Abstract
Prostate cancer often manifests as morphologically distinct tumour foci and is frequently found adjacent to presumed precursor lesions such as high-grade prostatic intraepithelial neoplasia (HGPIN). While there is some evidence to suggest that these lesions can be related and exist on a pathological and morphological continuum, the precise clonal and temporal relationships between precursor lesions and invasive cancers within individual tumours remain undefined. Here, we used molecular genetic, cytogenetic, and histological analyses to delineate clonal, temporal, and spatial relationships between HGPIN and cancer lesions with distinct morphological and molecular features. First, while confirming the previous finding that a substantial fraction of HGPIN lesions associated with ERG-positive cancers share rearrangements and overexpression of ERG, we found that a significant subset of such HGPIN glands exhibit only partial positivity for ERG. This suggests that such ERG-positive HGPIN cells either rapidly invade to form adenocarcinoma or represent cancer cells that have partially invaded the ductal and acinar space in a retrograde manner. To clarify these possibilities, we used ERG expression status and TMPRSS2–ERG genomic breakpoints as markers of clonality, and PTEN deletion status to track temporal evolution of clonally related lesions. We confirmed that morphologically distinct HGPIN and nearby invasive cancer lesions are clonally related. Further, we found that a significant fraction of ERG-positive, PTEN-negative HGPIN and intraductal carcinoma (IDC-P) lesions are most likely clonally derived from adjacent PTEN-negative adenocarcinomas, indicating that such PTEN-negative HGPIN and IDC-P lesions arise from, rather than give rise to, the nearby invasive adenocarcinoma. These data suggest that invasive adenocarcinoma can morphologically mimic HGPIN through retrograde colonization of benign glands with cancer cells. Similar clonal relationships were also seen for intraductal carcinoma adjacent to invasive adenocarcinoma. These findings represent a potentially undervalued indicator of pre-existing invasive prostate cancer and have significant implications for prostate cancer diagnosis and risk stratification.
Keywords: prostate cancer, prostatic intraepithelial neoplasia, ductal spreading, clonality, ERG, PTEN
Introduction
Prostate cancer is characterized by significant intra- and inter-tumour heterogeneity at the genomic and morphological levels [1–10]. Deciphering the clonal and temporal relationships between precursor lesions and morphologically and genetically distinct invasive carcinomas represents a major challenge in prostate cancer molecular pathology. The generally accepted precursor to the majority of invasive adenocarcinomas of the prostate is high-grade prostatic intraepithelial neoplasia (HGPIN). HGPIN is defined by the presence of neoplastic-appearing cells present within pre-existing acini and ducts that are confined by a surrounding layer of basal cells [11,12]. Several lines of evidence have suggested that HGPIN may represent a precusor to invasive cancer. HGPIN is often found in close proximity to invasive adenocarcinomas and it shares a number of defining morphological features with invasive adenocarcinomas such as nuclear and nucleolar enlargement [11,12]. Furthermore, the presence of extensive HGPIN on prostate needle biopsy is associated with an elevated risk of cancer on subsequent biopsies [13–16]; invasive ‘microcarcinomas’ at times appear to arise from HGPIN lesions [17]; HGPIN and adenocarcinoma share a number of molecular features [11,18–20]; and HGPIN lesions share a number of somatic genetic and epigenetic alterations with invasive prostatic adenocarcinomas [4,6,11,21–27]. It is important to note, however, that most of the previous studies evaluating somatic DNA alterations in HGPIN and adjacent cancers used low-resolution and mostly fluorescence in situ hybridization (FISH)-based approaches, which although suggestive of a common clonal relationship between HGPIN and invasive carcinomas, did not allow a definitive evaluation of clonal ancestry. Furthermore, the small size of most HGPIN lesions, their frequent tight physical proximity to invasive cancer, and the lack of refined analytical tools have hampered more in-depth molecular analyses. Finally, unlike potential precancerous lesions present in other organs, the temporal and clonal relationship of HGPIN progression to adenocarcinoma has proven difficult to assess due to the virtual impossibility of serially sampling individual HGPIN lesions in an individual prostate.
Among the most common genomic alterations in prostate cancer are structural rearrangements involving TMPRSS2 and ERG, which can be found in ~50% of cases [28,29]. TMPRSS2–ERG rearrangements result in the overexpression of the oncogenic transcription factor ERG and represent an early event in prostate cancer progression. This notion is based on the observation that TMPRSS2–ERG rearrangements are generally pervasive within an individual tumour such that virtually all invasive tumour cells have the same cytogenetically defined rearrangement [28,30–33]. At a higher resolution, genomic rearrangement breakpoints between TMPRSS2 and ERG can be used as a rigorous marker of clonality because, despite the presence of regional rearrangement hotspots [34,35], every TMPRSS2–ERG breakpoint identified thus far is unique within each individual, and each clonally distinct tumour focus [2,34,36]. To date, however, no studies showing clonal breakpoints in TMPRSS2–ERG or other ETS fusion genes have been reported in human HGPIN lesions.
Another frequently observed alteration in prostate cancer genomes involves the loss of the tumour suppressor gene PTEN. The most common mechanism of PTEN loss involves deletion [2,26]. Importantly for the present study, a number of different groups have shown that PTEN loss generally occurs subsequent to ETS gene fusions in those cancers that harbour ETS gene fusions [2,7,36,37]; PTEN loss often occurs subclonally in a subset of cancer glands within a cancer focus, further supporting the notion that PTEN loss is a later event in tumour progression [2,7,36,37] associated with more aggressive disease [38–41]. As genomic elements that have been eliminated by homozygous deletion cannot easily be regained, differing PTEN loss status between morphologically distinct, but clonally related lesions can provide information on the temporal vector that underlies the clonal evolutionary relationships between each lesion.
Several groups have previously noted that HGPIN lesions in close proximity to ERG-positive tumours display a high rate of ERG rearrangements. Isolated HGPIN lesions located at a distance from any invasive lesions, however, rarely contain ERG rearrangements [32,33,42,43]. This pattern of adjacent versus distal alterations in HGPIN lesions with regard to primary adenocarcinoma foci has also been noted with regards to PTEN status [32,41,44]. These findings of common ERG rearrangements in HGPIN lesions occurring in the immediate vicinity of an invasive carcinoma and infrequent ERG rearrangements in HGPIN lesions away from carcinoma suggest two main, yet distinct possibilities. First, supported by preclinical evidence of increased invasion in ERG-positive cell line models, HGPIN cells that undergo a TMPRSS2–ERG rearrangement could be more prone to invade [45]; therefore, ERG-positive HGPIN is closely associated with ERG-positive carcinoma as a result of the strong tendency for ERG-positive HGPIN to invade. Second, established carcinoma cells can invade, in a retrograde manner, back into the acinar or ductal structures of normal glands and thereby form lesions that morphologically resemble HGPIN, but actually represent a distinct pattern of invasion (eg retrograde glandular colonization).
It is widely accepted among genitourinary pathologists that intraductal carcinoma (IDC-P), a lesion that is diagnosed based on the presence of highly atypical neoplastic cells in a cribriform or solid architecture that may show acinar/glandular expansion and is surrounded by basal cells, most likely represents invasion of established invasive adenocarcinoma into normal glandular structures [44,46–50]. A recent case report provided the first direct evidence for the close clonal relationship between IDC-P, adjacent carcinoma, and concurrent metastatic lesions [51]. However, direct high-resolution genetic evidence showing that morphologically apparent IDC-P represents spread from an adjacent carcinoma has not been provided in a larger study. Nonetheless, there has been an extensive discussion on intraductal spreading pattern which morphologically shows defining features of IDC-P [46,49,50,52]. Cohen et al proposed a model in which invasive carcinoma and IDC-P exist in a dynamic relationship. This would suggest that invasive carcinoma can invade the ductal and acinar space, and vice versa, IDC-P can form invasive lesions [50]. However, only scant literature indicates the possibility that what appears morphologically to be HGPIN can in some cases represent invasive carcinoma that has subsequently spread into benign acini or ducts. The concept of retrograde glandular colonization for HGPIN challenges the prevailing view that virtually all morphologically recognizable HGPIN lesions represent solely precancerous lesions and highlights the necessity for a detailed delineation of the clonal evolutionary relationships between HGPIN and adjacent invasive carcinoma.
Here, we studied the clonal and temporal relationships of HGPIN and co-occurring invasive cancer lesions by using an integrated approach using immunohistochemistry, FISH, and molecular genetic approaches. Our results suggest that the majority of PTEN-negative HGPIN and IDC-P lesions, when present in close proximity to invasive carcinoma, more likely represent cancer cells that have invaded adjacent normal ductal and acinar structures than de novo precursor lesions.
Materials and methods
Patient samples
This study was approved by the Johns Hopkins Medicine Institutional Review Board. Forty-eight ERG-positive cancers were selected for this study to map topographical relationships between HGPIN and adenocarcinoma lesions by ERG IHC from the index lesion of totally embedded radical prostatectomy specimens. In addition to these cases, in seven additional cases for which TMPRSS2–ERG rearrangement breakpoints were previously determined from the index lesion using DNA obtained from macrodissected fresh frozen tumour material, (34) individual areas from adjacent FFPE tissues containing normal epithelium, HGPIN, IDC-P or invasive adenocarcinoma were microdissected and analysed as described below. It should be noted that in all seven cases, the invasive lesions showed uniform ERG positivity by IHC. HGPIN was classified according to Bostwick and Qian [18]. In contrast to HGPIN, IDC-P was defined as lesions confined by a partial or intact basal cell lining filling expanded acini/ducts in either a dense cribriform or micropapillary pattern with marked nuclear atypia (eg six times normal) or comedonecrosis [48]. Given that there is a spectrum of morphologies ranging from cribriform PIN to IDC-P, subsequent authors have suggested the inclusion of similar cribriform expansive lesions surrounded by basal cells, but that have less nuclear atypia, as IDC-P if they are in close proximity to carcinoma and we have used this definition here [47,53]. Lesions that showed cribriform formations without duct/acinar expansion and without six times nuclear enlargement were still considered cribriform PIN. Topographical relationships were scored as described by Putzi et al [54] with regard to proximity to an index carcinoma lesion, ‘adjacent’ being defined as being within 100 µm; ‘near’ as being between 100 µm and 1mm away; and ‘far’ as being more than 1mm away. It should be noted that the topographical proximity was measured on a single representative section of the index tumour. Since no 3D reconstruction was attempted, the distance measurements do not capture complex spatial relationships between different lesions. Clinicopathological characteristics are summarized in Table 1.
Table 1.
Summary of the histological characteristics and ERG expression status of the cases studied
| No of cases | |
|---|---|
| Histological characteristics | |
| Adenocarcinoma | 48 |
| Gleason grade | |
| 3+3 | 18 |
| 3+4 | 16 |
| 4+3 | 12 |
| 4+4 | 2 |
| HGPIN present | 41 |
| HGPIN adjacent (<100 µm) | 31 |
| HGPIN near (100 µm–1 mm) | 19 |
| HGPIN far (>1 mm) | 11 |
| Intraductal carcinoma (IDC-P) | 9 |
| ERG expression status | |
| ERG expression in carcinoma | 48 |
| HGPIN ERG-positive | 37 |
| ERG in HGPIN adjacent | 24 |
| ERG in HGPIN near | 11 |
| ERG in HGPIN far | 10 |
| HGPIN partial ERG-positive | 36 |
| IDC-P ERG-positive | 5 |
Note that a given case can have HGPIN in different topographical locations; therefore, the total number of all PIN lesions is greater than the number of cases with PIN lesions.
IHC and FISH
Immunolabelling for ERG and PTEN was performed as previously described [33,40]. Dual colour immunohistochemical staining was performed using mouse monoclonal anti-ERG or rabbit monoclonal anti-PTEN antibodies (PTEN, clone D4.3, 1:50; Cell Signaling Technologies, Beverly, MA, USA; ERG; CM421C; 1:50; BioCare Medical, Concord, CA, USA) in combination with basal cell-specific anti-p63 and 34β12 (CK903) antibodies (p63; #NB100-691; 1:50; Novus Biologicals, Littleton, CO, USA; CK903, #ENZ-C34903; 1:50, Enzo Life Sciences, Farmingdale, NY, USA). Immuno-complexes were labelled in red for basal cell markers p63 and 34Be12/903 using alkaline phosphatase (AP) with Vector Red (Vector Labs, Burlingame, CA, USA) as the chromogen, and in brown using horseradish peroxidase (HRP) (PowerVision; Leica Microsystems, Bannockburn, IL, USA) with DAB for ERG and PTEN (Supplementary methods). Micrographs shown in the Supporting information were taken from slides co-labelled for PTEN, ERG, p63, and 34Be12/903 as described previously [44]. PTEN status and nuclear ERG status were scored based on established criteria [33,40]. Fluorescence in situ hybridization (FISH) for PTEN deletion was performed on adjacent sections using commercially available probes (PTEN del-TECT Four Color FISH Probe; CymoGen Dx, LLC, Irvine, CA, USA) and was evaluated as previously described [7].
LCM, DNA extraction, and PCR detection of rearrangements
Representative areas from indicated lesions and matched normal glands from each patient were microdissected using a PALM MicroLaser System and PALM membrane slides (Carl Zeiss, Germany) and DNA was extracted as previously described [3]. DNA samples were analysed by PCR for the presence of previously identified case specific TMPRSS2–ERG rearrangements using breakpoint-flanking primers. PCR products were analysed by Sanger sequencing [34] (Supplementary Table 2).
Results
In order to investigate the clonal relationships between invasive adenocarcinomas and nearby HGPIN lesions, we evaluated their spatial distribution and their ERG staining pattern in a group of 48 ERG-positive prostate cancers using ERG-specific immunohistochemistry (Table 1). As documented previously by us and others [7,33], all of these ERG-positive cases showed immunolabelling in all tumour cells using ERG-specific antibodies (Figure 1A). Of these cases containing ERG-positive tumours, 41/48 (85%) contained HGPIN, of which 31/41 were ‘adjacent’ (<100 µm) to the carcinoma, 19/41 were ‘near’ the carcinoma (100 µm to 1 mm), and 11/41 were ‘far’ (>1 mm) from the index cancer lesion (Table 1). HGPIN was positive for ERG in 37/41 cases with 24/37 ERG-positive lesions adjacent, 11/37 lesions near, and 10/37 lesions far from the carcinoma (Table 1 and Figure 1B; note that a given case can harbour adjacent, near, and far HGPIN lesions).
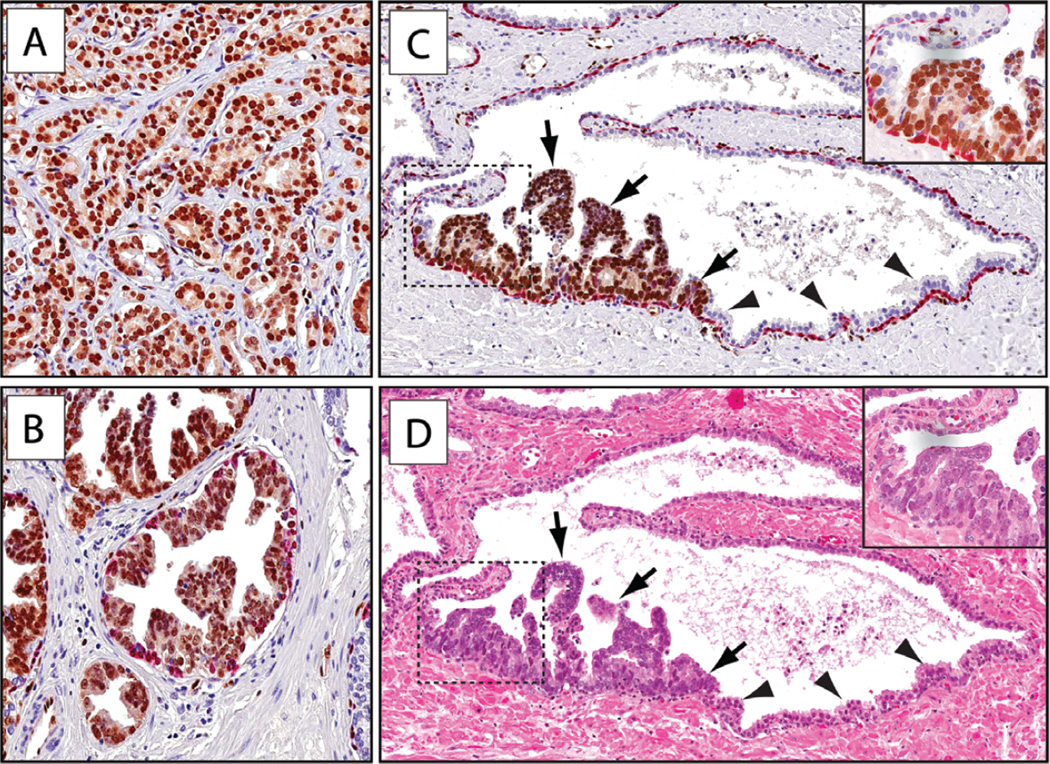
Figure 1.
ERG protein expression in carcinoma and HGPIN. Sections were subjected to immunohistochemical staining for ERG (brown) and basal cell-specific markers (p63/903, red) (original objective magnification 20×). (A) Representative micrograph of ERG-expressing tumour. (B) HGPIN (tufting type) lesion showing ERG expression. (C) Partial ERG-positive PIN lesion with ERG-expressing neoplastic cells (arrows) adjacent to normal prostatic epithelium (arrowheads). (D) H&E stain of adjacent section showing distinct HGPIN morphology of the ERG-positive cells.
In the majority of cases with HGPIN (36/37; 97%), we also observed glandular lesions with only partial involvement of ERG-positive HGPIN cells, distinguished by the presence of enlarged nuclei and distinct nucleoli and often with cytoplasmic basophilia characteristic of PIN. Such lesions occurred more frequently in close proximity to invasive carcinoma. In these cases, positive staining for ERG also occurred in the neoplastic-appearing cells, whereas the remaining benign-appearing cells within the acini had no detectable ERG expression (Figures 1C, 1D, and Supplementary Figure 1). Of note, neoplastic-appearing ERG-positive cells were also observed at times in atrophic glands (Supplementary Figure 2). For all of these lesions, the diagnosis of ‘HGPIN partially involving an individual gland’ was rendered. Overall, 36/48 (75%) cases showed partial ERG-positive glands. This phenotype showed no relationship to Gleason grade, and partially involved glands were found in both high-grade and low-grade lesions (Supplementary Figure 3).
ERG-positive HGPIN lesions, whether partially or fully involving glands that were in very close proximity to invasive carcinoma, suggest two main distinct temporal and topographical progression scenarios. In the first, these ERG-positive HGPIN-containing glands reflect de novo intraepithelial lesions, which at times have not yet fully replaced cells of the normal glandular structure (ie partially-positive HGPIN glands), but have nonetheless produced progeny cells that have already invaded into the surrounding stroma forming invasive adenocarcinoma. In the second scenario, ERG-positive cells with the appearance of HGPIN are derived from an established invasive adenocarcinoma that has invaded, in a retrograde manner, into the epithelial layer of a pre-existing normal acinus and/or duct.
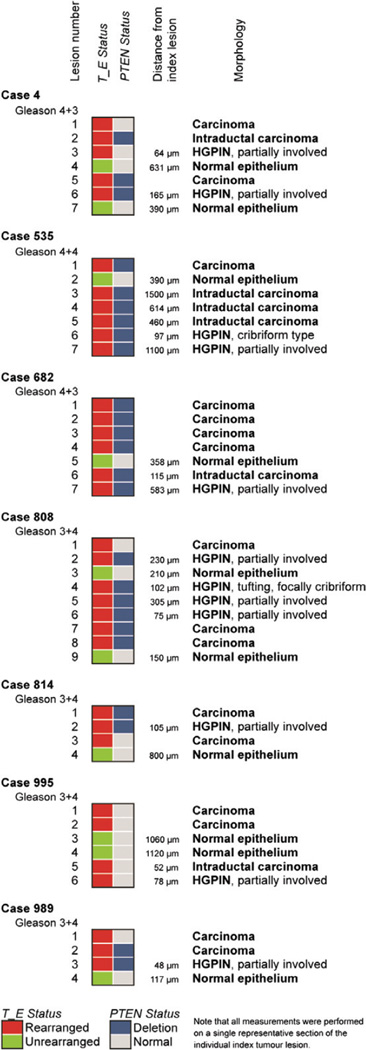
In order to distinguish between these possibilities, we performed high-resolution molecular pathology studies using the detection of TMPRSS2–ERG fusion genomic breakpoints by PCR as well as PTEN genomic loss detected by IHC and confirmed by FISH. We reasoned that if we found PTEN-deleted HGPIN lesions near a carcinoma in which all carcinoma and HGPIN cells showed the identical TMPRSS2–ERG rearrangement breakpoint, but only part of the invasive adenocarcinoma had PTEN deletion, then the HGPIN lesion would most likely represent retrograde glandular colonization of the PTEN-deleted invasive carcinoma, which itself arose after invasion and subsequent to TMPRSS2–ERG fusion. To formally test this, we made use of TMPRSS2–ERG breakpoint data at nucleotide resolution that were previously determined by our group (geBACS, Supplementary Figure 4) [34]. We identified seven independent cases for which nucleotide resolution TMPRSS2–ERG breakpoint information and FFPE material were available (Figure 3 and Supplementary Table 1). Guided by the immunohistochemistry for ERG, a total of 44 neoplastic lesions of different morphologies and adjacent normal glands were microdissected. Extracted DNA was amplified with rearrangement-specific primers and amplicons were sequence-verified (Figure 2 and Supplementary Figure 4). In 6/7 cases, ERG overexpression resulted from a fusion between TMPRSS2 intron 1 or 2 and ERG intron 3 [34]. A single case (case 989) harboured a fusion between a previously uncharacterized 5′ fusion partner, FAM177A1 (chromosome 14), and ERG, which was associated with ERG overexpression (Supplementary Figure 6).
Figure 3.
Summary of ERG and PTEN status in analysed cases.
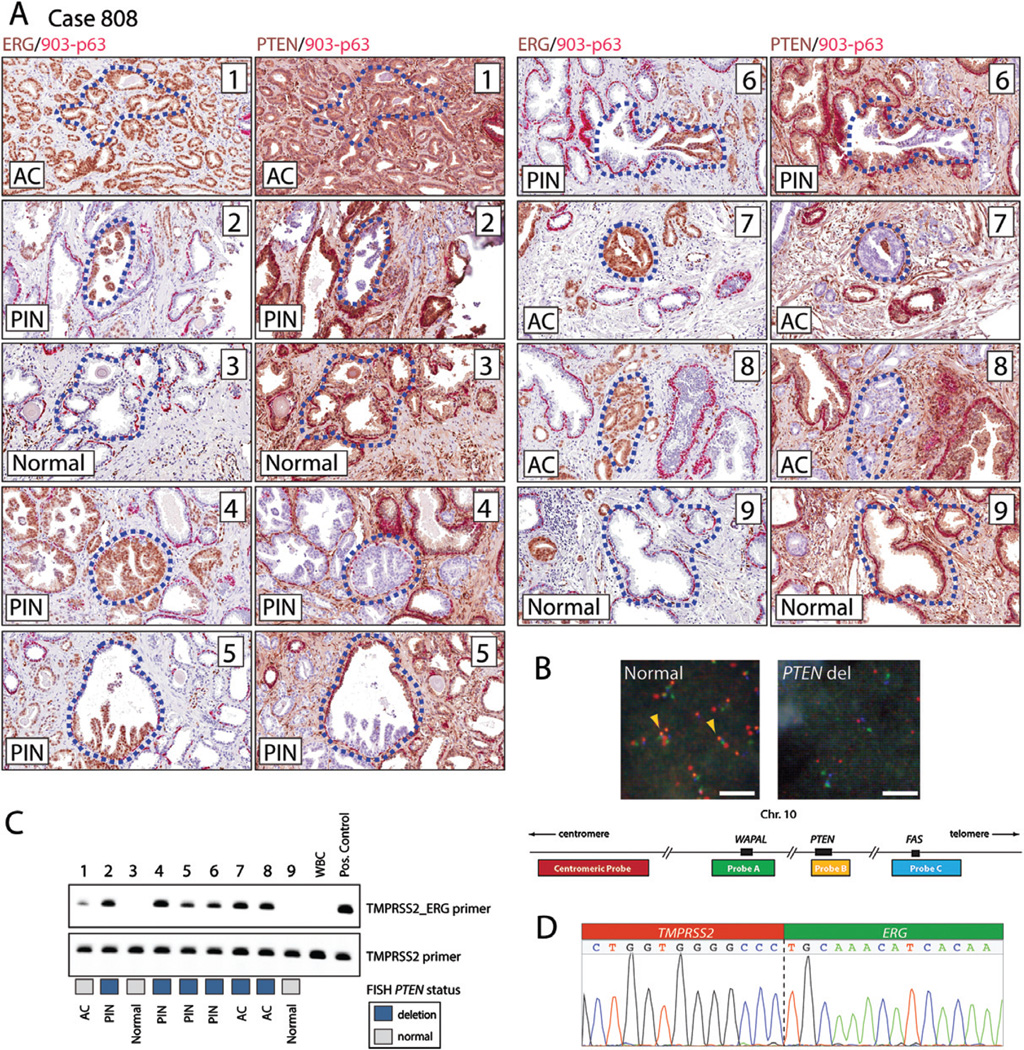
Figure 2.
Detailed analysis of representative case 808. Case 808 was immunohistochemically stained for ERG, PTEN, and basal cell markers. The locations of lesions that were microdissected and further analysed are indicated with dotted blue lines within each numbered lesion. (A) Slides were immunostained with a two-colour combination for ERG (brown) and basal cell markers (first and third columns of images; ERG in brown and a cocktail of 34BE12/ck903 and p63 in red), or PTEN and basal cell markers (second and fourth columns of images; PTEN in brown and 34BE12/ck903 and p63 in red). Individual lesions (1–9) are shown at 20× original objective magnification. The numbers in the upper right corner correspond to the lesion identifier. Lesions 1 and 8 are invasive adenocarcinomas. Lesion 7 represents cribriform adenocarcinoma. Lesions 2, 5, and 6 represent partial involvement by PIN. Lesion 4 represents PIN, tufting and focally cribriform pattern that does not expand the acinus. Lesions 3 and 9 contain normal prostate epithelium. The relative location and H&E-stained low- and high-magnification images of each lesion (1–9) are presented in Supplementary Figure 5. (B) Representative micrographs of cell nuclei with intact and homozygous deleted PTEN locus and schema of four colour FISH probes used to interrogate the PTEN locus. Note the homozygous loss of the PTEN probe (orange signal) in the right panel. (C) Gel image of PCR products amplified with either TMPRSS2–ERG rearrangement-specific or TMPRSS2 control genomic primers from DNA microdissected from individual lesions. PTEN FISH status is indicated in blue for homozygous loss and grey for no deletion. (D) Sequence chromatogram for the genomic rearrangement junction amplified from ERG-positive lesions. AC denotes adenocarcinoma; PIN denotes prostatic intraepithelial neoplasia.
In 7/7 analysed cases, all ERG-positive lesions within each case shared the same ERG breakpoint, indicating a common monoclonal origin (Figures 2, 3, and Supplementary Figures 6–11). Adjacent microdissected normal tissue or control white blood cell DNA (WBC) showed no evidence of TMPRSS2–ERG rearrangements (Figure 2 and Supplementary Figures 6–11) documenting the specificity of the assay. Notably, all microdissected partially ERG-positive HGPIN lesions, as well as stereotypical complete gland HGPIN lesions, showed the same genomic breakpoint as the adjacent index invasive carcinoma, establishing that HGPIN and partial ERG-positive HGPIN lesions share the same clonal origin as the invasive carcinoma lesions (Figures 2, 3, and Supplementary Figures 6–11). For example, these clonal relationships are evident in case 808 in lesions 2, 5, and 6, which represent partial positive lesions, and microdissected focus 4, which represents full gland HGPIN and cribriform HGPIN (Figure 2). Of note, in case 995 (Supplementary Figure 7), we observed complex genomic rearrangements which encompassed an intergenic TMPRSS2–ERG rearrangement and intragenic rearrangements within the TMPRSS2 gene [34]. Importantly, the genomic rearrangement breakpoints underlying these complex rearrangements were observed in all evaluated lesions of this case. This finding is consistent with the model of chromoplexy suggesting that a single event, occurring early during cancer progression, likely resulted in this genomic alteration [2,34]. In addition to HGPIN lesions, we found similar clonal relationships between invasive carcinoma lesions and adjacent IDC-P lesions (Supplementary Figures 7–9 and 11).
To assess PTEN deletion status, we used a previously validated immunohistochemical protocol as well as FISH. Homozygous loss of the tumour suppressor gene PTEN often occurs in a subclonal fashion, which allows delineation of temporal and spatial directionality of clonal evolutionary relationships between lesions [2,7,26,37,55]. We found regions of homozygous loss of PTEN and concomitant loss or marked decrease in PTEN protein expression in 6/7 cases in which we had nucleotide resolution ERG breakpoint data. As expected, in invasive carcinoma lesions, PTEN loss appeared more heterogeneous than ERG overexpression, as 4/6 ERG-positive cases (Figure 3, cases 4, 808, 814, and 989; Figure 4) showed PTEN loss in only a subset of tumour glands as opposed to ERG staining, which occurred in all tumour cells. We observed that in 4/4 cases with heterogeneous PTEN loss in the nearby invasive adenocarcinoma lesion, partial ERG-positive HGPIN, full gland ERG-positive HGPIN, and ERG-positive IDC-P lesions showed loss of PTEN (Figures 2 and 3). It is worth noting that, regardless of PTEN status, all lesions dissected from a given individual case harboured the identical TMPRSS2–ERG breakpoint, strongly supporting a common monoclonal origin and suggesting that the differences in PTEN status are most likely due to subclonal evolution and not to sampling of a collision tumour of phylogenetically diverse lesions. Based on these observations, the most likely model is that all neoplastic lesions in the analysed cases have the same clonal origin based on the fact that they share the identical TMPRSS2–ERG rearrangement breakpoint at the nucleotide level. Furthermore, since all of the invasive tumour cells harboured the TMPRSS2–ERG gene fusion and only a subset of the invasive tumour cells harboured PTEN loss (eg subclonal PTEN loss), the nearby lesions classified morphologically as HGPIN (full gland or partial gland) as well as IDC-P showing both TMPRSS2–ERG fusion and PTEN loss most likely arose after development of invasive carcinoma. Therefore, these cells likely do not represent a precancerous lesion but rather cancer cells that have invaded normal ductal and acinar structures (Figure 4).
Figure 4.
Proposed model of retrograde glandular colonization. All invasive adenocarcinoma cells are ERG-positive due to an early TMPRSS2–ERG fusion event, yet only part of the invasive adenocarcinoma shows PTEN loss and is therefore considered subclonal loss. In such an ERG-positive invasive adenocarcinoma with subclonal loss of PTEN, TMPRSS2–ERG genomic breakpoint analysis establishes the shared clonal origin of lesions. Subclonal PTEN loss can be used as a temporal vector. The observation that ERG-positive PTEN-negative cells populate normal acinar and ductal structures strongly suggests a retrograde spreading pattern of invasive carcinoma cells into benign glandular structures. PTEN-proficient normal prostate epithelial cells and adenocarcinoma are shown in brown. PTEN-deficient cells are drawn in grey. ERG overexpression is indicated by blue nuclei. Intact basal cells are highlighted in red.
Discussion
The spatial and temporal clonal relationship between different pre-neoplastic and invasive lesions in the prostate is an issue of great biological and clinical interest. Here, we present data suggesting that this tight association between ERG-positive HGPIN and ERG-positive adjacent invasive adenocarcinoma may arise from invasive adenocarcinoma cells colonizing normal glands and mimicking the histological appearance of HGPIN. In support of this notion, first, we found that HGPIN and nearby invasive adenocarcinoma lesions share identical ERG genomic breakpoints, providing very strong evidence of a common clonal origin. Second, we observed that HGPIN lesions in the vicinity of invasive adenocarcinomas showing homogeneous ERG rearrangements often shared loss of PTEN with part of the invasive carcinoma. This subclonal loss of PTEN in part of the invasive carcinoma as well as adjacent HGPIN strongly suggests that at least a subset of lesions that would be classified by histomorphological criteria as HGPIN do not represent precursors, but rather an intraductal and intra-acinar colonization and spreading of pre-existing invasive carcinoma (Figure 4).
This notion is indirectly supported by other findings: for example, a number of studies demonstrating that HGPIN lesions that are isolated, or distant from cancer lesions, show genomic alterations such as ERG rearrangements and PTEN copy number loss with much lower frequency than those in close proximity to invasive carcinomas [32,43,56]. Furthermore, we show that partially ERG-positive HGPIN lesions can be found frequently adjacent to invasive cancer lesions irrespective of tumour grade. Such lesions show identical ERG breakpoints with all adjacent invasive lesions, establishing in an unequivocal manner a direct clonal relationship. In addition, it is important to note that many ERG-positive HGPIN lesions often occurred as only a relatively small group of cells within an otherwise normal-appearing gland. Since this distribution pattern of ERG-positive cells is highly prevalent in lesions adjacent to invasive cancer (Table 1), and the majority of such lesions share the same ERG breakpoint, it is unlikely that such lesions represent de novo HGPIN lesions.
The misclassification of lesions representing invasive disease as pre-invasive has the potential for erroneous clinical decision-making, since it is possible that invasive carcinoma that is mimicking a precursor lesion could even represent an aggressive cancer. Although the diagnostic utility remains controversial, two recent studies provide evidence that cases with ERG-positive HGPIN are more likely to show invasive cancer on subsequent biopsies compared with cases that show no ERG expression in HGPIN [15,57]. Most notably, Park et al showed that the rate of cancer diagnosis in a subsequent biopsy is increased from 35% in patients with ERG-negative HGPIN to 53% in patients with ERG-positive HGPIN [15]. Our present findings suggest that a significant fraction of the ERG-positive HGPIN cases likely represent ductal/acinar spreading of an adjacent, but inadequately sampled, invasive carcinoma. This could explain the higher rates of cancer on repeat biopsy for ERG-positive HGPIN lesions. In our previous studies, and those of others, the rates of PTEN loss in isolated HGPIN lesions were very low [26,58], while those in IDC-P were very high [44,58]. In the present study, the HGPIN and partially involved HGPIN lesion that were in close proximity to invasive carcinoma showed high rates of PTEN loss. Therefore, it is important for additional studies to address whether IHC staining or FISH assays for ERG and PTEN could be helpful in determining which patients with isolated HGPIN on biopsy may require more intensive clinical follow-up.
There is still an ongoing evolution among pathologists about the precise morphological criteria and clinical relevance of intraepithelial cribriform lesions in the prostate [48–50,53]. It is important to note that the defining diagnostic criteria were established based on histomorphological evidence only. IDC-P, for instance, has been suggested as a defined morphological entity that is associated with worse prognosis [48–50]. More recent cytogenetic and IHC studies suggested a clonal relationship with adjacent invasive prostate cancer based on ERG [42,59]. In the present study, a subset of cases for which clonality was addressed using TMPRSS2–ERG breakpoint analysis contained IDC-P. The breakpoints in such cases were identical in invasive carcinoma and IDC-P, providing strong evidence for the shared clonal origin of these lesions (Figure 3). Furthermore, the majority of IDC-P lesions evaluated in this study also exhibited PTEN loss similar to the adjacent adenocarcinoma that showed subclonal PTEN loss, suggesting again that the IDC-P occurred subsequent to invasive carcinoma development. Retrograde spread of acinar carcinoma into ducts is widely accepted among pathologists to occur in IDC-P [44,50] – this study provides the first definitive evidence for this notion.
The present study has several limitations. Due to the small size of lesions and the lack of large-scale sequencing data, the clonal inferences were restricted to the assessment of only two index genomic alterations (ERG and PTEN). Despite offering a first opportunity to decipher both direct clonal and temporal evolutionary relationships, the restriction to two genomic markers did not allow a detailed phylogenetic analysis. Therefore, important questions about additional subclonal evolution and co-occurring genomic and epigenetic alterations could not be addressed. Future studies using a larger panel of genomic index alterations and genome-wide analytical tools will be helpful to validate and extend our findings. Furthermore, the use of ERG and PTEN alterations as clonal markers might also represent a bias. It therefore remains to be shown if the observed phenotype is strictly associated with ERG-positive cancers or if intraductal spreading mimicking HGPIN is a more general phenomenon of invasive cancers of other molecular subtypes. One possible reason for such a source of potential bias comes from preclinical studies in cell line models suggesting that ERG overexpression induces transcriptional programmes associated with cell invasion and produces an invasive phenotype along with disruption of 3D glandular structures in 3D in vitro model systems [45,60]. It is therefore possible that ERG-positive prostate cancers may be particularly prone to intra-acinar spread and retrograde glandular colonization. Finally, we focused on lesions that were in close proximity to cancer. It will be interesting in subsequent studies to determine how often lesions completely separate from cancer that morphologically resemble HGPIN might actually represent invasive carcinoma.
In conclusion, this paper provides the first evidence that a subset of HGPIN lesions might represent cancer cells that invade the ductal and acinar structures of normal prostate glands (Figure 4). This finding might explain previous observations suggesting that patients with ERG-positive HGPIN lesions more often show carcinoma on repeat biopsy, and add to our understanding of the order of molecular and morphological events and complex clonal relationships that can occur during the development and progression of primary prostate cancers. These findings have important implications for current practices in the biopsy-based diagnosis and for the optimization of histological risk stratification of prostate cancer.
Supplementary Material
Acknowledgments
We thank the members of the Johns Hopkins Urological Specimen Repository and Database, supported in part by NIH Prostate SPORE P50CA58236, The Department of Defense Prostate Cancer Biospecimen Network (PCBN). We would also like to thank members of the Oncology Tissue Services Center at the SKCCC (supported in part by NCI P30CA006973) for help with tissue samples and Dr Jonathan I Epstein and Dr Alan K Meeker for insightful comments on the manuscript. We also want to thank Peter Hartmayer from CymogenDX (Irvine, CA, USA) for providing PTEN FISH probe reagents and Lillian Dasko-Vincent from the SKCCC Cell Imaging Core Facility for technical support. This study received funding support from the National Institutes of Health/National Cancer Institute grants P50CA058236, R01CA070196, R01CA183965, and P30CA006973; The DOD W81XWH-10-2-0056; The Prostate Cancer Foundation research awards (to SY and WGN); The Prostate Cancer Foundation Young Investigator Award (to MCH); and The Patrick C Walsh Prostate Cancer Fund (to AMD).
Footnotes
Author contribution statement
MCH, CW, SY, WGN, and AMD conceived the study. MCH, CW, AV, DME, HT, and JH performed experiments. MCH, BG, BG, IK, TLL, and AMD performed histopathological evaluations. HF, WBI, and AMD provided tissue specimens. MX, MCH, and AMD conceived and executed illustrations. MCH, CW, SY, and AMD analysed all data and wrote the paper. All authors read and approved the final version of the manuscript.
SUPPORTING INFORMATION ON THE INTERNET
The following supporting information may be found in the online version of this article:
Supplementary methods. Immunohistochemistry.
Figure S1. Partial ERG-positive lesions show morphological similarities to HGPIN and can harbour loss of PTEN.
Figure S2. Partial ERG-positive PIN cells within atrophy lesions.
Figure S3. Partial ERG-positive PIN can be found adjacent to invasive carcinoma irrespective of tumour grade.
Figure S4. Summary of the analysis pipeline used in this study.
Figure S5. Haematoxylin and eosin stain of tissue used in case 808.
Figure S6. Summary of findings in case 989.
Figure S7. Summary of findings in case 995.
Figure S8. Summary of findings in case 535.
Figure S9. Summary of findings in case 682.
Figure S10. Summary of findings in case 814.
Figure S11. Summary of findings in case 4.
Table S1. Clinicopathological information of samples used in the microdissection study.
Table S2. Genomic coordinates of breakpoints and primers used in the study.
References
- 1.Lindberg J, Klevebring D, Liu W, et al. Exome sequencing of prostate cancer supports the hypothesis of independent tumour origins. Eur Urol. 2013;63:347–353. doi: 10.1016/j.eururo.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 2.Baca SC, Prandi D, Lawrence MS, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153:666–677. doi: 10.1016/j.cell.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest. 2013;123:4918–4922. doi: 10.1172/JCI70354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostwick DG, Shan A, Qian J. Matched foci of prostate carcinoma. Cancer. 1998;83:1995–2002. doi: 10.1002/(sici)1097-0142(19981101)83:9<1995::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Clark J, Attard G, Jhavar S. Prostate. Oncogene. 2007;27:1993–2003. doi: 10.1038/sj.onc.1210843. [DOI] [PubMed] [Google Scholar]
- 6.Brocks D, Assenov Y, Minner S, et al. Intratumor DNA methylation heterogeneity reflects clonal evolution in aggressive prostate cancer. Cell Rep. 2014;8:798–806. doi: 10.1016/j.celrep.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 7.Gumuskaya B, Gurel B, Fedor H, et al. Assessing the order of critical alterations in prostate cancer development and progression by IHC: further evidence that PTEN loss occurs subsequent to ERG gene fusion. Prostate Cancer Prostatic Dis. 2013;16:209–215. doi: 10.1038/pcan.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian J, Bostwick DG, Takahashi S, et al. Chromosomal anomalies in prostatic intraepithelial neoplasia and carcinoma detected by fluorescence in situ hybridization. Cancer Res. 1995;55:5408–5414. [PubMed] [Google Scholar]
- 9.Ruijter ET, Miller GJ, van de Kaa CA, et al. Molecular analysis of multifocal prostate cancer lesions. J Pathol. 1999;188:271–277. doi: 10.1002/(SICI)1096-9896(199907)188:3<271::AID-PATH359>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Cooper CS, Eeles R, Wedge DC, et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nature Genet. 2015;47:367–372. doi: 10.1038/ng.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bostwick DG, Cheng L. Precursors of prostate cancer. Histopathology. 2011;60:4–27. doi: 10.1111/j.1365-2559.2011.04007.x. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JI. Precursor lesions to prostatic adenocarcinoma. Virchows Arch. 2008;454:1–16. doi: 10.1007/s00428-008-0707-5. [DOI] [PubMed] [Google Scholar]
- 13.Lee MC, Moussa AS, Yu C, et al. Multifocal high grade prostatic intraepithelial neoplasia is a risk factor for subsequent prostate cancer. J Urol. 2010;184:1958–1962. doi: 10.1016/j.juro.2010.06.137. [DOI] [PubMed] [Google Scholar]
- 14.Netto GJ, Epstein JI. Widespread high-grade prostatic intraepithelial neoplasia on prostatic needle biopsy: a significant likelihood of subsequently diagnosed adenocarcinoma. Am J Surg Pathol. 2006;30:1184–1188. doi: 10.1097/01.pas.0000213324.97294.54. [DOI] [PubMed] [Google Scholar]
- 15.Park K, Dalton JT, Narayanan R, et al. TMPRSS2:ERG gene fusion predicts subsequent detection of prostate cancer in patients with high-grade prostatic intraepithelial neoplasia. J Clin Oncol. 2014;32:206–211. doi: 10.1200/JCO.2013.49.8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roscigno M, Scattoni V, Freschi M, et al. Diagnosis of isolated high-grade prostatic intra-epithelial neoplasia: proposal of a nomogram for the prediction of cancer detection at saturation re-biopsy. BJU Int. 2012;109:1329–1334. doi: 10.1111/j.1464-410X.2011.10532.x. [DOI] [PubMed] [Google Scholar]
- 17.McNeal JE, Villers A, Redwine EA, et al. Microcarcinoma in the prostate: its association with duct–acinar dysplasia. Hum Pathol. 1991;22:644–652. doi: 10.1016/0046-8177(91)90286-x. [DOI] [PubMed] [Google Scholar]
- 18.Bostwick DG, Qian J. High-grade prostatic intraepithelial neoplasia. Mod Pathol. 2004;17:360–379. doi: 10.1038/modpathol.3800053. [DOI] [PubMed] [Google Scholar]
- 19.Magi-Galluzzi C, Xu X, Hlatky L, et al. Heterogeneity of androgen receptor content in advanced prostate cancer. Mod Pathol. 1997;10:839–845. [PubMed] [Google Scholar]
- 20.Sakr WA, Partin AW. Histological markers of risk and the role of high-grade prostatic intraepithelial neoplasia. Urology. 2001;57(Suppl 1):115–120. doi: 10.1016/s0090-4295(00)00953-5. [DOI] [PubMed] [Google Scholar]
- 21.Bostwick DG. Prospective origins of prostate carcinoma. Prostatic intraepithelial neoplasia and atypical adenomatous hyperplasia. Cancer. 1996;78:330–336. doi: 10.1002/(SICI)1097-0142(19960715)78:2<330::AID-CNCR22>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 22.Meeker AK, Hicks JL, Platz EA, et al. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- 23.Qian J, Jenkins RB, Bostwick DG. Detection of chromosomal anomalies and c-myc gene amplification in the cribriform pattern of prostatic intraepithelial neoplasia and carcinoma by fluorescence in situ hybridization. Mod Pathol. 1997;10:1113–1119. [PubMed] [Google Scholar]
- 24.Brooks JD, Weinstein M, Lin X, et al. CG island methylation changes near the GSTP1 gene in prostatic intraepithelial neoplasia. Cancer Epidemiol Biomarkers Prev. 1998;7:531–536. [PubMed] [Google Scholar]
- 25.Nakayama M, Bennett CJ, Hicks JL, et al. Hypermethylation of the human glutathione S-transferase-π gene (GSTP1) CpG island is present in a subset of proliferative inflammatory atrophy lesions but not in normal or hyperplastic epithelium of the prostate: a detailed study using laser-capture microdissection. Am J Pathol. 2003;163:923–933. doi: 10.1016/s0002-9440(10)63452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bismar TA, Yoshimoto M, Vollmer RT, et al. PTEN genomic deletion is an early event associated with ERG gene rearrangements in prostate cancer. BJU Int. 2011;107:477–485. doi: 10.1111/j.1464-410X.2010.09470.x. [DOI] [PubMed] [Google Scholar]
- 27.Vukovic B, Park PC, Al-Maghrabi J, et al. Evidence of multifocality of telomere erosion in high-grade prostatic intraepithelial neoplasia (HPIN) and concurrent carcinoma. Oncogene. 2003;22:1978–1987. doi: 10.1038/sj.onc.1206227. [DOI] [PubMed] [Google Scholar]
- 28.Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29:3659–3668. doi: 10.1200/JCO.2011.35.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomlins SA, Bjartell A, Chinnaiyan AM, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol. 2009;56:275–286. doi: 10.1016/j.eururo.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 30.Mosquera J-M, Perner S, Genega EM, et al. Characterization of TMPRSS2–ERG fusion high-grade prostatic intraepithelial neoplasia and potential clinical implications. Clin Cancer Res. 2008;14:3380–3385. doi: 10.1158/1078-0432.CCR-07-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerveira N, Ribeiro FR, Peixoto A, et al. TMPRSS2–ERG gene fusion causing ERG overexpression precedes chromosome copy number changes in prostate carcinomas and paired HGPIN lesions. Neoplasia. 2006;8:826–832. doi: 10.1593/neo.06427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han B, Mehra R, Lonigro RJ, et al. Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol. 2009;22:1083–1093. doi: 10.1038/modpathol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furusato B, Tan S-H, Young D, et al. ERG oncoprotein expression in prostate cancer: clonal progression of ERG-positive tumor cells and potential for ERG-based stratification. Prostate Cancer Prostatic Dis. 2010;13:228–237. doi: 10.1038/pcan.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weier C, Haffner MC, Mosbruger T, et al. Nucleotide resolution analysis of TMPRSS2 and ERG rearrangements in prostate cancer. J Pathol. 2013;230:174–183. doi: 10.1002/path.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haffner MC, Aryee MJ, Toubaji A, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nature Genet. 2010;42:668–675. doi: 10.1038/ng.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowalsky AG, Ye H, Bubley GJ, et al. Clonal progression of prostate cancers from Gleason grade 3 to grade 4. Cancer Res. 2013;73:1050–1055. doi: 10.1158/0008-5472.CAN-12-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krohn A, Freudenthaler F, Harasimowicz S, et al. Heterogeneity and chronology of PTEN deletion and ERG fusion in prostate cancer. Mod Pathol. 2014;27:1612–1620. doi: 10.1038/modpathol.2014.70. [DOI] [PubMed] [Google Scholar]
- 38.Chaux A, Peskoe SB, Gonzalez-Roibon N, et al. Loss of PTEN expression is associated with increased risk of recurrence after prostatectomy for clinically localized prostate cancer. Mod Pathol. 2012;25:1543–1549. doi: 10.1038/modpathol.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonarakis ES, Keizman D, Zhang Z, et al. An immunohistochemical signature comprising PTEN, MYC, and Ki67 predicts progression in prostate cancer patients receiving adjuvant docetaxel after prostatectomy. Cancer. 2012;118:6063–6071. doi: 10.1002/cncr.27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lotan TL, Gurel B, Sutcliffe S, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res. 2011;17:6563–6573. doi: 10.1158/1078-0432.CCR-11-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshimoto M, Joshua AM, Cunha IW, et al. Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod Pathol. 2008;21:1451–1460. doi: 10.1038/modpathol.2008.96. [DOI] [PubMed] [Google Scholar]
- 42.Han B, Suleman K, Wang L, et al. ETS gene aberrations in atypical cribriform lesions of the prostate: implications for the distinction between intraductal carcinoma of the prostate and cribriform high-grade prostatic intraepithelial neoplasia. Am J Surg Pathol. 2010;34:478–485. doi: 10.1097/PAS.0b013e3181d6827b. [DOI] [PubMed] [Google Scholar]
- 43.Clark J, Attard G, Jhavar S, et al. Complex patterns of ETS gene alteration arise during cancer development in the human prostate. Oncogene. 2008;27:1993–2003. doi: 10.1038/sj.onc.1210843. [DOI] [PubMed] [Google Scholar]
- 44.Lotan TL, Gumuskaya B, Rahimi H, et al. Cytoplasmic PTEN protein loss distinguishes intraductal carcinoma of the prostate from high-grade prostatic intraepithelial neoplasia. Mod Pathol. 2013;26:587–603. doi: 10.1038/modpathol.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomlins SA, Laxman B, Varambally S, et al. Role of the TMPRSS2–ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–179. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNeal JE, Yemoto CE. Spread of adenocarcinoma within prostatic ducts and acini. Morphologic and clinical correlations. Am J Surg Pathol. 1996;20:802–814. doi: 10.1097/00000478-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Shah RB, Zhou M. Atypical cribriform lesions of the prostate: clinical significance, differential diagnosis and current concept of intraductal carcinoma of the prostate. Adv Anat Pathol. 2012;19:270–278. doi: 10.1097/PAP.0b013e31825c6c0e. [DOI] [PubMed] [Google Scholar]
- 48.Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: histologic features and clinical significance. Mod Pathol. 2006;19:1528–1535. doi: 10.1038/modpathol.3800702. [DOI] [PubMed] [Google Scholar]
- 49.Bonkhoff H, Wheeler TM, van der Kwast TH, et al. Intraductal carcinoma of the prostate: precursor or aggressive phenotype of prostate cancer? Prostate. 2012;73:442–448. doi: 10.1002/pros.22579. [DOI] [PubMed] [Google Scholar]
- 50.Cohen RJ, Wheeler TM, Bonkhoff H, et al. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch Pathol Lab Med. 2007;131:1103–1109. doi: 10.5858/2007-131-1103-APOTIH. [DOI] [PubMed] [Google Scholar]
- 51.Lindberg J, Kristiansen A, Wiklund P, et al. Tracking the origin of metastatic prostate cancer. Eur Urol. 2015;67:819–822. doi: 10.1016/j.eururo.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Kovi J, Jackson MA, Heshmat MY. Ductal spread in prostatic carcinoma. Cancer. 1985;56:1566–1573. doi: 10.1002/1097-0142(19851001)56:7<1566::aid-cncr2820560717>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 53.Shah RB, Magi-Galluzzi C, Han B, et al. Atypical cribriform lesions of the prostate: relationship to prostatic carcinoma and implication for diagnosis in prostate biopsies. Am J Surg Pathol. 2010;34:470–477. doi: 10.1097/PAS.0b013e3181cfc44b. [DOI] [PubMed] [Google Scholar]
- 54.Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology. 2000;56:828–832. doi: 10.1016/s0090-4295(00)00776-7. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimoto M, Ding K, Sweet JM, et al. PTEN losses exhibit heterogeneity in multifocal prostatic adenocarcinoma and are associated with higher Gleason grade. Mod Pathol. 2013;26:435–447. doi: 10.1038/modpathol.2012.162. [DOI] [PubMed] [Google Scholar]
- 56.Furusato B, Gao C-L, Ravindranath L, et al. Mapping of TMPRSS2–ERG fusions in the context of multi-focal prostate cancer. Mod Pathol. 2008;21:67–75. doi: 10.1038/modpathol.3800981. [DOI] [PubMed] [Google Scholar]
- 57.Gao X, Li LY, Zhou FJ, et al. ERG rearrangement for predicting subsequent cancer diagnosis in high-grade prostatic intraepithelial neoplasia and lymph node metastasis. Clin Cancer Res. 2012;18:4163–4172. doi: 10.1158/1078-0432.CCR-11-2449. [DOI] [PubMed] [Google Scholar]
- 58.Morais CL, Han JS, Gordetsky J, et al. Utility of PTEN and ERG immunostaining for distinguishing high-grade PIN from intraductal carcinoma of the prostate on needle biopsy. Am J Surg Pathol. 2015;39:169–178. doi: 10.1097/PAS.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider TM, Osunkoya AO. ERG expression in intraductal carcinoma of the prostate: comparison with adjacent invasive prostatic adenocarcinoma. Mod Pathol. 2014;27:1174–1178. doi: 10.1038/modpathol.2013.248. [DOI] [PubMed] [Google Scholar]
- 60.Becker-Santos DD, Guo Y, Ghaffari M, et al. Integrin-linked kinase as a target for ERG-mediated invasive properties in prostate cancer models. Carcinogenesis. 2012;33:2558–2567. doi: 10.1093/carcin/bgs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.