Abstract
Chronic inflammation is a risk factor for colorectal cancer. The MAPK-activated protein kinase 2 (MK2) pathway controls multiple cellular processes including p38-dependent inflammation. This is the first study to investigate the role of MK2 in development of colitis-associated colon cancer (CAC) using the AOM/DSS model. Herein, we demonstrate that MK2−/− mice are highly resistant to neoplasm development when exposed to AOM/DSS, while wild type C57BL/6 develop multiple neoplasms with the same treatment. MK2-specific cytokines IL-1, IL-6 and TNF-α were substantially decreased in AOM/DSS treated MK2−/− mouse colon tissues compared to wild type mice which coincided with a marked decrease in macrophage influx. Restoring MK2-competent macrophages by injecting wild type (WT) bone marrow derived macrophages into MK2−/− mice led to partial restoration of inflammatory cytokine production with AOM/DSS treatment; however, was not sufficient to induce neoplasm development. Our results indicate that MK2 functions as an inflammatory regulator to promote colonic neoplasm development and may be a potential target for CAC.
Keywords: MK2, Colorectal Cancer, IL-1, IL-6, TNF-α, macrophages
Introduction
Colorectal cancer will kill approximately 50,000 people every year in the US.(1) Patients with chronic colonic inflammation develop colorectal cancer (CRC) at a rate 2–6 times above normal and CAC is more difficult to treat than other CRCs.(2) Although the mechanisms of inflammation promoting cancer development are not fully understood, tissue damage and pro-survival mechanisms likely contribute to the growth of colon cancer. The protein kinase p38MAPK pathway has known pathogenic contributions to inflammatory bowel disease.(3) However, p38MAPK inhibition has resulted in poor outcomes; side effects of treatment can be severe and affect multiple organ systems, such as infection, dangerous increases in liver enzymes, skin disorders, in humans. (4;5) In animal models utilizing p38 inhibitors, gastrointestinal toxicity, destruction of lymphoid tissues, and promotion of cardiac plaques have been observed. (6;7)
MK2 is downstream of p38 MAPK and stabilizes the mRNAs of IL-1, IL-6, and TNF-α. Due to its stringent regulation of rather few genes compared to p38, it may be a better target. In LPS induction of inflammation in MK2−/− mice, IL-1, IL-6, and TNF-α were greatly reduced, (8) indicating a major role of this pathway in production of these cytokines. Additionally, these cytokines have been implicated in CAC development.(9;10) This is likely due to recruitment of immune cells to the site of inflammation and promotion of cell survival and proliferation.(11) MK2 is constitutively expressed at steady-state in multiple cell-types, both hematopoietic and non-hematopoietic origin. Recent evidence shows that intestinal macrophages maintain intestinal homeostasis, but are also major contributors to chronic inflammatory conditions in the colon.(12) Given this plasticity in function, it is crucial to understand the role of macrophages in CAC development. This functional plasticity may depend on MK2 activation and subsequent conditioning of the microenvironment to an inflammatory state. Pro-survival and pro-growth signals driven by inflammatory cytokine production from macrophages may be at the apex of inflammation and cancer development.
In this study, we set out to uncover the role of MK2 in CRC development as well as understand MK2 signaling in macrophages. Herein, we demonstrate MK2−/− mice were resistant to neoplasm development in the AOM/DSS CAC model. The complete loss of MK2 led to stunted production of cytokines and reduced macrophage infiltration into the colon. Transfer of MK2-proficient macrophages into MK2−/− mice ignited an inflammatory response, but was insufficient to restore tumorigenesis. Taken together, these results highlight the importance of MK2 in the inflammatory response and CAC development and highlight the importance of MK2 in multiple cell types is required to establish tumor development.
Materials and Methods
Mice
C57Bl/6 mice from Harlan Laboratories and the MK2−/−tm1Mgl (8) mouse strain were bred under pathogen free conditions. Animal procedures were approved by the UNM IACUC. Azoxymethane (AOM), (Sigma Aldrich, St. Louis, MO) was injected IP into 6–8 week old female mice at 12.5mg/kg. Dextran Sodium Sulfate (DSS), (MP Biomedicals, MW 36,000–50,000) was added to drinking water at 2.5% at days 5 and 26 and at 2.0% at day 47, 2.0% for 5 days sacrificed at day 80. Control mice received PBS IP and no DSS in water.
Mouse Colon Supernatants
8 mg (± 0.5 mg) of cleaned colon tissues were incubated in complete RPMI with antibiotics for 12 hours. Supernatants were analyzed for cytokines by Luminex bead array (Millipore, Billerica, MA) according to manufacturer’s instructions.
Flow Cytometry
Mouse colon tissues were treated with collagenase (I, II, and IV, Sigma Aldrich) and dispersed using the gentleMACs tissue dissociator (Miltenyi Biotech, Cologne, Germany). Cell suspensions were incubated overnight in media before staining for flow cytometry according to standard Biolegend protocols (Biolegend, San Diego, CA). Macrophages were stained with anti-F4/80-PE or FITC (Biolegend, BM8), anti-CD11b-APC or PE (eBioscience M1/70), anti-IL-1α-PE (eBioscience, ALF-161), anti-IL-1β-APC (eBioscience NJTEN3), anti-IL6 (eBioscience MP5-20F3), anti-TNF-α-PEcy5 (MP6-XT22 eBioscience), anti-IL-10-APC (eBioscience JES5-16E3), anti-Arg1-FITC (R&D Systems IC5868F) or isotype controls. All samples were run on a Guava easyCyte 8HT flow cytometer. Cells were gated on the forward and side scatter plot to remove debris, next on the F4/80+CD11b+ population and examined for cytokines. Macrophage numbers per colon were calculated by the percent of gated cells in relation to the overall number of cells per mouse colon.
Macrophage Culture
Macrophages were extracted and cultured as previously described. (13) For macrophage-treated mice, 1×106 macrophages were injected IP on the first day of each DSS treatment (days 5, 26, 47) and on day 68.
Statistics
Power analysis was performed to determine the sample size of the experimental and control groups to ensure that any effect, if one is in fact present, is statistically detectable. An alpha of 0.05 was used, and the minimum acceptable power was 0.80. A minimum of 5 animals per group (to allow for experimental error) at three independent experiments in vivo was used. Results were expressed as the mean ± SE. Differences between means were evaluated by one-way ANOVA in GraphPad Prism 5. Values of p <0.05 were considered statistically significant.
Results and Discussion
MK2 is essential for CAC development
Activation of MK2 leads to IL-1, IL-6, and TNF-α production. These cytokines are known to induce multiple cell survival and invasion pathways.(9–11) However, a specific role in cancer development has only been examined for MK2 in one study of skin cancer.(14) Thus, we set out to investigate pro-tumorigenic effects of MK2 in CAC. WT and MK2−/− mice were subjected to AOM and chronic treatments of DSS through three treatments and examined at day 80 as the commonly examined endpoint for this model. (15) Remarkably, MK2−/− mice did not develop neoplasms (Figure 1A), while wild type mice had 100% incidence. Also, upon H&E staining, AOM/DSS treated WT mice developed well defined neoplasms with dysplastic proliferation of the colonic epithelium (Figure 1B) and with DSS treatment had mild architectural disarray consistent with chronic injury (Figure 1C). In contrast, MK2−/− mice treated with AOM/DSS displayed no signs of dysplasia (Figure 1D). The complete absence of neoplasms in MK2-deficient mice after AOM and DSS-induced colitis indicate that MK2 is an important player in neoplasm development in CAC although the possibility exists that development could be delayed.
Figure 1. MK2−/− mice exposed to AOM/DSS do not develop neoplasms and have substantially decreased cytokine production compared to WT mice.
AOM/DSS treated mice develop A) multiple neoplasms, while MK2−/− mice do not. H&E staining indicates that B) AOM/DSS treated WT mice developed defined neoplasms with dysplastic proliferation of the colonic epithelium compared to C) architectural disarray of mucosal tissue consistent with chronic injury from multiple DSS treatments, while D) AOM/DSS treated MK2−/− mice displayed no visible signs of dysplasia or mucosal damage. AOM/DSS treated MK2−/− mice have significantly decreased E) IL-1α, F) IL-1β, G) IL-6, and H) TNF-α in organ culture supernatants compared to WT mice by multiplex bead array. N=7 for WT mice and 8 for MK2−/− mice in duplicate experiments.
Cytokine response is substantially reduced in MK2−/− mice exposed to AOM/DSS compared to wild type mice
IL-1, IL-6 and TNF-α are major factors in the establishment of the IBD promoting chronic inflammation, and are also known as tumor promoting cytokines in CRC (16;17). Interestingly, MK2−/− mice display decreased IL-1, IL-6 and TNF-α production in multiple models.(18;19) Since these cytokines may be the major inflammatory mediators driving inflammation and neoplasm development, we hypothesized the lack of neoplasms in MK2−/− mice could be the result of a dampened inflammatory response. Conditioned media collected from AOM/DSS treated wild type mouse colon organ cultures in a previously described tissue explant approach used by multiple groups (20;21) displayed a marked increase in IL-1α, IL-1β, IL-6 and TNF-α compared to control mice receiving one PBS injection and regular water (Figures 1E–H). To further support the induction of MK2-downstream cytokines in mouse colons, we found a similar pattern of increase in IL-1α, IL-1β, IL-6 and TNF-α gene expression in WT AOM/DSS treated mice, but minimal induction in MK2−/− mice (Figure S1). These high levels (compared to PBS groups) of cytokines indicate a chronic inflammatory response in the colon due to multiple DSS treatments. Conversely, supernatant from colon tissues of MK2−/− mice treated with AOM/DSS displayed a significant reduction in these cytokines compared to WT AOM/DSS treated mice. These findings emphasize the importance of MK2 in regulating the production of inflammatory mediators that promote colon neoplasm development.
MK2 deficiency reduces colonic macrophage accumulation and cytokine production in AOM/DSS treated mice
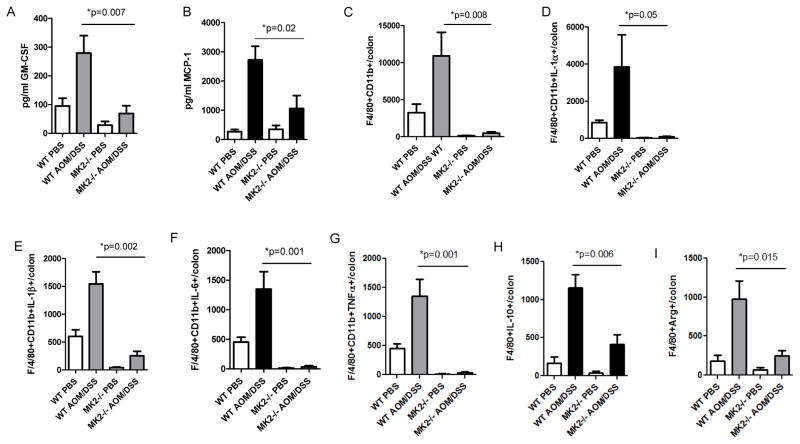
The MK2 downstream cytokines IL-1, IL-6 and TNF-α are produced by multiple cell types, including macrophages, in the AOM/DSS model.(13) To examine the impact of macrophages in MK2-dependent inflammation and neoplasm development, we first examined factors related to macrophage accumulation and activation in mouse colon supernatants. GM-CSF and MCP-1 were substantially decreased in MK2−/− mice compared to WT mice administered AOM/DSS (Figure 2A and B). The expression of these factors is important for macrophage development and accumulation.(22;23) Due to the role of GM-CSF, MCP-1, IL-1, IL-6 and TNF-α in macrophage accumulation and activation, we assessed the number of colonic macrophages by staining single cell colon suspensions for F4/80 and CD11b. The amount of F4/80+ CD11b+ cells found in the colon of AOM/DSS treated WT mice at day 80 was drastically increased in AOM/DSS treated WT mice compared to control groups, but markedly decreased in MK2−/− mice (Figure 2C). To further understand the role of MK2 in macrophages localized in the colon of mice that have developed neoplasms, intracellular cytokines were examined after isolation and ex vivo stimulation. A substantial amount of macrophages isolated from colon preps of AOM/DSS treated WT mice expressed IL-1α, IL-1β, IL-6 and TNF-α compared to macrophages isolated from AOM/DSS treated MK2−/− mice (Figures 2D–G). The considerable decrease in colonic macrophages during CAC development in MK2−/− mice indicate the proper cells are not present in the colon to mount an inflammatory response to contribute to neoplasm development. We also found an increase in IL-10-expressing macrophages in WT AOM/DSS treated mice (Figure 2H) and arginase-1 expressing macrophages (Figure 2I), both of which were significantly decreased in MK2−/− mice. These data suggest that MK2−/− mice not only show a decrease in macrophages expressing MK2 downstream mediators, but are also decreased in macrophages producing M2-like pro-tumorigenic factors. In addition to macrophages, there are also other myeloid-derived cells that are attracted to the mouse colon during inflammation. Myeloid-derived suppressor cells (MDSC) have been found to contribute to colitis and colitis-associated tumor development and growth. Suppressing trafficking to the colon, or knocking out MDSC-associated activity reduces inflammation and tumor burden in the AOM/DSS model. (24;25) Thus, we also stained for MDSC (CD11b+Gr1+ cells). These cells were increased in WT mice treated with AOM/DSS compared to PBS and present at higher levels in WT than MK2−/− mice, but the difference did not achieve significance (Figure S2).
Figure 2. Macrophages are a major source of MK2 downstream cytokines.
Cytokines related to macrophage influx and function, A) GM-CSF and B) MCP-1 are substantially increased in AOM/DSS treated mice, but significantly decreased in MK2−/− mice. WT mice treated with AOM/DSS have increased C) F4/80+CD11b+ macrophage influx, which is substantially decreased in MK2−/− mice treated with AOM/DSS. F4/80+CD11b+ cells in MK2−/− mice also displayed significantly less intracellular staining of D) IL-1α, E) IL-1β, F) IL-6, G) TNF-α, H) IL-10, and I) ARG1 compared to WT mice. N=7 for WT mice and 8 for MK2−/− mice in duplicate experiments.
Macrophages enhance pro-inflammatory cytokine production, but not neoplasm development in AOM/DSS treated mice
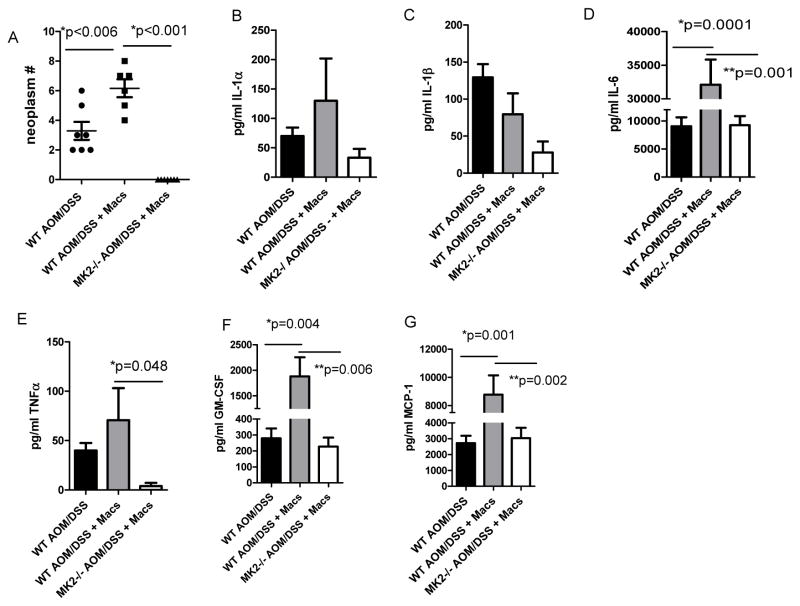
Macrophages harbor both anti- and pro-tumorigenic features that can hinder or enhance tumor formation. Our data above clearly indicate that macrophages accumulate and are producing MK2 downstream pro-inflammatory cytokines in tumor burden colons (Figure 2). To assess the contribution of macrophage to CAC, bone marrow-derived macrophages (BMM) from WT mice were adoptively transferred into WT and MK2−/− AOM/DSS treated mice at days 5, 26, 47, and 68. Interestingly, AOM/DSS treated MK2−/− mice receiving WT BMM displayed no neoplasms, while an increased number of neoplasms were found in WT mice injected with WT BMM (Figure 3A). WT mice receiving WT BMM had a mean of 6 neoplasms per mouse, while the WT mice had a mean of 3 neoplasms per mouse, which is a significant increase in number (p=0.006). These data indicate that macrophages promote neoplasm development in this system. Furthermore, addition of WT macrophages restored a proportion of the MK2 downstream cytokine production in MK2−/− mouse colons and also enhanced the amounts in WT mice receiving cells (Figures 3B–E). We further found an increase in the macrophage related cytokines GM-CSF and MCP-1 (Figure 3F and G). When comparing AOM/DSS treated MK2−/− mice supplemented with WT macrophages to AOM/DSS treated WT mice in Figures 1 and 2, IL-6, GM-CSF, and MCP-1 were drastically increased to similar levels, indicating the role of MK2 in promoting these responses. These data suggest that the number and frequency of BMM transfer into MK2−/− mice was adequate to mount a similar level of these cytokines as in wild type mice. IL-1α, IL-1β, and TNF-α were also increased upon WT BMM transfer into MK2−/− mice, but not to the same levels as WT mice suggesting that MK2 signaling in other cells may be responsible for production of these cytokines in WT mice. Introduction of WT macrophages into MK2−/− mice was not sufficient to restore neoplasm development indicating that MK2 is critical in other cells, perhaps epithelial cells, for neoplasm development. Furthermore, the significantly increased neoplasm development upon addition of extra macrophages into WT mice highlights the importance of MK2 signaling in macrophages in promoting tumor growth. Nevertheless, in our model, we have eliminated macrophages as being the primary determinant driving CAC development.
Figure 3. WT BMM injection in MK2−/− mice restores some cytokine production, but not neoplasm development.
BMM injections into WT and MK2−/− mice led to A) increased neoplasm development for WT mice, but not MK2−/− with AOM/DSS treatments. In organ culture, B) IL-1α, C) IL-1β, D) IL-6, E) TNF-α, F) GM-CSF, and G) MCP-1 production were found at higher levels in WT mice, but were also increased in MK2−/− mice supplemented with WT macrophages. N=6 for BMM supplementation experiments in duplicate experiments.
Our data highlight the importance of MK2 in CAC development and provide a target to hinder the inflammatory response. We demonstrate that restored MK2 signaling in a single cell type (macrophages) can restore the inflammatory response in MK2−/− mice and hence, this further substantiates the robustness of MK2 signaling pathway as a highly inflammatory event. Given the insurgence of reports demonstrating macrophages promote inflammation and cancer, we set out to the test hypothesis that MK2 in macrophages drives CAC development. The transfer of these cells was sufficient to restore the inflammatory response as determined by cytokine output (Figure 3B–G). Nonetheless, restoration of MK2 in these cells (as well as the proinflammatory cytokines detected) was not sufficient to re-establish tumor development in MK2−/− mice. This suggests a more complex role for MK2 in CAC development where restoration of the inflammatory cytokines is only one part of the equation. Upon supplementation of macrophages to WT mice, neoplasm number was significantly increased (Figure 3A). These data indicate that MK2-induced cytokines from macrophages promote increased tumor growth, but MK2 signaling is also needed in other cells, such as epithelial cells for tumor development. Thus, these studies raise the possibility that MK2 is a potential therapeutic target for patients with colitis or CAC that could prove beneficial.
Supplementary Material
Impact of work.
This is the first study to examine previously unreported mechanisms regarding the role of the MK2 pathway in colorectal cancer development in a mouse model. These mechanisms include: 1) a major role for MK2 downstream cytokines in colorectal cancer development, 2) a major role for the MK2 pathway in macrophages in the development of an inflammatory response in colorectal cancer, and 3) a major role for the MK2 pathway in macrophages in promoting tumor development.
Acknowledgments
This work was supported by the American Cancer Society (RSG-10-159-01-LIB), NIH 8UL1TR000041, the University of New Mexico Clinical and Translational Science Center, and NIH P30CA118100, UNM Cancer Center. A.L. Ray is a recipient of the T32AI007538-17-21 Predoctoral Fellowship.
Abbreviations
- MK2
MAPK-activated protein kinase 2
- CAC
colitis associated cancer
- WT
wild type
- CRC
colorectal cancer
- BMM
bone marrow-derived macrophages
- AOM
azoxymethane
- DSS
dextran sodium sulfate
Footnotes
The authors have no financial conflicts to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics for Hispanics/Latinos, 2012. CA Cancer J Clin. 2012;62:283–98. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 2.Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839–48. doi: 10.3748/wjg.v18.i29.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng YJ, Li YY. The role of p38 mitogen-activated protein kinase in the pathogenesis of inflammatory bowel disease. J Dig Dis. 2011;12:327–32. doi: 10.1111/j.1751-2980.2011.00525.x. [DOI] [PubMed] [Google Scholar]
- 4.Genovese MC. Inhibition of p38: has the fat lady sung? Arthritis Rheum. 2009;60:317–20. doi: 10.1002/art.24264. [DOI] [PubMed] [Google Scholar]
- 5.Xu JJ, Hendriks BS, Zhao J, de GD. Multiple effects of acetaminophen and p38 inhibitors: towards pathway toxicology. FEBS Lett. 2008;582:1276–82. doi: 10.1016/j.febslet.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 6.Morris DL, O’Neil SP, Devraj RV, Portanova JP, Gilles RW, Gross CJ, Curtiss SW, Komocsar WJ, Garner DS, Happa FA, Kraus LJ, Nikula KJ, et al. Acute lymphoid and gastrointestinal toxicity induced by selective p38alpha map kinase and map kinase-activated protein kinase-2 (MK2) inhibitors in the dog. Toxicol Pathol. 2010;38:606–18. doi: 10.1177/0192623310367807. [DOI] [PubMed] [Google Scholar]
- 7.Seimon TA, Wang Y, Han S, Senokuchi T, Schrijvers DM, Kuriakose G, Tall AR, Tabas IA. Macrophage deficiency of p38alpha MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J Clin Invest. 2009;119:886–98. doi: 10.1172/JCI37262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotlyarov A, Yannoni Y, Fritz S, Laass K, Telliez JB, Pitman D, Lin LL, Gaestel M. Distinct cellular functions of MK2. Mol Cell Biol. 2002;22:4827–35. doi: 10.1128/MCB.22.13.4827-4835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garlanda C, Riva F, Veliz T, Polentarutti N, Pasqualini F, Radaelli E, Sironi M, Nebuloni M, Zorini EO, Scanziani E, Mantovani A. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 2007;67:6017–21. doi: 10.1158/0008-5472.CAN-07-0560. [DOI] [PubMed] [Google Scholar]
- 10.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369–73. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 12.Steinbach EC, Plevy SE. The role of macrophages and dendritic cells in the initiation of inflammation in IBD. Inflamm Bowel Dis. 2014;20:166–75. doi: 10.1097/MIB.0b013e3182a69dca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14 doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen C, Vestergaard C, Kragballe K, Kollias G, Gaestel M, Iversen L. MK2 regulates the early stages of skin tumor promotion. Carcinogenesis. 2009;30:2100–8. doi: 10.1093/carcin/bgp238. [DOI] [PubMed] [Google Scholar]
- 15.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 16.Guven ME, Keskin O, Gursoy A, Nussinov R. The structural network of inflammation and cancer: merits and challenges. Semin Cancer Biol. 2013;23:243–51. doi: 10.1016/j.semcancer.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–67. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronkina N, Kotlyarov A, Dittrich-Breiholz O, Kracht M, Hitti E, Milarski K, Askew R, Marusic S, Lin LL, Gaestel M, Telliez JB. The mitogen-activated protein kinase (MAPK)-activated protein kinases MK2 and MK3 cooperate in stimulation of tumor necrosis factor biosynthesis and stabilization of p38 MAPK. Mol Cell Biol. 2007;27:170–81. doi: 10.1128/MCB.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tietz AB, Malo A, Diebold J, Kotlyarov A, Herbst A, Kolligs FT, Brandt-Nedelev B, Halangk W, Gaestel M, Goke B, Schafer C. Gene deletion of MK2 inhibits TNF-alpha and IL-6 and protects against cerulein-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1298–G1306. doi: 10.1152/ajpgi.00530.2005. [DOI] [PubMed] [Google Scholar]
- 20.Randall KJ, Turton J, Foster JR. Explant culture of gastrointestinal tissue: a review of methods and applications. Cell Biol Toxicol. 2011;27:267–84. doi: 10.1007/s10565-011-9187-5. [DOI] [PubMed] [Google Scholar]
- 21.Sheikh SZ, Matsuoka K, Kobayashi T, Li F, Rubinas T, Plevy SE. Cutting edge: IFN-gamma is a negative regulator of IL-23 in murine macrophages and experimental colitis. J Immunol. 2010;184:4069–73. doi: 10.4049/jimmunol.0903600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francisco-Cruz A, Aguilar-Santelises M, Ramos-Espinosa O, Mata-Espinosa D, Marquina-Castillo B, Barrios-Payan J, Hernandez-Pando R. Granulocyte-macrophage colony-stimulating factor: not just another haematopoietic growth factor. Med Oncol. 2014;31:774. doi: 10.1007/s12032-013-0774-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21:41–8. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–44. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res. 2011;9:133–48. doi: 10.1158/1541-7786.MCR-10-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.