Abstract
Although ghrelin and its cognate receptor GHSR1a are highly localized in the hypothalamic nuclei for the regulation of metabolic states and feeding, GHSR1a is also highly localized in the hippocampus suggesting its involvement in extra-hypothalamic functions. Indeed, exogenous application of ghrelin is reported to improve hippocampal learning and memory. However, the underlying mechanism of ghrelin regulation of hippocampal functions is poorly understood. Here we report ghrelin promoted phosphorylation of GluN1 and amplified NMDA receptor (NMDAR)-mediated EPSCs in the CA1 pyramidal cell of hippocampus in slice preparations. The ghrelin-induced responses were sensitive to a GHSR1a antagonist and inverse agonist, and were absent in GHSR1a homozygous (-/-) knock-out mice. These results indicated that activation of GHSR1a was critical in the ghrelin-induced enhancement of the NMDAR function. Interestingly, heterozygous (+/-) mouse hippocampi were also insensitive to ghrelin treatment, suggesting a slight reduction in the availability of GHSR1a may be sufficient to negate the effect of ghrelin on GluN1 phosphorylation and NMDAR channel activities. In addition, NMDAR-mediated spike current, which is of dendritic origin, was blocked by the GHSR1a antagonist, suggesting the presence of GHSR1a on the pyramidal cell dendrites with physical proximity to NMDAR. Together with our findings on the localization of GHSR1a in the CA1 region of the hippocampus, which was evidenced by fluorescent ghrelin binding, immunoreactivity, and eGFP reporter gene expression, we conclude that the activation of GHSR1a favors a rapid modulation of the NMDA receptor-mediated glutamatergic synaptic transmission by phosphorylating GluN1 subunit in the hippocampus.
Keywords: GHSR KO mouse, rat, ghrelin binding, immunohistochemistry, NMDA spikes Whole cell patch clamp recording
Graphical abstract
Introduction
It is widely acknowledged that learning can be accomplished more easily, accurately, and speedily when a subject is interested, motivated, and reward-driven. Numerous animal models of learning have demonstrated successful acquisition of specific tasks while providing food as a reward in fasted conditions. Ghrelin is a stomach hormone, released when the stomach is empty. It stimulates the brain's reward center (Malik, 2008). Accelerated acquisition of learning under the fasted condition suggests the potential importance of ghrelin as a key molecule for cellular and molecular mechanisms of learning and memory (Andrews, 2010; Albarran-Zeckler et al., 2011 Ferrini et al., 2009).
Ghrelin is a 28 amino acid peptide (Kojima et al., 1999). While its cognate receptor, i.e., ghrelin receptor (GHSR1a), is highly localized in the hypothalamic nuclei for the regulation of metabolic states and feeding (Cowley et al., 2003), GHSR1a is reported to be highly localized in the hippocampus as well (Zigman e al., 2006; Mani et al., 2014). The dentate gyrus, CA2, and CA3 show distinctively high expression of mRNAs of GHSR1a (Guan et al., 1997) while CA1 exhibits strong binding for biotinylated ghrelin (Diano et al., 2006) as well as the expression of eGFPs tagged to GHSR1a (Jiang et al., 2006). We previously reported that exogenous application of ghrelin upregulated the phosphorylation of CREB (cAMP response element binding protein) (Cuellar and Isokawa, 2011) and enhanced dendritic spine density (Berrout and Isokawa, 2012) through the activation of GHSR1a in the CA1 region of the hippocampus. Diano et al. (2006) reported that systemically-applied ghrelin crossed the blood-brain barrier and promoted dendritic spine-synapse formation and long-term potentiation (LTP) in the hippocampus. Synaptic incorporation of AMPA receptors was suggested as a result of GHSR1a-mediated plasticity (Ribeiro et al., 2013). In spite of the accumulating evidence for ghrelin's stimulatory roles on hippocampal functions, the fundamental mechanism of how ghrelin and its receptor regulate hippocampal synapses and neuron excitability is not fully understood.
The ghrelin gene generates various molecules besides ghrelin including des-acyl ghrelin, C-ghrelin, obestatin, and des-Gln14 ghrelin (Nishi et al., 2011). However, these molecules neither act as the endogenous ligand of GHSR1a (Holst et al., 2007; Lauwers et al., 2006), nor play any role in appetite control (Soares and Leite-Moreira, 2008). In order to activate GHSR1a, ghrelin has to be acyl-modified with a C8:0-group at serine 3 (Ser3) to become the n-octanoyl ghrelin (C8:0-ghrelin) (Hosoda et al., 2000). For this study, we used the 28-amino acid peptide acylated at Ser3 with C8:0 (from this point on, we refer to it as ghrelin). Our findings of immunohistochemically-detected GluN1 subunit phosphorylation and the whole-cell patch clamp recording of the NMDA receptor-mediated synaptic currents demonstrate ghrelin's stimulatory effect, through the activation of GHSR1a, on the NMDA receptor-mediated glutamatergic neurotransmission in the CA1 hippocampal pyramidal cells.
Materials and Methods
Animals
Sprague-Dawley rats, mice with eGFP reporter gene (Jiang, et al., 2006), and GHSR1a knock-out mice (Diano et al., 2006) were used. Both homozygous (GHSR1a-/-) and heterozygous (GHSR1a+/-) mice were examined. The rats were purchased from Charles River (Wilmington, MA), and the two strains of transgenic mice were purchased from Jackson Laboratory (B6;129S7-Ghsr<tm2Rgs>/J/Stock# 019908 and B6N(Cg)-Ghsrtm1.1(KOMP)Vlcg/J/Stock# 018595).
Acute hippocampal slice
Animals were decapitated based on the protocol approved by the University of Texas at Rio Grande Valley Institutional Animal Care and Use Committee (IACUC) in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23). Adequate measures were taken to minimize pain or discomfort. Brains were quickly immersed in ice cold artificial cerebrospinal fluid (ACSF) consisting of (in mM): 124 NaCl, 3 KCl, 2 CaCl2, 2 Mg2SO4, 1.24 NaH2PO4, 26 NaHCO3, and 10 glucose. Brains were sectioned in 400 μm thick slices using a vibroslicer (Ted Pella, Redding, CA) and maintained in an oxygenated ACSF at 35°C for up to 6 hours.
Hippocampal slice culture
Hippocampi from both hemispheres were dissected from P6 pups and sliced into 400μm thick sections using a chopper (Stoelting, Wood Dale, IL). Slices were placed on Transwell culture insert (Costar, Corning, NY) and incubated in culture media consisted of: 50% MEM, 25% HBSS, 24% horse serum, 0.5% penicillin/streptomycin solution, 0.5% glucose solution, and 25 mM HEPES with 5% CO2 at 35°C for up to 3 weeks (Isokawa, 2009). Slice culture was used because: 1) chemical effect of agonists and antagonists of GHSR1a can be assessed directly on the hippocampus in isolation while eliminating multi-synaptic circuit activities projected from extra-hippocampal regions (since those axon terminals projecting to the hippocampus die out in time during culturing); 2) acutely-prepared slices are expected to show fluctuating levels of endogenous ghrelin in the hippocampus among animals, since animals are fed ad libitum so that a systemic level of ghrelin likely varies at the time of decapitation. In contrast, slice culture can provide a stable control in order to test the effect of ghrelin on hippocampal neurons, independent of systemic ghrelin; and 3) transient elevation of dendritic spine density was reported as a possible result of decapitation and cardiac perfusion (O'Callaghan and Sriram, 2004).
Electrophysiology
Visualized whole-cell patch clamp recording was conducted with a recording pipette filled with a solution consisting of (in mM): 110 cesium methanesulphonate, 10 Hepes, 50 CsCl, 1 CaCl2, 1 MgCl2, 5 QX-314, 2 MgATP pH adjusted to 7.3 with CsOH. Pipette resistance was ∼5 MΩ when measured in the bath solution. The NMDA receptor-mediated EPSC (NMDAR-EPSC) was isolated at a holding potential of +40 mV in the presence of NBQX (10 μM; Tocris R&D, Bristol, UK) to block AMPA receptor-mediated EPSCs and picrotoxin (50 μM; Sigma, St. Luis, MO) to block GABAA receptor-mediated IPSCs, while stimulating the Stratum Radiatum with a concentric bipolar metal electrode at 0.05 Hz in order to avoid a run-down of NMDAR-EPSCs (Axopatch 200A and pClamp 10). Stimulus intensity was set to evoke 35-60% of the maximum amplitude. When access resistance changed more than 15% (checked by a small negative voltage pulse), data acquisition was discontinued. The following compounds were used: Ghrelin, D-Lys3-GHSR-6, and Substance P analogue ([D-Arg1,D-Phe5,D-Trp7,9,Leu11]Substance P), all from Phoenix Pharmaceuticals (Burlingame, CA), and L-629,585 from Tocris R&D (Bristol, UK). Inverse agonists and antagonists were bath-applied 0.5 h prior to the application of agonists. NMDAR-EPSCs were presented graphically as mean ± SEM (standard error of the mean) according to statistical methods set by the EJN Authors' Guideline (EJN vol. 28, pp. 2363-2364). Results were tested for statistical significance with a student t-test or ANOVA (analysis of variance). Differences were considered significant if P < 0.05.
Fluorescent ghrelin binding
Octanoylated form of FITC-conjugated ghrelin (F302) and non-octanoylated form of FITC-conjugated ghrelin (F203) were provided by Dr. Len Luyt at the University of Western Ontario in Canada. One μM of F302 or F203 were added to culture media, and hippocampal slices were incubated for 1 hour. At the end of application, slices were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS), glass-mounted, and examined with a confocal microscope (Fluoview 1000, Olympus, Center Valley, PA).
Immunohistochemistry
Slices were immersion-fixed with 4% paraformaldehyde in 1 M phosphate buffered saline (PBS), rinsed, treated with 0.2% Triton-X100 and 10% BSA for 1 hour, and incubated in a rabbit polyclonal anti-GHSR1a (1:200, Phoenix Pharmaceutical, Burlingame, CA; or 1:50, Santa Cruz Biotechnology, Santa Cruz, CA), or a goat polyclonal anti-pGluN1 (phosphorylated GluN1 subunit at Ser 896/897, 1:100, Santa Cruz Biotechnology, Santa Cruz, CA) for 48 hours at 4°C with gentle agitation, which was followed by Alexa 488-conjugated secondary antibody (1:200, Life Technologies, Grand Island, NY) for 1 hour at room temperature. No dual labeling was attempted. For control, we used a blocking peptide, omitted a primary antibody, or simultaneously applied primary and secondary antibodies. Slices were glass-mounted and imaged at a single cell resolution using a confocal microscope.
Quantification of pGluN1 immunoreactivity
Representative pGluN1 signals (Fig. 2A1) were manually selected (red circles in Fig. 2A2), and the area and intensity of the fluorescent signal were measured in each red circle using image analysis software (IPLab, BD Bioscience, San Jose, CA). The range (max/min) of areas and the range (max/min) of fluorescent intensities measured from a total of 236 red circles were used to establish selection criteria for pGluN1 immunoreactive puncta in 30 slices. The criteria, once established, were applied to immunofluorescent signals that were acquired from a total of 2024 images taken from 157 slices in 11 pups using auto-segmentation logic (Triangle), and only those that satisfied the criteria were accepted as pGluN1 immunoreactive puncta (in 512 images in 114 slices in 11 pups). pGluN1 immunoreactivity was quantified as a ratio between the summed total area of pGluN1 immunoreactive puncta over a total image area, and presented graphically as mean ± SEM (standard error of the mean) according to statistical methods set by the EJN Authors' Guideline (EJN vol. 28, pp. 2363-2364). Results were tested for statistical significance with a student t-test or ANOVA (analysis of variance). Differences were considered significant if P < 0.05.
Figure 2.
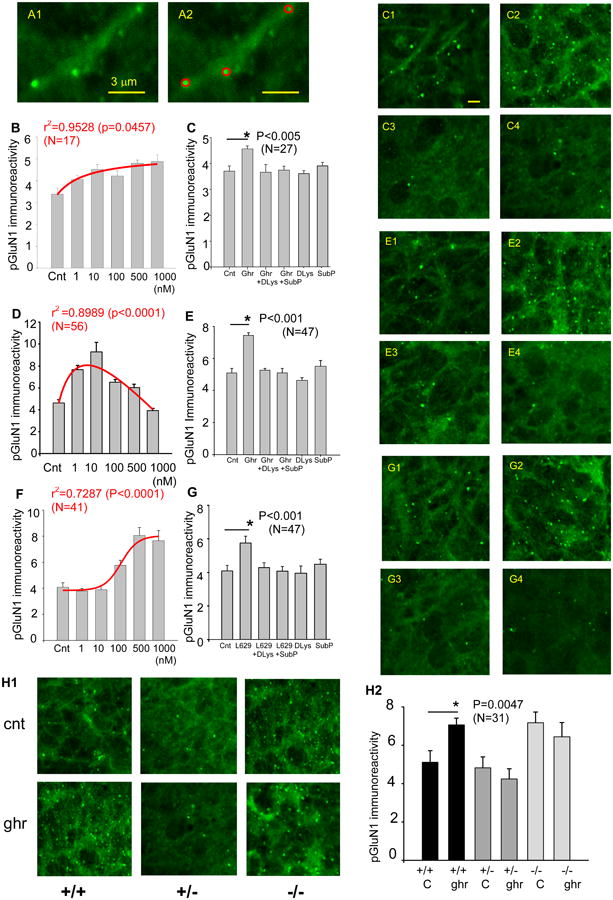
pGluN1 immunoreactivity. A. Representative pGluN1 signals (A1) were manually selected (red circles in A2) and processed for quantitative analysis as described in Methods. A2 shares the same calibration with A1. B-C. pGluN1 immunoreactivity in response to ascending doses of ghrelin in acutely-prepared rat hippocampal slices (B) and in response to GHSR1a antagonists, D-Lys3-GHSR6 (1 μM) and Substance P analogue (10 μM) with or without ghrelin (100 nM) (C). D-E. pGluN1 immunoreactivity in response to ascending doses of ghrelin in cultured rat hippocampal slices (D) and in response to GHSR1a antagonists, D-Lys3-GHSR6 (1 μM) and Substance P analogue (10 μM) with or without ghrelin (100 nM) (E). F-G. pGluN1 immunoreactivity in response to ascending doses of non-peptide agonist for GHSR1a, L-692,585 in cultured rat hippocampal slices (F) and in response to GHSR1a antagonists with or without L-692,585 (100 nM) (G). Photomicrographs in C1 (control), C2 (ghrelin), C3 (ghrelin and D-Lys3-GHRP6), and C4 (ghrelin and Substance P analogue) were taken from Experiment C. Photomicrographs in E1 (control), E2 (ghrelin), E3 (ghrelin and D-Lys3-GHRP6), and E4 (ghrelin and Substance P analogue) were taken from Experiment E. Photomicrographs in G1 (control), G2 (L-629,585), G3 (L-629,585 and D-Lys3-GHRP6) and G4 (L-629,585 and Substance P analogue) were taken from Experiment G. H. Representative pGluN1 immunoreactivity in control and ghrelin in wild-type (GHSR1a +/+), heterozygous (GHSR1a +/-), and homozygous (GHSR1a -/-) knock-out mice (H1). The result was quantified and summarized in H2. Calibration in C1 (5 μm) is shared by all photomicrographs except for A1 and A2. Data are mean ± SEM.
Results
Fluoescent ghrelin binding, GHSR1a immunohistochemistry, and eGFP reporter gene expression
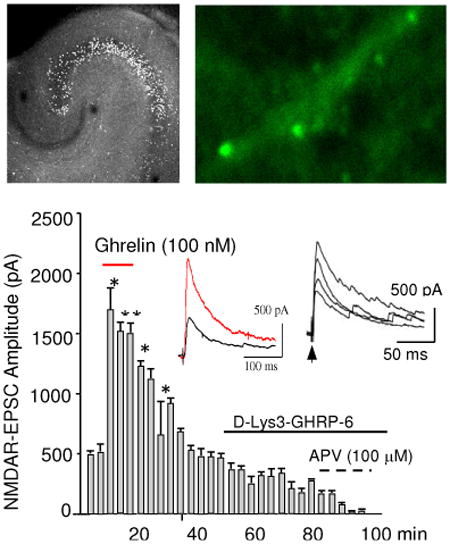
The octanoylated form of FITC-conjugated ghrelin (F302, 1 μM) exhibited strong binding in CA3 and CA2 of rat hippocampal slice culture (Fig. 1A), which extended to CA1 (Fig. 1B). The lack of binding with non-octanoylated form of FITC-conjugated ghrelin (F203, 1 μM) (Fig. 1C) confirmed F302 binding was specific to GHSR1a. Visualization of F302 binding with higher magnification revealed a clustered but randomly distributed GHSR1a in the CA1 pyramidal cell layer (Fig. 1D) in contrast with the lack of binding with F203 as a control (Fig. 1E).
Figure 1.
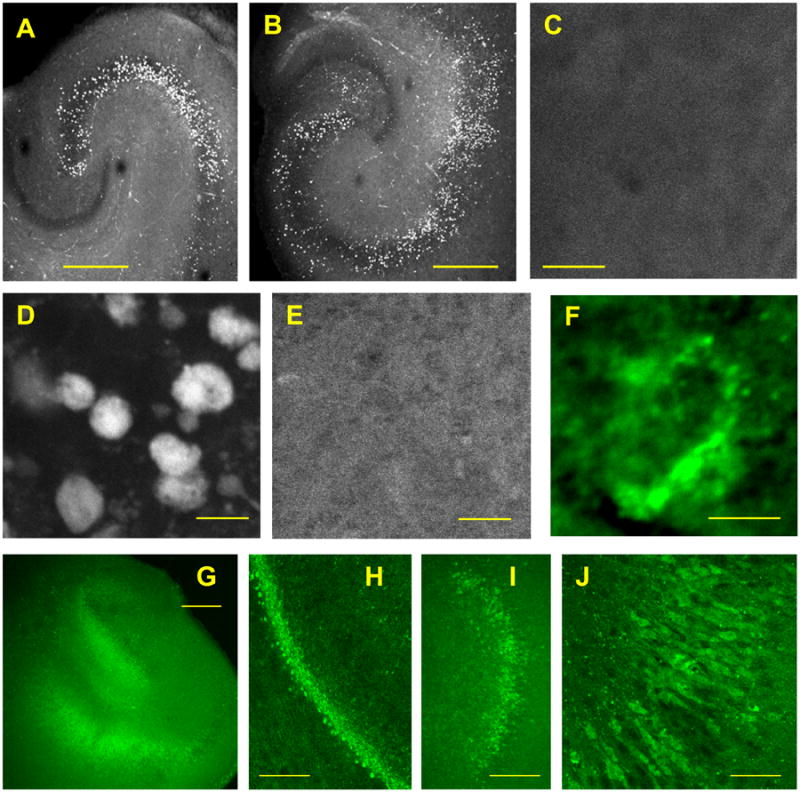
Localization of GHSR1a in cultured hippocampal slices. A-C. Binding of octanoylated form of FITC-conjugated ghrelin (F302, 1 μM) in the CA3 and CA2 of rat hippocampal slices (A), which extended to CA1 (B). The lack of binding with non-octanoylated form of FITC-conjugated ghrelin (F203, 1 μM) (C). D and E. Octanoylated ghrelin-binding (F302) (D) and non-octanoylated ghrelin-binding (F203) (E) in CA1 neurons. F. GHSR1a immunoreactivity. G-J. eGFP signals from GHSR1a-expressing cells in the RgS transgenic line of mouse hippocampal slices (G). The eGFP signals in the CA1 region with higher magnifications (H and I). Individual CA1 pyramidal cells expressing eGFPs (J). Images in A-F were obtained in fixed slices, and images in G-J were obtained in live slices. Calibrations: 500 μm in A, B, C and G; 200 μm in H; 100 μm in I; 10 μm in D, E and J; 5 μm in F.
Independent of ghrelin binding, the localization of GHSR1a was examined immunohistochemically using two different antibodies raised against GHSR1a. GHSR1a immunoreactivity was apparent with both antibodies in the hippocampal CA1 cell layer as discrete puncta that surrounded the soma (Fig. 1F), when compared with control specimens that received a blocking peptide or did not receive primary antibody (data not shown).
We also observed eGFP signals in the transgenic mouse hippocampus where the eGFP gene was inserted downstream of the GHSR1a promotor. In live slice culture specimens, eGFP signals were identified in the dentate gyrus and CA1 region (Fig. 1G). With higher magnifications, the CA1 pyramidal cell layer became clearly detectable with fluorescing signals (Fig. 1H and I), and individual CA1 pyramidal cells were identified as eGFP-expressing neurons (Fig. 1J).
Together these data demonstrate cellular and molecular evidence for the localization of GHSR1a in the CA1 pyramidal neurons of the hippocampus, and support the anatomical basis for our experiments on ghrelin-mediated phosphorylation of GluN1 and enhanced NMDA receptor-mediated synaptic transmission discussed in the following section of the present study.
pGluN1 immunoreactivity in response to ghrelin
Phosphorylation of GluN1 was studied at Ser 896 and Ser 897. Ser 896 phosphorylation is PKC-dependent, and Ser 897 phosphorylation is PKA-dependent. Since GHSR1a is a Gq-coupled receptor, the activation of the receptor can initiate IP3-mediated signaling pathways leading to PKC activation (Camiña, 2006). In addition, we previously reported the activation of cAMP signaling pathways including PKA in response to ghrelin (Cuellar and Isokawa, 2011). Phosphorylation of these sites was reported to potentiate NMDA receptor function (Roh et al., 2011).
Exogenous application of ascending doses of ghrelin (1-1000 nM) increased the magnitude of pGluN1 signal in both acutely-prepared and cultured hippocampal slices. This change was quantified as described in the methods section (Fig. 2A1 and A2). In acutely-prepared rat hippocampal slices, pGluN1 immunoreactivity increased in a dose-dependent manner by 20 % (r2=0.953, p<0.05, n=17, ANOVA) (Fig. 2B). The increase in pGluN1 was sensitive to and inhibited by GHSR1a antagonist, D-Lys3-GHSR6 (1 μM) and an inverse agonist, Substance P analogue (10 μM) in the presence of ghrelin (100 nM); however, the application of D-Lys3-GHSR6 (1 μM) or Substance P analogue (10 μM) alone did not have any effect when compared with control (p<0.005, n=27, unpaired t-test) (Fig. 2C). In cultured rat hippocampal slices, the ghrelin-induced increase in pGluN1 peaked at the concentration of 10 nM (r2=0.899, p<0.0001, n=56, ANOVA) (Fig. 2D) and started to decline with higher doses. This suggests that receptor desensitization or internalization might have occurred when the hippocampus was maintained in the form of cultured hippocampal slices. Similar to acute slices, ghrelin-mediated phosphorylation of GluN1 was inhibited by D-Lys3-GHSR6 (1 μM) and Substance P analogue (10 μM) in the hippocampal slice culture. However, the application of D-Lys3-GHSR6 (1 μM) or Substance P analogue (10 μM) alone did not have any effect when compared with control (Fig. 2E, ghrelin concentration: 100 nM) (p<0.001, n=47, unpaired t-test).
pGluN1 immunoreactivity in response to non-peptide agonist, L-692,585 (1-1000 nM) in the hippocampal slice culture exhibited a significant increase at 100 nM and plateaued between 500 and 1000 nM (r2 =0.729, p<0.001, n=41, ANOVA)(Fig. 2F) without any sign of receptor desensitization or internalization. This indicates that L-692,585 is effective within a narrow range of doses in our experimental conditions. L-692,585-induced increase in pGluN1 was inhibited by D-Lys3-GHRP-6 and Substance P analogue in the presence of L-692,585 (100 nM) (p<0.001, n=47, unpaired t-test) (Fig. 2G).
In cultured slices prepared from homozygous knock-out (GHSR1a -/-) mice, no changes were detected in pGluN1 immunoreactivity in response to exogenous application of ghrelin (100 nM). In contrast, the identical dose of ghrelin increased pGluN1 in wild-type (GHSR1a +/+) mouse hippocampal slices (p<0.005, n=31, unpaired t-test) (Fig. 2H1 and H2). Interestingly, cultured slices prepared from heterozygous (GHSR1a +/-) mice showed no changes in pGluN1 immunoreactivity in response to exogenous application of ghrelin (100 nM) (Fig. 2H1 and H2). This suggests that a slight reduction in the availability of GHSR1a may be sufficient to negate the effect of ghrelin on the phosphorylation of GluN1. Furthermore, the magnitude of pGluN1 was elevated in homozygous knock-out (-/-) mouse compared with the wild-type mouse in control and ghrelin. This suggests the possibility that some compensatory mechanisms might have turned on in the absence of physiological contribution of GHSR1a towards the homeostatic regulation of NMDA receptor function.
Ghrelin-induced and GHSR1a-dependent enhancement of NMDAR-EPSCs
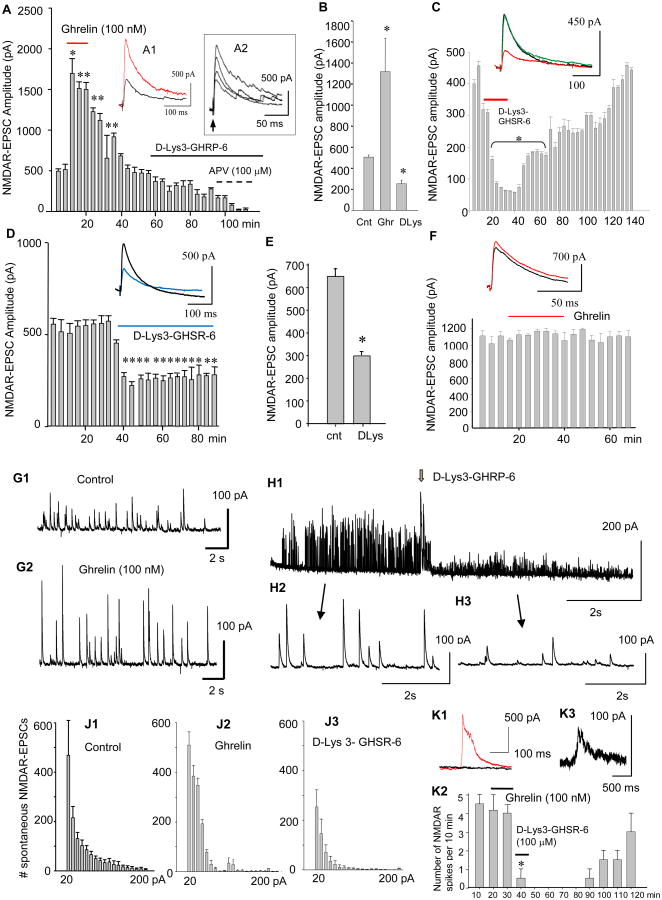
NMDAR-EPSCs were recorded with a patch electrode in the whole cell configuration from single CA1 pyramidal cells while stimulating the stratum radiatum every 20 s. A brief local application of ghrelin (100 nM) enhanced NMDAR-EPSCs (*F=29.12, p<0.001, **F=28.39, p<0.005, n=30, One Way Repeated Measure ANOVA) (Fig. 3A). The peak amplitude increased in a dose-dependent manner (Fig. 3A2). This increase was sensitive to D-Lys3-GHSR-6 (Fig. 3A1 and Fig. 3B) (* t=10.18, p<0.0006, ** t=14.96, p<0.0002, n=41, unpaired t-test). Interestingly, in the absence of exogenous ghrelin, NMDAR-EPSCs reduced peak amplitude in response to D-Lys3-GHSR-6 in a reversible manner (Fig. 3C and D) (* F=63.58, p<0.001, n=37, One Way Repeated Measure ANOVA in C, * F=16.06, P<0.001, n=18, One Way Repeated Measure ANOVA in D). A summary of the results shown in Fig. E (* t=9.87, p<0.0004, n=53, unpaired t-test) suggested either: 1) GHSR1a was constitutively active, or 2) endogenous ghrelin might be present in our cultured hippocampal slices. Finally, none of the responses observed above with ghrelin and D-Lys3-GHSR-6 were generated in slices prepared from GHSR1a knock-out mice (Fig. 3F) (F=0.772, p>0.77, n=18, One Way Repeated Measure ANOVA).
Figure 3.
NMDAR-mediated EPSCs (NMDAR-EPSCs) in the CA1 pyramidal cell in the presence of picrotoxin (50 μM) and NBQX (10 μM) at the holding potential of +40 mV. A. NMDAR-EPSCs in response to local puff application of ghrelin, and the subsequent bath application of D-Lys3-GHRP-6 and APV. Inset A1: NMDAR-EPSC in control (black trace) and ghrelin (red trace). Inset A2: Dose-responses of NMDAR-EPSCs to 0 nM (control), 20 nM, 50 nM, and 100 nM ghrelin. B. Summary of changes in the amplitude of NMDAR-EPSCs in control, ghrelin, and D-Lys3-GHSR-6. C. Reversible inhibition of NMDAR-EPSCs in response to D-Lys3-GHRP-6 in the absence of ghrelin. Inset in C: control (black trace), D-Lys3-GHSR-6 (100 μM, red trace), and wash (green trace). D. Effect of continual application of D-Lys3-GHSR-6 on NMDAR-EPSCs for over 40 min. Inset in D: control (black trace) and D-Lys3-GHSR-6 (100 μM, blue trace). E. Summary of changes in NMDAR-EPSCs in response to D-Lys3-GHSR-6 and Substance P analogue. F. Effect of ghrelin on NMDAR-EPSCs in GHSR1a knock-out (-/-) mice. Inset in F: control (black trace) and ghrelin (red trace). G. Spontaneous NMDAR-EPSCs in control (G1) and ghrelin (G2) in rat hippocampal CA1 pyramidal cell. H. Responses of spontaneous NMDAR-EPSCs to D-Lys3-GHRP-6 (H1). Individual waveforms of sEPSCs are shown before (H2) and during (H3) the application. J. The number of sEPSCs were quantified based on the amplitude in control (J1), ghrelin (J2), and D-Lys3-GHRP-6 (J3) during a period of 10 min of gap-free recordings obtained from 23 neurons (each neuron received ghrelin and the antagonist sequentially). K. NMDA spike currents in control (red trance in K1), D-Lys3-GHSR-6 (black trace in K1), and wash (K3). Each bar in the graph represents the frequency of occurrence per 10 min for a total of 120 min gap-free recording (K2). Data are mean ± SEM.
In addition to evoked NMDAR-EPSCs, spontaneously-occurring NMDAR-EPSCs (sEPSCs) were examined for the effect of ghrelin and a GHSR1a antagonist. The amplitude of sEPSCs was 100 pA in average in control ACSF (Fig. 3G1), which increased by 50-100 % in response to exogenous application of ghrelin (Fig. 3G2). However, the frequency of sEPSCs did not change significantly, suggesting the effect of ghrelin was likely postsynaptic. D-Lys3-GHRP-6 reduced the amplitude of sEPSCs (Fig. 3H1) without changing the frequency (Fig. 3H2 and 3). Cumulative frequency of sEPSCs with amplitudes ranging from 20 pA to 200 pA is summarized in control (J1), ghrelin (J2), and D-Lys3-GHRP-6 (J3) in the total of 23 neurons.
We recorded NMDA spike currents in the whole-cell configuration (Fig. 3K1). NMDA spikes are spontaneously-generated local electrical signals at dendritic branches (Antic et al., 2010) where NMDA receptors are highly localized (Ding et al., 2013). Its generation is promoted by glutamate spillover at any single point in the entire dendritic tree (Chalifoux and Carter, 2011) that may involve extra-synaptic receptors (Petralia, 2012). In the present study, NMDA spike currents were insensitive to exogenous application of ghrelin; however, the generation was blocked by the bath-application of D-Lys3-GHSR-6 in a reversible manner (Fig. 3K2) (Chi-square = 20.135, p<0.045, n=12, Friedman one way repeated measure ANOVA). This inhibitory effect of D-Lys3-GHRP-6 on the NMDA spike currents, independent of ghrelin, suggests potential influence of GHSR1a on extra-synaptic NMDAR and supports the interpretation that GHSR1a is likely present on pyramidal cell dendrites with physical proximity to the NMDA receptor.
Discussions
We have shown that ghrelin stimulates the NMDA receptor-mediated synaptic transmission in the hippocampus by phosphorylating the NMDA receptor subunit GluN1 through the activation of GHSR1a. This novel finding can provide a cellular and molecular mechanism to explain earlier reports on the improved memory retention by intra-hippocampal ghrelin application proposed by Carlini et al (2010). It also explains an increased dendritic spine density in the hippocampal slice culture in response to exogenous application of ghrelin (Berrout and Isokawa, 2012) and enhanced long-term potentiation and spine synapse formation induced by systemic application of ghrelin in vivo (Diano et al., 2006).
NMDA receptor function can be regulated by many factors. For example, protein kinase A (PKA) is a well-acknowledged molecule that enhances NMDA receptor function (Skeberdis et al., 2006). PKA does so by phosphorylating the NMDA receptor subunit (Leonard and Hell, 1997; Yaka et al., 2002). A typical way of increasing the availability of PKA signals is through the activation of cAMP. We previously reported, in fasted animals with an elevated serum ghrelin level, that phosphorylated CREB (cAMP-response element binding protein) was upregulated in the hippocampus and limbic cortex (Estrada and Isokawa, 2009). We also reported that exogenous application of ghrelin upregulated phosphorylated CREB (pCREB) in the CA1 pyramidal cell and this effect was blocked by the PKA antagonist, Rp-cAMP in cultured hippocampal slices (Cuellar and Isokawa, 2011). These findings are in agreement with our present findings and support our interpretation that ghrelin initiates GHSR1a-mediated signaling in the hippocampal CA1 neurons by targeting the NMDA receptor subunit GluN1 and enhancing NMDAR-mediated synaptic transmission through possible activation of PKA signaling.
A primary molecular constituent for GHSR1a is a Gq protein and Gq is not generally linked to the cAMP/PKA signaling cascade for CREB activation. However, GHSR1a can cause a robust activation of CRE-mediated gene transcription (Holst et al., 2003). For example, Gq activation can mobilize cytoplasmic calcium ([Ca2+]i) by translocating IP3 to the endoplasmic reticulum and initiate a release of Ca2+ from stores. Increase in cytosolic Ca2+ can stimulate cAMP production via the activation of Ca2+-dependent adenylate cyclase. Indeed, the upregulation of CREB by ghrelin has been reported in the hypothalamus (Petersen et al., 2009). In the hippocampus, the role of cAMP/PKA signaling in the induction and late (protein synthesis-dependent) phase of NMDA receptor-dependent LTP attracted considerable attention, and a gating role of PKA was proposed in the induction of hippocampal plasticity (Thompson et al., 2002). Thus, the ghrelin's stimulatory effect on the NMDA receptor via the activation of PKA and cAMP proposes a novel signaling pathway for the regulation of synaptic transmission in the hippocampal CA1 pyramidal neuron by a metabolic hormone.
In the present study, the dose-dependent increase in the magnitude of pGluN1 showed a unique pattern between different types of hippocampal preparation (i.e., acute vs. cultured slices) and different forms of agonist (acylated ghrelin vs. non-peptide agonist, L629,585). In acute slices, pGluN1 immunoreactivity continued to increase in response to ascending doses of ghrelin (Fig. 2B). On the other hand, in cultured slices, pGluN1 immunoreactivity peaked at the dose of 10 nM ghrelin, and declined thereafter with higher doses of ghrelin (Fig. 2D). This finding suggests a possible occurrence of GHSR1a receptor desensitization or internalization in cultured slices. Acutely-prepared hippocampal slices retain more of physiological conditions similar to those found in the in vivo system than cultured slices where the hippocampus is kept in isolation. Thus, physiological conditions that are likely retained in acute slices might have a mechanism to prevent GHSR1a receptor desensitization or internalization. One such possibility is the ample presence of fatty acids in the in vivo system. Unsaturated fatty acids were reported to prevent desensitization of the human GHSR1a by blocking its internalization (Delhanty et al., 2010). In this report, prolonged (96 h), but not acute, treatment of the GHSR cells in vitro with the 18C oligounsaturated fatty acids (OFAs) oleic and linoleic acid caused a significant increase (a factor of 2.5 to 3) in sensitivity of GHSR1a to ghrelin via a mechanism involving retardation of cellular internalization, rather than altered receptor affinity for the ligand. OFAs were found to block the inhibitory effects of ghrelin pretreatment on subsequent ghrelin responsiveness, suggesting that OFAs suppress desensitization of GHSR1a.
GHSR1a is desensitized via the classical clathrin-mediated pathway (Camiña et al., 2004; Holliday et al., 2007). The authors suggested there was specificity in the mechanism by which OFAs block entry of the GHSR1a into the endocytic pathway so that GHSR1a was retained on the cell surface, where it can continue to signal in response to ghrelin. In regard to the different forms of agonist, we observed no signs of receptor desensitization or internalization with a non-peptide agonist (L629,585) in cultured slices. Occurrence of receptor desensitization and/or internalization is often agonist-dependent; thus, L-692,585 may not cause desensitization or internalization as easily as ghrelin. On the other hand, receptor desensitization and/or internalization might have been observed if we had tested with higher (mM) ranges of doses.
After being released, ghrelin crosses the blood-brain barrier. The rate of crossing increases when serum ghrelin concentration increases (Diano et al., 2006), suggesting the possibility that ghrelin-mediated modulation of hippocampal synaptic transmission may be under the regulation of peripherally-circulating ghrelin. However, there is a report to show acylated ghrelin transport in the blood-to-brain direction is negligible (Banks et al., 2002) and vagotomy prevents peripheral ghrelin's effect on the hypothalamus (Date et al., 2002). Thus, ghrelin's direct effect on the brain may be of intrinsic origin (Nakazato et al., 2001). In the hippocampus, no cell bodies or processes were positive to ghrelin mRNAs except for septal neurons (Cowley et al., 2003) whose axons project to the hippocampus (Huh et al., 2010). This evidence suggests the possibility that septal neurons could be a source of hippocampal ghrelin. Further investigation on identifying the source(s) of ghrelin that can modulate hippocampal neuron function may provide additional support for our present findings of GHSR1a-mediated regulation of glutamatergic transmission.
We observed a decrease in the amplitude of NMDAR-mediated evoked EPSCs and spontaneous EPSCs by GHSR1a antagonists in the absence of ghrelin (Fig. 3C and D). We also observed the blockade of generation of NMDA spikes by GHSR1a antagonist in the absence of exogenous application of ghrelin (Fig. 3K2). These results suggest that GHSR1a might be constitutively active and involved in determining the peak amplitude of NMDAR-EPSCs as well as the generation of NMDA spikes. Indeed, the ghrelin receptor family including GHSR1a displays a high degree of ligand-independent signaling activity (Holst et al., 2003; Petersen et al., 2009). The receptor signals with around 50% of activity even in the absence of agonist (Holst et al., 2003). On the other hand, there is a report to show that D-Lys3-GHRP6 may interact with the NMDA receptor inhibiting its function independent of ghrelin, GHSR1a, or signaling cascade (Pinilla et al., 2003). In addition, although we reported a reduction in the NMDAR-mediated EPSC with the application of D-Lys3-GHRP6 alone, we found no effect of D-Lys3-GHRP6 alone on pGluN1. A similar finding was obtained with the application of Substance P analogue alone. One possible explanation for this discrepancy may be a difference in the sensitivity of detecting the effect of D-Lys3-GHRP6 or Substance P analogue between immunohistochemistry and electrophysiology. Patch clamp recordings of NMDAR-EPSCs were done from single pyramidal cells. Thus, a small reduction in the NMDAR-EPSC in response to D-Lys3-GHRP6 or Substance P analogue (200-300 pA) could be detected together with a large increase in the NMDAR-EPSC in response to ghrelin (over 1000 pA). On the other hand, pGluN1 immunohistochemistry was done on the whole hippocampus in slices (both acute and cultured), which included not only pyramidal cells but also GABAergic interneurons and non-neuronal cells such as astrocytes, microglia, and oligodendrocytes. Potential differences (or absence) in the expression of GHSR1a or the response(s) to D-Lys3-GHRP6/Substance P analogue in individual cells of different kinds were not addressed. Thus, even if pyramidal cells showed a small reduction in pGluN1, such reduction could have been masked by other cell types that did not show any change. Additional investigation will help further identify whether hippocampal GHSR1a signals constitutively or in the presence of endogenous ghrelin in order to regulate NMDA receptors.
Lastly, although our dose-response curve shows a direct correlation between GHSR1a-signaling and the GluN1 subunit phosphorylation (as depicted in Fig. 2B, D, and F), we cannot rule out the possibility that other subunits may also be involved. GluN2B subunits are abundant in the immature hippocampus and form triheteromeric NMDA receptors with GluN1 and GluN2A in the mature hippocampus (Rauner and Köhr, 2011; Tovar et al., 2013). The blockade of GluN2B decreases NMDAR-mediated tonic current (Papouin et al., 2012). Thus, the possibility for the involvement of GluN2A and 2B in the present study may need to be resolved.
In conclusion, we demonstrate in the present study that NMDA receptor functions are regulated by ghrelin in a GHSR1a-dependent manner in such a way that NMDAR-mediated synaptic currents are increased as a result of GluN1 subunit phosphorylation in the hippocampal pyramidal neurons and synapses. Our data propose a model to explain how a routinely-produced gut hormone that initiates innate behavior of feeding can act as a key molecule for the modulation of synaptic transmission in the hippocampus.
Acknowledgments
This work is supported by NIH grant 2R15DA021683. Brandon G. Muniz is a recipient of The American Physiological Society Undergraduate Summer Research Fellowship 2014. We thank Dr. Len Luyt at the University of Western Ontario for providing FITC-conjugated ghrelin. Editorial assistance was provided by Dr. Pamela E. Meredith at the University of Texas Rio Grande Valley.
Footnotes
There is no conflict of interest to declare for B.G. Muniz and M. Isokawa.
References
- Albarran-Zeckler RG, Sun Y, Smith RG. Physiological roles revealed by ghrein and ghrelin receptor deficient mice. Peptides. 2011;32:2229–2235. doi: 10.1016/j.peptides.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB. The extra-hypothalamic actions of ghrelin on neuronal function. Cell. 2010;34:31–40. doi: 10.1016/j.tins.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Antic SD, Zhou WL, Moore AR, Hort SM, Ikonomu KD. The decade of the dendritic NMDA spike. J Neurosci Res. 2010;88:2991–3001. doi: 10.1002/jnr.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Tschop M, Heiman ML. Extent and direction of ghrelin transport across the blood brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Berrout L, Isokawa M. Ghrelin promotes reorganization of dendritic spines in cultured rat hippocampal slices. Neurosci Lett. 2012;516:280–284. doi: 10.1016/j.neulet.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camiña JP. Cell biology of the ghrelin receptor. J Neuroendocrinol. 2006;18:65–76. doi: 10.1111/j.1365-2826.2005.01379.x. [DOI] [PubMed] [Google Scholar]
- Camiña JP, Carreira MC, El Messari S, Llorens-Cortes C, Smith RG, Casanueva FF. Desensitization and endocytosis mechanisms of ghrelinactivated growth hormone secretagogue receptor 1a. Endocrinology. 2004;145:930–940. doi: 10.1210/en.2003-0974. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Ghersi M, Schiöth HB, de Barioglio SR. Ghrelin and memory: differential effects on acquisition and retrieval. Peptides. 2010;31:1190–1193. doi: 10.1016/j.peptides.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. Glutamate spillover promotes the generation of NMDA spikes. J Neurosci. 2011;31:16435–16446. doi: 10.1523/JNEUROSCI.2777-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cuellar JN, Isokawa M. Ghrelin-induced activation of cAMP signal transduction and its negative regulation by endocannabinoids in the hippocampus. Neuropharmacology. 2011;60:842–851. doi: 10.1016/j.neuropharm.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay WC, da Silvo I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschöp MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Delhanty PJ, van Kerkwijk A, Huisman M, van de Zande B, Verhoef-Post M, Gauna C, Hofland L, Themmen AP, van der Lely AJ. Unsaturated fatty acids prevent desensitization of the human growth hormone secretagogue receptor by blocking its internalization. Am J Physiol Endocrinol Metab. 2010;299:E497–E505. doi: 10.1152/ajpendo.00414.2009. [DOI] [PubMed] [Google Scholar]
- Ding JD, Kennedy MB, Weinberg RJ. Subcellular organization of CaMKII in rat hippocampal pyramidal neurons. J Comp Neurol. 2013;521:3570–3583. doi: 10.1002/cne.23372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada NM, Isokawa M. Metabolic demand stimulates CREB signaling in the limbic cortex: Implication for the induction of hippocampal synaptic plasticity by intrinsic stimulus for survival. Front Syst Neurosci. 2009 doi: 10.3389/neuro.06.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Curr Neuropharmacol. 2009;7:37–49. doi: 10.2174/157015909787602779. http://www.ncbi.nlm.nih.gov/pubmed/19721816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJS, Smith RG, Van der Ploeg LHT, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Holliday ND, Holst B, Rodionova EA, Schwartz TW, Cox HM. Importance of constitutive activity and arrestin-independent mechanisms for intracellular trafficking of the ghrelin receptor. Mol Endocrinol. 2007;21:3100–3112. doi: 10.1210/me.2007-0254. [DOI] [PubMed] [Google Scholar]
- Holst B, Egerod KL, Schild E, Vickers SP, CHeetham S, Gerlach LO. GRP39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology. 2007;148:13–20. doi: 10.1210/en.2006-0933. [DOI] [PubMed] [Google Scholar]
- Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW. High constitutive signaling of the ghrelin receptor-Identification of a potent inverse agonist. Mol Endocrinol. 2003;17:2201–2210. doi: 10.1210/me.2003-0069. [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- Huh CY, Goutagny R, Williams S. Glutamatergic neurons of the mouse medial septum and diagonal band of Broca synaptically drive hippocampal pyramidal cells: relevance for hippocampal theta rhythm. J Neurosci. 2010;30:15951–61. doi: 10.1523/JNEUROSCI.3663-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isokawa M. Time-dependent induction of CREB phosphorylation in the hippocampus by the endogenous cannabinoid. Neurosci Lett. 2009;457:53–7. doi: 10.1016/j.neulet.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Betancourt L, Smith RG. Ghrelin amplifies dopamine signaling by cross talk involving formation of growth hormone secretagogue receptor/dopamine receptor subtype 1 heterodimers. Mol Endocrinol. 2006;20:1772–1785. doi: 10.1210/me.2005-0084. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Lauwers E, Landuyt B, Arckens L, Schoofs I, Luyten W. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem Biophys Res Commun. 2006;351:21–25. doi: 10.1016/j.bbrc.2006.09.141. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Hell JW. Cyclic AMP-dependent protein kinase and protein kinase C phosphorylate N-methyl-D-aspartate receptors at different sites. J Biol Chem. 1997;272:12107–15. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–409. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Mani BK, Walker AK, Lopez-Soto EJ, Raingo J, Lee CE, Perello M, Andrews ZB, Zigman JM. Neuroanatomical characterization of a growth hormone secretagogue receptor-green fluorescent protein reporter mouse. J Comp Neurol. 2014;522:3644–3666. doi: 10.1002/cne.23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Yoh J, Hiejima H, Kojima M. Structures and molecular forms of the ghrelin-family peptides. Peptides. 2011;32:2175–2182. doi: 10.1016/j.peptides.2011.07.024. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Sriram K. Focused microwave irradiation of the brain preserves in vivo protein phosphorylation: comparison with other methods of sacrifice and analysis of multiple phosphoproteins. J Neurosci Methods. 2004;135:159–68. doi: 10.1016/j.jneumeth.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SHR. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:6330646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Petersen PS, Woldbye DPD, Madsen AN, Egerod KL, Jin C, Lang M, Rasmussen M, Beck-Sickinger AG, Holst B. In Vivo Characterization of High Basal Signaling from the Ghrelin Receptor. Endocrinol. 2009;150:4920–4930. doi: 10.1210/en.2008-1638. [DOI] [PubMed] [Google Scholar]
- Petralia RS. Distribution of extrasynaptic NMDA receptors on neurons. ScientificWorldJournal. 2012 doi: 10.1100/2012/267120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinilla L, Barreiro ML, Tena-Sempere M, Aguilar E. Role of ghrelin in the control of growth hormone secretion in prepubertal rats: interactions with excitatory amino acids. Neuroendocrinology. 2003;77:83–90. doi: 10.1159/000068652. [DOI] [PubMed] [Google Scholar]
- Rauner C, Köhr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem. 2011;286:7558–66. doi: 10.1074/jbc.M110.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro LF, Catarino T, Santos SD, Benoist M, van Leeuwen JF, Esteban JA, Carvalho AL. Ghrelin triggers thesynaptic incorporation of AMPA receptors in the hippocampus. Proc Natl Acad Sci U S A. 2013;111:E149–158. doi: 10.1073/pnas.1313798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh DH, Choi SR, Yoon SY, Kang SY, Moon JY, Kwon SG, Han HJ, Beitz AJ, Lee JH. Spinal neuronal NOS activation mediates sigma-1 receptor-induced mechanical and thermal hypersensitivity in mice: involvement of PKC-dependent GluN1 phosphorylation. Br J Pharmacol. 2011;163:1707–20. doi: 10.1111/j.1476-5381.2011.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–10. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Soares JB, Leite-Moreira AF. des-acyl ghrelin and obestatin: three pieces of the same puzzle. Peptides. 2008;29:1255–1270. doi: 10.1016/j.peptides.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Drewery DL, Atkins HD, Stephenson FA, Chazot PL. Immunohistochemical localization of N-methyl-D-aspartate receptor subunits in the adult murine hippocampal formation: evidence for a unique role of the NR1D subunit. Brain Res Mol Brain Res. 2002;102:55–61. doi: 10.1016/s0169-328x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Tovar KR, McGinley MJ, Westbrook GL. Triheteromeric NMDA receptors at hippocampal synapses. J Neurosci. 2013;33:9150–9160. doi: 10.1523/JNEUROSCI.0829-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci USA. 2002;99:5710–5715. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–54. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]