Abstract
Legionella pneumophila , the intracellular pathogen that can cause severe pneumonia known as Legionnaire’s disease, translocates close to 300 effectors inside the host cell using Dot/Icm type IVB secretion system. The structure and function for the majority of these effector proteins remains unknown. Here, we present the crystal structure of the L. pneumophila effector Lem10. The structure reveals a multidomain organization with the largest C-terminal domain showing strong structural similarity to the HD protein superfamily representatives. However, Lem10 lacks the catalytic His-Asp residue pair and does not show any in vitro phosphohydrolase enzymatic activity, typical for HD proteins. While the biological function of Lem10 remains elusive, our analysis shows that similar distinct features are shared by a significant number of HD domains found in Legionella proteins, including the SidE family of effectors known to play an important role during infection. Taken together our data point to the presence of a specific group of non-catalytic Legionella HD domains, dubbed LHDs, which are involved in pathogenesis.
Keywords: X-ray crystallography, multidomain protein, gram-negative pathogen, secretion system substrate
INTRODUCTION
Legionella pneumophila is the causative agent of Legionnaires pneumonia. This Gram-negative intracellular pathogen has the largest known arsenal of bacterial effector proteins that are delivered inside the host organism upon infection through a Type 4B secretion system (T4BSS).1–3 While the T4BSS has been shown to be indispensable for infection,4,5 the majority of the effectors it secretes do not confer a discernible physiological phenotype in gene deletion studies.6,7 This finding complicates functional analysis for such effectors using genetic tools, while unique sequence content for many of the effector proteins further exacerbates the challenge of the functional assignment through bioinformatics and classical biochemical approaches.
Lem10 (Legionella effector identified by machine learning) is a known substrate for the L. pneumophila T4BSS, the function and structure of which remained unknown until this work. The Lem10 open reading frame (lpg1496) spans close to 600 residues; however, it does not show significant sequence similarity to any functionally characterized domain. Notably, the C-terminal half of the Lem10 protein sequence is conserved and widely distributed among other Legionella proteins suggesting that it constitutes a distinct functional domain. To shed light on the potential function of Lem10 and the reason for its distinct conservation within the Legionella spp. we undertook a structural characterization thereof. The de novo crystal structure of Lem10 reveals multidomain architecture of this protein, and the conserved C-terminal domain places this effector as a new member within the HD protein superfamily.
The HD domains are generally present in multidomain enzymes involved in nucleic acid metabolism and signal transduction through secondary messenger homeostasis.8 This function is accomplished via a metal-dependent phosphohydrolase activity with a strictly conserved His-Asp catalytic residue pair, key for metal coordination and giving the superfamily its name.8 Examples of well-characterized HD superfamily members from Gram-negative bacteria include RelA/SpoT proteins from Escherichia coli that are responsible for regulation of guanosine 5′-diphosphate 3′-diphosphate (ppGpp) concentrations in response to environmental cues9,10; and the regulation of biofilm formation by HD domain proteins through hydrolysis of the second messenger, cyclic-di-GMP, in Pseudomonas aeruginosa.11 Several members of HD domain superfamily, however, have been reported to lack active site residues required for catalytic activity while still being involved in signaling.11,12 Structural analysis of Legionella HD domain of Lem10 described in this work, suggests that this protein belongs to this particular group of HD proteins lacking catalytic residues. This structural information, representing, to our knowledge, the first report of an HD domain in the Legionella spp., provides direction for further exploration of the potential molecular role of the Legionella HD domain in this pathogen’s signaling mechanisms and physiology.
MATERIALS AND METHODS
Protein production and purification
Full-length lpg1496 sequence was cloned by ligase-independent cloning into the p15Tv-LIC vector, providing an N-terminal His6-tag fusion followed by a TEV protease cleavage site between the tag and residue 1 of Lem10. Selenomethionine (Se-Met)-substituted Lem10 was expressed using the standard M9 high yield growth procedure according to the manufacturer’s instructions (Shanghai Medicilon; catalog number MD045004-50), with E. coli BL21(DE3) codon plus cells, and purified by Ni-affinity chromatography. Cells were resuspended in binding buffer [100 mM HEPES pH 7.5, 500 mM NaCl, 5 mM imidazole, and 5% glycerol (v/v)], lysed using a sonicator, and cell debris were removed via centrifugation at 30,000g. Cleared lysate was loaded onto a 5 mL Ni-NTA column (QIAGEN) pre-equilibrated with binding buffer, extensively washed with binding buffer containing 30 mM imidazole, and proteins were eluted using the above buffer with 250 mM imidazole. Fractions containing the protein of interest were identified by SDS-polyacrylamide gel electrophoresis, pooled together and dialyzed overnight at 4°C with buffer: 0.3M NaCl, 50 mM HEPES, pH 7.5, 5% glycerol, and 0.5 mM tris[2-carboxyethyl]phosphine.
Protein crystallization
Se-Met-substituted Lem10 was concentrated to 98 mg/mL and treated with Trypsin at 1:2500 w/w ratio prior to crystallization set up. The crystallization trials were set up at room temperature using the hanging drop method, with 2 μL of protein solution mixed with 2 μL of reservoir solution. The crystallization solution that produced crystals contained 0.15M MgCl2, 0.1M Tris pH 8.4, 24% PEG3350, 5% DMSO, and 0.5 mM CaCl2. Crystals were cryo protected with 5%DMSO, 10% Ethylene Glycol, 2% Sucrose, 7% Glycerol, and flash frozen in liquid nitrogen for data collection.
X-ray data collection and structure determination
Diffraction data were collected at 100 K using an ADSC quantum Q315r charged coupled device detector at the 19ID beamline of the Structural Biology Center at the Advanced Photon Source, Argonne National Laboratory. Single wavelength anomalous dispersion (SAD) data at the selenium absorption peak were collected from a SeMet-substituted protein. The diffraction data were processed using the HKL3000 suite of programs.13
The initial phases were obtained with the SAD method using selenium peak data. All procedures for SAD phasing, phase improvement by density modification, and initial protein model building were performed through the structure module of the HKL3000 software package.13 The initial phases were obtained through the SHELX program suite included in the HKL3000 package; then the model-building module of HKL3000, ARP/wARP built 605 residues, with 505 of them placed correctly. The initial model was rebuilt with graphics program COOT14 and refined with PHENIX.15 The final model contained residues corresponding the N-terminal domain (1–126) and the C-terminal domain (297–590); residues 127–296 could not be built in the final model due to poor electron density. The geometrical properties of the model were assessed using COOT and Molprobity.16 Data collection and refinement statistics are summarized in Table I. Coordinates and structure factors for the crystal structures of Lem10 were deposited as a Protein Data Bank entry 5BQ9.
Table I.
X-ray Diffraction Data Collection and Refinement Statistics
| Wavelength (Å) | 0.97918 |
| Spacegroup | P21 |
| Cell parameters (Å, °) | a =74.4; b =76.6; c =76.7; β=96.7 |
| Resolution range (Å) | 50–2.28 (2.34–2.28) |
| Unique reflections | 38820 (1928) |
| Multiplicity | 6.8 (6.5) |
| Completeness (%) | 1. (98.8) |
| Mean I/sigma(I) | 1. (3.32) |
| R-merge | (0.861) |
| R-work | .207) |
| R-free | .252) |
| Number of atoms | 6950 |
| Nonsolvent | 6752 |
| Water | 198 |
| r.m.s.d. | |
| Bond length | 0.002 |
| Angles | 0.594 |
| Ramachandran | |
| Favored (%) | 95.8 |
| Outliers (%) | 0 |
| Molprobity clashscore | 2.63 |
| Average B-factor (Å2) | 45.2 |
Modeling of the middle domain
The middle domain of Lem10 (residues 127–296) was lacking in the refined crystal structure most likely due to the trypsinolysis treatment during crystallization trials. We then modeled the missing part of the protein using PHYRE2 server.17 Obtained model encompassed residues 203–284 (47% coverage of missing sequence) of Lem10 and had 69.8% confidence level.
Phosphodiesterase activity assay
The method for the phosphodiesterase assay was adapted from A. Yakunin et al.18 and comprised of a 2 step alkaline phosphatase coupled reaction. In step 1, 3 μg of purified Lem10 enzyme was incubated with 1 mM substrate (cAMP or cGMP) in the reaction buffer (30 mM KCl, 10 mM HEPES pH7.5, 5 mM MgCl2) for 1 h at room temperature. The final reaction volume was 100 μL set-up in 96-well plate format in triplicate. In step 2, a final concentration of 0.04 U/μL of shrimp alkaline phosphatase (Fermentas) in 100 μL of 1M CHES buffer pH 9.0, 1M MgCl2 was added to the reaction mixture, and incubated for additional 10 min at 37°C. The reaction was subsequently quenched with Malachite Green reagent, and detection of released phosphate carried out by measuring absorbance at 630 nm using SpectraMax plate reader (Molecular Devices). 4-Nitrophenyl phosphate was used as a substrate in positive control experiments to ensure efficient performance of shrimp alkaline phosphatase under the described assay conditions. A Legionella pneumophila effector protein Lpg439, not known to possess phosphodiesterase activity, was used as a negative background control. No Lem10-specific phosphodiesterase activity was detected (Supporting Information Fig. S1).
RESULTS AND DISCUSSION
Lem10 structure reveals multidomain protein organization
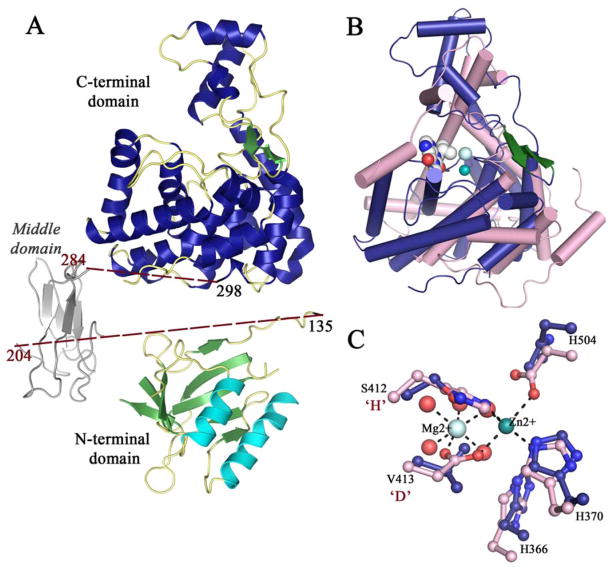
The crystal structure of Lem10, determined to 2.3 Å resolution, shows the presence of two protein monomers per asymmetric unit. Limited number of contacts between the monomers with an interface covering a surface area of 640 Å2 as well as asymmetric nature of these contacts suggest that the observed association is due crystal packing only, and that Lem10 exists as a monomer in solution. There are three separate domains within the protein monomer: the N-terminal (residues 1–130), middle (131–300), and C-terminal domain (300–590). The N-terminal domain forms an α,β-structure comprised of two β-sheets and an α-helical bundle forming a three layered sandwich [Fig. 1(A)]. Both β-sheets in the sandwich have antiparallel strand arrangement, with one sheet composed of five and the other of four strands. Structure classification analysis using CATH database19 revealed that the complete fold of the N-terminal domain does not show significant similarity to any known structure. However, the combination of the α-helical bundle and the central five stranded β-sheet forms the architectural unit known as a 2-layer sandwich with most similarity to proteins with α-β plaits topology.
Figure 1.
A: A ribbon diagram of the overall Lem10 structure. The multidomain organization is highlighted with helices colored in cyan for the N-terminal domain and in blue for the C-terminal domain. A model of the missing middle domain is in gray. B: A ribbon diagram of a superposition of the Lem10 C-terminal HD domain with a homologous PDE from Leishmania major (LmjPDEB1, PDB ID: 2R8Q).21 Lem10 is in blue and green, LmjPDEB1 in pink. The inhibitor bound in the LmjPDEB1 active site is in gray space-fill representation. C: A close-up view of the superposition of the conserved pocket of Lem10 and with the active site of LmjPDEB1. Strictly conserved residues are shown in ball-and-stick representation, color-coding as in (B). Metal ions from LmjPDEB1 are shown as spheres with coordination bonds shown as dotted lines. Residues from Lem10 are labeled, with the substitutions of the consensus “HD” indicated.
The middle domain is missing in the final structure most probably due to the protease treatment used during crystallization process (see Material and Methods for details). The effort to model this domain using the Phyre2 server identified weak homology thereof with forkhead-associated (FHA) domain proteins. FHA proteins follow a β-sandwich fold containing two antiparallel β-sheets, and are generally known to be involved in phosphopeptide-mediated signalling.20 However, due to high degree of uncertainty in the model prediction, caution is warranted in interpreting these observations in further detail. The C-terminal domain is almost exclusively comprised of α-helices except for a small β-hairpin [Fig. 1(A)].
Lem10 belongs to a new subgroup within HD domain superfamily
Remarkably, comparative structural analysis revealed that the C-terminal domain fold belongs to the HD superfamily of metal-dependent phosphohydrolases,8 and to our knowledge this is the first report of an HD domain structure from a Legionella spp. The closest structural homologues within this superfamily as identified by the DALI server21 are cyclic-nucleotide specific phosphodiesterases (PDEs; Supporting Information Table S1), with the best match belonging to the PDE from Leishmania major (LmjPDEB1, PDB ID: 2R8Q).22 While Lem10 shares only 10% identity with the LmjPDEB1 on sequence level, the RMSD for structural superposition is 3.0 Å [Fig. 1(B)] [mt]139 Cα positions. The superposition reveals that the core helices usually harboring residues responsible for metal coordination show the most similarity in conformation, with the two structures becoming more divergent away from the putative ligand binding pocket. This is especially apparent for the small three helix bundle subdomain located at the top of the structure in the orientation presented in Figure 1(B), where the equivalent of the long helix in LmjPDEB1 is absent in Lem10. Noteworthy is that the small β-hairpin observed in Lem10 is not present in LmjPDEB1.
Structural similarity with bona fide HD PDEs prompted us to search Lem10 structure for a putative active site. However, close examination of corresponding pocket in the Lem10 structure reveals that the residues known to be essential for metabolite hydrolysis are not conserved in its HD domain. More specifically, in the Lem10 structure, the positions corresponding to catalytic His and Asp are occupied by Ser412 and Val413, respectively; while another essential metal-coordinating position is occupied by His504 instead of the conserved Asp [Fig. 1(C)]. The observation of these alterations in the active site composition is consistent with a lack of any detectable cyclic nucleotide hydrolase activity in our experiments (Supporting Information Fig. S1). Reports of inactive HD domains have precedence in the literature. An inactive RelA hydrolase homolog, whose physiological relevance has not been explored, is found in E. coli,12 while in P. aeruginosa, a catalytically inactive HD domain affects bacteria’s motility through an unknown mechanism.11
Sequence analysis reveals more members of noncatalytic HD domain proteins in Legionella spp
To gain further insight into a potential functional role of the Lem10 HD domain an extensive sequence analysis was performed. A protein BLAST search of the NCBI database identified the presence of homologous HD domains in other effector proteins from L. pneumophila, with sequence identity of up to 30%. Noteworthy is the presence of the SidE family of effectors (SdeA, SdeB, SdeC, and SidE), important for L. pneumophila virulence, among the identified sequence homologs.23,24
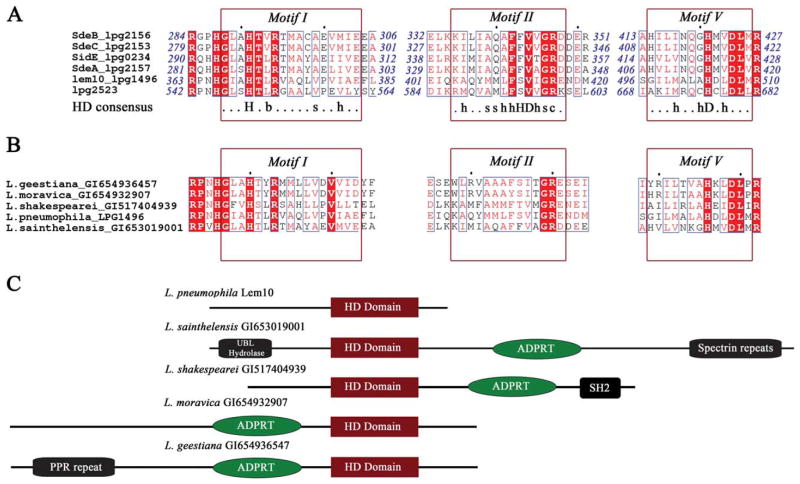
Sequence alignment of the homologous regions shows that residues comprising the canonical HD fingerprint regions are functionally conserved in Lem10 and its homologs, however, similarly to Lem10, these Legionella proteins lack the three aforementioned catalytic residues [Fig. 2(A)]. Of the three motifs that are conserved across the entire HD superfamily, Motif I shows complete conservation in the L. pneumophila HD domain, while motif II and V harbor the substitutions in catalytic residues with the amino acids around these conserved positions holding the general properties of the consensus motif.
Figure 2.
A: Sequence alignment of Lem10 with homologous regions of other L. pneumophila effector proteins. Strictly conserved residues are highlighted in red, and functionally conserved residues are shown in red font. Sequence regions that correspond to the conserved motifs of the HD superfamily are indicated by red boxes, and the HD consensus sequence6 is shown under the alignment. In the consensus sequence, capital letters stand for residue names; lower case letters designate the following: b: bulky, s: small, h: hydrophobic, c: charged, x: any residue. B: Sequence alignment of Lem10 with homologous regions of proteins from other Legionella species. Annotation and color-coding as in (A). C: A cartoon representation of the predicted domain organization for the full sequences of the proteins containing homologous HD domains shown in (B).
Furthermore, other species of the Legionella genus also contain proteins with HD domains similar to the one we described for Lem10. Of the 18 Legionella genomes available through the NCBI database, 5 contain at least one Lem10-like HD domain sequence. Representative homologous sequences were selected from each organism based on the highest degree of sequence identity with the Lem10 HD domain, and their alignment shows the same motif conservation as described above for the L. pneumophila proteins [Fig. 2(C)]. Altogether these observations suggest an existence of a unique subgroup of the HD superfamily proteins in the Legionella genus, which we termed “Legionella HD domain” or LHD domain in the analysis that follows. This subgroup contains the following motifs: GxxHxbR (consistent with HD consensus Motif I), xhxxsxhF(F/S)(V/I)hGR (Motif II), and xxxhxxsHx(V/L)DL (Motif V; nomenclature defined is Fig. 2).
Implications for Lem10 biological function
All of the identified LHD domain sequences are part of multi-domain ensembles [Fig. 2(B)]. This observation is common for the HD protein superfamily, where the domain architecture has been reported to include helicases, nucleotidyl transferases, and signaling proteins, among others.8 A well-characterized example of a frequently observed domain combination, is that of an HD domain associating with a two-component receiver domain, important for cyclic di-GMP homeostasis.25
Probing the domain organization of the examined Legionella spp. sequences bioinformatically with the HHpred server26 shows that the LHD domain does not appear to be part of a previously described domain architecture. The overall sequence length as well as the location of discrete domains within the sequence vary dramatically for the various multidomain proteins [Fig. 2(C)]. Noteworthy is the frequent association of the HD domain with a predicted ADP-ribosyltransferase (ADPRT) toxin domain, found in four out of five analyzed sequences. These toxins, generally found in Gram-positive pathogenic organisms, are known to misregulate host processes such as actin cytoskeleton and microtubule dynamics by post-translationally modifying key host small GTPases.27 The toxin-specific activity has not been reported for Legionella, however, the ADPRTs have been detected28 and a structure of a homolog, IcmQ, critical for T4BSS functionality, has been described.29 These reports confirm the presence of ADPRT domains in Legionella, however their specific function, especially of those associated with HD domains, remains to be investigated.
In conclusion, structural investigation of the Legionella effector Lem10 points to a yet unknown role in cell signaling. This hypothesis is supported by the presence of a potential FHA domain and that of a novel HD domain as part of a multi-domain ensemble within this protein. The wide distribution of the Lem10 HD domain in the Legionella genus, its strict conservation, and consistent occurrence within multi-domain proteins speak to its potential significance in the secondary messenger pathways and hence Legionella physiology and host interaction. These observations provide strong impetus for further scientific pursuit of the function of this domain, especially for the effectors from virulent species with known phenotypes such as those from the SidE effector family of L. pneumophila.
Supplementary Material
Acknowledgments
The results shown in this report are derived from work performed at Argonne National Laboratory, at the Structural Biology Center of the Advanced Photon Source. Argonne is operated by University of Chicago Argonne, LLC.
Grant sponsor: NIH PSI Grants; Grant numbers: GM74492 and GM094585; Grant sponsor: Canadian Institutes of Health Research; Grant number: MOP-13340; Grant sponsor: Natural Sciences and Engineering Research Council of Canada; Grant number: RGPIN-2014-03641; Grant sponsor: U.S. Department of Energy, Office of Biological and Environmental Research; Grant number: DE-AC02-06CH11357.
Footnotes
All authors declare no conflict of interests.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Burstein D, Zusman T, Degtyar E, Viner R, Segal G, Pupko T. Genome-scale identification of Legionella pneumophila effectors using a machine learning approach. PLoS Pathog. 2009;5:e1000508. doi: 10.1371/journal.ppat.1000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang L, Boyd D, Amyot WM, Hempstead AD, Luo ZQ, O’Connor TJ, Chen C, Machner M, Montminy T, Isberg RR. The E Block motif is associated with Legionella pneumophila translocated substrates. Cell Microbiol. 2011;13:227–245. doi: 10.1111/j.1462-5822.2010.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu W, Banga S, Tan Y, Zheng C, Stephenson R, Gately J, Luo ZQ. Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PloS One. 2011;6:e17638. doi: 10.1371/journal.pone.0017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel JP, Andrews HL, Wong SK, Isberg RR. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 5.Segal G, Purcell M, Shuman HA. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Pnas. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Connor TJ, Adepoju Y, Boyd D, Isberg RR. Minimization of the Legionella pneumophila genome reveals chromosomal regions involved in host range expansion. Pnas. 2011;108:14733–14740. doi: 10.1073/pnas.1111678108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor TJ, Boyd D, Dorer MS, Isberg RR. Aggravating genetic interactions allow a solution to redundancy in a bacterial pathogen. Science. 2012;338:1440–1444. doi: 10.1126/science.1229556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aravind L, Koonin EV. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci. 1998;23:469–472. doi: 10.1016/s0968-0004(98)01293-6. [DOI] [PubMed] [Google Scholar]
- 9.Heinemeyer EA, Richter D. Characterization of the guanosine 5′-triphosphate 3′-diphosphate and guanosine 5′-diphosphate 3′-diphosphate degradation reaction catalyzed by a specific pyrophosphorylase from Escherichia coli. Pnas. 1978;75:4180–4183. doi: 10.1021/bi00618a007. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson GC, Tenson T, Hauryliuk V. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PloS One. 2011;6:e23479. doi: 10.1371/journal.pone.0023479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan RP, Lucey J, O’Donovan K, McCarthy Y, Yang L, Tolker-Nielsen T, Dow JM. HD-GYP domain proteins regulate biofilm formation and virulence in Pseudomonas aeruginosa. Environ Microbiol. 2009;11:1126–1136. doi: 10.1111/j.1462-2920.2008.01842.x. [DOI] [PubMed] [Google Scholar]
- 12.Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 13.Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: the integration of data reduction and structure solution-from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- 14.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 15.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, III, Snoeyink J, Richardson JS, Richardson DC. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 wet portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yakunin AF, Proudfoot M, Kuznetsova E, Savchenko A, Brown G, Arrowsmith CH, Edwards AM. The HD domain of the Escherichia coli tRNA nucleotidyltransferase has 2′,3′-cyclic phosphodiesterase, 2′-nucleotidase, and phosphatase activities. J Biol Chem. 2004;279:36819–36827. doi: 10.1074/jbc.M405120200. [DOI] [PubMed] [Google Scholar]
- 19.Sillitoe I, Lewis TE, Cuff AL, Das S, Ashford P, Dawson NL, Furnham N, Laskowski RA, Lee D, Lees J, Lehtinen S, Studer R, Thornton JM, Orengo CA. CATH: comprehensive structural and functional annotations for genome sequences. Nucleic Acids Res. 2015;43:D376–381. doi: 10.1093/nar/gku947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao H, Byeon IJ, Tsai MD. Structure and function of a new phosphopeptide-binding domain containing the FHA2 of Rad53. J Mol Biol. 1999;294:1041–1049. doi: 10.1006/jmbi.1999.3313. [DOI] [PubMed] [Google Scholar]
- 21.Holm L, Rosenström P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545–549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Yan Z, Geng J, Kunz S, Seebeck T, Ke H. Crystal structure of the Leishmania major phosphodiesterase LmjPDEB1 and insight into the design of the parasite-selective inhibitors. Mol Microbiol. 2007;66:1029–1038. doi: 10.1111/j.1365-2958.2007.05976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong KC, Sexton JA, Vogel JP. Spatiotemporal regulation of a Legionella pneumophila T4SS substrate by the metaeffector SidJ. PLoS Pathog. 2015;11:e1004695. doi: 10.1371/journal.ppat.1004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang B, Kura F, Amemura-Maekawa J, Koizumi N, Watanabe H. Identification of a novel adhesion molecule involved in the virulence of Legionella pneumophila. Infect Immun. 2005;73:4272–4280. doi: 10.1128/IAI.73.7.4272-4280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 26.Söding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33:W244–248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koch-Nolte F, Reche P, Haag F, Bazan F. ADP-ribosyltransferases: plastic tools for inactivating protein and small molecular weight targets. J Biotechnol. 2001;92:81–87. doi: 10.1016/s0168-1656(01)00356-x. [DOI] [PubMed] [Google Scholar]
- 28.Belyi YF, Tartakovskii IS, Vertiev YV, Prosorovskii SV. Partial purification and characterization of ADP-ribosyltransferase produced by Legionella pneumophila. Biomed Sci. 1991;2:169–174. [PubMed] [Google Scholar]
- 29.Farelli JD, Gumbart JC, Akey IV, Hempstead A, Amyot W, Head JF, McKnight CJ, Isberg RR, Akey CW. IcmQ in the Type 4b secretion system contains an NAD+ binding domain. Structure. 2013;21:1361–1373. doi: 10.1016/j.str.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.