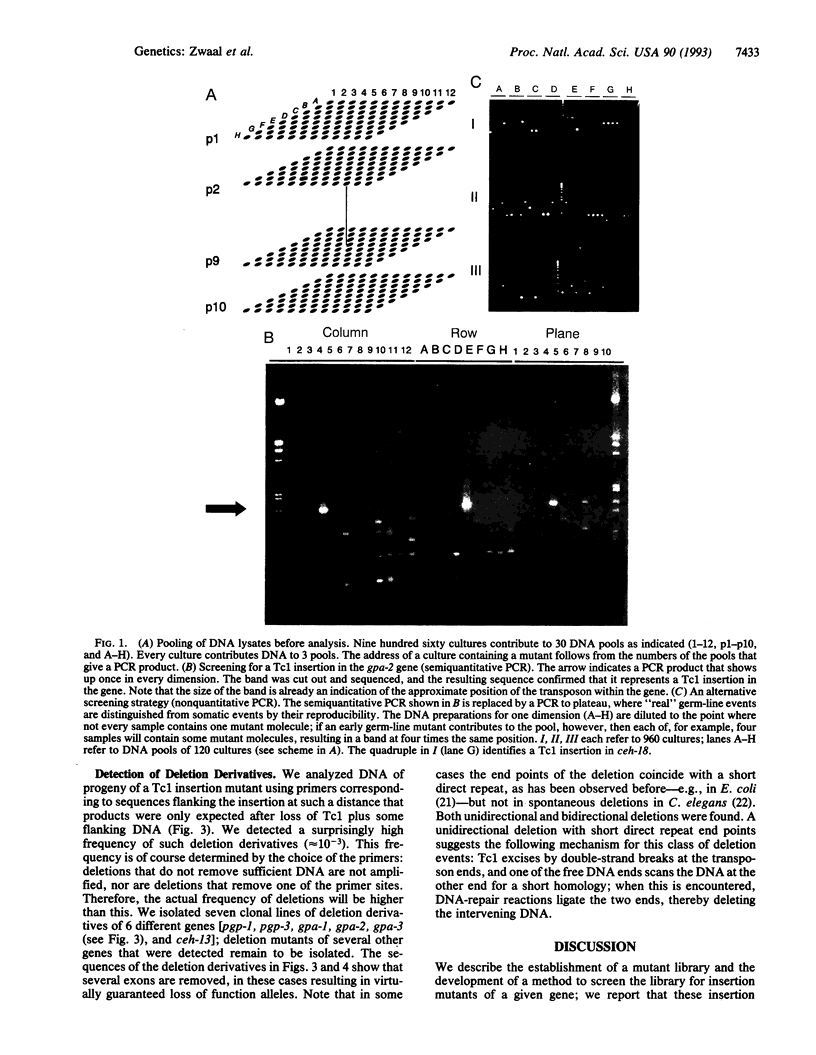
Abstract
To understand how genotype determines the phenotype of the animal Caenorhabditis elegans, one ideally needs to know the complete sequence of the genome and the contribution of genes to phenotype, which requires an efficient strategy for reverse genetics. We here report that the Tc1 transposon induces frequent deletions of flanking DNA, apparently resulting from Tc1 excision followed by imprecise DNA repair. We use this to inactivate genes in two steps. (i) We established a frozen library of 5000 nematode lines mutagenized by Tc1 insertion, from which insertion mutants of genes of interest can be recovered. Their address within the library is determined by PCR. (ii) Animals are then screened, again by PCR, to detect derivatives in which Tc1 and 1000-2000 base pairs of flanking DNA are deleted, and thus a gene of interest is inactivated. We have thus far isolated Tc1 insertions in 16 different genes and obtained deletion derivatives of 6 of those.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Ballinger D. G., Benzer S. Targeted gene mutations in Drosophila. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9402–9406. doi: 10.1073/pnas.86.23.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Saari B., Anderson P. Activation of a transposable element in the germ line but not the soma of Caenorhabditis elegans. Nature. 1987 Aug 20;328(6132):726–728. doi: 10.1038/328726a0. [DOI] [PubMed] [Google Scholar]
- Eide D., Anderson P. Insertion and excision of Caenorhabditis elegans transposable element Tc1. Mol Cell Biol. 1988 Feb;8(2):737–746. doi: 10.1128/mcb.8.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W., Yesner L. High-frequency excision of transposable element Tc 1 in the nematode Caenorhabditis elegans is limited to somatic cells. Cell. 1984 Mar;36(3):599–605. doi: 10.1016/0092-8674(84)90339-8. [DOI] [PubMed] [Google Scholar]
- Fino Silva I., Plasterk R. H. Characterization of a G-protein alpha-subunit gene from the nematode Caenorhabditis elegans. J Mol Biol. 1990 Oct 20;215(4):483–487. doi: 10.1016/s0022-2836(05)80160-3. [DOI] [PubMed] [Google Scholar]
- Kaiser K., Goodwin S. F. "Site-selected" transposon mutagenesis of Drosophila. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1686–1690. doi: 10.1073/pnas.87.5.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiff J. E., Moerman D. G., Schriefer L. A., Waterston R. H. Transposon-induced deletions in unc-22 of C. elegans associated with almost normal gene activity. Nature. 1988 Feb 18;331(6157):631–633. doi: 10.1038/331631a0. [DOI] [PubMed] [Google Scholar]
- Mazur P., Cole K. W., Hall J. W., Schreuders P. D., Mahowald A. P. Cryobiological preservation of Drosophila embryos. Science. 1992 Dec 18;258(5090):1932–1935. doi: 10.1126/science.1470915. [DOI] [PubMed] [Google Scholar]
- Moerman D. G., Kiff J. E., Waterston R. H. Germline excision of the transposable element Tc1 in C. elegans. Nucleic Acids Res. 1991 Oct 25;19(20):5669–5672. doi: 10.1093/nar/19.20.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Benian G. M., Moerman D. G., Waterston R. H. Transposable element Tc1 of Caenorhabditis elegans recognizes specific target sequences for integration. Proc Natl Acad Sci U S A. 1988 Feb;85(3):861–864. doi: 10.1073/pnas.85.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K. Searching for needles in haystacks via the polymerase chain reaction. Trends Genet. 1990 Jul;6(7):202–203. [PubMed] [Google Scholar]
- Plasterk R. H., Groenen J. T. Targeted alterations of the Caenorhabditis elegans genome by transgene instructed DNA double strand break repair following Tc1 excision. EMBO J. 1992 Jan;11(1):287–290. doi: 10.1002/j.1460-2075.1992.tb05051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk R. H. Reverse genetics of Caenorhabditis elegans. Bioessays. 1992 Sep;14(9):629–633. doi: 10.1002/bies.950140911. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H. The origin of footprints of the Tc1 transposon of Caenorhabditis elegans. EMBO J. 1991 Jul;10(7):1919–1925. doi: 10.1002/j.1460-2075.1991.tb07718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulak R. A., Anderson P. Structures of spontaneous deletions in Caenorhabditis elegans. Mol Cell Biol. 1988 Sep;8(9):3748–3754. doi: 10.1128/mcb.8.9.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig B., Liao L. W., Hirsh D. Sequence of the C. elegans transposable element Tc1. Nucleic Acids Res. 1983 Jun 25;11(12):4201–4209. doi: 10.1093/nar/11.12.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig B., Liao L. W., Hirsh D. Sequence of the C. elegans transposable element Tc1. Nucleic Acids Res. 1983 Jun 25;11(12):4201–4209. doi: 10.1093/nar/11.12.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushforth A. M., Saari B., Anderson P. Site-selected insertion of the transposon Tc1 into a Caenorhabditis elegans myosin light chain gene. Mol Cell Biol. 1993 Feb;13(2):902–910. doi: 10.1128/mcb.13.2.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz H. K., Cline T. W., Schedl P. Functional changes associated with structural alterations induced by mobilization of a P element inserted in the Sex-lethal gene of Drosophila. Genetics. 1987 Oct;117(2):221–231. doi: 10.1093/genetics/117.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J., Du Z., Thomas K., Wilson R., Hillier L., Staden R., Halloran N., Green P., Thierry-Mieg J., Qiu L. The C. elegans genome sequencing project: a beginning. Nature. 1992 Mar 5;356(6364):37–41. doi: 10.1038/356037a0. [DOI] [PubMed] [Google Scholar]
- Tsubota S., Schedl P. Hybrid dysgenesis-induced revertants of insertions at the 5' end of the rudimentary gene in Drosophila melanogaster: transposon-induced control mutations. Genetics. 1986 Sep;114(1):165–182. doi: 10.1093/genetics/114.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. D., Schrank B., Huynh C., Shownkeen R., Waterston R. H. A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics. 1992 Jul;131(3):609–624. doi: 10.1093/genetics/131.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]