Abstract
Background
Cancer patients have an approximately four-fold increased risk of venous thromboembolism (VTE) compared with the general population, and cancer patients with VTE have reduced survival. Tumor cells constitutively release small membrane vesicles called microvesicles (MVs) that may contribute to thrombosis in cancer patients. Clinical studies have shown that levels of circulating tumor-derived, tissue factor-positive (TF+) MVs in pancreatic cancer patients are associated with VTE.
Objectives
We tested the hypothesis that TF+ tumor-derived MVs (TMVs) activate platelets in vitro and in mice.
Materials and Methods
We selected two human pancreatic adenocarcinoma cell lines expressing high (BxPc-3) and low (L3.6pl) levels of TF as models to study the effect of TF+ TMVs on platelets and thrombosis.
Results and Conclusions
We found that both types of TF+ TMVs activated human platelets and induced aggregation in vitro in a TF- and thrombin-dependent manner. Further, injection of BxPc-3 TF+ TMVs triggered platelet activation in vivo and enhanced thrombosis in two mouse models of venous thrombosis in a TF-dependent manner. Importantly, BxPc-3 TF+ TMV-enhanced thrombosis was reduced in Par4-deficient mice and in wild-type mice treated with clopidogrel, suggesting that platelet activation was required for enhanced thrombosis. These studies suggest that TF+ TMV-induced platelet activation contributes to thrombosis in cancer patients.
Introduction
Cancer patients have a four-fold increased risk of venous thromboembolism (VTE) [1–4]. VTE in cancer patients is associated with increased mortality [5, 6]. Patients with pancreatic cancer have been shown to have a particularly high rate of VTE in comparison to other cancer types [5, 7, 8]. Cancer is a heterogeneous group of disorders and therefore it is likely that there are multiple mechanisms of cancer-associated VTE. Indeed, many mechanisms have been proposed to explain the enhancement of thrombosis with the development of cancer, including tissue factor (TF)+ microvesicles (MVs), neutrophils extracellular traps (NETs), leukocyte activation, and platelet activation [9].
TF expression in tumor cells has been shown to increase with histologic grade in many cancer types, including pancreatic cancer [10–12]. TF is the primary initiator of the extrinsic pathway of the coagulation cascade that culminates in thrombin generation and fibrin formation [13–15]. Thrombin is a particularly potent platelet agonist, mediating the activation of human platelets via Par1 and Par4 and mouse platelets via Par3 and Par4 [16].
MVs (also called microparticles or extracellular vesicles) are small membrane vesicles that are constitutively released by tumor cells [17, 18]. We will refer to these as tumor-derived MVs (TMVs). Importantly, the presence of TF makes MVs highly procoagulant [19, 20]. Several studies have shown that MVs released from human pancreatic tumor cell lines and human pancreatic tumors grown in mice contain TF [17, 21, 22]. Other studies have reported increased plasma TF+ MVs and MV TF activity in patients with different cancer types [23–27]. Further, increased levels of TF+ MVs and MV TF activity are associated with VTE in pancreatic cancer patients, which suggests a role for TF+ MVs in cancer-associated thrombosis [23, 24, 28, 29].
Previous studies by our lab and others have shown that TF+ TMVs from human breast and pancreatic adenocarcinoma cell lines trigger the activation of coagulation and platelets in mice [17, 30]. Thomas and colleagues showed that mice with TF+ Panc02 tumors, a mouse pancreatic adenocarcinoma cell line, had larger thrombi than control mice in a ferric chloride injury-induced mesenteric vessel model, and that TF+ TMVs from Panc02 cells enhanced thrombosis in wild-type (WT) mice [22]. We showed that mice with tumors derived from the human pancreatic adenocarcinoma cell line HPAF-II had shorter occlusion times than controls in ferric chloride injury-induced saphenous vein thrombosis, and that injection of exogenous TF+ TMVs enhanced thrombosis in the inferior vena cava (IVC) stenosis model in WT mice [30]. More recently, it was shown that mice containing TF+ Panc02 tumors had larger thrombi than control mice in the cremaster laser injury model and that this enhanced thrombosis was reduced by treatment with the anti-platelet drug clopidogrel [31]. In addition, mice bearing TF+ Panc02 tumors had increased thrombosis in the IVC stenosis model [32]. Tumor cells have been shown to induce platelet aggregation through a variety of mechanisms, including via thrombin generation [33]. Furthermore, MVs derived from SOJ6 pancreatic adenocarcinoima cells triggered aggregation of washed platelets in the presence of plasma [22].
In this study, we determined the effect of TF+ TMVs on platelets in vitro and in vivo. Our studies suggest that TF+ TMVs enhance thrombosis in mice, in part, in a platelet-dependent manner.
Materials and methods
Reagents and antibodies
We used the following reagents: clopidogrel (Bristol-Myers Squib, New York, NY, USA), prostaglandin E1 (PGE1) (Cayman Chem, Ann Arbon, MI, USA), anti-human TF antibody (HTF-1), anti-αvβ3 antibody (LM609), the substrate benzyloxycarbonyl-Gly-Gly-Arg-7-amido-4methylcoumarin• HCl (Z-GGR-AMC) (Bachem, Torrance, CA, USA), annexin V- Pac Blue (Life Technologies, Grand Island, NY, USA), Megamix beads (Biocytex, Marseille, France), anti-CD41a-R-Phycoerythrin (PE) (clone HIP8), anti-CD62P-allophycocyanin (APC) (clone AK-4), Pac-1-fluorescein (FITC), PE-IgG isotype control and anti-CD142-PE (clone HTF-1), anti-PSGL-1 (clone KPL-1), anti-CD24 (clone ML5), annexin V (BD Biosciences, San Jose, CA, USA), citrated mouse plasma, mouse IgG, apyrase, 2 Methylthioadenosine 5′-monophosphate (MesAMP), and acid citrate dextrose (ACD) (Sigma, Saint Louis, MO, USA), hirudin (Accurate Chem and Scientific, Westbury, NY, USA), active site-inhibited recombinant FVIIa (FVIIai) (American Diagnostica, Stamford, CT, USA), human α-thrombin (Thermo Scientific, Waltham, MA, USA), recombinant relipidated human TF (InnovinTM) (Dade Behring, Liederbach, Germany), 5(6)-Carboxyfluorescein diacetate succinimidyl ester (CFSE) (eBioscience, San Diego, CA, USA), anti-GPIX (clone Xia.B4) (Emfret Analytics, Eibelstdt, Germany), Enzygnost TAT micro ELISA kit (OWMG15) (Siemans Healthcare Diagnostics, Malvern, PA, USA), and the mouse CXCL4/PF4 DuoSet ELISA (R&D Systems, Minneapolis, MN, USA).
Cell Lines
The human pancreatic adenocarcinoma cell lines BxPc-3 and L3.6pl were obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and cultured in RPMI (Gibco, Grand Island, NY, USA) plus 10% fetal bovine serum (Omega Scientific, Tarzana, CA, USA) and 1% penicillin streptomycin (Gibco). MVs were isolated from 72 hour serum-free cell culture supernatant. Apoptotic bodies and cell debris were pre-cleared from culture supernatant by centrifugation at 2612 x g for 5 minutes. MVs were then pelleted at 20,000 x g for 20 minutes at 4°C, re-suspended in PBS, and stored at 80°C until use. MV concentration was determined by protein content using a BCA protein assay (Thermo Scientific, Rockford, IL, USA).
Human Subjects
Healthy non-pregnant adults were recruited into this study under IRB approval from the office of Human Research Ethics at University of North Carolina and informed consent was obtained. Blood was collected from the antecubital vein into ACD and 1 μg/mL PGE1. The first 3 mL of blood was discarded. Platelet-rich plasma (PRP) was isolated by centrifugation at 230 x g for 15 minutes at room temperature. PRP was then spun at 920 x g for 10 minutes with one Tyrode’s buffer wash step to isolate platelets. Platelets were re-suspended in Tyrode’s buffer without calcium.
Mice
All experiments used 8–22 week old C57Bl6/J male mice. Studies were approved by the Institutional Animal Care and Use Committee at the University of North Carolina and comply with NIH guidelines. C57Bl6/J and nude mice were obtained from Jackson laboratories (Bar Harbor, Maine, USA). Par4 knockout mice (−/−) and WT (+/+) littermate controls were obtained from Dr. Shaun Coughlin (University of California at San Francisco, CA, USA). WT mice were treated with clopidogrel (75 mg/kg) by oral gavage 24 hours and 1 hour before experimentation. Efficacy of this treatment was confirmed by the inability of platelets in clopidogrel treated mice to respond to 2.5 μm ADP (MFI of the anti-active αIIb/β3 antibody JonA-PE binding: saline without ADP 20.7 ±2.8; saline with ADP 38.9 ± 3.2; clopidogrel without ADP 20.7 ± 1.4; clopidogrel with ADP 13.4 ± 0.94). Orthotopic tumor injection of nude mice was performed as described [30]. Tumors were allowed to grow to 2 cm3 in size over 8–16 weeks.
Microscopy
Isolated MVs were visualized using a Tecnai 12 transmission electron microscopy (TEM) (FEI, Hillsboro, OR) at 80 kV, and images were captured on a Gatan Orius charge-coupled device (CCD) camera programmed with Digital Micrograph software (Gatan, Warrendale, PA).
TF activity
Cellular and MV TF activity assays were performed described [34, 35].
Flow Cytometry
Washed human platelets were re-suspended to a concentration of 5 × 108 plt/mL in Tyrode’s buffer containing 1 mM CaCl2. Platelets were incubated with 2 μL anti-CD41a-PE, anti-P-selectin-APC, and Pac-1-FITC. TMVs were incubated with platelets for 15 minutes at 37°C. Platelet reactions were then diluted in Tyrode’s buffer containing 0.5% formalin. For MV ligand studies, 0.1 μg of MVs were stained with 2 μL antibody in 100 μL of staining buffer (140 mM NaCl, 10 mM Hepes, 5 mM CaCl2) for 30 minutes on ice. Samples were diluted before analysis. Megamix beads were used to set an upper size limit of 1 μm for MVs. Platelet and MV samples were analyzed using a Stratedigm Ex1000 flow cytometer (Stratedigm, San Diego, CA). Data were analyzed using Flowjo version X.0.7 software.
Thrombin Generation Assay
We measured thrombin generation using a calibrated automated thrombogram (CAT) using PRP as described [36].
Platelet Aggregometry
Human or mouse washed platelets were re-suspended to 6 × 108 plt/mL in Tyrode’s buffer containing 1 mM CaCl2, 0.35% BSA, and 1:100 mouse or human plasma. Aggregation was triggered by the addition of BxPc-3 or L3.6pl MVs. Reactions were performed with stirring (1200 RPM) at 37°C in a Chrono-log 4-channel optical aggregation system (Chrono-log, Havertown, PA).
Mouse Thrombosis and Pulmonary Embolism Models
Femoral and IVC thrombosis models were performed as described [37, 38]. For the pulmonary embolism model, platelets were visualized with an infrared-labeled anti-GPIX antibody and platelet deposition in lungs imaged on an Odyssey Infrared imaging system (LI-COR, Lincoln, NE, USA) as described [39].
TF Expression in Tumors
Paraffin-embedded tumor tissue was incubated with either anti-human TF goat polyclonal antibody (R&D Systems, Minneapolis, MN) or a control IgG (Santa Cruz, Dallas, TX). A biotinylated anti-goat rabbit polyclonal antibody was used for detection and slides were incubated with DAB (DAKO, Carpinteria, CA) and counterstained with hematoxylin.
Statistical Analysis
Data are presented as mean ± SEM. TF activity and TF antigen expression data were analyzed by unpaired student’s t-test. Flow cytometry, thrombin generation, and thrombosis model data were analyzed by one- or two-way ANOVA as indicated with Bonferroni posttests comparing indicated pairs of data. Survival data were analyzed using Log-rank (Mantel-Cox) Test. Statistical significance is defined on each figure. Statistical analysis was performed on GraphPad Prism software version 5.01 (Graph Pad Software, Inc. La Jolla, CA, USA).
Results
TF expression by two human pancreatic cell lines
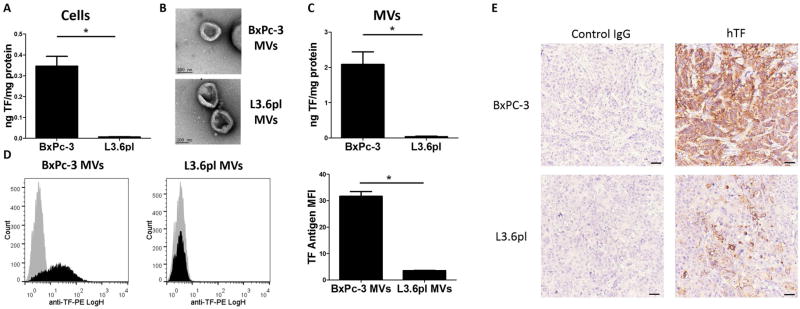
We focused our studies on two TF-expressing human pancreatic adenocarcinoma cell lines that expressed high (BxPc-3) and low (L3.6pl) levels of TF (Figure 1A). BxPc-3 and L3.6pl cells are moderately and poorly differentiated pancreatic adenocarcinoma cell lines, respectively [40]. The BxPc-3 cell line was established from a primary human tumor whereas the L3.6pl cell line was established from a more metastatic isolate of the human pancreatic adenocarcinoma cell line COLO 357 [41, 42]. MVs isolated from BxPc-3 and L3.6pl cells were imaged using electron microscopy to determine their size and purity (>95% vesicles <0.9 μm in size) (Figure 1B). MVs isolated from the cell culture supernatant of BxPc-3 cells had 58-fold more TF activity than MVs from L3.6pl cells (Figure 1C) and had more TF antigen (Figure 1D). Immunohistochemical analysis of TF expression in tumors demonstrated higher levels in BxPc-3 tumors than L3.6pl tumors (Figure 1E).
Figure 1. TF expression on tumor cells, tumor cell-derived microvesicles and tumors.
(A) Cellular tissue factor (TF) activity was measured using a one stage clotting assay. *P<0.001, n=6. (B) BxPc-3 and L3.6pl MVs were imaged by transmission electron microscopy. (C) Microvesicle (MV) TF activity was measured using a two-stage clotting assay. *P<0.005, n=4. TF activity was normalized to total protein. (D) TF antigen expression on MVs was analyzed by MV flow cytometry. Representative histograms are shown and the mean fluorescence intensity (MFI) of an anti-TF antibody is reported. Data were analyzed by unpaired student’s t-test. (E) Human TF expression in BxPc-3 and L3.6pl orthotopic tumors from mice.
Interaction of TF+ TMVs with platelets
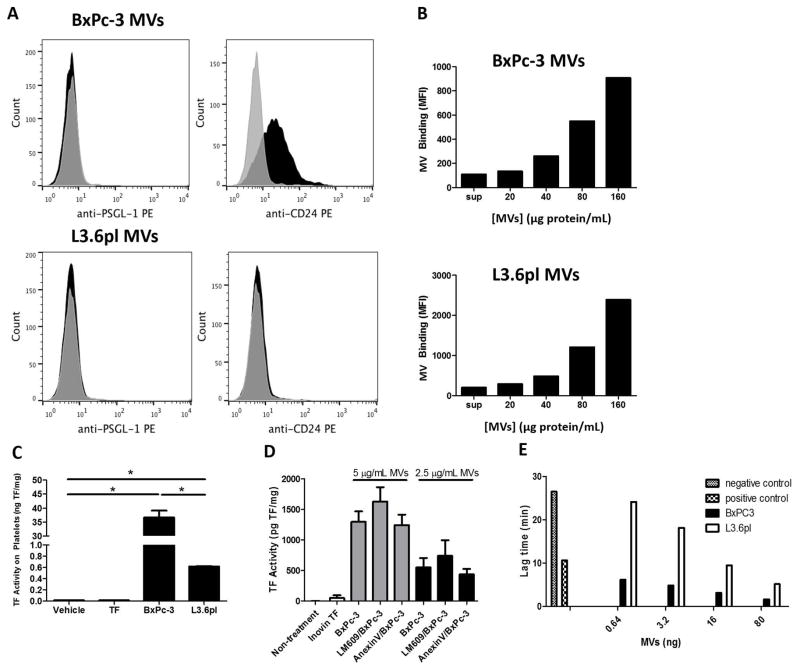
A previous study reported that the human pancreatic adenocarcinoma cell line SOJ-6 expressed PSGL-1 which was involved in the binding of TMVs to activated platelets [22]. We analyzed expression of the P-selectin ligands PSGL-1 and CD24 on MVs from BxPc-3 and L3.6pl cells. BxPc-3 MVs expressed CD24 but not PSGL-1 whereas neither ligand was present on L3.6pl MVs (Figure 2A). We evaluated the ability of BxPc-3 and L3.6pl TF+ MVs to interact with washed human platelets in vitro. Both MV types associated with platelets in a dose dependent manner (Figure 2B). Further, incubation of TF+ MVs with washed human platelets resulted in delivery of MV TF to platelets (Figure 2C). Platelets incubated with BxPc-3 MVs had 59-fold higher TF activity than platelets incubated with L3.6pl MVs. Blocking phosphatidylserine (PS) with annexin V on platelets or TMVs did not reduce the interaction of TMVs with platelets (Figure 2D and data not shown). Similarly, blocking αvβ3 with the antibody LM609 did not reduce the TMV-platelet interaction (Figure 2D). Finally, we analyzed the ability of TMVs to trigger thrombin generation in PRP. We have previously shown that adding TF shortens the lag time in the CAT assay in a dose-dependent manner [36]. Both BxPc-3 and L3.6pl MVs shortened the lag time (Figure 2E). We observed that the lag times were on average 3.9 +/- 0.9 times shorter with BxPc-3 MVs compared with L3.6pl MVs using PRP from 3 donors. These data indicate that TMVs from two pancreatic cell lines interact with resting platelets resulting in the delivery of TF.
Figure 2. Binding of TF positive tumor microvesicles to platelets.
(A) Expression of the P-selectin ligands PSGL-1 and CD24 was evaluated on MVs isolated from BxPc-3 and L3.6pl cells by flow cytometery. Representative histograms are shown. (B) BxPc-3 and L3.6pl TMVs were stained with 4 μM CFSE fluorescent dye followed by 2 wash steps at 20,000 x g for 20 minutes. The indicated concentrations of stained TMVs or washed supernatant were incubated with human platelets in the presence of 1:100 diluted human plasma for 15 minutes at 37°C in the presence of anti-human CD41a-PE. TMV-platelet binding was quantified by measuring the percentage of CFSE-positive platelet events by flow cytometry. A representative experiment of 3 independent experiments is shown. (C) Washed human platelets were incubated with 10 μg of BxPc-3 MVs (20 pg TF), 10 μg of L3.6pl MVs (0.4 pg TF), 0.5 pg of Innovin TF, or vehicle control for 15 minutes at 37°C. Platelets were then washed and cellular TF activity was determined to quantify the delivery of TMV TF to platelets. *P<0.0001, n=3. TF activity was normalized to total protein. (D) Incubation of platelets with either annexin V (2.5 μg/mL final concentration) or the anti-αvβ3 antibody LM609 (1:100 dilution) at room temperature for 15 minutes did not reduce the amount of TF delivered from either 5 or 2.5 μg/mL of BxPc-3 MVs. Data were analyzed by one-way ANOVA with Bonferroni posttests comparing indicated pairs of data. (E) BxPc-3 and L3.6pl MVs led to a dose-dependent shortening of lag time in a calibrated automated thrombogram with platelet-rich plasma. The PRP trigger reagent (1 pM TF) was added to the positive control and buffer was added to the negative control. Data are shown from one donor representative of 3 different donors.
TF+ TMVs activate platelets
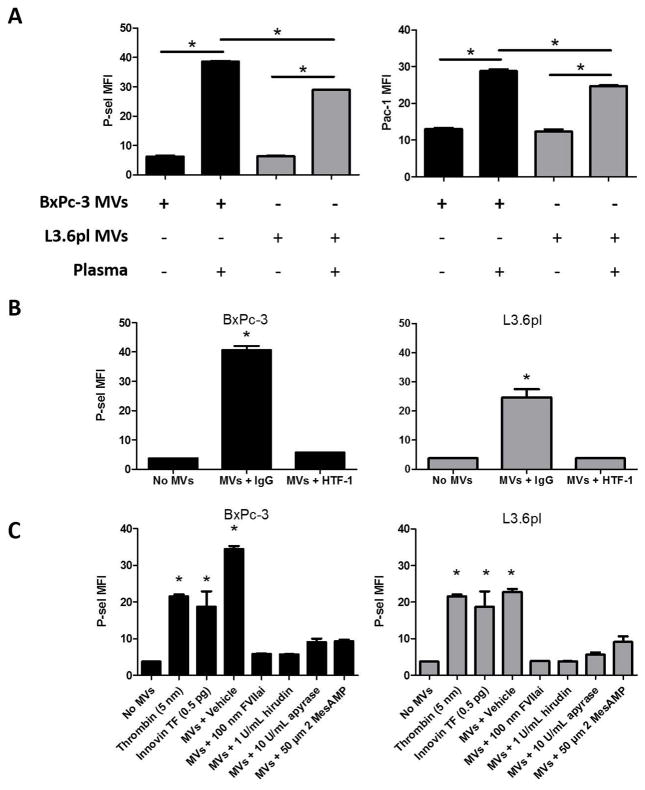
TF+ MVs from both BxPc-3 and L3.6pl cells activated platelets in the presence of plasma (Figure 3A). Inhibition of the TF/FVIIa complex with either HTF-1 or FVIIai blocked TF+ MV-induced platelet activation (Figure 3B and C). The percent inhibition of platelet activation by HTF-1 for BxPc-3 and L3.6pl MVs was 94.1% and 100.0%, and for FVIIai was 93.2% and 100.0%, respectively. As demonstrated above, TF+ MVs trigger the generation of thrombin in the presence of plasma, making thrombin the most likely candidate for the indirect mediator of TF+ TMV-induced platelet activation. To confirm this notion, BxPc-3 and L3.6pl MVs were incubated with platelets and diluted plasma in the presence of the thrombin inhibitor hirudin. Hirudin inhibited both BxPc-3 (percent inhibition 93.6%) and L3.6pl (percent inhibition 99.7%) MV-induced platelet activation (Figure 3C). MV-induced platelet activation was also inhibited by ADP scavenger apyrase or P2Y12 receptor inhibitor 2 MesAMP (Figure 3C). Representative dot plots are shown in Supplementary Figure 1.
Figure 3. Tumor microvesicles induce platelet activation in a tissue factor and thrombin-dependent manner.
(A) BxPc-3 (black bars) and L3.6pl (grey bars) microvesicles (MVs) (50 μg protein/mL) were incubated with washed human platelets in the presence and absence of diluted human plasma (1:100). Platelet activation was monitored by flow cytometry for Pac-1 binding to activated αIIb/β3 and P-selectin expression. Data are presented as the mean fluorescence intensity (MFI) of total CD41a-positive events (+/- SEM). Data are representative of 3 independent experiments. *P<0.01, n=3. Data were analyzed by one-way ANOVA with Bonferroni posttests comparing indicated pairs of data on Graphpad Prism software v5.01. (B) BxPc-3 and L3.6pl MVs (50 μg protein/mL) were pre-incubated with HTF-1 (50 μg/ml) or IgG control (50 μg/ml) for 30 minutes at 4°C. TMVs were then incubated with human platelets in the presence of diluted human plasma (1:100). Data are presented as the MFI of total CD41a-positive events (+/- SEM). Data are representative of 3 independent experiments. *P<0.0001, n=3. (C) Platelets were pre-incubated with inhibitors as indicated for 5 minutes at room temperature. BxPc-3 and L3.6pl MVs (50 μg/mL) were then incubated with human platelets in the presence of diluted plasma for 15 minutes at 37°C. Recombinant TF (InnovinTM) (0.5 pg) and alpha-thrombin (5 nM) were used as controls. After the activation reaction was complete, the reactions were diluted in Tyrode’s buffer + 1 mM CaCl2 + 0.5% formalin and P-selectin expression evaluated by flow cytometry. Data are presented as the MFI of total CD41a-positive events (± SEM). Data are representative of 3 independent experiments. *P<0.0001, n=3. Data were analyzed by one-way ANOVA with Bonferroni posttests comparing all data to the no MV control.
TF+ TMVs induce platelet aggregation
BxPc-3 and L3.6pl MVs induced human platelet aggregation in a dose-dependent manner in the presence of human plasma (Figure 4A). Since BxPc-3 MVs have a high level of TF activity they triggered platelet aggregation more quickly than L3.6pl MVs (Figure 4A). BxPc-3 and L3.6pl MV-induced platelet aggregation was dependent on the presence of both MV TF (Figure 4B) and thrombin (Figure 4C). A P-selectin blocking antibody had no effect on MV-induced platelet aggregation (data not shown). In order to confirm that these TMVs could be used in mice, aggregometry experiments were also performed using mouse platelets. BxPc-3 and L3.6pl MVs triggered thrombin-dependent mouse platelet aggregation in the presence of mouse plasma (Figure 4D).
Figure 4. Tumor microvesicles induce platelet aggregation in a tissue factor and thrombin-dependent manner.
Washed human platelets were re-suspended in Tyrode’s buffer containing human plasma at a dilution of 1:100. (A) Platelets were stimulated with the indicated concentrations of BxPc-3 or L3.6pl microvesicles (MVs). (B) MVs were pre-incubated with the inhibitory anti-TF antibody HTF-1 (50 μg/ml) or IgG control (50 μg/ml) for 30 minutes prior to being added to platelets. Platelets were then stimulated with either BxPc-3 or L3.6pl MVs (50 μg protein/mL). (C) The direct thrombin inhibitor hirudin (1 U/ml) or vehicle control were pre-incubated with platelets for 5 minutes at room temperature prior to the addition of MVs. Platelet aggregation was monitored by light transmittance and is reported as percent absorbance. Tracings are representative of 3 independent experiments. (D) Washed mouse platelets were re-suspended in Tyrode’s buffer containing mouse plasma at a dilution of 1:100. Hirudin, or vehicle control were pre-incubated with platelets for 5 minutes at room temperature. Platelets were then stimulated with BxPc-3 or L3.6pl MVs (50 μg protein/mL). Platelet aggregation was monitored by light transmittance and is reported as percent absorbance.
Effect of BxPc-3 tumors and exogenous TF+ TMVs on the activation of coagulation and thrombosis in mice
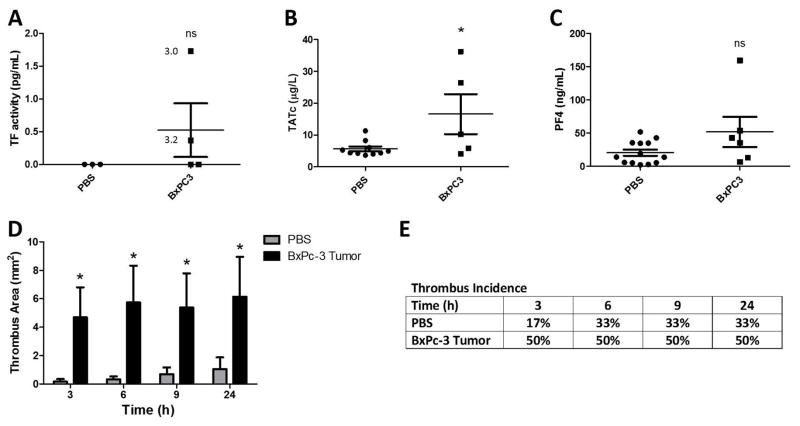
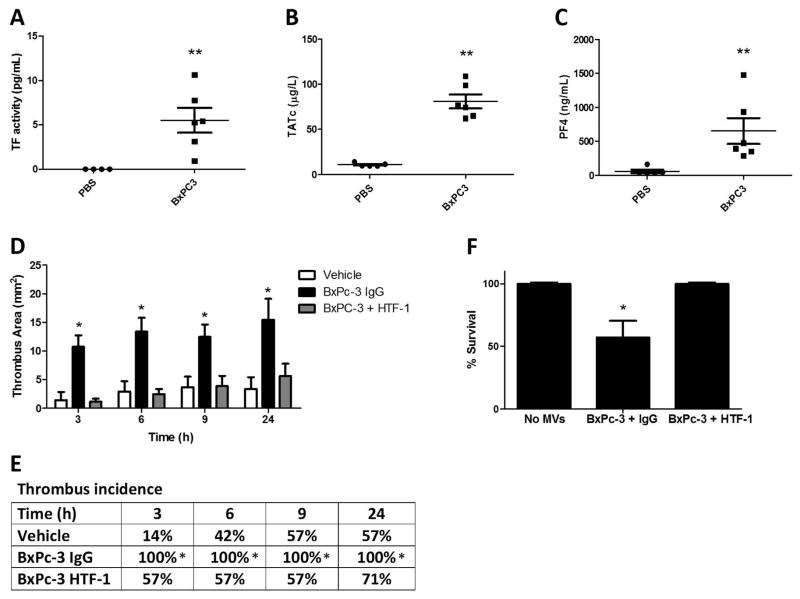
We determined the effect of orthotopic tumors on levels of MV TF activity, coagulation activation, platelet activation and venous thrombosis in mice. We used BxPc-3 MVs because they express higher levels of TF. We detected a low level of MV TF activity in the plasma of 2 out of 4 nude mice with BxPc-3 tumors (Figure 5A). Some of this variation appears to be due to tumor size. Tumor-bearing mice had increased plasma TATc but there was no increase in plasma PF4 (Figure 5B and C). In addition, tumor-bearing mice developed significantly larger thrombi in the IVC stenosis model in comparison to PBS controls with no significant increase in thrombus incidence (Figure 5D and E).
Figure 5. Effect of BxPc-3 tumors on thrombosis in mice.
(A–E) BxPc-3 cells or PBS were injected into the pancreas of nude mice. Tumors were allowed to develop for 8–16 weeks until tumors reached a maximum of 2 cm3 in size. Blood was drawn from the inferior vena cava (IVC) and plasma collected and stored at −80°C. Levels of (A) microvesicles (MV) tissue factor activity (TF), (B) thrombin-antithrombin complexes (TATc) and (C) platelet factor 4 (PF4) were measured in mouse plasma from tumor-bearing mice and controls. (*P<0.05, PBS n=10, BxPc-3 n=5). Data were analyzed by unpaired student’s t-test. Tumor weights (g) with detectable MV TF activity are indicated in panel A. Mice with tumor weights of 1.9 g and 0.3 g did not have detectable MV TF activity. (D) Thrombosis was induced in BxPc-3 tumor-bearing nude mice or PBS controls by stenosis of the IVC. Thrombus formation was quantified in mice using high-frequency ultrasonography. Data is presented as mean ± SEM. (p<0.001. PBS n=6, BxPc-3 n=5). Data were analyzed by two-way ANOVA with Bonferroni posttests comparing all data to the PBS control at each time point. (E) Percent incidence of thrombosis is shown from the thrombosis experiments in panel D. There was no difference in thrombus incidence found by log-rank sums test between tumor bearing and non-tumor bearing mice.
There may be several pathways that contribute to enhanced thrombosis in tumor-bearing mice. Therefore, we chose to specifically evaluate the role of TF+ TMVs by injecting exogenous MVs into mice. Injection of BxPc-3 MVs into WT mice without IVC stenosis significantly increased the level of MV TF activity in the plasma (Figure 6A). The level of MV TF activity observed with exogenous BxPc-3 MVs was ~10-fold higher than the levels observed in tumor bearing mice. Injection of BxPc-3 MVs into mice also increased plasma TATc and PF4 (Figure 6B and C) and lead to death of mice (data not shown). In contrast, injection of the same amount of L3.6pl TMVs into WT mice without IVC stenosis did not increase MV TF activity or induce death (data not shown). Injection of BxPc-3 TMVs into mice that also underwent IVC stenosis had an increase inthrombus size, thrombus incidence, and reduced survival (Figure 6D–F). In addition, TF+ TMVs-enhanced platelet and fibrin accumulation in a mouse femoral vein thrombosis model (Supplementary Figure 2).
Figure 6. Effect of BxPc-3 Tumor Microvesicles on Thrombosis in Mice.
(A–C) BxPc-3 tumor microvesicles (TMVs) (9 μg total) or the same volume of PBS control were injected into mice that did not undergo IVC stenosis at 5, 30, and 60 minutes. Immediately after the final injection, blood was drawn from the IVC and plasma collected and stored at −80°C. Levels of (A) MV TF activity, (B) TATc and (C) PF4 were measured in mouse plasma. *P<0.05, **P<0.0001, PBS n=5, BxPc-3 n=6. Data were analyzed by unpaired student’s t-test. (D-F) Thrombosis in mice was induced by IVC stenosis. Following stenosis, mice were injected intravenously with PBS, BxPc-3 MVs pre-incubated with IgG, or BxPc-3 MVs pre-incubated with the anti-TF antibody HTF-1 (1 mg/mL). (D) Thrombus formation was quantified in mice using high-frequency ultrasonography. Data is presented as mean ± SEM. *P<0.01. PBS n=7, BxPc-3 n=8, BxPc-3 + HTF-1 n=7. Data were analyzed by two-way ANOVA with Bonferroni posttests comparing all data to the no MV control at each time point. (E) Percent incidence of thrombosis is shown from the thrombosis experiment in panel D. Statistical significance was determined by log-rank sums test. (F) Mouse survival following MV injection in panel D is reported with death defined as permanent cessation of breathing within 5 minutes of MV injection. *P<0.05. PBS and BxPc-3 + HTF-1 n=7, BxPc-3 + IgG n=14. Survival data were analyzed by log-rank (Mantel-Cox) test.
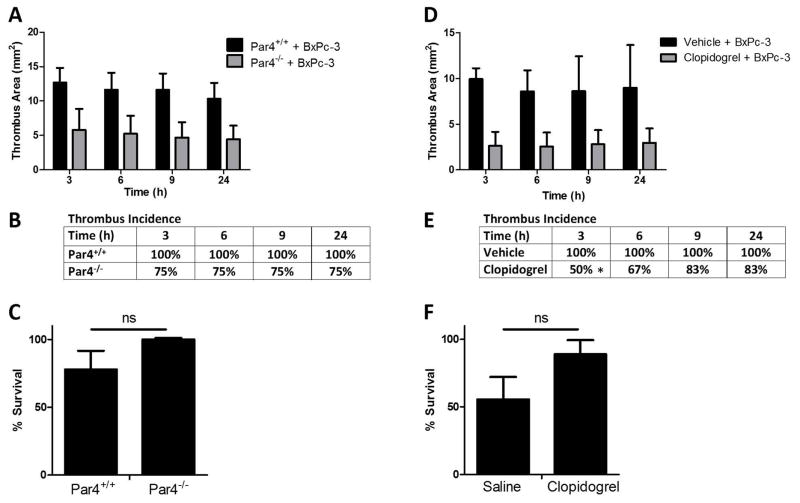
Role of TF and platelet activation in TMV-enhanced thrombosis in mice
Incubation of the BxPc-3 MVs with an anti-human TF antibody (HTF-1) prior to injection abolished the increase in thrombus size, decreased thrombus incidence and increased survival (Figure 6D–F). Similarly, both platelet and fibrin accumulation was significantly reduced in the femoral vein thrombosis model by pre-treatment of the TMVs with HTF-1 (Supplementary Figure 2). Interestingly, addition of the HTF-1-treated TMVs reduced fibrin accumulation levels to below that of the vehicle-treated mice.
Next, we determined the role of platelets in TF+ TMV-enhanced thrombosis. TF+ TMV-enhanced thrombosis was significantly reduced in Par4−/− mice compared to WT littermate controls (Figure 7A). A deficiency of Par4 did not affect thrombus incidence or survival (Figure 7B and C). Similarly, treatment of WT mice with the P2Y12 receptor inhibitor clopidogrel reduced the size in TMV treated mice at all time points (Figure 7D). Clopidogrel did not affect thrombus incidence at most time points and did affect survival (Figure 7E and F).
Figure 7. TF+ tumor microvesicles enhance thrombosis in mice in a platelet-dependent manner.
(A–C) Par4 KO mice (−/−) or WT (+/+) littermate controls underwent the inferior vena cava (IVC) stenosis model followed by injection of BxPc-3 MVs at 5 minutes, 30 minutes, and 1 hour (9 μg total). (A) Thrombus development was quantified by high-frequency ultrasonography. Data were analyzed by one-way ANOVA and are statistically significant for Par4+/+ + MVs versus Par4−/− + MVs. P<0.001, Par4+/+ n=6, Par4−/− n=5. (B) Percent incidence of thrombosis is shown from the thrombosis experiment in panel A. No difference was found by log-rank sums test. (C) Mouse survival following MV injection in panel A is reported with death defined as permanent cessation of breathing within 5 minutes of MV injection. Survival data were analyzed by log-rank (Mantel-Cox) test. (D-F) Clopidogrel or vehicle treated wild-type mice were subjected to IVC stenosis followed by BxPc-3 MV injection as above and (D) thrombus formation was quantified by high-frequency ultrasonography. Data were analyzed by one-way ANOVA and are statistically significant for MVs + clopidogrel versus MVs + vehicle control. P<0.001, BxPc-3 MVs + vehicle n=5, BxPc-3 MVs + clopidogrel n=6. (E) Percent incidence of thrombosis is shown from the thrombosis experiments in panel D. No difference was found by log-rank sums test. (F) Mouse survival following MV injection in panel D is reported with death defined as permanent cessation of breathing within 5 minutes of MV injection. Survival data were analyzed by log-rank (Mantel-Cox) test.
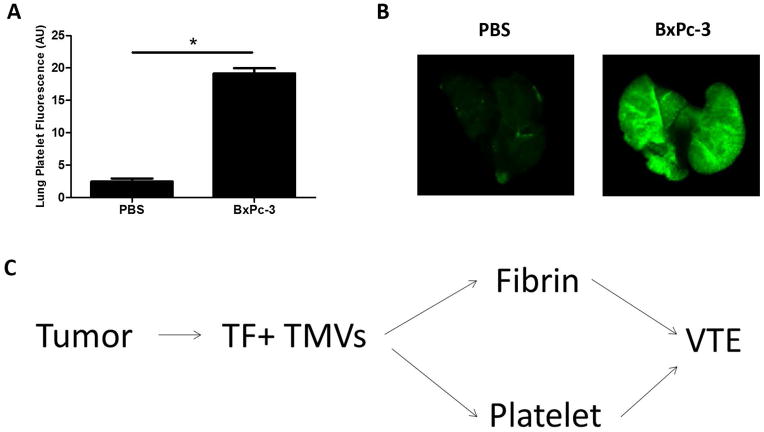
Intravenous Injection of TF+ TMVs increases lung platelet deposition in mice
We suspected that death of the mice receiving TF+ TMVs was due to formation of platelet-rich clots in the lungs. To examine this we injected BxPc-3 TMVs into WT mice without IVC stenosis and measured platelet deposition in the lungs. We found that platelet deposition in the lungs was increased with injection of BxPc-3 TMVs into mice, which suggests that TMV-induced platelet activation is occurring in vivo (Figure 8A and B). We have previously shown that accumulation of labeled platelets in the lung is associated with platelet-rich thrombi in the lung vasculature [39].
Figure 8. TF+ tumor microvesicles induce accumulation of platelets in the mouse lung.
(A) C57Bl6J mice were treated with an infrared-labeled anti-mouse GPIX antibody (0.125 μg/g). Mice were then injected intravenously with BxPc-3 microvesicles (MVs) at 5, 30, and 60 minutes (9 μg total) or the same volume of PBS control. Mice were sacrificed 5 minutes after the last injection, the lungs were removed and platelet deposition imaged on an Odyssey Infrared imaging system. Quantification of lung fluorescence was performed on ImageJ software. *P<0.0001, n=4 by unpaired student’s t-test. (B) Representative lung images are shown from mice injected with MVs or PBS. Some mice would start to gasp and then die within minutes of TMV injection. (C) Proposed pathways by which TF+ TMVs enhance venous thromboembolism (VTE).
Discussion
Cancer-associated thrombosis is a major clinical problem and is likely mediated by multiple mechanisms in different types of cancer [9]. In this study, we analyzed the role of TF+ TMV-enhanced platelet activation as a mechanism that contributes to cancer-associated thrombosis. We found that TF+ TMVs from two human pancreatic adenocarcinoma cell lines interact with resting platelets and activate them via thrombin generation.
What mediates the interaction between TMVs and platelets? Previous studies have shown that platelet binding of MVs derived from LPS stimulated human monocytes or the monocytic cell line THP-1 is mediated by PSGL-1 on the MV interacting with P-selectin on the activated platelet [43, 44]. Del Conde and colleagues found that 17% of the activated platelets acquired MV TF in a 30 minute incubation [43]. In contrast, little binding was observed when the monocyte MVs were incubated with unstimulated platelets. Ghosh and colleagues found that inhibition of CD36 reduced binding of endothelial, monocyte, and platelet MVs to resting platelets [45]. Moreover, annexin V or an antibody to PS also reduced MV binding to platelets, suggesting a role for MV PS in binding [45]. However, we did not observe any effect of annexin V on the binding of TMVs to resting platelets when incubated with the platelets or the MVs. Thomas and colleagues reported PSGL-1 expression by the human pancreatic cell line SOJ-6 and the mouse pancreatic cell line Panc02 and suggested that an interaction between MV PSGL-1 and platelet P-selectin mediated the docking of TMVs to the growing thrombus [22]. In contrast, a recent study found that neither P-selectin nor GP1b were required for TMV recruitment to the site of thrombosis in the IVC stenosis model [32]. We did not detect PSGL-1 expression on MVs derived from BxPc-3 and L3.6pl. Furthermore, inhibition of P-selectin did not reduce TMV-induced platelet aggregation (data not shown). This suggests that the observed interaction between BxPc-3 and L3.6pl MVs and resting platelets is not mediated by an interaction between PSGL-1 and P-selectin. More recently, it was reported that “Panc02 cancer cell-derived microparticles firmly adhere at the site of injury to fibrinogen present on activated platelets forming a thrombus” [31]. In addition, an inhibitor of the integrins αvβ1/β3 reduced the accumulation of Panc02 to the site of vessel injury in mice [31]. However, we did not observe any effect of either the integrin αvβ3 inhibitory antibody LM609 or annexin V on the interaction of BxPc-3 MVs with resting platelets. These differences may be due to the fact that MVs derived from different tumor lines have different cell surface receptors. Indeed, we have found marked differences in cell surface receptors on MVs derived from 4 human pancreatic cancer cells lines (BxPc-3, L3.6pl, HPAC and PANC-1) (Figure 2A and data not shown). Additional studies are needed to determine the mechanisms by which different TMVs interact with platelets.
We showed that TF+ TMVs activate washed platelets and induce platelet aggregation in the presence of plasma. This activation was dependent on both TF and thrombin. An earlier study showing that MVs from the human pancreatic cancer cell line SOJ-6 induced aggregation of washed platelets in the presence of plasma but not in the absence of plasma [22]. Furthermore, aggregation was abolished with FVII-depleted plasma but not with fibrinogen-depleted plasma. Taken together, these results indicate that TF+ TMVs from several different human pancreatic cell lines induce aggregation of platelets in vitro.
We found that injection of BxPc-3 MVs into mice increased plasma TATc, a marker of activation of coagulation, and PF4, a marker of platelet activation. Furthermore, BxPc-3 MV injection increased platelet accumulation in the lungs and resulted in death from suspected pulmonary embolism. A previous study found that injection of MVs from either TF+ human breast cancer cell line MDA-MB-231 or L3.6pl cells led to acute thrombocytopenia and signs of shock [17]. We found that TMV-enhanced thrombosis was abolished by inhibiting TF on the MV. In addition, TMV-enhanced thrombosis was reduced in mice lacking Par4 and in wild-type mice treated with clopidogrel. This suggests that platelet activation plays a role in TMV-enhanced thrombosis (Figure 8C). A limitation of these experiments is that deficiency of Par4 or administration of clopidogrel to WT mice will also reduce basal thrombosis. Low molecular-weight heparin (LMWH) or clopidogrel reversed the prothrombotic phenotype observed in mice bearing Panc02 tumors in a laser-injury model [31]. TF+ MVs have been detected in patients with various cancers, including breast and pancreatic cancer [23, 46]. Therefore, TF+ MVs may contribute to thrombosis in patients with different types of cancers.
LMWH is the recommended treatment for VTE in patients with cancer [47, 48]. However, VTE recurs in 6–9% of patients despite therapy [49–53]. Currently, the only evidence-based option for treating recurrent VTE in cancer patients despite secondary thromboprophylaxis is LMWH dose escalation [54]. Interestingly, there is clinical evidence for the involvement of platelets in cancer-associated thrombosis [55]. For instance, thrombocytosis is a risk factor for VTE in cancer patients, many of whom have increased plasma markers of platelet activation [56–61]. Although aspirin had a marginal effect (p=0.053) in ovarian cancer patients and had no effect on VTE risk in breast cancer patients in two retrospective studies, it reduced VTE as effectively as LMWH in multiple myeloma patients [62–64]. These data suggest that anti-platelet drugs may be beneficial in the prevention of cancer-associated thrombosis.
Non-cancer patients who develop a VTE are treated acutely with LMWH and then bridged to a vitamin K antagonist or treated with the new oral anticoagulants. However, clinical trials have found that long-term LMWH is more effective than vitamin K antagonists at reducing VTE recurrence in cancer patients [48, 51, 52]. These studies suggest that the pathophysiology of VTE in cancer patients is different from non-cancer patients. Our mouse study and the recent study by Mezouar and colleagues [31] suggest that anti-platelet drugs, such as clopidogrel, may reduce cancer-associated VTE in cancer patients with high levels of circulating TF+ TMVs.
Supplementary Material
Supplemental Figure 1. Representative scatter plots for platelet activation flow cytometry
Representative scatter plots are shown for the activation of platelets by BxPc-3 and L3.6pl microvesicles (MVs) (50 μg protein/mL) (A) MVs were pre-incubated with the anti-TF blocking antibody HTF-1 (50 μg/ml) or IgG control (50 μg/ml) for 30 minutes at 4°C. MVs were then incubated with human platelets in the presence of diluted human plasma (1:100). (B) Platelets were pre-incubated with inhibitors as indicated for 5 minutes at room temperature. BxPc-3 and L3.6pl MVs (50 μg/mL) were then incubated with human platelets in the presence of diluted plasma for 15 minutes at 37°C. Recombinant TF (InnovinTM) (0.5 pg) and alpha-thrombin (5 nm) were used as controls. After the activation reaction was complete, the reactions were further diluted in Tyrode’s buffer + 1 mM CaCl2 + 0.5% formalin and P-selectin expression evaluated by flow cytometry.
Supplementary Figure 2. TF+ tumor microvesicles enhance thrombosis in a femoral vein thrombosis model.
Thrombosis in mice was induced by electrolytic injury of the femoral vein. Mice were injected intravenously with vehicle (white circles), BxPc-3 MVs (6 μg) pretreated with IgG (50 μg/mL) (black line), or BxPc-3 MVs (6 μg) pre-treated with the anti-TF antibody HTF-1 (50 μg/ml) (grey line) immediately prior to injury and 30 minutes following injury. Thrombus size is shown as relative fibrin (A) and platelet (B) accumulation over time. *P<0.001, n=8 per group. Data were analyzed by one-way ANOVA with Bonferroni posttests comparing all data sets to the no MV control.
Essentials.
Cancer patients have a high rate of venous thrombosis but the underlying mechanisms are unkown.
The study investigates the role of tumor-derived, tissue factor-positive microvesicles in platelet activation in vitro and in vivo.
Tumor-derived, tissue factor microvesicles activated platelets in vitro and enhanced venous thrombosis in mice.
Platelets may contribute to venous thrombosis in some cancer patients and this could be prevented with anti-platelet drugs.
Acknowledgments
We would like to thank Drs. Christophe Dubois and Dougald M. Monroe III for helpful discussions and Wyeth Alexander and Lori Holle for technical support. Hirudin was a generous gift from Dougald M. Monroe III, PhD. HTF-1 was a generous gift from Dr. Ronald Bach (UMN). Electron Microscopy was performed by Dr. Sezgin Ozgur and Dr. Jack Griffith in the Electron Microscopy Core at the UNC Lineberger Cancer Center. Ultrasonography utilized equipment provided by the cardiovascular surgical animal models core facility at UNC MHI. The Stratedigm Ex1000 flow cytometer is supported by the UNC flow cytometry core facility (NIH S100DO12052). We would like to thank Dr. Ellie Tzima for providing LM609. This work was supported by grants from the NIH (J.G. F30HL117546, N.M. and N.K. HL095096, T.G and M.W. T32HL007169, W.B. P01HL120846), the Triangle Community Foundation, Gertrude B. Elion Mentored Medical Student Research Award, the Katherine Pryzwansky Young Investigator Award, and UNC Wagner Scholarship Fund.
Footnotes
Authorship Contributions
J. E. Geddings and N. Mackman designed the experiments and wrote the manuscript with significant input from Y. Hisada. Y. Boulaftali, T. Getz, M. Whelihan, N. S. Key, W. Bergmeier, B. C. Cooley, R. Dee, A. S. Wolberg and R. Fuentes, J. E. Geddings and Y. Hisada performed experiments and analyzed data.
Disclosure of Conflicts of Interest
The authors do not declare any conflicts of interest.
References
- 1.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. Jama. 2005;293:715–22. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 2.Cronin-Fenton DP, Sondergaard F, Pedersen LA, Fryzek JP, Cetin K, Acquavella J, Baron JA, Sorensen HT. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997–2006. British journal of cancer. 2010;103:947–53. doi: 10.1038/sj.bjc.6605883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160:809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 4.Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404–13. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–64. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–50. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 7.Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529–35. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 8.Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27:4839–47. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hisada Y, Geddings JE, Ay C, Mackman N. Venous Thrombosis and Cancer: from Mouse Models to Clinical Trials. J Thromb Haemost. 2015 doi: 10.1111/jth.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, Hostetter G, Harvey J, Taubman MB. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870–5. doi: 10.1158/1078-0432.CCR-06-2351. [DOI] [PubMed] [Google Scholar]
- 11.Kakkar AK, Lemoine NR, Scully MF, Tebbutt S, Williamson RC. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg. 1995;82:1101–4. doi: 10.1002/bjs.1800820831. [DOI] [PubMed] [Google Scholar]
- 12.Thaler J, Preusser M, Ay C, Kaider A, Marosi C, Zielinski C, Pabinger I, Hainfellner JA. Intratumoral tissue factor expression and risk of venous thromboembolism in brain tumor patients. Thromb Res. 2013;131:162–5. doi: 10.1016/j.thromres.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–93. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 14.Osterud B, Bjorklid E. Tissue factor in blood cells and endothelial cells. Front Biosci (Elite Ed) 2012;4:289–99. doi: 10.2741/e376. [DOI] [PubMed] [Google Scholar]
- 15.Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: Which? Where? When? Arterioscler Thromb Vasc Biol. 2009;29:1989–96. doi: 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest. 1999;103:879–87. doi: 10.1172/jci6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost. 2008;6:1517–24. doi: 10.1111/j.1538-7836.2008.02987.x. [DOI] [PubMed] [Google Scholar]
- 18.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 19.Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011;9:2251–61. doi: 10.1111/j.1538-7836.2011.04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biro E, Sturk-Maquelin KN, Vogel GM, Meuleman DG, Smit MJ, Hack CE, Sturk A, Nieuwland R. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–8. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 21.Yu JL, Rak JW. Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J Thromb Haemost. 2004;2:2065–7. doi: 10.1111/j.1538-7836.2004.00972.x. [DOI] [PubMed] [Google Scholar]
- 22.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206:1913–27. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873–80. doi: 10.1182/blood-2013-04-460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–5. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 26.Tesselaar ME, Romijn FP, van der Linden IK, Bertina RM, Osanto S. Microparticle-associated tissue factor activity in cancer patients with and without thrombosis. J Thromb Haemost. 2009;7:1421–3. doi: 10.1111/j.1538-7836.2009.03504.x. [DOI] [PubMed] [Google Scholar]
- 27.Manly DA, Wang J, Glover SL, Kasthuri R, Liebman HA, Key NS, Mackman N. Increased microparticle tissue factor activity in cancer patients with Venous Thromboembolism. Thromb Res. 2010;125:511–2. doi: 10.1016/j.thromres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bharthuar A, Khorana AA, Hutson A, Wang JG, Key NS, Mackman N, Iyer RV. Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb Res. 2013;132:180–4. doi: 10.1016/j.thromres.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830–40. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JG, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, Kirchhofer D, Bogdanov VY, Bach RR, Rak J, Church FC, Wolberg AS, Pawlinski R, Key NS, Yeh JJ, Mackman N. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119:5543–52. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mezouar S, Darbousset R, Dignat-George F, Panicot-Dubois L, Dubois C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int J Cancer. 2015;136:462–75. doi: 10.1002/ijc.28997. [DOI] [PubMed] [Google Scholar]
- 32.Thomas GM, Brill A, Mezouar S, Crescence L, Gallant M, Dubois C, Wagner DD. Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J Thromb Haemost. 2015 doi: 10.1111/jth.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet--cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. British journal of pharmacology. 2004;143:819–26. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawlinski R, Wang JG, Owens AP, 3rd, Williams J, Antoniak S, Tencati M, Luther T, Rowley JW, Low EN, Weyrich AS, Mackman N. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood. 2010;116:806–14. doi: 10.1182/blood-2009-12-259267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang JG, Manly D, Kirchhofer D, Pawlinski R, Mackman N. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. J Thromb Haemost. 2009;7:1092–8. doi: 10.1111/j.1538-7836.2009.03448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ollivier V, Wang J, Manly D, Machlus KR, Wolberg AS, Jandrot-Perrus M, Mackman N. Detection of endogenous tissue factor levels in plasma using the calibrated automated thrombogram assay. Thromb Res. 2010;125:90–6. doi: 10.1016/j.thromres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geddings J, Aleman MM, Wolberg A, von Bruhl ML, Massberg S, Mackman N. Strengths and weaknesses of a new mouse model of thrombosis induced by inferior vena cava stenosis: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:571–3. doi: 10.1111/jth.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooley BC. In vivo fluorescence imaging of large-vessel thrombosis in mice. Arterioscler Thromb Vasc Biol. 2011;31:1351–6. doi: 10.1161/atvbaha.111.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stolla M, Stefanini L, Andre P, Ouellette TD, Reilly MP, McKenzie SE, Bergmeier W. CalDAG-GEFI deficiency protects mice in a novel model of Fcgamma RIIA-mediated thrombosis and thrombocytopenia. Blood. 2011;118:1113–20. doi: 10.1182/blood-2011-03-342352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sipos B, Moser S, Kalthoff H, Torok V, Lohr M, Kloppel G. A comprehensive characterization of pancreatic ductal carcinoma cell lines: towards the establishment of an in vitro research platform. Virchows Archiv : an international journal of pathology. 2003;442:444–52. doi: 10.1007/s00428-003-0784-4. [DOI] [PubMed] [Google Scholar]
- 41.Tan MH, Nowak NJ, Loor R, Ochi H, Sandberg AA, Lopez C, Pickren JW, Berjian R, Douglass HO, Jr, Chu TM. Characterization of a new primary human pancreatic tumor line. Cancer Invest. 1986;4:15–23. doi: 10.3109/07357908609039823. [DOI] [PubMed] [Google Scholar]
- 42.Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, Fidler IJ. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–35. [PubMed] [Google Scholar]
- 43.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–11. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 44.Rauch U, Bonderman D, Bohrmann B, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Transfer of tissue factor from leukocytes to platelets is mediated by CD15 and tissue factor. Blood. 2000;96:170–5. [PubMed] [Google Scholar]
- 45.Ghosh A, Li W, Febbraio M, Espinola RG, McCrae KR, Cockrell E, Silverstein RL. Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in mice. J Clin Invest. 2008;118:1934–43. doi: 10.1172/jci34904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tesselaar ME, Osanto S. Risk of venous thromboembolism in lung cancer. Curr Opin Pulm Med. 2007;13:362–7. doi: 10.1097/MCP.0b013e328209413c. [DOI] [PubMed] [Google Scholar]
- 47.Lyman GH, Bohlke K, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE, Kakkar AK, Key NS, Levine MN, Liebman HA, Tempero MA, Wong SL, Somerfield MR, Falanga A. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update 2014. J Clin Oncol. 2015 doi: 10.1200/jco.2014.59.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee AY, Peterson EA. Treatment of cancer-associated thrombosis. Blood. 2013;122:2310–7. doi: 10.1182/blood-2013-04-460162. [DOI] [PubMed] [Google Scholar]
- 49.Carrier M, Khorana A, Zwicker J, Noble S, Lee A. Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH. J Thromb Haemost. 2013;27:12338. doi: 10.1111/jth.12338. [DOI] [PubMed] [Google Scholar]
- 50.Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7:760–5. doi: 10.1111/j.1538-7836.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 51.Hull RD, Pineo GF, Brant RF, Mah AF, Burke N, Dear R, Wong T, Cook R, Solymoss S, Poon MC, Raskob G. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062–72. doi: 10.1016/j.amjmed.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Lee AY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, Rickles FR, Julian JA, Haley S, Kovacs MJ, Gent M. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–53. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 53.Meyer G, Marjanovic Z, Valcke J, Lorcerie B, Gruel Y, Solal-Celigny P, Le Maignan C, Extra JM, Cottu P, Farge D. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729–35. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- 54.Ihaddadene R, Le Gal G, Delluc A, Carrier M. Dose escalation of low molecular weight heparin in patients with recurrent cancer-associated thrombosis. Thromb Res. 2014;134:93–5. doi: 10.1016/j.thromres.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 55.Connolly GC, Phipps RP, Francis CW. Platelets and cancer-associated thrombosis. Seminars in oncology. 2014;41:302–10. doi: 10.1053/j.seminoncol.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Caine GJ, Lip GY, Stonelake PS, Ryan P, Blann AD. Platelet activation, coagulation and angiogenesis in breast and prostate carcinoma. Thromb Haemost. 2004;92:185–90. doi: 10.1267/thro04070185. [DOI] [PubMed] [Google Scholar]
- 57.Cella G, Marchetti M, Vianello F, Panova-Noeva M, Vignoli A, Russo L, Barbui T, Falanga A. Nitric oxide derivatives and soluble plasma selectins in patients with myeloproliferative neoplasms. Thromb Haemost. 2010;104:151–6. doi: 10.1160/th09-09-0663. [DOI] [PubMed] [Google Scholar]
- 58.Li L, Li P, Yang YQ, Zhang H, Ai P, Wang F, Jiang Y, Zou LQ, Yan X, Luo F. sCD40L, sP-selectin and sICAM-1 plasma levels in nasopharyngeal carcinoma. Sichuan da xue xue bao Yi xue ban = Journal of Sichuan University Medical science edition. 2009;40:513–6. [PubMed] [Google Scholar]
- 59.Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822–9. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- 60.Jensvoll H, Blix K, Braekkan SK, Hansen JB. Platelet count measured prior to cancer development is a risk factor for future symptomatic venous thromboembolism: the Tromso Study. PloS one. 2014;9:e92011. doi: 10.1371/journal.pone.0092011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simanek R, Vormittag R, Ay C, Alguel G, Dunkler D, Schwarzinger I, Steger G, Jaeger U, Zielinski C, Pabinger I. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) J Thromb Haemost. 2010;8:114–20. doi: 10.1111/j.1538-7836.2009.03680.x. [DOI] [PubMed] [Google Scholar]
- 62.Palumbo A, Cavo M, Bringhen S, Zamagni E, Romano A, Patriarca F, Rossi D, Gentilini F, Crippa C, Galli M, Nozzoli C, Ria R, Marasca R, Montefusco V, Baldini L, Elice F, Callea V, Pulini S, Carella AM, Zambello R, Benevolo G, Magarotto V, Tacchetti P, Pescosta N, Cellini C, Polloni C, Evangelista A, Caravita T, Morabito F, Offidani M, Tosi P, Boccadoro M. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol. 2011;29:986–93. doi: 10.1200/jco.2010.31.6844. [DOI] [PubMed] [Google Scholar]
- 63.Shai A, Rennert HS, Lavie O, Ballan-Haj M, Bitterman A, Steiner M, Keren S, Rennert G. Statins, aspirin and risk of venous thromboembolic events in breast cancer patients. Journal of thrombosis and thrombolysis. 2014;38:32–8. doi: 10.1007/s11239-013-1015-8. [DOI] [PubMed] [Google Scholar]
- 64.Shai A, Rennert HS, Rennert G, Sagi S, Leviov M, Lavie O. Statins, aspirin and risk of thromboembolic events in ovarian cancer patients. Gynecologic oncology. 2014;133:304–8. doi: 10.1016/j.ygyno.2014.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Representative scatter plots for platelet activation flow cytometry
Representative scatter plots are shown for the activation of platelets by BxPc-3 and L3.6pl microvesicles (MVs) (50 μg protein/mL) (A) MVs were pre-incubated with the anti-TF blocking antibody HTF-1 (50 μg/ml) or IgG control (50 μg/ml) for 30 minutes at 4°C. MVs were then incubated with human platelets in the presence of diluted human plasma (1:100). (B) Platelets were pre-incubated with inhibitors as indicated for 5 minutes at room temperature. BxPc-3 and L3.6pl MVs (50 μg/mL) were then incubated with human platelets in the presence of diluted plasma for 15 minutes at 37°C. Recombinant TF (InnovinTM) (0.5 pg) and alpha-thrombin (5 nm) were used as controls. After the activation reaction was complete, the reactions were further diluted in Tyrode’s buffer + 1 mM CaCl2 + 0.5% formalin and P-selectin expression evaluated by flow cytometry.
Supplementary Figure 2. TF+ tumor microvesicles enhance thrombosis in a femoral vein thrombosis model.
Thrombosis in mice was induced by electrolytic injury of the femoral vein. Mice were injected intravenously with vehicle (white circles), BxPc-3 MVs (6 μg) pretreated with IgG (50 μg/mL) (black line), or BxPc-3 MVs (6 μg) pre-treated with the anti-TF antibody HTF-1 (50 μg/ml) (grey line) immediately prior to injury and 30 minutes following injury. Thrombus size is shown as relative fibrin (A) and platelet (B) accumulation over time. *P<0.001, n=8 per group. Data were analyzed by one-way ANOVA with Bonferroni posttests comparing all data sets to the no MV control.