Abstract
Objective
Laryngeal adductor muscle dysfunction is common cause of voice disorders. Reconstitution of adductor muscle function is often the target of therapy, but the effects of these muscles on voice production remain to be fully understood. This study investigated the differential roles of thyroarytenoid (TA) and lateral cricoarytenoid (LCA) muscles on voice production.
Study Design
Basic science study using an in vivo canine model of phonation.
Methods
The TA and LCA muscle nerve branches were stimulated to obtain 7 graded levels of muscle activation, from threshold to maximal contraction. The effects of LCA activation alone, TA activation alone, and combined TA and LCA activation on phonation onset parameters were investigated. Phonatory posture, phonation onset type, fundamental frequency (F0), phonation onset pressure, and airflow were evaluated.
Results
LCA activation closed the posterior glottis but mid-membranous gap remained. TA activation closed the membranous glottis but posterior gap remained. Complete glottal closure was obtained only with combined TA and LCA activation. Phonation onset with LCAs alone was characterized by multiple modes (soft, aperiodic, periodic), while with TAs alone was abrupt and periodic but had significant baseline noise. Combined muscle activation led to elimination of baseline noise with stable abrupt periodic onset of phonation. Combined muscle activation was also necessary for F0 variation. LCA assisted the TA in increasing subglottal pressure while concurrently reducing phonation onset airflow.
Conclusion
TA is necessary for F0 variation, stable onset phonation, and increased subglottal pressure but needs LCA for optimal effectiveness and to reduce airflow requirements with increased activation.
Keywords: Thyroarytenoid, Lateral cricoarytenoid, Speech production, In Vivo Phonation, Canine
INTRODUCTION
Voice production requires coordinated neuromuscular activation of the intrinsic laryngeal muscles (ILMs) to set up the glottal phonatory posture (shape) and stiffness. Aerodynamic energy then matches the subglottal pressure and trans-glottal airflow required for onset of phonation. Neuromuscular control plays a greater role than aerodynamic control in determining acoustic parameters such as fundamental frequency (F0).1–2 Among the ILMs, it is generally understood that the thyroarytenoid (TA), lateral cricoarytenoid (LCA), and interarytenoid (IA) muscles adduct the vocal folds to narrow the glottic inlet and facilitate rise of sufficient subglottal pressure for phonation, while the cricothyroid (CT) muscle elongates the vocal fold for fundamental frequency control (F0).3 However, in vivo studies evaluating the differential roles for TA and particularly LCA in phonation are severely lacking.
In many laryngeal diseases causing dysphonia the TA and LCA muscles are the most commonly affected. Reconstitution of the physiologic actions of these muscles is also the major goal in medical and surgical intervention. For example, these muscles are atrophic or hypo-functional in presbylarynx, paresis, and paralysis conditions and hyper-functional in adductor spasmodic dysphonia. Surgical intervention for hypo-function such as type 1 thyroplasty primarily mimics TA activation while arytenoid adduction mimics LCA activation. Other procedures such as laryngeal reinnervation using ansa cervicalis to recurrent laryngeal nerve (RLN) anastomosis for paralysis nonspecifically target both muscles.
If therapeutic interventions are geared towards reconstituting the actions and effects of laryngeal muscle activation then the roles of those muscles in phonation should be fully understood. For example, since both TA and LCA are laryngeal adductors, what are their respective roles and why are both muscles needed? Despite the widespread procedures targeting the LCA and TA muscles very little is known about their interactions and critical functions. If glottal closure alone is the critical requirement for normal phonation then LCA muscle procedures might be adequate. In that setting the role for TA activation is unclear. The fundamental question, how do TA and LCA muscles differ in their roles in control of voice production has not been investigated. How do they interact? What are the aerodynamic consequences? In this study the differential roles of the TA and LCA muscles on phonation onset was evaluated by systematic activation of these muscles in an in vivo canine model.
METHODS
In vivo canine model
This animal study protocol was approved by the Institutional Animal Research Committee of the University of California, Los Angeles. Surgical exposure of the larynx and the individual distal nerve branches of the individual laryngeal nerves was as described previously.2,4 Appropriately sized tripolar cuff electrodes (Ardiem Medical, Indiana, PA, USA) were applied to the respective nerve branches to stimulate the TA, LCA/IA, and the CT muscles separately. As described previously, the IA nerve branch cannot be divided without excessive laryngeal dissection that can potentially damage the muscles being tested.2 Thus, the LCA and IA were stimulated as a single complex, and the contribution of the IA is expected to be minimal compared to the LCA as previously described.5 The nerve branches to the posterior cricoarytenoid (PCA) muscle, Galen’s anastomosis, and the internal SLN branches were divided bilaterally to eliminate their effects during nerve stimulation.
A subglottal tube to provide rostral airflow for phonation was attached to the trachea at ring 2–3 and connected to an airflow controller (MCS Series Mass Flow Controller, Alicat Scientific, Tucson, Arizona, USA), which was used to increase the airflow rate linearly from 300 to 1600 ml/s during the 1500 milliseconds nerve stimulation duration. The airflow was increased in such manner to continuously increase subglottic pressure (Psub) to reach phonation onset pressure (Pth) and beyond until maximal airflow level was reached. The airflow at the glottic level was warmed to 37.5 degrees Celsius and 100% humidity using a heated humidifier (HumiCare 200, Gruendler Medical, Freudenstadt, Germany).
In this investigation the differential effects and interactions of LCA and TA muscles on phonation onset characteristics were investigated. First, the LCA muscles alone were symmetrically stimulated bilaterally over 7 levels of graded stimulation, from threshold muscle activation (level 1, where just a hint of vocal fold movement or strain change was observed) to maximal activation (level 7, where maximal vocal fold displacement or strain change was observed).6 Then the TA muscles alone were similarly stimulated over 7 levels. Subsequently, LCA activation was kept constant at several levels (levels 1, 3, 5) while TA activation was increased over 7 levels. Several LCA/TA combinations were repeated with CT activation but interactions with CT muscles was not a focus of this study and was not comprehensively studied. Neuromuscular stimulation duration for each condition was 1500 ms with 0.1 milliseconds long unipolar cathodic pulses at a repetition rate of 100 Hz. To allow muscle recovery and transfer of high speed video data to the host computer, each stimulation pulse train was followed by a 3.5 seconds pause prior to next stimulation with the next activation condition.
Measurement of experimental parameters (F0, Pth, Airflow)
Acoustic and aerodynamic data were recorded using a probe tube microphone (Model 4128, Bruel & Kjaer North America, Norcross, Georgia, USA) and a pressure transducer (MKS Baratron 220D, MKS Instruments, Andover, Massachusetts, USA) mounted flush with the inner wall of the subglottic inflow tube about 5 cm below the inferior border of the glottis. The subglottal acoustic pressure signal was used to determine the fundamental frequency (F0) at phonation onset using Sound Forge acoustic analysis software (Sonic Foundry Sound Forge Version 6.0, Sonic Foundry Inc., Madison, Wisconsin, USA) as described previously.2,4 The acoustic signal and digital video kymograms (DVK) of glottal vibration were simultaneously displayed on a computer screen to evaluate and categorize the characteristics of the acoustic signal as well as the vibratory pattern. The time instance of phonation onset was determined using the acoustic signal and confirmed by DVK to ensure that acoustic signal correlated with glottal vibration. The corresponding mean subglottal pressure (Psub) at phonation onset represented the phonation onset pressure (Pth). The corresponding airflow at phonation onset was also recorded.
A high-speed digital video camera (Phantom v210, Vision Research Inc., Wayne, New Jersey, USA) imaged laryngeal deformation and vibration at 3000 frames per second (fps) for the duration of nerve stimulation. The distance from the camera to the larynx remained constant for all conditions. India ink was used to mark several landmarks on the vocal fold surface, including the vocal processes. DVK was generated using custom programmed python software. The high speed video (HSV) was used to generate DVK.
To keep data scales consistent, data from one animal is presented. Findings are consistent from two other animals and where the role of ILMs in register control was studied.2,4 Results of this study shed new light in the mechanisms of voice production, as well as have applications for medical and surgical therapeutic procedures where the actions of the TA and LCA muscles are targeted.
RESULTS
Effects on Phonatory Posture
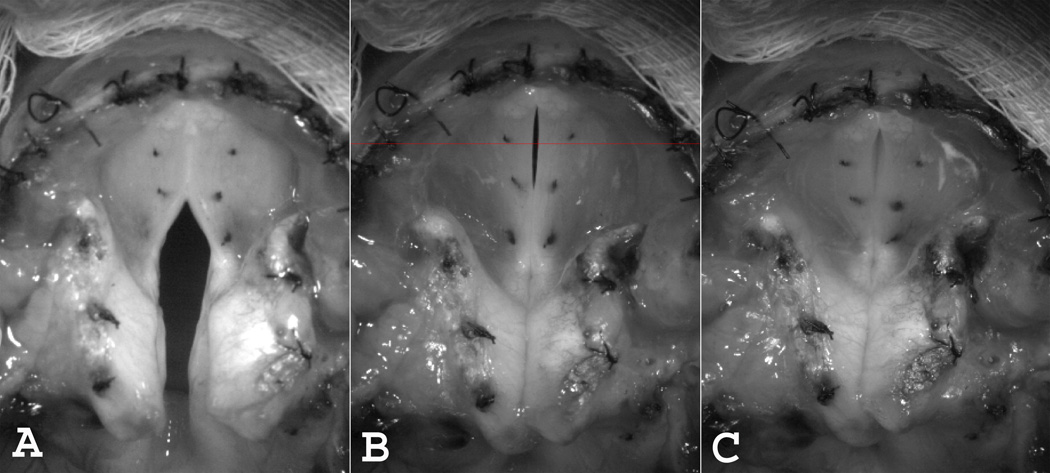
The two laryngeal adductor muscles affected the pre-phonatory posture differently (Figure 1). With symmetric TA activation alone there was mid-membranous closure but a large posterior glottal gap remained (A). In contrast, the posterior glottal gap was closed upon LCA/IA activation but a mid-membranous gap remained (B). Complete membranous and cartilaginous glottal closure was achieved with combined activation of both TA and LCA/IA muscles (C).
Figure 1.
Effects on pre-phonatory posture with symmetric activation of (A) Bilateral TA alone (level 3), (B) Bilateral LCA/IA alone (level 4), and (C) Combined bilateral TA (level 3) and bilateral LCA/IA (level 5). Thin horizontal line across mid-membranous area in (B) represents general location of line for digital kymography.
Effects on Vibration
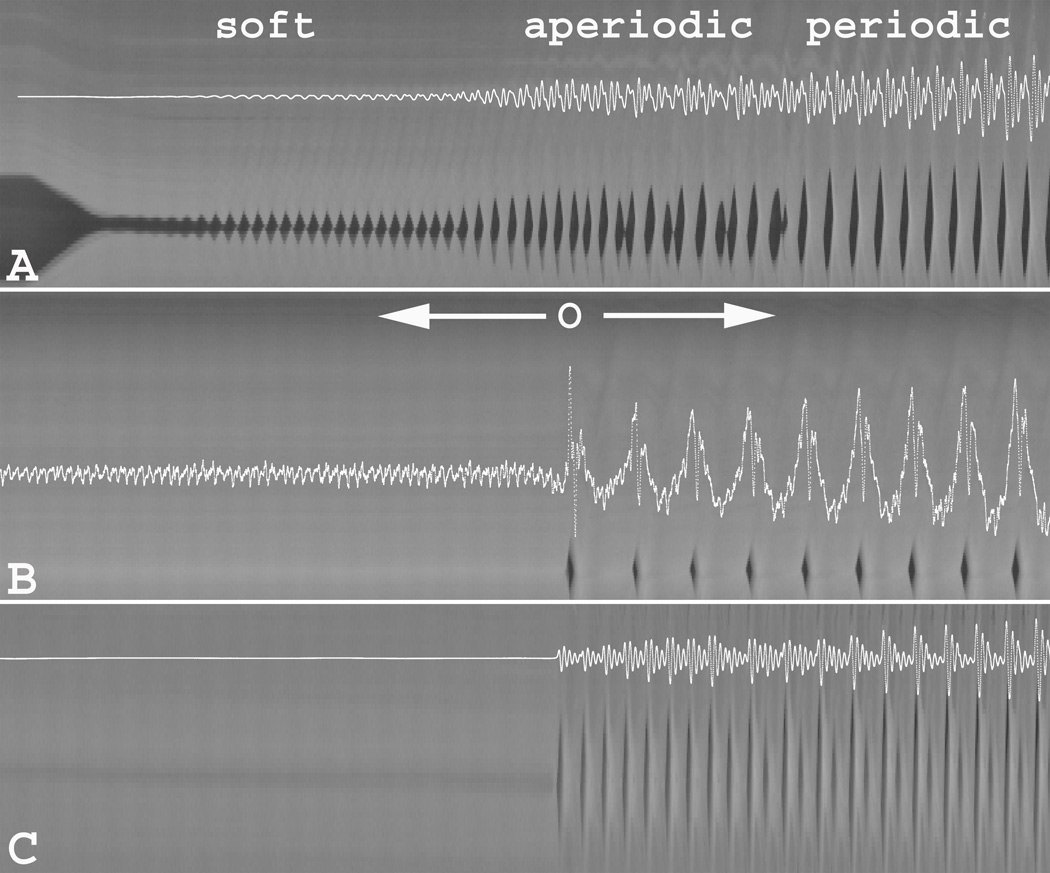
Glottal vibratory characteristics at phonation onset are presented in figure 2, where concurrent acoustic signal is overlaid with the corresponding DVK for three illustrative conditions. Phonation onset with activation of LCA/IA alone was characterized by three vibratory modes (Figure 2A). Immediately after adequate glottal closure was achieved a low-amplitude periodic oscillation commenced (“soft” onset). This soft onset vibration was limited to the medial edges of the glottis and was present for all but the highest (level 7) and the lowest (level 1) LCA activation levels. This vibratory mode was followed by aperiodic vibration of variable duration that was present in all but the lowest activation (level 1). Subsequently, as subglottal pressure increased, regular periodic vibration ensued until the end of nerve stimulation.
Figure 2.
Digital kymography (DKG) with concurrent overlay of acoustic signal illustrating vibratory characteristics at phonation onset upon activation of (A) bilateral LCA/IA alone (level 4), (B) bilateral TA alone (level 4), and (C) combined bilateral LCA/IA (level 5) and bilateral TA (level 3). See text for descriptions of vibratory characteristics. [O = phonation onset]
Unlike the tri-phasic phonation onset described above with LCA activation alone, vibration with symmetric TA activation alone was characterized by an abrupt onset (O) of periodic vibration (Figure 2B). However, prior to onset of membranous vocal fold vibration the acoustic signal displayed fine low-amplitude high-frequency baseline signal (“baseline noise”). Review of high speed video revealed fine mucosal oscillation of the open posterior cartilaginous glottis as the source of this noise (see open posterior glottis in Figure 1A). The baseline noise component disappeared completely upon activation of both TA and LCA/IA and posterior glottic closure was achieved. Phonation onset was abrupt and periodic throughout, even starting at TA activation level 1. Phonation onset was always abrupt and periodic when TA was active but when posterior glottic gap was present, the noise signal was always present.
Effects on fundamental Frequency (F0)
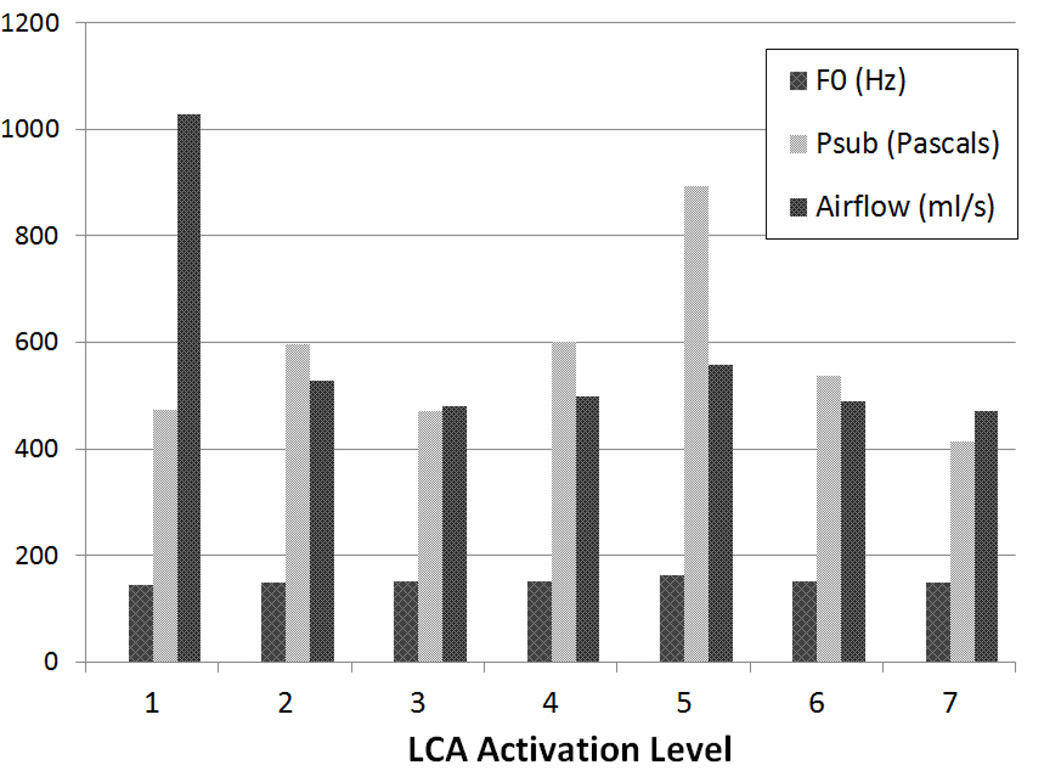
The F0 range of periodic vibration with LCAs alone was along a narrow and relatively flat range of 145–165 Hz (Figure 3). In contrast, the effects of TA activation on F0 depended on the LCA activation level (Table 1). When LCA activation was absent (level 0) or low (level 1), increasing TA activation led to decrease in F0. When LCA activation level was higher (levels 3 and 5), increasing TA activation resulted in F0 increase (range 137–285 Hz) (Table 1).
Figure 3.
Fundamental frequency (F0) and aerodynamic parameters at onset of periodic vibration with graded levels of symmetric LCA activation alone, from threshold activation (level 1) to maximal activation (level 7). [F0 = fundamental frequency at periodic voice onset, Psub = Subglottal pressure]
Table 1.
Fundamental Frequency (F0), Subglottic Pressure at Periodic Onset (Psub), and Airflow at Periodic Onset (Q) at various TA and LCA levels without CT activation: [100 Pascals is @ 1 cm H20)
| F0 (Hz) | ||||||||
| Level | TA 0 | TA 1 | TA 2 | TA 3 | TA 4 | TA 5 | TA 6 | TA 7 |
| LCA 0 | NP | 153 | 54 | 63 | 64 | 65 | 66 | 67 |
| LCA 1 | 144 | 96 | 104 | 79 | 74 | 82 | 97 | 89 |
| LCA 3 | 171 | 143 | 137 | 138 | 176 | 184 | 222 | 218 |
| LCA 5 | 163 | 137 | 154 | 167 | 191 | 242 | 265 | 285 |
| Phonation Onset Pressure (Pascals) | ||||||||
| Level | TA 0 | TA 1 | TA 2 | TA 3 | TA 4 | TA 5 | TA 6 | TA 7 |
| LCA 0 | NP | 366 | 859 | 989 | 1132 | 1387 | 1581 | 1550 |
| LCA 1 | 396 | 239 | 857 | 1151 | 1562 | 1871 | 2131 | 2191 |
| LCA 3 | 818 | 229 | 1029 | 1308 | 1747 | 2190 | 3117 | 3022 |
| LCA 5 | 460 | 289 | 1001 | 1322 | 1695 | 2127 | 2414 | 2977 |
| Phonation Onset Airflow (ml/s) | ||||||||
| Level | TA 0 | TA 1 | TA 2 | TA 3 | TA 4 | TA 5 | TA 6 | TA 7 |
| LCA 0 | NP | 878 | 1063 | 1260 | 1354 | 1272 | 1465 | 1486 |
| LCA 1 | 822 | 445 | 584 | 695 | 749 | 816 | 955 | 991 |
| LCA 3 | 583 | 376 | 547 | 592 | 667 | 711 | 835 | 827 |
| LCA 5 | 478 | 396 | 514 | 553 | 579 | 638 | 665 | 695 |
Effects on Phonatory Aerodynamics
As mentioned above, glottal vibration with bilateral LCAs alone was characterized by a triphasic vibratory mode at phonation onset. A small-amplitude vibration (“soft” onset) occurred at a very low Psub range of 20 – 144 Pascals (0.2 to 1.47 cm H20). As Psub increased the small amplitude vibration was followed by a short duration aperiodic vibration before settling into periodic vibration for the duration of the stimulation (Figure 2A). The range of Psub for periodic vibration with LCA activation alone was within a narrow range (416–603 Pascals) except for one outlier, level 5 at 895 Pascals (Figure 3). Onset airflow was higher at activation level 1 (1030 ml/s) due to large glottal gap but then settled to a narrow range for the rest of the activation levels (471–559 ml/s) as posterior glottic closure was achieved (Figure 3).
TA activation had a consistent effect on phonation onset Psuband airflow, regardless of LCA level (Table 1). Increasing TA activation at constant LCA level led to increase in both Pthand airflow. However, increasing LCA level at constant TA level led to increase in Psub but a decrease in airflow (Table 1). Thus, TA and LCA were synergistic for Pth but antagonistic for airflow. These results were more apparent with increasing levels of both TA and LCA activation.
DISCUSSION
In this study the differential roles of the TA and LCA muscles on phonation onset were investigated in an established in vivo model canine model of phonation.2,4,7 These results reveal specific roles for these adductor muscles. LCA is needed to close the posterior gap and reduce the phonation onset airflow. Maximal phonation time is limited by pulmonary vital capacity and therefore posterior glottal closure by LCA allows frugal use of pulmonary vital capacity. However, phonation onset with LCAs alone transitions through multiple vibratory modes, and also lacks F0 and subglottal pressure modulation over the range of activations. These acoustic and aerodynamic findings for LCA activation has been corroborated in human ex vivo larynx models, where LCA activation can be modeled but not TA activation.8 Thus, the voice with LCAs alone is monotonous in pitch and loudness. The TA muscles can modulate these latter voice parameters by introducing glottal tension: however they rely on the LCA muscles to most effectively accomplish these tasks. Vibration with TAs alone resulted in a contracted F0 range, reduced F0, increased subglottal pressure, increased airflow, with prominent baseline flow noise prior to phonation onset. Thus, voice production with TAs alone would also be low-pitch monotonous tone with reduced phonation time due to large posterior glottic gap.
The LCA acts as the “great assistant” to the TA. With the help of LCA, TA is able to generate a greater range of F0 and Psub. Most importantly, LCA allows TA to achieve these parameters with increased efficiency and without a penalty of increased airflow. LCA activation decreases phonation onset airflow and thus would be expected to play the most significant role in increasing maximal phonation time. An interesting finding in this study is that LCA and TA were synergistic in increasing F0. This makes sense anatomically as the LCA is able to hold the vocal fold “steady” at one end in an adducted positon while TA adds stiffness to the glottis. Without LCA activation, TA activation would simply shorten the glottis and the resulting laxity of the cover layer will lower F0, as seen this this study. As would be expected, LCA is more effective in this role as its activation level increases. Another important contribution of the TA is to generate an abrupt periodic phonation onset, an important consideration especially in singing. Phonation onset characteristics were best when both TA and LCA were activated.
These findings have implications for treatment of laryngeal dysfunction involving the TA and LCA muscles. In the treatment of vocal fold paralysis, this study would support combined procedures to close the membranous and cartilaginous glottis. For example, a combined type 1 thyroplasty and arytenoid adduction would be expected to have better results compared to either procedure alone. However, the study also points out some limitations of such static procedures. Since only one stiffness level can be introduced to the system with static procedures, interventions that control glottal stiffness dynamically such as laryngeal reinnervation would be expected to have improved F0 and intensity control. Procedures such as arytenoid adduction combined with laryngeal reinnervation currently have the best potential for vocal rehabilitation of unilateral paralysis, as adduction would statically mimic LCA action at the posterior glottis and reinnervation would provide some dynamic TA control. Further studies are needed to evaluate this hypothesis. In regards to the treatment of hyper-functional disorders it is clear that TA is necessary to increase Psub and LCA alone is unable to do so. However, LCA is synergistic with the TA in this regard and TA alone is not as effective. Thus both muscles are potential targets (e.g. for Botox injection).
CONCLUSION
The roles and interactions of the laryngeal adductor muscles in phonation have not been previously evaluated. Using an in vivo canine model of phonation the effects of TA and LCA muscles on phonation onset characteristics was studied. Alone, neither TA nor LCA were effective in F0 variation. The TA is able to add tension to the glottis and increase the Psub. However, LCA is essential for TA to be effective in varying the F0 and increasing Psub, while concurrently reducing the airflow requirement. Psub is one of the main physiological variables controlling vocal loudness.9 It would thus be expected that for voice production to be louder and efficient both muscle are necessary.
Acknowledgments
Financial Disclosure: This study was supported by Grant No. RO1 DC011300 from the National Institutes of Health.
Footnotes
The authors have no other financial disclosures to make.
Conflict of Interest: None
This article was presented as an oral presentation at The Triological Society Combined Sections Meeting, January 22–24, 2015, San Diego, CA, USA.
REFERENCES
- 1.Titze IR, Talkin DT. A theoretical study of the effects of various laryngeal configurations on the acoustics of phonation. J Acoust Soc Am. 1979;66(1):60–74. doi: 10.1121/1.382973. [DOI] [PubMed] [Google Scholar]
- 2.Chhetri DK, Neubauer J, Sofer E, Berry DA. Influence and interactions of laryngeal adductors and cricothyroid muscles on fundamental frequency and glottal posture control. J Acoust Soc Am. 2014;135(4):2052–2064. doi: 10.1121/1.4865918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin J, Zhang Z. Interaction Between the Thyroarytenoid and Lateral Cricoarytenoid Muscles in the Control of Vocal Fold Adduction and Eigenfrequencies. J Biomech Eng. 2014;136(11):111006. doi: 10.1115/1.4028428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chhetri DK, Neubauer J, Berry DA. Neuromuscular control of fundamental frequency and glottal posture at phonation onset. J Acoust Soc Am. 2012;131(2):1401–1412. doi: 10.1121/1.3672686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi HS, Ye M, Berke GS. Function of the interarytenoid(IA) muscle in phonation: in vivo laryngeal model. Yonsei Med J. 1995;36(1):58–67. doi: 10.3349/ymj.1995.36.1.58. [DOI] [PubMed] [Google Scholar]
- 6.Chhetri DK, Neubauer J, Berry DA. Graded activation of the intrinsic laryngeal muscles for vocal fold posturing. J Acoust Soc Am. 2010;127(4):EL127–EL133. doi: 10.1121/1.3310274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chhetri DK, Rafizadeh S. Young's modulus of canine vocal fold cover layers. J Voice. 2014;28(4):406–410. doi: 10.1016/j.jvoice.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mau T, Muhlestein J, Callahan S, Weinheimer KT, Chan RW. Phonation threshold pressure and flow in excised human larynges. Laryngoscope. 2011;121(8):1743-517. doi: 10.1002/lary.21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundberg J, Titze I, Scherer R. Phonatory control in male singing: a study of the effects of subglottal pressure, fundamental frequency, and mode of phonation on the voice source. J Voice. 1993;7(1):15–29. doi: 10.1016/s0892-1997(05)80108-0. [DOI] [PubMed] [Google Scholar]