Abstract
Aims
Determine how supercritical CO2 (scCO2) plus peracetic acid (PAA) inactivates Bacillus subtilis spores, factors important in spore resistance to scCO2-PAA, and if spores inactivated by scCO2-PAA are truly dead.
Methods and Results
Spores of wild-type B. subtilis and isogenic mutants lacking spore protective proteins were treated with scCO2-PAA in liquid or dry at 35°C. Wild-type wet spores (aqueous suspension) were more susceptible than dry spores. Treated spores were examined for viability (and were truly dead), dipicolinic acid (DPA), mutations, permeability to nucleic acid stains, germination under different conditions, energy metabolism and outgrowth. ScCO2-PAA-inactivated spores retained DPA, and survivors had no notable DNA damage. However, DPA was released from inactivated spores at a normally innocuous temperature (85°C), and colony formation from treated spores was salt sensitive. The inactivated spores germinated but did not outgrow, and these germinated spores had altered plasma membrane permeability and defective energy metabolism. Wet or dry coat-defective spores had increased scCO2-PAA sensitivity, and dry spores but not wet spores lacking DNA protective proteins were more scCO2-PAA sensitive.
Conclusions
These findings suggest that scCO2-PAA inactivates spores by damaging spores’ inner membrane. The spore coat provided scCO2-PAA resistance for both wet and dry spores. DNA protective proteins provided scCO2-PAA resistance only for dry spores.
Significance and Impact of Study
These results provide information on mechanisms of spore inactivation of and resistance to scCO2-PAA, an agent with increasing use in sterilization applications.
Keywords: spores, inner spore membrane, spore inactivation, spore resistance, supercritical carbon dioxide
INTRODUCTION
Dormant spores of a number of Bacillus and Clostridium species are extremely resistant to treatments that inactivate growing bacteria, and can therefore cause some severe human diseases. Consequently, there is significant interest in ways to inactivate spores, in particular using procedures that do not damage sensitive drugs, biomaterials or medical devices. One treatment that has attracted significant interest is supercritical carbon dioxide (scCO2) at pressures of 2-20 mega Pascals (mPa) and temperatures from 25-50°C (Spilimbergo et al. 2003; White et al. 2006; Spilimbergo et al. 2010; Park et al. 2013; Tamburini et al. 2014a,b; Bernhardt et al. 2015), conditions that alone are not especially damaging to drugs or devices. However, while such conditions can be efficient at inactivating growing bacteria of a variety of species, generally higher temperatures or pressures are needed to efficiently inactivate spores (Ishikawa et al. 1997; Rao et al. 2015).
Because of the poor efficiency of spore inactivation by scCO2 alone at moderate temperatures, a variety of chemicals have been added to scCO2 in order to increase spore inactivation, including acetic anhydride, hydrogen peroxide (H2O2), peracetic acid (PAA), PAA plus octanoic acid, trifluoroacetic acid, formic acid or alcohols (White et al. 2006; Nichols et al. 2009; Qiu et al. 2009; Shieh et al. 2009; Checinska et al. 2011; Howell et al. 2012; Russell et al. 2013; Yoganarasimha et al. 2014; Bernhardt et al. 2015;). Other variations on scCO2 have also been tested for inactivating spores of Bacillus species, including microbubbles of scCO2 and scCO2 treatment following a pulsed electric field treatment that sensitizes spores to scCO2 (Ishikawa et al. 1997; Spilimbergo et al. 2003).
A common additive to increase the efficacy of scCO2 inactivating spores is PAA (NovaSterilis U.S. patent 7,108,832), a compound that readily decomposes to acetic acid and water. PAA is an effective sporicide (Mohan et al. 2012; Buhr et al. 2013; Wehemeyer et al. 2015), but is much more effective when combined with scCO2 (White et al. 2006). Studies of spore inactivation by and resistance to PAA alone in liquid have shown that this agent does not inactivate spores by DNA damage, but perhaps by altering one or more of spores’ outer layers, possibly the inner membrane (IM), such that when spores germinate, this membrane ruptures to some degree (Setlow et al. 1997; Buhr et al. 2013; Park et al. 2014; Leggett et al. 2015). However, the precise mechanism of spore inactivation by PAA has not been identified. The spore coats seem to be very important in spore resistance to PAA in liquid, while DNA protection and DNA repair in spore outgrowth are not (Setlow et al. 1997; Leggett et al. 2015). The latter finding is consistent with spore inactivation by PAA in liquid not being by DNA damage.
There have been several studies of mechanisms of spore inactivation by scCO2 plus either hydrogen peroxide (Zhang et al. 2007; Cecinska et al. 2011) or ethanol (Park et al. 2013). Notably, scCO2 treatment with these agents appears to damage some spore permeability barrier, most likely the IM. However, spores treated with scCO2 plus hydrogen peroxide that were apparently dead could be revived to some degree if treated spores were artificially germinated. This suggests that some of these treated spores were not actually dead but only incapable of germination. In the current work we have examined Bacillus subtilis spores’ inactivation by and resistance to scCO2 plus PAA (scCO2-PAA). The scCO2-PAA treatment was carried out on spores in water (wet spores) and in the dry state (dry spores), since mechanisms of wet and dry spore inactivationcan differ (Setlow 2006). We have also investigated whether treated spores are truly inactivated, or just blocked in spore germination.
MATERIALS AND METHODS
B. subtilis strains and spore preparation and purification
The wild-type B. subtilis strain was PS533 (Setlow and Setlow 1996), an isogenic derivative of strain PS832, a laboratory 168 strain with plasmid pUB110 encoding resistance to kanamycin (10 mg l−1). Four isogenic derivatives of strain PS533 were also used: i) PS578, termed α−β− (Setlow and Setlow 1996), identical to PS533 but with deletions in the sspA and sspB genes encoding the two major α/β-type small, acid-soluble spore proteins (SASP) that protect spore DNA from damage (Setlow 2006); ii) PS3328 (Paidhungat et al. 2001), lacking the cotE gene resulting in a very defective spore coat, and also plasmid pUB110; iii) PS2318 (Young and Setlow 2003), lacking the recA gene essential for the repair of many types of DNA damage, and also plasmid pUB110 (Yasbin et al. 1993; Setlow and Setlow 1996; Setlow 2006); and iv) PS3379 (Setlow et al. 2001), lacking plasmid pUB110 and with the luxAB genes from Vibrio harveyi under the control of a strong forespore-specific promoter.
Spores of all strains were prepared on 2x Schaeffer's-glucose agar plates at 37°C and purified essentially as described (Nicholson and Setlow 1990; Paidhungat et al. 2000), but the plates for preparation of the recA spores were protected from UV light. All spores used were free (> 98%) of germinated spores, or growing or sporulating cells as observed by phase contrast microscopy, and were stored in water at 4°C protected from light.
Spore treatment with scCO2-PAA or PAA alone and assessment of spore inactivation
Spores were exposed to scCO2 in a 22 L Nova2200™ vessel at NovaSterilis (White et al. 2006). The supercritical phase was achieved by injecting liquid CO2 to a target pressure of 1,436 psi and temperature controlled at 35°C (CO2 reaches its supercritical point at 1,099 psi and 31.1°C). The bottom of the vessel is equipped with a propeller revolving at 650 rpm to provide constant movement of the fluid and thus homogeneity of scCO2 and additive. The sterilant additive NovaKill™ Gen2, containing PAA (certified 13.5%-18.5%) and hydrogen peroxide (H2O2) (certified 4.5%-6%), was added onto a cellulose pad (blotting paper cut into 3.5 × 20 cm strips; Ahlstrom, Helsinki, Finland) placed inside a stainless steel holder in a 1-inch tall stainless steel basket positioned directly over the propeller prior to injecting CO2. Based on the vessel volume, each ml of NovaKill™ Gen2 reagent should produce 7 ppm of PAA in the scCO2. The H2O2 stabilizes PAA, but appears to contribute minimally to spore inactivation (White et al. 2006); hence this reagent will be termed scCO2-PAA. 1-6•108 spores, unless otherwise noted, were added to 10 ml of reverse osmosis-purified (RO) water for scCO2 treatment in aqueous solution (wet spores), or 1-6•108 spores in 100 μl were air-dried on glass slides for dry treatment (dry spores). Wet or dry spore samples were placed in Poly-Tyvek pouches and sealed for scCO2 exposure in a second stainless steel basket. For inactivation of wet spores, the number of pouches containing 10 ml water was kept constant at 12 to avoid varying load effects. Following treatments, PAA and H2O2 were neutralized by adding one ml of 15 g l−1 sodium thiosulfate to each 10 ml in a pouch and glass slides were transferred to a 50 ml conical tube with 10 ml of 1.5 g l−1 sodium thiosulfate. Previous work (Cortezzo et al. 2004) has shown that thiosulfate treatment alone has no effect on B. subtilis spore viability. Dry spores were recovered from glass slides by vortexing for 5 min prior to pelleting spores by centrifugation. All samples were washed twice in water, suspended in 5 or 10 ml RO water, aliquots serially diluted in water, 10 μl aliquots spotted on LB medium agar plates with appropriate antibiotics, plates incubated for ~ 24 h at 30-37°C and colonies were counted. For treatments on glass slides, spore viability was compared to spores recovered from untreated glass slides. Spores (1-6•108) were also treated with aqueous PAA at 35°C in one ml of various concentrations of NovaKill™ Gen2. Neutralization, washes and assessment of spore viability were carried out as described above. All treated spores were stored at 4°C prior to further analyses.
To measure PAA accumulation in a solution after a sterilization run, one sachet of reagent RP9703PA-100 (Masters Company, Wood Dale, IL USA) was dissolved in 10 ml water, and 950 μl of this reagent was mixed with 50 μl of a test sample or serial dilutions of NovaKill™ Gen2 to produce a standard curve with absorbance measured at 550nm.
Analysis of scCO2-PAA-treated spore properties
Total DPA in spore preparations was assessed by extracting spore small molecules by boiling for 30 min, cooling on ice, centrifuging in a microcentrifuge, and assaying DPA in the supernatant fluid by its fluorescence with Tb+3 in a multi-well fluorometric plate reader (Molecular Devices, Sunnyvale, CA USA) (Yi and Setlow 2010). The release of DPA from spores upon high temperature incubations not lethal for untreated spores used spores at an optical density at 600 nm (O.D.600) of ~ 1.0 that were incubated in water at 85°C for 45 min, cooled on ice and then centrifuged in a microcentrifuge. Various amounts of the supernatant fluid up to 150 μl were then added to 50 μl of 200 μmol l−1 TbCl3 and 100 mmol l−1 K-Hepes buffer (pH 7.4) and the DPA released was quantitated from the Tb-DPA fluorescence (Yi and Setlow 2010; Yi et al. 2011).
Spore germination with nutrient germinants was routinely carried out with spores at an O.D.600 of ~ 0.5 in a multi-well fluorometric plate reader (Yi and Setlow 2010). Prior to germination, spores were heat activated for 30 min at 70°C (valine germination), or 1 h (AGFK germination) and cooled on ice. While longer heat activation times might have given more rapid AGFK germination (Luu et al. 2015), scCO2-PAA-treated spores released their DPA pool at normally sublethal temperatures (see Results), leading to use of 1 h of heat activation prior to AGFK germination. Spores were germinated at 37°C in 200 μl of 25 mmol l−1 K-Hepes buffer (pH 7.4), 50 μmol l−1 TbCl3, and either 10 mmol l−1 L-valine, or a mixture of 10 mmol l−1 L-asparagine – 10 mmol l−1 D-glucose – 10 mmol l−1 D-fructose – 10 mmol l−1 KCl (AGFK), and spore germination was monitored by Tb-DPA fluorescence. The degree of spore germination was also assessed at the end of experiments by phase contrast microscopy, and levels of germination determined by this method agreed with levels determined by monitoring DPA release. Spores were also germinated as described above but without TbCl3, and spore germination was followed by phase contrast microscopy every 30 min, with ~ 100 spores examined at each time point. For dodecylamine germination, spores at an O.D.600 of 0.5 were germinated at 50°C in 25 mmol l−1 K-Hepes buffer (pH 7.4) with 0.8 mmol l−1 dodecylamine. At various times 150 μl aliquots of the germination incubations were added to 50 μl of 200 μmol l−1 TbCl3, and fluorescence was measured.
For outgrowth spores were incubated at 37°C and an O.D.600 of ~ 1.0 in LB medium (Paidhungat and Setlow 2000) plus 10 mmol l−1 L-valine. At times out to four h, > 50 individual spores/cells were examined by phase contrast microscopy to distinguish dormant spores (phase bright), germinated spores (phase dark), outgrowing spores (swollen and beginning to elongate) and growing cells. Assessment of light production in relative light units from the V. harveyi LuxAB proteins during spore germination and outgrowth was carried out as described, but with PS3379 spores at an O.D.600 of 0.1-0.2, and light production was corrected for the relative amounts of spores in cultures (Setlow et al. 2001).
For assessment of mutagenesis following scCO2-PAA treatment of wild-type or α−β− spores, 200 μl aliquots of treated and untreated spores were spread on LB medium plates plus kanamycin (10 mg l−1). After overnight incubation at 37°C, individual colonies were toothpicked onto sporulation agar plates and Spizizen's minimal medium plates without Casamino acids (Spizizen 1958). The plates were incubated for two to three d at 37°C, and colonies were scored for auxotrophic and asporogenous mutations as described previously (Fairhead et al. 1993).
Assessment of viability of germinated spores
Untreated and scCO2-PAA-treated spores were incubated in LB medium plus 10 mmol l−1 L-valine as described above, but the spores were heat shocked (60 min; 70°C) prior to initiating germination. After 45 min, samples were incubated with the BacLight reagent (Thermo Fisher, Waltham, MA USA) to determine if the germinated spores were likely alive or dead, as live cells and live germinated spores stain green while dead cells and dead germinated spores stain red (Zhang et al. 2007). After incubation for ~ 15 min with the BacLight reagent spores were photographed on a fluorescence microscope (Murray et al. 1998; Coleman et al. 2007). Dormant untreated or scCO2-PAA-treated spores were also stained with the BacLight reagent and photographed as described above. In some experiments, dilutions of spores were spotted on LB medium plates containing either 150 mmol l−1 NaCl, 1 mol l−1 NaCl or 1 mol l−1 NaCl plus 10 mmol l−1 D-glucose, plates incubated at 30-37°C for 24-48 h, and colonies were counted.
Other tests
Untreated and scCO2-PAA-treated spores were incubated in hypertonic medium (Popham et al. 1996), germinated in LB medium as described above, and spotted on LB medium plates. In addition, at various times, lysozyme was added to 5 mg l−1 to germinated spore incubations that were incubated for a further 20 min, and samples were examined before and after lysozyme addition by phase contrast microscopy. Aliquots of these incubations were then spotted on LB medium agar plates made in hypertonic medium and with or without lysozyme (3 μg l−1), incubated overnight and colonies were counted. In another experiment, unheated and treated spores were chemically decoated (Bagyan et al. 1998), treated with lysozyme in hypertonic medium (Popham et al. 1996), and spores examined by microscopy and plating as described above. Spore resistance to hypochlorite was determined as described previously (Young and Setlow 2003) with a hypochlorite solution of 0.15 % available chlorine at pH ~ 11. In a few experiments the lysozyme sensitivity of scCO2- and scCO2-PAA-treated spores was examined as described previously (Klobutcher et al. 2006).
To determine if scCO2-PAA-treated spores’ IM became leaky when they germinated, treated and untreated spores were heat activated by incubation at 70°C for 30 min, cooled on ice and germinated at an O.D.600 of 2.5 in 2 ml of five mmol l−1 L-valine and 10 mmol l−1 K-Hepes buffer (pH 7.4). After incubation for 60 min at 37°C, the mix was harvested by centrifugation, and the supernatant fluid dialyzed for 48 h at 4°C in SpectraPor3 dialysis tubing (mol wt cutoff 3500 Da) against one l of distilled water with one change, and lyophilized. The dry residue was dissolved in 30 μl SDS polyacrylamide gel electrophoresis (PAGE) loading buffer with 50 mmol l−1 dithiothreitol, the sample incubated for 30 min at 60°C, aliquots run on SDS-PAGE (10% acrylamide), the gel stained with Coomassie Blue R, destained and photographed. The germinated spore pellet was suspended in 60 μl of SDS-PAGE loading buffer plus 50 mmol l−1 dithiothreitol, incubated at 90°C for 60 min, cooled to 23°C, centrifuged and aliquots of the supernatant fluid run on SDS-PAGE and stained as described above.
RESULTS
Spore inactivation by scCO2-PAA
ScCO2 alone does not exhibit sporicidal activity (White et al. 2006). Initial screening for sterilant additives found that 50% H2O2 added to scCO2 lowers spore viability only by 0.13-1.6 logs, while 5% PAA reduces spore viability by > 6.4 logs (White et al. 2006). This early work used 0.5 ml additive in a 600 ml prototype vessel, and was carried out at 1,500 psi for 1 h at ~55°C.
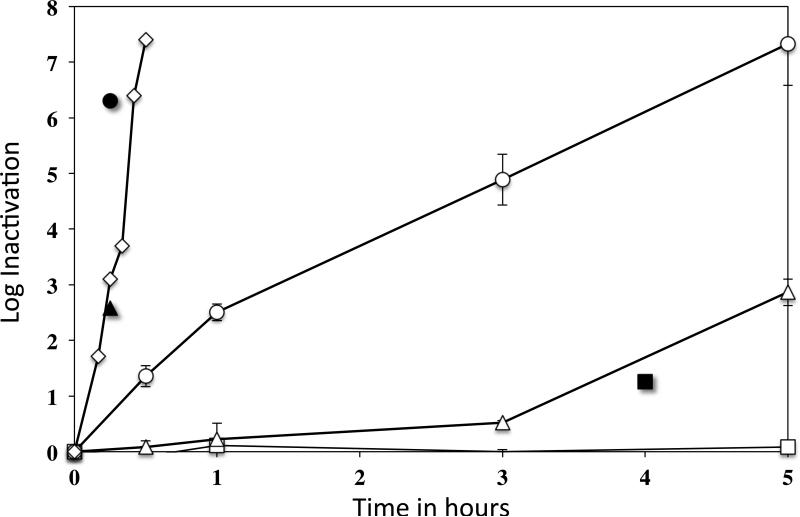
In current work, we found that scCO2 with 1 ml NovaKill™ Gen2 reagent produced very rapid inactivation of wet spores at 35°C, achieving greater than 6log10 inactivation in 25 min (Fig. 1). While 1 ml of the sterilant should, in theory, produce 7 ppm PAA throughout the supercritical vessel, PAA accumulation was measured at 35 to 55 ppm in the liquid spore samples in the Poly-Tyvek pouches. In comparison, treatments with aqueous PAA alone at 50 and 150 ppm resulted in minimal spore inactivation, and even 300 ppm of a PAA aqueous solution only achieved greater than 6log10 inactivation in 5 h (Fig. 1). Interestingly, scCO2-PAA inactivation required more NovaKill™ Gen2 reagent for dry spores. Thus 1 ml sterilant in the supercritical vessel gave only 90% spore inactivation after 4 h, although 16 ml sterilant (and a theoretical 112 ppm PAA throughout the supercritical vessel) gave greater than 6log10 spore inactivation in 15 min (Fig. 1).
Fig. 1.
scCO2-PAA inactivation of wet and dry PS533 (wild-type) B. subtilis spores, and by PAA alone in water. PS533 (wild-type) spores were treated for different times at 35°C with several levels of PAA from NovaKill™ either alone, in liquid with scCO2 or dry with scCO2, and spore viability was measured as described in Methods. The symbols for the different treatment conditions are: □, 50 ppm PAA alone; △, 150 ppm PAA alone; and ○, 300 ppm PAA alone; and scCO2 plus: ◇, 1 ml NovaKill™ Gen2 reagent with wet spores; ■, 1 ml NovaKill™ Gen2 reagent with dry spores; ▲, 8 ml NovaKill™ Gen2 reagent with dry spores; and ●, 16 ml NovaKill™ Gen2 reagent with dry spores.
Given the results described above, tests were carried out to ensure that the spores were truly dead, and not damaged such that they might be recoverable under special conditions. Thus spores were plated on medium plus lysozyme to assist in spore germination, and on hypertonic medium plus or minus lysozyme in case damage had rendered germinated spores osmotically fragile. Spores were also chemically decoated, and washed decoated spores were germinated artificially by lysozyme in hypertonic medium, and examined by phase contrast microscopy. The scCO2-PAA-treated spores were germinated well by this treatment, and were subsequently plated on normal or hypertonic LB medium plates. However, none of the methods noted above increased the recovery of viable wet or dry spores after scCO2-PAA treatment (data not shown).
Germination and outgrowth of scCO2-PAA-treated spores
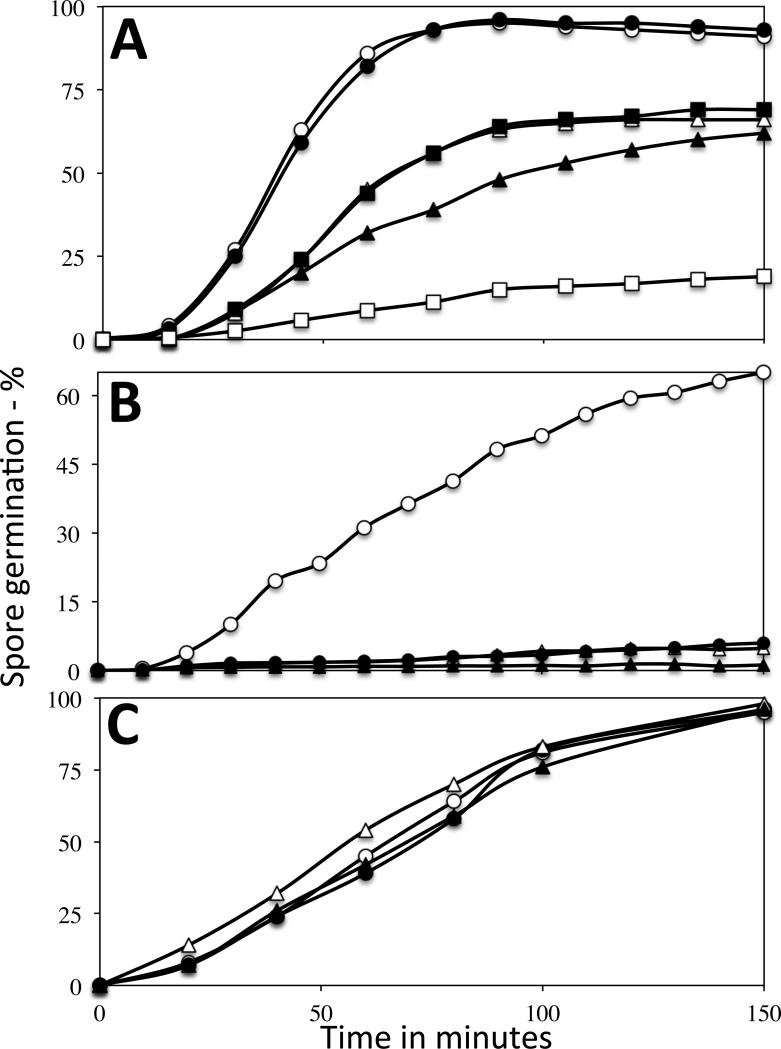
Since scCO2-PAA-treated spore viability was not increased by artificial germinants, presumably the treated spores were able to germinate fairly well. Indeed, this was the case when spore germination via the GerA germinant receptor (GR) trigger L-valine was measured by monitoring DPA release or phase contrast microscopy (Fig. 2A; and data not shown). Notably, when wet or dry spores treated with scCO2-PAA were inactivated 90-99%, 60-70% of the treated spores germinated with L-valine, albeit slightly slower than untreated spores. The germinated-treated spores also became dark in the phase contrast microscope, rather than the only slightly dimmer appearance if they had released their DPA but had not degraded their cortex peptidoglycan (Setlow et al. 2001). That cortex hydrolysis was complete in the germination of the treated spores was also indicated by the lack of effect of lysozyme in increasing recovery of scCO2-PAA-treated spores. The fact that wet spores inactivated 90-99% gave similar percentages of germination and germination kinetics was consistent with a germination defect not inactivating these spores. However, dry spores inactivated >99% germinated more poorly with L-valine.
Fig. 2A-C.
L-valine, AGFK and dodecylamine germination of untreated and scCO2-PAA-treated spores. Untreated (control) and scCO2-PAA-treated PS533 spores were germinated with either A) L-valine, B) AGFK or C) dodecylamine, and germination was measured by Tb-DPA fluorescence either A,B) continuously or C) by TbCl3 addition at various times, all as described in Methods. The symbols are: A) ○, control dry spores; ●, control wet spores; △, wet spores inactivated 99%; ■, wet spores inactivated 99.999%; ▲, dry spores inactivated ~ 90%; and □, dry spores inactivated 99.5%; B) ○, control wet spores; ●, wet spores inactivated 93%; △, dry spores inactivated 90%; and ▲, dry spores inactivated 99.5%; and C) ○, control dry spores; ●, control wet spores; △, dry spores inactivated ~ 90%; and ▲, wet spores inactivated 99%.
While there was some effect of scCO2-PAA treatment on spore germination with L-valine, a previous report indicated that PAA-treatment alone of wet B. subtilis spores results in much larger decreases in spore germination with the AGFK mixture via the GerB plus GerK GRs than on L-valine germination (Leggett et al. 2015). Notably, as reported for PAA alone, scCO2-PAA treatment also greatly decreased AGFK germination, and much more than L-valine germination, and this effect was seen for spores treated both wet and dry (Fig. 2B).
The germination of scCO2-PAA-treated spores with the GR-independent germinant dodecylamine was also examined (Fig. 2C). Dodecylamine causes spore germination by triggering DPA release from the spore core, likely by binding to a protein component of the DPA release channel in spores’ IM (Velásquez et al. 2014). Notably, rates and extents of dodecylamine germination of untreated spores and wet or dry spores inactivated extensively by scCO2-PAA were undistinguishable.
While spores inactivated >99% by scCO2-PAA germinated fairly normally with some agents, an obvious question is whether these spores initiate outgrowth. Consequently, wet spores inactivated ~ 99% by scCO2-PAA were germinated in LB medium plus L-valine, and were examined microscopically for evidence of outgrowth such as swelling and elongation. However, < two % of these germinated spores appeared to outgrow by four h, while > 95% of untreated spores exhibited spore swelling and elongation after 60 min (data not shown; and see below).
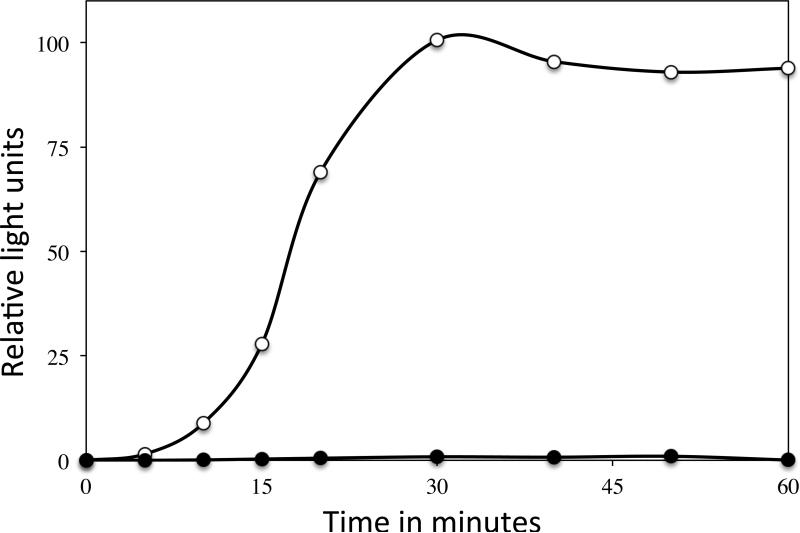
As further evidence that scCO2-PAA-treated spores had a severe outgrowth defect, light production was measured during germination and outgrowth of B. subtilis spores containing V. harveyi LuxAB proteins, using spores that had or had not been treated with scCO2-PAA (Fig. 3). Light production by the V. harveyi LuxAB proteins requires FMNH2, and thus energy metabolism by the outgrowing spores, and as expected the untreated spores gave significant light production. However, with wet spores inactivated ~99% by scCO2-PAA there was < one % of the light production seen with untreated spores, again consistent with minimal if any energy metabolism in spores inactivated by scCO2-PAA.
Fig. 3.
Light production during spore germination and outgrowth of B. subtilis spores with V. harveyi LuxAB proteins with or without prior scCO2-PAA treatment. Wet PS3379 (luxAB) spores with or without scCO2-PAA treatment giving 99% spore inactivation were germinated in a complete nutrient medium, and light production in relative light units was measured, all as described in Methods. The symbols are: ○, untreated spores; and ●, scCO2-PAA-treated spores.
Mechanisms of spore resistance to scCO2-PAA
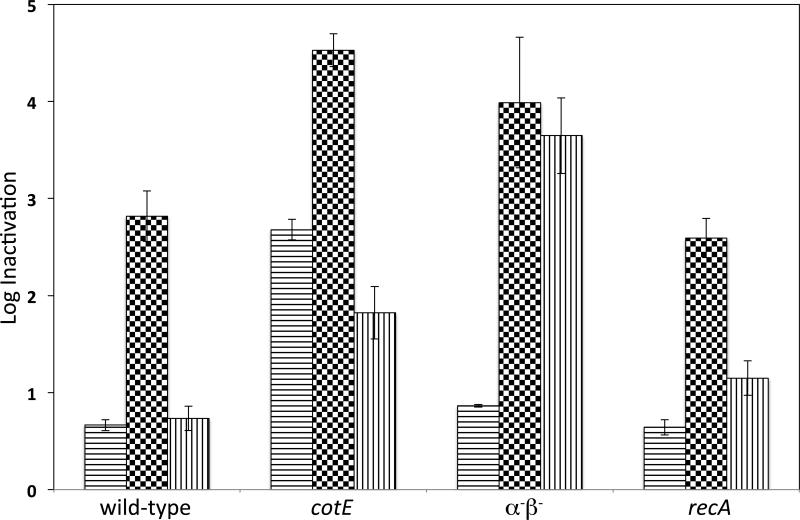
Previous work has shown that Bacillus spores are much more resistant to scCO2 +/− additives than are growing cells. To probe the mechanisms of spore resistance to scCO2-PAA, we examined the scCO2-PAA sensitivity of wet or dry spores of isogenic wild-type, cotE, recA and α−β− B. subtilis strains. As reported for spores treated with PAA alone (Leggett et al. 2015), the wet wild-type, recA and α−β− spores exhibited relatively similar scCO2-PAA sensitivity, while cotE spores were more sensitive (Fig. 4).
Fig. 4.
Inactivation of wet or dry B. subtilis spores of various strains by scCO2-PAA. Wet or dry spores of strains PS533 (wild-type), PS3328 (cotE), PS578 (α−β−) and PS2318 (recA) were treated with scCO2-PAA (1 ml NovaKill™ Gen2 reagent), and spore inactivation was determined as described in Methods. Data are the averages and standard deviations of triplicate determinations, and for dry spores have been corrected for spore recovery with no scCO2-PAA treatment. The symbols in the bars for the different treatment regimens are: horizontal lines, 20 min treatment of wet spores; black and white squares, 30 min treatment of wet spores; and vertical lines, 3 h treatment of dry spores.
As seen with wet spores treated with scCO2-PAA, dry cotE spores were also more sensitive to this agent than dry recA and wild-type spores (Fig. 4). However, dry α−β− spores were more sensitive to scCO2-PAA than even cotE spores. Overall these results point to major roles of the coat in protection of wet or dry spores against scCO2-PAA, while α/β-type SASP were only significant in scCO2-PAA resistance when dry spores were treated.
Mechanisms of spore inactivation by scCO2-PAA
Five different mechanisms of spore inactivation have been identified in previous work: i) rupture of spores’ IM such that small molecules, in particular DPA, are released; ii) DNA damage; iii) damage to one or more crucial spore core proteins; iv) damage to the IM such that this membrane is no longer a permeability barrier when spores germinate; and v) destruction of some essential germination component(s) (Setlow 2006; Setlow et al. 2014). Results given above demonstrated that a germination defect is not how scCO2-PAA inactivates spores. The germination results also suggested that scCO2-PAA-treated spores had retained much of their DPA. This point was tested directly by analyzing DPA in wet or dry spores inactivated by scCO2-PAA (Table 1). Strikingly, high levels of wet or dry spore inactivation by this reagent resulted in < 10% loss in DPA from these treated spores.
Table 1.
DPA level in and DPA release from untreated and scCO2-PAA treated spores*
| Spores | Inactivation % | Total spore DPA % of maximum | DPA release @ 85°C % |
|---|---|---|---|
| Dry untreated | 0 | 97 | < 2 |
| Wet untreated | 0 | 100 | < 2 |
| Dry treated | 95 | 92 | 80 |
| Wet treated | 99 | 94 | 63 |
| Wet treated | 99.99 | 91 | nt† |
| Wet treated | 99.999 | 92 | 47 |
Dry or wet PS533 (wild-type) spores were either untreated or treated with scCO2-PAA, CO2 removed, PAA neutralized, and spore inactivation was measured as described in Methods. Total DPA in spores or DPA released after incubation for 45 min at 85°C was measured as described in Methods. The total spore DPA is expressed relative to the value in the liquid untreated spores which was set at 100%.
nt – not tested.
The similar inactivation of wet wild-type and recA spores by scCO2-PAA suggested that scCO2-PAA was not inactivating wet spores by DNA damage, since lack of the major DNA repair protein, RecA, did not greatly affect spore resistance to scCO2-PAA. To test this spore inactivation mechanism further, we examined a large number of individual survivors of scCO2-PAA treatment of wet wild-type spores for mutations giving auxotrophic or asporogenous phenotypes (Table 2). However, there were no such mutations, while previous work has shown that agents that inactivate spores by DNA damage, such as UV radiation, give auxotrophic or asporogenous mutations in 5-10% of the survivors (Setlow 2006; Setlow et al. 2014). Similar to results with the inactivated wet spores, < 0.25% of the survivors of dry spores treated with scCO2-PAA had mutations (Table 2). In contrast, dry α−β− spores treated with scCO2-PAA had undergone significant mutagenesis, as ~ 7.5% of the survivors of this treatment carried auxotrophic or asporogenous mutations or both. This indicates that dry α−β− spores were almost certainly inactivated by DNA damage, and is further evidence that α/β-type SASP are crucial for DNA protection in dry spores against scCO2-PAA.
Table 2.
Mutations in untreated and scCO2-PAA treated spores*
| Numbers of mutants | ||||
|---|---|---|---|---|
| Spores treated | Inactivation - % | aux | spo | aux spo |
| PS533 (wild-type) | ||||
| Wet untreated | 0 | 0 | 0 | 0 |
| Dry treated | 90 | 1 | 1 | 0 |
| Dry treated | 99.5 | 0 | 0 | 0 |
| Wet treated | 92 | 0 | 0 | 0 |
| Wet treated | 99 | 0 | 0 | 0 |
| PS578 (α−β−) | ||||
| Untreated | 0 | 1 | 1 | 0 |
| Dry treated | 99.5 | 13 | 15 | 3 |
In each experiment colonies from 416 individual PS533 (wild-type) or PS578 (α−β−) spores either untreated or scCO2-PAA treated, were screened for auxotrophic (aux) or asporogenous (spo) mutants as described in Methods.
A variety of tests were also carried out to examine if there was IM damage in scCO2-PAA-treated spores. In the first, spores were given a normally innocuous heat treatment at 85°C and DPA release was measured (Table 1). Untreated spores released essentially no DPA, while wet or dry spores treated with scCO2-PAA released much of their DPA. As a further indication of IM damage that might be deleterious after spores were germinated, spores were spotted on LB plates that contained ~ 0.15 mol l−1 NaCl, and the viability on these plates was compared to that on LB plates with 1 mol l−1 NaCl or LB plates with 1 mol l−1 NaCl plus 50 mmol l−1 glucose (Table 3). The viability of the untreated spores varied less than 50% on the various media. In contrast, wet or dry spores treated with scCO2-PAA exhibited > 50-fold lower viability on high salt plates, although the presence of glucose on high salt plates increased the treated spores’ recovery on these plates. These results were similar to those found previously when spores surviving treatment with a variety of oxidizing agents were examined for their ability to give colonies on high salt plates and with or without glucose (Cortezzo et al. 2004).
Table 3.
Effect of high salt +/− glucose on the viability of untreated or scCO2-PAA-treated spores*
| Spore viability on various plates | ||||
|---|---|---|---|---|
| Spores treated | Inactivation (%) | LB1 | LB+NaCl | LB+NaCl+glucose |
| Dry untreated | 0 | 100 | 130 | 120 |
| Wet treated | 0 | 100 | 60 | 70 |
| Dry treated | 95 | 100 | 2 | 20 |
| Wet treated | 99 | 100 | 1.5 | 40 |
Various dilutions of untreated or scCO2-PA-treated PS533 (wild-type) spores were spotted in duplicate on normal LB medium (0.15 mol l−1 NaCl) plates with kanamycin (10 mg l−1) and with either 1 mol l−1 NaCl, or 1 mol l−1 NaCl plus 50 mmol l−1 glucose. Plates were incubated at 37°C for 16-72 h, and colonies were counted.
The viability of each spore preparation on normal LB medium plates was defined as 100%, and was measured as described in Methods.
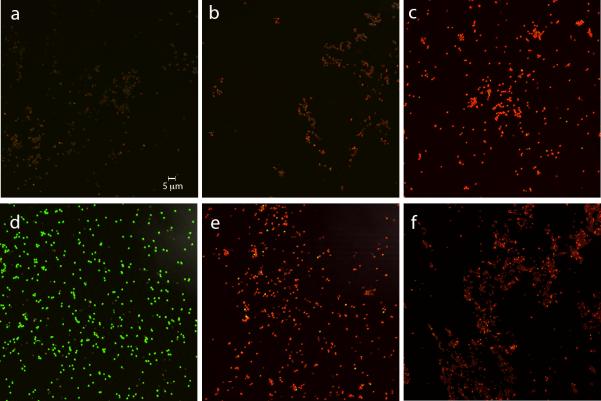
An additional test of IM damage was to examine the BacLight staining of dormant and germinated spores that had or had not been scCO2-PAA treated. The dye mixture in the BacLight reagent stains: i) intact dormant spores poorly and generally reddish; ii) dormant spores with a breached IM permeability barrier and dead germinated spores bright red; and iii) germinated spores that may be alive green (Coleman et al. 2007). As expected, dormant untreated spores stained only weakly red with BacLight (Fig. 5a; Table 4), and spores given a stringent autoclave treatment almost all stained bright red (Fig. 5c; Table 4). In addition, wet and dry spores inactivated 90% or 99%, respectively, by scCO2-PAA generally stained weakly, although a small percentage exhibited intermediate intensity red staining (Fig. 5b,c; Table 4; and data not shown). As expected ≥ 95% of germinated untreated spores stained green with BacLight (Fig. 5d; Table 4). However, with wet spores inactivated ~ 90% by scCO2-PAA, only ~ 6% of all spores stained green with BacLight while 63% stained dark red (Fig. 5e; Table 4), consistent with ~ 70% of these spores having germinated, as shown above (Fig. 1). With dry spores inactivated 99% by scCO2-PAA the level of germination was lower than with treated wet spores, as noted above (Fig 1). However, < 1% of the germinated spores stained green with BacLight, consistent with the level of spore inactivation in this spore preparation, with the great majority of germinated spores staining bright red (Fig. 5f; Table 4). Interestingly, Bacillus cereus spores in a biofilm are inactivated > 99.99% by treatment with scCO2 plus ethanol at 60°C, and when these treated spores are stained in essence with BacLight, essentially all of the spores stain dark red (Park et al. 2013), perhaps due in part to the rather high temperature used in this work. In addition, growing cells of Salmonella enterica treated with scCO2 alone and again stained in essence with BacLight also largely stained bright red, with the level of bright red-stained cells paralleling the level of cell inactivation (Kim et al. 2009).
Fig. 5a-f.
BacLight staining of dormant and germinated spores with or without prior scCO2-PAA treatment or autoclaving. Spores of strain PS533 (wild-type) either untreated, autoclaved (30 min; 250°F) or treated with scCO2-PAA wet and inactivated ~ 90% or treated dry and inactivated ~ 99% were stained with BacLight and photographed by fluorescence microscopy as described in Methods. scCO2-PAA treated and untreated spores were also heat activated for 60 min at 75°C, and germinated for 45 min at 37°C in LB medium plus 10 mmol l−1 L-valine, stained with BacLight and photographed by fluorescence microscopy. The various panels in the figure are: a) dormant untreated spores; b) wet dormant scCO2-PAA treated spores; c) dormant autoclaved spores; d) germinated untreated spores; e) germinated spores that had been treated with scCO2-PAA as wet spores; and f) germinated spores that had been treated with scCO2-PAA as dry spores. The scale bar in the panel is five microns and all panels have the same magnification.
Table 4.
Levels of various types of BacLight staining of different spore preparations*
| BacLight staining: | Poorly stained | Bright red | Bright green | Germination |
|---|---|---|---|---|
| Spore preparation | % of spores examined | |||
| Untreated dormant (327)† | 100 | 0 | 0 | - |
| Untreated germinated (509) | 6 | 3 | 91 | 94 |
| Treated wet – dormant (242) | 96 | 4‡ | 0 | - |
| Treated wet– germinated (472) | 31 | 63 | 6 | 69§ |
| Treated dry – dormant (380) | 95 | 5‡ | 0 | - |
| Treated dry – germinated (890) | 78 | 22 | 0.5 | 22§ |
| Autoclaved – dormant (370) | 3 | 97 | 0.4 | - |
Spores in the images shown in Fig. 5a-f (and data not shown) were scored for weak staining (see panel a), bright red staining (see panel c), or bright green staining (see panel d). The spores treated wet were inactivated 90%, and the spores treated dry were inactivated 99%.
Values in parentheses are the numbers of individual spores scored.
These spores generally exhibited an intermediate red staining intensity.
These values have not been corrected for any treated dormant spores that exhibited moderate red staining intensity, so may be lower than values shown.
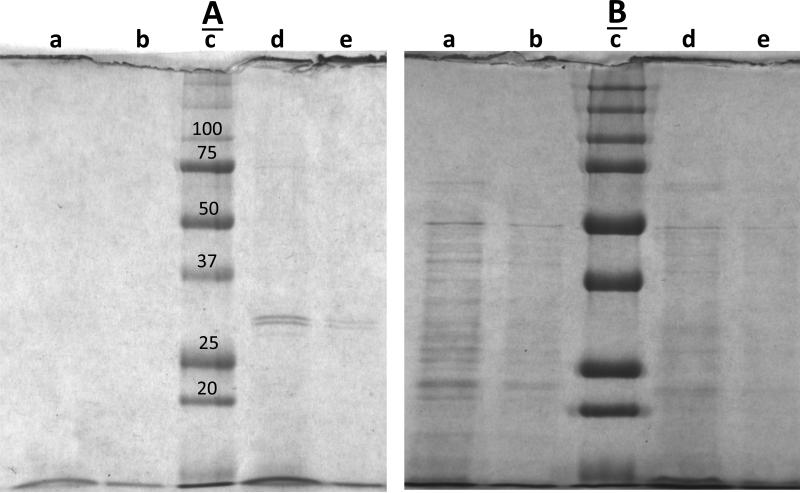
It was also notable that many germinated scCO2-PAA-treated spores appeared somewhat dim in the phase contrast microscope when they germinated in either LB medium or L-valine alone, suggesting that many of these germinated spores had lost cytoplasmic constituents. To test this point directly, the supernatant fluid from an L-valine germination of untreated and scCO2-PAA-treated spores was examined for proteins using SDS-PAGE (Fig. 6A,B). This analysis showed clearly that a significant amount of protein was released from the germinated scCO2-PAA-treated spores, while no significant amount of protein was released from the germinated untreated spores (Fig 6A; compare lanes a,b and d,e). This difference between the treated and untreated spores was seen even though while ~90% of the untreated spores germinated only ~25% of the treated spores germinated. In contrast, levels of protein in pellet fractions from germinated untreated spores and scCO2-PAA-treated spores were relatively similar, with perhaps more protein in the germinated untreated spores (Fig. 6B; compare lanes a,b and d,e). Notably, when differences in the staining intensities in the gels in Fig 6A and B were corrected for by the staining intensities of the mol wt marker bands, the germinated scCO2-PAA-treated spores were determined to have released >10% of their protein into the supernatant fluid.
Fig. 6A,B.
Proteins released from germinated untreated and scCO2-PAA-treated spores. Untreated wet spores of strain PS533 (wild-type) and wet spores inactivated ~ 90% by scCO2-PAA were heat activated, germinated, the supernatant fluid and pellet fractions from the germinated spores isolated and processed as described in Methods. Aliquots of processed A) germination supernatant fluid and B) spore pellets were run on SDS-PAGE, and the gels were stained and photographed as described in Methods. In A) the samples run in the various lanes were: a, 20 μl untreated spores; b, 5 μl untreated spores; c, mol wt markers (sizes in kDa just above the appropriate bands in panel A); d, 20 μl scCO2-PAA treated spores; and e, 5 μl scCO2-PAA-treated spores. In B) the samples run in the various lanes were: a, 10 μl untreated spores; b, 3 μl untreated spores; c, mol wt markers (sizes in kDa just above the same bands in panel A); d, 10 μl scCO2-PAA treated spores; and e, 3 μl scCO2-PAA-treated spores.
Effects of scCO2 treatment +/− PAA on the spore coat
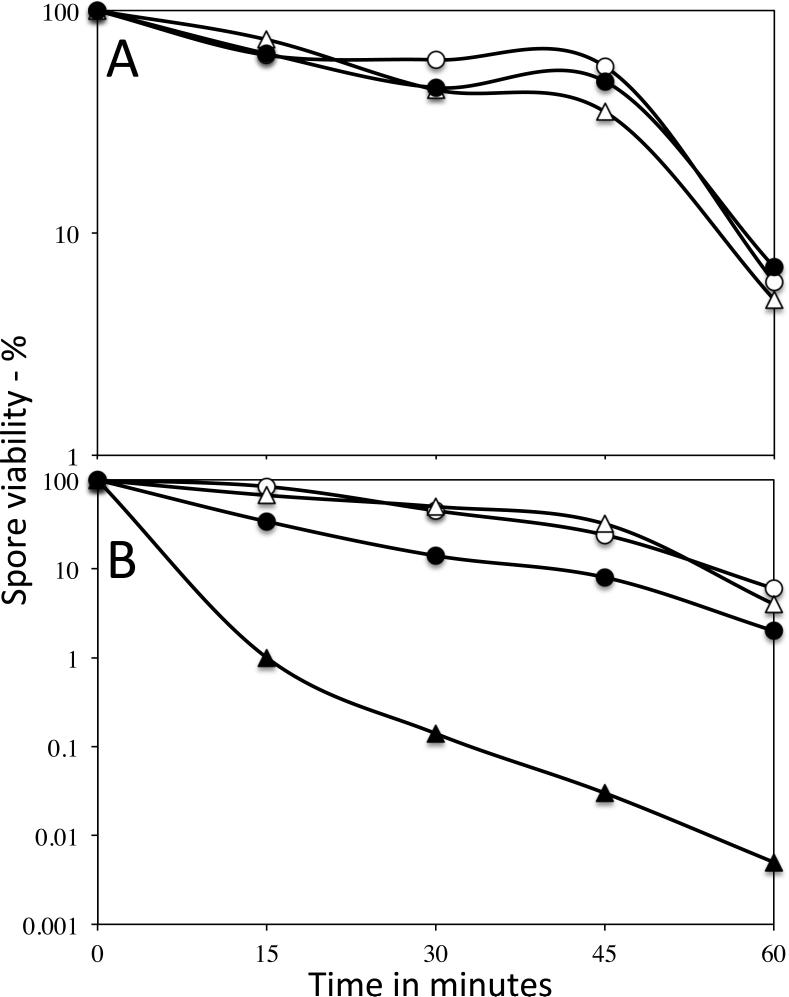
An obvious question about effects of scCO2-PAA on spores, in particular the large increase in the efficacy of scCO2-PAA inactivation of spores over that by PAA alone, is how does scCO2 provide this increased spore inactivation. One obvious possibility given the importance of the spore coat in spore resistance to PAA is that either scCO2 or scCO2-PAA treatments alter the spore coat, thus potentiating PAA action. To test whether the spore coat has been rendered more permeable by scCO2 treatment with or without PAA, the resistance of untreated and treated wild-type spores, as well as untreated cotE spores to sodium hypochlorite was tested (Fig. 7A,B). Previous work has shown that cotE spores are much more sensitive to hypochlorite than wild-type spores, and even spores with minor coat defects show significantly decreased hypochlorite resistance (Young and Setlow 2003; Klobutcher et al. 2006). Spores treated with scCO2 alone either wet or dry exhibited no differences in their hypochlorite resistance (Fig. 7A), and these spores’ viability was not decreased upon lysozyme treatment (data not shown). Survivors of dry spores inactivated ~99% by scCO2-PAA also exhibited no decrease in their hypochlorite resistance, although there was a small decrease in the hypochlorite resistance of wet spores treated with scCO2-PAA (Fig. 7B). However, this latter decrease was much smaller than that seen with cotE spores.
Fig. 7A,B.
Hypochlorite resistance of spores with or without treatment with scCO2 or scCO2-PAA. A) PS533 (wild-type) spores were either untreated or treated wet or dry with scCO2 alone for 1 h, and the hypochlorite inactivation of the untreated and treated spores was determined as described in Methods. The symbols used are: ○, untreated spores; ●, treated wet spores; and △, treated dry spores. B) PS533 or PS3328 (cotE) spores were either untreated or treated with scCO2-PAA, and hypochlorite inactivation was measured as described in Methods. The symbols are: ○, untreated PS533 spores; ●, PS533 spores treated wet and inactivated ~ 90%; △, PS533 spores treated dry and inactivated ~ 99%; and ▲, PS3328 untreated spores.
DISCUSSION
The work in this communication confirms reports showing that scCO2 can inactivate spores of Bacillus species if an appropriate additive, in this case PAA, is present. In addition, PAA is clearly much more effective in scCO2 than it is alone, as 50 ppm PAA alone gave < 10% inactivation of spores in water in 5 h while in ScCO2, there was ~ 3log10 inactivation of wet spores in 20 min. Dry spores were more resistant to scCO2-PAA, indicating that water potentiates the effect of this agent, possibly due to the measured PAA accumulation in solution, compared to the theoretical PAA levels in the vessel. Importantly, wet spores inactivated > 90% by scCO2-PAA exhibited levels of germination with L-valine that were ≥ one order of magnitude higher than the levels of spore inactivation. These results indicate that a spore germination defect is not the reason for the inactivation of wet spores by scCO2-PAA. Similar results were obtained with dry spores treated with scCO2-PAA, although the L-valine germination of dry-treated spores was decreased more by scCO2-PAA than was L-valine germination of treated wet spores. A variety of attempts using artificial spore germination to recover viability of wet or dry spores that appeared dead following scCO2-PAA treatment were unsuccessful. Thus spores apparently inactivated by scCO2-PAA are truly dead.
Major questions about scCO2-PAA treatment of spores then are: 1) what factors determine spore resistance to scCO2-PAA; 2) how does this treatment inactivate spores; and 3) do the answers to the first two questions vary for spores treated wet or dry. For spores treated in aqueous PAA, previous work shows that spore resistance to this agent is not dependent on DNA protection by α/β-type SASP or DNA repair in spore germination and outgrowth (Setlow et al. 1997; Leggett et al. 2015), and this was also true for scCO2-PAA treatment. However, disruption of the spore coat increased the inactivation of wet spores by scCO2-PAA, as found for wet spores treated with PAA alone (Leggett et al. 2015). Results with scCO2-PAA treatment of dry spores were similar, except that the absence of the DNA protective α/β-type SASP sensitized dry spores to scCO2-PAA and led to significant mutagenesis. Overall, these results indicate that the spore coat plays a major role in the scCO2-PAA resistance of wet or dry spores, as found for spore resistance to many toxic chemicals (Setlow 2006). In addition, DNA protection was very important in protecting dry spores from scCO2-PAA, and these protective proteins are presumably the reason there is little or no lethal DNA damage caused by this agent in dry spores, as seen by the lack of effect of a recA mutation on dry spore sensitivity to scCO2-PAA. However, the lack of effect of removal of most α/β-type SASP on scCO2-PAA resistance of wet spores indicates that this agent does not inactivate wet spores by DNA damage, even when the DNA protective α/β-type SASP are absent.
The second question noted above is how scCO2-PAA inactivates spores. This is not by rupture of spore permeability barriers, since wet or dry spore populations inactivated 99% retained almost all their DPA. scCO2-PAA inactivation of wet or dry spores was also not by DNA damage since a recA mutation did not decrease spore resistance to this agent, and there was minimal if any mutagenesis in survivors of treated spores. Treated spore populations also exhibited significant germination, certainly higher percentages than those of surviving spores, so destruction of spores’ ability to germinate is not how scCO2-PAA inactivates spores.
It is possible that scCO2-PAA damages one or more important spore core enzymes, as no data were obtained that indicated this was not the case. However, a number of results indicated that scCO2-PAA inactivates spores by IM damage, such that germinated spores’ plasma membrane that is derived from the dormant spore's IM is unable to function. In particular, this damage appears to cause a large increase in germinated spores’ plasma membrane permeability such that germinated scCO2-PAA-treated spores became leaky and were unable to carry out proper energy metabolism. Evidence consistent with this mechanism for scCO2-PAA inactivation spores is as follows: i) although DPA was retained by spore populations inactivated > 90% by scCO2-PAA, spore DPA was lost from these spores upon incubation at a temperature that has no effect on untreated spores, consistent with a weakened IM; ii) spores surviving scCO2-PAA treatment did not give colonies efficiently on medium with high salt, a phenomenon correlated with plasma membrane damage in bacteria and in germinated spores (Hurst 1977; Chilton et al. 2001; Seok-In and Pyun 2001; Cortezzo et al. 2004); iii) BacLight staining indicated that germinated scCO2-PAA spores largely stained red, indicating these spores’ IM permeability had increased, perhaps because of IM damage and a lack of membrane potential due to ineffective energy metabolism; and iv) germinated scCO2-PAA-treated spores released significant protein as if they had at least partially lysed. However, the precise IM damage caused by scCO2-PAA treatment is unknown, although this appears to be the mechanism whereby many other oxidizing agents also inactivate spores (Cortezzo et al. 2004; Setlow 2006).
In addition to the evidence for scCO2-PAA acting on the spore's IM, spore germination with L-valine via the GerA GR was decreased somewhat with spores treated with scCO2-PAA, consistent with this agent damaging some component of the spore germination apparatus. Crucial spore germination proteins include the GRs that recognize nutrient germinants, the SpoVA proteins essential for DPA release, and the cortex-lytic enzymes that degrade cortex peptidoglycan (Setlow 2013). From the effects of scCO2-PAA on L-valine germination, one cannot decide which germination protein is damaged by scCO2-PAA. Notably, AGFK germination via the GerB and GerK GRs was decreased more than L-valine germination. suggesting that scCO2-PAA treatment slows spore germination by acting on GRs, with the GerB or GerK GRs being more sensitive than GerA. Since the GRs are in spores’ IM, damage to the IM itself or some GR in the IM could cause the effects of scCO2-PAA on spore germination. This conclusion by no means proves that scCO2-PAA inactivates spores by IM damage; indeed this agent does not inactivate spores by damaging the germination apparatus. However, this conclusion indicates that scCO2-PAA damages IM proteins and perhaps the IM itself, and this damage has lethal effects on spores.
Previous work (White et al. 2006), as well as the current study have shown that scCO2-PAA is much more effective in inactivating spores than the comparable PAA concentration alone. An obvious question is what scCO2 does to potentiate PAA action? While the answer to this question is not clear, there appear to be two possible answers. One is that scCO2 treatment irreversibly damages the spore coat, making PAA more effective in inactivating spores. Indeed, scCO2-PAA treatment of wet spores caused a slight decrease in spore hypochlorite resistance, against which the spore coat is a major resistance factor. In contrast, there was no change in the hypochlorite resistance of dry spores treated with scCO2-PAA or of spores treated with scCO2 alone. A second possibility is that scCO2-PAA (and perhaps even scCO2 treatment) reversibly alters the spore coat, thus facilitating PAA movement through the coat and allowing PAA to access the IM. Indeed the spore coat does undergo reversible structural changes upon drying and rehydration (Westphal et al. 2003). Both of these possibilities certainly merit further study.
ACKNOWLEDGEMENTS
This work was supported by SBIR award 1R43AI112166-01A1 (NIH NIAID) to NovaSterilis Inc. and P. Setlow.
Footnotes
CONFLICT OF INTEREST
No conflict of interest declared
REFERENCES
- Bagyan I, Noback M, Bron S, Paidhungat M, Setlow P. Characterization of yhcN, a new forespore-specific gene of Bacillus subtilis. Gene. 1998;212:179–188. doi: 10.1016/s0378-1119(98)00172-3. [DOI] [PubMed] [Google Scholar]
- Bernhardt A, Wehrl M, Paul B, Hochmuth T, Schumacher M, Schütz K, Gelinsky M. Improved sterilization of sensitive biomaterials with supercritical carbon dioxide at low temperature. PLoS One. 2015;10:e0129205. doi: 10.1371/journal.pone.0129205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr TL, Wells CM, Young AA, Minter ZA, Johnson CA, Payne AN, McPherson DC. Decontamination of materials contaminated with Bacillus anthracis and Bacillus thuringiensis Al Hakam spores using PES-Solid, a solid source of peracetic acid. J Appl Microbiol. 2013;115:398–408. doi: 10.1111/jam.12253. [DOI] [PubMed] [Google Scholar]
- Checinska A, Fruth IA, Green TL, Crawford RL, Paszczynski AJ. Sterilization of biological pathogens using supercritical fluid carbon dioxide containing water and hydrogen peroxide. J Microbiol Meth. 2011;87:70–75. doi: 10.1016/j.mimet.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Chilton P, Isaacs NS, Mañas P, Mackey BM. Biosynthetic requirements for the repair of membrane damage in pressure-treated Escherichia coli. Int Sci Technol. 2001;37:2134–2138. doi: 10.1016/s0168-1605(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Coleman WH, Chen D, Li Y.-q., Cowan AE, Setlow P. How moist heat kills spores of Bacillus subtilis. J Bacteriol. 2007;189:8458–8466. doi: 10.1128/JB.01242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortezzo DE, Koziol-Dube K, Setlow B, Setlow P. Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes spores to subsequent stress. J Appl Microbiol. 2004;97:838–852. doi: 10.1111/j.1365-2672.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- Fairhead H, Setlow B, Setlow P. Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillus species. J Bacteriol. 1993;175:1367–1374. doi: 10.1128/jb.175.5.1367-1374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell J, Niu F, McCabe SE, Zhou W, Decedue CJ. Solvent removal and spore inactivation directly in dispensing vials with supercritical carbon dioxide and sterilant. Amer Assoc Pharm Sci Tech. 2012;13:582–589. doi: 10.1208/s12249-012-9777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst A. Bacterial injury: a review. Can J Microbiol. 1977;23:935–944. doi: 10.1139/m77-139. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Shimoda M, Tamaya K, Yonekura A, Kawano T, Osajima Y. Inactivation of Bacillus spores by the supercritical carbon dioxide micro-bubble method. Biosci, Biotechnol Biochem. 1997;61:1022–1023. doi: 10.1271/bbb.61.1022. [DOI] [PubMed] [Google Scholar]
- Kim TH, Choi JC, Kim KH. Flow cytometric analysis of Salmonella enterica serotype Typhimurium inactivated with supercritical carbon dioxide. J Microbiol Meth. 2009;78:155–160. doi: 10.1016/j.mimet.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Klobutcher LA, Ragkousi K, Setlow P. The Bacillus subtilis spore coat provides “eat resistance” during phagocytic predation by Tetrahymena thermophila. Proc Natl Acad Sci USA. 2006;103:165–170. doi: 10.1073/pnas.0507121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett MJ, Schwarz JS, Burke PA, Mcdonnell G, Denyer SP, Maillard J-Y. Resistance to and killing by the sporicidal microbicide peracetic acid. J Antmicrob Chemother. 2015;70:773–779. doi: 10.1093/jac/dku445. [DOI] [PubMed] [Google Scholar]
- Luu S, Cruz-Mora J, Setlow B, Feeherry FE, Doona CJ, Setlow P. The effects of heat activation on Bacillus spore germination, with nutrients or under high pressure, with or without various germination proteins. Appl Environ Microbiol. 2015;81:2927–38. doi: 10.1128/AEM.00193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A, Dunn J, Hunt MC, Sizer CE. Inactivation of Bacillus atrophaeus spores using surface-active peracids and characterization of formed free radicals using electron spin resonance spectroscopy. J Food Sci. 2012;74:M411–M4117. doi: 10.1111/j.1750-3841.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- Murray T, Popham DL, Pearson CB, Hand AR, Setlow P. Analysis of the outgrowth of Bacillus subtilis spores lacking penicillin-binding protein 2a. J Bacteriol. 1998;180:6493–6502. doi: 10.1128/jb.180.24.6493-6502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols A, Burns DC, Christopher R. Studies on the sterilization of human bone and tendon musculoskeletal allograft tissue using supercritical carbon dioxide. J Orthopaedics. 2009;6:e9. [Google Scholar]
- Nicholson WL, Setlow P. Sporulation, germination and outgrowth. In: Harwood CR, Cutting SM, editors. Molecular Biological Methods for Bacillus. United Kingdom: John Wiley & Sons; Chichester: 1990. pp. 391–450. [Google Scholar]
- Paidhungat M, Ragkousi K, Setlow P. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J Bacteriol. 2001;183:4886–4893. doi: 10.1128/JB.183.16.4886-4893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidhungat M, Setlow B, Driks A, Setlow P. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J Bacteriol. 2000;182:5505–5512. doi: 10.1128/jb.182.19.5505-5512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidhungat M, Setlow P. Role of Ger-proteins in nutrient and non-nutrient triggering of spore germination in Bacillus subtilis. J Bacteriol. 2000;182:2513–2519. doi: 10.1128/jb.182.9.2513-2519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Lee C, Bisesi M, Lee J. Efficiency of peracetic acid in inactivating bacteria, viruses, and spores in water determined with ATP bioluminescence, quantitative PCR, and culture-based methods. J Water Health. 2014;12:13–23. doi: 10.2166/wh.2013.002. [DOI] [PubMed] [Google Scholar]
- Park HS, Choi HJ, Kim M-D, Kim KH. Addition of ethanol to supercritical carbon dioxide enhances the inactivation of bacterial spores in the biofilm of Bacillus cereus. Int J Food Microbiol. 2013;166:207–212. doi: 10.1016/j.ijfoodmicro.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Popham DL, Helin J, Costello CE, Setlow, P. P. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc Natl Acad Sci USA. 1996;93:15405–15410. doi: 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q-Q, Leamy P, Brittingham J, Pomerleau J, Kabaria N, Connor J. Inactivation of bacterial spores and viruses in biological material using supercritical carbon dioxide with sterilant. J Biomed Mater Res B: Appl Biomater. 2009;91:572–578. doi: 10.1002/jbm.b.31431. [DOI] [PubMed] [Google Scholar]
- Rao L, Xu Z, Wang Y, Zhao F, Hu X, Liao X. Inactivation of Bacillus subtilis spores by high pressure CO2 with high temperature. Int J Food Microbiol. 2015;205:73–80. doi: 10.1016/j.ijfoodmicro.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Russell NA, Rives A, Pelletier MH, Bruce WJ, Walsh WR. The effect of sterilization on the mechanical properties of intact rabbit humeri in three-point bending, four-point bending and torsion. Cell Tissue Bank. 2013;14:231–242. doi: 10.1007/s10561-012-9318-0. [DOI] [PubMed] [Google Scholar]
- Seok-In H, Pyun Y-R. Membrane damage and enzyme inactivation of Lactobacillus plantarum by high pressure. Int J Food Microbiol. 2001;63:19–28. doi: 10.1016/s0168-1605(00)00393-7. [DOI] [PubMed] [Google Scholar]
- Setlow B, Melly E, Setlow P. Properties of spores of Bacillus subtilis blocked at an intermediate stage in spore germination. J Bacteriol. 2001;183:4894–4899. doi: 10.1128/JB.183.16.4894-4899.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Setlow CA, Setlow P. Killing bacterial spores by organic hydroperoxides. J Indust Microbiol Biotechnol. 1997;18:384–388. [Google Scholar]
- Setlow B, Setlow P. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol. 1996;178:3486–3495. doi: 10.1128/jb.178.12.3486-3495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B, Parish S, Zhang P, Li Y.-q., Neely WC, Setlow P. Mechanism of killing of spores of Bacillus anthracis in a high temperature gas environment. J Appl Microbiol. 2014;116:805–14. doi: 10.1111/jam.12421. [DOI] [PubMed] [Google Scholar]
- Setlow P. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J Appl Microbiol. 2006;101:514–525. doi: 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- Setlow P. When the sleepers wake: The germination of spores of Bacillus species. J Appl Microbiol. 2013;115:1251–1268. doi: 10.1111/jam.12343. [DOI] [PubMed] [Google Scholar]
- Shieh E, Paszczynski A, Wai CM, Lang Q, Crawford RL. Sterilization of Bacillus pumilus spores using supercritical fluid carbon dioxide containing various modifier solutions. J Microbiol Meth. 2009;76:247–252. doi: 10.1016/j.mimet.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Spilimbergo S, Dehghani F, Bertucco A, Foster NR. Inactivation of bacteria and spores by pulse electric field and high pressure CO2 at low temperature. Biotechnol Bioeng. 2003;82:118–125. doi: 10.1002/bit.10554. [DOI] [PubMed] [Google Scholar]
- Spilimbergo S, Quaranta A, Garcia-Gonzalez L, Contrini C, Cinquemani C, Van Ginnekin L. Intracellular pH measurement during high-pressure CO2 pasteurization evaluated by cell fluorescent staining. J Supercrit Fluids. 2010;53:185–191. [Google Scholar]
- Spizizen J. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci USA. 1958;44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburini S, Anesi A, Ferrentino G, Spilimbergo S, Guella G, Jousson O. Supercritical CO2 induces marked changes in membrane phospholipids composition in Escherichia coli K12. J Membrane Biol. 2014a;247:469–477. doi: 10.1007/s00232-014-9653-0. [DOI] [PubMed] [Google Scholar]
- Tamburini S, Ballarini A, Ferrentino G, Moro A, Foladori P, Spilimbergo S, Jousson O. Comparison of quantitative PCR and flow cytometry as cell viabity methods to study bacterial membrane permeabilization following supercritical CO2 treatment. Microbiology. 2014b;159:1056–1066. doi: 10.1099/mic.0.063321-0. [DOI] [PubMed] [Google Scholar]
- Velázquez J, Schuurman-Wolters G, Birkner JP, Abee T, Poolman B. Bacillus subtilis spore protein SpoVAC functions as a mechanosensitive channel. Mol Microbiol. 2014;92:813–823. doi: 10.1111/mmi.12591. [DOI] [PubMed] [Google Scholar]
- Wehmeyer JL, Natesan S, Christy RJ. Development of a sterile amniotic membrane tissue graft using supercritical carbon dioxide. Tissue Eng Part C Meth. 2015;21:649–59. doi: 10.1089/ten.TEC.2014.0304. [DOI] [PubMed] [Google Scholar]
- Westphal AJ, Price PB, Leighton TJ, Wheeler KE. Kinetics of size changes of individual Bacillus thuringiensis spores in response to changes in relative humidity. Proc Natl Acad Sci USA. 2003;100:3461–3466. doi: 10.1073/pnas.232710999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Burns D, Christensen TW. Effective terminal sterilization using supercritical carbon dioxide. J Biotechnol. 2006;123:504–15. doi: 10.1016/j.jbiotec.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Yasbin R, Cheo D, Bol D. DNA repair systems. In: Sonenshein AL, Hoch JA, Losick R, editors. Bacillus subtilis and Other Gram Positive Bacteria: Biochemistry, Physiology and Molecular Genetics. American Society for Microbiology; Washington, DC: 1993. pp. 529–538. [Google Scholar]
- Yi X, Bond C, Sarker MR, Setlow P. Multivalent cations including terbium (Tb3+) can efficiently inhibit the germination of coat-deficient bacterial spores. Appl Environ Microbiol. 2011;77:5536–5539. doi: 10.1128/AEM.00577-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X, Setlow P. Studies of the commitment step in the germination of spores of Bacillus species. J Bacteriol. 2010;192:3424–3433. doi: 10.1128/JB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoganarasimha S, Trahan WR, Best AM, Bowlin GL, Kitten TO, Moon PC, Madurantakam PA. Peracetic acid: a practical agent for sterilizing heat-labile polymeric tissue-engineering scaffolds. Tissue Eng Part C Meth. 2014;20:714–723. doi: 10.1089/ten.tec.2013.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SB, Setlow P. Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J Appl Microbiol. 2003;95:54–67. doi: 10.1046/j.1365-2672.2003.01960.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dalal N, Matthews MA, Waller LN, Saunders C, Fox KF, Fox A. Supercritical carbon dioxide and hydrogen peroxide cause mild changes in spore structures associated with high killing rate of Bacillus anthracis. J Microbiol Meth. 2007;70:442–451. doi: 10.1016/j.mimet.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]