Abstract
Background
Biomarkers for Huntington’s disease (HD) progression could accelerate therapeutic developments and improve patient care. Brain microRNAs (miRNA) relating to clinical features of HD may represent a potential HD biomarker in blood.
Objective
Examine candidate miRNAs in plasma to determine if changes observed in HD brains are detectable in peripheral samples.
Methods
Four miRNA from 26 manifest HD, 4 asymptomatic HD gene carriers and 8 controls were quantified in plasma using RT-qPCR. Linear regression was used to assess miRNA levels across control, asymptomatic gene carriers and manifest patients.
Results
miR-10b-5p (p= 0.0068) and miR-486-5p (p= 0.044) were elevated in HD plasma. miR-10b-5p was decreased in asymptomatic gene carriers as compared to HD patients (p= 0.049), but no difference between asymptomatic gene carriers and controls was observed (p= 0.24).
Conclusions
These findings suggest miRNA changes observed in HD brain may be detectable in plasma and have potential clinical utility.
Keywords: Huntington’s disease, microRNA, miRNA, blood, biomarker
Introduction
Huntington’s disease (HD) is caused by an expanded CAG trinucleotide repeat sequence in the huntingtin gene 1. Clinical onset of the disease is defined by the emergence of abnormal, choreiform movements, often accompanied by neurophysiological, psychiatric or cognitive impairments 2, and generally occurs around middle age 3. Neurodegeneration precedes clinical diagnosis, with as many as half of the neurons in the caudate nucleus lost before clinical onset occurs 4, and volumetric changes in the striatum as early as two decades before predicted onset age 5. Because motor and cognitive impairments correlate with the neuroanatomical changes in the striatum 6, to prevent neuronal loss and delay disease onset, therapeutic intervention would ideally occur prior to HD manifestation.
While genetic testing can reliably detect the presence of an expanded CAG repeat, the lack of validated biomarkers for HD onset and progression limits the evaluation of preventive and early-stage disease-modifying therapies. Current measures for prodromal and early-stage disease rely on ratings of functional decline, which are susceptible to inter-rater variability and limited sensitivity 2.
Large, multicenter, longitudinal studies comparing cohorts of asymptomatic gene carriers and early-stage HD to healthy controls have used a battery of clinical and neuroimaging based assessments. Aimed at identifying robust quantitative measures 7, 8, these studies have identified a number of image-based biomarkers that may relate to HD progression including morphometric changes 7, 9, the glial cell marker myo-inositol 10, the neuronal integrity marker N-acetyl aspartate 10, and recently, the medium spiny neuron marker PDE10A 11. Although these results are encouraging, neuroimaging is susceptible to reproducibility issues due to technical and analytical inconsistencies across centers, which must be addressed prior to clinical adoption 12.
Alternatively, disease mechanisms observed in the brain may be detectable in the blood. Studies of 8-OHdG levels 13, mutant HTT accumulation 14, inflammatory markers 15, and genome-wide RNA changes 16–18, have uncovered a number of concordant changes between brain and blood. While some are promising, the clinical utility of these measures is yet to be established19.
Our recent evaluation of altered miRNA levels obtained through small RNA sequencing in human HD and control prefrontal cortex identified 75 miRNAs significantly altered in HD 20. Several of these were associated with age at motor onset, or the level of neuropathology in the striatum 21, including miR-10b-5p, which associated with both 22. Furthermore, in asymptomatic HD gene carriers, miR-10b-5p levels were distinguishable from both the low expression observed in controls and higher levels seen among symptomatic HD patients, suggesting a progressive relationship between cortical levels of miR-10b-5p and disease stage.
Because brain-derived miRNAs may pass through the blood-brain-barrier by exosome transport 23 and are stable in serum 24, the miRNAs identified in postmortem brain tissue may be detectable in peripheral fluids 25–27, and thus potentially provide accessible biomarkers for disease stage and rate of progression in clinical trials.
The first step in the evaluation of clinical utility is to determine whether the observed HD brain-related miRNA alterations are detectable in peripheral samples. We therefore compared the levels of four miRNAs related to HD clinical features in postmortem brains (miR-10b-5p, miR-486-5p, miR-132-3p, and miR-363-3p) in HD, asymptomatic and healthy control plasma samples.
Methods
Study participants (n=38) were recruited through the Boston University Neurological Associates (BUNA) and Tewksbury State Hospital from 2012–2014, with appropriate IRB approval and consent (BUSM Protocol Number H-31052 and Massachusetts Department of Public Health Protocol Number 328647-2) (see Table 1). No significant differences were observed in the age or sex distribution between the 3 groups (HD cases, asymptomatic HD carriers, and controls). BD Vacutainer CPT Mononuclear Cell Preparation Tubes containing 0.1 mL sodium citrate anticoagulant and 0.1 M Ficoll medium were used to isolate plasma from 8 mL of whole blood drawn by a trained phlebotomist.
Table 1.
Summary of the samples used for the study
| Condition | N | Age | Onset age | Gender |
|---|---|---|---|---|
| Control | 8 | 46.1 ± 13.5 | 3M, 5F | |
| Asymptomatic HD | 4 | 42.5 ± 28.7 | 1M, 3F | |
| Manifest HD | 26 | 53.0 ± 8.7 | 47.6 ± 9.9 | 11M, 15F |
One mL of plasma was used for RNA extraction. To minimize platelet contamination 28, residual platelets were removed by centrifugation, collecting supernatant after spinning for 5 min at 16,000 × g. 0.22 um filtration was used to remove heterogeneous, phospholipid membrane bound microparticles, 0.05–1.5 um in size, shed from platelets and other blood cells 29. RNA was extracted using Qiazol and miRNeasy RNA isolation kit from Qiagen, according to manufacturer’s protocol. RNA purity and abundance was assessed by spectrophotometry.
Four miRNAs were selected based on the following criteria: (1) genome-wide significant changes in HD brain 22 (2) abundance in both brain and blood 30, and (3) nominal association in the cortical study (p<0.05) to clinical HD features (onset: miR-10b-5p, miR-486-5p miR-363-3p; striatal neuropathological involvement: miR-10b-5p, miR-132-3p).
Exiqon miRCURY LNA Universal RT miRNA PCR was used following the manufacturer’s protocol. UniSp6 synthetic spike-in was used to evaluate cDNA efficiency. Following cDNA synthesis, samples were diluted to 0.2 ng/ul in RNAse free water. Both SNORD44 and miR-451a were used for normalization. For quantitative PCR (qPCR), samples were assayed in triplicate across three 384-well plates, using Applied Biosystems 7900HT Real-Time PCR System. For analysis, threshold cycle (Ct) values for triplicate wells were normalized by average RNU44 and miR-451a values. Extreme outlier wells and samples (standard deviations above 10) were removed. miRNA levels were calculated using the ΔΔCt method 31, where positive ΔΔCt values indicated increased levels and negative ΔΔCt values indicated decreased levels as compared to controls.
Linear regression analyses predicting −ΔΔCt were used to test the association between miRNA levels and disease in HD cases and controls. One-tailed tests were used to test the a priori hypothesis of consistent direction of effects as observed in the brain study 22, thus relationships inconsistent with the cortical findings would not be identified as significant. The relationship of miR-10b-5p, miR-486-5p, miR-363-3p to age of motor onset in cases was assessed using linear regression (miR-132-3p did not show a relationship to onset in the cortical study). Bonferroni correction for four or three comparisons were applied respectively.
For miR-10b-5p, the relationship was further examined between asymptomatic HD gene carriers and HD cases and controls separately using linear regression, again using a one-tailed test of the previously observed relationships. In addition, a linear trend test was implemented in SAS (GLM Procedure) to test if an ordered relationship was observed between controls, asymptomatic gene carriers and HD cases.
Results
All four miRNAs were detected in plasma (average Ct range 25.7–33.8). After calculating −ΔΔCt levels, 26 HD patients were compared to the 8 controls to test whether miRNAs alterations in HD plasma resembled changes observed in HD brain. Increased levels of miR-10b-5p (one-sided p= 0.0068, β= 2.39) and miR-486-5p (one-sided p= 0.044, β= 1.44) were observed in manifest HD patients compared to control subjects, consistent with the changes in the brain 22 where both miRNAs were also increased. Levels of miR-132-3p, though not significant, were lower in HD plasma, consistent with the results in brain (one-sided p = 0.92, β= −0.62). miR-363-3p levels were not altered in blood, nor consistent with changes observed in HD brain (one-sided p = 1.00, β= −0.079).
Three previously observed associations to age at motor onset in the brain study were also tested. The relationship between plasma miR-10b-5p and onset age did not have the same direction as previously observed in HD brain. In fact, a positive association, opposite to that seen in brain and our tested a priori hypothesis, may exist (one-sided p=1.00, two-sided p=0.0096, beta=0.13). The nominal associations to age of onset observed in brain for miR-486-5p and miR-363-3p were not present in blood (miR-486-5p one-sided p=1, miR-363-3p one-sided p=1).
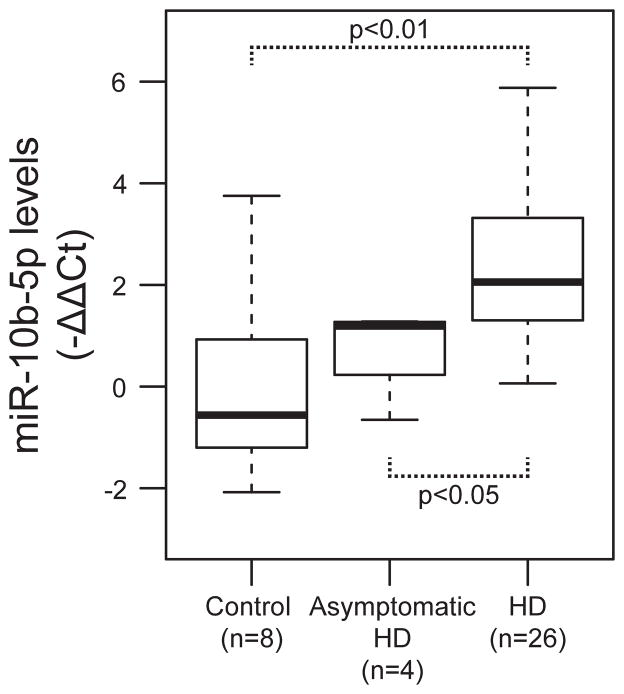
There was no statistical difference between asymptomatic HD gene carriers and controls in miR-10b-5p levels, (one-sided p= 0.24, β= 0.53), however miR-10b-5p levels were significantly elevated in manifest HD patients compared to asymptomatic gene carriers (one-sided p= 0.049, β= 1.15). A linear trend test of the ordered relationship of miRNA levels between controls, asymptomatic HD gene carriers and manifest HD patients found a significant positive association with miR-10b-5p levels (one-sided p= 0.0012) (see Figure 1), concordant with the direction of the effects observed in HD prefrontal cortex.
Figure 1. miR-10b-5p has an ordinal association with HD stage.
Boxplot of miR-10b-5p levels (−ΔΔCt) for control, asymptomatic HD gene, and manifest HD subjects. miR-10b-5p levels had a significant linear trend from controls, to asymptomatic HD gene carriers to manifest HD patients.
Discussion
The results from this study identify two candidate miRNAs, miR-10b-5p and miR-486-5p, as increased in both brain and blood in HD. In our previous HD brain study, miR-10b-5p had the strongest associations to disease stage, age of onset and extent of neuropathological involvement 22. While we did not replicate the relationship of miR-10b-5p levels to age of onset observed in brain, the levels of plasma miR-10b-5p were significantly elevated HD patients compared to asymptomatic HD gene carriers consistent with the brain study. While a consistent trend of increasing levels from controls, to asymptomatic HD to manifest HD was observed, plasma miR-10b-5p levels were not significantly different in asymptomatic subjects compared to controls, and given the small number of samples in the asymptomatic (N=4) and control (N=8) groups, caution should be taken in interpreting those findings. The significant differences between asymptomatic and manifest HD do however suggest pre-clinical HD miRNAs changes may occur.
The concordance of these changes in both HD brain and blood supports the role of miR-10b-5p in the disease and suggests potential utility as a clinically useful biomarker for HD. Longitudinal within-subject tests of miRNA levels over time, as well as tests of the relationship of miRNA levels to motor, cognitive and functional ratings may be warranted for miR-10b-5p in particular. The identification of two of the four candidate miRNA examined also suggests that a full examination of all 75 brain-identified miRNA may reveal additional miRNA with potential clinical utility.
Blood-based biochemical assays are minimally invasive and relatively simple compared to neuroimaging-based diagnostics. The strength of this study and overall approach is that the blood-based assays described here are based on miRNA alterations observed in HD cortex, providing greater probability that these alterations are representative of the disease process. This is in contrast to signals detected primarily in peripheral tissues, which are more likely to represent systemic physiological responses independent of neurodegeneration and other disease features 32. Because of this strategy, we did not test miR-34b which has been previously reported as changed in premanifest HD plasma, but is not altered in HD brain 20, 22.
These findings are a key first step towards the evaluation of the potential utility of miRNA as biomarkers of disease progression and for use in examination of treatment efficacy in early premanifest patients.
Acknowledgments
Funding sources for study
This study was funded by Jerry McDonald HD Research Fund; US National Institutes of Health R01 NS073947 Epigenetic Markers in Huntington’s disease Brain.
A patent application has been submitted.
Footnotes
Relevant conflicts of interest/financial disclosures: None to report.
Authors' Roles
Conceived and designed the experiments: AGH JCL RHM. Performed the experiments:
AGH RHM VL. Analyzed the data: AGH JCL RHM VL. Contributed reagents/materials/analysis tools and critically reviewed the manuscript: TCH RHM SF.
Wrote the paper: AGH JCL RHM.
Full Financial Disclosures of all Authors for the Past Year
Information concerning all sources of financial support and funding for the preceding twelve months, regardless of relationship to current manuscript, must be submitted with the following categories suggested: Stock Ownership in medically-related fields, Intellectual Property Rights, Consultancies, Expert Testimony, Advisory Boards, Employment, Partnerships, Contracts, Honoraria, Royalties, Grants, Other
AGH: None to report.
VNL had the following grant support:
Howard Hughes Medical Institute Summer Research Fellowship through Boston University Department of Neuroscience, 5510106
SF had the following grant support: Auspex Pharmaceuticals, a wholly owned subsidiary of Teva Pharmaceuticals.
TCH was employed by GNS Healthcare
RHM had the following grant support:
Jerry McDonald HD Research Fund, US National Institutes of Health, R01-NS073947, Epigenetic Markers in Huntington’s Disease Brain, US National Institutes of Health, R01-NS076843, Characterization of the Role of Cyclin G-associated Kinase in Parkinson Disease, US National Institutes of Health, R01-HL117078, Probing the Dark Matter of the Genome in the NHLBI Family Heart Study, US National Institutes of Health, R01-NS088538, An IPSc based platform for functionally assessing genetic and environmental Risk in PD, National Science Foundation PHY-1444389 EArly-concept Grants for Exploratory Research (EAGER).
JCL had the following grant support: R03 NS082601-01, R01 NS088538-01, R01 HL117078-01, R01 NS073947-01, R01 NS076843-01
References
- 1.HDCRG. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72(6):971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Unified Huntington’s Disease Rating Scale: reliability and consistency. Huntington Study Group. Movement disorders : official journal of the Movement Disorder Society. 1996;11(2):136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 3.Myers RH. Huntington’s disease genetics. NeuroRx. 2004;1(2):255–262. doi: 10.1602/neurorx.1.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44(6):559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Aylward EH, Sparks BF, Field KM, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63(1):66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 6.Kloppel S, Henley SM, Hobbs NZ, et al. Magnetic resonance imaging of Huntington’s disease: preparing for clinical trials. Neuroscience. 2009;164(1):205–219. doi: 10.1016/j.neuroscience.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabrizi SJ, Scahill RI, Durr A, et al. Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: the 12-month longitudinal analysis. The Lancet Neurology. 2011;10(1):31–42. doi: 10.1016/S1474-4422(10)70276-3. [DOI] [PubMed] [Google Scholar]
- 8.Paulsen JS, Long JD, Ross CA, et al. Prediction of manifest Huntington’s disease with clinical and imaging measures: a prospective observational study. The Lancet Neurology. 2014;13(12):1193–1201. doi: 10.1016/S1474-4422(14)70238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington DL, Liu D, Smith MM, et al. Neuroanatomical correlates of cognitive functioning in prodromal Huntington disease. Brain and behavior. 2014;4(1):29–40. doi: 10.1002/brb3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sturrock A, Laule C, Wyper K, et al. A longitudinal study of magnetic resonance spectroscopy Huntington’s disease biomarkers. Movement disorders : official journal of the Movement Disorder Society. 2015;30(3):393–401. doi: 10.1002/mds.26118. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad R, Bourgeois S, Postnov A, et al. PET imaging shows loss of striatal PDE10A in patients with Huntington disease. Neurology. 2014;82(3):279–281. doi: 10.1212/WNL.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 12.McArthur RA. Translational neuroimaging : tools for CNS drug discovery, development and treatment. London ; Waltham, MA: Academic Press; 2013. [Google Scholar]
- 13.Long JD, Matson WR, Juhl AR, et al. 8OHdG as a marker for Huntington disease progression. Neurobiology of disease. 2012;46(3):625–634. doi: 10.1016/j.nbd.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wild EJ, Boggio R, Langbehn D, et al. Quantification of mutant huntingtin protein in cerebrospinal fluid from Huntington’s disease patients. The Journal of clinical investigation. 2015;125(5):1979–1986. doi: 10.1172/JCI80743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjorkqvist M, Wild EJ, Thiele J, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J Exp Med. 2008;205(8):1869–1877. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borovecki F, Lovrecic L, Zhou J, et al. Genome-wide expression profiling of human blood reveals biomarkers for Huntington’s disease. Proc Natl Acad Sci U S A. 2005;102(31):11023–11028. doi: 10.1073/pnas.0504921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mastrokolias A, Ariyurek Y, Goeman JJ, et al. Huntington’s disease biomarker progression profile identified by transcriptome sequencing in peripheral blood. European journal of human genetics : EJHG. 2015 doi: 10.1038/ejhg.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Chopra V, Chopra R, et al. Transcriptional modulator H2A histone family, member Y (H2AFY) marks Huntington disease activity in man and mouse. Proc Natl Acad Sci U S A. 2011;108(41):17141–17146. doi: 10.1073/pnas.1104409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borowsky B, Warner J, Leavitt BR, et al. 8OHdG is not a biomarker for Huntington disease state or progression. Neurology. 2013;80(21):1934–1941. doi: 10.1212/WNL.0b013e318293e1a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoss AG, Kartha VK, Dong X, et al. MicroRNAs located in the Hox gene clusters are implicated in huntington’s disease pathogenesis. PLoS genetics. 2014;10(2):e1004188. doi: 10.1371/journal.pgen.1004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadzi TC, Hendricks AE, Latourelle JC, et al. Assessment of cortical and striatal involvement in 523 Huntington disease brains. Neurology. 2012;79(16):1708–1715. doi: 10.1212/WNL.0b013e31826e9a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoss AG, Labadorf A, Latourelle JC, et al. miR-10b-5p expression in Huntington’s disease brain relates to age of onset and the extent of striatal involvement. BMC medical genomics. 2015;8:10. doi: 10.1186/s12920-015-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature biotechnology. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song SJ, Poliseno L, Song MS, et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell. 2013;154(2):311–324. doi: 10.1016/j.cell.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu M, Zhang Q, Deng M, et al. An analysis of human microRNA and disease associations. PloS one. 2008;3(10):e3420. doi: 10.1371/journal.pone.0003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sassen S, Miska EA, Caldas C. MicroRNA: implications for cancer. Virchows Archiv : an international journal of pathology. 2008;452(1):1–10. doi: 10.1007/s00428-007-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng HH, Yi HS, Kim Y, et al. Plasma processing conditions substantially influence circulating microRNA biomarker levels. PloS one. 2013;8(6):e64795. doi: 10.1371/journal.pone.0064795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simak J, Gelderman MP. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Transfusion medicine reviews. 2006;20(1):1–26. doi: 10.1016/j.tmrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Burgos KL, Javaherian A, Bomprezzi R, et al. Identification of extracellular miRNA in human cerebrospinal fluid by next-generation sequencing. Rna. 2013;19(5):712–722. doi: 10.1261/rna.036863.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(11846609):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Aziz NA, van der Burg JM, Landwehrmeyer GB, et al. Weight loss in Huntington disease increases with higher CAG repeat number. Neurology. 2008;71(19):1506–1513. doi: 10.1212/01.wnl.0000334276.09729.0e. [DOI] [PubMed] [Google Scholar]