Abstract
Background
Chronic rhinosinusitis (CRS) has a broad range of co-morbidities. Due to a lack of longitudinal studies, it is not known whether these co-morbidities cause CRS, are promoted by CRS, or share a systemic disease process with CRS.
Objective
To determine the risk of incident disease within five years after a new diagnosis of CRS with nasal polyps (CRSwNP) and without nasal polyps (CRSsNP).
Methods
We conducted a case-control study nested within the longitudinal cohort of primary care patients in the Geisinger Clinic using electronic health record data. We evaluated incident disease over 5 years in newly diagnosed CRSwNP and CRSsNP cases compared to controls using multivariable Cox regression models.
Results
CRSsNP (n=3612) cases were at greater risk (HR, 95% confidence interval) than controls for incidence of: upper airway diseases, including adenotonsillitis (3.29, 2.41–4.50); lower aerodigestive tract diseases, including asthma (2.69, 2.14–3.38); epithelial conditions, including atopic dermatitis (2.75, 1.23–6.16); and hypertension (1.38, 1.19–1.61). CRSwNP (n=241) cases were at greater risk for obesity than controls (1.74, 1.08–2.80), but CRSwNP was not associated with other diseases.
Conclusion
The risk of other diseases associated with CRS adds to the burden of an already highly burdensome condition, and suggests either that CRS promotes onset of other diseases or is an indicator of systemic disease processes. Different patterns of association with diseases by CRS phenotype may be due to CRSwNP sample size imitations or reflect a different pattern of disease onset by phenotype. These findings have implications for screening guidelines and care of CRS patients.
Keywords: CRS, nasal polyps, epidemiology
INTRODUCTION
Chronic rhinosinusitis (CRS) is a highly prevalent and burdensome condition of the nose and paranasal sinuses. In the United States CRS is estimated to affect 31 million people, more than diseases such as asthma and diabetes.1–4 Co-morbidities associated with CRS, including asthma and chronic obstructive pulmonary disease (COPD), are highly burdensome, and in some cases, associated with high morbidity and mortality.5–7 It is not known whether these relationships are causal or the result of shared etiology. Observations of co-occurrence might also reflect part of a natural progression of diseases, analogous to the atopic march, whereby an exposure could initiate a systemic response and predispose individuals to development of one or more diseases over time.8 Insights into the reasons for the co-occurrence of disease with CRS could provide guidance on the prevention and management of this highly prevalent disease.
There are a number of potential explanations for the co-morbidities associated with CRS. Consistent with the unified airway model, the pathophysiological processes of CRS could expand to involve other parts of the airways, leading to the onset of other airway diseases.9,10 Defects in the epithelial barrier that may predispose individuals to develop CRS could put CRS patients at risk for other diseases involving epithelial surfaces such as atopic dermatitis and psoriasis.11,12 Finally, exposure to inflammatory cytokines found in the sinuses of CRS patients could increase the risk for inflammatory conditions outside of the airway.13,14
Most of the studies that have reported co-morbid associations lack the longitudinal data needed to assess the temporal relationship between incident CRS and other diseases, limiting any conclusions about what accounts for these associations.5–7,15 Retrospective electronic health record (EHR) datasets provide the longitudinal data needed to study the natural history of the occurrence of CRS relative to other diseases. We previously used EHR data to determine what diseases precede a diagnosis of CRS. We reported that patients with CRS had a higher prevalence of acute rhinosinusitis, allergic rhinitis, asthma, and other conditions before diagnosis than controls.16 The objective of the present study was to use EHR data to learn what diseases occur after a CRS diagnosis. We used longitudinal data from the EHR of Geisinger Clinic (GC) to determine the risk of incident diseases within five years after a new diagnosis of CRS with nasal polyps (CRSwNP) or CRS without nasal polyps (CRSsNP) in a primary care population.
METHODS
Study Overview
We conducted a case-control study nested within the longitudinal cohort of GC’s primary care patients using EHR data. We identified patients with a new diagnosis of a CRS phenotype (CRSwNP or CRSsNP) between 2004 and 2007. We then evaluated incident disease over a five year period in the CRS cases compared to controls without CRS. Prior to analysis we selected diseases with plausible pathophysiological links to CRS, including upper airway diseases, lower aerodigestive diseases, epithelial diseases, systemic autoimmune diseases, and conditions linked to inflammation, including heart and vascular diseases. This study was approved by Geisinger’s Institutional Review Board.
Study Setting and Data Source
GC provides care to more than 400,000 primary care patients residing across a 44-county region of Pennsylvania. The primary care population is representative of the general population in the region. Age and sex distribution of the GC population is similar to that of the census data for the region.17 We used EHR data available from 2001 to 2012 to identify CRS cases and controls and compare these populations. All data used in this analysis was extracted from the GC EHR.
Identification of CRS patients and controls
We confined our study to patients with an initial diagnosis of a CRS subtype between 2004 and 2007. To be considered an incident case, primary care patients had to have at least two ICD-9 codes for a CRS subtype (CRSwNP – 471.X; CRSsNP – 473.X) associated with an outpatient, inpatient, or emergency department encounter, with the first ICD-9 code appearing between 2004 and 2007. A comparison group of primary care patients with no ICD-9 code for CRS was frequency matched to each of the CRS subtypes on age strata and visit year.
Outcome measurement: Incident disease
We evaluated associations between new diagnosis of CRS and incident development of selected relevant diseases among patients with CRS and controls. For chronic conditions, patients were classified as having a new diagnosis of disease if they had a disease ICD-9 code associated with at least two encounters (inpatient, outpatient, or emergency department) in the record within 5 years following the CRS diagnosis, and the ICD-9 code had not appeared in the record previously. For acute conditions, such as conjunctivitis, we required only one ICD-9 code. Exceptions included hypertension, obesity, myocardial infarction (MI), and stroke. We classified patients as having hypertension if they met any of the following criteria: at least 2 hypertension diagnoses associated with outpatient encounters; at least one antihypertensive medication prescribed in association with a diagnosis of hypertension; or at least three measures of systolic blood pressure greater than 140 or diastolic blood pressure greater than 90. We defined obesity as a body mass index (BMI) ≥ 30 kg/m2, calculated using weight and height data recorded in the EHR. MI and ischemic stroke were defined as at least one ICD-9 code associated with an inpatient discharge diagnosis. To capture ischemic stroke we included codes for both ischemic and ill-defined stroke.
Statistical Analysis
We used multilevel, multivariable Cox regression models to estimate hazard ratios for the development of each incident disease for each CRS phenotype. Patient-level variables were derived from EHR data and included age, sex, race/ethnicity (black, Hispanic, white), smoking status (current, previous, never use), alcohol use (current, previous, never use), socioeconomic status (SES) (ever vs. never on medical assistance), and BMI. Diabetes, hypertension, and asthma status (each ever vs. never) were also included in the models. However, the co-morbid variable was removed from the model when estimating the hazard ratio (HR) for the same disease (e.g., diabetes was removed when estimating the HR for diabetes). SES was a binary variable, where a patient was considered as residing in a household with low SES if he or she used a medical assistance program as health insurance for at least one healthcare encounter.18 Community-level variables, based on the geocoded home addresses of patients, were also included in the model. A continuous community-level socioeconomic deprivation score was derived using previously described methods and community type was categorized as township, borough, or census tract.19 The community type was taken as a random factor in the model to control the variability within each community type. We tested the above variables for linearity. After determining the final models, we further tested for effect modification by family SES, community-level deprivation, age, and sex by adding cross-product (“interaction”) terms one at a time to the final model.
The majority of cases had no objective evaluation of their sinus symptoms (e.g., sinus CT, endoscopy procedure) recorded in the EHR. In the absence of objective testing, symptom reporting can alter the likelihood of diagnosis. Thus, we conducted univariate analysis to determine whether cases and controls differed in prevalence of co-morbid depression or anxiety, as negative affect increases reporting of symptoms, regardless of the presence of physical illness.10,20 However, no significant differences between cases and controls in these diagnoses were observed. Therefore, in the interest of developing parsimonious models, we did not include these diagnoses in our analysis.
Potential pathways between CRS and subsequent disease include exposure to medications used to treat CRS. Oral corticosteroid (OCS) use has been linked to side effects such as obesity and hypertension.21–23 To distinguish between the effect of CRS versus the effect of treatment for CRS on onset of incident disease, we created two variables to account for OCS’s effect. The first variable was a binary variable to indicate an OCS prescription at time of onset of post-morbid disease and the second variable was a continuous variable that indicated the “intensity” of OCS prescription. The continuous variable was based on the cumulative number of OCS prescription orders during the time between diagnosis of CRS and onset of disease divided by the time interval. For conditions that have been linked to OCS use, including hypertension and obesity, we added this variable to the model and assessed whether this addition impacted the association between CRS and post-morbid disease.
Our final definition for CRS required that a patient have at least 2 ICD-9 codes associated with outpatient, inpatient, or emergency department visits. We conducted sensitivity analysis of our EHR-based CRS case criteria by repeating the statistical modeling across several EHR-based definitions for CRS. First, we evaluated whether requiring at least one patient interaction with GC (e.g., outpatient visit, inpatient admission) at least 12 months prior to the incident CRS diagnosis changed our results. Next, we repeated our univariate and multivariate analyses using several definitions that required evidence of CRS in addition to the encounter diagnoses: a definition requiring at least one diagnostic procedure relevant to CRS (e.g., sinus CT, endoscopy) prior to diagnosis; a definition that required the co-occurrence of asthma; a definition that required the presence of aspirin sensitivity in the EHR; and a definition that required the presence of both an asthma diagnosis and aspirin sensitivity in the medical record.
RESULTS
Study Population Characteristics
We identified 241 patients who met the criteria for incident CRSwNP and 3612 patients who met the criteria for incident CRSsNP between 2004 and 2007. We frequency-matched CRSwNP cases to 240 controls and CRSsNP cases to 3567 controls. On average at the time of the first CRS ICD-9 code, CRSsNP cases were 37.6 years of age and CRSwNP were 46.7 years of age. Nearly 60% of CRSsNP patients and 40% of CRSwNP were women. Approximately 13% of CRSsNP cases and 4% of CRSwNP cases had ever received medical assistance during the study period. More than 97% of CRS patients were Caucasian (Table I).
Table I.
Characteristics of the patients with CRSwNP (471.X), patients with CRSsNP (473.X), and matched controls.
| Variable | CRSsNP patients |
Control patients (matched to CRSsNP) |
p-value | CRSwNP patients |
Control patients (matched to CRSwNP) |
p- value |
|---|---|---|---|---|---|---|
| Number | 3612 | 3567 | 241 | 240 | ||
| Age, years: mean (sd) | 37.60 (21.54) | 38.57 (21.55) | 0.02 | 46.74 (18.14) | 46.06 (18.17) | 0.43 |
| Age group, number (%) | 0.42 | 0.99 | ||||
| 0–14 | 774 (21%) | 737 (21%) | 9 (4%) | 12 (5%) | ||
| 15–24 | 282 (8%) | 288 (8%) | 23 (10%) | 22 (9%) | ||
| 25–34 | 436 (12%) | 400 (11%) | 28 (12%) | 24 (10%) | ||
| 35–44 | 631 (17%) | 596 (17%) | 54 (22%) | 50 (21%) | ||
| 45–54 | 649 (18%) | 665 (19%) | 46 (19%) | 48 (20%) | ||
| 55–64 | 473 (13%) | 486 (14%) | 35 (15%) | 35 (15%) | ||
| 65–74 | 260 (7%) | 259 (7%) | 31 (13%) | 34 (14%) | ||
| >75 | 107 (3%) | 136 (4%) | 15 (6%) | 15 (6%) | ||
| Female, number (%) | 2105 (58%) | 2039 (57%) | 0.39 | 97 (40%) | 130 (54%) | 0.002 |
| Caucasian, number (%) | 3538 (98%) | 3463 (97%) | 0.02 | 236 (98%) | 236 (98%) | 0.74 |
| Medical assistance1, number (%) | 463 (13%) | 411 (12%) | 0.09 | 10 (4%) | 13 (5%) | 0.51 |
Considered to have medical assistance if one of 14 need-based medical assistance programs as health insurance for at least one healthcare encounter
Associations of CRSsNP with Incident Disease
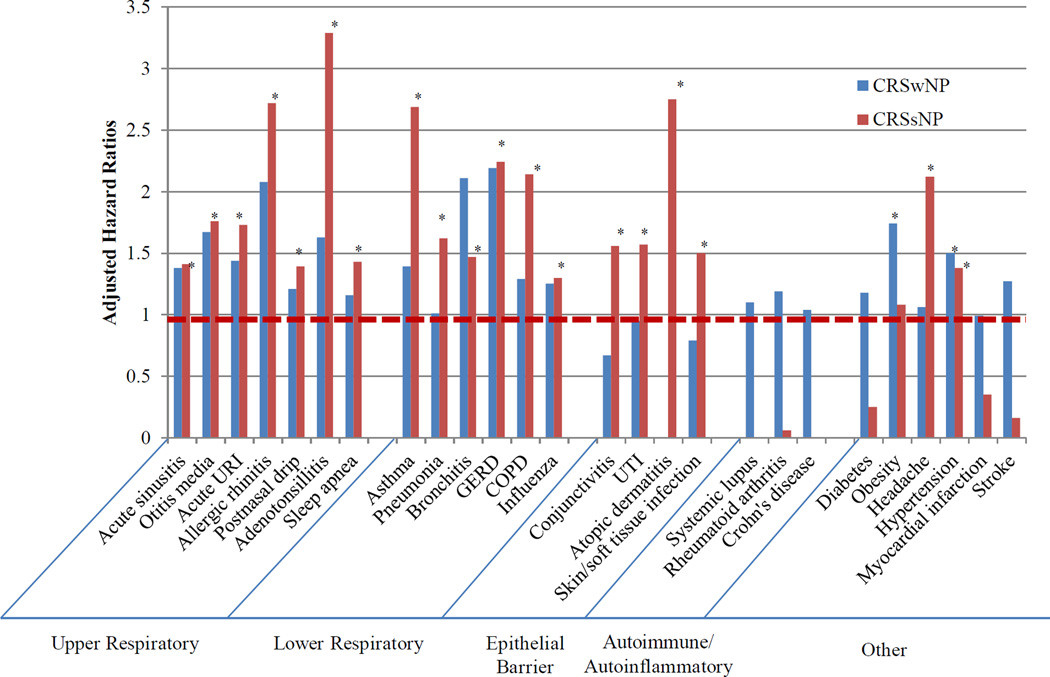
HRs were evaluated for associations of new CRSsNP (n = 2026) compared to controls for incident development of the majority of the 33 conditions we studied, adjusting for age, sex, race/ethnicity, smoking, alcohol use, medical assistance, BMI, community-level deprivation, community-type, and co-morbidities (Table II). With the exception of sleep apnea, CRSsNP cases were at significantly greater risk (HR, 95% confidence interval) than controls for incidence of all of the upper airway diseases we studied, including adenotonsillitis (3.29, 2.41–4.50) and allergic rhinitis (2.72, 2.24–3.30). Similarly, CRSsNP cases were at significantly higher risk for onset of all of the lower aerodigestive tract diseases examined, including asthma (2.69, 2.14–3.38), COPD (2.14, 1.51–3.03), and gastroesophageal reflux disease (GERD) (2.24, 1.85–2.72). Among epithelial conditions, CRSsNP cases had a greater risk than controls for nearly all of the diseases studied, including atopic dermatitis (2.75, 1.23–6.16) and conjunctivitis (1.56, 1.27–1.90). There was no association with development of Crohn’s disease, however the incidence of Crohn’s in both cases and controls was low. Incidence of systemic lupus and rheumatoid arthritis were also low in both CRSsNP cases and controls. There were no associations with risk of development of obesity, heart failure, stroke, or myocardial infarction. CRS was associated with development of hypertension (1.38, 1.19–1.61). (Figure 1) This association was not explained by OCS use, as controlling for cumulative orders for OSC before hypertension diagnosis strengthened this relationship (2.05, 1.76–2.39.)
Table II.
Unadjusted and adjusted associations of CRSsNP (ICD-9 473.x) case status and post-morbid conditions diagnosed within five years after CRS diagnosis
| Post-morbid condition |
Cases (N = 3612) N (%) |
Controls (N = 3567) N (%) |
Un-adjusted Chi-square P-value |
Adjusted Hazard ratio1 |
P-value |
|---|---|---|---|---|---|
| Acute rhinosinusitis | 705 (19.52) | 505 (14.16) | < 0.001 | 1.41 (1.26–1.59) | < 0.001 |
| Otitis media | 392 (10.85) | 215 (6.03) | < 0.001 | 1.76 (1.49–2.09) | < 0.001 |
| Acute URI2 | 692 (19.16) | 402 (11.27) | < 0.001 | 1.73 (1.52–1.96) | < 0.001 |
| Allergic rhinitis | 398 (11.02) | 144 (4.04) | < 0.001 | 2.72 (2.24–3.30) | < 0.001 |
| Chronic rhinitis | 299 (8.28) | 53 (1.49) | < 0.001 | 5.68 (4.23–7.64) | < 0.001 |
| Postnasal drip/wheeze | 838 (23.20) | 594 (16.65) | < 0.001 | 1.39 (1.25–1.55) | < 0.001 |
| Adenotonsillitis | 192 (5.32) | 54 (1.51) | < 0.001 | 3.29 (2.41–4.50) | < 0.001 |
| Sleep apnea | 65 (1.80) | 54 (1.51) | 0.34 | 1.16 (0.8–1.68) | 0.44 |
| Asthma3 | 311 (8.61) | 102 (2.86) | < 0.001 | 2.69 (2.14–3.38) | < 0.001 |
| Pneumonia | 705 (19.52) | 432 (12.11) | < 0.001 | 1.62 (1.44–1.83) | < 0.001 |
| Bronchitis | 269 (7.45) | 172 (4.82) | < 0.001 | 1.47 (1.21–1.79) | < 0.001 |
| GERD2 | 352 (9.75) | 152 (4.26) | < 0.001 | 2.24 (1.85–2.72) | < 0.001 |
| Influenza | 131 (3.63) | 90 (2.52) | 0.007 | 1.25 (0.95–1.64) | 0.11 |
| Conjunctivitis | 266 (7.36) | 160 (4.49) | < 0.001 | 1.56 (1.27–1.90) | < 0.001 |
| UTI2 | 453 (12.54) | 283 (7.93) | < 0.001 | 1.57 (1.34–1.83) | < 0.001 |
| Atopic dermatitis | 27 (0.75) | 8 (0.22) | 0.002 | 2.75 (1.23–6.16) | 0.01 |
| Crohn’s disease | 4 (0.11) | 4 (0.11) | 0.98 | 1.04 (0.26–4.18) | 0.96 |
| Ulcerative colitis | 26 (0.72) | 13 (0.36) | 0.04 | 1.90 (0.97–3.73) | 0.06 |
| Skin/soft tissue infections | 605 (16.75) | 383 (10.74) | < 0.001 | 1.50 (1.31–1.71) | < 0.001 |
| Systemic lupus | 3 (0.08) | 2 (0.06) | 0.66 | 1.10 (0.15–8.30) | 0.92 |
| Rheumatoid arthritis | 16 (0.44) | 11 (0.31) | 0.35 | 1.19 (0.54–2.61) | 0.66 |
| Diabetes4 | 125 (3.46% | 104 (2.92) | 0.19 | 1.18 (0.90–1.54) | 0.24 |
| Obesity | 615 (17.03) | 543 (15.22) | 0.04 | 1.08 (0.95–1.22) | 0.23 |
| Headache | 277 (7.67) | 123 (3.45) | < 0.001 | 2.12 (1.70–2.63) | < 0.001 |
| Hypertension5 | 411 (11.38) | 301 (8.44) | < 0.001 | 1.38 (1.19–1.61) | < 0.001 |
| Heart failure | 11 (0.30) | 13 (0.36) | 0.66 | 0.73 (0.32–1.66) | 0.45 |
| Myocardial infarction | 33 (0.91) | 32 (0.90) | 0.94 | 0.99 (0.61–1.62) | 0.99 |
| Ischemic stroke | 31 (0.86) | 25 (0.70) | 0.45 | 1.27 (0.74–2.17) | 0.39 |
| COPD2 | 104 (2.88) | 47 (1.32) | < 0.001 | 2.14 (1.51–3.03) | < 0.001 |
p-value from Cox regression model, controlling for age, sex, race/ethnicity, smoking status, alcohol status, medical assistance, body mass index (BMI), community deprivation, community type, diabetes, hypertension, and asthma – unless otherwise noted below.
URI: Upper respiratory infection; GERD: Gastrointestinal esophageal reflux disease; UTI: Urinary tract infection; COPD: Chronic obstructive pulmonary disease.
Adjusting for age, sex, race/ethnicity, smoking status, alcohol status, family SES (medical assistance,) BMI, DEP, MCD Type, diabetes, and hypertension.
Adjusting for age, sex, race/ethnicity, smoking, alcohol, medical assistance, BMI, DEP, MCD Type, hypertension, and asthma.
Adjusting for age, sex, race/ethnicity, smoking, alcohol, medical assistant, BMI, DEP, MCD Type, diabetes, and asthma
Figure 1.
Adjusted hazard ratios comparing incident disease among patients with CRSwNP and patients with CRSsNP compared to control patients (dotted red line) GERD: Gastrointestinal esophageal reflux disease; UTI: Urinary tract infection; COPD: Chronic obstructive pulmonary disease
*p<0.05
Associations of CRSwNP with Incident Disease
With the exception of obesity (1.74, 1.08–2.80), there were no associations of new diagnosis of CRSwNP (compared to controls) with subsequent development of any other disease (Table III). The association with obesity is not explained by OCS use, as controlling for cumulative OCS use between CRS diagnosis and onset of obesity strengthened the relationship (2.13, 1.32–3.46). When the upper airway diseases were collapsed into one category, there was a greater risk of disease among cases than controls (1.67, 1.23–2.26) (Table IV). With only 241 CRSwNP cases, there were several diseases for which there were fewer than 5 cases or controls, including adenotonsillitis, influenza, atopic dermatitis, and rheumatoid arthritis. There were no incident cases of Crohn’s disease, ulcerative disease, or lupus in CRSwNP or matched controls.
Table III.
Unadjusted and adjusted associations of CRSwNP (ICD-9 471.x) case status and post-morbid conditions diagnosed within five years after CRS diagnosis
| Post-morbid condition | Cases (N=241) N (%) |
Controls (N = 240) N (%) |
Un- adjusted Chi- Square P-value |
Adjusted Hazard ratio1 |
P-value |
|---|---|---|---|---|---|
| Acute rhinosinusitis | 51 (21.16) | 34 (14.17) | 0.04 | 1.38 (0.86–2.23) | 0.18 |
| Otitis media | 15 (6.22) | 12 (5.00) | 0.56 | 1.67 (0.69–4.05) | 0.26 |
| Acute URI2 | 40 (16.60) | 25 (10.42) | 0.05 | 1.44 (0.82–2.53) | 0.20 |
| Allergic rhinitis | 19 (7.88) | 10 (4.17) | 0.09 | 2.08 (0.90–4.8) | 0.09 |
| Chronic rhinitis | 10 (4.15) | 1 (0.42) | 0.006 | 12.77 (1.42–114.75) | 0.02 |
| Postnasal drip/Wheeze | 38 (15.77) | 40 (16.67) | 0.79 | 1.21 (0.74–1.98) | 0.45 |
| Adenotonsillitis | 3 (1.24) | 2 (0.83) | 0.20 | 1.63 (0.19–14.28) | 0.66 |
| Sleep apnea | 8 (3.32) | 6 (2.50) | 0.59 | 1.43 (0.45–4.57) | 0.55 |
| Asthma3 | 9 (3.88) | 6 (2.56) | 0.42 | 1.39 (0.44–4.42) | 0.58 |
| Pneumonia | 42 (17.43) | 37 (15.42) | 0.55 | 1.01 (0.62–1.64) | 0.96 |
| Bronchitis | 17 (7.05) | 9 (3.75) | 0.11 | 2.11 (0.86–5.14) | 0.10 |
| GERD2 | 20 (8.30) | 9 (3.75) | 0.04 | 2.19 (0.91–5.27) | 0.08 |
| Influenza | 3 (1.24) | 4 (1.67) | 0.70 | 1.30 (0.18–9.58) | 0.80 |
| Conjunctivitis | 10 (4.15) | 12 (5.00) | 0.66 | 0.67 (0.26–1.71) | 0.40 |
| UTI2 | 19 (7.88) | 20 (8.33) | 0.86 | 0.95 (0.47–1.92) | 0.88 |
| Atopic dermatitis | 1 (0.41) | 1 (0.42) | 1.0 | - | - |
| Crohn’s disease | - | - | - | - | - |
| Ulcerative colitis | - | - | - | - | - |
| Skin/soft tissue infections | 32 (13.28) | 37 (15.42) | 0.50 | 0.79 (0.47–1.33) | 0.37 |
| Systemic lupus | - | - | - | - | - |
| Rheumatoid arthritis | 1 (0.41) | 3 (1.25) | 0.31 | 0.06 (0.0–154.9) | 0.48 |
| Diabetes3 | 4 (1.66) | 9 (3.75) | 0.16 | 0.25 (0.06–1.08) | 0.06 |
| Obesity | 51 (21.16) | 37 (15.42) | 0.10 | 1.74 (1.08–2.80) | 0.02 |
| Headache | 13 (5.39) | 10 (4.17) | 0.53 | 1.06 (0.43–2.63) | 0.90 |
| Hypertension4 | 37 (15.35) | 26 (10.83) | 0.14 | 1.50 (0.87–2.6) | 0.15 |
| Heart failure | 5 (2.07) | 3 (1.25) | 0.48 | 1.90 (0.38–9.51) | 0.43 |
| Myocardial infarction | 2 (0.83) | 3 (1.25) | 0.65 | 0.35 (0.02–1.84) | 0.19 |
| Ischemic stroke | 2 (0.83) | 5 (2.08) | 0.25 | 0.16 (0.02–6.16) | 0.48 |
| COPD | 6 (2.49) | 4 (1.67) | 0.53 | 1.29 (0.33–5.02) | 0.71 |
p-value from Cox regression model, controlling for age, sex, race/ethnicity, smoking status, alcohol status, medical assistance, body mass index (BMI), community deprivation, community type, diabetes, hypertension, and asthma – unless otherwise noted below.
URI: Upper respiratory infection; GERD: Gastrointestinal esophageal reflux disease; UTI: Urinary tract infection; COPD: Chronic obstructive pulmonary disease.
Adjusting for age, sex, race/ethnicity, smoking status, alcohol status, family SES (medical assistance,) BMI, DEP, MCD Type, diabetes, and hypertension.
Adjusting for age, sex, race/ethnicity, smoking, alcohol, medical assistance, BMI, DEP, MCD Type, hypertension, and asthma.
Adjusting for age, sex, race/ethnicity, smoking, alcohol, medical assistant, BMI, DEP, MCD Type, diabetes, and asthma
Exploratory and Sensitivity Analyses
To determine whether the relationships between CRS and other diseases changed based on demographic factors (age, sex, race) or community factors (urban/rural, community type) we added interaction terms to the models. These factors did not modify the relations between CRS and incident morbidities (results not shown). Confining analysis to adult patients also did not change these relationships. We conducted sensitivity analysis using alternative EHR-based criteria for CRS. While the overall case counts changed, inferences and magnitude of associations did not.
DISCUSSION
Our study determined that patients with CRS are at an increased risk for development of incident disease within five years after a CRS diagnosis. To our knowledge, this is the first study to evaluate post-morbid risk associated with airway, epithelial, inflammatory, and autoimmune conditions in CRS and the first to study post-morbid risk at the phenotype level. Our findings are an important first step towards understanding the temporal associations between CRS and co-morbid disease. This study provides support for targeted screening of CRS patients with the goal of determining whether aggressive treatment of CRS can prevent the onset of these subsequent health conditions.
Patients with CRSsNP were found to be at increased risk for airway diseases, consistent with the unified airway conceptual framework.9 Similarly, we found an association between CRSwNP and post-morbid upper airway diseases. There is already a large body of literature that has reported the co-occurrence of CRS with other diseases of the airway, however many of these studies are cross-sectional and, therefore, do not consider temporality, making causal inferences impossible.5–7 One of the exceptions is our prior report on relationship between CRS and pre-morbid conditions of the airway, or conditions diagnosed prior to CRS, including acute sinusitis, allergic rhinitis, asthma, pneumonia, and GERD.10 Our post-morbid findings, paired with these pre-morbid associations demonstrate the bidirectional relationship expected under the unified airway model, where the same system-wide effects that put someone with asthma at risk for CRS, for example, could have the reverse effect.9
Diagnosis of CRSsNP was also consistently associated with epithelial conditions, such a conjunctivitis, UTI, atopic dermatitis, and skin/soft tissue diseases. These epidemiological findings complement genetic and immunological support for the barrier defect model, where defects in barrier function serve as one of the primary causative mechanisms in the pathogenesis of CRS.24,25 We have reported an association between pre-morbid conjunctivitis, UTI and atopic dermatitis and CRS. This bidirectional relationship is expected under the barrier defect model, as the co-occurrence of disease under this model is due to a common cause (i.e., systemic defect in the epithelial barrier.)10
While our findings provide support for the unified airway and epithelial defect models, they call into question other previously posited explanations for associations. Based on prior reports of a relationship between GERD and CRS, for example, it has been proposed that the direct mucosal injury from refluxed or aerosolized acid resulting from GERD may trigger CRS.26–28 Consistent with this theory, our previous work has found that GERD precedes the onset of CRS.10 Our finding of post-CRS diagnosis risk of GERD does not negate the potential role of GERD in the development of CRS, however the onset of GERD both before and after a CRS diagnosis indicates that there may also be common causal pathway to these diseases.
We did not find an association between CRS and post-morbid vascular conditions, other than hypertension.5 Chung et al. also recently reported a co-morbid association between CRSsNP and hypertension using cross-sectional data. However, our findings on MIs and stroke contrast with prior work by Kan et al., who recently reported an increased risk of stroke within five years of a CRS diagnosis and Hao et al., who reported an increased risk of myocardial infarction within three years.13,29 There are a few possible explanations for the differences in our findings. First, both Kan’s and Hao’s studies were based on claims data, while our study used data from an EHR system. Previous studies have demonstrated differences in how diagnoses recorded in claims versus medical records have the potential to produce different results even when similar methods are applied.30,31 Second, across the three studies, three different methods of identifying CRS patients were applied. Kan required one diagnosis in ambulatory care for CRS using an ICD-9 code of 473.0, 473.1, 473.2, 473.3, 473.8, 473.9. We required at least two encounter diagnoses using these codes, but also included inpatient and emergency department diagnoses. Hao and colleagues applied these codes but also included a code of 461 for acute sinusitis. Ours was the only study to look separately at CRSwNP using ICD-9 code 471. Third, our case groups did not differ from our control groups in terms of co-morbid diabetes, hypertension, asthma, COPD, hyperlipidemia, or coronary artery disease at the time of CRS diagnosis, while cases and controls in the other studies differed by several of these conditions. While Hao and Kang controlled for these factors in their analyses, residual confounding may account, in part, for their findings of stroke and MI risk. The study populations also differed by race/ethnicity. In both Kang’s and Hao’s study, over 98% of the population was Han Chinese, while more than 97% of our study population was Caucasian. Significant race/ethnic differences have been reported in the presentation of CRS and may account for the inconsistent findings across studies.13,32–34 Finally, our study focused on ischemic stroke as an outcome, while Kang collapsed all stroke types.
While we suspect our analysis of patients with CRSwNP was underpowered for a number of disease areas, we did find a modest association between CRSwNP and obesity. Bhattacharyya also reported an association between obesity and CRS. Bhattacharyya studied the relationship using cross-sectional data, therefore temporality of the conditions could not be determined.15 Ours is the first study, to our knowledge, to report a link between CRSwNP and subsequent onset of obesity. Potential pathways include side effects of OCS used to treat CRSwNP or a reduction in physical activity that has been associated with a diagnosis of chronic sinus disease.1,21–23 However, our analysis determined that OCS use was not a mediator of the relation between new CRS diagnosis and incident development of obesity.
While both patients with CRSwNP and CRSsNP were at risk for upper airway diseases, we found different patterns of association with other diseases by CRS phenotype. In some cases, the sample size of the CRSwNP may not have been adequate to detect an association. The results may also reflect a different pattern of disease onset by CRS phenotype. Just as allergic rhinitis precedes asthma in the atopic march, our studies indicate that asthma precedes onset of CRSwNP.16,35 CRSsNP appears to have a different “march” pattern, where asthma both precedes and proceeds a CRSsNP diagnosis. Differences in post-morbid risk are consistent with other differences in CRS phenotypes, including inflammatory profiles and treatment outcomes.36,37
Among the strengths of this study was the ability to test longitudinally and determine the timing of disease onset relative to CRS diagnosis. This study design generates insights into the pathophysiology of disease and provides some clarification as to the relationship between CRS and other diseases. This is also one of the few studies to look at the risk associated with both CRSsNP and CRSwNP separately, allowing us to observe differences between these phenotypes. The study had several limitations, however. First, the study population is nearly all Caucasian patients and there are known racial/ethnic differences in CRS.32 Second, in order to study a population-based sample, we did not limit our study to patients with objective evidence of CRS. Instead, we used an EHR-based algorithm. As a result, there may have been a degree of case misclassification, both in terms of CRS status and CRS phenotype classification. Sensitivity testing revealed that the method used to identify patients, however, did not substantively change inferences or associations. If misclassification of case status occurred, it likely would have biased our results to the null. Future studies should repeat this analysis in a population-based sample of patients with a CRSwNP or CRSsNP diagnosis confirmed using validated diagnostic criteria, rather than EHR-based diagnoses. Finally, EHR data allows us to consider the temporal relationship between the diagnosis of disease, diagnosis date is not synonymous with onset. Diseases that appear for the first time in the record after CRS may have started prior to the CRS diagnosis. Therefore, causal conclusions must be tempered.
CONCLUSION
The risks associated with a new diagnosis of CRS have important implications for the management of this disease. Differences in risk between phenotypes may be due to sample size limitations but may also reflect differences in the natural history of the conditions and support the notion that each of these phenotypes may be part of a different “march.” To better tailor treatment to the CRS phenotype, further research should explore these differences to gain insight into the unique sets of predictors and risks associated with CRSsNP and CRSwNP, and evaluate whether optimal CRS treatment can prevent the development of these subsequent health conditions.
Acknowledgments
Funding: This publication was made possible by Grants U19AI106683 and R37HL068546 from the NIH, and the Ernest S. Bazley Foundation. These study sponsors did not play a role in the study design, analysis, interpretation, or writing of the report and did not take part in the decision to submit this article for publication.
Abbreviations
- BMI
Body Mass Index
- COPD
Chronic Obstructive Pulmonary Disease
- CRS
Chronic Rhinosinusitis
- CRSwNP
Chronic Rhinosinusitis with Nasal Polyps
- CRSsNP
Chronic Rhinosinusitis without Nasal Polyps
- EHR
Electronic Health Record
- GC
Geisinger Clinic
- GERD
Gastroesophageal reflux disease
- HR
Hazard Ratio
- ICD-9
International Classification of Diseases
- MI
Myocardial infarction
- OCS
Oral Corticosteroid
- SES
Socioeconomic Status
- UTI
Urinary Tract Infection
Footnotes
Drafting/revision of the manuscript, interpretation of data, final approval of the manuscript, and agree to be accountable for all aspects of the work: All. Conception, design, and interpretation of results: AGH, XY, BKT, RPS, RCK, BSS. Acquisition and analysis: AGH, XY, BSS.
Conflict of Interest: The authors have no conflicts of interest to report.
Clinical Implications: We found an increased risk of other diseases after a new diagnosis of CRS. These findings have implications for managing patients with newly diagnosed CRS.
REFERENCES
- 1.Gliklich R, Metson R. The health impact of chronic sinusits in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113(1):104–109. doi: 10.1016/S0194-59989570152-4. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald K, McNally J, Massoud E. The health and resource utilization of canadians with chronic rhinosinusitis. Laryngoscope. 2009;119(1):184–189. doi: 10.1002/lary.20034. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the united states. Ann Otol Rhinol Laryngol. 2011;120(7):423–427. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 4.Soler Z, Wittenberg E, Schlosser R, Mace J, Smith T. Health state utility values in patients undergoing endoscopic sinus surgery. Laryngoscope. 2011;121(12):2672–2678. doi: 10.1002/lary.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung S, Chen P, Lin H, Hung S. Comorbidity profile of chronic rhinosinusitis: A population-based study. Laryngoscope. 2014;214:1536–1541. doi: 10.1002/lary.24581. [DOI] [PubMed] [Google Scholar]
- 6.Lin D, Chandra R, Tan B, et al. Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25(4):205–208. doi: 10.2500/ajra.2011.25.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: The GA2LEN survey in europe. Allergy. 2012;67(1):91–98. doi: 10.1111/j.1398-9995.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 8.Spergel J. From atopic dermatitis to asthma: The atopic march. Ann Allergy Asthma Immunol. 2010;105:99–106. doi: 10.1016/j.anai.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Krouse J. The unified airway - conceptual framework. Otolaryngol Clinc North Am. 2008;41(2):257–266. doi: 10.1016/j.otc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Tan B, Chandra R, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131(5):1350–1360. doi: 10.1016/j.jaci.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan B, Schleimer R, Kern R. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head and Neck Surg. 2010;18(1):21–26. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kern R, Conley D, Walsh W, et al. Perspectives on the etiology of chronic rhinosinusitis: An immune barrier hypothesis. Am J Rhinol. 2008;22(6):549–559. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang J, Wu C, Keller J, Lin H. Chronic rhinosinusitis increased risk of stroke: A 5-year follow-up study. Laryngoscope. 2013;123(4):835–840. doi: 10.1002/lary.23829. [DOI] [PubMed] [Google Scholar]
- 14.Roidman I, Beck P, Anderson T, Eisenberg M, Genest J. Chronic inflammatory diseases and cardiovascular risk: A systematic review. Can J Cardio. 2011;27(2):174–182. doi: 10.1016/j.cjca.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharyya N. Associations between obesity and inflammatory sinonasal disorders. Laryngoscope. 2013;123:1840–1844. doi: 10.1002/lary.24019. [DOI] [PubMed] [Google Scholar]
- 16.Tan B, Chandra R, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131(5):1350–1360. doi: 10.1016/j.jaci.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Bureau of the Census. Census 2000. [Accessed February, 2015];Censtats Databases Web site. http://censtats.census.gov/data/PA/About_the_profile.pdf. Updated 2002.
- 18.Schwartz B, Bailey-Davis L, Bandeen-Roche K, et al. Attention deficit disorder, stimulant use, childhood body mass index trajectory. Pediatrics. 2014;133(4):668–676. doi: 10.1542/peds.2013-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nau C, Schwartz B, Bandeen-Roche K, et al. Community socio economic deprivation and obesity trajectories in children using electronic health records. Obesity. 2014;23(1):207–212. doi: 10.1002/oby.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa PJ, McCrae R. Neuroticism, somatic complaints, and disease: Is the bark worse than the bite? J Pers. 1987;55:299–316. doi: 10.1111/j.1467-6494.1987.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 21.McDonough A, Curtis J, Saag K. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol. 2008;20(2):131–137. doi: 10.1097/BOR.0b013e3282f51031. [DOI] [PubMed] [Google Scholar]
- 22.Leslie W, Hankey C, Lean M. Weight gain as an adverse effect of some commonly prescribed drugs: A systematic review. QJM. 2007;100(7):395–404. doi: 10.1093/qjmed/hcm044. [DOI] [PubMed] [Google Scholar]
- 23.Buchman A. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33(4):289–294. doi: 10.1097/00004836-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Kern R, Conley D, Walsh W, et al. Perspectives on the etiology of chronic rhinosinusitis: An immune barrier hypothesis. Am J Rhinol. 2008;22(6):549–559. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tieu D, Kern R, Schleimer R. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:37–42. doi: 10.1016/j.jaci.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lupa M, DelscGuadio J. Evidence-based practice: Reflux in sinusitis. Otolaryngol Clinc North Am. 2012;45(5):983–992. doi: 10.1016/j.otc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Coelho M, Spolaor M, Filho E, Sirena E, Oliveira M. Incidence of gastroesophageal reflux symptoms in patients with refractory chronic sinusitis upon clinical treatment. Int Arch Otorhinolaryngol. 2009;13(3):300–303. [Google Scholar]
- 28.Bohnhorst I, Jawad S, Lange B, Kjeldsen J, Hansen J, Kjeldsen A. Prevalence of chronic rhinosinusitis in a population of patients with GERD. Am J Rhinol Allergy. 2015;29(3):e70–e74. doi: 10.2500/ajra.2015.29.4167. [DOI] [PubMed] [Google Scholar]
- 29.Hao W, Lin H, Chao P, et al. Risk of myocardial infarction in patients with rhinosinusitis. Atherosclerosis. 2013;226(1):263–268. doi: 10.1016/j.atherosclerosis.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 30.Tang P, Ralston M, Arrigotti M, Qureshi L, Graham J. Comparison of methodologies for calculating quality measures based on administrative data versus clinical data from an electronic health record system: Implications for performance measures. J Am Med Inform Assoc. 2007;14(1):10–15. doi: 10.1197/jamia.M2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeVoe J, Gold R, McIntire P, Puro J, Chauvie S, Gallia C. Electronic health records vs. medicaid claims: Completeness of diabetes preventive care data in community health centers. Ann Fam Med. 2011;9(4):351–358. doi: 10.1370/afm.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soler Z, Mace J, Litvack J, Smith T. Chronic rhinosinusitis, race and ethnicity. Am J Rhinol Allergy. 2012;26:110–116. doi: 10.2500/ajra.2012.26.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahdavina M, Suh L, Carter R, et al. Increased noneosinophilic nasal polyps in chronic rhinosinusitis in US second-generation asians suggest genetic regulation of eosinophilia. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.08.031. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao P, Li H, Wang B, et al. Distinct immunopatholoic characterstics of various type of chronic rhinosinusitis in adult chinese. J Allergy Clin Immunol. 2009;124(3):478–484. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Almqyist C, Li Q, Britton W, et al. Early predictors for developing allergic disease and asthma: Examining separate steps in the 'allergic march'. Clin Exp Allergy. 2007;37(9):1296–1302. doi: 10.1111/j.1365-2222.2007.02796.x. [DOI] [PubMed] [Google Scholar]
- 36.Bachert C, van Bruaene N, Toskala E, et al. Important research questions in allergy and related diseases: 3-chronic rhinosinusitis and nasal polposis - a GALEN study. Allergy. 2009;64(4):520–533. doi: 10.1111/j.1398-9995.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 37.Bachert C, Zhang N, van Zele T, Gevaert P. Chronic rhinosinusitis: From one disease to different phenotypes. Pediatr Allergy Immunol. 2012;23(Suppl 22):2–4. doi: 10.1111/j.1399-3038.2012.01318.x. [DOI] [PubMed] [Google Scholar]