Summary
Background
Case-control studies have created genetic risk scores of single nucleotide polymorphisms (SNPs) associated with venous thromboembolism (VTE) and documented their ability to predict VTE, but prospective data are lacking.
Objective
To test the ability of a genetic risk score to predict VTE incidence in a prospective study, particularly in African Americans.
Methods
We computed a previously proposed genetic risk score, based on five established VTE SNPs in the F5, F2, ABO, FGG, and F11 genes, in 9,520 whites and 3,049 African Americans initially free of VTE. We followed them a median of 22.6 years for VTE occurrence (n=380 events in whites and n=187 in African Americans).
Results
In whites, the 5-SNP weighted genetic risk score ranged from 0 to 5.8, and VTE risk increased 1.41 fold (95% CI 1.27, 1.56) per allele increment. In African Americans, the weighted genetic risk score ranged 0 to 4.6 and the hazard ratio per risk allele was 1.14 (95% CI 0.94, 1.38), with adjustment for 10 principal components of ancestry. The area under the receiver operating characteristic curve (AUC) for 20-year prediction of VTE from the weighted genetic risk score was 0.59 (95% CI 0.56, 0.63) in whites and 0.56 (95% CI 0.51, 0.61) in African Americans. Adding nongenetic factors increased the AUC to 0.67 in whites and to 0.66 in African Americans.
Conclusions
Higher values for a 5-SNP genetic risk score helped identify white adults at risk of VTE. The genetic risk score did not identify future VTE occurrence in African Americans.
Keywords: deep vein thrombosis, pulmonary embolism, prospective studies, genetics, risk factors
Introduction
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), has a moderately strong genetic component, as evidenced by family, candidate gene, and genome-wide association studies. Recently, de Haan et al showed that a genetic risk score (GRS) based on 31 single nucleotide polymorphisms (SNPs) was strongly predictive of VTE in two large Dutch case-control studies [1]. A GRS based on just the five top SNPs, in the F5 (Leiden), F2, F11, FGG, and ABO genes, was nearly as predictive [area under the receiver operating characteristic curve (AUC) = 0.69] as the 31-SNP score (AUC=0.71). Adding non-genetic variables improved the AUC to 0.82 in this Dutch sample. Bruzelius et al showed the same 5-SNP GRS predicted VTE in a Swedish case-control study (AUC = 0.65) but was slightly stronger when two additional SNPs (PROC and KNG1) were included (AUC = 0.66) and even stronger including lifestyle predictors (AUC = 0.84) [2]. Another report involving two European case-control studies showed comparable to somewhat weaker prediction of VTE using several similar GRSs [3]. A GRS for VTE, if sufficiently predictive, might have utility for the primary or secondary prevention of VTE.
To our knowledge, no U.S. prospective study has yet examined the ability of a GRS to predict VTE. The published GRSs may be less predictive in African Americans, in whom some genetic variants for thrombophilia are rare, but who have a greater incidence rate of VTE than do whites [4]. In this paper, we therefore attempted to replicate the 5-SNP GRS association with VTE [1, 2] in ARIC whites and African Americans.
Methods
Study population
We reported the ARIC study design, methods, and VTE incidence rates in detail elsewhere [5, 6]. In brief, 15,792 men and women aged 45 to 64 years enrolled in the ARIC study in 1987–1989, and had subsequent examinations in 1990–92, 1993–95, 1996–98, and 2011–13, with annual telephone contact since visit one. The institutional review committees at each study center approved the methods and staff obtained informed participant consent.
Measurement of SNPs and risk factors at baseline
ARIC isolated genomic DNA from buffy coat specimens. ARIC measured the five SNPs for the GRS reported by de Haan et al [1]: F5 Leiden rs6025, F2 rs1799963, ABO rs8176719 (O vs. non-O groups), FGG rs2066865, and F11 rs2036914. The ARIC DNA laboratory at the University of Texas-Houston genotyped rs6025 and rs2066865 using the iPLEX multiplex assay which utilizes the MassARRAY system (Sequenom, Inc., San Diego, CA), rs8176719 using the functionally tested TaqMan Assay-by-Design system (Applied Biosystems, Foster City, CA), rs1799963 using the pre-validated TaqMan Assay-on-Demand system (Applied Biosystems, Foster City, CA), and rs2036914 using the ITMAT-Broad-CARe custom array (Illumina, San Diego, CA) [7]. In order to control for population stratification, we estimated ten principal components of ancestry in African Americans using Eigenstrat [8] with genotypes from the Affymetrix Genome-wide Human SNP array 6.0. For a supplemental analysis, we also included the two additional SNPs in the GRS of Bruzelius et al [2], namely PROC rs1799810 and KNG1 rs710446, measured on the same platforms. Supplemental Table 1 shows quality and other metrics related to the SNPs studied.
ARIC had several non-genetic VTE risk factors, although fewer VTE “triggers” than the cited case-control studies [1, 2]. Participants reported race, hormone replacement therapy (HRT) use and history of cancer. We measured body mass index as weight (kg)/height (m)2. We defined diabetes as a fasting blood glucose of 126 mg/dl or higher, non-fasting blood glucose of 200 mg/dl or higher, a physician diagnosis of diabetes, or use of antidiabetic medication in the past 2 weeks.
VTE occurrence
Staff contacted ARIC participants annually by phone and asked about all hospitalizations in the previous year. They retrieved hospital records for possible VTE events through 2011. To validate VTE events, two physicians reviewed the records using standardized criteria [6], requiring positive imaging tests for diagnosis of DVT and PE. We restricted DVTs for this analysis to those in the lower extremity or vena cava, because upper extremity DVTs were relatively few and almost always the result of venous catheters. The reviewers sub-classified VTEs as unprovoked (no obvious cause) or provoked (associated with cancer, major trauma, surgery, marked immobility).
Statistical methods
Our hypothesis was that the 5-SNP GRS created by de Haan et al [1] would be associated positively with incidence of VTE in whites and African Americans. From the ARIC baseline cohort (n = 15,792), we successively excluded 48 who were of other race groups; 276 who reported a history of VTE at baseline; 73 who were taking anticoagulants at baseline; and 145 who declined use of DNA or had none of the five SNPs measured. We additionally excluded 48 African Americans with incomplete principal components of ancestry data. A total of 12,569 (9,520 whites, 3,049 African Americans) had all five SNPs measured for the GRS. There was little difference in the race-specific crude VTE incidence rate per 1,000 person-years for the 12,569 with the GRS computed (2.0 [95% CI 1.8, 2.2] whites, 3.1 [95% CI 2.7, 3.6] African Americans) compared with the 15,250 with any VTE data (2.0 [95% CI 1.8, 2.2] whites, 3.3 [95% CI 2.9, 3.7] African Americans).
We computed the 5-SNP GRS using the same five SNPs and method employed by de Haan et al [1]. The unweighted GRS counted the total number of risk-increasing alleles for each person and thus has a theoretical range of 0 to 10. The weighted GRS assigned weights to the risk alleles for each SNP (shown in Supplemental Table 1), corresponding to the logarithm of the average odds ratio reported in the literature for that SNP [1]. The weighted GRS was rescaled by dividing by the mean of the log odds ratios in order to be within the theoretical range of the unweighted score. In a supplemental analysis, we created a 7-SNP GRS including the two additional SNPs identified by Bruzelius et al [2].
Follow-up for VTE extended from ARIC baseline to the first of: VTE date, loss to follow-up, death, or December 31, 2011. For Figures 1 and 2, we plotted the race-specific distribution of risk alleles in the unweighted GRS and the crude incidence rates of VTE by number of risk alleles. We performed race-specific proportional hazards regression to estimate the hazard ratio of VTE for individual SNPs as well as the GRS. The main race-specific models included just the GRS, but for African Americans the analysis also included ten principal components of ancestry. We computed the AUC for predicting VTE from the GRS, overall and stratified by earlier and later follow-up. We also computed the AUC after adding in several baseline nongenetic risk factors for VTE in ARIC, namely age, HRT, sex, diabetes, BMI, and history of cancer.
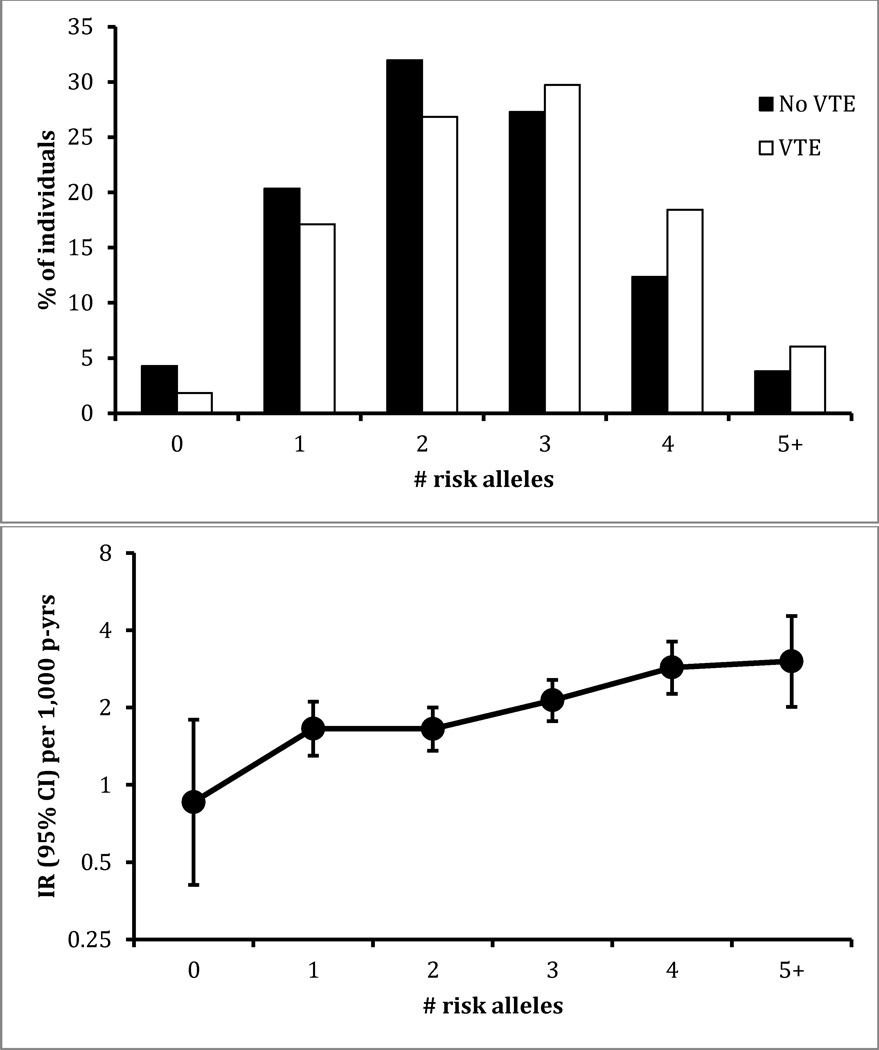
Figure 1.
Distribution of unweighted genetic risk score for those who did or did not develop venous thromboembolism (VTE), and incidence rate (IR) according to number of risk alleles present, ARIC whites (n = 9,520), 1987–2011.
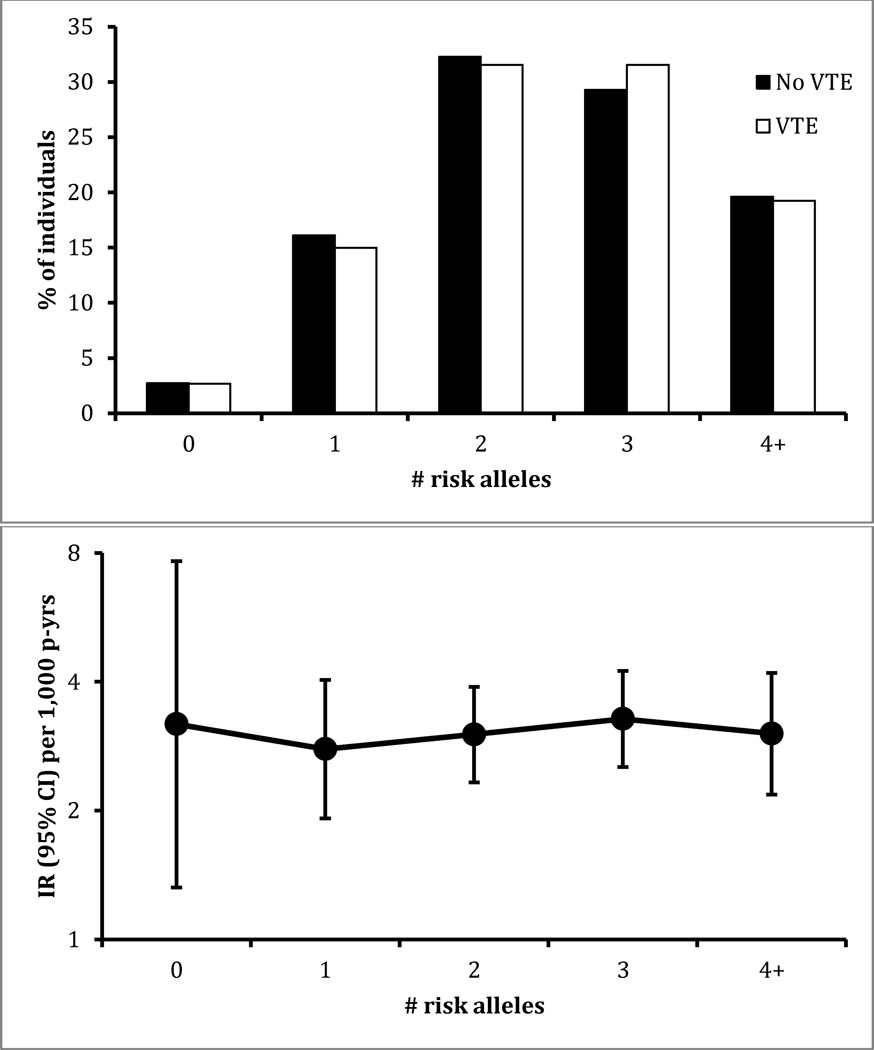
Figure 2.
Distribution of unweighted genetic risk score for those who did or did not develop venous thromboembolism (VTE), and incidence rate (IR) according to number of risk alleles present, ARIC African Americans (n = 3,049), 1987–2011.
In a final supplemental analysis, we computed race-specific mean values of several hemostatic factors according to the number of risk alleles present for the 5-SNP GRS. These were measured by standardized methods in research laboratories using plasma samples from ARIC baseline in 1987–89 (fibrinogen, factor VIIc, factor VIIIc, von Willebrand factor, activated partial thromboplastin time) or ARIC visit 3 in 1993–95 (factor XI, D-dimer).
Results
The ARIC cohort was followed for a median of 22.6 years (25.1 years maximum) for VTE events. Among the 9,520 whites at risk with complete GRS data, we identified 380 VTEs; among the 3,049 African Americans, we identified 187 VTEs. As shown by individual SNP in Table 1, for whites, VTE incidence was statistically significantly greater per risk allele of F5 (Leiden), ABO (non-O blood type), and F11, but not for FGG or the F2 SNP, the latter being rare in ARIC. The hazard ratios for whites for F5, F2, and F11 were somewhat stronger for earlier follow-up (when the cohort was younger) than later follow-up. In African Americans, as expected the F5 and F2 risk alleles were quite rare. Yet, F5 Leiden conveyed increased VTE risk in African Americans, as did non-O blood type. The two supplemental SNPs identified by Bruzelius et. al [2] in PROC and KNG1 were not significantly associated with VTE in either whites or African Americans (data not shown).
Table 1.
Race-specific SNP frequencies and hazard ratios (HRs) of venous thromboembolism (VTE) per risk allele, ARIC, 1987–2011
| Risk allele frequency, % | All follow-up | First 10 years | Second 10 years | Remaining ~5 years | ||||
|---|---|---|---|---|---|---|---|---|
| Race | Gene | SNP | No VTE | VTE | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Whites* | ||||||||
| N events --> | 380 | 90 | 198 | 92 | ||||
| F5 | rs6025 | 3 | 7 | 2.89 (2.19, 3.80) | 4.60 (2.91, 7.26) | 2.78 (1.88, 4.10) | 1.47 (0.69, 3.14) | |
| F2 | rs1799963 | 1 | 2 | 1.11 (0.63, 1.96) | 1.56 (0.58, 4.21) | 1.05 (0.47, 2.34) | 0.80 (0.20, 3.19) | |
| ABO | rs8176719 | 37 | 41 | 1.17 (1.02, 1.36) | 1.04 (0.77, 1.41) | 1.27 (1.04, 1.54) | 1.12 (0.83, 1.50) | |
| FGG | rs2066865 | 24 | 26 | 1.10 (0.93, 1.29) | 1.06 (0.76, 1.49) | 1.09 (0.87, 1.37) | 1.15 (0.83, 1.60) | |
| F11 | rs2036914 | 53 | 57 | 1.18 (1.02, 1.36) | 1.29 (0.96, 1.74) | 1.12 (0.92, 1.37) | 1.20 (0.89, 1.60) | |
| African Americans† | ||||||||
| N events --> | 187 | 34 | 112 | 41 | ||||
| F5 | rs6025 | 0.4 | 2 | 3.88 (1.69, 8.90) | 9.13 (2.01, 41.4) | 2.14 (0.52, 8.81) | 6.11 (1.41, 26.5) | |
| F2 | rs1799963 | 0.3 | 0 | -- | -- | -- | -- | |
| ABO | rs8176719 | 29 | 34 | 1.26 (1.02, 1.56) | 1.28 (0.78, 2.10) | 1.14 (0.86, 1.51) | 1.62 (1.03, 2.56) | |
| FGG | rs2066865 | 30 | 28 | 0.90 (0.71, 1.12) | 0.95 (0.56, 1.61) | 0.85 (0.63, 1.15) | 0.97 (0.60, 1.57) | |
| F11 | rs2036914 | 65 | 63 | 0.91 (0.74, 1.12) | 1.38 (0.81, 2.34) | 0.79 (0.61, 1.04) | 0.94 (0.60, 1.50) | |
Based on 9,520 whites at risk of VTE.
Based on 3,049 African Americans at risk of VTE. HRs for African Americans adjusted for ten principal components of ancestry.
The unweighted 5-SNP GRS ranged from 0 to 7 in whites, and the more risk alleles present, the higher the VTE risk was (Figure 1). In African Americans, the unweighted 5-SNP GRS ranged 0 to 6, and there was no apparent association of the GRS with VTE risk (Figure 2).
In whites, the weighted 5-SNP GRS ranged from 0 to 5.8, and VTE risk increased 1.41 fold (95% CI 1.27, 1.56) per risk unit increment, somewhat stronger for the first 20 years in comparison to the entire follow-up (Table 2). In African Americans, the weighted 5-SNP GRS ranged 0 to 4.6 and the hazard ratio per risk unit was 1.14 (95% CI 0.94, 1.38) with adjustment for 10 principal components of ancestry. There was no evidence in either race group that the association of weighted 5-SNP GRS differed between men and women (interaction p > 0.70).
Table 2.
Race-specific hazard ratio (HR) and area under receiver operating characteristic curve (AUC) for prediction of venous thromboembolism (VTE) during follow-up, ARIC
| Outcome Model |
Whites (n = 9,520) |
African Americans (n = 3,049) |
||
|---|---|---|---|---|
| HR (95% CI)* | AUC (95% CI) | HR (95% CI)* | AUC (95% CI) | |
| Total VTE | ||||
| All follow-up | ||||
| Weighted GRS | 1.41 (1.27, 1.56) | -- | 1.14 (0.94, 1.38) | |
| Nonweighted GRS | 1.22 (1.12, 1.32) | -- | 1.02 (0.90, 1.16) | |
| First 20 years, 20-yr risk | ||||
| Weighted GRS | 1.47 (1.31, 1.66) | 0.59 (0.56, 0.63) | 1.06 (0.85, 1.32) | 0.56 (0.51, 0.61) |
| Nonweighted GRS | 1.24 (1.13, 1.36) | 0.57 (0.54, 0.61) | 0.98 (0.85, 1.14) | 0.56 (0.51, 0.61) |
| Nongenetic factors† | 0.64 (0.61, 0.67) | 0.66 (0.61, 0.70) | ||
| Nongenetic + weighted GRS | 0.67 (0.64, 0.70) | 0.66 (0.62, 0.70) | ||
| Unprovoked VTE | ||||
| All follow-up | ||||
| Weighted GRS | 1.65 (1.40, 1.93) | -- | 1.25 (0.92, 1.69) | |
| Nonweighted GRS | 1.33 (1.16, 1.51) | -- | 1.03 (0.84, 1.27) | |
| First 20 years, 20-yr risk | ||||
| Weighted GRS | 1.81 (1.52, 2.16) | 0.65 (0.60, 0.71) | 1.13 (0.80, 1.59) | 0.61 (0.54, 0.68) |
| Nonweighted GRS | 1.41 (1.21, 1.64) | 0.62 (0.56, 0.67) | 0.96 (0.77, 1.21) | 0.60 (0.53, 0.68) |
| Nongenetic factors† | 0.67 (0.62, 0.72) | 0.67 (0.60, 0.74) | ||
| Nongenetic + weighted GRS | 0.72 (0.68, 0.77) | 0.67 (0.60, 0.74) | ||
HR per 1 allele increase in 5-SNP genetic risk score (GRS). HR for African Americans adjusted for 10 principal components of ancestry.
Nongenetic factors include: age (continuous), sex/HRT (men, women not on HRT, women on HRT), BMI (continuous), diabetes (yes, no), and history of cancer (yes, no) at baseline.
As further shown in Table 2, the AUC for 20-year prediction of VTE from the 5-SNP weighted GRS was 0.59 (95% CI 0.56, 0.63) in whites and 0.56 (95% CI 0.51, 0.61) in African Americans. These AUCs for the GRS were slightly larger in the first 10 years of follow-up than in the second 10 years. In the supplemental analysis, the AUC was not significantly improved including the two additional SNPs of Bruzelius et. al [2]; the 7-SNP GRS increased the AUC by only 0.005 in whites and 0.001 in African Americans. In contrast with the AUC values for the GRS, the AUC for 20-year VTE prediction from nongenetic factors was 0.64 in whites and 0.66 in African Americans. Adding the 5-SNP GRS to nongenetic factors increased the AUC to 0.67 in whites but it remained 0.66 in African Americans.
The hazard ratios and AUCs in relation to the GRS were uniformly stronger for unprovoked VTE than for total VTE (Table 2). In contrast, the hazard ratios and AUCs for DVT only and for PE (with or without DVT) were quite similar to those for total VTE (data not shown).
As shown in Supplemental Table 2, in both African Americans and whites, the number of risk alleles in the 5-SNP GRS that a participant carried was associated positively (p<0.0001) with plasma Factor VIIIc, von Willebrand factor, Factor XI, and inversely with activated partial thromboplastin time and fibrinogen (only whites significant).
Discussion
This large U.S. population-based prospective study documented that a previously published 5-SNP GRS [1] was associated positively with incident VTE in whites over more than two decades of follow-up, although stronger for earlier than later follow-up. The GRS was more strongly associated with unprovoked VTE than total VTE. In contrast with whites, in African Americans the GRS was not significantly associated with VTE.
Our results for whites replicate to some degree the findings of two reports of European case-control studies using the same GRS [1, 2]. However, the F2 rs1799963 risk allele was rare in ARIC, and most of the other SNPs had weaker associations than in the case control studies. As a result, the GRS added little predictive value beyond the nongenetic factors in this study with very long follow-up. In the de Haan et al study [1], VTE risk increased by 61% (54–68%) per risk allele in the weighted 5-SNP GRS, and the AUC was 0.66. In ARIC whites, VTE risk increased by 41% (27–56%) per risk allele in the weighted GRS, but the AUC was 0.59, suggesting poorer discrimination in ARIC than in all the three previous reports [1–3]. Several factors might explain this difference. Firstly, the five SNPs in the GRS chosen by de Haan et al were optimized for their Dutch sample and might be expected to predict VTE less well in a U.S. sample of whites with more diverse ancestry. Secondly, the VTE cases in our 20-year multicenter prospective study are likely to be more heterogeneous than cases in a single population case-control sample taken over a shorter period [1] or in a single sex group [2]. Our prediction for the more homogeneous group of “unprovoked” VTEs was better than for total VTE. Thirdly, the non-cases in VTE case-control studies may be less representative of their study base than in cohort studies. For example, the MEGA Study used acquaintances or partners of the VTE patients as controls [1]. Despite differences among studies, all in all, the 5-SNP score does help identify adults at risk of VTE in multiple white populations.
There are several possible reasons why the 5-SNP GRS did not work in African Americans. The score was derived in whites. Some variants (e.g., F5 Leiden rs6025 and F2 rs1799963) are rare in African Americans, and it is possible that other variants are important for a GRS in African Americans. On average, haplotype blocks are smaller in African Americans than whites and linkage disequilibrium patterns frequently differ between populations, which may limit transferability of disease-associated SNPs from whites to African Americans [9]. However, there are few genomic studies of VTE in African Americans, and they have often been underpowered. We had a moderately large sample of African Americans, but given the weak GRS association with VTE, our statistical power also was limited. Individually F5 Leiden and non-O blood group were risk factors for VTE in African Americans, whereas the other SNPs were not. Supplemental Table 2 also showed that the 5-SNP GRS was associated with several hemostatic factors quite similarly in whites and African Americans, suggesting the GRS has similar race-specific associations with multiple intermediate hemostasis phenotypes. Yet, clearly the 5-SNP GRS, which predicts VTE reasonably well in whites, cannot be applied to African Americans. To derive a better VTE GRS for African Americans, a larger study than ARIC, and a replication sample, will be required.
We are uncertain why the hazard ratios of VTE for some genetic variants decreased over time (Table 1). Most likely explanations are that heritability of traits, such as VTE, likely decreases with age [10, 11], that the proportion of provoked VTEs (presumably less genetically determined) increases with age, and that other competing health risks become important.
The nongenetic factors we studied in ARIC did not predict VTE as well as in the case-control studies [1, 2], for several likely reasons. Firstly, lifestyle factors over a two decade prospective study may change over time, leading to their misclassification [12], whereas exposures collected retrospectively by case-control studies may be more accurate and better reflect the risk period relevant to VTE cases. Secondly, ARIC was a general cardiovascular cohort study and did not capture some VTE specific exposures such as VTE family history, immobilization, and other VTE triggers. Case-control studies can more readily study VTE triggers, having such information on both cases and controls, whereas cohort studies cannot easily capture this information for non-cases. Thirdly, prediction of VTE from nongenetic factors inevitably is more accurate in a case-control study, which focuses on a short period between exposure and outcome, than in a long-term cohort study [13].
Another limitation of our study is that we identified hospitalized VTE patients only. Pilot data suggest the vast majority of patients with first VTEs in ARIC between 1987 and 2011 were hospitalized.
In conclusion, in the ARIC cohort, higher values for a GRS comprising five SNPs consistently associated with VTE helped identify white adults at risk of VTE, particularly unprovoked VTE. The 5-SNP GRS did not identify future VTE occurrence in African Americans. Further research on VTE risk prediction in this high risk ethnic group is needed. Clinical trials may be warranted to evaluate whether estimation of VTE risk based on genetic variants may have clinical utility in the primary prevention of VTE in whites.
Supplementary Material
Essentials.
There is little prospective information on genetic risk scores to predict venous thromboembolism.
Community based cohort followed a median of 22.6 years for venous thromboembolism occurrence.
A 5-SNP risk score identified whites at risk of venous thromboembolism, but not African Americans.
The utility of genetic risk scores for venous thromboembolism is yet to be established.
Acknowledgements
The authors thank the staff and participants of the ARIC Study for their important contributions.
Sources of Funding
This study was supported by National Heart, Lung, and Blood Institute (NHLBI) R01 HL59367, R01 HL103706, and ARIC contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C.
Footnotes
Addendum
All authors contributed to critical revision of the manuscript and approved the final version. In addition, A. R. Folsom contributed to concept and design, classified VTE cases, and drafted the manuscript; W. Tang contributed to concept and design; L-C. Weng analyzed the data; N. S. Roetker analyzed the data; M. Cushman contributed to concept and design, and classified VTE cases; S. Basu provided statistical advice; and J. S. Pankow contributed to concept and design.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.de Haan HG, Bezemer ID, Doggen CJM, Le Cessie S, Reitsma PH, Arellano AR, Tong CH, Devlin JJ, Bare LA, Rosendaal FR, Vossen CY. Multiple SNP testing improves risk prediction of first venous thrombosis. Blood. 2012;120:656–663. doi: 10.1182/blood-2011-12-397752. [DOI] [PubMed] [Google Scholar]
- 2.Bruzelius M, Bottai M, Sabater-Lleal M, Strawbridge RJ, Bergendal A, Silveira A, Sundström A, Kieler H, Hamsten A, Odeberg J. Predicting venous thrombosis in women using a combination of genetic markers and clinical risk factors. J Thromb Haemost. 2015;13:219–227. doi: 10.1111/jth.12808. [DOI] [PubMed] [Google Scholar]
- 3.Soria JM, Morange PE, Vila J, Souto JC, Moyano M, Trégouët DA, Mateo J, Saut N, Salas E, Elosua R. Multilocus genetic risk scores for venous thromboembolism risk assessment. J Am Heart Assoc. 2014;3:e001060. doi: 10.1161/JAHA.114.001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckner TW, Key NS. Venous thrombosis in blacks. Circulation. 2012;125:837–839. doi: 10.1161/CIRCULATIONAHA.111.073098. [DOI] [PubMed] [Google Scholar]
- 5.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 6.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the Longitudinal Investigation of Thromboembolism Etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Keating BJ, Tischfield S, Murray SS, Bhangale T, Price TS, Glessner JT, Galver L, Barrett JC, Grant SF, Farlow DN, Chandrupatla HR, Hansen M, Ajmal S, Papanicolaou GJ, Guo Y, Li M, Deronhannessian S, de Bakker PI, Bailey SD, Montpetit A, et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS One. 2008;3:e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 9.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 10.Marenberg ME, Risch N, Berkman LF, Floderus B, de Faire U. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med. 1994;330:1041–1046. doi: 10.1056/NEJM199404143301503. [DOI] [PubMed] [Google Scholar]
- 11.Zöller B, Li X, Sundquist J, Sundquist K. Age- and gender-specific familial risks for venous thromboembolism: a nationwide epidemiological study based on hospitalizations in Sweden. Circulation. 2011;124:1012–1020. doi: 10.1161/CIRCULATIONAHA.110.965020. [DOI] [PubMed] [Google Scholar]
- 12.Wattanakit K, Lutsey PL, Bell EJ, Gornik H, Cushman M, Heckbert S, Rosamond W, Folsom AR. Association between cardiovascular disease risk factors and occurrence of venous thromboembolism: A time-dependent analysis. Thromb Haemost. 2012;108:508–515. doi: 10.1160/TH11-10-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.