Abstract
Organochlorine insecticides have been studied extensively in relation to breast cancer incidence and results from two meta-analyses have been null for late-life residues, possibly due to measurement error. Whether these compounds influence survival remains to be fully explored. We examined associations between organochlorine insecticides (p,p’-DDT, its primary metabolite, p,p’-DDE, and chlordane) assessed shortly after diagnosis and survival among women with breast cancer. A population-based sample of women diagnosed with a first primary invasive or in situ breast cancer in 1996–1997 and with available organochlorine blood measures (n=633) were followed for vital status through 2011. After follow-up of 5 and 15 years, we identified 55 and 189 deaths, of which 36 and 74, respectively, were breast cancer-related. Using Cox regression models, we estimated the multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for lipid-adjusted organochlorine concentrations with all-cause and breast cancer-specific mortality. At 5 years after diagnosis, the highest tertile of DDT concentration was associated with all-cause (HR=2.19; 95%CI: 1.02, 4.67) and breast cancer-specific (HR=2.72; 95%CI: 1.04, 7.13) mortality. At 15 years, middle tertile concentrations of DDT (HR=1.42; CI 0.99, 2.06) and chlordane (HR=1.42; 95%CI: 0.94, 2.12) were modestly associated with all-cause and breast cancer-specific mortality. Third tertile DDE concentrations were inversely associated with 15-year all-cause mortality (HR=0.66; 95%CI: 0.44, 0.99). This is the first population-based study in the United States to show that DDT may adversely impact survival following breast cancer diagnosis. Further studies are warranted given the high breast cancer burden and the ubiquity of these chemicals.
Keywords: Organochlorine compounds, pesticides, DDT, DDE, chlordane, breast cancer, survival
INTRODUCTION
In the United States (US), the organochlorine insecticide dichlorodiphenyltrichloroethane (DDT) was first used during World War II to combat malaria, typhus, and other diseases among military populations.1 Widespread use began shortly after in 1945. DDT use increased until 1959 and declined steadily until its ban in 1972 because of growing environmental and wildlife concerns.2,3 Other countries restricted its use several years earlier, including Canada in 1969, while others continued to use DDT until much later, including Mexico which halted the use of DDT in 2000.2,4 Today, DDT production continues in China, India, and North Korea, as does indoor residual spraying for malaria control, which involves coating the walls and other surfaces of a house with a residual insecticide,5 in countries such as India, Ethiopia, and South Africa.6 Continued DDT use ensures continued direct and indirect exposure to DDT and its metabolites.7 Another organochlorine insecticide, chlordane, was used agriculturally in the US from 1948 until 1983 and then restricted to use for termite control until its ban in 1988.8
DDT and its primary metabolite, dichlorodiphenyldichloroethylene (DDE), have been extensively studied in relation to breast cancer incidence because they are highly lipophilic and have long biological half-lives.9,10 While DDT shows estrogenic activity in breast cells in vitro,11 DDE is an anti-androgen.11–13 Furthermore, organochlorine chemicals are stored in adipose tissue, including the breast.14 Importantly, breast tissue levels can be estimated validly and less invasively with peripheral blood measures.15 Additionally, it is important to consider weight and weight change since body mass index (BMI), a surrogate for adiposity, can alter tissue and blood concentrations and can cause slower elimination of organochlorine compounds and thus result in extended exposures in the body.10 While several studies have found significant associations between DDT, DDE, and chlordane and breast cancer incidence,16–21 results of most,22–27 including two meta-analyses,28,29 have largely been null, possibly because the measurements obtained may not correctly reflect the exposures during the etiologically relevant period(s). Although data on early life exposures are limited, results of a two-generation cohort studies found that blood measures of DDT ascertained prenatally or prior to a woman's reproductive years were associated with subsequent risk of developing breast cancer;21,30,31 however, the role of early life exposures to DDT remains unresolved.32
Whether organochlorine pesticides impact breast cancer survival remains a largely unexplored topic, with only one research group in Denmark publishing a positive association between the organochlorine insecticide dieldrin and all-cause and breast cancer-specific mortality following a breast cancer diagnosis.33–35 This potential association is particularly important given the high breast cancer incidence and mortality among women in the US and globally.36,37
The present study aimed to examine the associations of the organochlorine insecticides, DDT and chlordane, and the DDT metabolite DDE with survival among US women with breast cancer. We hypothesized that organochlorine compounds would be positively associated with mortality, particularly breast cancer-specific mortality. Additionally, we were interested in examining whether weight-related measures modified the relationships observed given the potential for adiposity to alter the elimination rate of organochlorine compounds.10
METHODS
Study design and study population
The Long Island Breast Cancer Study Project (LIBCSP) was a population-based study that was initiated as a case-control study to identify environmental factors associated with developing breast cancer, and then continued as a follow-up study to identify factors associated with survival. Details of the LIBCSP have been published previously.24,38 Briefly, adult female residents of Nassau and Suffolk counties with a first diagnosis of invasive or in situ breast cancer between August 1, 1996, and July 31, 1997, confirmed by physicians and medical records, were identified for inclusion through daily/weekly contact of pathology departments of 31 hospitals on Long Island and New York City, NY.
At baseline, on average within three months of the participant’s diagnosis, 1,508 women with breast cancer, with signed informed consent, completed an interviewer-administered questionnaire; 1,102 provided blood samples for laboratory analyses. The present study uses data from 633 women with breast cancer for whom blood levels of DDT (n=622), DDE (n=632), or chlordane (n=586) and lipids were available.24 Participants with available organochlorine measures were primarily white (92%) with a mean age of 58 years (range=29–89 years), post-menopausal (66%), and diagnosed with a first primary invasive breast cancer (71%), as described in Table 1.
Table 1.
Distribution of the selected baseline characteristics of the LIBCSP women diagnosed with breast cancer in 1996–1997 (n=633).
| p,p’-DDTa (n=622) | ||||
|---|---|---|---|---|
| Total (n=633) |
Tertile 1 (n=207) |
Tertile 2 (n=208) |
Tertile 3 (n=207) |
|
| n (%) | n (%) | n (%) | n (%) | |
| Age | ||||
| <35 | 14 (2%) | 5 (2%) | 5 (2%) | 11 (5%) |
| 35–44 | 90 (14%) | 27 (13%) | 32 (15%) | 42 (20%) |
| 45–54 | 160 (25%) | 59 (29%) | 55 (26%) | 44 (21%) |
| 55–64 | 163 (26%) | 55 (27%) | 60 (29%) | 64 (31%) |
| 65–74 | 155 (24%) | 51 (25%) | 40 (19%) | 39 (19%) |
| 75+ | 51 (8%) | 10 (5%) | 16 (8%) | 7 (3%) |
| BMI | ||||
| <25kg/m2 | 283 (45%) | 94 (46%) | 100 (49%) | 85 (42%) |
| 25–30kg/m2 | 206 (33%) | 78 (38%) | 53 (26%) | 74 (36%) |
| 30+kg/m2 | 136 (22%) | 34 (17%) | 50 (25%) | 46 (22%) |
| Missing | 8 | 1 | 5 | 2 |
| Income | ||||
| <$15,000–$24,999 | 125 (20%) | 28 (14%) | 40 (19%) | 54 (26%) |
| $25,000–$49,999 | 188 (30%) | 66 (32%) | 60 (29%) | 59 (29%) |
| $50,000-$90,000+ | 318 (50%) | 113 (55%) | 107 (52%) | 93 (45%) |
| Missing | 2 | 0 | 1 | 1 |
| Education | ||||
| <HS-HS graduate | 277 (44%) | 89 (43%) | 85 (41%) | 99 (48%) |
| Some college/-College graduate | 253 (40%) | 82 (40%) | 93 (45%) | 73 (36%) |
| Post college | 100 (16%) | 36 (17%) | 29 (14%) | 33 (16%) |
| Missing | 3 | 0 | 1 | 2 |
| Parity/Lactation history | ||||
| Nulliparous | 67 (11%) | 27 (13%) | 17 (8%) | 22 (11%) |
| Parous/never lactated | 350 (55%) | 115 (56%) | 108 (52%) | 121 (58%) |
| Parous/ever lactated | 216 (34%) | 65 (31%) | 83 (40%) | 64 (31%) |
| Menopausal status | ||||
| Premenopausal | 212 (34%) | 71 (35%) | 78 (38%) | 60 (30%) |
| Postmenopausal | 409 (66%) | 133 (65%) | 128 (62%) | 140 (70%) |
| Missing | 12 | 3 | 2 | 7 |
Long Island Breast Cancer Study Project (LIBCSP) participants diagnosed with breast cancer between August 1, 1996 and July 31, 1997 and followed-up through December 31, 2011.
Lipid-adjusted p,p’-DDT concentration cut-points: Tertile 1 (<56.8ng/g), Tertile 2 (≥56.8–<91.2ng/g), Tertile 3 (≥91.2ng/g)
The LIBCSP study protocol was approved by the Institutional Review Board of all participating institutions and in accordance with an assurance filed with and approved by the US Department of Health and Human Services.
Laboratory assays
Blood sample collection, analytic methods, and QA/QC procedures in the LIBCSP have been previously published.10,38 Briefly, approximately 73% of participants provided 40-mL non-fasting blood samples, of which 77% were collected prior to the initiation of chemotherapy. Samples for assaying were selected as follows: (1) randomly sampled from among women with invasive breast cancer (n=415); (2) all women with tumors initially categorized as in situ that were subsequently determined to be invasive (n=42); (3) all women with in situ tumors (n=184); and (4) all African-American participants who were not selected in the first three steps (n=5).
Gas chromatography/electron capture detection was conducted as outlined by Brock et al.39 to estimate concentrations of p,p’-DDT (DDT), p,p’-DDE (DDE) and chlordane (the sum of oxychlordane and trans-nonachlor). Positive and zero values of individual organochlorine levels below the detection limit (0.2ng/ml) were set to the lowest observed positive value for that compound, rather than being assigned a censored value. The proportions of observations that were below the detection limit were 8% for DDT, 1% for DDE, and 8% for chlordane.
Lipid profiles were determined for use in adjustment of DDT/DDE concentrations to account for non-fasting variations and to more closely approximate adipose tissue levels.40,41 We also present results for models in which we include total lipids as a covariate (Table S1). In the main analyses, continuous concentrations of lipid-adjusted organochlorines were divided into tertiles using the following cut-points (in ng/g) for: p,p’-DDT, <56.82, ≥56.82–<91.22 and ≥91.22; p,p’-DDE, <467.86, ≥467.86–<1,058.20 and ≥1,058.20; and chlordane, <81.08, ≥81.08–<131.00, and ≥131.00.
Follow-up for mortality
The National Death Index (NDI), a centralized database of death record information maintained by the National Center for Health Statistics,42 was used to ascertain date and cause of death. International Statistical Classification of Diseases codes 174.9 and C-50.9 listed anywhere on the death certificate were used to identify breast cancer-related deaths. Participants were followed from diagnosis in 1996–1997 until December 31, 2011. The maximum duration of follow-up was 15.42 years. Among our 633 participants after 5-years of follow-up, 55 (9%) deaths occurred, of which 36 were due to breast cancer; and, after 15 years, 189 (30%) deaths occurred, with 74 due to breast cancer.
Interview and medical record data
Prior to data collection, participants provided signed informed consent and permission for medical record release. Participants completed a 2-hour interviewer-administered questionnaire to assess demographic characteristics and potential and established risk/prognostic factors for breast cancer. Medical records were abstracted to obtain information on tumor hormone receptor status, primarily estrogen and progesterone receptor (ER and PR, respectively) status and first course of treatment.
Statistical analyses
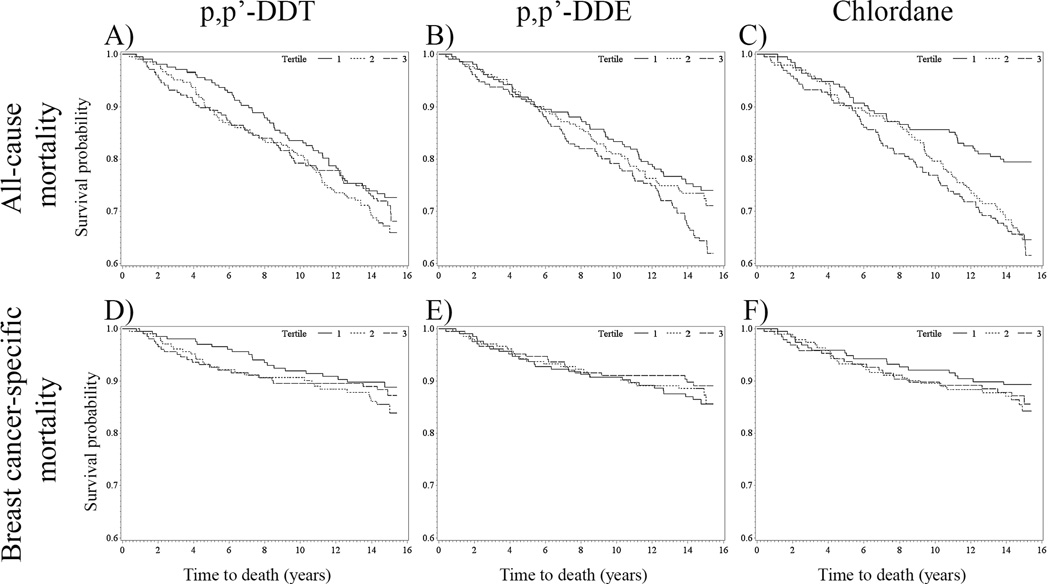
Kaplan-Meier survival curves were used for preliminary examination of the unadjusted data (Figure 1). The proportional hazards assumption was assessed by testing interaction terms of the exposure variables with time and natural log of time and by Schoenfeld residuals for all covariates. No violations of the proportional hazards assumption were evident based on these tests; however, several of the p-values for the interaction terms of the exposure variables with time at 15 years were marginally significant for all-cause [log(time)*log(DDT) p=0.07] and breast cancer-specific [log(time)*log(DDT) p=0.09; log(time)*log(DDE) p=0.12] mortality. Multivariable Cox models43 were fit to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for increasing tertiles of lipid-adjusted DDT, DDE, and chlordane concentrations in association with all-cause and breast cancer-specific mortality at 5 and 15 years after diagnosis. Models were re-run restricted to cases with invasive tumors. Tests for trend used continuous natural log-transformed lipid-adjusted concentrations in regression models.
Figure 1.
Kaplan-Meier survival curves for all-cause mortality for (A) p,p’-DDT, (B) p,p’-DDE, and (C) chlordane and for breast cancer-specific mortality for (D) p,p’-DDT, (E) p,p’-DDE, and (F) chlordane stratified by organochlorine concentration tertiles 1 (solid line), 2 (dotted line), and 3 (dashed line) among LIBCSP women diagnosed with breast cancer in 1996–1997 (n=633). The x-axis shows times to death in years; the y-axis shows proportion of participants alive.
To assess for effect modification interaction terms between continuous organochlorine concentrations and body size were included in Cox models. Additionally, models were stratified by: (1) body mass index (BMI; weight (kg)/height (m)2), in the year prior to diagnosis, categorized as “BMI<25kg/m2” and “BMI≥25kg/m2;” and (2) percent weight-gain since age 20, categorized as “0–<20% weight-gain”, “20–<40% weight-gain” and “≥40% weight-gain” 44. Few women reported a decrease in adult weight change; therefore, associations within the strata of weight-loss were not examined. Also, at 5 years after diagnosis there were too few deaths to examine the associations stratified by body size; therefore, only 15-year associations are presented.
Possible confounders were selected based on previous studies of organochlorines and breast cancer incidence and survival10,45 and directed acyclic graphs,46 and included: age at diagnosis (5-year age groups), parity/lactation (nulliparous, parous/never lactated, and parous/ever lactated); menopausal status (premenopausal and postmenopausal), hormone replacement therapy use (continuous, number of months of use), BMI (continuous); annual household income (categorical); cigarette smoking (never, former, and current smokers).
Hormone receptor status was not included as a covariate in the models since ER/PR status may mediate the association between organochlorine compounds and breast cancer survival47 precluding adjustment by stratification for receptor status.48 Treatment undergone was also not included in our models as a covariate, given treatment is also a possible causal intermediate since ER/PR status is directly related to treatment.49
All statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
5-year all-cause and breast cancer-specific mortality
In the Kaplan-Meier survival curves, compared to tertile 1, tertiles 2 and 3 of DDT were associated with a higher probability of 5-year all-cause and breast cancer-specific mortality (Figure 1, Panels A and D). As shown in Table 2, multivariable-adjusted HRs for 5-year all-cause mortality were more than doubled for women with DDT concentrations in the middle (HR=2.55, 95%CI: 1.20, 5.45) and highest (HR=2.19, 95%CI: 1.02, 4.67)) tertiles, as compared to women with DDT concentrations in the lowest tertile (p-trend=0.02). The magnitude appeared modestly larger for the corresponding DDT estimates for 5-year breast cancer-specific mortality (HR=2.94; 95%CI: 1.12, 7.67; HR=2.72; 95%CI: 1.04, 7.13, respectively; p-trend=0.02). Estimates were slightly more pronounced when we restricted our analyses to women diagnosed with invasive breast cancer (Table S2) and post-menopausal women (Table S3). Additionally, these estimates were relatively unchanged when BMI was excluded from the adjustment set (Table S4).
Table 2.
Cox regression hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between blood levels of organochlorines and mortality in the LIBCSP women diagnosed with breast cancer in 1996–1997 (n=633).
| 5-Year All-Cause Mortality |
5-Year Breast Cancer Specific-Mortality |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age- Adjusteda |
Multivariable- Adjustedb |
Age- Adjusteda |
Multivariable- Adjustedb |
|||||||
| Deaths | Censored | HR (95% CI) | HR (95% CI) | p-trend | Deaths | Censored | HR (95% CI) | HR (95% CI) | p-trend | |
| p,p’-DDTc | ||||||||||
| Tertile 1 | 10 | 197 | 1 (Reference) | 1 (Reference) | 6 | 201 | 1 (Reference) | 1 (Reference) | ||
| Tertile 2 | 22 | 186 | 2.24 (1.06, 4.73) | 2.55 (1.20, 5.45) | 15 | 193 | 2.53 (0.98, 6.51) | 2.94 (1.12, 7.67) | ||
| Tertile 3 | 22 | 185 | 2.19 (1.03, 4.64) | 2.19 (1.02, 4.67) | 0.02 | 15 | 192 | 2.65 (1.03, 6.86) | 2.72 (1.04, 7.13) | 0.02 |
| p,p’-DDEd | ||||||||||
| Tertile 1 | 17 | 193 | 1 (Reference) | 1 (Reference) | 12 | 198 | 1 (Reference) | 1 (Reference) | ||
| Tertile 2 | 19 | 192 | 0.98 (0.50, 1.92) | 0.95 (0.48, 1.89) | 13 | 198 | 1.14 (0.51, 2.54) | 1.10 (0.48, 2.52) | ||
| Tertile 3 | 19 | 192 | 0.85 (0.41, 1.78) | 0.72 (0.33, 1.56) | 0.78 | 11 | 200 | 1.04 (0.42, 2.61) | 0.85 (0.32, 2.23) | 0.38 |
| Chlordanee | ||||||||||
| Tertile 1 | 13 | 182 | 1 (Reference) | 1 (Reference) | 9 | 186 | 1 (Reference) | 1 (Reference) | ||
| Tertile 2 | 19 | 177 | 1.40 (0.69, 2.87) | 1.26 (0.61, 2.61) | 13 | 183 | 1.59 (0.67, 3.76) | 1.53 (0.63, 3.69) | ||
| Tertile 3 | 19 | 176 | 1.34 (0.63, 2.86) | 1.26 (0.58, 2.71) | 0.13 | 12 | 183 | 1.70 (0.67, 4.33) | 1.68 (0.65, 4.35) | 0.13 |
| 15-Year All-Cause Mortality |
15-Year Breast Cancer Specific-Mortality |
|||||||||
| Age- Adjusteda |
Multivariable- Adjustedb |
Age- Adjusteda |
Multivariable- Adjustedb |
|||||||
| Deaths | Censored | HR (95% CI) | HR (95% CI) | p-trend | Deaths | Censored | HR (95% CI) | HR (95% CI) | p-trend | |
| p,p’-DDTc | ||||||||||
| Tertile 1 | 56 | 151 | 1 (Reference) | 1 (Reference) | 21 | 186 | 1 (Reference) | 1 (Reference) | ||
| Tertile 2 | 69 | 139 | 1.28 (0.90, 1.82) | 1.42 (0.99, 2.06) | 29 | 179 | 1.43 (0.81, 2.50) | 1.59 (0.90, 2.83) | ||
| Tertile 3 | 61 | 146 | 0.98 (0.68, 1.41) | 0.99 (0.68, 1.44) | 0.70 | 24 | 183 | 1.20 (0.67, 2.15) | 1.23 (0.68, 2.24) | 0.30 |
| p,p’-DDEd | ||||||||||
| Tertile 1 | 54 | 156 | 1 (Reference) | 1 (Reference) | 28 | 182 | 1 (Reference) | 1 (Reference) | ||
| Tertile 2 | 58 | 153 | 0.84 (0.57, 1.22) | 0.81 (0.56, 1.19) | 25 | 186 | 0.90 (0.52, 1.57) | 0.92 (0.53, 1.62) | ||
| Tertile 3 | 77 | 134 | 0.79 (0.54, 1.18) | 0.66 (0.44, 0.99) | 0.10 | 21 | 190 | 0.78 (0.41, 1.47) | 0.70 (0.36, 1.37) | 0.43 |
| Chlordanee | ||||||||||
| Tertile 1 | 40 | 155 | 1 (Reference) | 1 (Reference) | 20 | 175 | 1 (Reference) | 1 (Reference) | ||
| Tertile 2 | 68 | 128 | 1.48 (1.00, 2.20) | 1.42 (0.94, 2.12) | 27 | 169 | 1.46 (0.81, 2.62) | 1.47 (0.81, 2.67) | ||
| Tertile 3 | 70 | 125 | 1.23 (0.81, 1.87) | 1.31 (0.86, 2.00) | 0.07 | 24 | 171 | 1.43 (0.75, 2.72) | 1.45 (0.76, 2.75) | 0.35 |
Long Island Breast Cancer Study Project (LIBCSP) participants diagnosed with breast cancer between August 1, 1996 and July 31, 1997 and followed-up through December 31, 2011.
Adjusted for age at diagnosis
Adjusted for age at diagnosis, smoking status, income, body mass index, and parity/lactation history
Lipid-adjusted p,p’-DDT concentration cut-points: Tertile 1 (<56.8ng/g), Tertile 2 (≥56.8–<91.2ng/g), Tertile 3 (≥91.2ng/g)
Lipid-adjusted p,p’-DDE concentration cut-points: Tertile 1 (<467.1ng/g), Tertile 2 (≥467.1–<1,058.2ng/g), Tertile 3 (≥1,058.2ng/g)
Lipid-adjusted chlordane (Σoxychlordane and trans-nonachlor) concentration cut-points: Tertile 1 (<81.1ng/g), Tertile 2 (≥81.1–<131.0ng/g), Tertile 3 (≥131.0ng/g)
In the Kaplan-Meier survival curves, the highest tertiles of DDE and chlordane did not appear to be associated with higher 5-year all-cause and breast cancer specific mortality (Figure 1, Panels B, C, E, and F). However, in the multivariable models associations with all-cause and breast cancer-specific mortality 5 years after breast cancer diagnosis were positively associated with chlordane, and inversely associated with DDE, but the confidence intervals for the modest hazards included the null value.
15-year all-cause and breast cancer-specific mortality
In the Kaplan-Meier curves, the DDT survival curves for all-cause mortality appeared to converge after 8 years while the survival curves for chlordane after 5 years diverged. In the multivariable models, as shown in Table 2, 15-year all-cause mortality hazards were elevated for DDT (HR=1.42, 95%CI: 0.99, 2.06) and chlordane (HR=1.42, 95%CI: 0.94, 2.12) concentrations in the middle tertiles. The highest tertile of chlordane was elevated though closer to the null (HR=1.31; 95%CI 0.86, 2.00). Corresponding 15-year hazards for breast cancer-specific mortality were similarly elevated for DDT (HR=1.59, 95%CI: 0.90, 2.83) and chlordane (HR=1.47; 95%CI: 0.81, 2.67). In contrast, 15-year all-cause and breast cancer-specific mortality risk decreased with increasing DDE concentrations (p-trend=0.10). Most confidence intervals, however, included the null value. These inverse associations were not apparent for all-cause mortality in the Kaplan-Meier curves; however, the inverse association was apparent after 8 years for breast cancer-specific mortality (Figure 1, Panels A and E)
BMI-stratified 15-year all-cause mortality
As shown in Table 3, among women with BMI<25kg/m2, but not among women with BMI≥25kg/m2, hazards for all-cause mortality were increased in association with: DDT concentrations in the middle (HR=2.32; 95%CI: 1.23, 4.38) and highest (HR=1.43; 95%CI: 0.73, 2.79) tertiles (p-interaction=0.03; p-trend=0.19); and with chlordane concentrations in the middle (HR=1.79; 95%CI: 0.92, 3.47) and highest (HR=1.42; 95%CI: 0.69, 2.92) tertiles (p-interaction<0.01; p-trend=0.27). In contrast, within both strata of categorized BMI, increasing DDE concentrations were associated with decreasing hazard of all-cause mortality, but estimates were strongest among women with BMI<25kg/m2 (p-interaction=0.09).
Table 3.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between blood levels of organochlorines and mortality in the LIBCSP women diagnosed with breast cancer in 1996–1997 (n=625), stratified by BMI.
| BMI <25kg/m2 |
15-Year All-Cause Mortality |
15-Year Breast Cancer Specific-Mortality |
||||||
|---|---|---|---|---|---|---|---|---|
| Multivariable-Adjusteda | Multivariable-Adjusteda | |||||||
| Deaths | Censored | HR (95% CI) | p-trend | Deaths | Censored | HR (95% CI) | p-trend | |
| p,p’-DDTb | ||||||||
| Tertile 1 | 16 | 78 | 1 (Reference) | 8 | 86 | 1 (Reference) | ||
| Tertile 2 | 29 | 71 | 2.32 (1.23, 4.38) | 12 | 88 | 1.92 (0.76, 4.88) | ||
| Tertile 3 | 20 | 65 | 1.43 (0.73, 2.79) | 0.19 | 9 | 76 | 1.46 (0.55, 3.89) | 0.56 |
| p,p’-DDEc | ||||||||
| Tertile 1 | 25 | 96 | 1 (Reference) | 15 | 106 | 1 (Reference) | ||
| Tertile 2 | 23 | 73 | 0.72 (0.39, 1.33) | 11 | 85 | 0.82 (0.34, 1.99) | ||
| Tertile 3 | 18 | 48 | 0.53 (0.24, 1.14) | 0.26 | 3 | 63 | 0.23 (0.05, 1.09) | 0.18 |
| Chlordaned | ||||||||
| Tertile 1 | 15 | 84 | 1 (Reference) | 6 | 93 | 1 (Reference) | ||
| Tertile 2 | 26 | 65 | 1.79 (0.92, 3.47) | 15 | 76 | 2.93 (1.10, 7.79) | ||
| Tertile 3 | 20 | 51 | 1.42 (0.69, 2.92) | 0.27 | 17 | 64 | 1.72 (0.54, 5.48) | 0.57 |
|
BMI ≥25kg/m2 |
15-Year All-Cause Mortality |
15-Year Breast Cancer Specific-Mortality |
||||||
| Multivariable-Adjusteda | Multivariable-Adjusteda | |||||||
| Deaths | Censored | HR (95% CI) | p-trend | Deaths | Censored | HR (95% CI) | p-trend | |
| p,p’-DDTb | ||||||||
| Tertile 1 | 39 | 73 | 1 (Reference) | 13 | 99 | 1 (Reference) | ||
| Tertile 2 | 40 | 63 | 1.10 (0.69, 1.75) | 17 | 86 | 1.51 (0.71, 3.19) | ||
| Tertile 3 | 40 | 80 | 0.73 (0.46, 1.17) | 0.47 | 15 | 105 | 1.11 (0.51, 2.41) | 0.37 |
| p,p’-DDEc | ||||||||
| Tertile 1 | 29 | 59 | 1 (Reference) | 13 | 75 | 1 (Reference) | ||
| Tertile 2 | 35 | 77 | 0.77 (0.46, 1.29) | 14 | 98 | 0.98 (0.45, 2.14) | ||
| Tertile 3 | 57 | 84 | 0.67 (0.40, 1.11) | 0.14 | 18 | 123 | 0.93 (0.42, 2.08) | 0.98 |
| Chlordaned | ||||||||
| Tertile 1 | 25 | 71 | 1 (Reference) | 14 | 82 | 1 (Reference) | ||
| Tertile 2 | 40 | 62 | 1.13 (0.66, 1.94) | 12 | 90 | 0.94 (0.41, 2.14) | ||
| Tertile 3 | 50 | 70 | 1.09 (0.63, 1.89) | 0.24 | 17 | 103 | 1.36 (0.60, 3.08) | 0.47 |
Long Island Breast Cancer Study Project (LIBCSP) participants diagnosed with breast cancer between August 1, 1996 and July 31, 1997 and followed-up through December 31, 2011.
Adjusted for age at diagnosis, smoking status, income and parity/lactation history
Adjusted for age at diagnosis, smoking status, income, body mass index, and parity/lactation history
Lipid-adjusted p,p’-DDT concentration cut-points: Tertile 1 (<56.8ng/g), Tertile 2 (≥56.8–<91.2ng/g), Tertile 3 (≥91.2ng/g)
Lipid-adjusted p,p’-DDE concentration cut-points: Tertile 1 (<467.1ng/g), Tertile 2 (≥467.1–<1,058.2ng/g), Tertile 3 (≥1,058.2ng/g)
Lipid-adjusted chlordane (Σoxychlordane and trans-nonachlor) concentration cut-points: Tertile 1 (<81.1ng/g), Tertile 2 (≥81.1–<131.0ng/g), Tertile 3 (≥131.0ng/g)
BMI-stratified 15-year breast cancer-specific mortality
As shown in Table 3, hazards for breast cancer-specific mortality 15 years after diagnosis were increased among women with BMI<25kg/m2 for: DDT concentrations in the middle (HR=1.92; 95%CI: 0.76, 4.88) and highest (HR=1.46; 95%CI: 0.55, 3.89) tertiles (p-interaction<0.01; p-trend=0.56); and chlordane concentrations in the middle (HR=2.93; 95%CI: 1.10, 7.79) and highest (HR=1.72; 95%CI: 0.54, 5.48) tertiles (p-interaction=0.82; p-trend=0.57). In contrast, among women with BMI<25kg/m2, but not among women with BMI≥25kg/m2, breast cancer-specific mortality was reduced in association with the middle (HR=0.82; 95%CI: 0.34, 1.99) and highest (HR=0.23; 95%CI: 0.05, 1.09) DDE tertiles, but confidence intervals included the null value (p-interaction=0.14; p-trend=0.18).
Adult-lifetime percent weight gain-stratified 15-year all-cause mortality
As shown in Table 4, among women with 0–<20% lifetime weight-gain, 15-year all-cause mortality was positively associated with DDT concentrations in the middle tertile (HR=2.11; 95%CI: 1.03, 4.32). The DDT-mortality association decreased in magnitude with increasing percent weight gain for concentrations in the middle tertile (20–<40% weight gain, HR=1.30; 95%CI: 0.67, 2.51; ≥40% weight gain, HR=1.11; 95%CI: 0.54, 2.25; p-interaction=0.25).
Table 4.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between blood levels of organochlorines and mortality in the LIBCSP women diagnosed with breast cancer in 1996–1997 (n=558), stratified by adult-lifetime weight-gain.
| 0-<20% gain | 15-Year All-Cause Mortality |
|||
|---|---|---|---|---|
| Multivariable-Adjusteda | ||||
| Deaths | Censored | HR (95% CI) | p-trend | |
| p,p’-DDTb | ||||
| Tertile 1 | 15 | 47 | 1 (Reference) | |
| Tertile 2 | 21 | 53 | 2.11 (1.03, 4.32) | |
| Tertile 3 | 15 | 53 | 0.81 (0.37, 1.75) | 0.56 |
| p,p’-DDEc | ||||
| Tertile 1 | 15 | 57 | 1 (Reference) | |
| Tertile 2 | 18 | 54 | 0.91 (0.43, 1.94) | |
| Tertile 3 | 18 | 45 | 0.92 (0.39, 2.16) | 0.86 |
| Chlordaned | ||||
| Tertile 1 | 12 | 56 | 1 (Reference) | |
| Tertile 2 | 17 | 39 | 1.55 (0.70, 3.41) | |
| Tertile 3 | 19 | 44 | 1.30 (0.58, 2.93) | 0.22 |
| 20-<40% gain | 15-Year All-Cause Mortality |
|||
| Multivariable-Adjusteda | ||||
| Deaths | Censored | HR (95% CI) | p-trend | |
| p,p’-DDTb | ||||
| Tertile 1 | 19 | 48 | 1 (Reference) | |
| Tertile 2 | 21 | 31 | 1.30 (0.67, 2.51) | |
| Tertile 3 | 25 | 45 | 0.98 (0.50, 1.90) | 0.82 |
| p,p’-DDEc | ||||
| Tertile 1 | 21 | 44 | 1 (Reference) | |
| Tertile 2 | 19 | 40 | 0.71 (0.37, 1.38) | |
| Tertile 3 | 26 | 40 | 0.49 (0.24, 0.99) | 0.09 |
| Chlordaned | ||||
| Tertile 1 | 15 | 39 | 1 (Reference) | |
| Tertile 2 | 28 | 40 | 1.34 (0.69, 2.60) | |
| Tertile 3 | 20 | 39 | 0.72 (0.34, 1.52) | 0.23 |
| ≥40% gain | 15-Year All-Cause Mortality |
|||
| Multivariable-Adjusteda | ||||
| Deaths | Censored | HR (95% CI) | p-trend | |
| p,p’-DDTb | ||||
| Tertile 1 | 17 | 41 | 1 (Reference) | |
| Tertile 2 | 20 | 32 | 1.11 (0.54, 2.25) | |
| Tertile 3 | 13 | 33 | 0.63 (0.28, 1.41) | 0.68 |
| p,p’-DDEc | ||||
| Tertile 1 | 11 | 32 | 1 (Reference) | |
| Tertile 2 | 16 | 42 | 0.95 (0.42, 2.15) | |
| Tertile 3 | 23 | 36 | 0.93 (0.40, 2.14) | 0.40 |
| Chlordaned | ||||
| Tertile 1 | 11 | 38 | 1 (Reference) | |
| Tertile 2 | 16 | 34 | 1.17 (0.50, 2.75) | |
| Tertile 3 | 19 | 32 | 1.01 (0.41, 2.50) | 0.51 |
Long Island Breast Cancer Study Project (LIBCSP) participants diagnosed with breast cancer between August 1, 1996 and July 31, 1997 and followed-up through December 31, 2011.
Adjusted for age at diagnosis, smoking status, income, body mass index and parity/lactation history
Lipid-adjusted p,p’-DDT concentration cut-points: Tertile 1 (<56.8ng/g), Tertile 2 (≥56.8–<91.2ng/g), Tertile 3 (≥91.2ng/g)
Lipid-adjusted p,p’-DDE concentration cut-points: Tertile 1 (<467.1ng/g), Tertile 2 (≥467.1–<1,058.2ng/g), Tertile 3 (≥1,058.2ng/g)
Lipid-adjusted chlordane (Σoxychlordane and trans-nonachlor) concentration cut-points: Tertile 1 (<81.1ng/g), Tertile 2 (≥81.1–<131.0ng/g), Tertile 3 (≥131.0ng/g)
A similar pattern was observed for chlordane concentrations, where 15-year all-cause mortality was positively associated with middle tertile levels among women with 0–<20% lifetime weight-gain (HR=1.55; 95%CI: 0.70, 3.41) and a decrease in magnitude with increasing percent weight gain was observed (20–<40% weight gain HR=1.34; 95%CI: 0.69, 2.60; ≥40% weight-gain HR=1.17; 95%CI: 0.50, 2.75; p-interaction=0.65).
In contrast, DDE concentrations were inversely associated with 15-year all-cause mortality among women with 20–<40% adult-lifetime percent weight-gain (HR=0.71; 95%CI: 0.37, 1.38; HR=0.49; 95%CI: 0.24, 0.99; p-interaction=0.84).
DISCUSSION
In this first US population-based study to examine the association between blood levels of organochlorine compounds and survival following breast cancer, we observed a greater than two-fold increase in all-cause and breast cancer-specific mortality after 5 years of follow-up in association with DDT concentrations measured within a few month of diagnosis. Estimates were more pronounced when we restricted our analysis to women with invasive breast cancer only. Slightly attenuated DDT HRs remained elevated after 15 years of follow-up, but CIs were imprecise. The more pronounced association 5 years after breast cancer diagnosis, rather than after 15, may reflect susceptible women dying within 5 years. The risk of death from breast cancer within 5 years of diagnosis, while low (10%), decreases after 6–8 years during which death from other causes increases.50
To date, only one research group in Denmark has published on the association between organochlorine compounds and breast cancer mortality.33–35 In their initial report 33, among the compounds examined in 195 Danish women only dieldrin was associated with increased all-cause and breast cancer-specific mortality. In the present study, we were unable to examine dieldrin due to insufficient number of cases with levels above detectable limits;24 however, we were able to examine DDT, DDE and chlordane. In the Danish study, there was a suggestion of elevated mortality following breast cancer for p,p’-DDT, which is consistent with the results reported here. In contrast to the Danish study, we also observed an increased risk of all-cause mortality 15 years after a breast cancer diagnosis for chlordane, but a decreased risk for DDE despite the much lower concentrations of organochlorine compounds observed in this study. In the US, one study has examined whether organochlorine levels were associated with recurrence among women of Long Island, NY.51 In their study, the highest tertiles of total PCBs and the middle tertiles of DDE were positively associated with recurrence. However, their study was limited by the small case-control (n=224 women, 30 of which were diagnosed with a recurrence), hospital-based study design.
Although endocrine disrupting chemicals may act through multiple, complex, and unknown pathways, several biological mechanisms related to the estrogenic and hormone-antagonistic potential of these compounds may explain the associations observed here. The first is that organochlorine compounds may directly affect tumor cell proliferation by interacting with important receptors.52 The second proposed mechanism is that estrogenic organochlorine compounds stored in breast adipose tissue adjacent to the breast carcinoma may affect the tumor microenvironment making it more estrogenic favoring cell proliferation among hormone receptor positive neoplasms.47 Our positive findings between the estrogenic compounds DDT and chlordane and mortality support these mechanisms. We also observed an inverse association between DDE, a known anti-androgen, and mortality.13
Our stratified results that women with lower BMI and less adult-lifetime weight gain had the highest HRs of all-cause mortality, may reflect the competing estrogenic effects associated with high BMI. Our observation may be analogous to the situation where the positive association between breast cancer incidence and hormone replacement is only evident among non-obese women.53 Third, organochlorines compounds with known endocrine disruption effects may also result in metabolic disruption54 leading to pre- and post-diagnosis weight changes which may influence survival.44,55
At least two studies21,31 have shown that timing of exposure, especially during critical developmental windows, may be important for breast cancer etiology. Failure to account for exposure windows may partially explain the lack of association observed for breast cancer incidence. In contrast, for survival following breast cancer, the relevant windows of exposure to environmental contaminants, including continued endogenous exposure from the release of stored toxins, may extend from as early as 5 years before diagnosis56 through death.55 Thus, a possible explanation for our observation of stronger associations with 5-year mortality, rather than 15-year mortality, may be that biomarker concentrations assessed at baseline are more likely to reflect the relevant exposure period influencing mortality closer to diagnosis, rather than the exposures that are likely to have changed over the 15-year period of follow-up; although we were not able to assess how organochlorine levels changed over time as a result of ongoing low-level dietary exposure.57 However, it is also possible that exposures in early life may influence the characteristics of the tumor (e.g. receptor status, grade, lymph node involvement, and stage at diagnosis),34,47,58,59 which are known impact the effectiveness of chemotherapy, aggression of the cancer, and probability of survival. Approximately one-third (212 of 633) of the women included in our study were born in or after 1945 when DDT was widely introduced in the US. These women could have been potentially exposed to technical DDT in utero, a window of exposure that has been shown to be associated with breast cancer incidence.31 Additionally, almost half (295 of 633) of the women in our study were of younger reproductive age, between the ages of 14 and 25, during the years of DDT peak use in the US, from 1955 through 1962.
Our study has several strengths including biomarker assessments of organochlorine compounds in blood samples that were collected from a population-based sample of American women within a few months following diagnosis of their first primary breast cancers, who were then subsequently followed using the NDI, which provides high quality ascertainment of vital status. Nonetheless, this study has several limitations. While the largest HR of mortality was observed 5 years after breast cancer diagnosis, the low frequency of deaths at 5 years in our study population precluded us from examining whether these associations varied by a priori covariates of interest or by other potentially important characteristics such as birth cohort, although even with larger numbers we would be unable to say with certainty that birth cohort directly corresponds to age of exposure.
Another potential study concern is that we adjusted for BMI, which may have resulted in model misspecification. A recent study suggests that organochlorines may influence the metabolic system, including weight.60 Given that weight is a strong predictor of breast cancer survival,44,55 BMI is a possible causal intermediate for the organochlorine-breast cancer survival association. Thus, we also fit Cox models excluding BMI as a covariate. However, removal of BMI from the model did not appreciably alter our HR estimates; any potential bias of our results due to inclusion of BMI in our models is likely to be low.
Our study included only one organochlorine measurement. While concentrations measured at baseline are likely to be more temporally relevant to the outcomes, we were unable to account for changes in exposure to organochlorine compounds. Additionally, the biomarker assessment does not provide any information about the source of exposure and whether women were exposed to technical DDT and chlordane or whether they were exposed through other sources such as diet. Although we considered associations stratified by weight gain, we were not able to fully account for changes in organochlorine concentrations over time due to BMI changes, which may result in changes in the amount of chemicals stored in fatty breast tissue.10
Finally, determination of breast cancer-related deaths may have resulted in outcome misclassification. However, this misclassification is likely to be non-differential with respect to organochlorine levels. This non-differential misclassification would attenuate the risk estimates for breast cancer-specific mortality.61
Results of this first US population-based study indicate that exposure to organochlorine insecticides, especially those with known estrogenic properties, may negatively impact survival following breast cancer. In our study, DDT was associated with a more than two-fold increase in 5-year all-cause and breast-cancer specific mortality; and the mortality hazards, while attenuated, remained elevated 15 years after the first primary breast cancer diagnosis. Our finding that DDE was inversely associated with mortality may be suggestive of an anti-androgenic pathway that requires further investigation. Given the limited research on breast cancer survival conducted to date, our findings require replication in future studies, which should explore additional organochlorine compounds. Our study findings, which are consistent with the results from Denmark,35 emphasize the importance of environmental exposures in cancer survival and have important policy implications since further restriction of the use of these and other similar chemicals may be warranted due to the high burden of breast cancer worldwide.
Supplementary Material
Novelty and Impact.
While organochlorine compounds have been extensively studied in relation to breast cancer incidence, only one research group to date has examined whether these compounds are associated with survival. In this first population-based study in the United States, we show that DDT may adversely impact survival following breast cancer diagnosis. These results emphasize the importance of environmental exposures in cancer survival.
Acknowledgements
The authors gratefully acknowledge grant support from: the National Cancer Institute and the National Institutes of Environmental Health and Sciences Grant nos. UO1CA/ES66572, UO1CA66572, T32ES007018; and the Babylon Breast Cancer Coalition.
Abbreviations
- BMI
Body Mass Index
- CI
Confidence Interval
- DDE
dichlorodiphenyldichloroethylene
- DDT
dichlorodiphenyltrichloroethane
- ER
Estrogen Receptor
- ERR
Estrogen-Related Receptor
- HR
Hazard Ratio
- ICD
International Statistical Classification of Diseases
- LIBCSP
Long Island Breast Cancer Study Project
- NDI
National Death Index
- PR
Progesterone Receptor
- US
United States
Footnotes
Conflicts of interest: None declared
REFERENCES
- 1.US EPA. DDT - A Brief History and Status [Internet] 2012 Available from: http://www2.epa.gov/ingredients-used-pesticide-products/ddt-brief-history-and-status.
- 2.Smith D. Worldwide trends in DDT levels in human breast milk. Int J Epidemiol. 1999;28:179–188. doi: 10.1093/ije/28.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Carson R. Silent Spring. Houghton M. Greenwich. Conn: Fawcett Publications, Inc; 2002. [Google Scholar]
- 4.Commission for Environmental Cooperation of North America. DDT no longer used in North America [Internet] 2003 Available from: http://www3.cec.org/islandora/en/item/1968-ddt-no-longer-used-in-north-america-en.pdf.
- 5.Centers for Disease Control and Prevention. Indoor Residual Spraying [Internet] 2012 [cited 2015 May 13];Available from: http://www.cdc.gov/malaria/malaria_worldwide/reduction/irs.html.
- 6.Van den Berg H, Zaim M, Yadav RS, Soares A, Ameneshewa B, Mnzava A, Hii J, Dash AP, Ejov M. Global trends in the use of insecticides to control vector-borne diseases. Environ Health Perspect. 2012;120:577–582. doi: 10.1289/ehp.1104340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer KS, Kegley SE. Persistent toxic chemicals in the US food supply. J Epidemiol Community Health. 2002;56:813–817. doi: 10.1136/jech.56.11.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US EPA. Chlordane [Internet] 2013 Available from: http://www.epa.gov/ttn/atw/hlthef/chlordan.html.
- 9.Wolff MS. Half-lives of organochlorines (OCs) in humans. Arch Environ Contam Toxicol. 1999;36:504. doi: 10.1007/pl00006624. [DOI] [PubMed] [Google Scholar]
- 10.Wolff MS, Britton JA, Teitelbaum SL, Eng S, Deych E, Ireland K, Liu Z, Neugut AI, Santella RM, Gammon MD. Improving organochlorine biomarker models for cancer research. Cancer Epidemiol biomarkers Prev. 2005;14:2224–2236. doi: 10.1158/1055-9965.EPI-05-0173. [DOI] [PubMed] [Google Scholar]
- 11.Welch RM, Levin W, Conney AH. Estrogenic action of DDT and its analogs. Toxicol Appl Pharmacol. 1969;14:358–367. doi: 10.1016/0041-008x(69)90117-3. [DOI] [PubMed] [Google Scholar]
- 12.Kim TS, Kim CY, Lee HK, Kang IH, Kim MG, Jung KK, Kwon YK, Nam H-S, Hong SK, Kim HS, Yoon HJ, Rhee GS. Estrogenic Activity of Persistent Organic Pollutants and Parabens Based on the Stably Transfected Human Estrogen Receptor-α Transcriptional Activation Assay (OECD TG 455) Toxicol Res. 2011;27:181–184. doi: 10.5487/TR.2011.27.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 14.National Pesticide Information Center. DDT (Technical Fact Sheet) [Internet] 2000 Available from: http://npic.orst.edu/factsheets/ddttech.pdf.
- 15.Stellman SD, Djordjevic MV, Muscat JE, Gong L, Bernstein D, Citron ML, White A, Kemeny M, Busch E, Nafziger AN. Relative abundance of organochlorine pesticides and polychlorinated biphenyls in adipose tissue and serum of women in Long Island, New York. Cancer Epidemiol Biomarkers Prev. 1998;7:489–496. [PubMed] [Google Scholar]
- 16.Wolff MS, Paolo G, Lee EW, Dubin N. Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst. 1993;85:648–652. doi: 10.1093/jnci/85.8.648. [DOI] [PubMed] [Google Scholar]
- 17.Romieu I. Breast cancer, lactation history, and serum organochlorines. Am J Epidemiol. 2000;152:363–370. doi: 10.1093/aje/152.4.363. [DOI] [PubMed] [Google Scholar]
- 18.Krieger N, Wolff MS, Hiatt RA, Rivera M, Vogelman J, Orentreich N. Breast cancer and serum organochlorines: a prospective study among White, Black, and Asian women. J Natl Cancer Inst. 1994;86:589–599. doi: 10.1093/jnci/86.8.589. [DOI] [PubMed] [Google Scholar]
- 19.Güttes S, Failing K, Neumann K, Kleinstein J, Georgii S, Brunn H. Chlororganic pesticides and polychlorinated biphenyls in breast tissue of women with benign and malignant breast disease. Arch Environ Contam Toxicol. 1998;35:140–147. doi: 10.1007/s002449900361. [DOI] [PubMed] [Google Scholar]
- 20.Charlier C. Breast cancer and serum organochlorine residues. Occup Environ Med. 2003;60:348–351. doi: 10.1136/oem.60.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007;115:1406–1414. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter DJ, Hankinson SE, Laden F, Colditz GA, Manson JE, Willett WC, Speizer FE, Wolff MS. Plasma organochlorine levels and the risk of breast cancer. N Engl J Med. 1997;337:1253–1258. doi: 10.1056/NEJM199710303371801. [DOI] [PubMed] [Google Scholar]
- 23.López-Carrillo L, Blair A, López-cervantes M, Lãpez-carrillo L, Mohar A, Bravo J. Dichlorodiphenyltrichloroethane serum levels and breast cancer risk: a case-control study from Mexico. Cancer Res. 1997;57:3728–3732. [PubMed] [Google Scholar]
- 24.Gammon MD, Wolff MS, Neugut AI, Eng SM, Teitelbaum SL, Britton JA, Terry MB, Levin B, Stellman SD, Kabat GC, Hatch M, Senie R, et al. Environmental toxins and breast cancer on Long Island. II. Organochlorine compound levels in blood. Cancer Epidemiol Biomarkers Prev. 2002;11:686–697. [PubMed] [Google Scholar]
- 25.Millikan R, DeVoto E, Duell EJ, Tse CK, Savitz Da, Beach J, Edmiston S, Jackson S, Newman B. Dichlorodiphenyldichloroethene, polychlorinated biphenyls, and breast cancer among African-American and white women in North Carolina. Cancer Epidemiol Biomarkers Prev. 2000;9:1233–1240. [PubMed] [Google Scholar]
- 26.Høyer AP, Grandjean P, Jørgensen T, Brock JW, Hartvig HB. Organochlorine exposure and risk of breast cancer. Lancet. 1998;352:1816–1820. doi: 10.1016/S0140-6736(98)04504-8. [DOI] [PubMed] [Google Scholar]
- 27.Engel LS, Hill DA, Hoppin JA, Lubin JH, Lynch CF, Pierce J, Samanic C, Sandler DP, Blair A, Alavanja MC. Pesticide use and breast cancer risk among farmers’ wives in the agricultural health study. Am J Epidemiol. 2005;161:121–135. doi: 10.1093/aje/kwi022. [DOI] [PubMed] [Google Scholar]
- 28.Ingber SZ, Buser MC, Pohl HR, Abadin HG, Edward Murray H, Scinicariello F. DDT/DDE and breast cancer: a meta-analysis. Regul Toxicol Pharmacol. 2013;67:421–433. doi: 10.1016/j.yrtph.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López-Cervantes M, Torres-Sánchez L, Tobías A, López-Carrillo L. Dichlorodiphenyldichloroethane burden and breast cancer risk: a meta-analysis of the epidemiologic evidence. Environ Health Perspect. 2004;112:207–214. doi: 10.1289/ehp.112-1241830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Interagency Breast Research Cancer and Environmental (IBCERCC) Coordinating Committee. Prioritizing Prevention [Internet] Research Triangle Park, NC: 2013. Breast Cancer and the Environment. Available from: http://www.niehs.nih.gov/about/assets/docs/ibcercc_full_508.pdf. [Google Scholar]
- 31.Cohn BA, La Merrill M, Krigbaum NY, Yeh G, Park J-S, Zimmermann L, Cirillo PM. DDT Exposure in Utero and Breast Cancer [published online ahead of print June 16, 2015] J Clin Endocrinol Metab. 2015 doi: 10.1210/jc.2015-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loomis D, Guyton K, Grosse Y, El Ghissasi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet. 2015;16:891–892. doi: 10.1016/S1470-2045(15)00081-9. [DOI] [PubMed] [Google Scholar]
- 33.Høyer AP, Jørgensen T, Brock JW, Grandjean P. Organochlorine exposure and breast cancer survival. J Clin Epidemiol. 2000;53:323–330. doi: 10.1016/s0895-4356(99)00165-1. [DOI] [PubMed] [Google Scholar]
- 34.Høyer AP, Jorgensen T, Rank F, Grandjean P. Organochlorine exposures influence on breast cancer risk and survival according to estrogen receptor status: a Danish cohort-nested case-control study. BMC Cancer. 2001;1:8. doi: 10.1186/1471-2407-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Høyer AP, Gerdes A-M, Jørgensen T, Rank F, Hartvig HB. Organochlorines, p53 mutations in relation to breast cancer risk and survival. A Danish cohort-nested case-controls study. Breast Cancer Res Treat. 2002;71:59–65. doi: 10.1023/a:1013340327099. [DOI] [PubMed] [Google Scholar]
- 36.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 37.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 38.Gammon MD, Neugut AI, Santella RM, Teitelbaum SL, Britton JA, Terry MB, Eng SM, Wolff MS, Stellman SD, Kabat GC, Levin B, Bradlow HL, et al. The Long Island Breast Cancer Study Project: description of a multi-institutional collaboration to identify environmental risk factors for breast cancer. Breast Cancer Res Treat. 2002;74:235–254. doi: 10.1023/a:1016387020854. [DOI] [PubMed] [Google Scholar]
- 39.Brock JW, Burse VW, Ashley DL, Najam AR, Green VE, Korver MP, Powell MK, Hodge CC, Needham LL. An improved analysis for chlorinated pesticides and polychlorinated biphenyls (PCBs) in human and bovine sera using solid-phase extraction. J Anal Toxicol. 1996;20:528–536. doi: 10.1093/jat/20.7.528. [DOI] [PubMed] [Google Scholar]
- 40.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 41.López-Carrillo L, Torres-Sánchez L, López-Cervantes M, Blair A, Cebrián ME, Uribe M. The adipose tissue to serum dichlorodiphenyldichloroethane (DDE) ratio: some methodological considerations. Environ Res. 1999;81:142–145. doi: 10.1006/enrs.1999.3961. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. National Death Index [Internet] 2014 Available from: http://www.cdc.gov/nchs/ndi.htm.
- 43.Allison P. Survival Analysis Using SAS: A Practical Guide. 2nd ed. Cary, NC: 2010. [Google Scholar]
- 44.Cleveland RJ, Eng SM, Abrahamson PE, Britton Ja, Teitelbaum SL, Neugut AI, Gammon MD. Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol biomarkers Prev. 2007;16:1803–1811. doi: 10.1158/1055-9965.EPI-06-0889. [DOI] [PubMed] [Google Scholar]
- 45.Soerjomataram I, Louwman MWJ, Ribot JG, Roukema JA, Coebergh JWW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107:309–330. doi: 10.1007/s10549-007-9556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 47.Muñoz-de-Toro M, Durando M, Beldoménico PM, Beldoménico HR, Kass L, García SR, Luque EH. Estrogenic microenvironment generated by organochlorine residues in adipose mammary tissue modulates biomarker expression in ERα-positive breast carcinomas. Breast Cancer Res. 2006;8:1–9. doi: 10.1186/bcr1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd Editio. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008. [Google Scholar]
- 49.Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version) Arch Pathol Lab Med. 2010;134:e48–e72. doi: 10.5858/134.7.e48. [DOI] [PubMed] [Google Scholar]
- 50.Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular disease mortality is higher among long-term breast cancer survivors than among a population-based sample of women without breast cancer: The Long Island Breast Cancer Study. Epidemiology. in press. [Google Scholar]
- 51.Muscat JE, Britton JA, Djordjevic M V, Citron ML, Kemeny M, Busch-Devereaux E, Pittman B, Stellman SD. Adipose Concentrations of Organochlorine Compounds and Breast Cancer Recurrence in Long Island, New York. Cancer Epidemiol Biomarkers Prev. 2003;12:1474–1478. [PubMed] [Google Scholar]
- 52.Zhuang S, Zhang J, Wen Y, Zhang C, Liu W. Distinct mechanisms of endocrine disruption of DDT-related pesticides toward estrogen receptor α and estrogen-related receptor γ. Environ Toxicol Chem. 2012;31:2597–2605. doi: 10.1002/etc.1986. [DOI] [PubMed] [Google Scholar]
- 53.Endogenous Hormones Breast Cancer Collaborative Group. Body Mass Index, Serum Sex Hormones, and Breast Cancer Risk in Postmenopausal Women. JNCI. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 54.Casals-Casas C, Desvergne B. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol. 2011;73:135–162. doi: 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- 55.Bradshaw PT, Ibrahim JG, Stevens J, Cleveland R, Abrahamson PE, Satia JA, Teitelbaum SL, Neugut AI, Gammon MD. Postdiagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology. 2012;23:320–327. doi: 10.1097/EDE.0b013e31824596a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trivers KF, Gammon MD, Abrahamson PE, Lund MJ, Flagg EW, Moorman PG, Kaufman JS, Cai J, Porter PL, Brinton LA, Eley JW, Coates RJ. Oral contraceptives and survival in breast cancer patients aged 20 to 54 years. Cancer Epidemiol biomarkers Prev. 2007;16:1822–1827. doi: 10.1158/1055-9965.EPI-07-0053. [DOI] [PubMed] [Google Scholar]
- 57.US DHHS. Toxicological profile for DDT, DDE, and DDD [Internet] Atlanta, GA: 2002. Available from: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=81&tid=20. [Google Scholar]
- 58.Dewailly E, Dodin S, Verreault R, Ayotte P, Sauvé L, Morin J, Brisson J. High organochlorine body burden in women with estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1994;86:232–234. doi: 10.1093/jnci/86.3.232. [DOI] [PubMed] [Google Scholar]
- 59.Demers A, Ayotte P, Brisson J, Concentrations O, Dodin S, Robert J, Dewailly E. Risk and aggressiveness of breast cancer in relation to plasma organochlorine concentrations. Cancer Epidemiol Biomarkers Prev. 2000;9:161–166. [PubMed] [Google Scholar]
- 60.Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127:204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wacholder S, Hartge P, Lubin JH, Dosemeci M. Non-differential misclassification and bias towards the null: a clarification. Occup Env med. 1995;52:557–558. doi: 10.1136/oem.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.