Abstract
Future therapies for the treatment of dental decay have to consider the importance of preserving bacterial ecology while reducing biofilm adherence to teeth. A multi-species plaque derived (MSPD) biofilm model was used to assess how concentrations of N-acetyl-L-cysteine (0, 0.1%, 1%, 10%) affected the growth of complex oral biofilms. Biofilms were grown (n=96) for 24 hours on hydroxyapatite disks in BMM media with 0.5% sucrose. Bacterial viability and biomass formation was examined on each disk using a microtiter plate reader. In addition, fluorescence microscopy and Scanning Electron Microscopy was used to qualitatively examine the effect of NAC on bacterial biofilm aggregation, extracellular components, and bacterial morphology. The total biomass was significantly decreased after exposure of both 1% (from 0.48, with a 95% confidence interval of (0.44, 0.57) to 0.35, with confidence interval (0.31, 0.38)) and 10% NAC (0.14 with confidence interval (0.11, 0.17)). 16S rRNA amplicon sequencing analysis indicated that 1% NAC reduced biofilm adherence while preserving biofilm ecology.
Keywords: Biofilms, Colonization, Disease, Dental, Caries, Microbiome
Introduction
Dental caries (decay) is a biofilm mediated disease where short term antibiotic or conventional antibiotic therapy is not appropriate for management. Antibiotic approaches to eradicate bacterial species, especially those that can have commensal properties, can have potentially counterproductive effects. Future therapies for the treatment of dental decay have to consider the importance of preserving bacterial ecology while reducing biofilm adherence to teeth. Furthermore, pathogenic oral species exist at detectable levels even in healthy individuals (Aas et al., 2008; Tanner et al., 2011). These healthy individuals also possess commensal bacteria that have the potential virulence factors, such as acid production in the case of dental caries (Svensäter et al., 1997). A multitude of oral bacteria are capable of producing damaging acid following high/repeated sucrose challenges (Rudney et al., 2012). The acidic challenge is related to both the composition of dental plaque and also the thickness of the adherent biofilm (Ilie et al., 2012). Based on mathematical modeling, biofilm thickness can dramatically influence both the immediate amount of acid generation and the amount of time that the surface is exposed to a lower pH (Ilie et al., 2012). Tooth demineralization in dental caries is augmented with thicker biofilms exposed to carbohydrate sources. Reducing the thickness and coverage of these oral biofilms has potential prevention benefits for patients (Firestone and Muhlemann, 1985; Wolff and Larson, 2009).
With increased concerns over antibiotic resistance in medicine, there is renewed interest in the anti-biofilm properties of N-acetyl-L-cysteine (NAC). NAC is a mucolytic compound with a long history of use and wide margin of safety as a therapeutic(Atkuri et al., 2007; Millea, 2009). The compound has found multiple applications in medicine(Bernard, 1991; Kory et al., 1968; Maayan et al., 1989). NAC is most frequently used to reduce the viscosity of mucus in pulmonary compromised patients, including pediatric patients with cystic fibrosis(Henke and Ratjen, 2007). For those patients, it has been found that NAC has additional therapeutic benefits(Balsamo et al., 2010). NAC has bacteriostatic effects on in vitro single species biofilms(Zhao and Liu, 2010). These biofilms have the potential in vivo to complicate pulmonary function(Zhao and Liu, 2010). Additionally, by disrupting the mucus secretion and microbial biofilms, NAC can potentiate the effectiveness of antibiotic treatment (Mansouri et al., 2013; Parry and Neu, 1977). The results of NAC based studies in pulmonary medicine have elicited investigation into other biofilm-mediated diseases (Cammarota et al., 2012; Drago et al., 2013; Mansouri et al., 2013).
The purpose of this study was to examine the anti-biofilm properties of NAC on controlling oral biofilm formation while examining the effect on the bacterial ecology of these multi-species biofilms. The clinical significance of this approach is to investigate NAC as a potential non-bactericidal, non-antibiotic, topical agent that can be used to control biofilm/dental plaque formation in high caries risk individuals. Given the high safety margin of NAC, this caries management approach may have the potential to be utilized in pediatric populations, where high fluoride intake is an ongoing concern.
Results and Discussion
Effect of Biofilm Formation
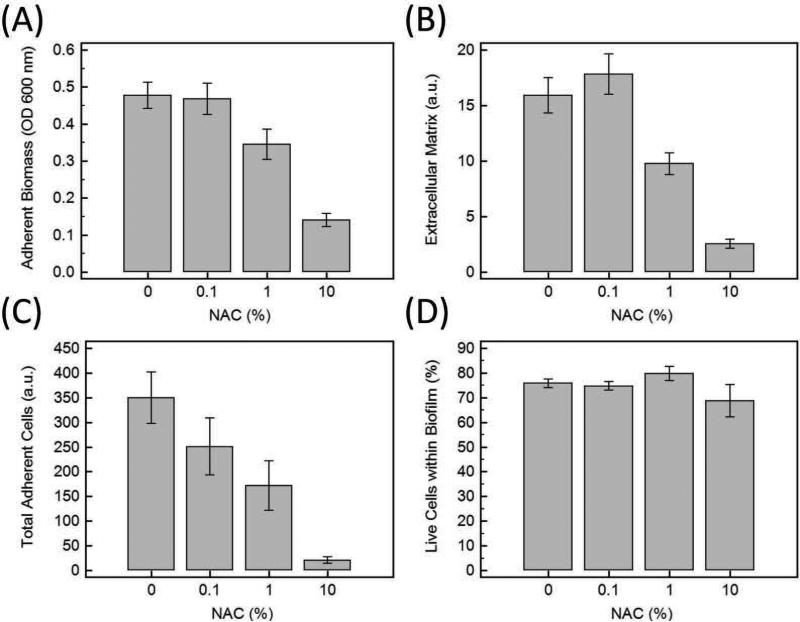
When assessing the biomass accumulation measured by the crystal violet assay (Figure 1A), N-acetyl-L-cysteine (NAC) had a dose-dependent effect on the biomass accumulation. Both 1% and 10% NAC was shown to significantly reduce the adherent biomass of the biofilms compared to 0% NAC (Figure 1A). The extracellular matrix component of the biofilm was also reduced at 1% and 10% concentrations of NAC (Figure 1B). When examining the cellular component of the biofilm, the number of total adherent cells (live and dead) quantified by the live/dead assay (SYTO® 9 /PI stain) showed a dose response effect to NAC on bacterial colonization (Figure 1C). The total adherent cells measured by LIVE/DEAD® BacLight™ Bacterial Viability Assay were reduced at concentrations of 1% and 10 % NAC. This assay emphasized the lateral coverage of the cellular components of the biofilm. To evaluate if NAC was exerting a bactericidal effect, the percentage of live bacteria within the biofilm was calculated and graphed (Figure 1D). Although there was an overall reduction in the number of adherent bacteria, which is seen in Figure 1C, the proportion of live bacteria compared to dead bacteria did not markedly decrease when exposed to 0.1%, 1 %, and 10% NAC (Figure 1D). Exposure to 10% NAC resulted in a greater variability of the percentage of live cells within the biofilm but the confidence intervals overlap with the confidence intervals of 0% (Figure 1D).
Figure 1.
A) Adherent Biomass on HA disks measured by crystal violet assay and the absorbance of the dye at 600 nm. B) Quantifying Extracellular Matrix with FilmTracer™ SYPRO® Ruby biofilm matrix stain at the excitation/emission (450 nm/610 nm). C) Quantifying total adherent bacteria (Live plus dead cells) using SYTO®9 and propidium iodide (PI). D) Although NAC caused a decrease in the amount of biofilm, the percentage of live bacteria within the biofilm was nearly unchanged with increased NAC concentrations. Error bars are 95% confidence intervals for the means (n=24 for each condition).
Imaging the Effect on Biofilm Formation
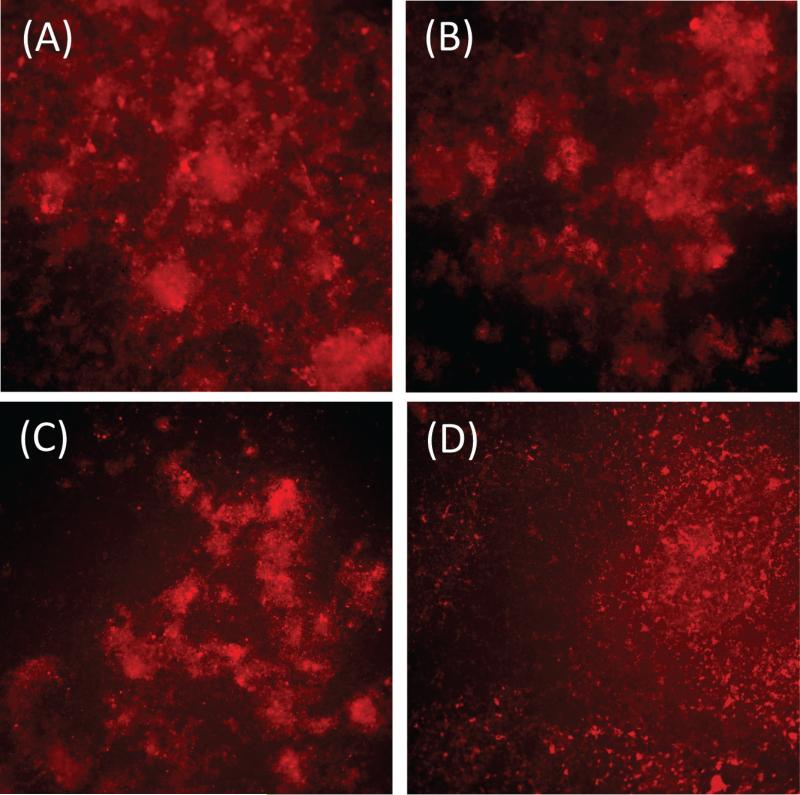
Fluorescence microscopy images of the MSPD biofilm demonstrated how NAC reduced the bacteria aggregation and matrix production. Since the BMM media broth nourished the biofilm bacteria with hemin, an iron containing porphyrin, there was non-specific red light fluorescence (near 580 nm) for all the bacteria in these biofilms. Other authors have also shown this non-specific fluorescence for oral bacteria exposed to porphyrin nutrients(Volgenant et al., 2013). In this case, we also added the SYPRO® Ruby matrix stain (at the outer portion of the stain's excitation bandwidth) to fluoresce the extracellular matrix protein components. With the 540-580 nm/600-660 nm excitation/emission (filter), the MSPD biofilms (cellular and extracellular) components were visualized. At 1 % and 10% NAC, the bacteria and the matrix components were clearly dispersed (Figure 2). A large proportion of the bacteria showed reduced co-aggregation.
Figure 2.
Fluorescence microscopy images of the bacterial biofilm matrix at the excitation/ emission wavelength of 560±20 nm/630±30 nm using SYPRO® Ruby biofilm matrix stain. A-D 10× magnification. A) 0% NAC B) 0.1% NAC C) 1% NAC D) 10% NAC. Increase concentrations of NAC at 1% and 10% caused a marked reduction of the biofilm matrix with the dispersed colonies of bacteria.
Imaging the Effect on Bacterial Aggregation
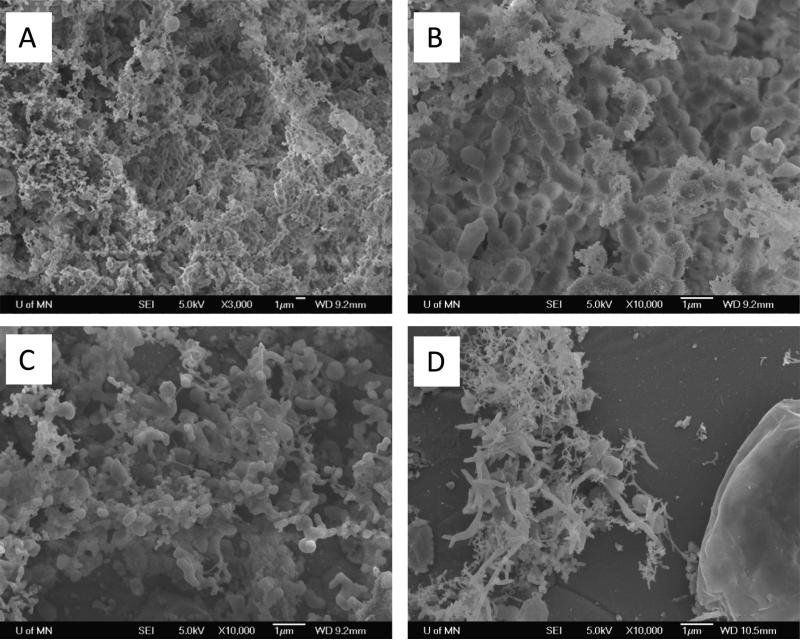
SEM analysis of the MSPD biofilms further investigated the bacteria aggregation effects of NAC, in addition to qualitatively examining the extracellular components and the bacterial morphology. Images of the controls (0% NAC) showed the dense network of bacteria surrounded by extracellular components of the matrix (Figure 3). At 1% NAC, SEM images showed a loss of the EM components and lower amounts of adherent bacterial cells. In addition, the structure of the co-aggregation was changed. There was a loss of long chain aggregation, which is common to Streptococci species common in oral biofilms. There was also both a higher abundance of small cell structures and the presence of dysmorphic globular cell structures. This became even more evident at 10% NAC concentrations. At 10% NAC, the few adherent structures were evident with a reduction of bacterial cells and extracellular matrix. Bacterial aggregation was rare with an almost complete loss of the presence of elongated chains. Adherent bacteria were associated with the presence of extracellular matrix, and a number of these bacteria also possessed filamentous form morphology.
Figure 3.
Scanning Electron Microscopy. A) 0% NAC (3000X). Biofilms showed the characteristic co-aggregation and extracellular matrix. B) 0% NAC (10,000X). Chains structures are evident within the co-aggregated matrix with surrounding matrix. C) 1% NAC (10,000X) show a loss of the EM, lower density of co-aggregation, and chain aggregation was mostly lost. An increase in small cell structures and dysmorphic globular large cell structures were seen. D) 10% NAC (10,000X) the few adherent bacteria have high presence of filamentous form morphology. Adherent cells were more likely to be associated with a small matrix. Bacterial aggregation was rare with a full loss of elongation chains.
Examining the Effect on Bacterial Ecology
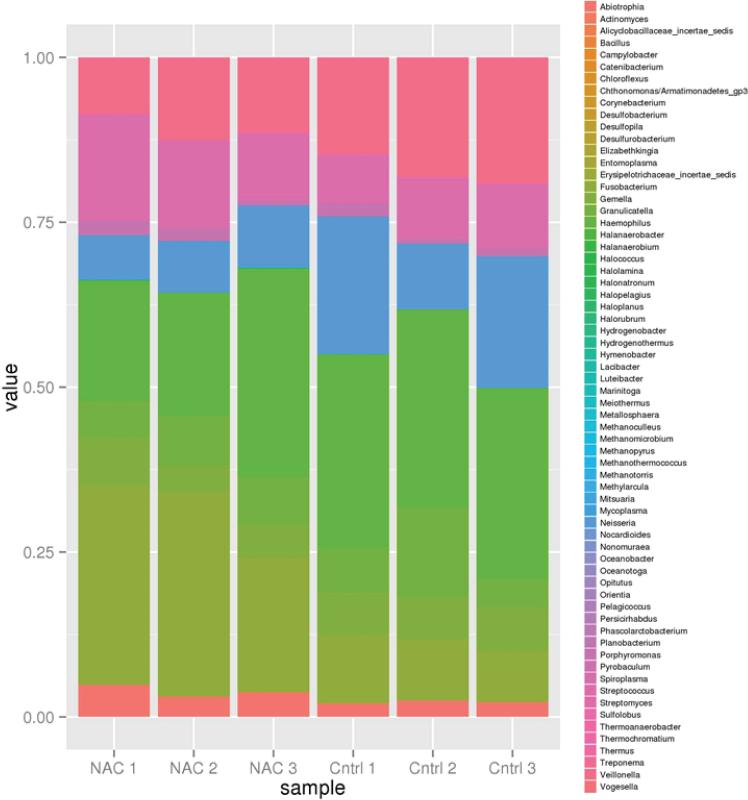
The MSPD biofilm model used in this study was composed of a diverse array of bacteria. We examined the effect of 1% NAC exposure versus the control (0% NAC) on the bacterial composition within these biofilms adherent on hydroxyapatite discs. 1% was chosen based on the SEM analysis results that confirmed that the bacteria morphology was not significantly altered at this concentration whereas bacteria exposed to 10% NAC showed abnormal filamentous morphologies. Examining the bacterial composition within the three control MSPD biofilms showed the diverse composition of these bacteria and the relative stability of the bacterial proportions at the genus level across replicates (Figure 4). The primary genera were: Abiotrophia, Fusobacterium, Gemella, Granulicatella, Haemophilus, Neisseria, Porphyromonas, Streptococcus, and Veillonella. 1% NAC (n=3) caused little variation versus 0% (n=3, controls) on the bacterial ecology within the biofilm. The only genera bordering on significantly changing was Fusobacterium which differed between the 2 samples. Fusobacterium was lower in the sample without NAC with a fold difference of 0.35 and an adjusted p-value of 0.05358 (using the Benjamini Hochberg adjustment). Overall, this supports the hypothesis that NAC had mostly a non-specific biofilm inhibitory affect.
Figure 4.
Effect of 1% NAC (n=3) versus 0% (n=3, controls) on the bacterial ecology within the biofilm. The primary genera are: Abiotrophia, Fusobacterium, Gemella, Granulicatella, Haemophilus, Neisseria, Porphyromonas, Streptococcus, and Veillonella. The bacterial ecology was nearly unchanged with 1% NAC. The only genera bordering on significantly changing was Fusobacterium which differed between the 2 samples. Fusobacterium was lower in the sample without NAC with a fold difference of 0.35 and an adjusted p-value of 0.05358 (using the Benjamini Hochberg adjustment).
Translational Significance
N-Acetyl-L-cysteine (NAC) inhibited multi-species biofilm formation on hydroxyapatite. It is important to consider the results of this study in the context of NAC at 1-10% concentration as a potential anti-plaque and caries management agent rather than as an antibiotic. NAC is a safe and biocompatible topical agent with potential to reduce biofilm formation rather than be used to ‘wipe’ out a large infection. This study showed how exposure to NAC can reduce total biofilm biomass. Importantly, NAC is different from triclosan and other anti-biofilm agents. Triclosan's emerging concerns come from its ubiquitous use in medicine, cosmetics, and cleaning agents and its high chemical stability that allows extended action to disrupt unintended microbial ecologies. NAC, on the other hand, is an acetylated amino acid that is mostly metabolized in the human body into cysteine and can then, in turn, be metabolized into glutathione (an anti-oxidant), and is proven to be extremely safe (Gillissen et al., 1997). There is potential for NAC to be used often and to add other active agents. The concentrations of NAC used in this study did not demonstrate an effect that would make it a profound oral antibiotic. A traditional antibiotic based assay such as examining the minimal bactericidal concentration would require reducing the bacteria by 99.9% which is relevant for endocarditis but not generally for oral biofilms. Caries management topical agents should be expected to reduce rather than eradicate biofilm formation. Recent computer modeling of oral biofilms illustrate how reducing the biofilm's mass may have profound effects in reducing the extended low pH challenge at the tooth interface(Ilie et al., 2012).
NAC at 1% and 10% led to a reduction of the total biomass and total adherent cells (live and dead). However, for the few cells that remain on the surface of the HA, the percentage of live bacterial cells for most samples still remained above 50%. This indicates that NAC did not selectively remove live or dead cells. It indicates that NAC was not exerting a bactericidal effect on the bacterial cells. This effect on biofilm formation was seen with 0.5% sucrose exposure. This sucrose concentration is a clinically relevant addition to the model system since it promotes both acid and polysaccharide production. More work is needed to examine the effect at higher sucrose concentrations.
Studying a diverse multi-species biofilm in vitro provides important insight on the potential of NAC to affect the bacterial ecology in vivo. We chose to the exam the effect of 1% NAC compared to a control since 1% NAC did not markedly change the bacterial morphology. Our results showed 1% NAC compared to the control of 0% NAC did not substantially alter the bacterial ecology within the biofilm.
The clinical significance of our study is that since NAC has the capability to be used over an extended period of time based on its ideal safety profile, which is especially important for pediatric use, a NAC based topical therapeutic can be made in rinse or gel formulations. This study leads to further investigation if NAC has the potential to be used as an anti-plaque/anti-biofilm and caries management agent (secondary prevention) for children and adults with chronic caries. Our results further explain results from a pilot study that showed NAC formulated in a pH 6.5 aqueous rinse, used several times a day for 7 days, reduced plaque levels 25.56%(Bowels and Goral, 1985). This is similar to the effect we saw in our 24 hour incubation experiment. The author of the clinical pilot was unsure of the mechanism of action for reducing plaque levels so significantly in such a short time. The fluorescence microscopy and SEM images of the MSPD biofilm results, along with our microtiter biofilm assays, clearly demonstrate that NAC is not just affecting the matrix production or aggregation but affecting the cellular function and morphology of the bacteria in the biofilm. This further supports that NAC has both extra- and intracellular effects on biofilm formation. More work is needed to investigate the ideal concentration that reduces biofilm formation while preserving biofilm ecology. Additional studies should examine the effect of N-Acetyl-L-cysteine on low-abundant potentially pathogenic species such as Streptococcus mutans within developing biofilms. A NAC-based therapeutic, which needs to be formulated with taste improving additives (Goyal et al., 2010) such as stevia(Das et al., 1992) or xylitol(Ly et al.,), may reduce dental plaque build-up by decreasing both biofilm matrix formation and bacterial growth.
Materials and Methods
Multi-Species Plaque Derived In Vitro Biofilms
In affiliate studies, we have characterized and banked a wide collection of multispecies biofilms derived from dental plaque in children (Reilly et al., 2014; Rudney et al., 2012). The biofilm model used in this study was chosen based on its ability to grow substantial adherent biofilm under the media conditions used in this study. The Multi-Species Plaque Derived (MSPD) in vitro biofilm model used in this study originated from a dental plaque sample taken from a high caries risk child. The process of collecting the dental plaque followed U of M Institutional Review Board (IRB) procedures. The dental plaque samples was taken from the supragingival (above the gumline) region of the tooth (lingual interproximal region of teeth #24 and 25). After the supragingival plaque was isolated, the plaque sample was placed in a vial of 0.5ml of 10 % (1:9 v/v) glycerol/modified Gibbons’ buffer solution and then frozen (−80°C)(Zhang et al., 2005). The protocol for developing multi-species plaque derived biofilms is found elsewhere (Reilly et al., 2014).
N-acetyl-L-cysteine Effects on Biofilm Formation
The stock MSPD biofilm was used to assess how concentrations of N-acetyl-L-cysteine (NAC, Sigma-Aldrich, St. Louis, MO) affected the growth of complex biofilms in basal medium mucin (BMM) media.
The biofilm stocks were recovered by use of an inoculation loop into the frozen stock samples. The inoculation loop immediately placed in a 100 ml 100% BMM solution overnight in an anaerobic chamber (10% Co2, 10% H, 80% N2, Coy Labs, Grass Lake, MI). BMM (pH 6.8) contains 2.5 g l−1 partially purified pig gastric mucin (type III; Sigma-Aldrich, St. Louis, MO), 5.0 g l−1 trypticase peptone (Fisher Scientific, Hampton, NH), 10.0 g l−1 proteose peptone (Fisher Scientific, Hampton, NH), 5.0 g l−1 yeast extract (Sigma-Aldrich, St. Louis, MO), 5 mg l−1 hemin (Fisher Scientific, Hampton, NH), 33.5 mmol l−1 KCl, 5.8 mmol l−1 menadione, 1 mmol l−1 urea and 1 mmol l−1 arginine (Sissons et al., 1991).
Separate NAC/BMM media with 0.5% sucrose was prepared. We tested 0%, 0.1%, 1%, and 10% NAC in BMM mixtures adjusted to pH 6.2-7.1 with 5M NaOH. Sterile compressed HA disks (Clarkson Chromatography Products Inc, USA) were placed at the bottom of 96 well plates. 135 μl (150 μl to control wells) of 0%, 0.1%, 1%, 10% of NAC in BMM were added to the wells. The OD600nm of the overnight inoculations were tested the following morning and the final OD600nm readings were adjusted to 0.2 ± 0.01. 15 μl of the adjusted inoculum was added to each well. Microplates were placed in the anaerobic chamber for 24 hour incubation to test the effect of NAC on biofilm formation.
A total of 96 HA disks were assessed. After disks were washed 3× with 200ml of 1×PBS, assays testing bacterial viability and subsequently biomass accumulation were performed with samples for each condition (n=24 for each condition 0%, 0.1%, 1%, 10%). Additional samples were used for qualitative analysis using fluorescent microscopy and scanning electron microscopy.
Bacterial Viability and Biofilm Formation
To assess the bacterial viability of cells adherent to the hydroxyapatite (HA) surfaces after 24 hours, we used a LIVE/DEAD® BacLight™ Bacterial Viability Assay using the green-fluorescent nucleic acid stain of SYTO®9 and the red-fluorescent nucleic acid stain, propidium iodide (PI). The fluorescence plate reader (Synergy HT, Biotek) that quantified components of the biofilm in this study used a beam thickness (FWHM) of 3 mm. Concurrently, FilmTracer™ SYPRO® Ruby biofilm matrix stain (Life Technologies, USA) was used to assess the biofilms. After these fluorescent assays, total biomass was immediately assessed with a crystal violet assay on matched disks(Chen et al., 2012). To visualize the bacteria and matrix aggregation incubated at different concentrations of NAC, fluorescence microscopy (Eclipse E800, Nikon) was used at the excitation/ emission wavelength of 560±20 nm/630±30 nm. This targeted both the SYPRO® Ruby biofilm matrix stain and the natural red fluorescence of these oral bacteria themselves with heme as a one of the nutrients within the media.
Scanning Electron Microscopy
Visualization of bacteria and grown biofilms was performed using a field-emission scanning electron microscope (6500 FE-SEM, JEOL, Japan). Bacteria directly on the HA disks were fixed with 2% glutaraldehyde and 0.15% alcian blue in 0.1 mol l−1 sodium cacodylate buffer (pH 7.4) for 1h at room temperature and then overnight at 4°C. They were secondarily fixed with 1% OsO4 in 0.1 mol l−1 sodium cacodylate buffer for 1 h. Then, samples were dehydrated with ethanol solutions at increasing concentrations, critical-point dried, and sputter coated with 5 nm Pt.
Biofilm Ecology Analysis
Biofilms exposed to 0% (n=3) and 1% (n=3) NAC were examined using 16S rRNA amplicon sequencing of the V4 region (MiSeq-Illumina). DNA isolation is described elsewhere (Reilly et al., 2014). 16S library preparation workflow followed Illumina technical guidelines with the notable exception of using qPCR to reduce amplification bias. qPCR was done prior to sequencing to aid in normalizing the initial loading concentration of our six samples. The forward primer (Meta_V4_515F) used was TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGCCAGCMGCCGCGGTAA, and the reverse primer (Meta_V4_806R) used was GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT. Upon obtaining FASTQ files from the sequencing center, we used the collection of programs packaged as UPARSE. We followed the default analysis algorithm with the exception that we allowed for staggered merges since many of the read pairs had total length greater than the amplicon lengths. Details of the UPARSE pipeline are described elsewhere (Edgar, 2013). We then used custom R scripts to create a data matrix in R.
Statistical Analysis
To estimate the effect of NAC concentration on biofilm formation and composition, 3 generalized linear models (GLMs) were fitted and confidence intervals were computed based on the parameter estimates. All models treated concentration as a factor since there was strong evidence against a linear fit. To examine NAC concentration on total biomass a GLM with a linear link was fit. To examine how NAC concentration impacted the number of total adherent cells a Poisson regression model was fit. To estimate the effect of concentration on the proportion of live cells a logistic regression was fit. To test for differences between bacterial levels as obtained from our data analysis pipeline using UPARSE the edgeR package was used (with trended and tagwise dispersion estimates). Parameter estimates for all GLMs were obtained using the customary iteratively reweighted least squares algorithm implemented in R (a statistical software package) v. 3.1.0.
Significance and Impact of the Study.
As a compound with a wide safety margin, N-acetyl-L-cysteine (NAC) has the potential to be used as a long term anti-plaque bacteriostatic agent for managing chronic dental decay without substantially altering biofilm's bacterial ecology. The potential anti-caries benefit of NAC is directly related to reducing the biofilm coverage which reduces the degree of acid generation and the amount of time that the surface is exposed to a lower pH.
Acknowledgements
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Conrado Aparicio for his help in our SEM assay.
Footnotes
Conflict of Interest
The authors have no financial involvement or affiliation with any entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
- Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, Dewhirst FE, Leys EJ, Paster BJ. Bacteria of dental caries in primary and permanent teeth in children and young adults. J. Clin. Microbiol. 2008;46:1407–17. doi: 10.1128/JCM.01410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. N-Acetylcysteine-a safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007;7:355–9. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo R, Lanata L, Egan CG. Mucoactive drugs. Eur. Respir. Rev. 2010;19:127–33. doi: 10.1183/09059180.00003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard GR. N-acetylcysteine in experimental and clinical acute lung injury. Am. J. Med. 1991;91:54S–59S. doi: 10.1016/0002-9343(91)90284-5. [DOI] [PubMed] [Google Scholar]
- Bowels QH, Goral V. Clinical trials of the anti-plaque activity of a mucolytic agent, N-Acetyl Cysteine. Dent. Hygene. 1985;59:454–6. [PubMed] [Google Scholar]
- Cammarota G, Sanguinetti M, Gallo A, Posteraro B. Review article: biofilm formation by Helicobacter pylori as a target for eradication of resistant infection. Aliment. Pharmacol. Ther. 2012;36:222–30. doi: 10.1111/j.1365-2036.2012.05165.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Rudney J, Aparicio C, Fok A, Jones RS. Quantifying dental biofilm growth using cross-polarization optical coherence tomography. Lett Appl Microbiol. 2012;54:537–542. doi: 10.1111/j.1472-765X.2012.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Das AK, Murphy RA, Punwani IC, Nasution MP, Kinghorn AD. Evaluation of the cariogenic potential of the intense natural sweeteners stevioside and rebaudioside A. Caries Res. 1992;26:363–6. doi: 10.1159/000261469. [DOI] [PubMed] [Google Scholar]
- Drago L, De Vecchi E, Mattina R, Romanò CL. Activity of N-acetyl-L-cysteine against biofilm of Staphylococcus aureus and Pseudomonas aeruginosa on orthopedic prosthetic materials. Int. J. Artif. Organs. 2013;36:39–46. doi: 10.5301/ijao.5000135. [DOI] [PubMed] [Google Scholar]
- Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Meth. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Firestone AR, Muhlemann HR. In vivo pH of plaque-covered and plaque-free interdental surfaces in humans following a sucrose rinse. Clin. Prev. Dent. 1985;7:24–26. [PubMed] [Google Scholar]
- Gillissen A, Jaworska M, Orth M, Coffiner M, Maes P, App EM, M CA, Schultze-Werninghaus Nacystelyn, a novel lysine salt of N-acetylcysteine, to augment cellular antioxidant defence in vitro. Respir. Med. 1997;91:159–168. doi: 10.1016/s0954-6111(97)90052-4. [DOI] [PubMed] [Google Scholar]
- Goyal SK, Samsher, Goyal RK. Stevia (Stevia rebaudiana) a bio-sweetener: a review. Int. J. Food Sci. Nutr. 2010;61:1–10. doi: 10.3109/09637480903193049. [DOI] [PubMed] [Google Scholar]
- Henke MO, Ratjen F. Mucolytics in cystic fibrosis. Paediatr. Respir. Rev. 2007;8:24–9. doi: 10.1016/j.prrv.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Ilie O, van Loosdrecht MCM, Picioreanu C. Mathematical modelling of tooth demineralisation and pH profiles in dental plaque. J. Theor. Biol. 2012;309:159–75. doi: 10.1016/j.jtbi.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Kory RC, Hirsch SR, Giraldo J. Nebulization of N-acetylcysteine combined with a bronchodilator in patients with chronic bronchitis. A controlled study. Dis. Chest. 1968;54:504–9. doi: 10.1378/chest.54.6.504. [DOI] [PubMed] [Google Scholar]
- Ly KA, Milgrom P, Rothen M. Xylitol, sweeteners, and dental caries. Pediatr. Dent. 2006;28:154–63. [PubMed] [Google Scholar]
- Maayan C, Bar-Yishay E, Yaacobi T, Marcus Y, Katznelson D, Yahav Y, Godfrey S. Immediate effect of various treatments on lung function in infants with cystic fibrosis. Respiration. 1989;55:144–51. doi: 10.1159/000195725. [DOI] [PubMed] [Google Scholar]
- Mansouri MD, Hull R a, Stager CE, Cadle RM, Darouiche RO. In vitro activity and durability of a combination of an antibiofilm and an antibiotic against vascular catheter colonization. Antimicrob. Agents Chemother. 2013;57:621–5. doi: 10.1128/AAC.01646-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millea PJ. N-acetylcysteine: multiple clinical applications. Am. Fam. Physician. 2009;80:265–9. [PubMed] [Google Scholar]
- Parry MF, Neu HC. Effect of N-acetylcysteine on antibiotic activity and bacterial growth in vitro. J. Clin. Microbiol. 1977;5:58–61. doi: 10.1128/jcm.5.1.58-61.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly C, Rasmussen K, Selberg T, Stevens J, Jones RS. Biofilm community diversity after exposure to 0·4% stannous fluoride gels. J. Appl. Microbiol. 2014;117:1798–1809. doi: 10.1111/jam.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudney JD, Chen R, Lenton P, Li J, Li Y, Jones RS, Reilly C, Fok AS, Aparicio C. A reproducible oral microcosm biofilm model for testing dental materials. J. Appl. Microbiol. 2012;113:1540–53. doi: 10.1111/j.1365-2672.2012.05439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissons CH, Cutress TW, Hoffman MP, Wakefield JS. A multi-station dental plaque microcosm (artificial mouth) for the study of plaque growth, metabolism, pH, and mineralization. J. Dent. Res. 1991;70:1409–16. doi: 10.1177/00220345910700110301. [DOI] [PubMed] [Google Scholar]
- Svensäter G, Larsson UB, Greif EC, Cvitkovitch DG, Hamilton IR. Acid tolerance response and survival by oral bacteria. Oral Microbiol. Immunol. 1997;12:266–73. doi: 10.1111/j.1399-302x.1997.tb00390.x. [DOI] [PubMed] [Google Scholar]
- Tanner ACR, Mathney JMJ, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, Papadopolou E, Dewhirst FE. Cultivable anaerobic microbiota of severe early childhood caries. J. Clin. Microbiol. 2011;49:1464–74. doi: 10.1128/JCM.02427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgenant CMC, van der Veen MH, de Soet JJ, ten Cate JM. Effect of metalloporphyrins on red autofluorescence from oral bacteria. Eur. J. Oral Sci. 2013;121:156–61. doi: 10.1111/eos.12045. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Larson C. The cariogenic dental biofilm: good, bad or just something to control? Braz. Oral Res. 2009;23:31–8. doi: 10.1590/s1806-83242009000500006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lei Y, Nobbs A, Khammanivong A, Herzberg MC. Inactivation of Streptococcus gordonii SspAB Alters Expression of Multiple Adhesin Genes. Infect. Immun. 2005;73:3351–3357. doi: 10.1128/IAI.73.6.3351-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Liu Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010;10:140. doi: 10.1186/1471-2180-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]