Abstract
Background
ABT-126 is a novel, safe and well-tolerated α7 nicotinic receptor agonist in a Phase 2 Alzheimer's disease study. Here we test the antidyskinetic effect of ABT-126 in MPTP-treated squirrel monkeys with moderate and more severe nigrostriatal damage.
Methods
Monkeys (n=21, Set 1) were lesioned with MPTP 1-2×. When parkinsonian, they were gavaged with levodopa (10 mg/kg)/carbidopa (2.5 mg/kg) twice daily and dyskinesias rated. They were then given nicotine in drinking water (n=5), or treated with vehicle (n=6) or ABT-126 (n=10) twice daily orally 30 min before levodopa. Set 1 was then re-lesioned 1-2 times for a total of 3-4 MPTP injections. The antidyskinetic effect of ABT-126, nicotine and the β2* nicotinic receptor agonist ABT-894 was re-assessed. Another group of monkeys (n=23, Set 2) was lesioned with MPTP only 1-2×. They were treated with levodopa/carbidopa, administered the α7 agonist ABT-107 (n=6), ABT-894 (n=6), nicotine (n=5) or vehicle (n=6) and dyskinesias evaluated. All monkeys were euthanized and the dopamine transporter measured.
Results
With moderate nigrostriatal damage (MPTP 1-2×), ABT-126 dose-dependently decreased dyskinesias (~60%), with similar results with ABT-894 (~60%) or nicotine (~60%). With more severe damage (MPTP 3-4×), ABT-126 and nicotine reduced dyskinesias, but ABT-894 did not. The dopamine transporter was 41% and 8.9% of control with moderate and severe nigrostriatal damage, respectively. No drug modified parkinsonism.
Conclusion
The novel α7 nicotinic receptor drug ABT-126 reduced dyskinesias in monkeys with both moderate and severe nigrostriatal damage. ABT-126 may be useful to reduce dyskinesias in both early and later stage Parkinson's disease.
Keywords: dyskinesia, levodopa, ABT-126, nicotinic, Parkinson's disease
A major limitation of levodopa (L-3,4-dihydroxyphenylalanine) therapy for Parkinson's disease (PD) is the development of abnormal involuntary movements or dyskinesias 1-5. Levodopa-induced dyskinesias (LIDs) arise in the majority of patients with continued use and can become debilitating. The only drug currently available for the treatment of LIDs is the N-methyl-D-aspartic acid receptor antagonist amantadine; however, it has limited efficacy and serious side effects can arise, including hallucinations and confusion 6-9. The development of more effective treatments for LIDs is therefore critical.
Although the mechanisms underlying LIDs are uncertain, numerous neurotransmitter systems have been implicated as potential therapeutic targets including the serotonergic, glutamatergic, GABAergic, opioid, adenosine and other systems 2, 10-16. Additionally, recent observations indicate a role for the nicotinic cholinergic system in LIDs. Evidence for this possibility initially stemmed from studies showing that the general nicotinic receptor (nAChR) agonist nicotine reduced LIDs in parkinsonian mice, rats and monkeys without worsening motor symptoms on or off levodopa 17-19. This reduction persisted for months, with no development of tolerance.
Nicotine exerts its effect primarily through nAChRs, a family of pentameric ligand-gated ion channels composed of homomeric arrangements of α (α7 or α9) subunits or heteromeric combinations of α (α2-α6) and β (β2-β4) subunits. Multiple receptors exist with α1β1*, α3β4* and α7 nAChRs comprising the major subtypes in the mammalian peripheral nervous system and α4β2*, α6β2* and α7 nAChRs the primary ones in the brain 20, 21. The asterisk denotes the possible presence of other nAChR subunits in the receptor.
The question thus arose whether drugs targeting nAChR subtypes in the brain may reduce LIDs, with fewer peripheral side effects. Preclinical studies showed that β2* nAChR agonists, including varenicline, A85380, sazetidine, and a series of Targacept, Inc. agonists reduced LIDs in 6-hydroxydopamine-lesioned rats 22, 23. Additionally, the β2* selective nAChR agonists TC-8831, ABT-894 and ABT-089 decreased LIDs in parkinsonian monkeys without affecting the therapeutic efficacy of levodopa 24-26. We recently also showed that the α7 nAChR agonist ABT-107 attenuated LIDs in parkinsonian monkeys 27.
The first objective of this study was to test the antidyskinetic effect of the novel α7 nAChR agonist ABT-126 ((1R, 4R, 5S)-4-(5-Phenyl-[1, 3, 4] thiadiazol-2-yloxy)-1-azatricyclo[3.3.1.1]decane) in MPTP-lesioned monkeys. The advantage of this drug is that it is reported to be safe and well tolerated in subjects with mild to moderate Alzheimer's disease 28. Thus it could readily be transitioned for use in PD patients. The second goal was to determine if ABT-126 reduced LIDs in monkeys with varying nigrostriatal damage. The present results are the first to show that the α7 nicotinic receptor agonist ABT-126 reduces LIDs in monkeys with both moderate and more severe nigrostriatal damage, suggesting it may be useful in both early and later stage PD.
Materials and Methods
Animal treatment and behavioral assessments
Adult squirrel monkeys (Saimiri sciureus) weighing 0.6-1.2 kg were obtained from World Wide Primates (Miami, FL). They were quarantined for one month, according to California state regulations. Animals were housed at 27 ± 3°C with a 12:12-h light/dark cycle. Monkey chow, fruits, and vegetables were provided throughout the day, with water freely available. All studies were done according to the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee at SRI.
After quarantine, all monkeys were singly housed and trained to perform various motor tasks17, 25, 29, 30. They were then injected with 2.0 mg/kg sc MPTP (Sigma-Aldrich, St. Louis, MO) dissolved in saline 17, 25, 29, 30. Parkinsonism was rated 3 to 4 wk later once weekly using a scale from 0 (normal) to 4 (severely parkinsonian). The maximum possible score is 28 based on seven parameters. These include spatial hypokinesia, body bradykinesia, manual dexterity in both hands, balance, freezing, and action tremor as described17, 25, 29, 30. Parkinsonism stabilized ~4 wk after MPTP treatment. Parkinsonism was rated immediately before and 90 min after L-dopa administration once weekly on Fridays throughout the study. A score of 3-4 was considered moderately parkinsonian. MPTP injection was repeated (1.8-2.0 mg/kg sc per time), if the monkey was not parkinsonian. All monkeys were injected with MPTP 1-2× (see timelines).
The monkeys were then gavaged with levodopa (10 mg/kg)/carbidopa (2.5 mg/kg) (unless otherwise indicated) twice daily 4.5 h apart 5 d per wk, as described 17, 25, 29, 30. The monkeys had previously received nAChR drugs 24, 25, with the present studies done after 1-2 months washout when LIDs were similar to vehicle-treated monkeys.
Animals were then divided into Set 1 and Set 2. Set 1 (n = 12 males and 9 females) was treated with ABT-126 (n=10), nicotine (n=5) or vehicle (n=6), after which the experiments in Figs. 1 and 2 were done. Set 1 monkeys were subsequently re-injected with MPTP for a total of 3-4 MPTP treatments (Fig. 3). The antidyskinetic effect of ABT-126, nicotine and ABT-894 was re-assessed (Fig. 3). The monkeys were subsequently euthanized, and the striatal dopamine transporter and α6β2* nAChRs measured (Figs. 4 and 5).
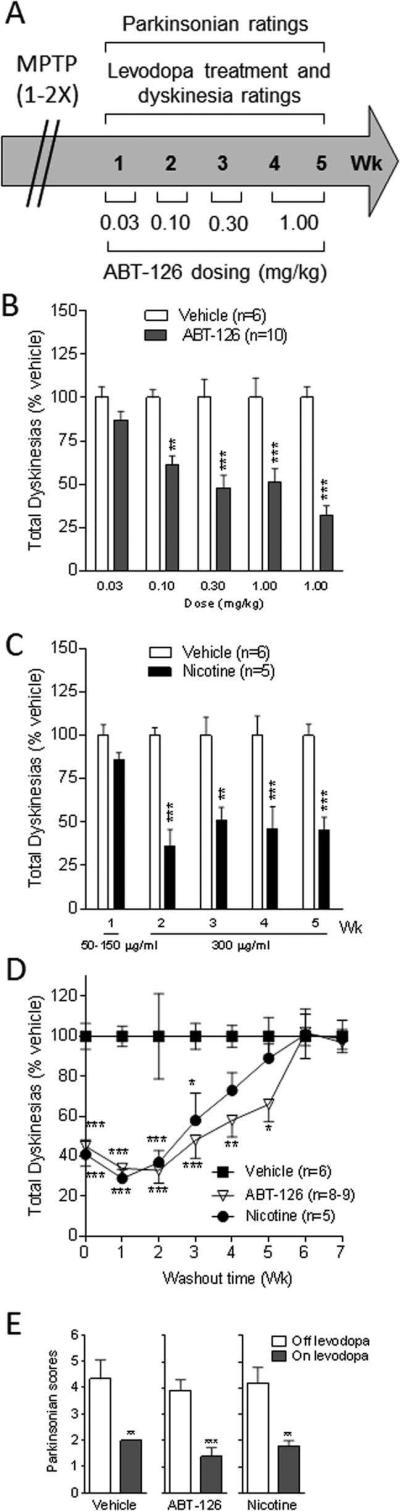
FIG. 1.
Dose-dependent decline in LIDs with the α7 nAChR agonist ABT-126 in Set 1 monkeys after they were treated with MPTP only 1-2×. Treatment timeline (A). Dose dependent decline in LIDs with ABT-126 (B). Nicotine reduces LIDs (C). Discontinuation of ABT-126 led to a return of LIDs to vehicle-treated levels (D). ABT-126 had no effect on parkinsonism off and on levodopa (E). Values are the mean ± SEM of the number of monkeys. Significantly different from vehicle, *P < 0.05, **P < 0.01, ***P < 0.001 using two-way ANOVA (A-D) or paired t-test (E).
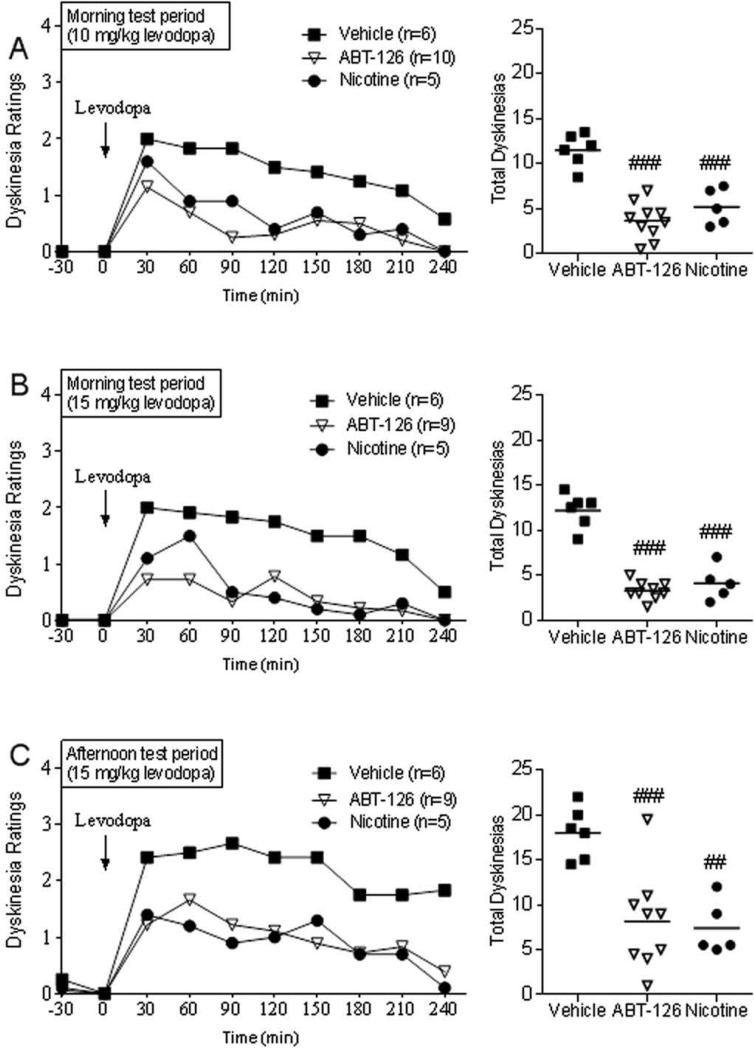
FIG. 2.
ABT-126 administration reduces the hourly time course of LIDs to a similar extent with 10 (A) or 15 mg/kg levodopa after either the morning or afternoon levodopa dose (B,C) in Set 1 monkeys (MPTP 1-2×). Values were averaged over 2 sessions per wk. Both ABT-126 (1 mg/kg) and nicotine treatments are significantly different from vehicle (P < 0.05) using a Friedman test. Effect of drugs on total LID scores (right), with the line depicting the mean. Significantly different from vehicle, ##P < 0.01, ###P < 0.001 using one-way ANOVA.
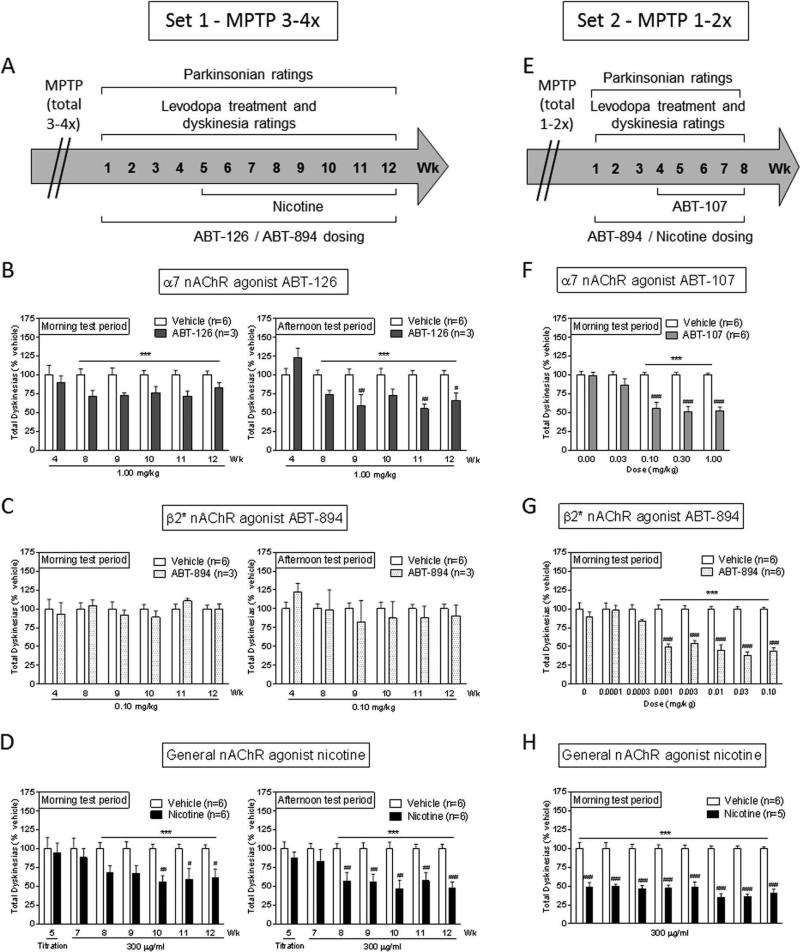
FIG. 3.
The α7 nAChR agonist ABT-126, but not the β2* nAChR agonist ABT-894, decreases LIDs in monkeys with severe (MPTP 3-4×) nigrostriatal damage. The data in the left panels is from Set 1 monkeys administered MPTP a total of 3-4× (A-D). The data in the right panels is from Set 2 monkeys given MPTP a total of 1-2× (E-H). Treatment timelines (A,E). α7 agonists and nicotine decrease LIDs in both sets of monkeys (B,D,F,H). ABT-894 reduces LIDs only in monkeys treated with MPTP 1-2× (C,G). Values are the mean ± SEM of the number of monkeys in parenthesis. Significantly different from vehicle, #P < 0.05, ##P < 0.01, ###P < 0.001 using two-way ANOVA. ***P < 0.001, main effect of drug treatment.
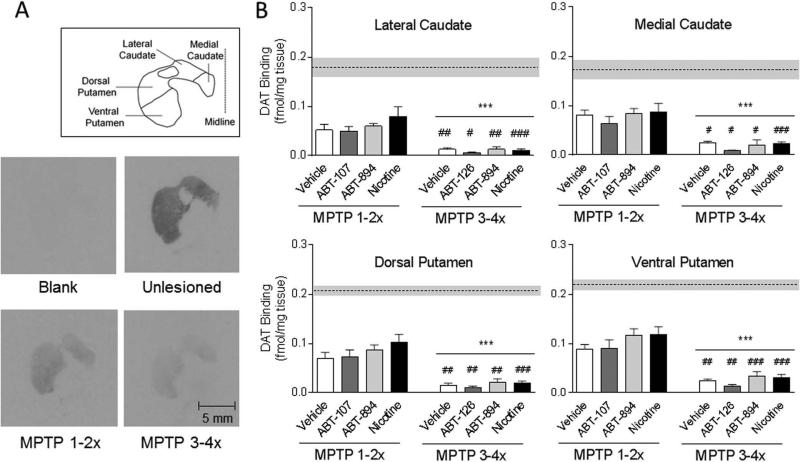
FIG. 4.
Significantly greater decline in the dopamine transporter in all striatal areas in monkeys treated with MPTP 3-4× compared to 1-2×. Autoradiograms are shown in A and the quantitative data in B. The dotted horizontal line and grey bar represent the mean ± SEM of the transporter levels in the unlesioned monkeys (n=5). Significantly different from MPTP 1-2×, #P < 0.05, ##P < 0.01, ###P < 0.001 using two-way ANOVA. ***P < 0.001, main effect of lesioning between MTP 1-2× compared to MPTP 3-4× group.
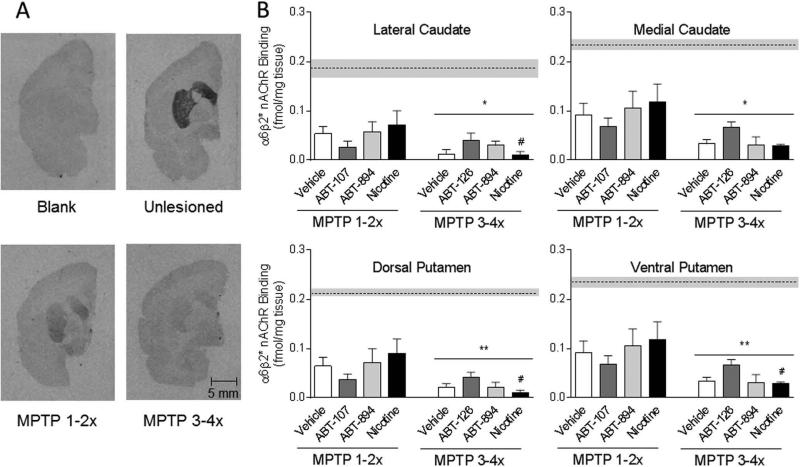
FIG. 5.
Significantly greater decline in α6β2* nAChRs in all striatal areas in monkeys treated with MPTP 3-4× compared to 1-2× times. Autoradiograms are shown in A and the quantitative data in B. The dotted horizontal line and grey bar represent the mean ± SEM of α6β2* nAChR levels in unlesioned monkeys (n=5). Significantly different from MPTP 1-2×, #P < 0.05 using two-way ANOVA. *P < 0.05, **P < 0.01, main effect of lesioning between MPTP 1-2× compared to MPTP 3-4× group.
Set 2 monkeys (n = 12 males and 11 females) were injected with MPTP for a total of only 1-2× (Fig. 3). They were then given levodopa until stably dyskinetic. Set 2 monkeys had received other nAChR drugs prior to the study depicted in Fig. 3 24, 25, with the present studies done after 1-2 months washout at which time LIDs were similar to vehicle-treated monkeys. Set 2 monkeys were next administered the α7 agonist ABT-107 (n=6), ABT-894 (n=6), nicotine (n=5) or vehicle (n=6) (Fig. 3), after which they were euthanized and the striatal dopamine transporter and α6β2* nAChRs assayed (Figs. 4 and 5).
ABT-107, ABT-126 and ABT-894 were administered orally in a small cracker at the doses indicated 30 min before levodopa/carbidopa twice daily 5 days/wk for 1 or more wk. Nicotine, included as a positive control, was provided in the drinking water starting at 50 μg/ml for 2 days, increased to 150 μg/ml for another two days and then maintained at 300 μg/ml 7 days/wk.
The monkeys were video-recorded at 8:00 AM, 30 min before their daily levodopa treatment to assess baseline dyskinetic movement. They were then rated for 4 h after levodopa treatment from the video-recordings for 1 min every 30 min by a blinded rater. Ratings of dyskinetic movements were on a scale of 0 (no dyskinesias) to 4 (severely dyskinetic) with: 1 = subtle dyskinesias that were not sustained (< 3 trunk movements in a row); 2 = sustained dyskinesias (≥ 3 trunk movements in a row); 3 = moderate dyskinesias that impair the ability to remain stationary; and 4 = severe dyskinesias that were generalized and incapacitating 17, 25, 29, 30. Dystonia was not evaluated. The LID scores for the different groups of monkeys were equivalent at the start of all vehicle/drug dosing regimens. The effect of the drugs on LIDs was tested for 2-3 days every wk, with the values shown per wk representing the average of 2 sessions during each wk.
Tissue preparation and autoradiography
Monkeys were euthanized as recommended by the Panel of Euthanasia of the American Veterinary Medical Association 10-30 d after discontinuation of nAChR drug treatment. They were injected with 1.5 ml Euthasol (390 mg sodium pentobarbital and 50 mg phenytoin sodium/ml ip; Butler Schein, Chicago, IL) for sedation, followed by 1.5 ml Euthasol iv for euthanasia. The brains were rapidly removed, rinsed in cold phosphate-buffered saline and cut into 2 mm-thick blocks using a squirrel monkey brain mold. These were quick frozen in isopentane on dry ice. Fourteen μm sections were prepared at −20°C using a cryostat. Frozen sections were mounted onto poly-L-lysine coated slides, dried and stored at −80°C.
Dopamine transporter autoradiography was done using 125I-RTI-121 (2200 Ci/mmol; PerkinElmer Life and Analytical Sciences, Boston, MA), as described 31. Thawed sections were pre-incubated in buffer and then re-exposed to the same buffer containing 0.025% bovine serum albumin, 1.0 μM fluoxetine and 5 pM 125I-RTI-121 for 2 h. Sections were washed as described 31. Background binding was evaluated in the presence of 100 μM of the dopamine uptake inhibitor nomifensine.
125I-α-CtxMII (2200 Ci/mmol) autoradiography was done as described 31. Sections were preincubated in buffer followed by incubation in binding buffer plus 0.5% bovine serum albumin, also containing 5 mM EDTA, 5 mM EGTA, and 10 μg/ml each of aprotinin, leupeptin, pepstatin A, and 0.4 nM 125I-α-CtxMII. To terminate the assay, slides were washed as described 31. Nicotine (100 μM) was used to determine nonspecific binding.
For both assays, sections were air dried and exposed to Kodak MR film for 7-10 d together with 125I-standards (American Radiolabeled chemicals, Inc., Saint Louis, MO). The ImageQuant program from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK) was used to obtain optical density values from autoradiographic images, which were converted to fmol/mg tissue using standard curves generated from the standards.
Statistics
Statistics were performed with GraphPad Prism using paired t-tests or ANOVA for multiple group comparisons followed by Newman-Keuls post hoc test (parametric statistics). Rating data is presented as scores or % vehicle. Values represent the mean ± SEM of the number of monkeys. Differences in rating scores between groups were analyzed using nonparametric tests (Friedman test) followed by Dunnett's multiple comparison test, with the results provided as the median (nonparametric). A value of P ≤ 0.05 was considered statistically significant.
Results
Moderately lesioned, levodopa-primed dyskinetic monkeys (Set 1, MPTP 1-2×) were divided into the following groups; vehicle-treated (n = 6), ABT-126-treated (n = 10) and nicotine-treated (n = 5) (Fig. 1). ABT-126 (0.1, 0.3 and 1.0 mg/kg) significantly reduced LIDs by 40-70% compared to the vehicle-treated group (Fig. 1B). Nicotine significantly attenuated LIDs (Fig. 1C), in agreement with previous studies 17, 32. To determine whether the ABT-126-induced reduction in LIDs persisted with drug removal, ABT-126 treatment was discontinued (Fig. 1D). LIDs returned to vehicle-treated levels after 4-6 wk of drug washout. ABT-126 did not affect parkinsonism either off or on levodopa, nor did nicotine (Fig 1E), consistent with previous results17, 29, 30.
The hourly time course of the ABT-126-induced decrease in LIDs is shown in Fig. 2A, with the effect of nicotine provided for comparison. The data points represent the average of two separate sessions within the same week, with the data derived from Fig. 1B and 1C. ABT-126 reduced LIDs over the entire time course. All monkeys were next given a higher dose of levodopa (15 mg/kg) to determine if ABT-126 could still reduce dyskinesias with increased drug. A comparable improvement in LIDs was observed with the 15 mg/kg levodopa dose (Fig. 2B). Additionally, we tested ABT-126's antidyskinetic effect with the morning and afternoon dose of levodopa since LIDs are generally higher in the afternoon (Fig. 2B and C). As expected, LID scores were higher (P < 0.01) in the afternoon (18.0 ± 1.8, n = 6) compared to the morning (12.2 ± 0.8, n = 6) (Fig. 2B, C) due to a carry-over effect from the morning levodopa dose. ABT-126 treatment resulted in a comparable decline in LIDs in the morning (71% reduction) and the afternoon (57% reduction), with similar results for nicotine.
We next examined whether the severity of nigrostriatal damage influenced the ability of ABT-126 to reduce LIDs. To approach this, the monkeys in Set 1 were administered 1 or 2 more doses of MPTP over a 2 month period so that all animals received a total of 3-4 doses of MPTP (Fig. 3). They were then orally given the α7 nAChR agonist ABT-126, the β2* nAChR agonist ABT-894 or nicotine (Fig. 3A-D). LIDs were scored both after the morning and afternoon dose of levodopa. The first data points shown in Fig. 3B and 3C are with 4 wk of treatment since there were no statistically significant differences between vehicle and drug in wk 1-3. ABT-126 and nicotine significantly reduced LIDs (40-50%); however, ABT-894 did not.
We also did a study in a second set (Set 2) of monkeys that were injected with MPTP for a total of 1-2× (Fig. 3 E-H). In this study, monkeys were only rated for LIDs following the morning dose of levodopa. The α7 nAChR agonist ABT-107, the β2* nAChR agonist ABT-894 and nicotine all reduced LIDs to ~60%. These data suggest β2* nAChR agonists exert a greater antidyskinetic effect in monkeys with moderate nigrostriatal damage.
Parkinsonism was measured 45 min before and 90 min after levodopa treatment. None of the nAChR drugs affected parkinsonism with or without levodopa treatment, consistent with previous results in monkeys 17, 19, 24, 29, 33. Values for monkeys treated with MPTP 3-4× (Set 1) are as follows (mean ± SEM): vehicle off levodopa 3.8±0.6(n=6), vehicle on levodopa 0.3±0.2(n=6) (***P<0.001); ABT-126 (1.0 mg/kg) off levodopa 3.7±0.5(n=3), ABT-126 (1.0 mg/kg) on levodopa 2.3±0.4(n=3) (*P<0.05); ABT-894 (0.1 mg/kg) off levodopa 3.4±0.1(n=3), ABT-894 (0.1 mg/kg) on levodopa 2.3±0.3(n=3) (*P<0.05); nicotine off levodopa 4.2±0.8(n=6), nicotine on levodopa 2.4±0.2(n=6) (*P<0.05). Values for monkeys treated with MPTP 1-2× (Set 2) are as follows (mean ± SEM): vehicle off levodopa 4.3±0.8(n=6), vehicle on levodopa 2.7±0.5(n=6) (**P<0.01); ABT-107 (0.1 mg/kg) off levodopa 4.8±0.4(n=6), ABT-107 (0.1 mg/kg) on levodopa 2.8±0.2(n=6) (**P<0.01); ABT-894 (0.1 mg/kg) off levodopa 3.4±0.1(n=3), ABT-894 (0.1 mg/kg) on levodopa 2.3±0.3(n=3) (*P<0.05); nicotine off levodopa 3.6±0.5(n=5), nicotine on levodopa 2.6±0.4(n=5) (*P<0.05).
The dopamine transporter was measured to determine the extent of nigrostriatal damage in the Set 1 (overall MPTP treatment 3-4×) and Set 2 monkeys (overall MPTP treatment 1-2×) (Fig. 4). α6β2* nAChRs were also measured since they are present on striatal dopaminergic terminals and thus represent another index of nigrostriatal integrity (Fig. 5). There were no statistically significant differences in the transporter or α6β2* nAChR levels between vehicle and nAChR drug within either the MPTP 1-2× lesion group or the MPTP 3-4× lesion group. However, the decline in both the transporter and α6β2* nAChRs was significantly greater in all striatal areas with the more extensive MPTP treatment (3-4×). Fifty to 70% decreases were observed in the transporter and α6β2* nAChRs with 1-2 MPTP injections but 75-95% declines with exposure to 3-4 MPTP treatments. These data suggest that a partially intact nigrostriatal dopaminergic system, possessing α6β2* nAChRs, is essential for the antidyskinetic effect of β2* nAChR drugs.
α7 nAChRs were not measured in the current study because these receptors are not detectable in monkey striatum using available radioactive ligands (Quik et al., unpublished data), consistent with results in rats 34.
Discussion
The present results are the first to show that the novel α7 nAChR agonist ABT-126 reduces LIDs without worsening parkinsonism in a nonhuman primate model of PD. Tolerance to the antidyskinetic effect of ABT-126 did not develop over the course of the current study. ABT-126 administration did not cause side effects such as emesis, an adverse response common to other nAChR drugs 25, 35. The decline in LIDs persisted for several wk after ABT-126 discontinuation indicating that long-term molecular changes are involved. ABT-126 reduced LIDs in monkeys with both moderate and more severe nigrostriatal damage. These findings suggest that ABT-126 may be a good antidyskinetic agent in both early and later stage PD.
ABT-126 is an agonist that binds with high affinity to α7 nAChRs in human brain (Ki = 12.3 nM) and activates currents in Xenopus oocytes expressing recombinant human α7 nAChRs (EC50 = 2.0 μM; intrinsic activity of 74% relative to acetylcholine) 36. In contrast, ABT-126 has low affinity for α4β2* nAChRs in human cortex (Ki = 1740 nM). ABT-126 does bind to α3β4* nAChRs in human IMR-32 neuroblastoma cells (Ki = 60 nM), but has only 12% efficacy at 100,000 nM in a calcium flux assay in these cell. Like some other α7 nAChR agonists, ABT-126 is also a 5-HT3 receptor antagonist, but it has >10-fold lower affinity for this receptor than for α7 nAChRs (Ki of 140 nM). Based on this profile, the agonist activity of ABT-126 appears restricted to α7 nAChRs and binding at other nAChRs or other receptor types is not expected to contribute to its efficacy profile.
The demonstration that an α7 nAChR targeted drug can modulate the occurrence of LIDs is consistent with previous studies in parkinsonian mice and monkeys. Deletion of the α7 nAChR increased levodopa-induced abnormal involuntary movements in mutant mice compared to wild type littermates 37. In addition, the present and previous studies 27 showed that another α7 nAChR agonist ABT-107 reduced LIDs by ~60% without affecting parkinsonism on and off levodopa, similar to ABT-126. The novelty of the current study lies in the fact that ABT-126 may represent a viable clinical candidate for PD patients. This latter drug demonstrated an adequate safety profile and an efficacy signal in a randomized double-blind, placebo- and active-controlled, multicenter Phase 2a study in subjects with mild to moderate Alzheimer's disease 28. By contrast, ABT-107 was discontinued from clinical development based on safety signals from preclinical studies (http://www.euroinvestor.dk/pdf/cse/263716-0.pdf).
Our studies showed that ABT-126 reduced LIDs with both moderate and severe nigrostriatal damage. This is not unexpected since α7 nAChRs are primarily present in the cortex, cerebellum, thalamus and hippocampus, which are not affected by MPTP treatment 21. In fact, there are few α7 nAChRs in striatum, although they are present at low density in the nigra 20, 21. Nigral α7 nAChRs may be involved in the ABT-126 observed decline in LIDs. However, a more likely explanation is that other brain areas are involved 38. Accumulating pre-clinical experiments indicate a role for the cortex, with LIDs correlating with supersensitive excitatory transmission at corticostriatal synapses 39 and high-frequency cortical oscillations 40. Additionally, human neuroimaging studies suggest that abnormal dopaminergic modulation of striato-cortical networks underlies LIDs 41, and that alterations in activity of frontal cortex areas were linked to LIDs in PD patients 42, 43. Dyskinetic patients exhibited decreased activity of the right inferior frontal cortex after levodopa, whereas patients without dyskinesias showed a reverse effect. Accumulating evidence also implicates the cerebellum in LIDs possibly mediated through the primary motor cortex 44, 45. In fact, there appears to be a dysregulation of function in numerous brain regions with the occurrence of LIDs, any of which may disrupt motor execution 46. Overall, LIDs appear to be complex in origin with the involvement of numerous brain circuits.
By contrast to the results with ABT-126, ABT-894 did not reduce LIDs in severely lesioned monkeys. This is probably because ABT-894 is a selective agonist at β2* nAChR, which are present on dopaminergic terminals that are markedly reduced in severely lesioned monkeys. Thus, the lack of effectiveness of ABT-894 may be because β2* nAChRs are greatly reduced in more severely lesioned monkeys.
The present studies also show that nicotine reduced LIDs in severely-lesioned monkeys, in contrast to previous results 30. A possible explanation may relate to the fact that the monkeys in the severely-lesioned group in the present experiments were less parkinsonian (average scores = 4) compared to the earlier study (average score = 9.4) 30, despite the low transporter levels in this study. Another difference may relate to the experimental duration, which was 16 months compared to only 8 months for the prior study 17, 30. Monkeys become much more responsive to nicotine with continued treatment 17. Indeed, in both Set 1 and in Set 2 monkeys the ability of nicotine to reduce LIDs appeared more pronounced than other nAChR drugs. However, this may have been due to the fact that nicotine was included in the drinking water ad libitum 7 d per wk, while other drugs were administered twice daily 5 d per wk.
The finding that LIDs remain depressed for several weeks after nAChR drugs are discontinued suggest that long term adaptations are involved. This may include altered signaling via α7 nAChRs present presynaptically on cortical glutamatergic efferents to modulate neurotransmitter release, postsynaptically to mediate intracellular transduction mechanisms and also at perisynaptic sites where the receptors exert a variety of modulatory effects 47-50. The initial step most likely involves alterations in calcium influx and/or release from internal stores 51, 52. This may lead to an activation of Ca2+/calmodulin-dependent protein kinase (CaMK) and mitogen-activated protein kinase (MAPK), which in turn activate various transcription factors including cAMP response element-binding protein (CREB) and others with long-term alterations in gene expression 53, 54. These changes lead to α7 nAChR-mediated alterations in synaptic plasticity and structural remodeling.
Other cellular changes that underlie LIDs may include alterations in striatal dopamine release. It is well established that LIDs are associated with an aberrant dopamine release from striatal terminals 62. Nicotine decreases dopamine release via β2* nAChR-mediated desensitization and down regulation 63. Striatal α7 nAChRs on glutamatergic afferents from the cortex may be similarly involved. Stimulation of these α7 nAChRs increases glutamate release, which in turn acts at glutamate receptors on dopamine terminals to modulate dopamine release/turnover 64. Additionally, α7 nAChRs in the substantia nigra may influence the release of striatal dopamine 65.
The decline in LIDs with the α7 nAChR agonist ABT-126 was not complete, with an average 60% reduction compared to vehicle-treated monkeys. These results resemble those with the α7 nAChR agonist ABT-107, which also yielded a 60% decline in LIDs 27. Similarly, β2* nAChR agonist resulted in comparable maximal reductions in LIDs 24. These findings support the idea that multiple neurotransmitter systems regulate the occurrence of LIDs, with the nicotinic cholinergic system representing only one. Therapies targeting several neurotransmitter systems may therefore be necessary to completely block LIDs. In fact, current clinical and preclinical research is focused on developing and testing drug combinations that stimulate more than one neurotransmitter systems 66-69.
In summary, the results show that the α7 nAChR agonist ABT-126 significantly reduces LIDs in parkinsonian monkeys, with no development of tolerance or worsening of parkinsonism. Moreover, ABT-126 was nearly as effective in monkeys with severe and moderate nigrostriatal damage. These data indicate that ABT-126 may represent a good candidate for the treatment of LIDs in PD. An added advantage is that α7 nAChR agonists improve memory and learning deficits in preclinical animal models 70-72 and have been shown to improve some cognitive components in schizophrenia and/or Alzheimer disease 28, 73-75.
Acknowledgement
We thank AbbVie, Inc. for providing ABT-107, ABT-126 and ABT-894.
Funding sources: This study was supported by NIH grant NS59910 and AbbVie, Inc.
Footnotes
Financial disclosures/Conflict of interest: MWD is employed by and owns stock in AbbVie, Inc. There are no other conflicts of interest or disclosures.
-
1)Research project: A. Conception, M. Quik B. Organization, M. Quik, D. Zhang, M. McGregor, C. Execution, D. Zhang, M. McGregor, X. Perez, T. Bordia
-
2)Statistical Analysis: D. Zhang, M. McGregor, M. Quik,
-
3)Manuscript: A. Writing of the first draft, M. Quik, D. Zhang B. Review and Critique, M. Quik, D. Zhang, M. McGregor, X. Perez, T. Bordia, J.M. McIntosh, M.W. Decker
References
- 1.Iravani MM, McCreary AC, Jenner P. Striatal plasticity in Parkinson's disease and L-DOPA induced dyskinesia. Parkinsonism Relat Disord. 2012;18(Suppl 1):S123–125. doi: 10.1016/S1353-8020(11)70038-4. [DOI] [PubMed] [Google Scholar]
- 2.Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. The pharmacology of L-DOPA-induced dyskinesia in Parkinson's disease. Pharmacol Rev. 2013;65(1):171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- 3.Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson's disease. Mov Disord. 2011;26(6):1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 4.Meissner WG, Frasier M, Gasser T, et al. Priorities in Parkinson's disease research. Nat Rev Drug Discov. 2011;10(5):377–393. doi: 10.1038/nrd3430. [DOI] [PubMed] [Google Scholar]
- 5.Obeso JA, Rodriguez-Oroz MC, Goetz CG, et al. Missing pieces in the Parkinson's disease puzzle. Nat Med. 2010;16(6):653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- 6.Crosby NJ, Deane KH, Clarke CE. Amantadine for dyskinesia in Parkinson's disease. Cochrane Database Syst Rev. 2003;(2):CD003467. doi: 10.1002/14651858.CD003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luginger E, Wenning GK, Bosch S, Poewe W. Beneficial effects of amantadine on L-dopa-induced dyskinesias in Parkinson's disease. Mov Disord. 2000;15(5):873–878. doi: 10.1002/1531-8257(200009)15:5<873::aid-mds1017>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Rodnitzky RL, Narayanan NS. Amantadine's role in the treatment of levodopa-induced dyskinesia. Neurology. 2014;82(4):288–289. doi: 10.1212/WNL.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of amantadine benefit on dyskinesia of severe Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75(1):141–143. [PMC free article] [PubMed] [Google Scholar]
- 10.Rylander D. The serotonin system: a potential target for anti-dyskinetic treatments and biomarker discovery. Parkinsonism Relat Disord. 2012;18(Suppl 1):S126–128. doi: 10.1016/S1353-8020(11)70039-6. [DOI] [PubMed] [Google Scholar]
- 11.Gasparini F, Di Paolo T, Gomez-Mancilla B. Metabotropic glutamate receptors for Parkinson's disease therapy. Parkinsons Dis. 2013;2013:196028. doi: 10.1155/2013/196028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blandini F, Armentero MT. New pharmacological avenues for the treatment of L-DOPA-induced dyskinesias in Parkinson's disease: targeting glutamate and adenosine receptors. Expert Opin Investig Drugs. 2012;21(2):153–168. doi: 10.1517/13543784.2012.651457. [DOI] [PubMed] [Google Scholar]
- 13.Sgambato-Faure V, Cenci MA. Glutamatergic mechanisms in the dyskinesias induced by pharmacological dopamine replacement and deep brain stimulation for the treatment of Parkinson's disease. Prog Neurobiol. 2012;96(1):69–86. doi: 10.1016/j.pneurobio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Duty S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson's disease. CNS Drugs. 2012;26(12):1017–1032. doi: 10.1007/s40263-012-0016-z. [DOI] [PubMed] [Google Scholar]
- 15.Brotchie J, Jenner P. New approaches to therapy. Int Rev Neurobiol. 2011;98:123–150. doi: 10.1016/B978-0-12-381328-2.00005-5. [DOI] [PubMed] [Google Scholar]
- 16.Fox SH, Chuang R, Brotchie JM. Serotonin and Parkinson's disease: On movement, mood, and madness. Mov Disord. 2009 doi: 10.1002/mds.22473. [DOI] [PubMed] [Google Scholar]
- 17.Quik M, Mallela A, Ly J, Zhang D. Nicotine reduces established levodopa-induced dyskinesias in a monkey model of Parkinson's disease. Mov Disord. 2013;28(10):1398–1406. doi: 10.1002/mds.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Grady SR, Quik M. Nicotine Reduces L-Dopa-Induced Dyskinesias by Acting at {beta}2 Nicotinic Receptors. J Pharmacol Exp Ther. 2011;338:932–941. doi: 10.1124/jpet.111.182949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bordia T, Campos C, Huang L, Quik M. Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson's disease. J Pharmacol Exp Ther. 2008;327(1):239–247. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- 20.Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56(1):237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 21.Quik M, Wonnacott S. {alpha}6{beta}2* and {alpha}4{beta}2* Nicotinic Acetylcholine Receptors As Drug Targets for Parkinson's Disease. Pharmacol Rev. 2011;63(4):938–966. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang LZ, Campos C, Ly J, Carroll FI, Quik M. Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in moderately lesioned parkinsonian rats. Neuropharmacology. 2011;60:861–868. doi: 10.1016/j.neuropharm.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quik M, Campos C, Bordia T, et al. alpha4beta2 nicotinic receptors play a role in the nAChR-mediated decline in l-dopa-induced dyskinesias in parkinsonian rats. Neuropharmacology. 2013;71:191–203. doi: 10.1016/j.neuropharm.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang D, Bordia T, McGregor M, McIntosh JM, Decker MW, Quik M. ABT-089 and ABT-894 reduce levodopa-induced dyskinesias in a monkey model of Parkinson's disease. Mov Disord. 2014;29(4):508–517. doi: 10.1002/mds.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, Mallela A, Sohn D, et al. Nicotinic receptor agonists reduce L-DOPA-induced dyskinesias in a monkey model of Parkinson's disease. J Pharmacol Exp Ther. 2013;347(1):225–234. doi: 10.1124/jpet.113.207639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston TH, Huot P, Fox SH, et al. TC-8831, a nicotinic acetylcholine receptor agonist, reduces L-DOPA-induced dyskinesia in the MPTP macaque. Neuropharmacology. 2013;73:337–347. doi: 10.1016/j.neuropharm.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, McGregor M, Decker MW, Quik M. The α7 nicotinic receptor agonist ABT-107 decreases L-dopa-induced dyskinesias in parkinsonian monkeys. Society for Neuroscience Abstr. 2014:2014. doi: 10.1124/jpet.114.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gault L, Ritchie CW, Robieson WZ, Pritchett Y, Othman AA, Lenz RA. A phase 2 randomized, controlled trial of the α7 agonist ABT-126 in mild-to-moderate Alzheimer's dementia. Alzheimer's & Dementia: Alzheimer's & Dementia: Translational Research & Clinical Interventions. 2015 doi: 10.1016/j.trci.2015.06.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quik M, Cox H, Parameswaran N, O'Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Annals of neurology. 2007;62:588–596. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- 30.Quik M, Mallela A, Chin M, McIntosh JM, Perez XA, Bordia T. Nicotine-mediated improvement in l-dopa-induced dyskinesias in MPTP-lesioned monkeys is dependent on dopamine nerve terminal function. Neurobiol Dis. 2013;50:30–41. doi: 10.1016/j.nbd.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quik M, Polonskaya Y, Kulak JM, McIntosh JM. Vulnerability of 125I-alpha-conotoxin MII binding sites to nigrostriatal damage in monkey. J Neurosci. 2001;21(15):5494–5500. doi: 10.1523/JNEUROSCI.21-15-05494.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quik M, Cox H, Parameswaran N, O'Leary K, Langston JW, Di Monte D. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Annals of neurology. 2007;62(6):588–596. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- 33.Bordia T, Campos C, McIntosh JM, Quik M. Nicotinic receptor-mediated reduction in L-dopa-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther. 2010;333:929–938. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke PB, Pert A. Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain research. 1985;348(2):355–358. doi: 10.1016/0006-8993(85)90456-1. [DOI] [PubMed] [Google Scholar]
- 35.Swan GE, Javitz HS, Jack LM, et al. Varenicline for smoking cessation: nausea severity and variation in nicotinic receptor genes. Pharmacogenomics J. 2011 doi: 10.1038/tpj.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gopalakrishnan M, Bitner R, Anderson D, et al. Pharmacological Characterization of ABT-126: A Novel α7 Cholinergic Receptor Agonist for the Potential Treatment of Cognitive Deficits Associated with Alzheimer's Disease and Schizophrenia. Submitted. [Google Scholar]
- 37.Quik M, Campos C, Grady SR. Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias: studies with parkinsonian nicotinic receptor knockout mice. Biochem Pharmacol. 2013;86(8):1153–1162. doi: 10.1016/j.bcp.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothwell JC, Obeso JA. Can levodopa-induced dyskinesias go beyond the motor circuit? Brain. 2015;138(Pt 2):242–244. doi: 10.1093/brain/awu365. [DOI] [PubMed] [Google Scholar]
- 39.Ueno T, Yamada J, Nishijima H, et al. Morphological and electrophysiological changes in intratelencephalic-type pyramidal neurons in the motor cortex of a rat model of levodopa-induced dyskinesia. Neurobiol Dis. 2014 doi: 10.1016/j.nbd.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Richter U, Halje P, Petersson P. Mechanisms underlying cortical resonant states: implications for levodopa-induced dyskinesia. Rev Neurosci. 2013;24(4):415–429. doi: 10.1515/revneuro-2013-0018. [DOI] [PubMed] [Google Scholar]
- 41.Herz DM, Haagensen BN, Christensen MS, et al. Abnormal dopaminergic modulation of striato-cortical networks underlies levodopa-induced dyskinesias in humans. Brain. 2015;138(Pt 6):1658–1666. doi: 10.1093/brain/awv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cerasa A, Donzuso G, Morelli M, et al. The motor inhibition system in Parkinson's disease with levodopa-induced dyskinesias. Mov Disord. 2015 doi: 10.1002/mds.26378. [DOI] [PubMed] [Google Scholar]
- 43.Cerasa A, Koch G, Donzuso G, et al. A network centred on the inferior frontal cortex is critically involved in levodopa-induced dyskinesias. Brain. 2015;138(Pt 2):414–427. doi: 10.1093/brain/awu329. [DOI] [PubMed] [Google Scholar]
- 44.Kishore A, Popa T. Cerebellum in levodopa-induced dyskinesias: the unusual suspect in the motor network. Front Neurol. 2014;5:157. doi: 10.3389/fneur.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kishore A, Popa T, Balachandran A, et al. Cerebellar sensory processing alterations impact motor cortical plasticity in Parkinson's disease: clues from dyskinetic patients. Cereb Cortex. 2014;24(8):2055–2067. doi: 10.1093/cercor/bht058. [DOI] [PubMed] [Google Scholar]
- 46.Engeln M, De Deurwaerdere P, Li Q, Bezard E, Fernagut PO. Widespread Monoaminergic Dysregulation of Both Motor and Non-Motor Circuits in Parkinsonism and Dyskinesia. Cereb Cortex. 2015;25(9):2783–2792. doi: 10.1093/cercor/bhu076. [DOI] [PubMed] [Google Scholar]
- 47.Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283(3):1396–1411. [PubMed] [Google Scholar]
- 48.Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18(20):8485–8495. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383(6602):713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- 50.Radcliffe KA, Fisher JL, Gray R, Dani JA. Nicotinic modulation of glutamate and GABA synaptic transmission of hippocampal neurons. Ann N Y Acad Sci. 1999;868:591–610. doi: 10.1111/j.1749-6632.1999.tb11332.x. [DOI] [PubMed] [Google Scholar]
- 51.Barrantes GE, Murphy CT, Westwick J, Wonnacott S. Nicotine increases intracellular calcium in rat hippocampal neurons via voltage-gated calcium channels. Neuroscience letters. 1995;196(1-2):101–104. doi: 10.1016/0304-3940(95)11859-u. [DOI] [PubMed] [Google Scholar]
- 52.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13(2):596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson's disease. Mov Disord. 2012;27(8):947–957. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25(6):317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Fieblinger T, Cenci MA. Zooming in on the small: The plasticity of striatal dendritic spines in l-DOPA-Induced dyskinesia. Mov Disord. 2015;30:484–493. doi: 10.1002/mds.26139. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Meredith GE, Mendoza-Elias N, Rademacher DJ, Tseng KY, Steece-Collier K. Aberrant restoration of spines and their synapses in L-DOPA-induced dyskinesia: involvement of corticostriatal but not thalamostriatal synapses. J Neurosci. 2013;33(28):11655–11667. doi: 10.1523/JNEUROSCI.0288-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suarez LM, Solis O, Carames JM, et al. L-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiatry. 2014;75(9):711–722. doi: 10.1016/j.biopsych.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Nishijima H, Suzuki S, Kon T, et al. Morphologic changes of dendritic spines of striatal neurons in the levodopa-induced dyskinesia model. Mov Disord. 2014;29(3):336–343. doi: 10.1002/mds.25826. [DOI] [PubMed] [Google Scholar]
- 59.Coronas V, Durand M, Chabot JG, Jourdan F, Quirion R. Acetylcholine induces neuritic outgrowth in rat primary olfactory bulb cultures. Neuroscience. 2000;98(2):213–219. doi: 10.1016/s0306-4522(00)00143-3. [DOI] [PubMed] [Google Scholar]
- 60.Pugh PC, Berg DK. Neuronal acetylcholine receptors that bind alpha-bungarotoxin mediate neurite retraction in a calcium-dependent manner. J Neurosci. 1994;14(2):889–896. doi: 10.1523/JNEUROSCI.14-02-00889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan J, Quik M. A role for the nicotinic alpha-bungarotoxin receptor in neurite outgrowth in PC12 cells. Neuroscience. 1993;56(2):441–451. doi: 10.1016/0306-4522(93)90344-f. [DOI] [PubMed] [Google Scholar]
- 62.Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007;30(5):236–243. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Bordia T, McIntosh JM, Quik M. The nicotine-mediated decline in l-dopa-induced dyskinesias is associated with a decrease in striatal dopamine release. J Neurochem. 2013;125:291–302. doi: 10.1111/jnc.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaiser S, Wonnacott S. alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58(2):312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- 65.Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21(5):1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hill MP, Ravenscroft P, Bezard E, et al. Levetiracetam potentiates the antidyskinetic action of amantadine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primate model of Parkinson's disease. J Pharmacol Exp Ther. 2004;310(1):386–394. doi: 10.1124/jpet.104.066191. [DOI] [PubMed] [Google Scholar]
- 67.Kobylecki C, Hill MP, Crossman AR, Ravenscroft P. Synergistic antidyskinetic effects of topiramate and amantadine in animal models of Parkinson's disease. Mov Disord. 2011;26(13):2354–2363. doi: 10.1002/mds.23867. [DOI] [PubMed] [Google Scholar]
- 68.Bezard E, Tronci E, Pioli EY, et al. Study of the Antidyskinetic Effect of Eltoprazine in Animal Models of Levodopa-Induced Dyskinesia. Mov Disord. 2013 doi: 10.1002/mds.25366. [DOI] [PubMed] [Google Scholar]
- 69.Ko WK, Pioli E, Li Q, et al. Combined fenobam and amantadine treatment promotes robust antidyskinetic effects in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primate model of Parkinson's disease. Mov Disord. 2014 doi: 10.1002/mds.25859. [DOI] [PubMed] [Google Scholar]
- 70.Levin ED. alpha7-Nicotinic receptors and cognition. Curr Drug Targets. 2012;13(5):602–606. doi: 10.2174/138945012800398937. [DOI] [PubMed] [Google Scholar]
- 71.Callahan PM, Hutchings EJ, Kille NJ, Chapman JM, Terry AV., Jr Positive allosteric modulator of alpha7 nicotinic-acetylcholine receptors, PNU-120596 augments the effects of donepezil on learning and memory in aged rodents and non-human primates. Neuropharmacology. 2013;67:201–212. doi: 10.1016/j.neuropharm.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pirttimaki TM, Codadu NK, Awni A, et al. alpha7 Nicotinic receptor-mediated astrocytic gliotransmitter release: Abeta effects in a preclinical Alzheimer's mouse model. PloS one. 2013;8(11):e81828. doi: 10.1371/journal.pone.0081828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Freedman R, Olincy A, Buchanan RW, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165(8):1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olincy A, Harris JG, Johnson LL, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63(6):630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- 75.Lieberman JA, Dunbar G, Segreti AC, et al. A randomized exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. 2013;38(6):968–975. doi: 10.1038/npp.2012.259. [DOI] [PMC free article] [PubMed] [Google Scholar]