brief advice to reduce or cease their alcohol consumption. However, we have also found evidence of under-reporting in this setting;14,15 therefore, it is plausible that these trends were at least partially attributable to the social desirability bias inherent in self-reported measures of alcohol and drug use.16 Socially desirable responding may be especially prevalent in Africa, where persons infected with HIV who are hoping to receive ART may under-report their alcohol consumption in fear of being denied treatment.17 While there have been no recommendations for withholding ART from drinkers, such fear may be an outgrowth of the original scarcity of ART prior to 2004, after which the provision of ART dramatically increased18 In preparing to start ART, providers discuss with their patients their readiness to begin lifelong treatment, and clinic counselors frequently promote healthy living, including avoiding tobacco and alcohol and maintaining good nutrition; these may influence patients’ reporting. To help overcome this challenge, metabolites of alcohol consumption can serve as objective biomarkers of alcohol consumption.19 Phosphatidylethanol (PEth), a phospholipid formed only in the presence of alcohol, has shown high sensitivity and specificity for heavy alcohol consumption in several studies.20–22 Thus, we utilized this biomarker to augment self-report to objectively measure unhealthy alcohol use.
The goals of this study were (1) to determine whether unhealthy alcohol consumption changes over the first year of HIV care in Uganda, using PEth to augment self-report, (2) to determine whether trends in unhealthy alcohol use differ by health factors such as perceived health, HIV symptoms, and ART status, as well as variables that have been consistently associated with unhealthy alcohol use in Uganda, namely gender and prior unhealthy alcohol use,13 and (3) to determine whether our findings using PEth to augment self-report differ from using self-report alone to measure unhealthy alcohol use.
Methods
This manuscript reports the results of a one-year prospective cohort study that is part of the BREATH (Biomarker Research on Ethanol Among Those with HIV) Study. Other components of the BREATH Study included qualitative observations of HIV clinic visits to examine communication about alcohol consumption, semi-structured qualitative interviews among a randomly selected subset of the cohort study,23 and a randomly allocated minimally assessed arm to examine assessment reactivity (to be reported elsewhere).
Study participants
Participants were recruited from the Mbarara Regional Referral Hospital Immune Suppression Syndrome (ISS) Clinic in southwest Uganda. ISS clinic patients were eligible for the study if they were 18 years or older, their primary language was Runyankole or English, they had not previously received HIV care, they resided within 60 km of the clinic, and either they reported any alcohol consumption in the prior year to HIV clinic counselors during the AUDIT-C at their initial clinic visit or the counselors suspected they were alcohol consumers (n=13).
Study procedures
We conducted an interviewer-administered structured assessment at baseline and quarterly for one year. Breath alcohol concentration (BAC) testing and phlebotomy were conducted at each visit. Institutional Review Board approvals were obtained from the University of California San Francisco, Mbarara University of Science and Technology (MUST), and the Uganda National Council for Science and Technology.
Measures
Laboratory measures
HIV viral load testing (bDNA, Bayer, USA) was performed at baseline, CD4+ cell count testing (Beckman Coulter, USA) was performed at baseline, 6, and 12 months, and PEth was tested at baseline and each quarterly study visit. The PEth testing was performed using liquid chromatography-tandem mass spectrometry (LC-MS/MS) following extraction into methanol on dried blood spots (DBS) by the United States Drug Testing Laboratory.24 The lower limit of quantification was 8 ng/ml, and the most common PEth homologue (16:0/18:1) was detected. We previously found, in a study of 77 persons with HIV in Uganda, high area under the receiver-operating curve (AUC) of PEth for any alcohol use in the prior 21 days (AUC=0.92, 95% CI: 0.86–0.97) which did not differ substantially by ART status.22 PEth results, are considered experimental and were not shared with patients or providers.
Outcome variables
The main outcome measure was unhealthy alcohol use, in which we used the PEth biomarker result to augment self-reported unhealthy alcohol use. Self-reported unhealthy alcohol consumption was obtained via the AUDIT-C, modified to elicit responses for drinking in the prior 3 months, with cutoffs of ≥ 3 for women and ≥ 4 for men.13 PEth was considered positive if the value was ≥ 50 ng/ml. We chose this PEth cutoff based on previous studies. Using daily breathalyzer tests and self-reported daily drinking over 21 daily home/bar visits in 77 persons with HIV in Uganda, PEth was 95% sensitive and 73% specific for detecting any risky alcohol consumption (NIAAA cutoffs25) over 21 days using a cutoff of ≥ 10 ng/ml.22 Other studies, using higher cutoffs, demonstrated higher specificity, as expected. A study of 80 non-pregnant reproductive-aged women found that a PEth cutoff of ≥ 45 ng/ml was 62% sensitive and 95% specific for detecting an average of >1 drink per day over the prior 2 weeks,26 while using a cutoff of 50 ng/ml was highly sensitive (93%) and reasonably specific (83%) for detecting daily drinking of 2 or more drinks per day in a study of 222 patients with liver disease (S. Stewart, personal communication). Thus, we chose 50 ng/ml as the cutoff for PEth that would yield good sensitivity and high specificity for unhealthy drinking. We defined unhealthy drinking in the prior 3 months, as either PEth+ or AUDIT-C+ at each study visit.
Predictor variables
Months since baseline was the main predictor of interest. We also examined whether the trend in unhealthy alcohol consumption differed by the following covariates: gender (male vs. female), prior unhealthy alcohol consumption, general health perception, number of HIV-related symptoms, and current ART use.
Prior self-reported unhealthy alcohol use was determined by asking the participants to consider the period in their lives in which they had consumed the most alcohol, and to answer the AUDIT-C questions with that period in mind. Scores above the usual cutoff (≥3 for women, ≥ 4 for men) indicated prior unhealthy drinking.
At each interview, we measured general health perception using the first question of the Medical Outcomes Study HIV Survey (MOS-HIV), “In general would you say your health is excellent, very good, good, fair, or poor?”. The MOS-HIV has been shown to validly measure quality of life and is a good marker of health status in persons with HIV in Uganda.27 This single question has not been validated in persons with HIV, however the question predicted one-year mortality as well as a validated physical health scale in persons receiving Veteran’s Administration outpatient care, 28 with mortality rate increasing linearly with the perception of poorer health. Therefore, we included the general health perception measure as a continuous variable, with scores from 1=excellent through 5=poor.
At each visit, we elicited HIV and HIV treatment-related severe symptoms lasting at least 4 weeks, from a list of 9 of the most prevalent symptoms chosen from the AIDS Clinical Trials Group Index.29 ART status was whether the participant reported being on ART in the prior 3 months; there was 97% agreement between clinic records and self-report on this variable. All questions were translated and back-translated and underwent cognitive interviewing to confirm that the intended meanings were conveyed.30
Statistical analyses
The primary analysis was based on evaluating temporal trends in the proportion of participants engaging in unhealthy drinking, as measured by the measure described above. We used mixed effects logistic regression models (as implemented in the meqrlogit procedure31 in Stata), including a random intercept for each participant.32 The initial models included months since baseline, gender, self-reported prior unhealthy alcohol consumption; time-dependent measures were ART status, general health rating, and HIV symptoms. Next, we evaluated evidence for interaction between months since baseline and each of these predictors individually, using a 10% significance level as the criterion for retaining the associated product term in the model. The only variable that met this criterion was ART status, as reflected in our reported results. For comparison purposes, we ran additional analyses using AUDIT-C+ as the outcome. We also investigated possible sample size-related instabilities in estimated parameters by refitting models using alternate optimization routines and varying quadrature points, and found no issues with the system defaults.
The study sample size of 212 was selected to obtain a 95% confidence interval (CI) for the proportion of prior year drinkers who become abstinent over the year of follow up of with a width of at most 15%. Assuming a true proportion of 50% and up to 15% loss to follow up yielded a sample size of 180. This sample size was also sufficient to yield a similar degree of precision for engaging in unhealthy drinking, which we subsequently selected as the outcome more relevant to HIV outcomes.
Results
Recruitment and retention
From July, 2011 through July, 2013, 3747 new patients were screened and 621 were considered eligible for the BREATH study. Sixty-one percent (n=381) agreed to enroll in the study. Similar proportions of those who enrolled compared to those who declined participation were female (45% versus 47%, respectively, p=0.59). Of the 381 persons enrolled, 213 were randomized to the cohort described here and 168 to the minimally assessed arm. Of the 213, 5 were found ineligible after enrollment, leaving a sample size of 208, with a total of 937 visits. The 3, 6, 9, and 12 month visit completion proportions were 89%, 88%, 88%, and 87%. Reasons for non-completion included death (n=7), study withdrawal (n=3) and loss to follow up (n=18); 3 additional data points were excluded because PEth testing was not performed.
Participant characteristics
The majority (57%) of the participants were male, the median age was 30 (interquartile range [IQR]: 25–38), the median CD4+ cell count at baseline was 347 cells/mm3 (IQR: 219–533), and 72% of those enrolled initiated ART during the study (Table 1). At baseline, AUDIT-C was positive in 44% of the participants, while PEth was positive (≥50 ng/ml) in 52%. Median PEth at baseline was 57.8 ng/ml (IQR: 1.0–211.0). PEth was positive in 36% of those who were AUDIT-C negative, and 73% of those who were AUDIT-C positive (p<0.01). The baseline proportion of unhealthy drinking, i.e. PEth+ or AUDIT-C+, was 64%. Three participants had attended an alcohol support group or sought help for their alcohol problems in the 3 months prior to baseline.
Table 1.
Characteristics of persons with HIV who reported prior year alcohol use at entry into HIV care (baseline) (n=208).
| N (%) | Median (IQR) | ||
|---|---|---|---|
| Demographic characteristics | |||
| Sex | = Male | 119 (57.2) | |
| Age (years) | 30 (25–38) | ||
| Religion | = Protestant | 115 (55.0) | |
| = Catholic | 86 (41.4) | ||
| = Other (Moslem/Saved) | 7 (3.4) | ||
| Education | = Greater than primary | 73 (35.1) | |
| Marital status | = Married/common law spouse | 109 (52.4) | |
| Literacy | = Can read partial/entire sentence | 160 (77.7) | |
| Household Asset Index | = Low | 81 (38.9) | |
| = Medium | 85 (40.9) | ||
| = High | 42 (20.2) | ||
| Health characteristics | |||
| General health perception, on a scale of 1=excellent to 5=poor | 3.5 (1–4) | ||
| Number of HIV symptoms (of a list of 9) | 2 (0–4) | ||
| Months since HIV diagnosis | 0.3 (0.1–1.3) | ||
| CD4 cell count (cells/mm3) | 347 (219–533) | ||
| Initiated antiretroviral therapy during the one-year study | 150 (72.1) | ||
| Alcohol consumption | |||
| Unhealthy alcohol use (self-reported), any time prior to baseline | 155 (74.5) | ||
| Unhealthy alcohol use (self-reported), 3 months prior to baseline (AUDIT-C+) | 92 (44.2) | ||
| PEth+, at baseline (n=207) | 108 (52.2) | ||
| Unhealthy alcohol use (PEth+ or AUDIT-C+), overall (n=207) | 133 (64.3) | ||
| PEth+, by AUDIT-C (n=207) | |||
| PEth+, among AUDIT-C negative participants | 41 (35.7) | ||
| PEth+, among AUDIT-C positive participants | 67 (72.8) | ||
| PEth ng/ml, median (IQR) (n=207) | 57.8 (1.0–211.0) | ||
AUDIT-C+: Alcohol Use Disorders Identification Test – Consumption, covering prior 3 months, score ≥ 3 for women, score ≥ 4 for men PEth+: Phosphatidylethanol ≥ 50 ng/ml
Unhealthy drinking (PEth+ or AUDIT-C+)
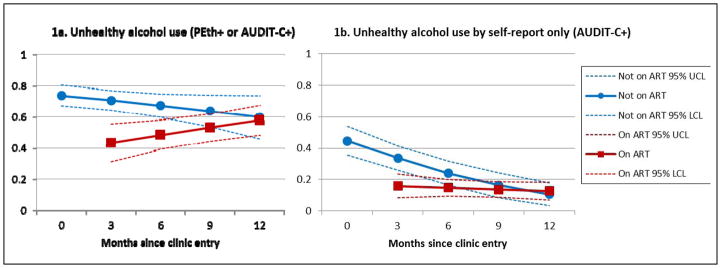
The random effects mixed model showed no significant reduction in unhealthy drinking by months since baseline (Table 2, model A). The odds of unhealthy drinking were significantly lower for those visits in which patients were on ART versus not on ART, reported more HIV symptoms, reported poorer general health, and the odds were increased for men and for those reporting prior unhealthy drinking at baseline. The only significant interaction was between months since baseline and being on ART (Table 2 model B and Figure 1a), demonstrating a decline in unhealthy drinking over time for those not on ART, and an increase on ART.
Table 2.
Random effects multivariable logistic regression models of unhealthy drinking among persons at quarterly visits during their first year of HIV care in Mbarara, Uganda (n=208)
|
Adjusted Odds Ratio Unhealthy drinking defined as PEth+ or AUDIT-C+ |
Adjusted Odds Ratio Unhealthy drinking defined as AUDIT-C+ (self-report alone) |
|||
|---|---|---|---|---|
| A. Main effects model | B. Interaction model (months X ART status) | C. Main effects model | D. Interaction model (months X ART status) | |
| Months since baseline (Δ+1 month), overall | 1.01 (0.94–1.08) | --- | 0.89 (0.85–0.95)* | --- |
| Months since baseline (Δ+1 month), no ART | --- | 0.91 (0.83–1.00)* | --- | 0.84 (0.78–0.91)* |
| Months since baseline (Δ+1 month), on ART prior 3 months | --- | 1.11 (1.01–1.22)* | --- | 0.97 (0.89–1.05) |
| On ART=Yes | 0.22 (0.11–0.43)* | --- | 0.49 (0.29–0.83)* | --- |
| Sex=male | 3.28 (1.26–8.57)* | 3.32 (1.24–8.86)* | 0.81 (0.43–1.52) | 0.80 (0.43–1.52) |
| Unhealthy alcohol use prior to baseline=yes | 26.99 (8.26–88.22)* | 28.70 (8.52–96.69)* | 5.50 (2.48–12.22)* | 5.59 (2.50–12.47)* |
| HIV symptoms (Δ+1 symptom) | 0.82 (0.72–0.95)* | 0.82 (0.71–0.95)* | 0.88 (0.78–0.99)* | 0.88 (0.78–0.99)* |
| General health perception (Δ+1 on a 5-point scale where 1=excellent and 5=poor) | 0.75 (0.61–0.92)* | 0.74 (0.60–0.91)* | 0.92 (0.78–1.09) | 0.92 (0.77–1.08) |
AUDIT-C+: Alcohol Use Disorders Identification Test – Consumption, covering prior 3 months, score ≥ 3 for women, score ≥ 4 for men
PEth+: Phosphatidylethanol ≥ 50 ng/ml
ART: antiretroviral therapy
p<0.05
Figure 1.
Estimated proportions of unhealthy drinking over time and by antiretroviral (ART) status, among 208 persons entering HIV care in Mbarara, Uganda, obtained from multivariable random effects logistic regressions (see Table 2). A. Unhealthy drinking is defined as phosphatidylethanol (PEth)≥50 ng/ml or 3-months AUDIT-C positive. B. Unhealthy drinking is defined as 3-months AUDIT-C positive.
Self-reported unhealthy drinking (AUDIT-C+)
The proportion self-reporting unhealthy alcohol consumption by AUDIT-C declined over the first year of HIV care (Table 2, model C), and the proportion AUDIT-C+ declined in the first year of HIV care for those not on ART, but there was no significant change for those on ART (Table 2, model D, and Figure 1b). Multivariable models of AUDIT-C+ as the outcome demonstrated attenuated covariate associations compared to models using PEth+ or AUDIT-C+, and the associations of gender and the general health rating with unhealthy drinking were no longer statistically significant.
Discussion
The main finding of this study is that we found no evidence of an overall decline in unhealthy alcohol use among persons in their first year of HIV care in rural Uganda. Unhealthy alcohol consumption did decrease prior to ART initiation, but then increased over study visits in which the patients were on ART, returning to high levels. The clinical implication is that while patients may initially decrease their alcohol consumption upon entry into HIV care and prior to ART initiation, such reductions are unlikely to be maintained over time without more systematic interventions, and unhealthy alcohol use is likely to negatively impact ART adherence and HIV outcomes. The majority of participants, 75%, reported unhealthy drinking in the past, and the extremely large odds of continued unhealthy drinking for this group suggests that reducing drinking for most current drinkers may be very challenging.
The initial decline in unhealthy alcohol use among those visits when the patients were not on ART may be related to the advice given by the ISS clinic counselors at intake visits that alcohol and ART do not mix. We found in a qualitative sub-study23 that patients were motivated to reduce their drinking by this advice. However, many reported that it was difficult to maintain these reductions. Our finding of increasing unhealthy alcohol use when on ART is consistent with a finding of decreasing and then increasing sexual risk behavior among men initiating ART in another cohort of persons with HIV in southwestern Uganda.33 This finding is also consistent with the larger literature proposing that different processes influence initiating health behavior change and maintaining change over time.34 Given the sometimes dramatic improvement in physical health subsequent to ART initiation, the cues to action for avoiding unhealthy alcohol use may taper over time among HIV-positive persons on treatment. When this study was conducted, ART was initiated when CD4 cell counts declined below 350 cells/mm3. The current cutoff for initiation is 500 cells/mm3 and thus many patients now begin ART at their first visit, often prior to the development of HIV symptoms; therefore effective interventions that do not rely on symptoms as cues to action are needed.
Further work is needed to reduce alcohol consumption during the course of ART. There is a paucity of trained mental health professionals in SSA; however, brief alcohol interventions, which can be delivered by lay personnel and therefore may be feasible for use in health clinics in low resource settings, have shown promising results for reducing unhealthy alcohol consumption in primary care clinics in resource-rich countries.35 Recent trials of single-session brief interventions conducted in health clinics in South Africa have yielded inconclusive findings (both the intervention and control arms reported large reductions in alcohol use),36,37 while trials of multi-session (4–6 sessions) alcohol interventions conducted in Kenya38,39 and South Africa40 have shown efficacy to reduce self-reported unhealthy drinking. Thus, while the latter results are encouraging, more work is needed to determine whether multi-session interventions can feasibly be integrated into HIV care in SSA, and whether briefer interventions, which are likely to be more scalable in resource constrained settings, are also efficacious.
Another important finding is that the trends over time were quite different when PEth was used to augment self-report, compared to when self-report alone was used. This is consistent with previous findings of under-reporting of alcohol use among persons with HIV in Uganda.41,42 This is the first study that we are aware of that used biomarkers to examine trends in drinking. Our results suggest that prior findings of declines in self-reported drinking after HIV testing11 and ART initiation12 in Uganda may be invalid. When the AUDIT-C was used as the outcome variable, a declining trend was seen, and the regression coefficients for the covariates all attenuated towards the null (one), the expected result of misclassification of outcome variables.43 The dramatic attenuation of the odds ratio for gender from 3.28 to 0.81 is consistent with our recent finding that the odds of under-report are much higher for men compared to women.15 It is likely that patients in HIV care, in which they are told to avoid alcohol, feel the need to represent themselves as good candidates for ART or simply want to report what they feel is the “correct” behavior. The implication of this finding is that in the setting of HIV care in Uganda, clinicians should consider that unhealthy drinking is possible, especially among men, even if a patient denies unhealthy alcohol use. Statistics about unhealthy alcohol use in persons with HIV, and possibly other populations in which alcohol use is discouraged, should be viewed cautiously. However, self-reported prior unhealthy alcohol use was a consistent and strong predictor of current unhealthy alcohol use. This suggests that patients may be more willing to report past use and this variable may provide a way to identify current unhealthy drinkers. Another implication of socially desirable reporting is the obfuscating of the results of clinical trials. For example, socially desirable reporting recently undermined a large trial of HIV pre-exposure prophylaxis in women in Zimbabwe, in which participants told researchers they were using the study products but biologically confirmed compliance was later discovered to be very low.44 Thus our findings extend beyond the area of alcohol use to serve as a reminder that research studies should be especially alert to the potential for social desirability bias, and should utilize objective biological markers and other techniques to avoid this bias wherever possible.
There are several limitations of note. First, this study was limited to those who had reported consuming any alcohol in the prior year at their initial clinic visit; therefore, drinkers who denied prior year alcohol use, if they exist, were omitted. The AUDIT-C has been sensitive and specific for detecting alcohol misuse in patients in primary care in the U.S.;13 however, there have been no validation studies of the AUDIT-C in Africa, nor studies of appropriate cut-points, despite its frequent use.45 In addition, while our classification of unhealthy drinking was improved by the use of PEth, this biomarker is not 100% sensitive or specific; therefore, we may have misclassified unhealthy drinking in either direction. In addition, prior unhealthy alcohol consumption was a novel study variable and therefore has not been validated. Furthermore, the study was powered to examine the proportion with the outcome at one year, thus the sample sizes for our analyses of effect modifiers of the trend were not large. However, we used a significance level of 0.10 to counter-act the lower power to detect interaction. Lastly, the study covered the first year of HIV care, therefore there was less than one year of follow up of ART use, and we cannot extrapolate our unhealthy drinking results beyond this period.
In summary, using PEth to augment self-report, we found that unhealthy alcohol use declined prior to the start of ART but then rebounded with time. This has serious implications for the long-term success of ART, as unhealthy alcohol consumption adversely impacts treatment success via reduced ART adherence. While unhealthy drinkers entering HIV care may be initially motivated to reduce their alcohol consumption, that may not be enough to maintain reductions in drinking over the long term. Systematic strategies to reduce unhealthy alcohol consumption among those on ART that are feasible for implementation in limited-resource settings should be explored. Interventions such as brief alcohol interventions, possibly with the assistance of technology for intervention delivery,46,47 should be considered, and biomarker measurement will be necessary to objectively examine their impact.
Acknowledgments
We would like to thank the study participants for their time and energy in participating, and the members of the BREATH Study team for their hard work. This work was funded by the U.S. National Institutes of Health grants R01AA018631, U01AA020776, K24AA022586, K01AA021671, and P30DK026743.
Footnotes
Conflict of interest declaration: None.
References
- 1.Global Status Report on Alcohol and Health. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.Nemtsov AV. [Accessed July 03, 2015];A Contemporary History of Alcohol in Russia. 2011 http://www.diva-portal.org/smash/get/diva2:425342/FULLTEXT01.pdfArchived by WebCite® at http://www.webcitation.org/6aAUG728v.
- 3.UNAIDS. HIV and AIDS estimates (2013) 2013 http://www.unaids.org/en/regionscountries/countries/uganda (Archived by WebCite® at http://www.webcitation.org/6aAUnnT9M)
- 4.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiser SD, Palar K, Frongillo EA, et al. Longitudinal assessment of associations between food insecurity, antiretroviral adherence and HIV treatment outcomes in rural Uganda. AIDS. 2014;28(1):115–120. doi: 10.1097/01.aids.0000433238.93986.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 8.Kirscht JP. The health belief model and illness behavior. Health Education & Behavior. 1974;2(4):387–408. [Google Scholar]
- 9.Moskowitz JT, Wrubel J, Hult JR, Maurer S, Acree M. Illness appraisals and depression in the first year after HIV diagnosis. PLoS One. 2013;8(10):e78904. doi: 10.1371/journal.pone.0078904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouellette JA, Wood W. Habit and intention in everyday life: The multiple processes by which past behavior predicts future behavior. Psychological Bulletin. 1998;124(1):54–74. [Google Scholar]
- 11.Hahn JA, Fatch R, Wanyenze RK, et al. Decreases in self-reported alcohol consumption following HIV counseling and testing at Mulago Hospital, Kampala, Uganda. BMC Infect Dis. 2014;14:403. doi: 10.1186/1471-2334-14-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos GM, Emenyonu NI, Bajunirwe F, et al. Self-reported alcohol abstinence associated with ART initiation among HIV-infected persons in rural Uganda. Drug Alcohol Depend. 2014;134:151–157. doi: 10.1016/j.drugalcdep.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 14.Hahn JA, Fatch R, Kabami J, et al. Self-Report of Alcohol Use Increases When Specimens for Alcohol Biomarkers Are Collected in Persons With HIV in Uganda. J Acquir Immune Defic Syndr. 2012;61(4):e63–64. doi: 10.1097/QAI.0b013e318267c0f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajunirwe F, Haberer J, Boum Y, et al. Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV infected patients initiating antiretroviral treatment in south western Uganda. PLoS ONE. 2014 doi: 10.1371/journal.pone.0113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson T, Fendrich M. Modeling sources of self-report bias in a survey of drug use epidemiology. Ann Epidemiol. 2005;15(5):381–389. doi: 10.1016/j.annepidem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Papas RK, Gakinya BN, Baliddawa JB, et al. Ethical issues in a stage 1 cognitive-behavioral therapy feasibility study and trial to reduce alcohol use among HIV-infected outpatients in western Kenya. Journal of empirical research on human research ethics : JERHRE. 2012;7(3):29–37. doi: 10.1525/jer.2012.7.3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UNAIDS. Access to Antiretroviral Therapy in Africa: Status Report on Progress towards the 2015 Targets. 2015 http://www.unaids.org/sites/default/files/media_asset/20131219_AccessARTAfricaStatusReportProgresstowards2015Targets_en_0.pdf. (Archived by WebCite® at http://www.webcitation.org/6aAV5hUT7)
- 19.Hannuksela ML, Liisanantti MK, Nissinen AE, Savolainen MJ. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45(8):953–961. doi: 10.1515/CCLM.2007.190. [DOI] [PubMed] [Google Scholar]
- 20.Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C. PHosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol. 2006;41(4):431–437. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann S, Aradottir S, Graf M, et al. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12(1):81–84. doi: 10.1111/j.1369-1600.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 22.Hahn JA, Dobkin LM, Mayanja B, et al. Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa. Alcohol Clin Exp Res. 2012;36(5):854–862. doi: 10.1111/j.1530-0277.2011.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundararajan R, Wyatt MA, Woolf-King S, et al. Qualitative Study of Changes in Alcohol Use Among HIV-Infected Adults Entering Care and Treatment for HIV/AIDS in Rural Southwest Uganda. AIDS Behav. 2014 doi: 10.1007/s10461-014-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones J, Jones M, Plate C, Lewis D. The detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Analytical Methods. 2011;3(5):1101. [Google Scholar]
- 25.Helping patients who drink too much: A clinician’s guide. National Institutes of Health; National Institute on Alcohol Abuse and Alcoholism; 2005. [Google Scholar]
- 26.Stewart SH, Law TL, Randall PK, Newman R. Phosphatidylethanol and alcohol consumption in reproductive age women. Alcohol Clin Exp Res. 2010;34(3):488–492. doi: 10.1111/j.1530-0277.2009.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stangl AL, Bunnell R, Wamai N, Masaba H, Mermin J. Measuring quality of life in rural Uganda: reliability and validity of summary scores from the medical outcomes study HIV health survey (MOS-HIV) Qual Life Res. 2012;21(9):1655–1663. doi: 10.1007/s11136-011-0075-5. [DOI] [PubMed] [Google Scholar]
- 28.DeSalvo KB, Fan VS, McDonell MB, Fihn SD. Predicting mortality and healthcare utilization with a single question. Health Serv Res. 2005;40(4):1234–1246. doi: 10.1111/j.1475-6773.2005.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Justice AC, Holmes W, Gifford AL, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54 (Suppl 1):S77–90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 30.Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: Sage Publications; 2005. [Google Scholar]
- 31.Stata 14 Manual: Multilevel Mixed-Effects Reference Manual. Multilevel mixed-effects logistic regression (QR decomposition) 2015 http://www.stata.com/manuals14/memeqrlogit.pdf. (Archived by WebCite® at http://www.webcitation.org/6aAVLdMK6)
- 32.McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear, and Mixed Models. 2. Hoboken: John Wiley & Sons; 2008. [Google Scholar]
- 33.Kembabazi A, Bajunirwe F, Hunt PW, et al. Disinhibition in risky sexual behavior in men, but not women, during four years of antiretroviral therapy in rural, southwestern Uganda. PLoS One. 2013;8(7):e69634. doi: 10.1371/journal.pone.0069634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothman AJ. Toward a theory-based analysis of behavioral maintenance. Health Psychol. 2000;19(1 Suppl):64–69. doi: 10.1037/0278-6133.19.suppl1.64. [DOI] [PubMed] [Google Scholar]
- 35.O’Donnell A, Anderson P, Newbury-Birch D, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol. 2014;49(1):66–78. doi: 10.1093/alcalc/agt170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pengpid S, Peltzer K, Skaal L, Van der Heever H. Screening and brief interventions for hazardous and harmful alcohol use among hospital outpatients in South Africa: results from a randomized controlled trial. BMC Public Health. 2013;13:644. doi: 10.1186/1471-2458-13-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peltzer K, Naidoo P, Louw J, et al. Screening and brief interventions for hazardous and harmful alcohol use among patients with active tuberculosis attending primary public care clinics in South Africa: results from a cluster randomized controlled trial. BMC Public Health. 2013;13:699. doi: 10.1186/1471-2458-13-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papas RK, Sidle JE, Gakinya BN, et al. Treatment outcomes of a Stage 1 cognitive-behavioral trial to reduce alcohol use among HIV-infected outpatients in western Kenya. Addiction. 2011 [Google Scholar]
- 39.L’Engle KL, Mwarogo P, Kingola N, Sinkele W, Min D, Weiner DH. A randomized controlled trial of a brief intervention to reduce alcohol use among female sex workers in Mombasa, Kenya. J Acquir Immune Defic Syndr. 2014 doi: 10.1097/QAI.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 40.Zule W, Myers B, Carney T, Novak SP, McCormick K, Wechsberg WM. Alcohol and drug use outcomes among vulnerable women living with HIV: results from the Western Cape Women’s Health CoOp. AIDS Care. 2014;26(12):1494–1499. doi: 10.1080/09540121.2014.933769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahn JA, Fatch R, Kabami J, et al. Self-Report of Alcohol Use Increases When Specimens for Alcohol Biomarkers Are Collected in Persons With HIV in Uganda. J Acquir Immune Defic Syndr. 2012;61(4):e63–e64. doi: 10.1097/QAI.0b013e318267c0f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bajunirwe F, Haberer J, Boum Y, et al. Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV infected patients initiating antiretroviral treatment in south western Uganda. 2014 doi: 10.1371/journal.pone.0113152. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Copeland KT, Checkoway H, McMichael AJ, Holbrook RH. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105(5):488–495. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- 44.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. New England Journal of Medicine. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peltzer K, Simbayi L, Kalichman S, Jooste S, Cloete A, Mbelle Alcohol Use in Three Different Inner Cities in South Africa: AUDIT-C and CAGE. Journal of Psychology in Africa. 2007;17(1–2) [Google Scholar]
- 46.Hasin DS, Aharonovich E, Greenstein E. HealthCall for the smartphone: technology enhancement of brief intervention in HIV alcohol dependent patients. Addict Sci Clin Pract. 2014;9(1):5. doi: 10.1186/1940-0640-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasin DS, Aharonovich E, O’Leary A, et al. Reducing heavy drinking in HIV primary care: a randomized trial of brief intervention, with and without technological enhancement. Addiction. 2013;108(7):1230–1240. doi: 10.1111/add.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]