Abstract
Background
Voriconazole (VOR) levels are highly variable, with potential implications to both efficacy and safety. We hypothesized that VOR therapeutic drug monitoring (TDM) will decrease the incidence of treatment failures and adverse events (AEs).
Methods
We initiated a prospective, randomized, non-blinded multicenter study to compare clinical outcomes in adult patients randomized to standard dosing (clinician-driven) vs. TDM (doses adjusted based on levels). VOR trough levels were obtained on day 5, 14, 28, and 42 (or at completion of drug; ± 3 days). Real-time dose adjustments were made to maintain a range between 1–5 μg/mL on the TDM-arm, while levels were assessed retrospectively in the standard arm. Patient questionnaires were administered to assess subjective AEs.
Results
The study was discontinued prematurely, after 29 patients were enrolled. Seventeen (58.6%) patients experienced 38 AEs: visual changes (22/38, 57.9%), neurological symptoms (13/38, 34.2%), and liver abnormalities (3/38, 7.9%). VOR was discontinued in 7 (25%) patients because of an AE (4 standard-arm, 3 TDM-arm). VOR levels were frequently out of range in the standard-arm (8 tests >5 μg/mL; 9 tests < 1 μg/mL). Three dose changes occurred in the TDM-arm for VOR levels <1 μg/mL. Levels decreased over time in the standard-arm, with mean VOR levels lower at end of therapy compared to TDM (1.3 vs. 4.6 μg/mL, P = 0.008).
Conclusions
VOR TDM has become widespread clinical practice, based on known variability in drug levels, which impaired accrual in this study. Although comparative conclusions are limited, observations of variability and waning levels over time support TDM.
Keywords: voriconazole, therapeutic drug monitoring, transplant monitoring
Voriconazole (VOR) is a mold-active triazole with activity against Aspergillus species and other filamentous fungi, including Fusarium and Scedosporium species. It is available in both oral and intravenous formulations and acts by inhibiting the fungal cytochrome P450-dependent 14-alpha-sterol demethylase, like other azole antifungal agents. VOR undergoes extensive hepatic metabolism by the following P450 system enzymes: CYP2C19, 2C9, and 3A4. Genetic polymorphisms in CYP2C19, drug-drug interactions, and underlying liver disease may affect the metabolism of VOR. The use of VOR has been associated with a number of adverse events (AEs), including: (i) a skin rash and/or photosensitivity reactions (3.4–15.4%); (ii) visual changes and hallucinations (4–44.8%), ranging from enhanced light perception, blurred vision, wavy or zigzag lines, increased “brightness” perception, altered visual or color perception, to photophobia; and (iii) abnormal liver function tests (3.8–23.0%) (1–12).
Multiple reports have underscored the variability of VOR plasma levels and possible associations between levels and outcomes (treatment failures and toxicities) (13–16). In small retrospective studies, maintaining a trough VOR level >1 μg/mL was associated with improved clinical outcomes (15). In contrast, plasma VOR levels >5.5 μg/mL were associated with toxicities. These observations have generated the proposal that VOR levels should be monitored both to assure efficacy and to minimize toxicities, targeting blood levels between 1 and 5.5 μg/mL (17).
We hypothesized that a level-guided approach of VOR dosing would decrease the incidence of AEs and treatment failures. This report details results of a randomized trial in which Patients ≥ 18 years of age were observed on VOR administered according to ‘standard’ dosing suggestions, or with monitoring of drug levels (therapeutic drug monitoring, TDM).
Materials and methods
Study design
This was a prospective, randomized, non-blinded multicenter study, conducted at 4 academic institutions in North America: The Johns Hopkins University, Hôpital Maisonneuve-Rosemont at University of Montreal, University of Florida, and University of Pittsburgh. The study was approved by the institutional review board at each institution. Patients with an underlying immunocompromising condition (e.g., stem cell transplant, hematologic malignancy, etc.) were enrolled if they were ≥ 18 years of age; initiated on VOR for possible, probable, or proven invasive fungal infection (IFI); had signed an informed consent form; and were willing and able to comply with the protocol’s treatment plan and visits.
Patients were excluded for any of the following criteria: (i) history of allergy or serious reactions to azoles, (ii) aspergilloma or allergic bronchopulmonary aspergillosis, (iii) chronic invasive aspergillosis with duration of symptoms or radiological findings for >4 weeks, (iv) receipt of drugs known to interact with azoles, such as rifampin and sirolimus, (v) receipt of >5 days of VOR and/or >7 days of systemic antifungal treatment prior to enrollment, (vi) severe liver dysfunction (defined as total bilirubin, aspartate aminotransferase, alanine aminotransferase, or alkaline phosphatase [ALP] >3 times the upper limit of normal), (vii) any condition which could affect patient safety or make it unlikely that the proposed course of therapy can be completed, and (viii) body weight <45 and >120 kg.
Randomization and interventions
Patients were randomly assigned 1:1 to receive standard therapy with VOR, doses adjusted according to clinician determination (“standard-arm”) vs. intensive therapeutic monitoring of drug levels (“TDM-arm”), with doses adjusted according to levels. Randomization was performed within the first 5 days of VOR initiation, through the REDCap electronic data capture system, hosted at the Johns Hopkins Bloomberg School of Public Health.
VOR administration
Patients were stratified at entry according to whether they were initiated on therapy with oral or intravenous VOR. A loading dose of VOR was not a requirement for enrollment. Intravenous VOR was administered at a maintenance dose of 4 mg/kg every 12 h. For patients treated with oral VOR, a weight based regimen (4 mg/kg every 12 h) was used, rounded to the closest 50 mg dose: e.g., for patients weighing 67 and 72 kg, the mg/kg dose (268 mg and 288 mg) was rounded to 250 mg and 300 mg, respectively.
TDM-arm
Patients who were randomized to TDM had baseline trough VOR levels obtained within 5 (± 3) days after start of the drug. Levels were again drawn on day 14 (± 3), 28 (± 3), and 42 (or at completion of drug, ± 3). Safety assessments were performed 4 weeks after discontinuation of the drug. Members of the treating teams were notified about trough VOR levels and dose adjustments were made. Specifically, for patients with trough VOR level <1 or >5 μg/mL, the VOR dose was increased or decreased, respectively, by 1 mg/kg per dose. Each time a dose adjustment was made, a repeat trough VOR level was obtained in 7 (±2) days.
Standard-arm
Patients who were randomized to the standard-arm received VOR according to the clinician’s dosing judgment, without levels available for guidance. However, sera were collected at the same time scale as for TDM-arm subjects, and stored frozen for batched analysis of VOR trough levels. If the primary team requested and obtained a VOR level, the patient was discontinued from the study. Dose escalation was performed at the discretion of the treating physician.
Data collection
The following data were collected: demographics, underlying disease, transplant characteristics, immunosuppressive agents and other concomitantly administered medications, and IFI specifics. Laboratory tests were collected to assess renal and liver function on day 5, 14, 28, 42, and at end of treatment (± 3). All VOR levels in the TDM-arm were performed by high-performance liquid chromatography assays, in clinical pathology laboratories of the 4 participating centers. Before the study, a validation analysis was performed to document reproducibility of results generated in each center (data not shown). Levels from patients in the standard-arm were performed after completion of enrollment, by batched analysis at one site (Johns Hopkins).
Adverse events were collected at each time point by review of medical records; investigators coded events according to likelihood of relation to VOR, with consideration of known anticipated effects on vision, neurological symptoms (hallucinations, mental status changes), hepatotoxicities, and skin rashes. To obtain more subjective understanding of each patient’s experience, a questionnaire was administered to determine the presence of visual changes, difficulties in concentration, thought impairment, changes in sleep patterns, etc.
Endpoints and statistics
IFI were defined as possible, probable, and proven based on the revised EORTC/MSG consensus definitions (18). Clinical response was assessed at day 42 after study entry and coded based on adjusted definitions (19) as complete (resolution of all clinical signs and symptoms and >90% of the lesions caused by IFI on radiologic studies at baseline), partial (clinical improvement and >50% improvement in radiological findings compared to baseline), stable (no change from baseline or <50% improvement in radiological findings compared to baseline), and worse (worsening disease, either clinically or radiographically) (19). For the purposes of this study, complete, partial, and stable clinical responses were coded as “clinical success,” whereas worsening (or death) were coded as “clinical failure.”
Targeted enrollment was estimated to assess the hypothesis that VOR TDM reduces the risk of failure (defined using a composite of clinical success and safety), down from 45% to 20% (i.e., a difference of 25%). A goal of 146 patients was established to yield 122 evaluable patients to provide 80% power to detect this difference with a 2-sided alpha of 0.05. However, the study was discontinued prematurely after 29 patients were enrolled, because of widespread reluctance of clinicians to enroll patients into a study that utilized a standard, non-TDM approach. Thus, data presented include descriptive analyses of secondary objectives: assessment of the frequency and timing of VOR-related AEs, clinical failure rates, and serial VOR levels.
Appropriate descriptive statistics (mean and standard deviation for continuous variables; frequency and percentage for categorical variables) were used to summarize the baseline demographics, overall responses, and AEs over time. Comparisons were performed with chi-square tests for categorical variables and t-tests or ANOVA for continuous variables. P-values <0.05 were considered significant. Analyses were performed using GraphPad Prism 6 statistical computing software (GraphPad Software, San Diego, California, USA).
Results
Patient characteristics
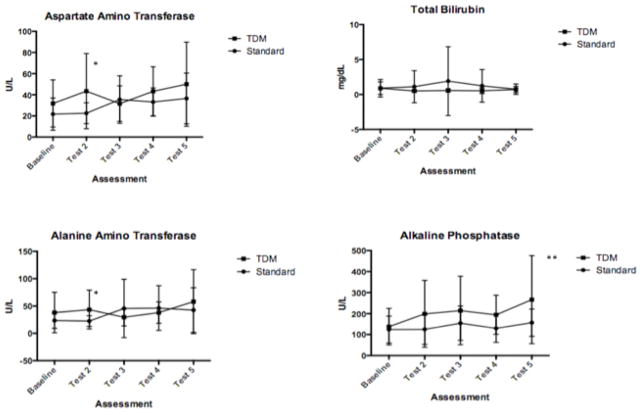
Between March 2012 and October 2013, 29 people (15 in the standard-arm and 14 in the TDM-arm) were enrolled at 4 different medical centers (Johns Hopkins University n = 20, University of Montreal n = 6, University of Florida n = 2, and University of Pittsburgh n = 1). Demographics, underlying diseases, and conditions at start of VOR therapy were similar between groups (Table 1). The starting VOR dose was higher in the standard-arm (mean 5.6, range 2.2–9.1 mg/kg) compared to the TDM-arm (mean 3.9, range 2.3–4.5 mg/kg). No difference was found in baseline liver function tests obtained from patients between the 2 arms (data not shown) (Fig. 1). One person with baseline ALP and transaminases > 5 times the upper limit of normal was excluded from the TDM-arm after randomization, and not included in subsequent analyses.
Table 1.
Demographic and clinical characteristics at baseline
| Variable | Standard n = 15 (%) | TDM n = 14 (%) |
|---|---|---|
|
| ||
| Demographics | ||
|
| ||
| Mean age (range) | 59.1 (28–84) | 63.4 (49–83) |
|
| ||
| Mean weight (kg, range) | 74.3 (45–103) | 86.6 (48–118) |
| Ethnicity | ||
| White | 14 (93) | 14 (100) |
| Hispanic | 1 (7) | 0 |
|
| ||
| Gender, Female | 5 (33) | 5 (36) |
|
| ||
| Medical conditions, n (%) | ||
|
| ||
| Underlying disease | ||
| Acute leukemia | 7 (47) | 7 (47) |
| Chronic leukemia | 3 (15) | 4 (27) |
| Lymphoma | 1 (7) | 1 (7) |
| Other solid tumors | 5 (33) | 2 (14) |
|
| ||
| SCT (n = 11) | ||
| Allogeneic, HLA MRD | 4 (27) | 2 (14) |
| Allogeneic, HLA URD / Haploidentical | 1 (7) | 4 (29) |
|
| ||
| Diagnosis of IFI, n (%)1 | ||
|
| ||
| Possible invasive aspergillosis | 11 (73) | 9 (64) |
| Probable invasive aspergillosis | 4 (27) | 3 (21) |
| Proven invasive aspergillosis | 0 | 1 (7) |
|
| ||
| Treatment initiation | ||
|
| ||
| Oral | 10 (77) | 6 (43) |
| Intravenous | 5 (33) | 8 (57) |
| Mean mg/kg at starting dose (range) | 5.6 (2.2–9.1) | 3.9 (2.3–4.5) |
One patient in the therapeutic drug monitoring (TDM) arm received the drug without a diagnosis of invasive fungal infection (IFI). This patient was enrolled after the trial was expanded to include patients who received voriconazole as prophylaxis.
SCT, stem cell transplant; HLA, histocompatibility antigen; MRD, matched related donor; URD, unmatched related donor.
Fig. 1. Serial liver function testing.
Levels were obtained at baseline (enrollment) and at specified time intervals (7, 14, 28, and 42 days) after start of therapy (corresponding to baseline, tests 2–5). Point estimates of means with 2-sided standard deviations are shown. P-value calculated from 2-sided t- test adjusted for multiple comparisons using Holm-Sidak method are shown (*P = 0.08, **P = 0.09).
Adverse events
Median duration of therapy did not differ between standard-arm (40 days, range 2–126) and TDM-arm (42 days, range 4–110) patients. Eleven (39%) patients experienced no AE potentially related to VOR. The remaining 17 patients experienced 38 AEs thought to be possibly or probably related to VOR, with an average of 1.3 per patient in the standard-arm and 1.4 per patient in the TDM-arm. Most patients reported changes in vision as their primary symptom (22 of 38 AEs, 57.9%), including blurry vision (n = 12), increased color or light sensitivity (n = 8), or flashing lights (n = 2). Neurological symptoms were frequent (13 of 38, 34.2%), including visual hallucinations (n = 5), visual and auditory hallucinations (n = 1), changes in thinking (n = 3), difficulties in memory or concentration (n = 2), difficulties in speech (n = 1), or insomnia (n = 1). Serial measurements of liver function tests are shown in Figure 1. No differences in parameters were seen at baseline, but trends were noted towards differences in transaminase and ALP levels with later testing. However, no difference in means reached statistical significance. Three patients developed changes in liver function testing that were > 3 times the upper limit of normal.
These AEs were largely early in onset after start of VOR (27 AEs were reported within 7 days of treatment initiation, and 11 were reported after 1 week), with a trend to earlier onset in people who received standard dosing (median 1 day, range 1–53) compared to TDM (median 5 days, range 1–10). Seven people (25%) had VOR stopped because of development of an AE, with no difference in standard therapy arm (n = 4) and TDM-arm (n = 3).
Clinical response
Clinical responses were assessed at day 42 after study enrollment. Three people had died (1/15, 6.7% standard-arm, 2/14, 14.3% TDM-arm; P = 0.6); 6 people were judged to have failed clinical responses (5/15, 33.3% standard-arm, 1/14, 7.1% TDM-arm; P = 0.17); and 19 were considered to have had a relatively positive outcome of antifungal therapy (stable, partial, or complete responses, reported in 7/15, 46.7% standard-arm and 12/14, 85.7% TDM-arm recipients; P = 0.05).
VOR trough levels
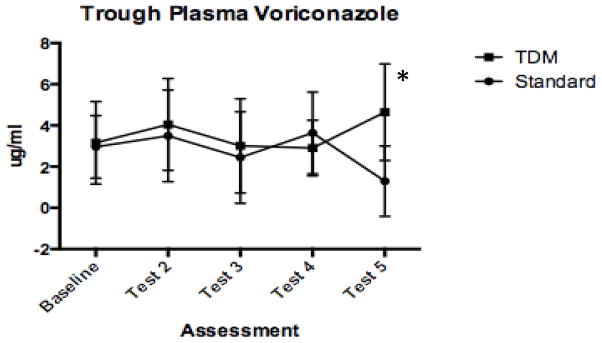
VOR levels were measured with real-time dose adjustments to maintain a range between 1 and 5 μg/mL on the TDM-arm, but levels were not measured on the standard-arm until completion of the study. During the study period, doses were changed in 3 (20%) standard-arm and in 7 (50%) TDM-arm patients (P = 0.09). It was unusual for results to fall out of range with weight-based dosing in the TDM-arm. Three patients had levels that required an escalation in dose to yield a result >1 μg/mL, and these results occurred after the third testing. VOR levels were noted to be between 5 and 6 μg/mL in 4 tests from 3 patients in the TDM-arm. VOR levels were frequently out of range in patients on the standard-arm, with 8 tests from 4 patients reported as > 5 μg/mL and 9 tests from 5 patients being < 1 μg/mL. In patients with >1 VOR trough levels out of the 1–5 μg/mL range, low (<1 μg/mL) and high (>5 μg/mL) VOR trough levels were observed in the same patients over time. Figure 2 shows results of trough values over time. Levels went down in the standard-arm; at completion of study, the mean level in standard-arm had decreased (1.3 ± 1.7 μg/mL) relative to TDM-arm levels (4.6 ± 2.4 μg/mL, P = 0.008). It is notable that a small number of patients were on therapy before test 5 (4 patients in standard-arm, 5 patients in TDM-arm), likely leading to the large standard deviations demonstrated in Figure 2.
Fig. 2. Serial voriconazole levels.
Levels obtained in real-time (TDM) and measured by HPLC (standard-arm), and levels obtained from batched sampling at protocol-specified time intervals are shown. Point estimates of means with 2-sided standard deviations are shown. P-value calculated from 2-sided t-test adjusted for multiple comparisons using Holm-Sidak method is shown (*P = 0.008). TDM, therapeutic drug monitoring; HPLC, high-performance liquid chromatography.
Discussion
This randomized trial was performed to test the hypothesis that monitoring of VOR levels can decrease drug-related AEs and assist in clinical outcomes in patients treated for an IFI. Unfortunately, the study was stopped prematurely owing to inability to enroll patients after the availability of VOR levels in clinical microbiology laboratories resulted in the widespread perception that TDM is helpful in directing dosing. Results described herein are therefore not definitive owing to few patients enrolled. However, with this limitation in mind, the novel observation generated from sequential, blinded testing of VOR levels includes the wide variability in levels resulting from standard dosing, and the waning of levels over time.
After this study was opened to enrollment in 2012, several studies were published to support the role of TDM for VOR dosing (20, 21). These studies showed that multiple variables, including genotype, drug interactions, age, and even inflammation, play a role in dictating variable drug levels (22–25). VOR level monitoring was also studied in 1 small randomized trial, in which 110 adult patients were randomized in a non-blinded comparison; results of that analysis suggested that TDM reduces drug discontinuation and AEs (26). We sought to perform a randomized trial to measure the clinical impact of TDM on safety and efficacy of the drug, but failed to complete enrollment. In order to continue the trial, we amended the design of the study twice, expanding indications for VOR (to include prophylaxis), including patients with other underlying diseases (e.g., solid organ transplant recipients), and lowering the age of inclusion (to 12 years). Despite these changes, and with TDM becoming more ingrained into clinical practice, we were unable to recruit patients into this trial. Results thus do not provide definitive data on the impact of TDM on clinical care, but add to conclusions drawn from observational studies.
Patients’ initial VOR doses ranged widely, as detailed in Table 1. These doses were initiated by treating physicians, as patients were identified for enrollment in the study after the drug had begun (within 5 days). This variation in dosing outlines frequent inconsistencies and miscalculations in dosing based on clinician practice. The combination of variable VOR dosing without monitoring of results over time in the standard-arm may, in part, explain the decreasing trend of VOR levels observed on standard dosing during the study period. Although levels were numerically higher in the TDM-arm after 42 days of therapy, definitive comparisons, and implications on clinical significance cannot be assessed, given the small numbers of patients sampled. It is, however, of interest that few studies have been performed to assess serial VOR levels over long periods of time. The potential of auto-induction of metabolism has been postulated in humans (27), and studied in greater depth in rodents, the latter of which demonstrate auto-induction by mechanisms that involve pregnane X receptor- and constitutive androstane receptor-mediated induction of CYP3A11 (28). However, this mechanism is thought to be species-specific and not involved in human metabolism of the drug. These observations suggest that periodic level monitoring may enable dose adjustment based on low trough values over time.
We used questionnaires to elicit feedback from patients regarding potential drug-related toxicities, and found that reported AEs that involved vision, hallucinations, and cognitive functioning were relatively common. This questionnaire is similar to the survey-methodology used by investigators who recently reported high rates of alopecia and nail changes in people who received VOR for treatment of iatrogenic fungal meningitis (29). This methodology has both strengths and limitations, especially with regards to the potential of over-estimating events elicited by survey bias. Although we cannot conclude that the reported experiences are directly associated with the drug, the timing of onset was typically early and thus consistent with prior reports of VOR-related neurologic toxicities, and most likely reflects the effect of a loading VOR dose in a number of these patients. Notably, a trend was seen to earlier AE onset in the standard-arm group. As a higher starting dose (mean of 5.6 mg/kg) of VOR was observed in the latter group of patients, this may, in part, suggest more frequent use of a loading dose in this group associated with early AEs.
In conclusion, this small randomized trial failed to achieve targeted goals of enrollment owing to waning enthusiasm to follow patients in a non-TDM-arm after the TDM test was made available by clinical laboratories. Weight-based dosing within the TDM-arm was generally successful in achieving levels within the 1–5 μg/mL range. Given the decreased VOR levels observed late after start of therapy, and cumulative reports of variability, it seems wise to periodically test VOR troughs after initiating weight-based dosing. We cannot comment on the impact of VOR levels on either clinical efficacy or toxicities, given the limitations in study enrollment.
Acknowledgments
Support: This study was supported by an unrestricted medical school grant provided by Pfizer (IIR WS878026) and National Institutes of Health grant K24 AI085118.
Footnotes
Potential conflicts of interest: D.N. has received research grants from Pfizer and served as a consultant and/or advisory board member for Roche and Astellas. He is currently employed by Roche Diagnostics.
S.S. is the recipient of research grants from Pfizer, Merck, and Astellas and has performed data review committee work for Astellas. All funds from these entities have been paid to Johns Hopkins University School of Medicine and managed as per that institution’s policies.
M.L. has received research grants from Pfizer, Astellas, and Merck & Co. He served as a consultant and or as advisory board member for Pfizer Canada, and Merck Canada
M.H.N. has received research grants from Pfizer, Merck, and Astellas.
K.A M. has served as a consultant and received research funding from Astellas, Merck, and Pfizer.
D.O., J.H., W.C., L.B., and N.L.: No conflicts of interest.
References
- 1.Denning DW, Griffiths CE. Muco-cutaneous retinoid-effects and facial erythema related to the novel triazole antifungal agent voriconazole. Clin Exper Dermatol. 2001;26(8):648–653. doi: 10.1046/j.1365-2230.2001.00909.x. [DOI] [PubMed] [Google Scholar]
- 2.Boyd AE, Modi S, Howard SJ, et al. Adverse reactions to voriconazole. Clin Infect Dis. 2004;39(8):1241–1244. doi: 10.1086/424662. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein M, Levy ML, Metry D. Voriconazole-induced retinoid-like photosensitivity in children. Pediatr Dermatol. 2004;21(6):675–678. doi: 10.1111/j.0736-8046.2004.21614.x. [DOI] [PubMed] [Google Scholar]
- 4.Racette AJ, Roenigk HH, Jr, Hansen R, Mendelson D, Park A. Photoaging and phototoxicity from long-term voriconazole treatment in a 15-year-old girl. J Am Acad Dermatol. 2005;52(5 Suppl 1):S81–85. doi: 10.1016/j.jaad.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 5.McCarthy KL, Playford EG, Looke DF, Whitby M. Severe photosensitivity causing multifocal squamous cell carcinomas secondary to prolonged voriconazole therapy. Clin Infect Dis. 2007;44(5):e55–56. doi: 10.1086/511685. [DOI] [PubMed] [Google Scholar]
- 6.Malani AN, Aronoff DM. Voriconazole-induced photosensitivity. Clin Med Res. 2008;6(2):83–85. doi: 10.3121/cmr.2008.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ally R, Schurmann D, Kreisel W, et al. A randomized, double-blind, double-dummy, multicenter trial of voriconazole and fluconazole in the treatment of esophageal candidiasis in immunocompromised patients. Clin Infect Dis. 2001;33(9):1447–1454. doi: 10.1086/322653. [DOI] [PubMed] [Google Scholar]
- 8.Walsh TJ, Pappas P, Winston DJ, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002;346(4):225–234. doi: 10.1056/NEJM200201243460403. [DOI] [PubMed] [Google Scholar]
- 9.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 10.Denning DW, Ribaud P, Milipied N, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 2002;34(5):563–571. doi: 10.1086/324620. [DOI] [PubMed] [Google Scholar]
- 11.Kullberg BJ, Sobel JD, Ruhnke M, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet. 2005;366(9495):1435–1442. doi: 10.1016/S0140-6736(05)67490-9. [DOI] [PubMed] [Google Scholar]
- 12.Ostrosky-Zeichner L, Oude Lashoff AM, Kullberg BJ, Rex JH. Voriconazole salvage treatment of invasive candidiasis. Eur J Clin Microbiol Infect Dis. 2003;22(11):651–655. doi: 10.1007/s10096-003-1014-3. [DOI] [PubMed] [Google Scholar]
- 13.Trifilio S, Singhal S, Williams S, et al. Breakthrough fungal infections after allogeneic hematopoietic stem cell transplantation in patients on prophylactic voriconazole. Bone Marrow Transplant. 2007;40(5):451–456. doi: 10.1038/sj.bmt.1705754. [DOI] [PubMed] [Google Scholar]
- 14.Trifilio S, Pennick G, Pi J, et al. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer. 2007;109(8):1532–1535. doi: 10.1002/cncr.22568. [DOI] [PubMed] [Google Scholar]
- 15.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46(2):201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 16.Howard A, Hoffman J, Sheth A. Clinical application of voriconazole concentrations in the treatment of invasive aspergillosis. Ann Pharmacother. 2008;42(12):1859–1864. doi: 10.1345/aph.1L243. [DOI] [PubMed] [Google Scholar]
- 17.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 18.De Pauw B, Walsh TJ, Donnelly JP, et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORT/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47(5):674–683. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2014;69(5):1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laverdiere M, Bow EJ, Rotstein C, et al. Therapeutic drug monitoring for triazoles: a needs assessment review and recommendations from a Canadian perspective. Can J Infect Dis Med Microbiol. 2014;25(6):327–343. doi: 10.1155/2014/340586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolton MJ, Ray JE, Chen SC, Ng K, Pont LG, McLachlan AJ. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob Agents Chemother. 2012;56(9):4793–4799. doi: 10.1128/AAC.00626-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolton MJ, Mikus G, Weiss J, Ray JE, McLachlan AJ. Understanding variability with voriconazole using a population pharmacokinetic approach: implications for optimal dosing. J Antimicrob Chemother. 2014;69(6):1633–1641. doi: 10.1093/jac/dku031. [DOI] [PubMed] [Google Scholar]
- 24.van Wanrooy MJ, Span LF, Rodgers MG, et al. Inflammation is associated with voriconazole trough concentrations. Antimicrob Agents Chemother. 2014;58(12):7098–7101. doi: 10.1128/AAC.03820-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartelink IH, Wolfs T, Jonker M, et al. Highly variable plasma concentrations of voriconazole in pediatric hematopoietic stem cell transplantation patients. Antimicrob Agents Chemother. 2013;57(1):235–240. doi: 10.1128/AAC.01540-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park WB, Kim NH, Kim KH, et al. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin Infect Dis. 2012;55(8):1080–1087. doi: 10.1093/cid/cis599. [DOI] [PubMed] [Google Scholar]
- 27.Moriyama B, Elinoff J, Danner RL, et al. Accelerated metabolism of voriconazole and its partial reversal by cimetidine. Antimicrob Agents Chemother. 2009;53(4):1712–1714. doi: 10.1128/AAC.01221-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohbuchi M, Yoshinari K, Kaneko H, Matsumoto S, et al. Coordinated roles of pregnane X receptor and constitutive androstane receptor in autoinduction of voriconazole metabolism in mice. Antimicrob Agents Chemother. 2013;57(3):1332–1338. doi: 10.1128/AAC.01900-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malani AN, Kerr L, Obear J, Singal B, Kauffman CA. Alopecia and nail changes associated with voriconazole therapy. Clin infect Dis. 2014;59(3):e61–65. doi: 10.1093/cid/ciu275. [DOI] [PubMed] [Google Scholar]