Abstract
Background
Type 2 cytokine-related (i.e., type 2) immune responses associated with development of antigen-specific Immunoglobulin E antibodies (IgE) can contribute to pathology in allergic diseases and to fatal anaphylaxis. However, recent findings in mice indicate that IgE also can enhance defense against honeybee venom.
Objective
We tested whether IgE antibodies, IgE-dependent effector mechanisms, and a local anaphylactic reaction to an unrelated antigen can enhance defense against Russell's viper venom (RVV) and determined whether such responses can be influenced by immunization protocol or mouse strain.
Methods
We compared the resistance of RVV-immunized wild-type, IgE-deficient, and Fcer1a-deficient mice following injection of a potentially lethal dose of RVV.
Results
A single prior exposure to RVV enhanced the ability of wild-type mice, but not mice lacking IgE or functional FcεRI, to survive challenge with a potentially lethal amount of RVV. Moreover, IgE-dependent local passive cutaneous anaphylaxis in response to challenge with an antigen not naturally present in RVV significantly enhanced resistance to the venom. Finally, we observed different effects on resistance to RVV or honeybee venoms in BALB/c versus C57BL/6 mice which had received a second exposure to that venom prior to challenge with a high dose of that venom.
Conclusion
These observations illustrate the potential benefit of IgE-dependent effector mechanisms in acquired host defense against venoms. The extent to which type 2 immune responses against venoms can decrease pathology associated with envenomation seems to be influenced by the type of venom, the frequency of venom exposure, and the genetic background of the host.
Keywords: Acquired resistance, allergy, Daboia russelii, Russell's viper, FcεRIα, honeybee, immunoglobulin E, toxin hypothesis, venom, mast cells, type 2 immunity
Introduction
Venoms are complex mixtures of toxic molecules1-3 employed by many different animal species to fulfill functions of deterrence, defense, and/or predation4, 5. Millions of years of co-evolution with venomous animals have allowed certain mammals, including those that eat or are the prey of venomous creatures, to develop innate defense mechanisms that can increase their basal (or “innate”) resistance against venoms and their toxins. Such specialized defense strategies include producing circulating serum proteins that efficiently neutralize venom components6, 7 and conserving mutations, e.g., in proteins targeted by toxins8, which confer increased resistance to that toxin.
We and others have been interested in the possibility that mast cells (MCs) can represent an important component of the innate defense of vertebrates to animal venoms. MCs populate virtually all vascularized mammalian tissues9-11. When appropriately activated, MCs can release cytoplasmic granules containing a broad spectrum of pre-formed mediators into the surrounding tissues11. Notably, many components of animal venoms can induce such MC degranulation12 and some mediators stored in MC granules have the ability to neutralize the toxicity of components of animal venoms13-17. Higginbotham and colleagues showed that the venoms of the honeybee15 and the highly poisonous18, 19 Russell's viper14 can induce degranulation of mouse MCs in vivo14, 15, and that the toxicity of those venoms was significantly reduced upon their ex vivo incubation with heparin, the serglycin proteoglycan stored in MC cytoplasmic granules20. More recently, mice deficient in MCs or certain MC-associated proteases were used to show that MCs can importantly contribute to innate host defense against venoms13, 16, 17, 21, or toxic venom components13, 16, 17, of honeybees16, 21, two scorpions13, and various reptiles13, 16, 17.
Since IgE antibodies can enhance MC sensitivity and responsiveness against specific antigens, and in light of evidence that MCs can enhance innate resistance to venoms13, 16, 17, 21, Profet22, Metz et al.16, and Palm et al.23 speculated that IgE antibodies may also play a protective role in acquired resistance to venoms. However, it is well known that humans and other mammals which develop IgE antibodies to venom components from honeybees2, 24, reptiles25-29, or other animals30-33 can exhibit anaphylaxis, a catastrophic and potentially fatal acute allergic reaction, upon subsequent venom exposure34, 35. Such observations suggested that the development of an acquired T helper cell type 2 (TH2 or type 2) immune response and associated IgE directed against venom components probably would increase, not decrease, the pathology associated with envenomation.
Recently, our group21 and Palm et al.36 reported that the development of a type 2 immune response to honeybee venom (BV)21 or BV phospholipase A2 (bvPLA2)36 could increase the resistance of mice (as quantified by body temperature21, 36 and/or survival21) against a near-lethal dose challenge of whole BV21 or bvPLA236. This effect was dependent on the high affinity IgE receptor (i.e., FcεRIα21, 36) and IgE antibodies21. In addition, we also observed that injection of mice with sublethal amounts of Russell's viper venom (RVV), a snake venom of high clinical relevance18, 19, induced a type 2 immune response that enhanced the survival of mice injected with a potentially lethal amount of that venom21.
In the present study, we aimed to define the importance of IgE antibodies, FcεRIα, FcεRIα+ IgE effector cells, and local IgE-mediated MC activation in the orchestration of systemic resistance against RVV. In addition, we evaluated the influence of repeated exposure to venom and the genetic background of the host on acquired protection against challenge with a potentially lethal amount of RVV or BV.
Methods
Mice
All animal care and experiments were carried out in accord with current National Institutes of Health guidelines and with the approval of the Stanford University Institutional Animal Care and Use Committee. Age-matched 5 to 7 week-old WT C57BL/6J or BALB/cJ female mice were purchased from Jackson Laboratories. All transgenic mouse strains were bred and housed with the respective (in house-bred) control mice in the Stanford Animal facilities under specific pathogen free conditions. Details regarding transgenic strains can be found in this article's Online Repository at www.jacionline.org.
Reagents
Russell's viper (Daboia russelii) venom was obtained from Sigma (Lots SLBB5602V and SLBK7058V). Details regarding additional reagents can be found in this article's Online Repository at www.jacionline.org.
Venom injections
Briefly, mice were shaved at the injection sites 24 h before injections and were consistently treated in the morning (without anesthesia) by administering subcutaneous (s.c.) injections of 50 μL PBS alone or containing indicated amounts of RVV or BV. Additional details regarding injections, mouse handling, quantification of scratching behavior and descriptions of experiments involving serum transfer or multiple exposure to venoms prior to high dose venom challenge are provided in this article's Online Repository at www.jacionline.org.
Other methods
Detailed descriptions of the following methods are provided in the Online Repository at www.jacionline.org: histology and assessment of MC degranulation; analysis of skin and white blood cells by flow cytometry; measurement of RVV-specific IgG1 and IgE, BV-specific IgG1, bvPLA2-specific IgE and total IgE antibodies; anti-dinitrophenol-conjugated human serum albumin (DNP-HSA)-specific IgE-dependent passive cutaneous anaphylaxis; antibody-mediated neutrophil depletion; generation and degranulation analysis of bone marrow-derived cultured mast cells.
Statistical analysis
Statistical tests were performed using GraphPad PRISM 6 software. Two-tailed Student's t-test (unpaired), Mann-Whitney test, Mantel-Cox, or Chi-Square tests were performed as noted in the figure legends. ns, not significant (P>0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Results
Mast cells rapidly degranulate upon injection of RVV and contribute to enhanced innate resistance to RVV
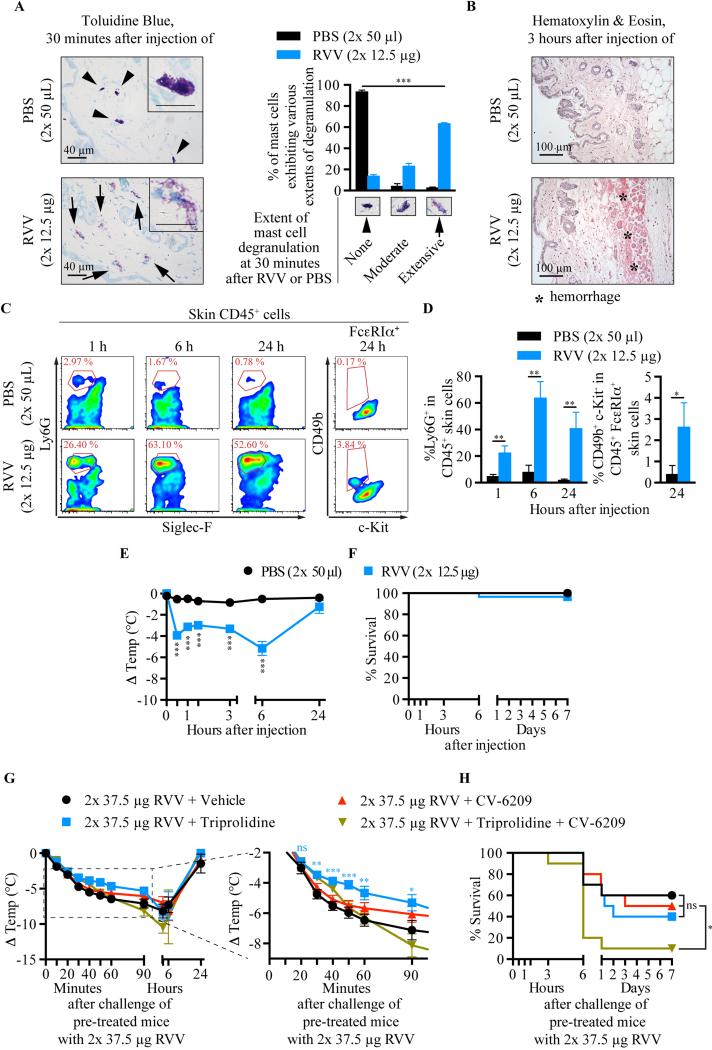
Injection of RVV s.c. into naïve C57BL/6 WT mice elicited intense scratching of that site (data not shown), rapid degranulation of skin MCs (Fig 1, A), local hemorrhage (Fig 1, B), and tissue infiltration with neutrophils and basophils (Fig 1, C,D), whereas the small numbers of eosinophils at such sites were not significantly different in sites injected with RVV versus PBS (data not shown). Systemically, RVV injection induced an increased percentage of blood neutrophils (see Fig. E1 in the Online Repository) and marked hypothermia (Fig 1, E). However, almost all mice appeared to recover fully within 24 h (Fig 1, E-F). Pre-treatment of C57BL/6 and BALB/c mice with the H1 anti-histamine, triprolidine, but not with the platelet-activating factor (PAF) receptor antagonist, CV-6209, significantly decreased RVV-induced hypothermia without affecting mortality (Fig. 1, G-H and see Fig. E2 in the Online Repository). However, in C57BL/6 mice, combined treatment with the anti-histamine and PAF receptor antagonist did not protect against RVV-induced hypothermia and significantly increased RVV-induced mortality (Fig. 1, G-H), while such treatment decreased hypothermia but did not influence mortality in RVV-injected BALB/c mice (see Fig. E2 in the Online Repository). These findings suggest that there might be strain-dependent differences in the mechanisms contributing to responses to RVV in naïve mice.
Fig 1.
RVV can induce local MC degranulation, recruitment of innate inflammatory cells, and hypothermia. A,B, Toluidine Blue- (A) and Hematoxylin & Eosin- (B) stained back skin sections; A, Extent of MC degranulation (mean+SD). C,D, flow cytometry plots (C) and quantification (D) (mean+SD, from 3 mice, representative of 2 experiments) of CD45+ skin cells. E,F, temperature (E) and survival (F) after RVV injection. G,H, temperature (right panel magnifies the area in the dashed box) (G) and survival (H) of RVV-treated mice pretreated with anti-histamine and/or PAF-receptor antagonist. P values: Chi-Square test (A); Student's t test (D,E,G); Mantel-Cox test (H). Symbols in (E): comparison of group in that color with vehicle-treated mice for that time point. E-H, data pooled from 2-3 experiments.
We next evaluated the possible contributions of MCs, basophils and neutrophils to the type 2 humoral response induced by RVV. Injection of a sub-lethal dose of RVV in basophil deficient Mcpt8-Crehet;DTAfl/− mice or MC- and basophil-deficient Cpa3-Cre+;Mcl-1fl/fl mice (which are markedly deficient in MCs and have an ~75% reduction in blood basophils37) induced serum levels of IgG1 and IgE antibodies not significantly different from those in the corresponding littermate controls (see Fig. E3, B-E in the Online Repository). Interestingly, anti-GR-1-treated neutrophil-depleted C57BL/6 mice developed similar levels of IgG1 antibodies but significantly higher levels of IgE antibodies than did the isotype control antibody-treated mice (see Fig. E3, G-H in the Online Repository). These results provide evidence that neither mast cells, basophils nor neutrophils are necessary for the induction of a type 2 humoral immune response to RVV.
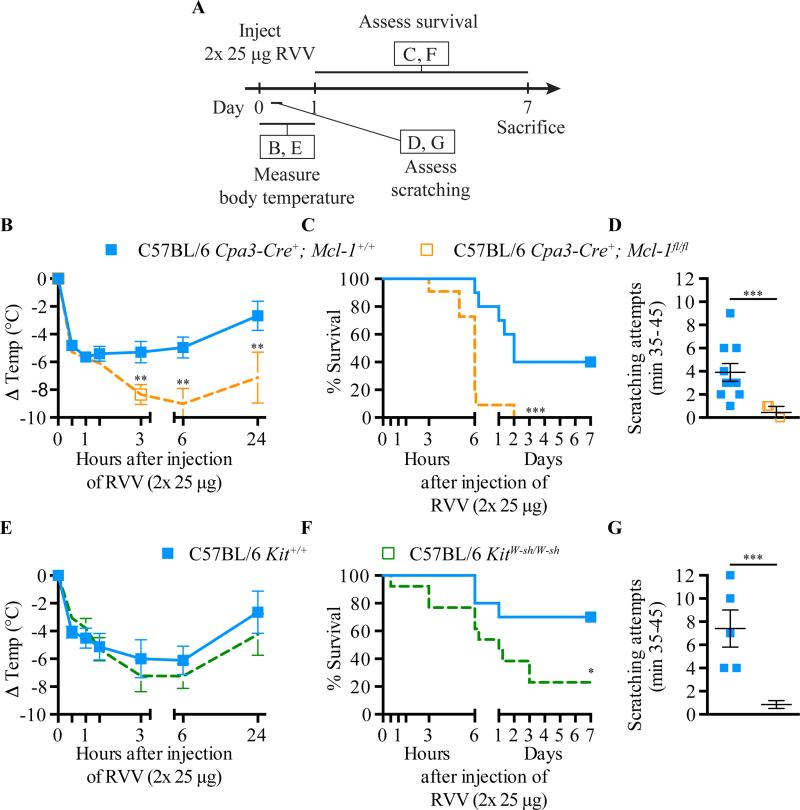
We next evaluated the possible contribution of MCs to innate resistance against RVV by testing two different types of MC-deficient mice (Fig 2, A). C57BL/6-KitW-sh/W-sh mice virtually lack MCs (but exhibit moderately increased numbers of blood basophils38) due to a mutation in c-kit, the gene encoding stem cell factor receptor39, 40. The MC deficiency of C57BL/6-Cpa3- Cre+;Mcl-1fl/fl mice is independent of c-kit and accompanied by decreased blood basophil numbers37. We found that each type of MC-deficient mouse exhibited significantly more susceptibility to RVV toxicity, assessed by extent of hypothermia (Fig 2, B-E) and/or survival (Fig 2, C-F), than did the corresponding control mice. MC-deficient mice also exhibited an almost complete absence of the scratching that was elicited in control mice (Fig 2, D-G).
Fig 2.
MCs can contribute to innate resistance and behavioral responses to RVV. A, Experimental outline. B and E, body temperature; C and F, survival; D and G, scratching attempts, of MC-deficient Cpa3-Cre+; Mcl-1fl/fl (B-D) and KitW-sh/W-sh (E-G) mice and corresponding control mice after RVV injection. P values: (B,D,E,G) Student's t test; (C,F) Mantel-Cox test. Data pooled from 2-4 experiments (n=5-21/group).
IgE- and FcεRIα-dependent effector mechanisms contribute to increased survival of mice challenged with RVV
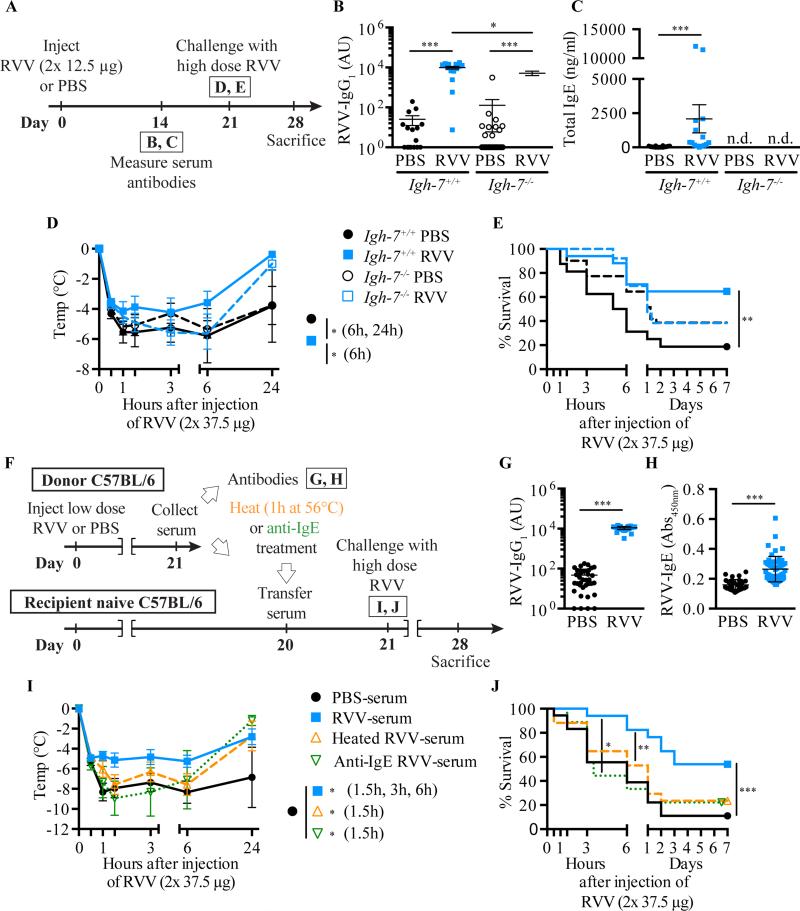
To assess whether IgE antibodies can contribute to acquired host resistance to RVV21, we injected a low dose of RVV into IgE-deficient C57BL/6-Igh7−/− and IgE-sufficient C57BL/6-Igh7+/+ mice (Fig 3, A). RVV induced RVV-specific IgG1 antibodies in both Igh7−/− and Igh7+/+ mice (Fig 3, B), but no detectable serum IgE in Igh7−/− mice (Fig 3, C). When challenged s.c. with a potentially lethal dose of RVV 3 weeks after their first exposure to RVV, C57BL/6-Igh7+/+ mice, but not C57BL/6-Igh7−/− mice, exhibited enhanced resistance to the RVV-induced hypothermia and mortality (Fig 3, D-E). The same was true for the comparison between IgE-deficient and IgE-sufficient BALB/c mice (see Fig E4 in the Online Repository).
Fig 3.
IgE can contribute to acquired resistance to RVV. A Outline of experiments with IgE-deficient (Igh-7−/−) and control (Igh-7+/+) C57BL/6 mice (B-E). B,C, Serum RVV-specific IgG1 (B) and total IgE (C). D,E, Body temperature (D) and survival (E). F, Outline of serum transfer experiments in C57BL/6 mice (G-J). G,H, Serum RVV-specific IgG1 (G) and total IgE (H). I,J, Body temperature (I) and survival (J). Data pooled from 3-4 experiments (n= 9-25/group). P values: Mann-Whitney test (B,C,G,H), Student's t test (D,I) and Mantel-Cox test (E,J).
Serum transfer studies also supported a critical role for IgE antibodies in acquired resistance to RVV; enhanced protection could be transferred passively to naive C57BL/6 mice (Fig 3, F) by injecting them with 250 μl of serum collected from RVV-exposed WT donor mice (RVV-serum) that contained significantly increased levels of RVV-specific IgG1 and IgE antibodies (Fig 3, G-H, respectively), but not with the same amount of serum obtained from PBS mock-immunized mice (PBS-serum) (Fig 3, F, I-J). Moreover, RVV-serum from WT mice lost its protective potential when the contained IgE antibodies were neutralized either by heating (which destroys the ability of IgE to bind to FcεRI and induce passive cutaneous anaphylaxis without affecting the function of other antibody isotypes41, 42) or treatment with an anti-IgE antibody (Fig 3, F, I -J).
Immune functions of IgE are primarily mediated by effector cells, including MCs and basophils, which express FcεRI43, 44. Both C57BL/6-Fcer1a−/− mice (that lack the IgE-binding component of FcεRI [i.e., FcεRIα]) and WT animals developed similar type 2 humoral responses after s.c. injection of RVV (Fig 4, A-C), but enhanced resistance to RVV challenge could only be detected in mice expressing the complete IgE receptor (Fig 4, D-E). Furthermore, in passive immunization experiments, C57BL/6-Cpa3-Cre+;Mcl-1fl/fl mice exhibited no difference in survival after RVV challenge whether they had received untreated RVV-serum from C57BL/6 WT mice (which contained functionally active venom-specific IgE antibodies) versus control serum from PBS-mock-sensitized C57BL/6 WT mice (Fig 4, F-H).
Fig 4.
FcεRIα and FcεRIα–bearing cells can contribute to acquired resistance to RVV. A, Outline of experiments with Fcer1a−/− and control (Fcer1a+/+) C57BL/6 mice (panels B-E). B,C, Serum RVV-specific IgG1 (B) and total IgE (C). D,E, Body temperature (D) and survival (E). F, Outline of serum transfer experiments involving MC-deficient C57BL/6 mice (G,H). G,H, Body temperature (G) and survival (H). Data pooled from 3 experiments (n=9-17/group). P values: Mann-Whitney test (B,C); Student's t test (D,G); Mantel-Cox test (E,H).
Taken together, these results demonstrate that IgE antibodies and FcεRIα-bearing effector cells contribute importantly to the acquired resistance of RVV-immunized mice against a high dose RVV challenge.
A local anaphylactic reaction to an unrelated antigen can increase survival of mice challenged with a potentially lethal amount of RVV
Immunization with honeybee venom-derived PLA2 (i.e., bvPLA2), which represents approximately 10% of the dry weight of whole BV45, can reduce the toxicity-related hypothermia induced by subsequent challenge of the mice with a high dose of the same allergen in an antibody- and FcεRIα-dependent manner36. However, it is not clear whether an IgE response to a single constituent of an animal venom would be able to enhance resistance to the entire group of toxins contained in that venom.
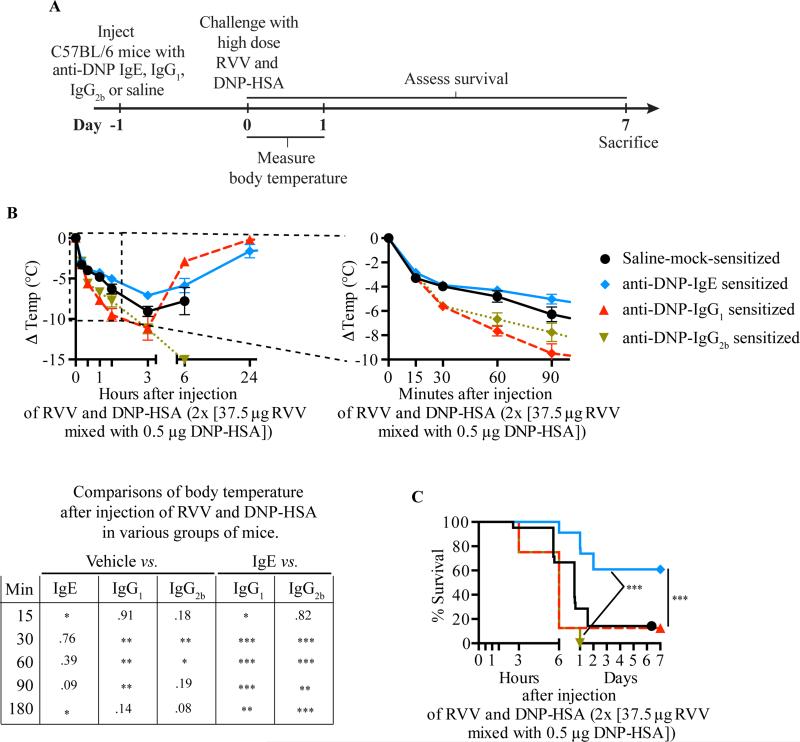
To investigate this, we used a well-characterized monoclonal mouse anti-dinitrophenol (DNP) IgE antibody46, which can sensitize mouse MCs to degranulate in response to challenge in vivo with DNP coupled to human serum albumin (DNP-HSA)47, 48. Specifically, we passively sensitized WT C57BL/6 and BALB/c mice against DNP-HSA by s.c. injections of anti-DNP IgE (or with anti-DNP IgG1 or IgG2b as controls), or mock-sensitized them with saline, then challenged the mice s.c. at the same site 24 h later by injecting a mixture of RVV and DNP-HSA (Fig 5, A). We used amounts of anti-DNP IgE and DNP-HSA which were able to induce a local increase in vascular permeability at the DNP-HSA injection site without resulting in systemic hypothermia, and showed that the amount of DNP-HSA used did not by itself influence the toxicity of RVV (see Fig E5 in the Online Repository). We found that pre-sensitization with anti-DNP IgE significantly increased the resistance of C57BL/6 (Fig 5, B,C) or BALB/c (see Fig E5, H-I in the online repository) mice to challenge with a potentially lethal amount of RVV admixed with DNP-HSA. However, pre-sensitization of mice with anti-DNP IgG1 or IgG2b, DNP-specific IgG isotypes with the capacity to activate effector cells via Fcγreceptors49, not only failed to increase protection but also resulted in increased hypothermia at early time points compared to vehicle-treated or IgE-sensitized mice (Fig 5, B,C). Compared to passive sensitization with a 10 fold higher amount of anti-OVA IgE, anti-DNP IgE significantly enhanced the survival of IgE-deficient mice challenged with a potentially lethal amount of RVV admixed with DNP-HSA (Fig E6). By contrast, IgE-deficient mice passively sensitized with a 10:1 mixture of anti-OVA IgE and anti-DNP IgE exhibited a level of survival that was intermediate between that observed in mice which received either anti-DNP IgE alone or anti-OVA IgE alone (Fig E6, C). This result suggests that the effect on survival of antigen-specific IgE in this model may depend on the proportion of antigen-specific vs. antigen non-specific IgE on FcεRI-bearing effector cells.
Fig 5.
IgE-dependent passive cutaneous anaphylaxis to an irrelevant antigen can increase resistance to a potentially lethal challenge with RVV. A, Experimental outline. B,C, Body temperature (B) and survival (C) of C57BL/6 mice treated with 3 s.c. injections of saline alone or containing 50 ng anti-DNP IgE, IgG1 or IgG2b antibody and challenged 18 h later with 2 s.c. injections, each containing 37.5 μg RVV and 0.5 μg DNP-HSA. Data pooled from 2-5 independent experiments (n=10-25/group). P values: Student's t test (B); Mantel-Cox test (C).
These findings show that local tissue responses mediated by IgE and antigen can enhance host resistance against RVV even when that antigen is not a native constituent of the venom, and are consistent with the general idea that the host needs only to generate an IgE response against a limited number of the components of a complex venom (perhaps as few as one component) in order to manifest enhanced acquired resistance to that venom.
Influence of venom type, genetic background, and venom exposure protocol on the protective effects of type 2 immune responses
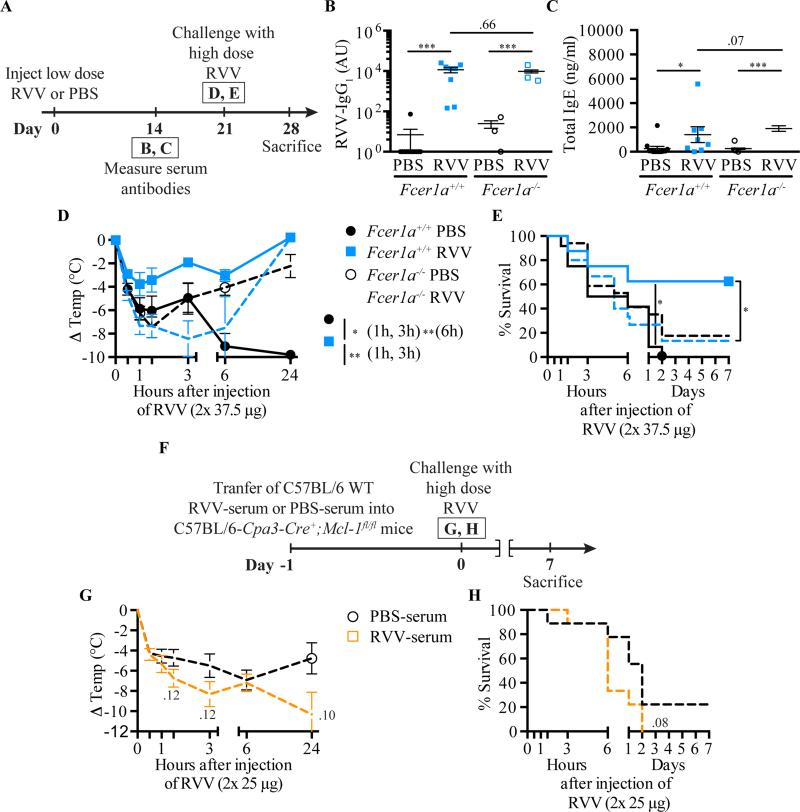
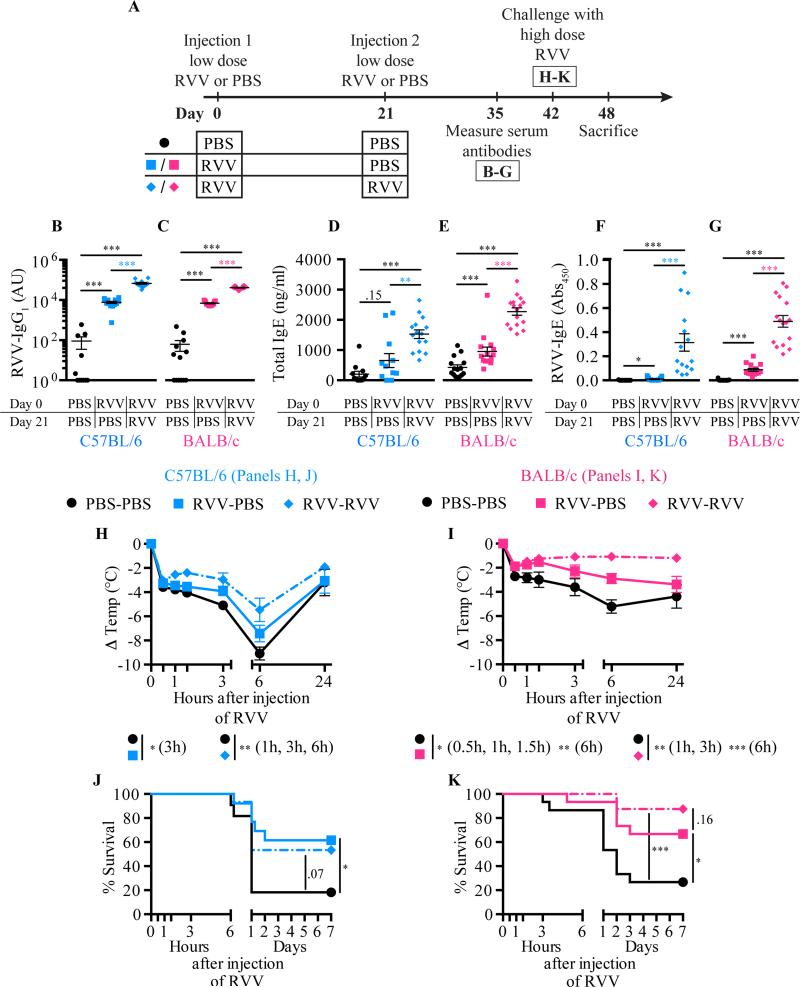
IgE-associated type 2 immune responses induced by a single exposure to honeybee venom21 or Russell's viper venom (this study) can increase the resistance of C57BL/6 or BALB/c mice to challenge with a potentially lethal amount of that venom. However, in nature, some animals may be exposed to the same venom more than twice. To analyze the potential effects of multiple venom exposures on acquired resistance to that venom, we injected mice s.c. with RVV (or PBS as a control) once at day 0, then some RVV-injected mice received a second RVV s.c. injection on day 21 (or got PBS as a control), and then all mice were challenged with a high dose of RVV at day 42 (Fig 6, A). C57BL/6 or BALB/c mice that had received 2 prior RVV injections (RVV-RVV mice) had significantly higher levels of RVV-specific IgG1 (Fig 6, B-C), total IgE (Fig 6, D-E), and RVV-specific IgE (Fig 6, F-G) at day 35 than did the mice that received only a single RVV injection (RVV-PBS mice). Both RVV-RVV and RVV-PBS C57BL/6 mice developed less hypothermia upon RVV challenge than did control mice that had received two mock immunizations with PBS (PBS-PBS mice) (Fig 6, H). However, while survival of C57BL/6 mice injected once or twice with RVV was similar, the survival of the RVV-RVV mice did not quite achieve statistical significance versus that in the pooled PBS-PBS group (P = 0.07) (Fig 6, J). In BALB/c mice, animals injected once or twice with RVV were significantly more resistant than the PBS-PBS control mice to the hypothermia and the mortality induced by challenge with a potentially lethal dose of RVV (Fig 6, I-K).
Fig 6.
Influence of genetic background and immunization regimen on acquired resistance to RVV. A, Experimental outline. B-G, Serum RVV-specific IgG1 (B,C); total IgE (D,E); and RVV-specific IgE (F,G). H,I, Body temperature. J,K, Survival. Data pooled from 3-4 experiments (n=11-16/group). P values: Mann-Whitney test (B-G), Student's t test (H,I) or Mantel-Cox test (J,K).
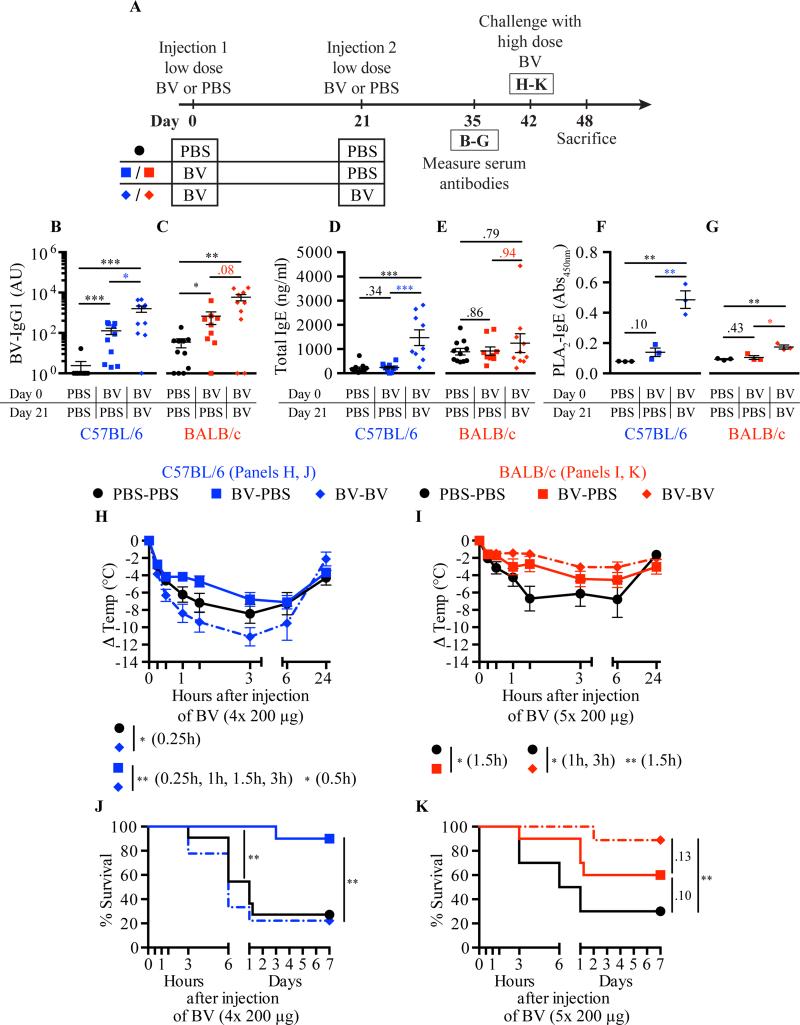
We also tested the consequences of a second exposure to BV on responses to challenge with a potentially lethal amount of BV in C57BL/6 versus BALB/c mice (Fig 7, A). Serum levels of BV-specific IgG1 (Fig 7, B,C), total IgE (Fig. 7, D,E) and bvPLA2-specific IgE (Fig 7, F,G) were significantly higher in the serum of C57BL/6 mice that had received 2 exposures to BV (BV-BV mice) as compared to mice that had received only one (BV-PBS mice), whereas the differences in antibody levels in the two corresponding groups of BALB/c mice only were statistically significant in the case of bvPLA2-specific IgE. C57BL/6 mice that had been injected once with BV prior to potentially lethal challenge showed significantly increased survival as compared to PBS-PBS control animals, confirming our prior findings21, but this was not true for the C57BL/6 mice which were injected twice with BV prior to high dose BV challenge (Fig 7, J). Moreover, these BV-BV C57BL/6 mice exhibited a drop in body temperature in response to high dose BV challenge that was significantly more profound than that observed in the BV-PBS mice over the entire first 3 h of the response and that was even significantly worse than that of the PBS-PBS mice at 15 min after BV challenge (Fig 7, H). In contrast to the results with C57BL/6 mice, in BALB/c mice challenged with high dose BV, hypothermia was not significantly exacerbated in BV-BV versus BV-PBS mice (Fig 7, I) and survival was significantly enhanced by two BV exposures whereas the effect on survival did not reach statistical significance in the BVPBS mice (P = 0.1) (Fig 7, K). Notably, the strain-dependent differences observed in the responses to high dose BV in mice immunized once or twice with low dose BV did not appear to reflect differences in the ability of IgE antibodies from these mice to sensitize MCs to degranulate in response to BV challenge in vitro (see Fig E7 in the online repository).
Fig 7.
Influence of genetic background and immunization regimen on acquired resistance to BV. A, Experimental outline. B-G, Serum BV-specific IgG1 (B,C); total IgE (D,E); and bvPLA2-specific IgE (F,G). H-I, Body temperature. J-K, Survival. Data pooled from 3 experiments (n=9-11/group). P values: Mann-Whitney test (B-G); Student's t test (H,I); Mantel-Cox test (J,K).
Discussion
Antigen-specific IgE antibodies and FcεRI-expressing effector cells constitute a sensitive, specific, and powerful module of acquired immunity that can respond within minutes to exposure to small amounts of antigen by initiating local or systemic inflammatory reactions9, 11. It appears plausible that this rapid and efficient, but also potentially dangerous, effector mechanism evolved primarily to operate in situations that represent a substantial threat for the organism. In her “toxin hypothesis of allergy”, Margie Profet proposed that toxins and venoms represent examples of such substantial threats and that “allergic reactions” originally evolved as immune defense mechanisms against such noxious substances22.
Recently, our lab21 and others36 provided in vivo experimental evidence that IgE antibodies can indeed contribute to protective immunity in mice against either whole BV21 or the potentially toxic BV enzyme, bvPLA236. The results of the current study indicate that acquired IgE-mediated immune resistance is not restricted to BV, but can also be deployed as a potent adaptive immune defense mechanism against a reptile venom of high clinical relevance19. Notably, the immunization and challenge doses of RVV used in this study (25 μg and 50-100 μg, respectively), in relation to the body weight of a mouse, are similar to the amounts of venom that a human might be exposed to if bitten by a Russell's viper19. Taken together, our findings support the idea that IgE antibodies and FcεRIα-bearing effector cells may constitute part of a general defense strategy against animal venoms, in addition to having roles in host responses to certain macroparasites50.
We also found that a local IgE-dependent reaction to an unrelated antigen (i.e., DNPHSA) not ordinarily contained in RVV can enhance the survival of mice subjected to challenge with a potentially lethal amount of RVV. Our results thus support the conclusion that mounting an IgE-dependent reaction to a single antigen can be sufficient to enhance host resistance to the complex mixture of toxins contained in the venom51. The effector mechanisms involved in such enhanced resistance remain to be defined, but may include MC-mediated venom detoxification13-17 and the local dilution and/or interference with the systemic spread of the toxins22, 23.
Ever since it was discovered that IgE antibodies can mediate anaphylactic reactions52-55, the development of IgE antibodies specific for certain antigens, including components of venoms25, 26, 29, 31, 33, 56, 57, has primarily been regarded as a risk factor for the development of deleterious IgE-mediated hyperreactivity upon subsequent antigen exposure. Yet a recent survey of more than 7,000 German adults58, 59 showed a prevalence of 22.6 % for sensitization (i.e., having specific serum IgE antibodies) against hymenoptera (wasp and bee) venom in the general public58, while the lifetime prevalence of diagnosed insect venom allergy in that group is only 2.8 %59. Indeed, it is well known from other studies that the vast majority (~80%) of people who have demonstrable IgE antibodies specific for hymenoptera venoms have no history of manifesting systemic reactions to such venoms56, 60 and that the presence of antigen-specific IgE antibodies, taken in isolation, is not predictive of severe clinical reactivity to the recognized antigens61-66. It is therefore possible that, in some humans, the presence of anti-venom IgE antibodies may be beneficial, e.g., by decreasing venom toxicity and tissue damage upon subsequent venom exposure.
It is thought that multiple factors, such as differences in pathogen exposure during childhood, the characteristics of the host's microbiome, and many other environmental influences, as well as genetic background and the nature and frequency of exposure to potential allergens, can contribute to the variation in individual susceptibilities to develop clinical allergies67-70. Here we compared the resistance of C57BL/6 and BALB/c mice to RVV or BV following one versus two sublethal exposures to the same venom. In contrast to BALB/c mice, C57BL/6 mice that were immunized twice with BV rapidly developed increased hypothermia upon subsequent BV challenge. Importantly, such twice-immunized C57BL/6 mice, in striking contrast to singly immunized C57BL6 mice or twice-immunized BALB/c mice, did not exhibit enhanced resistance against high dose BV challenge. Taken together, our data indicate that, depending on the mouse strain and the type of venom, a second exposure to venom can either increase (BV in BALB/c mice) or eliminate (BV in C57BL/6 mice) the enhanced protection to venom challenge that is observed after a single exposure to that venom. While many factors might contribute to such strain-dependent differences, including genetically-determined differences in end organ sensitivity to MC-derived mediators71-74, our data suggest that such factors probably don't include differences in the ability of IgE antibodies from these mice to sensitize MCs to degranulate in response to BV challenge.
Our findings are consistent with the hypothesis that the co-evolution of mammals with venomous animals provided positive evolutionary pressure to conserve IgE antibodies and IgE-effector cells as survival advantages. However, it seems likely that sustaining the beneficial functions of this “allergy module” of immunity critically requires regulatory mechanisms which can keep this potentially dangerous effector mechanism under tight control. We therefore speculate that anaphylaxis represents only the most extreme end of a spectrum of acquired TH2 immunity to venom and that appropriately regulated TH2 immune responses can actually enhance resistance, rather than susceptibility, to venoms.
In fact, the occurrence of potentially dangerous allergic TH2 responses in some individuals may represent the price paid to maintain, for the species, the benefits of IgE-associated TH2 immune responses. For example, beekeepers, who are frequently exposed to bee venom, can exhibit high levels of BV-specific IgG and IgE antibodies, associated, in some of these individuals, with the danger of anaphylaxis75. However, in many beekeepers, exposure to multiple bee stings as the season progresses induces the development of BV-specific, IL-10-producing, inducible type 1 T regulatory (TR1) cells, which suppress T cell responses to BV in vitro and which, in vivo, may contribute to the observed reduction in cutaneous late phase responses to bee stings which occur as the beekeeping season progresses76. Mechanisms of antigen-induced, regulatory T cell-dependent immune tolerance also are thought to contribute to the success of venom specific immunotherapy in patients with hymenoptera venom allergy77. It is therefore tempting to speculate that IgE-dependent enhanced resistance to the toxicity of BV may represent an initial phase of a beneficial adaptive immune response to BV which, in individuals frequently exposed to the venom, then can be supplemented or supplanted by T regulatory cell-dependent immune tolerance to BV, one important function of which is to restrain the development of an overly excessive, and therefore potentially dangerous, IgE response to BV.
Supplementary Material
Key Messages.
IgE and IgE effector mechanisms can limit Russell's viper venom toxicity in mice.
A local anaphylactic reaction elicited by an unrelated antigen at the site of Russell's viper venom injection can increase resistance against that venom.
The extent of IgE-associated acquired resistance to venom can be influenced by venom type, mouse genetics, and the number of exposures to that venom.
Capsule Summary.
IgE and IgE effector cells, and local anaphylactic reactions, can increase resistance to a snake venom in mice. Such acquired venom resistance is influenced by type of venom, host genetics, and number of venom exposures.
Acknowledgements
We thank all members of the Galli lab for helpful discussions, Hans C. Oettgen and Mitchell Grayson for generously providing IgE-deficient mice, and Chen Liu and Mariola Liebersbach for excellent technical assistance.
P.S. was supported by a Max Kade Fellowship of the Max Kade Foundation and the Austrian Academy of Sciences and a Schroedinger Fellowship of the Austrian Science Fund (FWF): J3399-B21. T.M. was supported by a Marie Curie International Outgoing Fellowship for Career Development (Grant number 299954). N.G was supported by fellowships from the French “Fondation pour la Recherche Médicale FRM”. L.L.R. was supported by the Arthritis National Research Foundation (ANRF) and National Institutes of Health grant K99AI110645. R.S. was supported by a fellowship from the Lucile Packard Foundation for Children's Health and the Stanford NIH/NCRR CTSA, award number UL1 RR025744. This work was supported by National Institutes of Health grants AI023990, CA072074 and AI070813 (to S.J.G.) and by the Department of Pathology of Stanford University.
Abbreviations
- BMCMCs
bone marrow-derived cultured mast cells
- BV
honeybee venom
- bvPLA2
honeybee venom phospholipase A2
- DNP
dinitrophenol
- DNP-HSA
dinitrophenol-conjugated human serum albumin
- IgE
Immunoglobulin E (antibody)
- MC(s)
mast cell(s)
- PAF
platelet activating factor
- RVV
Russell's viper venom
- s.c.
subcutaneous
- TH2
T helper cell type 2
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that no conflicts of interest exist.
References
- 1.Berger BJ, Bhatti AR. Snake venom components and their cross-reactivity: a review. Biochem Cell Biol. 1989;67:597–601. doi: 10.1139/o89-092. [DOI] [PubMed] [Google Scholar]
- 2.Habermann E. Bee and wasp venoms. Science. 1972;177:314–22. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 3.Meier J, White J. Handbook of clinical toxicology of animal venoms and poisons. CRC Press; Boca Raton: 1995. p. c1995. [Google Scholar]
- 4.Fry BG, Roelants K, Champagne DE, Scheib H, Tyndall JD, King GF, et al. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annu Rev Genomics Hum Genet. 2009;10:483–511. doi: 10.1146/annurev.genom.9.081307.164356. [DOI] [PubMed] [Google Scholar]
- 5.Brodie ED., 3rd Toxins and venoms. Curr Biol. 2009;19:R931–5. doi: 10.1016/j.cub.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Perales J, Neves-Ferreira AG, Valente RH, Domont GB. Natural inhibitors of snake venom hemorrhagic metalloproteinases. Toxicon. 2005;45:1013–20. doi: 10.1016/j.toxicon.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 7.Voss RS, Jansa SA. Snake-venom resistance as a mammalian trophic adaptation: lessons from didelphid marsupials. Biol Rev Camb Philos Soc. 2012;87:822–37. doi: 10.1111/j.1469-185X.2012.00222.x. [DOI] [PubMed] [Google Scholar]
- 8.Rowe AH, Xiao Y, Rowe MP, Cummins TR, Zakon HH. Voltage-gated sodium channel in grasshopper mice defends against bark scorpion toxin. Science. 2013;342:441–6. doi: 10.1126/science.1236451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–86. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- 11.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 12.Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–28. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 13.Akahoshi M, Song CH, Piliponsky AM, Metz M, Guzzetta A, Abrink M, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest. 2011;121:4180–91. doi: 10.1172/JCI46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higginbotham RD. Mast cells and local resistance to Russell's viper venom. J Immunol. 1965;95:867–75. [PubMed] [Google Scholar]
- 15.Higginbotham RD, Karnella S. The significance of the mast cell response to bee venom. J Immunol. 1971;106:233–40. [PubMed] [Google Scholar]
- 16.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–30. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 17.Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007;204:2629–39. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson ID, Norris RL. Snakes of medical importance in India: is the concept of the “Big 4” still relevant and useful? Wilderness Environ Med. 2007;18:2–9. doi: 10.1580/06-weme-co-023r1.1. [DOI] [PubMed] [Google Scholar]
- 19.Warrell DA. Snake venoms in science and clinical medicine. 1. Russell's viper: biology, venom and treatment of bites. Trans R Soc Trop Med Hyg. 1989;83:732–40. doi: 10.1016/0035-9203(89)90311-8. [DOI] [PubMed] [Google Scholar]
- 20.Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14:478–94. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 21.Marichal T, Starkl P, Reber LL, Kalesnikoff J, Oettgen HC, Tsai M, et al. A beneficial role for immunoglobulin E in host defense against honeybee venom. Immunity. 2013;39:963–75. doi: 10.1016/j.immuni.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Profet M. The function of allergy: immunological defense against toxins. Q Rev Biol. 1991;66:23–62. doi: 10.1086/417049. [DOI] [PubMed] [Google Scholar]
- 23.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–72. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilo BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339–49. doi: 10.1111/j.1398-9995.2005.00963.x. [DOI] [PubMed] [Google Scholar]
- 25.de Medeiros CR, Barbaro KC, Lira MS, Franca FO, Zaher VL, Kokron CM, et al. Predictors of Bothrops jararaca venom allergy in snake handlers and snake venom handlers. Toxicon. 2008;51:672–80. doi: 10.1016/j.toxicon.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Kopp P, Dahinden CA, Mullner G. Allergic reaction to snake venom after repeated bites of Vipera aspis. Clin Exp Allergy. 1993;23:231–2. doi: 10.1111/j.1365-2222.1993.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 27.Reimers AR, Weber M, Muller UR. Are anaphylactic reactions to snake bites immunoglobulin E-mediated? Clin Exp Allergy. 2000;30:276–82. doi: 10.1046/j.1365-2222.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmutz J, Stahel E. Anaphylactoid reactions to snakebite. Lancet. 1985;2:1306. doi: 10.1016/s0140-6736(85)91589-2. [DOI] [PubMed] [Google Scholar]
- 29.Wadee AA, Rabson AR. Development of specific IgE antibodies after repeated exposure to snake venom. J Allergy Clin Immunol. 1987;80:695–8. doi: 10.1016/0091-6749(87)90289-2. [DOI] [PubMed] [Google Scholar]
- 30.deShazo RD, Butcher BT, Banks WA. Reactions to the stings of the imported fire ant. N Engl J Med. 1990;323:462–6. doi: 10.1056/NEJM199008163230707. [DOI] [PubMed] [Google Scholar]
- 31.Leynadier F, Hassani Y, Chabane MH, Benguedda AC, Abbadi MC, Guerin L. Allergic reactions to North African scorpion venom evaluated by skin test and specific IgE. J Allergy Clinical Immunol. 1997;99:851–3. doi: 10.1016/s0091-6749(97)80022-x. [DOI] [PubMed] [Google Scholar]
- 32.Portier MM, Richet C. De l'action anaphylactique de certains venims. C R Soc Biol. 1902;54:170–2. [Google Scholar]
- 33.Togias AG, Burnett JW, Kagey-Sobotka A, Lichtenstein LM. Anaphylaxis after contact with a jellyfish. J Allergy Clin Immunol. 1985;75:672–5. doi: 10.1016/0091-6749(85)90092-2. [DOI] [PubMed] [Google Scholar]
- 34.Beyer K, Eckermann O, Hompes S, Grabenhenrich L, Worm M. Anaphylaxis in an emergency setting -elicitors, therapy and incidence of severe allergic reactions. Allergy. 2012;67:1451–6. doi: 10.1111/all.12012. [DOI] [PubMed] [Google Scholar]
- 35.Lee JK, Vadas P. Anaphylaxis: mechanisms and management. Clin Exp Allergy. 2011;41:923–38. doi: 10.1111/j.1365-2222.2011.03779.x. [DOI] [PubMed] [Google Scholar]
- 36.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39:976–85. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lilla JN, Chen CC, Mukai K, BenBarak MJ, Franco CB, Kalesnikoff J, et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–8. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, et al. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol. 2010;176:926–38. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duttlinger R, Manova K, Chu TY, Gyssler C, Zelenetz AD, Bachvarova RF, et al. W-sash affects positive and negative elements controlling c-kit expression: ectopic c-kit expression at sites of kit-ligand expression affects melanogenesis. Development. 1993;118:705–17. doi: 10.1242/dev.118.3.705. [DOI] [PubMed] [Google Scholar]
- 40.Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33:613–25. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prouvost-Danon A, Binaghi RA, Abadie A. Effect of heating at 56 degrees C on mouse IgE antibodies. Immunochemistry. 1977;14:81–4. doi: 10.1016/0019-2791(77)90284-1. [DOI] [PubMed] [Google Scholar]
- 42.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and FcγRIIb cross-linking. J Clin Invest. 2006;116:833–41. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinet JP. The high-affinity IgE receptor (FcƐRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–72. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 44.Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Habermann E, Walsch P, Breithaupt H. Biochemistry and pharmacology of the cortoxin complex. II. Possible interrelationships between toxicity and organ distribution of phospholipase A, crotapotin and their combination. Naunyn Schmiedebergs Arch Pharmacol. 1972;273:313–30. doi: 10.1007/BF00499666. [DOI] [PubMed] [Google Scholar]
- 46.Liu FT, Bohn JW, Ferry EL, Yamamoto H, Molinaro CA, Sherman LA, et al. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980;124:2728–37. [PubMed] [Google Scholar]
- 47.Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991;87:446–53. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schafer B, Piliponsky AM, Oka T, Song CH, Gerard NP, Gerard C, et al. Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice. J Allergy Clin Immunol. 2013;131:541–8. e1–9. doi: 10.1016/j.jaci.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–9. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 50.Artis D, Maizels RM, Finkelman FD. Forum: Immunology: Allergy challenged. Nature. 2012;484:458–9. doi: 10.1038/484458a. [DOI] [PubMed] [Google Scholar]
- 51.Risch M, Georgieva D, von Bergen M, Jehmlich N, Genov N, Arni RK, et al. Snake venomics of the Siamese Russell's viper (Daboia russelli siamensis) --relation to pharmacological activities. J Proteomics. 2009;72:256–69. doi: 10.1016/j.jprot.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Ishizaka K, Ishizaka T, Hornbrook MM. Physico-chemical properties of human reaginic antibody. IV. Presence of a unique immunoglobulin as a carrier of reaginic activity. J Immunol. 1966;97:75–85. [PubMed] [Google Scholar]
- 53.Johansson SG, Bennich H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology. 1967;13:381–94. [PMC free article] [PubMed] [Google Scholar]
- 54.Stanworth DR. The discovery of IgE. Allergy. 1993;48:67–71. doi: 10.1111/j.1398-9995.1993.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 55.Stanworth DR, Humphrey JH, Bennich H, Johansson SG. Specific inhibition of the Prausnitz-Kustner reaction by an atypical human myeloma protein. Lancet. 1967;2:330–2. doi: 10.1016/s0140-6736(67)90171-7. [DOI] [PubMed] [Google Scholar]
- 56.Bilo BM, Bonifazi F. Epidemiology of insect-venom anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8:330–7. doi: 10.1097/ACI.0b013e32830638c5. [DOI] [PubMed] [Google Scholar]
- 57.Russo AJ, Calton GJ, Burnett JW. The relationship of the possible allergic response to jellyfish envenomation and serum antibody titers. Toxicon. 1983;21:475–80. doi: 10.1016/0041-0101(83)90125-3. [DOI] [PubMed] [Google Scholar]
- 58.Haftenberger M, Laussmann D, Ellert U, Kalcklosch M, Langen U, Schlaud M, et al. [Prevalence of sensitisation to aeraoallergens and food allergens: results of the German Health Interview and Examination Survey for Adults (DEGS1)] [Article in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:687–97. doi: 10.1007/s00103-012-1658-1. [DOI] [PubMed] [Google Scholar]
- 59.Langen U, Schmitz R, Steppuhn H. [Prevalence of allergic diseases in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)] [Article in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:698–706. doi: 10.1007/s00103-012-1652-7. [DOI] [PubMed] [Google Scholar]
- 60.Sturm GJ, Heinemann A, Schuster C, Wiednig M, Groselj-Strele A, Sturm EM, et al. Influence of total IgE levels on the severity of sting reactions in Hymenoptera venom allergy. Allergy. 2007;62:884–9. doi: 10.1111/j.1398-9995.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 61.Bousquet J, Anto JM, Bachert C, Bousquet PJ, Colombo P, Crameri R, et al. Factors responsible for differences between asymptomatic subjects and patients presenting an IgE sensitization to allergens. A GA2LEN project. Allergy. 2006;61:671–80. doi: 10.1111/j.1398-9995.2006.01048.x. [DOI] [PubMed] [Google Scholar]
- 62.Golden DB, Marsh DG, Kagey-Sobotka A, Freidhoff L, Szklo M, Valentine MD, et al. Epidemiology of insect venom sensitivity. JAMA. 1989;262:240–4. [PubMed] [Google Scholar]
- 63.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–7. e1–13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Schafer T, Przybilla B. IgE antibodies to Hymenoptera venoms in the serum are common in the general population and are related to indications of atopy. Allergy. 1996;51:372–7. [PubMed] [Google Scholar]
- 65.Sturm GJ, Kranzelbinder B, Schuster C, Sturm EM, Bokanovic D, Vollmann J, et al. Sensitization to Hymenoptera venoms is common, but systemic sting reactions are rare. J Allergy Clin Immunol. 2014;133:1635–43. e1. doi: 10.1016/j.jaci.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 66.Hamilton RG. Allergic sensitization is a key risk factor for but not synonymous with allergic disease. J Allergy Clin Immunol. 2014;134:360–1. doi: 10.1016/j.jaci.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 67.Blumenthal MN. Genetic, epigenetic, and environmental factors in asthma and allergy. Ann Allergy Asthma Immunol. 2012;108:69–73. doi: 10.1016/j.anai.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Holgate ST. Genetic and environmental interaction in allergy and asthma. J Allergy Clin Immunol. 1999;104:1139–46. doi: 10.1016/S0091-6749(99)70005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holloway JW, Yang IA, Holgate ST. Genetics of allergic disease. J Allergy Clin Immunol. 2010;125:S81–94. doi: 10.1016/j.jaci.2009.10.071. [DOI] [PubMed] [Google Scholar]
- 70.McGowan EC, Bloomberg GR, Gergen PJ, Visness CM, Jaffee KF, Sandel M, et al. Influence of early-life exposures on food sensitization and food allergy in an inner-city birth cohort. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arumugam M, Ahrens R, Osterfeld H, Kottyan LC, Shang X, Maclennan JA, et al. Increased susceptibility of 129SvEvBrd mice to IgE-Mast cell mediated anaphylaxis. BMC Immunol. 2011;12:14. doi: 10.1186/1471-2172-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–77. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–53. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vaz NM, de Souza CM, Hornbrook MM, Hanson DG, Lynch NR. Sensitivity to intravenous injections of histamine and serotonin in inbred mouse strains. Int Arch Allergy Appl Immunol. 1977;53:545–54. doi: 10.1159/000231796. [DOI] [PubMed] [Google Scholar]
- 75.Muller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343–7. doi: 10.1097/01.all.0000173783.42906.95. [DOI] [PubMed] [Google Scholar]
- 76.Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887–98. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. Mechanisms of immunotherapy to wasp and bee venom. Clin Exp Allergy. 2011;41:1226–34. doi: 10.1111/j.1365-2222.2011.03812.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.