Abstract
Myosin Ia (Myo1a), the most prominent plus-end directed motor and myosin VI (Myo6) the sole minus-end directed motor, together exert opposing tension between the microvillar (MV) actin core and the apical brush border (BB) membrane of the intestinal epithelial cell (IEC). Mice lacking Myo1a or Myo6 each exhibit a variety of defects in the tethering of the BB membrane to the actin cytoskeleton. Double mutant (DM) mice lacking both myosins revealed that all the defects observed in either the Myo1a KO or Snell’s waltzer (sv/sv) Myo6 mutant mouse are absent. In isolated DM BBs, Myo1a cross links between MV membrane and MV actin core are absent but the gap (which is lost in Myo1a KO) between the MV core and membrane is maintained. Several myosins including Myo1c, d and e and Myo5a are ectopically recruited to the BB. Consistent with the restoration of membrane tethering defects by one or more of these myosins, upward ATP-driven shedding of the BB membrane, which is blocked in the Myo1a KO, is restored in the DM BB. However, Myo1a or Myo6 dependent defects in expression of membrane proteins that traffic between the BB membrane and endosome (NaPi2b, NHE3, CFTR) are not restored. Compared to controls, Myo1a KO, sv/sv mice exhibit moderate and DM high levels of hypersensitivity to dextran sulfate sodium-induced colitis. Consistent with Myo1a and Myo6 playing critical roles in maintaining IEC integrity and response to injury, DM IECs exhibit increased numbers of apoptotic nuclei, above that reported for Myo1a KO.
Keywords: Snell’s waltzer, plus end-, minus end-directed motors, CFTR, NaPi2b, DSS
INTRODUCTION
Myosin Ia (Myo1a) and myosin VI (Myo6) are the principle and sole plus-end and minus-end [Wells et al. 1999] directed motors, respectively, associated with the actin cytoskeleton of the intestinal epithelial cell (IEC) brush border (BB) membrane [Crawley et al. 2014; Heintzelman et al. 1994]. Myo1a comprises the spiral array of lateral bridges between the microvillar (MV) actin core and the BB membrane [Mooseker and Cheney 1995]. Myo6 is present at the base of MV within the subapical terminal web domain [Hegan et al. 2012; Heintzelman et al. 1994]. Ultrastructural studies of IECs in mice lacking either Myo1a or Myo6 indicate that both of these myosins serve to tether the BB membrane to the underlying actin cytoskeleton [Hegan et al. 2012; Tyska et al. 2005]. The BB membrane of Myo1a KO IECs exhibits a variety of perturbations of BB membrane tethering to the underlying MV actin core. These include extrusion of MV membrane from the distal, plus-end tips of MV and prominent herniations of the BB membrane in which lateral attachments are lost from multiple adjacent MV cores. The crucial role of Myo1a in membrane tethering has been directly measured by comparing tethering force required to pull the membrane away from isolated wild type (WT) and Myo1a KO. Much less force is required to untether the membrane in the Myo1a KO BB, and the distance the membrane can be displaced is significantly greater [Nambiar et al. 2009]. There is also frequent vesiculation of MV that is likely due to Ca2+-activated villin severing of the MV core, a consequence of absence of intraMV Ca2+ buffering by Myo1a-associated calmodulin light chains. In the Snell’s waltzer (sv/sv) homozygous mutant mouse (a functional Myo6 null mutant [Avraham et al. 1995]) there is detachment of the MV membrane from the MV core at the base of MV resulting in partially fused MV [Hegan et al. 2012].
Interestingly, the association of Myo6 with the BB membrane is dependent on Myo1a. In the Myo1a KO, the subapical terminal web localization of Myo6 with the BB is depleted [Tyska et al. 2005]. One potential mechanism for this reduction of Myo6 BB localization is that the opposing plus-end mediated tension of the BB membrane is required to stabilize the association of Myo6 with the BB. Indeed there is some evidence that Myo6 is a mechano-sensor in that the mechanochemical properties of both two-headed and single-headed Myo6 are affected by opposing tension [Altman et al. 2004; Chuan et al. 2011; Ramamurthy et al. 2012]. We propose (see Discussion) that it is Myo1a-dependent association of Myo6 with the BB cytoskeleton that is the basis for Myo1a tumor suppression [Mazzolini et al. 2012].
There is also evidence for functional synergy between Myo1a and Myo6 in membrane traffic between the BB membrane and the subapical endosomal compartment that is localized just below the terminal web. For example, in the sv/sv mouse, traffic of the cystic fibrosis transmembrane conductance regulator (CFTR) channel from the BB membrane to subapical endosomes is inhibited [Ameen and Apodaca 2007]. Conversely, traffic of CFTR from subapical endosomes to the BB membrane is impaired in the Myo1a KO [Kravtsov et al. 2012]. Myo6 has also been implicated in the endocytic trafficking of the Na+/H+ exchanger NHE3 as well as movement of NHE3 down the MV prior to endocytic uptake [Chen et al. 2014]. High phosphate (Pi) diet-induced redistribution of the Na+ /Pi transporter, NaPi2b, from the BB to the subapical endosome is inhibited in the sv/sv mouse [Hegan et al. 2012]. Whether Myo1a plays a role in endosome-to-BB membrane trafficking for either NHE3 or NaPi2b, as it does for CFTR, has not been examined.
To evaluate the potential mechanical and functional synergy between Myo1a and Myo6, we have examined the consequences of loss of function of both of these myosins in Myo1a KO-sv/sv double mutant (DM) mice. Surprisingly, essentially all the architectural defects in BB membrane organization observed in either Myo1a KO or sv/sv mice are absent in the DM mouse. Evidence is presented that this structural/mechanical rescue is due to ectopic recruitment of other myosins to the BB that occurs in the absence of both motors. However, Myo1a and Myo6 dependent functions in trafficking of membrane proteins between the BB membrane and subapical endosome are not restored. In addition, the DM mice are extremely sensitive to dextran sulfate sodium (DSS) induced colitis, indicating that these two myosins play essential roles in response to intestinal mucosal injury.
RESULTS
BB membrane tethering defects and perturbations in MV packing and core/membrane organization are absent in the Myo1a-sv/sv DM mouse
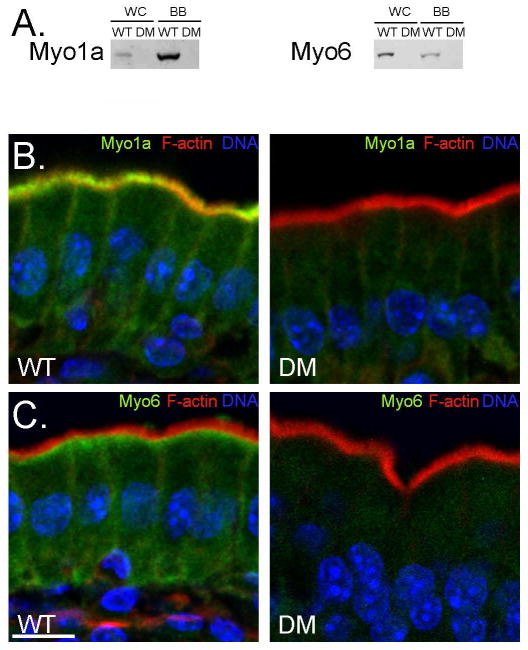
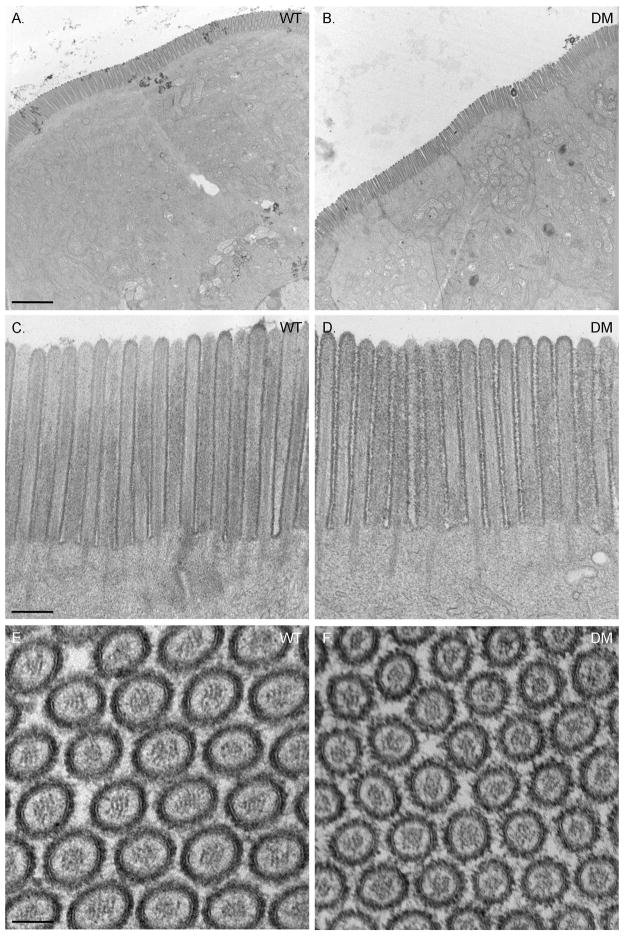
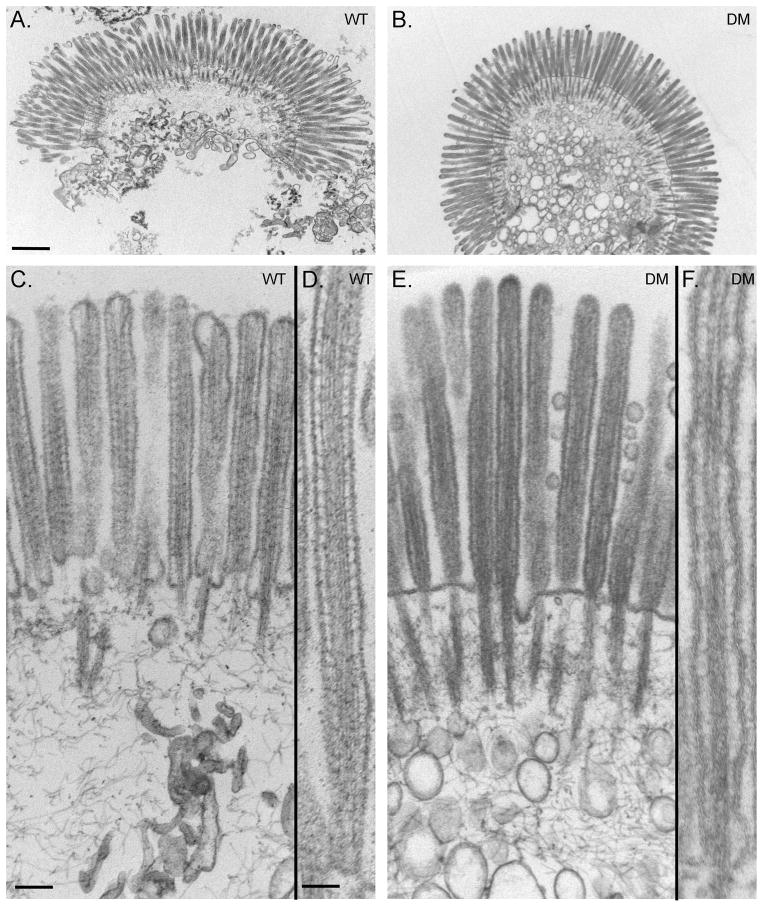
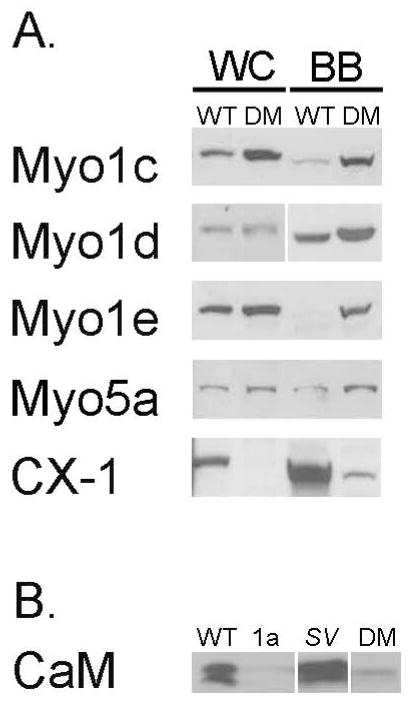
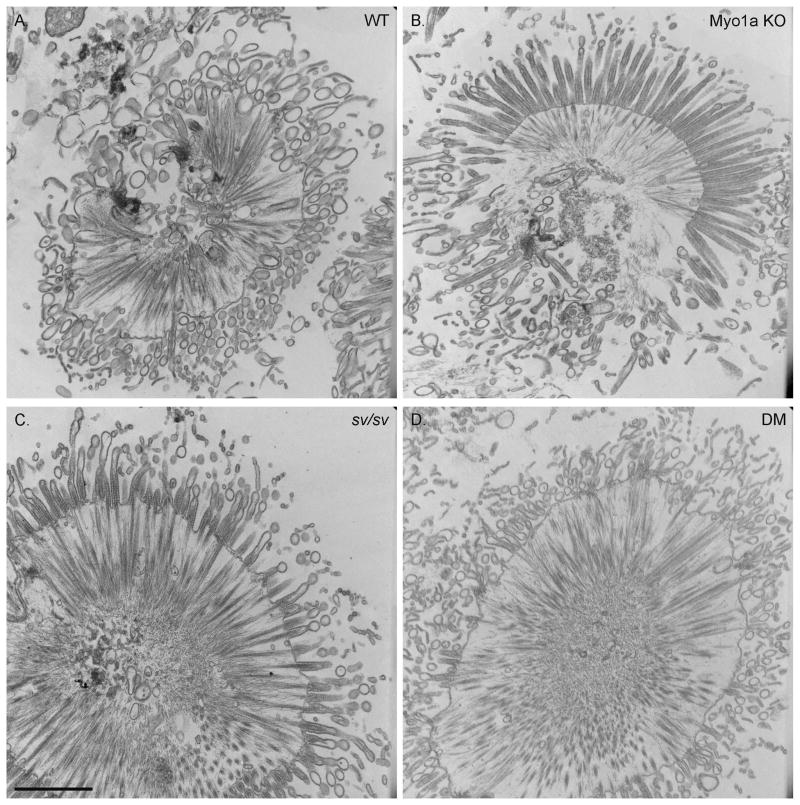
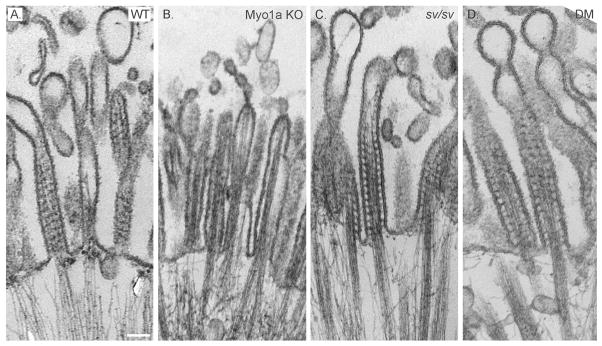
As detailed in Materials and Methods, Myo1a KO-sv/sv DM mice were generated through a series of crosses between Myo1a KO and sv/sv mice. Immunoblot analysis of DM whole IEC homogenates and isolated BBs verified that the DM mice lack both Myo1a and Myo6 (Fig. 1A); nor did the DM BB domain exhibit detectable MV localization of Myo1a (Fig. 1 B) or subapical expression of Myo6 (Fig. 1C). Thin section TEM analysis of the DM intestinal epithelium revealed that overall cellular organization was WT-like in appearance (Fig. 2). The dilation of endoplasmic reticulum and vesiculation of MV observed in Myo1a KO IECs (see Fig. 6 in ref. [Tyska et al. 2005]) was absent (Fig 2 A). Ultrastructural examination of the BB domain of the DM intestinal epithelium indicated that none of the defects in membrane tethering observed in either the Myo1a KO or sv/sv mouse are present in the DM (Fig. 2 B) including restoration of MV hexagonal packing (Fig. 2 F). The BB domain is ultrastructurally indistinguishable from that of WT. The restoration of BB membrane tethering in the DM is particularly evident in isolated BBs (Fig. 3). In isolated Myo1a KO BBs, the periodic lateral bridges are absent and the gap between the BB membrane and underlying MV core is collapsed. There is also extrusion of membrane at the tips of MV (Tyska et al. 2005). Isolated BBs from DM mice (Fig. 3 B, E, F) closely resemble those from WT mice (Fig. 3 A, C, D) except that the periodic lateral Myo1a bridges between the MV core and membrane are absent although some possible lateral but non-periodic links between the MV core are present (Fig. 3 E, F). The gap between the membrane and the core, absent in Myo1a KO BBs, is present (Fig. 3 F).
Fig. 1. Myo1a and Myo6 are not expressed in the DM intestinal epithelium.
(A) Immunoblots of whole cell homogenates (WC) and isolated BBs (BB) from WT and DM mice for Myo1a and Myo6 expression. (B) Localization of F actin (red), Myo1a and Myo6 (green) in WT and DM mice. Hoechst stained nuclei are shown in blue. Bar: 10 μm
Fig. 2. The ultrastructure of the DM intestinal epithelial cell and its apical BB is WT-like in organization.
(A) Low magnification TEMs of WT and DM intestinal epithelium. (B) Ultrastructure of the WT and DM BB region. (C) Cross section through BB MV in WT and DM. Bars (A): 2 μm (B): 250 nm (C): 75 nm
Fig. 3. Except for lack of cross bridges between MV core and MV membrane, isolated DM BBs exhibit WT-like ultrastructural features.
Low (A, B) intermediate (C, E) and high (D, F) magnification TEM images of WT (A, C, D) and DM (B, E, F) isolated BBs. Bars: (A, B): 500 nm; (C, E): 200 nm; (D, F): 100 nm
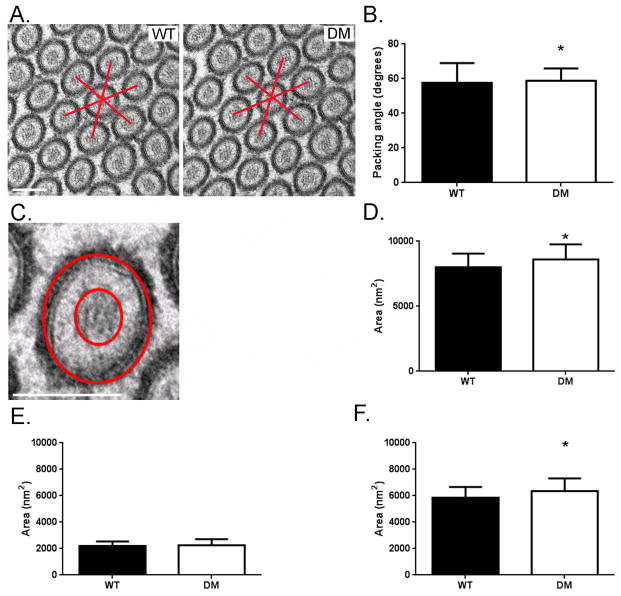
The tight hexagonal packing of MV within the BB is disrupted in the Myo1a KO as quantified by deviation from a 60° angle between adjacent MV as viewed in en face sections [Tyska et al. 2005]. In contrast, the packing organization of adjacent MV in the DM BB is comparable to that of WT (Fig. 2 C, Fig. 4 A-B). In the Myo1a KO, the area of “free” cytosol between the MV core and surrounding membrane is significantly reduced (~30%; [Tyska et al. 2005]), presumably because of the lack of Myo1a links, allowing movement of the MV membrane closer to the core. These periodic links are clearly absent in the DM BB, based on ultrastructural analysis of isolated DM BBs (Fig. 3 E, F). However, total MV cross sectional area (Fig. 4D) and the area of free cytosol between core and membrane (Fig. 4F) are both significantly (p>0.005) somewhat greater than WT MV indicating that on average, DM MV are a few nm greater in diameter than WT. This might be expected if the restoration of MV tethering to the core were effected by ectopically recruited membrane linkers (e.g.Myo5a; see below) that are longer than the ~ 30 nm Myo1a molecule [Conzelman and Mooseker 1987] The increase in free cytosolic area is not due to a reduction in MV actin core diameter (Fig. 4 E).
Fig. 4. MV hexagonal packing and intramembrane space between the MV membrane and MV core is restored in the DM.
(A) TEM cross sectional views of MV in WT and DM BB. Bar: B) Histogram of measured angles between adjacent WT and DM MV measured from multiple (n=606 angle measurements per genotype) cross sectional TEM images. Both WT and DM MV exhibit hexagonal packing with DM exhibiting even tighter packing than WT (WT, 57.7° ± 11.3°; DM, 58.7° ± 7.2°; *p < 0.05). C) High magnification cross sectional view of a DM MV showing the MV core, inter-MV membrane-core space and MV membrane. MV and MV core cross sectional areas were determined by measuring the area within the outer and inner circles respectively; intra-MV free space area was determined by subtracting the inner circle area from the outer circle area. Bar: 100 nm D) Histogram of mean MV cross sectional area shows that DM microvilli have slightly increased diameter (~102 nm vs. 105 nm) as compared to WT (WT, 8025 nm2 ± 1033 nm2, n = 46 vs. DM, 8596 nm2 ± 1171nm2, n = 46 ; *p < 0.005). E) Histogram of mean MV actin core area shows that there is no statistical difference between WT and DM (WT, 2198 nm2 ± 836 nm2, n = 46 vs. DM, 2269 nm2 ± 445 nm2, n = 46). F) Histogram of mean MV free space area between plasma membrane and actin core shows that there is slightly increased free space in the DM as compared to WT consistent with the increase and total MV diameter and comparable MV core diameter (WT, 5826 nm2 ± 836 nm2, n = 46 vs. DM, 6327 nm2 ± 998 nm2, n = 46; *p < 0.005).
Multiple myosins are recruited to the MV of the DM BB
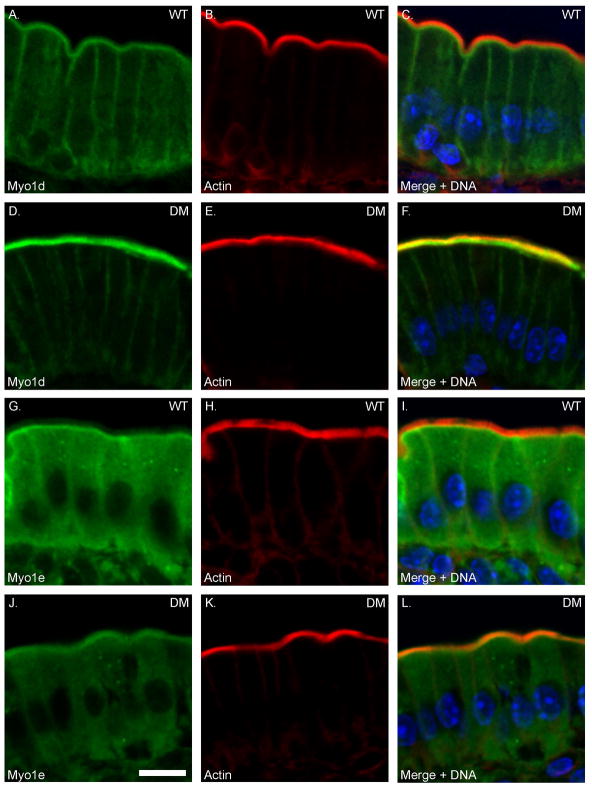
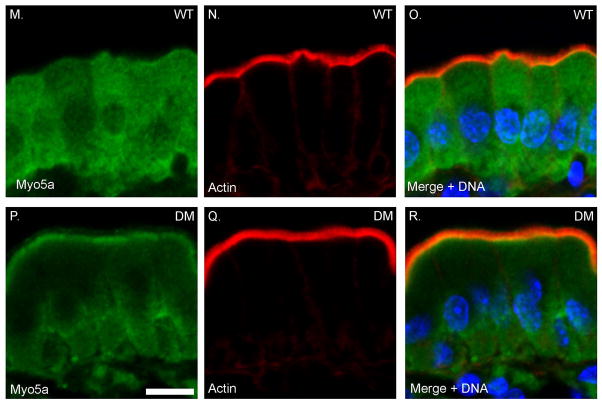
One possible mechanism for the “rescue” of tethering defects in the DM BB, is that there is ectopic recruitment of other membrane-actin filament tethers such as P-ezrin (see below) or other myosins expressed in the intestinal epithelium. Indeed both increased as well as decreased BB association of several myosins has been observed for both sv/sv and Myo1a KO BBs. In the Myo1a KO, there is increased MV association of Myo1c and Myo1d, while both Myo1e and Myo6 expression is reduced [Benesh et al. 2010; Tyska et al. 2005]. In sv/sv BBs there is increased association of Myo1e and Myo5a, and decreased Myo1d [Hegan et al. 2012]. Immunoblot analysis of isolated DM BBs indicates that multiple myosins are recruited to the BB including Myo1c, Myo1d, Myo1e and Myo5a (Fig. 5A). However, immunoblot analysis with a monoclonal antibody, CX-1, that recognizes multiple class I myosins [Carboni et al. 1988; Peterson et al. 1993], indicates that there is still a significant decrease in total class I myosins because of the absence of Myo1a. Immunolocalization of Myo1d, e and Myo5a (Fig. 6) indicate that these localize to the MV domain of the DM BB (commercially available Myo1c antibodies were not suitable for immuno-localization). There is a marked increase in the BB association of Myo1d compared to WT, with a concomitant reduction in basolateral localization. In the case of Myo1e, compared to WT, there is a striking redistribution from the subapical terminal web domain (Fig. 6 G-I) to the MV domain (Fig. 6. J-L). However, unlike Myo1d, the overall increase in Myo1e staining intensity is not as pronounced as one might expect based on the immunoblot data. This could be due to differential loss of subapical Myo1e during isolation of WT BBs and/or preferential retention of MV associated Myo1e during DM BB isolation. The diffuse cytoplasmic distribution of Myo5a seen in WT (Fig. 6 M-O) is reduced in the DM, presumably due to the concentration of Myo5a in the BB, although unlike the recruited Myo1s, Myo5a is concentrated at the base of MV and is not distributed uniformly along the full length of BB MV (Fig. 6 P-R).
Fig. 5. Multiple myosins are recruited to the DM BB.
(A) Immunoblot analysis of isolated WT and DM BBs for Myo1c, d, e and Myo5a indicate that these myosins exhibit increased expression/association in the isolated DM BB. Total class I myosin expression, based on immunoblot with the monoclonal antibody CX-1, is still less than in WT BB. (B) Comparison of calmodulin content in isolated BBs from WT, sv/sv, Myo1a KO and DM. The 20-17 kDa doublet bands seen in the WT and sv/sv lanes reflect the differential migration of apo- and Ca2+-calmodulin (Chacko et al. 1994).
Fig. 6. Myo1d, Myo1e and Myo5a exhibit increased BB expression levels in the DM.
Localization of Myo1d (A-F) and Myo1e (G-L) and Myo5a (M-R) in WT and DM IECs. Bar: 10 μm
One of the “side effects” of loss of Myo1a in the Myo1a KO BB, is the depletion of Myo1a calmodulin light chains from the intra-MV compartment, and as a consequence, reduced intraMV Ca2+ buffering. This is potentially an important function for Myo1a since the BB membrane is a site of vitamin D-dependent dietary uptake of Ca2+ [Christakos 2012]. As noted in the Introduction, this loss of Ca2+ buffering capacity is presumably the basis for the increased villin severing-mediated MV vesiculation observed in the Myo1a KO. In contrast, despite the absence of Myo1a calmodulin light chains, MV vesiculation does not occur in the DM, perhaps because of the ectopic recruitment of myosins and their associated calmodulin light chains to the BB. Immunoblot analysis of calmodulin content in isolated BBs from WT, sv/sv, Myo1a KO and DM indicates that there is an increase in calmodulin in the DM BB in comparison to the Myo1a MO BB, but calmodulin content is far below that in either WT or sv/sv BBs (Fig. 5B).
ATP-dependent BB membrane shedding is restored in the DM BB
Addition of ATP to isolated WT BBs has been shown to drive the upward shedding of the BB membrane from the underlying MV actin cores [Howe and Mooseker 1983; McConnell and Tyska 2007; Tyska et al. 2005]. This membrane shedding is inhibited in isolated Myo1a KO BBs [McConnell and Tyska 2007; Tyska et al. 2005] . Myo1a-dependent upward membrane movement has also been shown to drive the release of alkaline phosphatase (AP) -enriched vesicles from MV tips in vivo. These vesicles have been shown to have AP-based antimicrobial activity [McConnell et al. 2009]. These vesicles are not released in the Myo1a KO [McConnell et al. 2009]. To determine if the ectopic recruitment of myosins restores the membrane shedding activity to the DM BB, ultrastructural analysis on BBs isolated from WT, Myo1a KO, sv/sv and DM mice treated at 37° for 10 min. in the absence (Supplementary Fig. 1) and presence of ATP (Fig. 7) was performed. Both Myo1a KO and DM BBs fixed after 10 min. at 37° in the absence of ATP exhibited more membrane vesiculation at tips of MV than observed for BBs fixed without warming (Fig. 3; [Tyska et al. 2005]), indicative that the linkage of the BB membrane to the underlying MV actin core in the DM BB may be less stable than in WT. As expected, ATP-dependent upward BB membrane shedding was observed for WT, (Fig. 7 A) and sv/sv BBs (Fig. 7 C). Consistent with previous results [McConnell and Tyska 2007; Tyska et al. 2005] membrane shedding was inhibited in Myo1a KO BBs although some BBs exhibited some slight upward membrane movement not previously observed (Fig. 7 B); this may be due to the higher temperatures (37° vs. RT) at which the current experiments were performed. Consistent with our ectopic myosin rescue hypothesis, DM BBs exhibited robust ATP dependent BB membrane shedding (Fig. 7 D). In regions of unshed membrane at the tips of MV, periodic (~20–30 nm), Myo1a-like bridges are seen in WT (Fig. 8 A) and sv/sv BBs (Fig. 8 C) but not at tips of MV in the Myo1a KO BB (Fig. 8 B). Despite the absence of Myo1a, similar bridges are also present at the tips of DM BBs (Fig. 8 D), presumably reflective of the upward movement and tip-ward concentration of one or more of the myosins ectopically recruited to the MV of the DM BB.
Fig. 7. ATP-dependent upward shedding of the BB membrane is restored in the DM BB.
TEMs of isolated WT (A), Myo1a KO (B), sv/sv (C) and DM (D) BBs treated with ATP at 37° for 10 minutes. Bar: 500 nm
Fig. 8. Periodic membrane-MV actin core crosslinks are present at regions of unshed membrane at the tips of MV cores in ATP treated WT, sv/sv and DM BBs.
TEMs of tip regions of ATP treated BBs from WT (A), Myo1a KO (B), sv/sv (C) and DM (D) ATP-treated isolated BBs. Bar: 100 nm.
There are numerous compositional changes in the molecular components of the DM BB
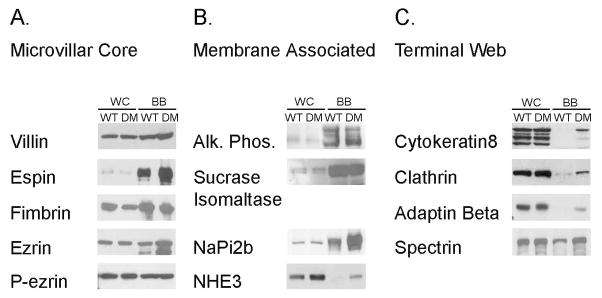
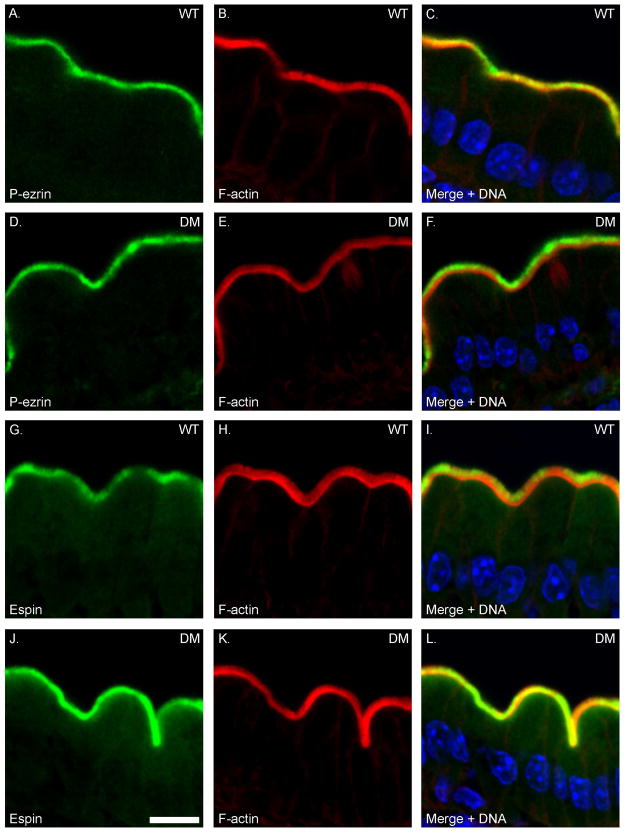
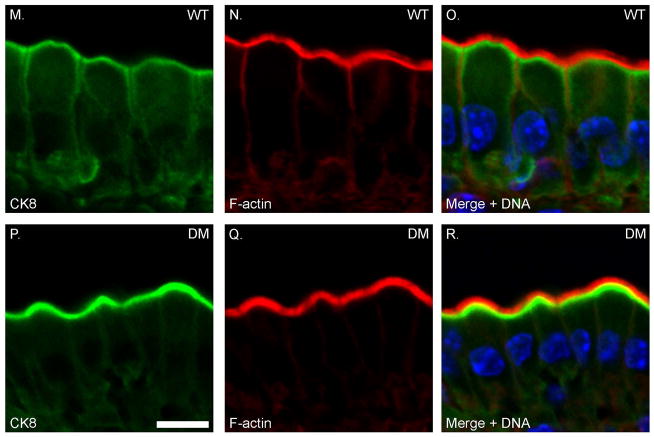
In addition to the changes in myosin expression, there are also changes in levels of expression of a number of key components of the BB, based on immunoblot analysis of isolated WT and DM BBs (Fig. 9). There is increased expression of the MV core proteins espin and ezrin, although unlike the sv/sv BB [Hegan et al. 2012], the level of the active membrane tethering form of ezrin, P-ezrin is not increased (Fig. 9 A). Thus the restoration of BB membrane tethering in the DM is not due to changes in activation of ezrin in the DM BB. Myo1a is required for the BB membrane localization of the lipid raft associated hydrolase, sucrase isomaltase (SI) [Tyska and Mooseker 2004] and there is significant reduction of BB associated SI (~ 50%) , and mis-localization of SI to the basolateral membrane in the Myo1a KO [Tyska et al. 2005]. In contrast, BB-associated SI is expressed at nearly WT levels in the DM BB membrane; as in the Myo1a KO BB, there is no change in BB-associated AP (Fig. 9 B). There is increased BB associated NaPi2b and the Na+/H+ exchanger NHE3 in the DM (Fig. 9 B). With respect to subapical terminal web components (Fig. 9 C), there is increased expression of clathrin and clathrin adaptor adaptin β, as well as spectrin which together with myosin II, forms crosslinks between adjacent MV core rootlets [Mooseker 1985). There is a slight increase in cytokeratin associated with the isolated BB, which is much more striking based on immunolocalization (see below). Immunolocalization studies of these components on intestinal WT and DM tissue confirm the above results (Fig. 10) including WT levels of P-ezrin localization in the BB and increased MV associated espin (Fig. 10 A, B) with the exception that the increased association of cytokeratin with the terminal web domain is much more pronounced (Fig. 10 C). A similar, but less dramatic increase in cytokeratin association with the BB is seen in the Myo1a KO [Tyska et al. 2005].
Fig. 9. The DM BB exhibits altered protein compositions associated with the MV core and terminal web.
Immunoblot analysis of whole cell homogenates (WC) and isolated BBs (BB) from WT and DM mice for (A) MV core proteins villin, espin, fimbrin, ezrin, P-ezrin (B) BB membrane proteins sucrase-isomaltase (SI), alkaline phosphatase (AP), NaPi2b, NHE3 and (C) terminal web proteins cytokeratin 8, clathrin, adaptin beta and spectrin.
Fig. 10. There is enhanced recruitment of espin, cytokeratin but not P-ezrin to the BB domain of the DM.
Localization of F-actin and P-ezrin (A–F), Espin (G–L) and cytokeratin 8 (M–R) in WT and DM IECs. Bar: 10 μm
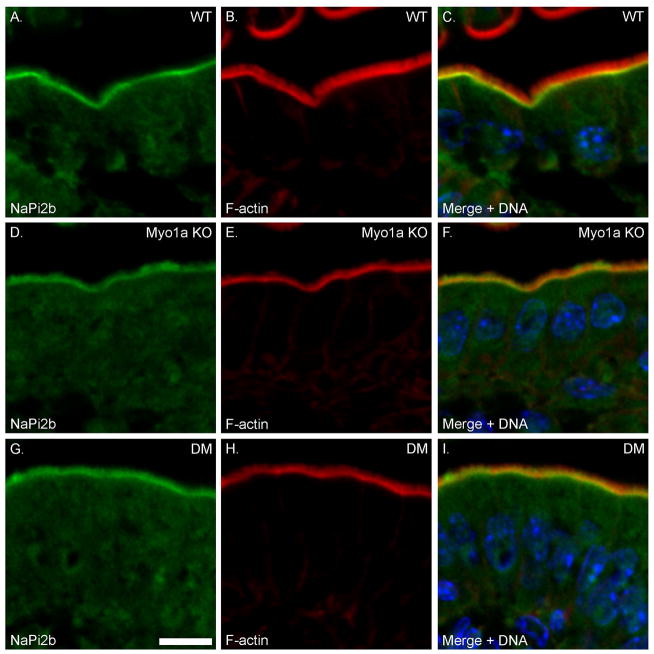
High Pi-induced redistribution of NaPi2b to subapical endosomes is impaired in Myo1a KO and DM mice
Under high Pi diet conditions, NaPi2b is largely absent from the MV membrane compartment and is concentrated sub-apically (see Fig. 11 A), presumably in a subapical endosomal compartment [Giral et al. 2009; Hegan et al. 2012]. Conversely, low Pi diet induces the recruitment of NaPi2b to the BB membrane [Giral et al. 2009; Hegan et al. 2012]. In the sv/sv mouse, NaPi2b remains concentrated in the BB membrane under both low and high Pi conditions indicating that the endocytic retrieval of this transporter is dependent on Myo6 [Hegan et al. 2012]. However, Myo1a may also play a role in the physiologic regulation of NaPi2b expression/localization since it is retained in the BB membrane of Myo1a KO mice maintained on a high Pi diet (Fig. 11 B) This may be due to the depletion of Myo6 from the subapical domain in the Myo1a KO [Tyska et al. 2005]. As in sv/sv [Hegan et al. 2012] and Myo1a KO mice, NaPi2b remains largely localized to BB MV in the DM under high Pi conditions. (Fig. 11 C). WT, Myo1a KO and DM mice maintained on low Pi diet all exhibit localization to the apical BB membrane (Supplementary Fig. 2). Thus unlike the rescue of Myo1a/Myo6 membrane tethering defects, the high Pi diet-induced redistribution of NaPi2b to subapical endosomes is not rescued in the DM, and both DM and Myo1a KO phenocopy the sv/sv mouse. Similarly, another protein that traffics between the BB membrane and endosome is the Na+/H+ transporter NHE3 [He and Yun 2010]. Under steady state conditions, there is increased accumulation of NHE3 in the BB membrane of sv/sv mice [Hegan et al. 2012]. Immunoblot analysis of isolated DM BBs (Fig. 9 B) and immunolocalization also indicate increased BB-associated NHE3 (Supplementary Fig. 3).
Fig. 11. High phosphate diet-induced trafficking of NaPi2b from the BB membrane to the subapical endosome is inhibited in the Myo1a KO and DM.
Localization of F-actin and NaPi2b in WT (A–C), Myo1a KO (D–F) and DM (G–I) intestinal epithelium. Bar: 10 μm
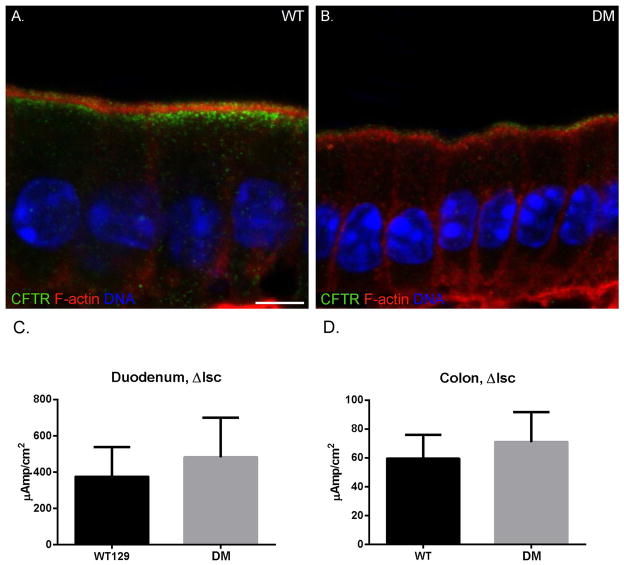
The BB localization and cAMP-stimulated anion transport of CFTR of the DM is comparable to that of WT, but endosomal expression/localization is reduced
In the sv/sv mouse, there is excess functional BB surface accumulation of CFTR [Ameen and Apodaca 2007], while in the Myo1a KO, the opposite is observed, with CFTR residing largely in the subapical endosomal compartment, resulting in reduced cAMP stimulated anion transport [Kravtsov et al. 2012]. These results suggest that Myo1a is required for apical trafficking of CFTR to the BB membrane and Myo6 effects endocytic retrieval to subapical endosomes. Unlike the BB expression of NaPi2b or NHE3 in the DM, the localization of CFTR in the DM duodenal intestinal epithelium is distinct from the phenotypes of either sv/sv or Myo1a KO. (Fig. 12). Based on fluorescence intensity, there is greatly reduced subapical CFTR compared to WT, indicative of a sv/sv-like phenotype. However, apical BB fluorescence intensities in the DM and WT are comparable (Fig. 12 A, B). Consistent with this observation, measurement of cAMP-stimulated anion transport in WT and DM duodenum sheets revealed only a slight, but not significant increase in transport in the DM (Fig. 12 C). No statistically significant differences in cAMP-stimulated anion transport were observed for WT and DM colon (Fig. 12 D). As has been reported previously for WT, sv/sv and Myo1a [Ameen and Apodaca 2007; Kravtsov et al. 2012], CFTR is apically localized in crypts of both WT and DM in both duodenum and colon (Supplementary Fig. 4).
Fig. 12. The BB MV localization and cAMP-stimulated anion transport of CFTR of the DM are comparable to that of WT, but subapical endosomal expression/localization is reduced.
Localization of actin (red) and CFTR (green) in WT (A) and DM (B) duodenum. DAPI stained nuclei are shown in blue. Total CFTR staining intensity is greatly reduced in the DM due to loss of subapical localization. Bar: 5 μm. (C, D) No significant differences in the increase in isc (μamps/cm2) after forskolin stimulation in (WT n=2) and DM (n=2) duodenum (C; P=0.49) and colon (D; P=0.42) were observed.
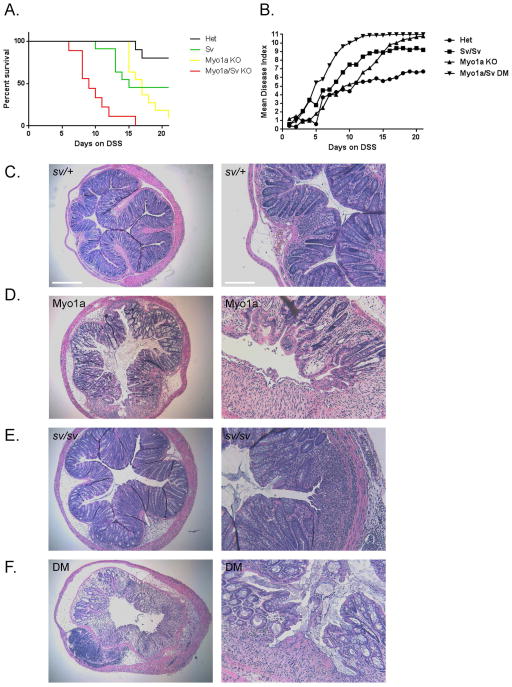
The DM mouse is hypersensitive to DSS-induced colitis
The DSS-induced colitis model has been used to assess the potential role of components of the intestinal epithelium in response to DSS induced mucosal injury [Kiesler et al. 2015]. For example, although KO mice for the MV core protein villin exhibit no obvious phenotype under normal conditions, the villin KO mouse is hypersensitive to DSS treatment [Ferrary et al. 1999; Wang et al. 2008]. It has been proposed that villin plays a key role in the cytoskeletal remodeling events required for epithelial responses to injury [Athman et al. 2002; Khurana and George 2008; Ubelmann et al. 2013]. To determine if loss of either Myo1a or Myo6 function, or loss of both motors differentially contributes to the extent and rate of DSS induced mucosal injury, sets of animals were treated with DSS over a 21 day period. Three assessments were performed a) percent animals surviving b) daily progression of disease index and c) histologic examination of colons at either day 21 (sv/+ control, sv/sv) or from mice that reached the 20% weight loss end point (Myo1a, DM) (Fig. 13). Both sv/sv and Myo1a KO mice exhibited enhanced sensitivity to DSS compared to control mice based on all three metrics: increased mortality rate (Fig. 13 A), more rapid onset of elevated disease index (13 B), and increased mucosal injury at experimental end point (Fig. 13 C-E). However, the onset of elevated disease index and mortality was most dramatically increased in the DM mutant (Fig. 13 A, B, F)
Fig. 13. Myo1a KO and sv/sv mice exhibit intermediate and DM mice high levels of hypersensitivity to DSS induced colonic mucosal injury.
(A). Survival index of sv/+ (black), sv/sv (yellow) , Myo1a KO (green) and DM (red) mice as a function of days on drinking water with 3% DSS. (B) Disease index scores as a function of days on DSS. (C–F) H&E stained sections of most affected regions of colon from sv/+ and sv/sv mice at the 21 day end point for treatment (C, E), or from Myo1a KO (D) and DM (F) mice that reached the 20% weight loss end point (for the images of mice colons shown, day 15 for the Myo1a KO and day 13 for the DM). Note that in comparison to the sv/+ control, mucosal injury, edema and submucosal immune cell infiltration is greater in all three myosin mutants. Left bar B–F: 375 μm; Right bar (B–F): 150 μm
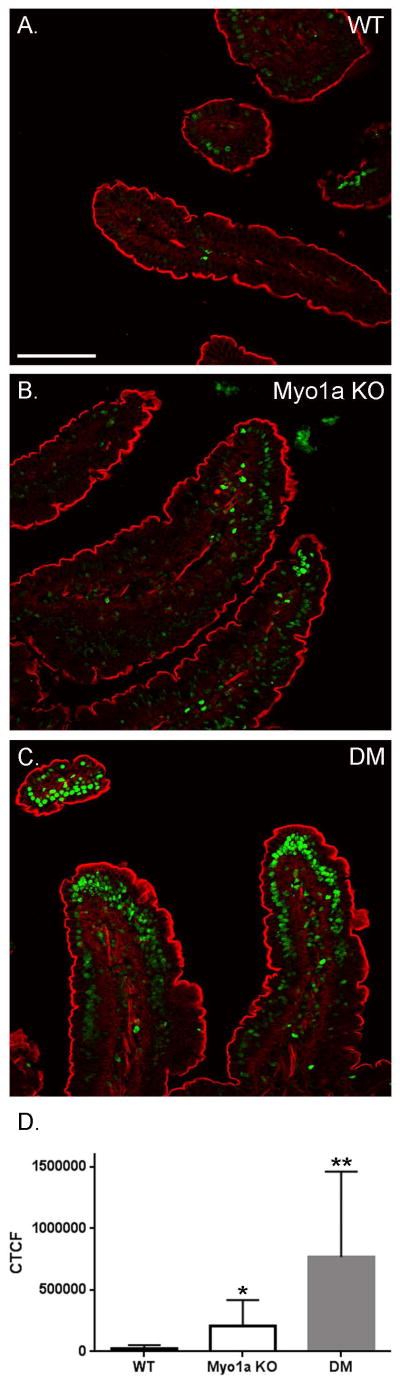
The DM mouse exhibits highly elevated levels of IEC apoptosis compared to WT and Myo1a KO mice
A possible explanation for the increased sensitivity of the DM to DSS treatment is that these mice may be pro-apoptotic. Studies on the villin KO and villin-gelsolin double KO demonstrated greatly increased levels of epithelial cell apoptosis in response to DSS treatment [Wang et al. 2012; Wang et al. 2008]. Moreover, it has been previously shown that there are elevated levels of IEC apoptosis along the villus axis in the Myo1a KO [Tyska et al. 2005]. Surprisingly, particularly given the normal mucosal epithelial ultrastructure observed in the DM, TUNEL staining of duodenal mucosal nuclei (Fig. 14) revealed levels of apoptosis much greater than that observed in the Myo1a KO in the DM (note WT levels of apoptotic nuclei, primarily at villus tips was observed in sv/sv; [Hegan et al. 2012]).
Fig. 14. The DM duodenal intestinal epithelium exhibits elevated numbers of TUNEL positive nuclei.
(A) TUNEL stained duodenal intestinal villi in WT, Myo1a KO and DM. Bar: 100 μm (B) Histogram quantifying TUNEL positive signal as the mean corrected total cell fluorescence (CTCF) of the epithelial cells in WT, Myo1a KO, and DM villi. The DM duodenum has a significantly higher level of apoptosis than both WT and the Myo1a KO. The Myo1a KO has a significantly higher level of apoptosis than WT (WT, CTCF = 31,842 ± 21,409, n = 18 vs. Myo1a KO, CTCF = 204,370 ± 213,873, n = 18; *p < 0.005 vs. DM, CTCF = 768,311 ± 693,423, n = 18; **p < 0.0005 for DM compared to WT, **p < 0.005 for DM compared to Myo1a KO).
DISCUSSION
The results presented here provide novel insights into the functional synergy between Myo1a and Myo6 in the BB of the IEC, particularly given the unexpected consequences of loss of both of these motors on the restoration of BB structural organization and integrity. On the other hand, restoration of BB membrane-cytoskeleton structural integrity is not sufficient to return the DM to a fully WT phenotype since Myo1a and Myo6 dependent functions in BB membrane traffic are not rescued. Moreover, the hyper-elevated apoptotic status of the DM intestinal mucosa, and hypersensitivity of the DM mouse to DSS-induced intestinal injury clearly indicate that these two motors are essential for IEC health and integrity. These aspects of the consequences of loss of Myo1a and Myo6 function are discussed below.
Based on the plethora of ultrastructural and compositional defects observed within the BB domain in the absence of either Myo1a or Myo6 (as summarized in the Introduction), we expected that removal of both of these motors would result in even greater, if not catastrophic perturbations in BB organization and IEC viability. Instead, with the exception of the absence of Myo1a MV membrane-actin core crosslinks, all of the striking ultrastructural defects observed in the absence of either motor are gone when both Myo1a and Myo6 are removed from this complex membrane cytoskeletal apparatus (Figs 2–4). One possible explanation that perhaps raises more questions than answers, is that the restoration of BB membrane-actin organization is effected by the ectopic recruitment of other myosins including Myo1c, d, e and Myo5a to the MV membrane-actin core interface (Fig. 5), which results in at least partial restoration of both mechanical and mechanochemical tethering interactions between the membrane and MV actin core. The restoration of ATP/motor dependent upward shedding of the BB membrane in the DM BB provides substantive functional evidence for this hypothesis.
This hypothesis begs the question as to why the removal of both Myo1a and Myo6 is required for such ectopic rescue. This is particularly puzzling given that in the Myo1a KO, the steady state localization of Myo6 within the subapical terminal web domain is greatly reduced. Moreover both Myo1c and Myo1d do redistribute to the MV domain in the Myo1a KO [Benesh et al. 2010; Tyska et al. 2005], yet membrane tethering (as measured in isolated BBs under rigor conditions) is lost and motor-dependent upward BB membrane shedding is inhibited. A partial answer may be found in the fact that the high duty ratio, processive motor Myo5a [Mehta et al. 1999] is recruited to the MV domain in the DM but not in the Myo1a KO. Recruitment of a relatively low number concentration of Myo5a motors to the MV membrane-MV core interface might be sufficient to restore motor-driven BB membrane shedding. But is the total number concentration of ectopic myosin tethers recruited to the DM MV sufficient to explain the dramatic restoration of the mechanical integrity of the BB membrane-actin cytoskeleton? In this regard it would be of great interest to directly measure the untethering forces required to detach the DM BB membrane from the underlying cytoskeleton as has been so elegantly determined for the Myo1a KO BB [Nambiar et al. 2009].
Another question raised by these studies is whether there are differences in the structural /physical and/or compositional properties of either the MV actin core or cytoplasmic domain of the MV membrane that allows restoration of membrane tethering (e.g. via recruitment of Myo5a) only in the absence of both Myo1a and Myo6? One possibility, albeit highly speculative, is that in the WT, in the presence of both Myo1a and Myo6, there is a balance of plus- and minus-end directed tension on the MV membrane that physically tunes the optimal membrane binding interactions between the membrane and the tail domains of Myo1a and Myo6. This seems particularly feasible for the subapical membrane binding of Myo6 since the mechanochemical properties of this motor are altered by opposing tension. Under laser trap applied high load, two-headed Myo6 stalls in a “latched”, actin-anchored state [Altman et al. 2004; Chuan et al. 2011]. The velocity of single-headed Myo6 is substantially increased when motility is assayed in the presence of the plus end-directed motors myosin II or Myo5a [Ramamurthy et al. 2012]. Thus in the absence of Myo1a-mediated tension on the MV membrane, binding of Myo6 to the MV core and/or membrane may be impaired. By removal of both the major plus end motor, Myo1a, and sole minus-end directed motor, there may be a loss of this tension imbalance between the MV membrane and MV actin core that provides alterations in the either the MV membrane or MV actin core that promotes the ectopic recruitment Myo5a and/or other myosins for restoration of membrane tethering. For example, there could be alterations in either phospholipid organization or composition that promote Myo5a tail binding to the MV membrane lipid bilayer. Alternatively, perhaps there is redistribution of as yet unidentified Myo5a tail binding proteins into the MV. Finally, there could be changes in the affinity of Myo5a and/or the recruited Myo1s for MV core actin filaments due to the loss of tension imbalance that results in structural changes in the core filaments.
Another unexpected “rescue” feature in the DM is the increased level of SI associated with the BB membrane compared to the ~ 50% reduction in the Myo1a KO BB (Fig. 9). Unfortunately we were unable to determine if the basolateral mis-targeting of SI to the basolateral membrane observed in the Myo1a KO was reduced in the DM because none of the currently available commercial antibodies were reliable. The SI antibody used in our previous studies [Tyska et al. 2005] is no longer available. Myo1a has been shown to be required for SI localization to the BB membrane, based on expression of the dominant negative Myo1a tail domain and expression of the N-terminal cytoplasmic domain of SI, both of which completely inhibit SI BB localization in the intestinal cell line Caco-2 BBe [Tyska and Mooseker 2004]. In the Myo1a KO, there is only a ~ 50% reduction in BB-associated SI, as well as mistargeting of SI to the basolateral membrane [Tyska et al. 2005]. It was proposed that the ectopic recruitment of other class I myosins to the BB was responsible for the partial retention of SI in the BB membrane. However, even with the recruitment of Myo1e, in addition to Myo1 c and d, the number concentration of total class I myosins associated with the DM BB is far less than the concentration of Myo1a in the WT BB. This is based on immunoblot analysis with the pan-Myo1 antibody CX-1 as well the greatly reduced amount of BB-associated Myo1-associated calmodulin light chains in the DM compared to either WT or sv/sv (Fig. 5). Thus, one should also consider that the apical targeting and retention of SI in the DM BB could also be due to the absence of tension imbalance effects on the BB membrane. For example, in the Myo1a KO there may be perturbations in the stability of BB membrane lipid rafts, with which SI is associated [Danielsen and Hansen 2006], that are absent in the DM BB.
While apical targeting of SI to the BB membrane is apparently at least partially restored in the DM, this is not the case for membrane proteins that traffic between the BB membrane and the subapical endosomal compartment such as NaPi2b, NHE3 and CFTR. The inhibition of high Pi diet-induced redistribution of NaPi2b to endosomes and higher steady state level of NHE3 BB expression in the DM are equivalent to that observed in sv/sv mice. Thus the endocytic function for Myo6 in trafficking of membrane proteins from the BB membrane to the endosome is not restored in the DM.
In contrast, the altered steady state distributions of CFTR in the BB and endosomal membrane compartments are quite distinct from that observed in either sv/sv or Myo1a KO. While there appears to be WT-levels of BB membrane surface expression in the DM based both on fluorescence intensity (Fig. 12 A, B) and cAMP-stimulated anion transport in the duodenum (Fig. 12 C), steady state levels of CFTR in the subapical endosomal compartment are markedly reduced compared to that in WT (or Myo1a KO [Kravtsov et al. 2012]). This suggests that there must be a Myo1a-independent exocytic delivery of CFTR to the BB membrane operative in the DM, but that at steady state, the excess BB accumulation of CFTR expected in the absence of Myo6 [Ameen and Apodaca 2007] does not occur. There are multiple candidates to effect apical delivery of CFTR including the myosins ectopically recruited to the BB in the DM, as well as Myo5b, which has been implicated in apical BB membrane targeting based on its identification as the target gene responsible for MV inclusion disease (reviewed in [Thoeni and Cutz 2014]). One possible explanation for the depletion of CFTR from the endosomal compartment is that there is increased turnover of BB membrane-associated CFTR delivered to the BB by the putative Myo1a independent mechanism proposed above. This could result in net depletion of the endosomal pool of CFTR due to loss of Myo6 recycling of CFTR to endosomes and also reduction in total BB-associated to WT levels compared to that observed in the absence of just Myo6 [Ameen and Apodaca 2007].
The intermediate and hypersensitive responses of sv/sv, Myo1a KO and DM mice to DSS treatment seem consistent with expected and additive, if not synergistic, roles for these two myosins in response to mucosal injury. These results are quite analogous to those obtained for the villin KO, in which there are no obvious phenotypic defects in these mice until they are challenged with mucosal injury [Ferrary et al. 1999; Khurana and George 2008]. Like villin [Athman et al. 2002; Khurana and George 2008; Ubelmann et al. 2013], Myo6 and Myo1a could play multiple roles in the cytoskeletal remodeling that must occur in the intestinal epithelium in response to injury. Moreover, even though the intestinal epithelium of the DM looks normal under unstressed conditions, the injury response functions for these two myosins uncovered under DSS induced injury, may also be required for intestinal mucosal homeostasis under normal conditions given the high levels of apoptosis observed in the DM.
Does Myo1a suppress the oncogenic activity of Myo6?
Potentially the most important aspect of the functional synergy between Myo1a and Myo6 yet to be explored is whether it is the suppression of Myo6 oncogenic activity that is the molecular basis for the function of Myo1a as a tumor suppressor. The progression of both genetically and carcinogen-initiated tumor grown is greatly accelerated in the Myo1a KO mouse. Moreover, loss of Myo1a expression in patients diagnosed with colorectal cancers is associated shorter disease free intervals and overall rates of survival compared to patients with high levels of Myo1a expression [Mazzolini et al. 2012]. We propose that Myo1a acts as a tumor suppressor by sequestering Myo6 to the BB cytoskeleton, preventing it from entering the nucleus to transcriptionally activate tumor progression genes and/or down regulate expression of tumor suppressors. Evidence that Myo6 may have oncogenic activity includes the following studies. Elevated levels of Myo6 expression have been observed in breast cancer [Su et al. 2001], prostate cancer [Dunn et al. 2006], high grade ovarian carcinomas [Yoshida et al. 2004] and in the colon carcinoma cell line HT 116 (http://www.genecards.org/cgi-bin/carddisp.pl?gene=MYO6&search=Myo6). Moreover, RNAi knock down (KD) of Myo6 in ovarian carcinoma cells results in defects in cell migration as well as tumor dissemination [Yoshida et al. 2004]. KD of Myo6 in LNCaP cells, a prostate cancer cell line, inhibits cell migration and colony formation. A microarray analysis of these KD cells identified a tenfold induction of the tumor suppressor VDUP1, a protein also decreased in prostate cancer specimens [Dunn et al. 2006]. VDUP1 was also identified as the most highly upregulated gene in a microarray analysis of 5-fluorouracil treated colon cancer cell line, SW620 [Takahashi et al. 2002]. VDUP1 has also been shown to act as a tumor suppressor modulating the stability of the cyclin dependent kinase inhibitor, p27kip1 and causing cell cycle arrest [Jeon et al. 2005]. Although most Myo6 is cytoplasmic, it has also been localized to the nucleus. Nuclear Myo6 colocalizes with RNA Pol II and is recruited to the promoters of actively transcribed genes [Vreugde et al. 2006]. Myo6 has been shown to be induced and localized to the nucleus in a p53 and DNA damage dependent manner in the non small cell lung carcinoma cell line H1299. KD of Myo6 in these cells decreased p53 activation and causes susceptibility to apoptosis [Jung et al. 2006].
The availability of the Myo1a KO, sv/sv and DM mouse lines provides a robust experimental platform to test this hypothesis. For example, if Myo1a directly suppresses the oncogenic activity of Myo6, then carcinogen-induced tumor progression in sv/sv and DM mice should be comparable to that observed in WT. Conversely, if Myo1a-mediated tumor suppression acts independently of Myo6, then tumor progression rates in the DM should be comparable to the accelerated rate observed in the Myo1a KO mouse.
MATERIALS AND METHODS
Mice
Mice of the strain B6 x STOCK Tyrc-ch Bmp5se +/+ Myo6sv/J (stock #000578) were purchased from Jackson Labs (Bar Harbor, ME) and crossed to the 129X1/SvJ wild-type (WT) mouse to create a Snell’s waltzer mouse with a 129 genetic background. These mice were then crossed to the Myo1a KO mouse (129X1/SvJ background)[Tyska et al. 2005] to establish mice that were mutant for both Myo1a and Myo6. DM mice displayed the circling, head tossing, and hyperactivity phenotype that is associated with the Myo6 mutation. 129X1/SvJ wild-type (WT) mice were used as controls for the double mutant mouse with the exception of the DSS trials in which sv/+ mice were used. All data shown were generated using male mice. All procedures involving mice were performed in accordance with an approved Yale – IACUC animal protocol.
Electron microscopy
Tissues were processed for transmission electron microscopy (TEM) as described in [Mooseker and Tilney 1975]. WT and DM tissue from 4 different pairs of age matched (ages ranged from 4 –12 months) male mice were processed over an ~ 2 year period.) Segments of proximal intestine were dissected and flushed with 4° C phosphate buffered saline (PBS). The gut was everted, cut into small pieces and fixed in 2% glutaraldehyde, 0.2% tannic acid in 0.1M sodium phosphate buffer, pH 7.0 for 10 minutes at room temperature (RT), and then shifted to 4°C for an additional 50 minutes. Following fixation the tissue was washed three times with 0.1M sodium phosphate buffer, pH 7.0, and then incubated on ice for 1 hour in 1% OsO4 in 0.1M sodium phosphate buffer, pH 6.0. The tissue was then washed three times with cold water and incubated overnight at 4°C in 1% uranyl acetate. The samples were dehydrated with a 25 – 100% ethanol series at RT, followed by two 10 minute incubations in propylene oxide. The tissue was embedded in EmBed 812 (Electron Microscopy Sciences (EMS), Hatfield, PA) following established protocols recommended by the manufacturer. Pellets of isolated BBs were fixed and processed for TEM following the same protocols. Imaging was done on a JEOL 1230 transmission electron microscope (JEOL, Tokyo, Japan), and digital images were recorded with a Hamamatsu ORCA-HR digital camera (Hamamatsu, Hamamatsu City, Japan).
Morphometry of MV ultrastructure
Micrographs of cross sections through MV from WT and DM were used to measure the angle projected from the center of each MV to the center of the six MV surrounding it using ImageJ (http://rsb.info.nih.gov/ij/; NIH). Cross sectional areas of MV, MV cores and the intra-membrane MV free space was determined by measuring the area within circles drawn around the MV, the MV core, and subtraction of the core area from the MV area to determine the intra-MV free space area. Measurements were made using ImageJ.
Tissue preparation for immunofluorescence microscopy
For immunolocalization studies segments of intestine were flushed with PBS, everted, and segments were cut into small pieces and fixed with 4 % paraformaldehyde (prepared from a freshly opened vial of 16% PFA from EMS) in PBS for 10 minutes at RT and then shifted to 4°C for an additional 50 minutes. The tissue was washed twice with PBS, followed by 30 minutes in each of 5%, 8%, 12%, and 16% sucrose in PBS at RT. The tissue was incubated overnight in 20% sucrose in PBS at 4°C, and the following day was incubated in 12% sucrose, 35% OCT (Sakura Finetek USA, Torrance, CA) in PBS for 1 hour at RT. The tissue was embedded in 12% sucrose + 35% OCT in PBS and stored at -80°C. Five micron sections were cut on a Leica CM 3050S cryostat (Leica, Wetzlar, Germany) and mounted on Superfrost + slides (Esco, Portsmouth, NH). The following steps were done with either PBS or TRIS buffered saline, with 0.05% Tween 20 as the buffer, depending on the primary antibody used. The sections were washed 3x5 minutes with room temperature PBS to remove OCT. The tissue was blocked for 1 hour at RT in 10% normal goat serum (NGS) in PBS. Primary antibodies were diluted in PBS + 5% NGS and incubated at RT for 1 hour, or overnight at 4°C. The slides were then washed 3x10 minutes with PBS. Alexa fluor labeled secondary antibodies (Invitrogen, Carlsbad,CA) were diluted 1:200 in PBS containing 5% NGS , 0.33μM rhodamine phalloidin (Invitrogen) and 1μg/ml Hoechst dye (Sigma, St. Louis, MO) and incubated for 1 hour at RT in the dark. Slides were then washed 3x10 minutes with PBS and coverslips were mounted with Prolong Gold anti-fade media (Invitrogen). Localization of CFTR was performed as described in (Kravtsov et al. 2012). All immunofluorescence microscopy was done using a Zeiss LSM510 META laser scanning confocal microscope, and digital images were captured using ZEN 2009 software (Zeiss). All antigen localizations to be compared were prepared at the same time and were imaged with identical microscope settings. Contrast enhancement, cropping, and all other image manipulation was done using either Adobe Photoshop or ImageJ.
Isolation of whole mucosal cell homogenates and BBs
BBs were purified from the entire length of the small intestine as previously described (Mooseker et al. 1978) with modifications detailed in (Hegan et al. 2012). WT and DM BBs were isolated at the same time and the compositional data reported here was confirmed through analysis of 3 different BB preparations made from pairs of age-matched WT and DM mice. Whole mucosal gel samples were prepared by trichloroacetic acid precipitation of mucosal homogenates as described in [Hegan et al. 2012]. For immunoblot analysis, SDS sample buffer was added to whole mucosal homogenates and isolated BBs (equal protein for each genotype) and then electrophoresed on 5–20% gradient gels. The gels were then electo-transferred to Hybond nitrocellulose membrane (GE Healthcare, Piscataway, NJ) for immunoblot analysis. Horseradish peroxidase conjugated secondary antibodies (Pierce, Rockford, IL) were visualized by Enhanced Chemo Luminescence (GE Healthcare).
BB membrane shedding assay
Pellets of isolated BBs from WT, Myo1a KO, sv/sv and DM small intestines were suspended in 9 volumes of 10 mM Imidazole pH 7.1, 75 mM KCl, 4 mM MgCl2, 1 mM EGTA, 0.2 mM DTT with or without 2 mM ATP and incubated at 37° for 10 min., chilled to 4°, pelleted at 10,000xg for 5 min. and then fixed and processed for thin section EM as described above for pellets of isolated BBs.
Pi diet effects on NaPi2b expression
Low (0.1%) and high (1.2%) Pi mouse diets were purchased from Harlan Teklad (Madison, WI) and fed ad libitum to both WT, Myo1a and DM mice for a minimum of 14 days (Giral et al. 2009]. Mice were then sacrificed and tissue was collected from duodenum, jejunum, proximal ileum, and distal ileum. These tissue samples were fixed and processed for immunofluorescence microscopy of NaPi2b localization as described above.
Ussing chamber electrophysiological analysis
Electrophysiological analysis of cAMP-stimulated, CFTR mediated anion transport was performed on strips of duodenum and colon from WT and DM mice exactly as described in [Kravtsov et al. 2012].
DSS-induced colitis
Sets ( 9–11 mice/genotype each) of sv/+, Myo1a KO, sv/sv and DM male mice were transferred from drinking water to distilled water containing 3 % w/v DSS (MP Biomedicals, Solon, OH) for a maximum of 21 days. Mice were monitored daily using a disease index score that included the following assessments: 1. Weight loss: no change or weight increase; score of 0; weight loss of 1–3% of body weight at day 0, score of 1; weight loss of greater than 3 to 6%, score of 2; weight loss greater than 6% to 9%, score of 3; weight loss of greater than 9%, score of 4; weight loss of equal or greater than 20%, score of 5—and mortality end point. 2. Stool consistency: no change in consistency compared to that at day 0, score of 0; slight change in firmness, score of 1; soft, no blood; score of 2; soft with blood, score of 3; bloody diarrhea 4. 3. Fur, activity/ posture: no change compared to that at day 0; score of 0; slight change in fur scruff and/or posture, score of 1; significant change in fur scruff and activity/posture (very scruffy fur, hunched posture, reduced movement/activity); score of 2. Note that pairs of mock control mice maintained on water never achieved a disease index score greater than 1 (for transient slight weight loss).
Histology
For histologic analysis, segments of colon were taken from DSS treated mice at either the 21 day end point for treatment (sv/+ control, sv/+) or mice that reached 20% weight loss end point ( Myo1a KO and DM) The colons were flushed with ice cold PBS, cut into large pieces, and fixed for 24 hours at 4°C in 10% paraformaldehyde (PFA) in PBS. Tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E) using standard methods (Research Histology, Yale University School of Medicine). Histological sections were viewed with a Nikon Diaphot 300 inverted microscope (Nikon Instruments, Melville, NY) equipped with a Zeiss Axiocam (Zeiss, Thornwood, NY), and digital images were captured with AxioVision v.4.6 (Zeiss).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) labeling
Cryosections from WT, Myo1a, sv/sv and DM duodenum were washed 3x5 minutes with PBS. Tissue sections from all genotypes were mounted on the same slide in order to ensure equal treatment during the assay. The tissue sections were then blocked / permeabilized in 10% NGS, 0.1% Triton X-100 in PBS. The TUNEL assay (Roche Diagnostics, Indianapolis, IN) was done according to manufacturer’s instructions; enzyme and label were prepared at a 1:10 ratio and the tissue was incubated at 37°C for 1 hour, and then washed 3x5 minutes with PBS. F-actin was stained with 0.33μM rhodamine phalloidin (Invitrogen) for 30 minutes at RT in the dark, followed by 3x5 minute washes with PBS. Coverslips were mounted with Prolong Gold anti-fade media (Invitrogen), and confocal images were taken and processed as described above. To quantify the results of the TUNEL assay, fluorescence intensity per um2 of intestinal epithelium was determined using Image J (function for determining corrected total cell fluorescence).
Primary antibodies used for immunolocalization and/or immunoblot analysis
Myo1a and Myo1e, 1 μg/ml [Skowron et al. 1998], Myo1d and sucrase isomaltase, 1.5μg/ml (Santa Cruz Biotechnology, Inc., Dallas, TX), Myo1c and alkaline phosphatase, 1μg/ml (Sigma, St. Louis, MO), villin, 1μg/ml (AMAC, Westbrook, ME), espin, 1μg/ml (Gift from J.R. Bartles, Northwestern University), fimbrin, 1μg/ml [Shibayama et al. 1987], Myo5a and Myo6, 1μg/ml [Heintzelman et al. 1994], Myo1a and Myo1e, 1 μg/ml [Skowron et al. 1998], ezrin, phosphoezrin, and clathrin, 1:1000 (Cell Signaling Technology, Danvers, MA), cytokeratin 8, 1:500 (TROMA-1, DSHB, Iowa City, IA), adaptin beta, 1:500 (BD Biosciences, Franklin Lakes, NJ), NHE3, 1μg/ml (Alpha Diagnostic, San Antonio, TX), NaPi2b 1 μg/ml [Giral et al. 2009], spectrin, 1:1000 dilution of serum (gift from J. Morrow, Yale School of Medicine). CFTR, AME 4991 CFTR, 1:1000 [Kravtsov et al. 2012]
Supplementary Material
Acknowledgments
This work was supported by NIH grants DK 25387 (MSM), DK 077065 (N.A.A.) and NIH grant DK 34969 to the Digestive Diseases Research Core at Yale University.
References
- Altman D, Sweeney HL, Spudich JA. The mechanism of myosin VI translocation and its load-induced anchoring. Cell. 2004;116(5):737–49. doi: 10.1016/s0092-8674(04)00211-9. [DOI] [PubMed] [Google Scholar]
- Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic. 2007;8(8):998–1006. doi: 10.1111/j.1600-0854.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- Athman R, Louvard D, Robine S. The epithelial cell cytoskeleton and intracellular trafficking. III. How is villin involved in the actin cytoskeleton dynamics in intestinal cells? Am J Physiol Gastrointest Liver Physiol. 2002;283(3):G496–502. doi: 10.1152/ajpgi.00207.2002. [DOI] [PubMed] [Google Scholar]
- Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet. 1995;11(4):369–75. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- Benesh AE, Nambiar R, McConnell RE, Mao S, Tabb DL, Tyska MJ. Differential localization and dynamics of class I myosins in the enterocyte microvillus. Mol Biol Cell. 2010;21(6):970–8. doi: 10.1091/mbc.E09-07-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni JM, Conzelman KA, Adams RA, Kaiser DA, Pollard TD, Mooseker MS. Structural and immunological characterization of the myosin-like 110-kD subunit of the intestinal microvillar 110K-calmodulin complex: evidence for discrete myosin head and calmodulin-binding domains. J Cell Biol. 1988;107(5):1749–57. doi: 10.1083/jcb.107.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko S, Jacob SS, Horiuchi KY. Myosin I from mammalian smooth muscle is regulated by caldesmon-calmodulin. J Biol Chem. 1994;269(22):15803–7. [PubMed] [Google Scholar]
- Chen T, Hubbard A, Murtazina R, Price J, Yang J, Cha B, Sarker R, Donowitz M. Myosin VI mediates the movement of NHE3 down the microvillus in intestinal epithelial cells. J Cell Sci. 2014;127(Pt 16):3535–45. doi: 10.1242/jcs.149930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakos S. Recent advances in our understanding of 1,25-dihydroxyvitamin D(3) regulation of intestinal calcium absorption. Arch Biochem Biophys. 2012;523(1):73–6. doi: 10.1016/j.abb.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuan P, Spudich JA, Dunn AR. Robust mechanosensing and tension generation by myosin VI. J Mol Biol. 2011;405(1):105–12. doi: 10.1016/j.jmb.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelman KA, Mooseker MS. The 110-kD protein-calmodulin complex of the intestinal microvillus is an actin-activated MgATPase. J Cell Biol. 1987;105(1):313–24. doi: 10.1083/jcb.105.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley SW, Mooseker MS, Tyska MJ. Shaping the intestinal brush border. J Cell Biol. 2014;207(4):441–51. doi: 10.1083/jcb.201407015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen EM, Hansen GH. Lipid raft organization and function in brush borders of epithelial cells. Mol Membr Biol. 2006;23(1):71–9. doi: 10.1080/09687860500445604. [DOI] [PubMed] [Google Scholar]
- Dunn TA, Chen S, Faith DA, Hicks JL, Platz EA, Chen Y, Ewing CM, Sauvageot J, Isaacs WB, De Marzo AM, et al. A novel role of myosin VI in human prostate cancer. Am J Pathol. 2006;169(5):1843–54. doi: 10.2353/ajpath.2006.060316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrary E, Cohen-Tannoudji M, Pehau-Arnaudet G, Lapillonne A, Athman R, Ruiz T, Boulouha L, El Marjou F, Doye A, Fontaine JJ, et al. In vivo, villin is required for Ca(2+)-dependent F-actin disruption in intestinal brush borders. J Cell Biol. 1999;146(4):819–30. doi: 10.1083/jcb.146.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giral H, Caldas Y, Sutherland E, Wilson P, Breusegem S, Barry N, Blaine J, Jiang T, Wang XX, Levi M. Regulation of rat intestinal Na-dependent phosphate transporters by dietary phosphate. Am J Physiol Renal Physiol. 2009;297(5):F1466–75. doi: 10.1152/ajprenal.00279.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Yun CC. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol. 2010;2010:238080. doi: 10.1155/2010/238080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegan PS, Giral H, Levi M, Mooseker MS. Myosin VI is required for maintenance of brush border structure, composition, and membrane trafficking functions in the intestinal epithelial cell. Cytoskeleton. 2012;69:235–51. doi: 10.1002/cm.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzelman MB, Hasson T, Mooseker MS. Multiple unconventional myosin domains of the intestinal brush border cytoskeleton. J Cell Sci. 1994;107( Pt 12):3535–43. doi: 10.1242/jcs.107.12.3535. [DOI] [PubMed] [Google Scholar]
- Howe CL, Mooseker MS. Characterization of the 110-kdalton actin-calmodulin-, and membrane-binding protein from microvilli of intestinal epithelial cells. J Cell Biol. 1983;97(4):974–85. doi: 10.1083/jcb.97.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JH, Lee KN, Hwang CY, Kwon KS, You KH, Choi I. Tumor suppressor VDUP1 increases p27(kip1) stability by inhibiting JAB1. Cancer Res. 2005;65(11):4485–9. doi: 10.1158/0008-5472.CAN-04-2271. [DOI] [PubMed] [Google Scholar]
- Jung EJ, Liu G, Zhou W, Chen X. Myosin VI is a mediator of the p53-dependent cell survival pathway. Mol Cell Biol. 2006;26(6):2175–86. doi: 10.1128/MCB.26.6.2175-2186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, George SP. Regulation of cell structure and function by actin-binding proteins: villin's perspective. FEBS Lett. 2008;582(14):2128–39. doi: 10.1016/j.febslet.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesler P, Fuss IJ, Strober W. Experimental Models of Inflammatory Bowel Diseases. Cell Mol Gastroenterol Hepatol. 2015;1(2):154–170. doi: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravtsov DV, Caputo C, Collaco A, Hoekstra N, Egan ME, Mooseker MS, Ameen NA. Myosin Ia is required for CFTR brush border membrane trafficking and ion transport in the mouse small intestine. Traffic. 2012;13(8):1072–82. doi: 10.1111/j.1600-0854.2012.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzolini R, Dopeso H, Mateo-Lozano S, Chang W, Rodrigues P, Bazzocco S, Alazzouzi H, Landolfi S, Hernandez-Losa J, Andretta E, et al. Brush border myosin Ia has tumor suppressor activity in the intestine. Proc Natl Acad Sci U S A. 2012;109(5):1530–5. doi: 10.1073/pnas.1108411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell RE, Higginbotham JN, Shifrin DA, Jr, Tabb DL, Coffey RJ, Tyska MJ. The enterocyte microvillus is a vesicle-generating organelle. J Cell Biol. 2009;185(7):1285–98. doi: 10.1083/jcb.200902147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell RE, Tyska MJ. Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J Cell Biol. 2007;177(4):671–81. doi: 10.1083/jcb.200701144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AD, Rock RS, Rief M, Spudich JA, Mooseker MS, Cheney RE. Myosin-V is a processive actin-based motor. Nature. 1999;400(6744):590–3. doi: 10.1038/23072. [DOI] [PubMed] [Google Scholar]
- Mooseker MS. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol. 1985;1:209–41. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- Mooseker MS, Cheney RE. Unconventional myosins. Annu Rev Cell Dev Biol. 1995;11:633–75. doi: 10.1146/annurev.cb.11.110195.003221. [DOI] [PubMed] [Google Scholar]
- Mooseker MS, Pollard TD, Fujiwara K. Characterization and localization of myosin in the brush border of intestinal epithelial cells. J Cell Biol. 1978;79(2 Pt 1):444–53. doi: 10.1083/jcb.79.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker MS, Tilney LG. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol. 1975;67(3):725–43. doi: 10.1083/jcb.67.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar R, McConnell RE, Tyska MJ. Control of cell membrane tension by myosin-I. Proc Natl Acad Sci U S A. 2009;106(29):11972–7. doi: 10.1073/pnas.0901641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MD, Bement WM, Mooseker MS. An in vitro model for the analysis of intestinal brush border assembly. II. Changes in expression and localization of brush border proteins during cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci. 1993;105( Pt 2):461–72. doi: 10.1242/jcs.105.2.461. [DOI] [PubMed] [Google Scholar]
- Ramamurthy B, Cao W, De La Cruz EM, Mooseker MS. Plus-end directed myosins accelerate actin filament sliding by single-headed myosin VI. Cytoskeleton (Hoboken) 2012;69(1):59–69. doi: 10.1002/cm.21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama T, Carboni JM, Mooseker MS. Assembly of the intestinal brush border: appearance and redistribution of microvillar core proteins in developing chick enterocytes. J Cell Biol. 1987;105(1):335–44. doi: 10.1083/jcb.105.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowron JF, Bement WM, Mooseker MS. Human brush border myosin-I and myosin-Ic expression in human intestine and Caco-2BBe cells. Cell Motil Cytoskeleton. 1998;41(4):308–24. doi: 10.1002/(SICI)1097-0169(1998)41:4<308::AID-CM4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Su AI, Welsh JB, Sapinoso LM, Kern SG, Dimitrov P, Lapp H, Schultz PG, Powell SM, Moskaluk CA, Frierson HF, Jr, et al. Molecular classification of human carcinomas by use of gene expression signatures. Cancer Res. 2001;61(20):7388–93. [PubMed] [Google Scholar]
- Takahashi Y, Nagata T, Ishii Y, Ikarashi M, Ishikawa K, Asai S. Up-regulation of vitamin D3 up-regulated protein 1 gene in response to 5-fluorouracil in colon carcinoma SW620. Oncol Rep. 2002;9(1):75–9. [PubMed] [Google Scholar]
- Thoeni C, Cutz E. Pediatric and Perinatal Pathology: SY21-3 Recent advances in molecular pathology of microvillus inclusion disease (MVID) Pathology. 2014;46(Suppl 2):S34. [Google Scholar]
- Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell. 2005;16(5):2443–57. doi: 10.1091/mbc.E04-12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyska MJ, Mooseker MS. A role for myosin-1A in the localization of a brush border disaccharidase. J Cell Biol. 2004;165(3):395–405. doi: 10.1083/jcb.200310031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubelmann F, Chamaillard M, El-Marjou F, Simon A, Netter J, Vignjevic D, Nichols BL, Quezada-Calvillo R, Grandjean T, Louvard D, et al. Enterocyte loss of polarity and gut wound healing rely upon the F-actin-severing function of villin. Proc Natl Acad Sci U S A. 2013;110(15):E1380–9. doi: 10.1073/pnas.1218446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugde S, Ferrai C, Miluzio A, Hauben E, Marchisio PC, Crippa MP, Bussi M, Biffo S. Nuclear myosin VI enhances RNA polymerase II-dependent transcription. Mol Cell. 2006;23(5):749–55. doi: 10.1016/j.molcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Wang Y, George SP, Srinivasan K, Patnaik S, Khurana S. Actin reorganization as the molecular basis for the regulation of apoptosis in gastrointestinal epithelial cells. Cell Death Differ. 2012;19(9):1514–24. doi: 10.1038/cdd.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Srinivasan K, Siddiqui MR, George SP, Tomar A, Khurana S. A novel role for villin in intestinal epithelial cell survival and homeostasis. Journal of Biological Chemistry. 2008;283(14):9454–64. doi: 10.1074/jbc.M707962200. [DOI] [PubMed] [Google Scholar]
- Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, Hasson T, Carragher BO, Milligan RA, Sweeney HL. Myosin VI is an actin-based motor that moves backwards. Nature. 1999;401(6752):505–8. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Cheng W, Hung J, Montell D, Geisbrecht E, Rosen D, Liu J, Naora H. Lessons from border cell migration in the Drosophila ovary: A role for myosin VI in dissemination of human ovarian cancer. Proc Natl Acad Sci U S A. 2004;101(21):8144–9. doi: 10.1073/pnas.0400400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.