Abstract
An ideal tissue engineering scaffold should not only promote, but take an active role in, constructive remodeling and formation of site appropriate tissue. ECM-derived proteins provide unmatched cellular recognition, and therefore influence cellular response towards predicted remodeling behaviors. Materials built with only these proteins, however, can degrade rapidly or begin too weak to substitute for compliant, matrix-dense tissues. The focus of this review is on biohybrid materials that incorporate polymer components with ECM-derived proteins, to produce a substrate with desired mechanical and degradation properties, as well as actively guide tissue remodeling. Materials are described through four fabrication methods: (1) polymer and ECM-protein fibers woven together, (2) polymer and ECM proteins combined in a bilayer, (3) cell-built ECM on polymer scaffold, and (4) ECM proteins and polymers combined in a single hydrogel. Scaffolds from each fabrication method can achieve characteristics suitable for different types of tissue. In vivo testing has shown progressive remodeling in injury models, and suggests ECM-based biohybrid materials promote a prohealing immune response over single component alternatives. The prohealing immune response is associated with lasting success and long term host maintenance of the implant.
Keywords: extracellular matrix, biohybrid materials, scaffolds, scaffold remodeling
1. Introduction
This review will discuss biohybrid materials, or materials with both a synthetic and natural component, being developed to rebuild compliant, matrix-dense tissues. These materials have great potential for tissue engineering strategies since they combine the advantage of a natural substrate, to aid in site specific tissue regrowth, as well as a synthetic polymer component, to provide strength to withstand the force from environment while the material is remodeled. The applications for these materials include skin wound healing, vascular repair and grafts, tendon replacement, and intestinal wall reconstruction. We divided materials fitting this description into four groups based on fabrication techniques, and report on biological and mechanical property progress in subsequent sections. We conclude with a summary of the current work evaluating immune response to the biohybrid material, and suggest the need for a more in depth investigation into the relationship.
2. Role of ECM in Engineered Tissues
No longer casted with a merely supportive role, the extracellular matrix (ECM) is now considered to play a major part in tissue morphology and function, defining the microenvironment through controlling migration, behavior, and phenotype of resident cells.[1] The dynamic interaction between ECM and local cells is responsible for mechanical, chemical and physical changes in the tissue.[2, 3] As such, the ECM must be in constant state of deconstruction and specific rebuilding, based on cellular secretions, physical and chemical cues.
An ideal scaffold for tissue engineering would not only promote but take active part in this constructive remodeling and formation of site appropriate tissue. Material choice can be very influential in the overall success and incorporation of an implantable scaffold into the rebuilding process. Many implantable materials have been engineered to prioritize one of two main characteristics: biological relevancy or mechanical consistency to the tissue they are intended to replace.
Biologically relevant materials, such as naturally produced ECM structures, have been shown to help define the microenvironment and signal the building of site appropriate functional tissue. Matrix molecules represent a diverse set of structural and functional proteins, glycoproteins, and glycosaminoglycans that are arranged in an ultrastructure that is unique to each anatomic location. A variety of growth factors and binding sites have been described in nearly every tissue, specific to the environment and contained or transported through the ECM, as well. As described, the ECM provides a naturally occurring and highly conserved substrate for cell viability and growth through biological and chemical cues. Fragments of parent molecules, including collagen and fibronectin, have been shown to promote activities including angiogenesis, anti-angiogenesis, antimicrobial effects, and chemotactic effects. It is hypothesized that the specialized composition including structural and functional proteins and growth factors, together with the formation of chemotactic cryptic peptides, can recruit stem and progenitor cells and contribute to modulating the immune response. Successful stem and progenitor cell recruitment together with a moderate immune response have significant influence when determining the success of an implantable scaffold.[2, 4–6]
Due to this highly specified nature, ECM based materials have great and perhaps unparalleled potential to control the biological activities of cellular recognition and colonization. In addition to, or in fact because of, the particular composition of material, mechanical and physical characteristics of ECM are unique and highly specific. In fact, many of the ECM-based implantables that are commercially available today are utilized only for mechanical properties, intentionally blocking any chemical or biological identifiers.[7, 8]
The complexity of ECM makes identifying and understanding the minute components challenging, and because the constitution is only partially understood, it becomes a difficult material to replicate. ECM protein identification was pursued by Hansen et al, who used a novel combination of rapid ultrasonication and surfactant assisted digestion to aid in proteonomic analysis of tissue derived ECM.[9] Their results reveal hundreds of previously unidentified proteins in rat mammary glands that were substantially different from those in matrigel alternatives. Human mammary epithelial cells cultured in each of the two mediums demonstrate significantly different behavior, indicating that inaccuracies of mimicking cellular ECM constitution could significantly alter the nature of the resultant tissue.[9] The physical presentation of ECM derived tissue has been implicated in cellular behavior influences as well. In a study conducted by Xu et al. to mimic the ECM using a synthetic component, they found that minor changes in fiber diameters (1.28–1.50 μm), fiber density (22.2–46.1 # of fibers/100μm2), and fiber alignment (0.45–0.60 angular distribution), as well as resulting changes to the construct modulus, had significant effects on cardiac cell differentiation and growth.[10] This study agrees with many, that the precise composition and physical appearance of cell substrate, natural ECM or synthetic scaffold, can drastically direct cell and therefore tissue function.[11]
Considering the unique advantages of ECM based materials, and the complexity to replicating the naturally composed tissue, a hypothetical ideal tissue engineering scaffold would utilize ECM already produced, on a site and person specific basis. Unfortunately, ECM if left untreated has several challenges to consider before including it in an implantable scaffold material.
ECM-based material is observed to degrade rapidly when implanted in a foreign body. It has been reported to have degraded significantly in 2–3 weeks in vivo in a subdermal implant,[12] 60% in 30 days and 100% at 90 days in an in vivo tendon repair model.[13] The incorporation into self-sustained material through constructive remodeling can be a long process, and the material needs to retain designed physical properties until that time. A synthetic material, on the other hand, is much less vulnerable to proteolytic enzymes and degradation tactics, and can be tuned to retain certain physical parameters.
As pointed out by Zhu et al, synthetic polymer constituents allow for manipulation of a scaffold on a molecular level by controlling polymerization, crosslinking and functionalization.[14] These are important considerations when engineering soft tissue components, as the physical properties of the bulk tissue determine its ability to carry out its main function. There has been much development in polymer manufacturing techniques to produce materials that can mimic ECM in strength, elasticity, and structure of interconnected networks. However, synthetic polymers are usually utilized as passive scaffolds, and do not naturally create active cell interactions.
By combining blocks of natural tissue ECM with synthetic polymer components, the best of both materials can be included in one scaffold. This combination will be termed an ECM-based biohybrid material. A biohybrid material for the purpose of this review must contain significant blocks of both natural ECM-based material as well as synthetic fibers, for the purpose influencing the biology of the microenvironment or providing strength and resistance to degradation. Small-scale natural components such as growth factors and small adherent proteins are excluded from the natural material category, since they do not play a major role in ECM material bulk or mechanical properties.
3. Methods
In order to prepare a comprehensive review of this topic, a search was conducted through Thompson Reuters Web of Science and PubMed to compile recent and relevant publications. Searches covered 10 years, and the following search terms were used to conduct three independent searches of title, abstract, author key words, and key words plus®: “extracellular matrix” near scaffold and poly*, biohybrid and polymer and material, and biohybrid and scaffold. Each search yielded 800, 108, and 85 publications, respectively. Searches were designed to include any material containing physiologically built components that was used in combination with a polymer as a scaffold. The term biohybrid can be applied in the literature to a material that has any part of a biologically created material, including components as small as functional molecular groups and growth factors. The search terms were kept intentionally broad, and publications that did not involve large ECM-based structural proteins were excluded by hand. From the remaining pool, publications were selected for review that matched the above definition of an ECM-based biohybrid material, and were used for a compliant, matrix-dense tissue application. Relevant citations from or of said papers were reviewed as well.
4. Types of Biohybrid Materials
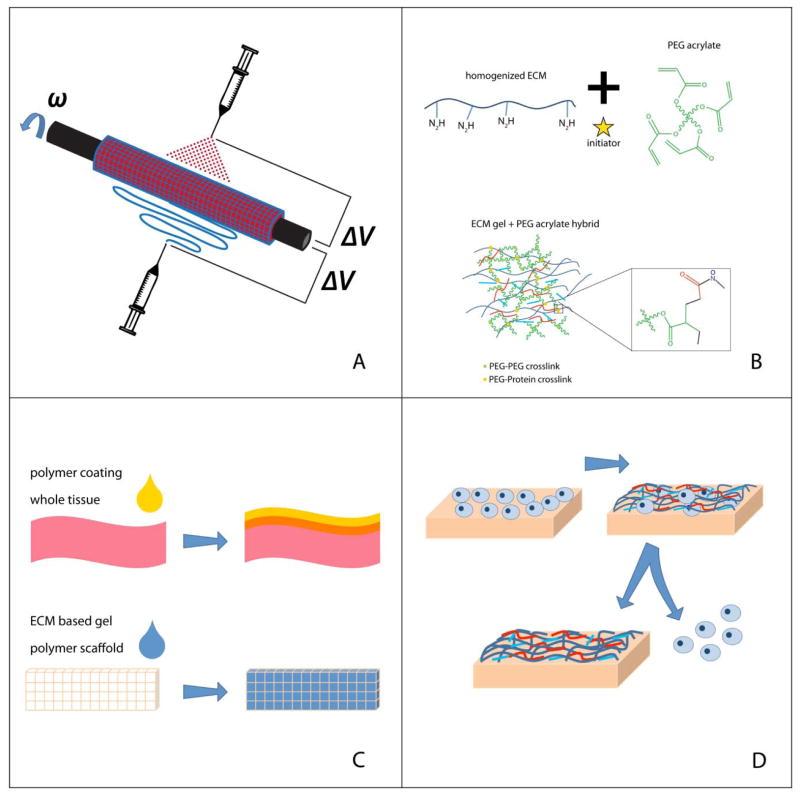
Upon review, we see four classes that can describe ECM-based biohybrid materials, separated by fabrication and ultimate landscape of the material. Selection of fabrication technique is in part dictated by the choice of both polymer and natural constituent. The fabrication techniques covered in this review are illustrated in Figure 1.
Figure 1.
Methods of Fabricating ECM-Based Biohybrid Materials. (A) Materials are fabricated with interwoven fibers, using either electrospinning, electrospraying, or a combination of the two. (B) Materials are fabricated by blending hydrogel components of ECM derived proteins and polymer chains. (C) Materials are built using a layering technique, with either a whole tissue or a polymer scaffold as a base. (D) Cell-built ECM layer fabrication, where cells are cultured on a polymer scaffold, and then removed, leaving an ECM layer on the polymer surface.
Materials composed primarily of intermingled fibers of both the polymer and natural component: The first technique covered in this report is the building of a composite by interweaving fibers. Typically, this is achieved by electrospinning or electrospraying one or both components although other methods like co-extrusion can produce this effect as well. Even though electrodeposition is capable of producing independent fibers on a micro and nano level, the deposition can be random, and cannot account for organized bioactive factor incorporation or placement.[15, 16]
Materials fabricated using the layering technique: The next method covered results in a bilayered scaffold, with distinct layers or regions of polymer and natural constituents. Regions of each constituent must be large enough to locally retain physical properties of the independent constituent. Fabricating this way can incorporate whole, decellularized tissue, which allows for the inclusion of many (even many unidentified) proteins and minerals.
Cell-built ECM layer fabrication method: The third method covered utilizes a cell layer to build a matrix on the surface of a polymer scaffold, leaving behind a biologically active interface when the cells are removed. This cell laid matrix is the primary biological contribution to the scaffold. This is also an effective means to ensure complete inclusion of proteins. For some tissue applications, this method is perhaps the closest to reality, if site appropriate cells can be cultured. Although, the resources and time to culture could to prevent this method from being a scalable procedure.
Materials fabricated by blending hydrogel components: The approach is to blend together homogenized tissue or ECM components with a soluble polymer phase, in order to create one cohesive hydrogel. The blending in this method mostly occurs on a molecular level. This method allows for a high level of user control, but eliminates natural structure and organization of the tissue components.
Based on the variety of material components, and in cases significant contribution from biological material, it can difficult to define biohybrid material scaffolds within traditional categories for standardized surgical implants. With regard to mechanical assessment, appropriate testing and measurement protocols to represent the most important characteristics of the material could be unavailable. ASTM International describes this problem for new biomaterials of tissue engineering scaffolds for growth, support, or delivery of cells and/or biomolecules in standard F2027. The standard explains that although there are no specific protocols set for novel materials, important properties that should be specified include elastic modulus, ultimate tensile strength and compressive modulus, among other physical properties such as viscosity, density and contact angles when appropriate. In standard F2150, Standard Guide for Characterization and Testing of Biomaterial Scaffolds Used in Tissue-Engineered Medical Products, a list is compiled of standards to consult for methodology that would be perhaps appropriate based on material characteristics. These recommendations are included in Table 1. Most of the protocols are designed for plastics, which neglects the natural tissue component.
Table 1.
Selection of Relevant ASTM Standards for Testing Biomaterials
| Standard | Title | Test Notes | |
|---|---|---|---|
| Tensile Testing | D412 | Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension |
|
| D638 | Standard Test Method for Tensile Properties of Plastics |
|
|
| D882 | Standard Test Method for Tensile Properties of Thin Plastic Sheeting |
|
|
| D1623 | Standard Test Method for Tensile and Tensile Adhesion Properties of Rigid Cellular Plastics |
|
|
| D1708 | Tensile Properties of Plastics microtensile specimens |
|
|
| D3039 | Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials |
|
|
| Compressive Testing | D695 | Standard Test Method for Compressive Properties of Rigid Plastics |
|
| D1621 | Standard Test Method for Compressive Properties of Rigid Cellular Plastics |
|
|
| F2977 | Standard Test Method for Small Punch Testing of Polymeric Biomaterials Used in Surgical Implant |
|
Following a standard protocol to test these new biohybrid materials would help identify the effect of various constituents and fabrication techniques to create material physically suited for several compliant tissue applications. To summarize the current mechanical data on biohybrid materials, mechanical properties, if reported, are often under testing conditions unique to the specific application of the study. For example, the elastic modulus of a polypropylene and collagen mesh from a tracheal implant was evaluated using compressive testing after 6 months implanted.[17] It is possible, however, that this combination of materials would be well suited for another tissue as well if evaluated using tension or in a different sample shape. In a discussion on the mechanical properties of a polypropylene mesh with dermal ECM for hernia repair, one group suggests that a standard should include an analysis of planar biaxial mechanics, instead of merely stretching a sample until failure.[18] Protocols that may have been originally designed for plastics and metals expose tissue to super-physiological conditions, which may not record the most relevant behavior. To design materials for compliant tissue engineering, a portrayal of stress and strain in a multi-axis relationship could be the closest to realistic physiological challenges.
4.1. Materials Fabricated with Interwoven Fibers
Building a scaffold by weaving or spinning together various compositions of fibers and polymer chains is a popular technique for creating hybrid scaffolds. Electrospinning and electrospraying provide a controlled way to deposit fibers in a designed shape. By controlling the speed and charge of material as it is deposited, the resulting woven material can resemble the structure of natural ECM, by producing single strand diameters between 50 and 500 nm.[15] Additionally, scaffolds manufactured on an individual fiber basis can have an open and interconnected porous architecture, which can help cells easily penetrate to the inner part of the scaffold. The single fiber identity can also provide cell attachment points at each fiber junction, similar to collagen fibrous strands in vivo structures.[19] This ease of infiltration, migration and adherence results in homogenous cell distribution and tissue formation in these types of grafts.[20]
By incorporating both synthetic and natural components into interpenetrating networks, each region of the scaffold has identical cell attachment and signaling opportunities, and at the same time provides equal stiffness or strength at each point of the scaffold. There are typically few to no concentrations of either component, which could create a polar or nonbioactive side of the scaffold. As a result, scaffolds built in this manner often see uniform degradation and infiltration, as well as uniform remodeling.[21]
4.1.1. Material Selection
The primary determinant in selecting materials for this fabrication technique is the potential for electric deposition. Common materials include poly(ε-caprolactone) (PCL), poly(lactic acid) (PLA), or poly(lactic-co-glycolic acid) (PLGA), which have been extensively developed to electrically deposit, and are biocompatible, strong, and biodegradable. Using an electro-spun fiber as a base building block, the biological components can incorporated with either casting, electrospraying, or in some cases electrospinning along with the synthetic component. Casting methods have been used to incorporate water-soluble collagen nanofibers[22] and ECM-based polysaccharides and proteins[23] into a PCL electrospun meshes. Since casting methods can avoid harsh solvents and conditions, they can be adapted to transport cellular constituents to the scaffold. In a study by Koch et al., we see the development of vascular grafts by casting fibrin gel with resident cells in a mold around a poly(D,L-lactide) mesh cylinder.[21]
To prioritize an organized, fiber-based deposition, ECM components can be incorporated into the polymer phase before it is electrospun. In a study by Hong et al, porcine small intestine mucosa (SIS) powder was blended with PCL before it was co-electrospun with silk fibroin. This method of incorporation improved hydrophilicity of the polymer, and effectively incorporated the biological component.[24] Other successful applications that rely solely on electrospinning include a blend of poly(vinyl alcohol), (PVA), with type I collagen into a single fiber constituent,[25] and a blend solution of PLGA, gelatin and elastin that was electrospun into vascular grafts.[26]
In contrast, using electrospraying techniques in combination with electrospinning can maintain the single fiber integrity of the polymer phase, but can apply the control of deposition more broadly to the collection of water based biological components. In one study, a complete tissue ECM is homogenized and then sprayed onto a poly(ester urethane) urea (PEUU) mesh scaffold resulting in interconnected fibrous layers embedded in a ECM gel.[27] Similarly, by developing a dual head, co-electrospinning device, a human-like collagen/chitosan blend was able to be co-spun into a vascular graft with PLA,[14] demonstrating the feasibility of using two different solutions to construct a scaffold for blood vessel tissue engineering. Finally, by combining the above mentioned methods, a hyaluronic acid (HA) hydrogel (Heprasil™) was added via a dual electrodeposition system to PCL-collagen blend (PCL/Col), mesh. Collagen was blended with PCL prior to deposition, resulting in an apparent surface coating of the deposited fibers, with obvious collagen domains and segregations. Simultaneous deposition of HA and PCL/Col allowed HA gel to be electrosprayed onto the mesh of electrospun PCL/Col, creating a mesh with hydrogel pockets interspersed within a matrix of polymer microfibers.[28] This double-team of electrospraying and electrospinning is conceivably capable of producing the closest replica of the ECM in vasculature and barrier tissues compared to an independent technique, since it combines fibers with softer gel pockets for a heterogeneous but consistent material.
4.1.2. Mechanical and Physical Properties
The geometric properties of scaffolds formed using this fabrication method are highly similar to the fibrous network of ECM. The thinly sprayed woven fibers can resemble specific components of ECM through intentional size-control by altering composition and deposition parameters. As shown by Hong et al., among others, increasing the concentration of the SIS powder in the PCL/silk fibroin solution resulted in a diameter reduction of the fibers due to increased electrical conductivity[24]. The increased electrical conductivity appeared to improve the size uniformity as well, compared to that of pure PCL fibers [24]. Results from incorporating human-like collagen/chitosan blend to PCL agree; Zhu et al found the electrospun collagen/chitosan/PCL had a more biomimetic structure than pure PLA, as the fiber diameters approached the size of the extracellular matrix.[14]
The deliberate deposition of fibers in the manufacturing process makes this method well suited to form highly defined cylindrical, or other self-supporting shapes. Since most methods contain a polymer phase that solidifies after deposition, fibers can usually support themselves. Woven fibers that possess strength individually, when combined and held together at junction points in a mesh, form materials with noted high flexibility and strength. In one case, blended fibers of PVA and collagen type I are gathered into a bundle and three bundles were twisted into a braid with the diameter of ~4.5 mm, building a flexible rope.[25] It is as a result of this strength in a variety of formations that we see many free standing scaffolds where structural integrity is crucial. This high structural integrity is an indicator of success in applications such as vascular grafts, intestinal lining, skin and even tendon or ligament grafts. [14, 21, 25, 26]
The mechanical strength is usually determined by the contents polymer phase, not the components contained in the hydrogel or biological fibers. As such, maximum mechanical strength of the composite can be attributed to the choice of polymer component and configuration. In some cases, the nature of the polymer phase can be altered by incorporation of biological or other components.[24] A table comparing mechanical strength of scaffolds fabricated using the woven fibers methods, as well as other methods, is shown in Table 2. While it is by no means to draw specific comparisons between the scaffolds, as they are all tested in their own specific shapes and thicknesses under different parameters, it can provide a vision of the broad range of parameters achieved by various materials and fabrication methods.
Table 2.
Mechanical Properties of Select ECM-Based Biomaterials
| Variety | Tensile Strength | Young’s Modulus | ||
|---|---|---|---|---|
| Fibers Woven | Composite of poly(ester urethane) urea (PEUU) and ECM gel (electrospun/sprayed in cylindrical conduits) [25] | 67 PEUU/33 ECM | 80 kPa longitudinal axis, 41 kPa circumferential | |
| 80 PEUU/20 ECM | 187 kPa longitudinal axis, 91 kPa circumferential | |||
| Collagen type 1 (Col-1) and polyvinyl alcohol (PVA) scaffold (braided fibers 4.5mm diameter)[23] | Col-1/PVA scaffold | 33.63 ± 3.10 MPa | 0.26 ± 0.05 GPa | |
| Regenerated ligament | 29.71 ± 0.96 MPa | 0.25 ± 0.02 GPa | ||
| Native ACL | 37.43 ± 2.13 MPa | 0.01 ± 0 GPa | ||
| Small intestine submuscosa (SIS) powder blended with poly(ε-caprolactone) (PCL), co-electrospun with silk fibroin (SF)[24] | PCL | 13 MPa | 11 MPa | |
| PCL/SF | 13.5 MPa | 12.5 MPa | ||
| PCL/SF/SIS | 14 MPa | 14 MPa | ||
| Poly(lactic-co-glycolic acid) (PLGA), gelatin, and elastin electrospun fiber mats, (PGE)[72] | 1 PLGA : 2 gelatin : 1 elastin | 16 ±7 kPa | 134 ± 51 kPa | |
| 2 PLGA : 2 gelatin : 1 elastin | 102 ± 26 kPa | 427 ± 41 kPa | ||
| 3 PLGA : 2 gelatin : 1 elastin | 130 ± 7 kPa | 770 ±131 kPa | ||
| Electrospun PCL/collagen nanofiber meshes (tested parallel to fibers)[29] | Random fiber orientation | 4.01 ± 0.29 MPa | 4.33 ±0.57 MPa | |
| Aligned fiber orientation | 4.88 ± 0.18 MPa | 4.43 ± 0.37 MPa | ||
| Layer Method | Poly-L-lactide (PLLA) mesh and collagen[3] | PLLA mesh | 33.8 ± 0.7 MPa warpa, 32.3 ± 0.1 MPa wefta | 212 ± 11 MPa warp, 177 ± 28 MPa weft |
| PLLA-collagen | 5.0 ± 0.4 MPa warp, 4.9 ±0.8 MPa weft | 55 ±12 MPa warp, 43±4 MPa weft | ||
| PLLA-gelatin | 5.8 ± 0.4 MPa warp, 4.7 ± 1.7 MPa weft | 43 ± 5 MPa warp, 41 ± 3 MPa weft | ||
| Collagen sponge | 0.01 ±0.00 MPa | 0.11 ± 0.05 MPa | ||
| Poly(propylene fumarate) (PPF) and bovine pericardium [33] | PPF reinforced pericardium | 14.17 ± 4.59 MPa | 54.00 ± 12.67 MPa | |
| GA-crosslinked pericardium | 11.51 ± 3.42 MPa | 67.87 ± 18.48 MPa | ||
| Untreated pericardium | 12.34 ± 1.49 MPa | 58.79 ± 10.17 MPa | ||
| Poly(L-lactic acid)-co-poly-(e-caprolactone (PLACL) nanofibers and GA-bovine pericardium [39] | PLACL and GA-pericardium | 2.39 ± 0.17 MPa | ||
| PLACL-Gel and GA-pericardium | 1.22 ± 0.17 MPa | |||
| GA-pericardium | 7.04 ± 0.51 MPa | |||
| ECM Polymer Hydrogel | Compressive Modulus | |||
| Carbon nanotube (CNT)-gelatin methacrylate (GelMA) hydrogels [61] | GelMA, no CNT | 10 kPa | ||
| CNT-GelMA | 32 kPa | |||
| Myocardial Matrix poly(ethylene glycol) (PEG) hydrogels [54] | ECM gel | 5 Pa | ||
| ECM- PEG NHS | 5–30 Pa | |||
| ECM- PEG diacrylate | 5–30 Pa | |||
| ECM- StarPEG acrylate | 719 Pa | |||
| Gelatin methacrylamide (GelMA) polymerized within a PEG framework [54] | GelMA(5%) PEG (5%) | 10.8 kPa | ||
| GelMA (20%) PEG (20%) | 327.7 kPa | |||
| Gelatin (5%) physically mixed with PEG (5%) | 8.2 kPa | |||
| Gelatin (20%) physically mixed with PEG (20%) | 66.3 kPa | |||
Textile terms referring to the orientation of the horizontal and vertical woven fibers, the lengthwise fibers that hold tension on a frame is the warp, and the set of fibers that is woven over and under the warp fibers is called the weft.
4.1.3. Biological Properties
While the woven polymer addition to biological components can improve strength of the scaffold, the incorporation of biological elements has a positive impact on the biocompatibility of the material. Electrospun pure polymer scaffolds alone have been plagued by low cell infiltration, cell adhesion, proliferation, and differentiation, due to minimal porosity, hydrophobicty acidic degrading sections, among other problems. By increasing the presence of a biological component, these problems can be mitigated.[28–31] Improved cell adhesion, infiltration and migration are seen as a result of incorporating both natural and synthetic components in many of these studies.
By incorporating a significant biological component, cell viability and metabolism are seen to improve over the pure polymer control in several studies.[14, 32] As an example, a biohybrid composite of a ratio of 72 PEUU: 28 ECM gel and a control mesh of PEUU were implanted into Lewis rats, replacing a full-thickness abdominal wall defect. After 4 weeks of implantation, histological staining showed extensive cellular infiltration into the biohybrid scaffold. The newly developed tissue was well integrated with the native periphery, while minimal cellular ingress into the electrospun PEUU scaffold was observed.[27] Similarly, by increasing the SIS concentration in the PCL/SF/SIS polymer blend, a high level of initial cell attachment was achieved. The result of incorporating the ECM-based material SIS was compared to plain PCL/SF fibers, which showed a low level of initial cell attachment compared to that of even the pure PCL fibers.[24]
Histological evidence from scaffolds extracted from in vivo experiments in trachea,[22] arteries,[27] abdominal wall,[27] and ligaments,[25] all show increased number of recruited cells over the polymer scaffold alone. The speed and numbers of migrating cells can help determine the success of the implant, especially in cases of trachea, vessels, and abdominal wall, where a complete lining is essential to success. PCL grafts electrospun and then cast with collagen nanofiber gel showed significantly higher cell number stained for nuclei and f actin than PCL alone in a tracheal wall implant. In contrast to the control PCL group, the PCL- collagen fiber group showed complete regeneration of the tracheal wall, with the mucosal epithelium of the trachea completely covered.[22]
In addition to cell infiltration and cell attachment, many studies cite an improvement in ECM production when ECM-based materials are included. Improved cell attachment and ECM production usually results in beneficial remodeling of the construct. Specifically, a study by Hong et al. discovered in their in vivo assessment of an abdominal wall that biohybrid material started to remodel so significantly that its mechanical properties mimicked those of the native abdominal wall. Histological methods revealed dense homogenous cell layers, extensive collagen formation, no calcification, absence of thrombus, and no evidence of aneurysm. These results were compared to polytetrafluoroethylene grafts (that have no ECM-based material component), which were occluded with thrombus formation.[27] The ability of the biohybrid graft to create rapid endothelium is an important success indicator in other applications as well, particularly in vascular grafts.[21] Vascular grafts electrospun from a blend solution of PLGA, gelatin, and elastin (PGE), were seen to support human aortic endothelial cells (HAECs) in forming a confluent, nonthrombogenic, and physiologically competent monolayer, as assessed by tissue factor gene expression and protein activity assays. The levels of mRNA/protein activity in HAECs grown on PGE scaffolds were similar to those on gelatin or collagen IV-coated 2-D surfaces. However, analysis of a microarray containing 84 ECM-related cDNA probes demonstrated that HAECs on PGE scaffolds expressed an ECM-related “transcriptome”, where cells were less activated on 2-D gelatin. This study highlights important role of substrate composition, and suggests that that substrate composition plays a greater role than surface topography in affecting the endothelial ECM-related “transcriptome”.[26] Comparatively, in a study on the hybrid mesh PCL/collagen and HA hydrogel loaded with two potent angiogenic growth factors (VEGF165 and PDGF-BB) the growth factor-loaded hybrid meshes were shown to not only support cellular attachment, but also their infiltration and the recapitulation of primitive capillary network in the scaffold’s architecture.[28]
It is apparent that PCL is a popular choice for electrospun polymers, used in biohybrid material scaffolds for muscular,[29] nervous,[31] dermal,[30] and vascular tissue repair.[28] Certainly through this frequent review, protocols to electrospin the polymer have been optimized, and use of the polymer in biomedical implantables has been well accepted. It is possible however, that the popularity and progress is reinforcing the repeated selection of PCL, as it may appear as a well-established go-to scaffold material. In fact, as a linear hydrophobic polymer, it’s degradation rate through hydrolysis is fairly slow, cited at longer than 24 months.[33] This timeline is not ideal for most soft tissue engineering applications. Degradation, as well as wettability can be improved through the mixing with ECM based proteins (gelatin and collagen), [29, 30] but the rate is still slower than other hydrophilic, electrospinable polymers, such as PVA and polyurethane.[33] It may be beneficial to continue to develop electrospinning techniques with these polymers, or investigate the use of others with alternate physical properties.
4.2. Materials Fabricated Using the Layering Technique
In this fabrication technique, two components are considered as distinct entities that are combined in a layered fashion to create one composite material. Because the components are not integrated on fiber by fiber basis, the outcomes are evaluated as two separate materials. In some cases, the materials are defined by a hierarchy of influence, one material as the main focus, and the other material as a support of the primary constituent. As such, the primary material often has an outstanding intrinsic quality that makes it well suited for the application, such as elasticity or strength, but requires additional support in the form of biocompatibility or resistance to degradation. Material fabricated in this way is being developed for skin,[34] cardiac and vascular tissue,[35, 36] and abdominal muscle injury.[27]
4.2.1 Material Selection
As the biological component, in some cases, it is beneficial to use an already intact tissue. Some tissues, like pericardium, dermis, and intestinal submucosa have very unique mechanical properties that are unmimicable by manmade techniques. In these applications, the synthetic component is layered on top of or around the tissue to add strength to the composite and to prevent against degradation.
Pericardium, one unique material, is well suited for use in a layering application. It is clinically used in a chemically fixed form, usually with glutaraldehyde, in heart valves, vascular and intestinal patches.[37] However, it is a tissue primarily composed of extracellular matrix proteins, with few resident cells, and unmatched mechanical properties which make it an ideal candidate for an ECM-based biohybrid material. In previous work, we have shown that a thin layer of poly(propylene fumarate) (PPF) married to the surface of bovine pericardium can provide initial physical protection from proteolytic enzymes and degradation, but leaves the original collagen and elastin matrix unaltered.[35] Porcine pericardium has also been investigated by other groups, using a polyurethane oligomer coating layer, which is found to crosslink with the top surface of pericardial tissue. This protective layer is predicted to also prevent against degradation, similar to chemical fixation agent, but with less alteration of the underlying tissue.[38] Other coatings have been investigated with bovine pericardium, including titanium,[36] polyurethane prepolymer,[39] and chitosan or silk fibroin film.[40] In one study, 3D nanofibers of poly(L-lactic acid)-co-poly-(e-caprolactone) (PLACL) were coated on the surface of bovine pericardium. This method introduced valuable porosity and cell attachment points, as covered earlier. However, the tissue was also treated with glutaraldehyde to crosslink the constituents, which would severely mask biological components and alter cell interaction with the tissue.[41] In a final example, equine pericardium that is surface-crosslinked with woven polymer (commercially, orthADAPT PR Bioimplant) has FDA clearance for rotator cuff repair,[42] and is reported to have performed better than its pure pericardium predecessor in in vivo studies. This tissue source can provide rich amounts of organized ECM around 0.5mm thick as a substrate for infiltrating and recruited cells to an injury cite.[35] The thickness provides a substantial reservoir for hosting cells, but can take time to remodel, and requires support through remodeling. Methods that crosslink the tissue reduce the space for cell recognition and habitation, as well as alter the time to remodel, since crosslinked tissues do not break down. To fully utilize the benefits of the tissue, techniques should be pursued to use pericardium in a way that keeps it as close to unaltered as possible.
In other applications, the polymer is the base component, and a weaker natural component gel is layered on top or through the pre-formed polymer scaffold. PLGA scaffolds filled with a layer of fibrin gel,[23] or modified with a layer coating of artificial extracellular matrices (aECM) consisting of collagen type I, chondroitin sulphate, and sulphated hyaluronan,[43] and laminin, fibronectin, vitronectin, collagen type IV and poly(lysine) are examples of this type of combination.[44] Other polymer components reported use porous elastomeric scaffolds filled with self-assembling peptide gel.[45, 46]
Utilizing more of a traditional layer technique, some scaffolds are first formed in a mesh, and then a hydrogel component is gelled on top. In one study, a poly(caprolactone-co-lactide)/Poloxamer nanofiber membrane was made using electrospinning, and then a hydrogel of gelatin (20%) and dextran (10%) was layered on the bottom of the fiber mesh to create a bilayer scaffold.[34] In other studies, hybrid scaffolds were constructed by forming funnel-like collagen or gelatin sponges on one side of a poly-L-lactide (PLLA) woven mesh,[3] or layered around a PCL electrospun mesh.[11] Neutralized dermal ECM digest has also been used in this method, forming a layer to coat a polypropylene mesh.[47] This method is particularly adaptable to 3D printing techniques, since the two layers can be formed separately with reconciling potentially complex printing parameters. For example, materials that are not conducive self-supporting structures can be extrusion printed directly on the surface of polymer meshes that would be created using stereolithography, extrusion under harsher conditions, or a method separate from 3D printing altogether.[48]
4.2.2. Mechanical and Physical Properties
Investigations of mechanical properties are often conducted in comparison to the individual components of the layered material. Hydrogels such as collagen, gelatin and HA are considered weak independently, but show improved mechanical strength with the addition of the polymer layer. This relationship highlights the benefit of the fabrication method. For example, when collagen or gelatin is gelled within a PLLA scaffold with interconnected pore structures, mechanical strength is increased by the presence of PLLA, but interconnected regions of the hydrogel remain unaltered if examined in isolation.[3] Using a similar fabrication technique, PLGA–collagen hybrid scaffold formed from collagen microsponges in the openings of a PLGA knitted mesh were evaluated in a tracheal wall reconstruction. The elastic modulus of engineered tissues was tested after 6 months of implantation. Incorporating collagen, gelatin, and basic fibroblast growth factor resulted in a significantly higher modulus than the pure polymer (7.52 ± 1.60 × 10−2 and 3.00 ± 1.60 × 10−2), although was still significantly less than that of the native trachea (10.79 ± 1.49 × 10−2).[17]
With similar conclusions, some have investigated PCL porous scaffolds fabricated using a salt leaching technique, and injected with HA hydrogel. As expected, the storage modulus of the composite is greater than the hydrogel, and reduced when compared to the PCL porous scaffold alone. However, the composite does not show major changes in Young’s modulus over the 6 week testing period from plain PCL scaffolds.[49] The results from this study suggest that the hybrid scaffolds provide the potential for high stiffness properties in tension and compression, (presumably from the PCL component), while exhibiting the viscoelastic response found in hydrogels (HA) and native cartilage tissue. For softer tissue applications however, regional mechanical characteristics from a horizontal layered technique appear to accurately approximate target tissue. If skin, for example, was approximated as a bilayer consisting of the epidermis (modulus, 140 to 600 kPa; thickness, 0.05 to 1.5 mm) and the dermis (modulus, 2 to 80 kPa; thickness, 0.3 to 3 mm), a bilayer scaffold of PLCL/Poloxamer nanofibers with dextran/gelatin hydrogel was found to be a suitable substitute when both constituents where mechanically tested independent layers.[34]
4.2.3. Biological Properties
Parallel to the mechanical analysis of these constructs, cellular response is often investigated in comparison with the individual components of the layered material. It is important to show that since this method could involve incorporating a polymer with a biological scaffold there is no statistically significant difference between the bilayer composite and a control group of pure biological component. As seen in a comparison between PLCL/Poloxamer nanofibers with dextran/gelatin hydrogel and plain dextran/gelatin hydrogel, there was no significant difference when the polymer was incorporated, indicating that the bilayer scaffold has no detriment on cell viability.[34] Fortunately, several studies show improved cell viability, cell adhesion and proliferation, compared to polymer alternatives or control collagen sponges.[3, 49] The porous nature of some composites, supported by an overall porous, or funnel like structure[3] of the polymer base, is suggested to aid in the improved adhesion and proliferation.[41]
Perhaps more convincing of a scaffold’s benefit than unaffected viability is the potential to promote cell behavior towards reconstruction. In an in vivo comparison after 180 days post implantation in a rat abdominal muscle injury, the ECM-coated polypropylene mesh showed decreased density of collagen and amount of mature type I collagen deposited between mesh fibers when compared to the uncoated mesh devices. This study confirmed and extends previous findings that an ECM coating supports healing and reconstruction of the injury site, and mitigates associated scar tissue deposition characteristic of polypropylene expected when used for ventral hernia repair.[47] In another example, the combination of PLLA and collagen for skin wound healing, resulted in better regeneration of dermal tissue and epidermis than either of the materials independently when evaluated in a nude mouse subdermal study.[3] Other studies support this finding, citing the production of increased amount and specificity of matrix proteins by recruited cells on biohybrid materials when compared to the plain polymer alternative.[23, 41, 43]
4.3. Cell-Built ECM Layer Fabrication Method
This method describes utilizing a cell population to lay down a tissue specific matrix on a polymer scaffold. When the cells are removed, the polymer scaffold is left with a bioactive ECM layer, prepared to host recruited cells. As described, this method could be an effective means to ensure complete inclusion of proteins based on cellular production, assuming the physiological niche can be appropriately simulated.
This method appears more frequently used in bone and cartilage engineering applications,[50] which are beyond the scope of this report. However, there has been promising results in soft tissue engineering applications as well. In a study by Shtrichman et al, an electropsun PCL/PLGA mesh served as mechanical support for cell seeding and ECM generation. By decellularizing the composite after a fixed time, the result is self termed an available “off-the-shelf” implantable product. This composite demonstrated biodegradability and biocompatibility in a rat subcutaneous model, and supported advanced cellular infiltration and habitation compared to uncoated PCL/PLGA scaffolds.[51] Along similar lines, another study utilized a Vicryl knitted mesh made of polyglactin 910 (a 90:10 copolymer of glycolic acid and lactic acid (PLGA)), and cultured either mesenchymal stem cells, normal human articular chondrocytes, or normal human dermal fibroblasts onto both sides of the PLGA mesh. When these discs were decellularized, they also showed promising results for supporting cell viability and ingrowth.[3] In an in vivo study, a similar approach utilizing hMSCs to build a cell derived matrix within a collagen/HA scaffold showed excellent results in mimicking the bone marrow niche. ECM-Col/HA scaffolds formed less bone than Col/HA with or without hMSCs, suggesting the appropriate environment for bone marrow cells.[52]
For some tissue applications, this method is perhaps the closest to reality, if site appropriate cells can be cultured. Although, the resources and time to culture could to prevent this method from being a scalable procedure, and so far, success appears mostly in 2-dimensional constructs. Relying on cells to build an amount of ECM that would produce a significant, 3-dimensional layer could be challenging. It may be more feasible to obtain 3-dimensional scaffolds by designing the polymer base to support a 3D culture structure with pores or mesh. If a decellularizing technique could be developed that effectively removed cell fragments and other possible inflammatory debris, this method has the potential to add very specific ECM to geometrically relevant soft tissue implants.
4.4. Materials Fabricated By Blending Hydrogel Components
In this approach, presentation of site-specific proteins and extracellular matrix components are the main focus for the material. The objective of most design plans is to improve mechanical properties of biologically based matrices, and ensure retained cytocompatability. In order to alter mechanical properties, yet leave the natural portion bioactive and retain cellular recognition, inert polymers are doped into the material as structural components. Inert polymers are often chosen over the addition of other proteins to avoid disruption of the tissue specific composition of the ECM.
4.4.1. Material Selection
To create a cohesive and consistent material, a synthetic polymer network can physically and covalently incorporate a protein phase. In this way, ECM proteins are secured in 3D space without modification while the biological inert polymer serves to maintain consistent mechanical integrity and transport properties. As a bioinert and biodegradable material, the source of polymer component is most commonly poly(ethylene glycol) (PEG), or PEG with some modifications. PEG gels are often selected due to high water solubility, nontoxicity, low protein adhesion, and non immunogenitic properties.[53] If modified through common techniques, PEG molecules can be functionalized using NHS and acrylate groups, for example, making them suited to chemically crosslink with side amine groups in ECM proteins.
The source of the biological component in this fabrication technique is often either non-purified ECM homogenate, or artificially constructed ECM-like gel, made from individual purified components. Non-purified ECM homogenate may more accurately emulate the microscale heterogeneity of natural ECM.[54] As we have described, using regionally specific tissue has great effects on the influence on the resulting tissue, and the inclusion of possibly unknown structural and chemical components could aid in tissue regeneration. It follows that the choice of tissue for this ECM homogenate should reflect the ultimate cite for the material. Popular choices include, myocardial matrix (heart tissue homogenized), alternative ECM tissue derived from the umbilical cord (Wharton’s jelly), homogenized intestinal lining, and homogenized dermal tissue.[32, 55, 56]
On the other hand, creating an ECM-based hydrogel from scratch by using building blocks with known concentration and composition creates a consistent material. These constituents include collagen, elastin, glycosaminoglycan, as well as gelatin and chitosan. Gelatin, the partially hydrolyzed form of collagen, is not naturally present active ECM. However, in many respects including chemical structure, degradation mechanisms, and byproducts, can closely resemble its parent molecule collagen. Modified gelatin (gelatin methacrylate) hyrdogels are a popular choice for the biological component in these scaffolds, combined with silk,[57] with PEG,[58] PEG diacrylate (PEGDA),[10] and with chondroitin sulfate,[59] cellulose.[60]
Blending of the materials requires optimization techniques based on unique properties. In addition to the chemical crosslinking referred to above that utilizes functionalized PEG or gelatin, physical entrapment or freeze-thaw cycles to form cryogels are also possible techniques shown to produce evenly dispersed materials.[60] By incorporating fibrillar type I collagen and reticular laminin, Jung et al were able to physically entrap these two ECM proteins within chemoselectively crosslinked PEG. The interpenetrating networks were confirmed, and it was determined that the PEG component is capable of slowing degradation of the bulk material, although did not chemically alter the proteins.[32] In another example, silk fibroin protein and chitosan hydrogels are formed using physical entrapment through ultrasonication to avoid harsh solvents or chemical crosslinking. The ultrasonication of silk and chitosan induce a conformational change of the silk from random coil to beta sheet. This results in the self-assembly of the hyrophobic peptide segments in the protein, entrapping chitosan chains in the silk networks.[61] Freeze thaw cycles were employed by several groups to form cryogels of glycosaminoglycan-PEG,[62] and ECM/PVA hydrogel. In this technique, scaffolds were created with a thin ECM layer upon polymer solution poured into a mold, and then freeze-thaw cycles are introduced to physically cross-link the hydrogel and to embed the lyophilized matrix upon it.[55]
A unique material inclusion by Shin et al. highlights the possibility of one additional material characteristic, electroconductivity, otherwise unmentioned in the research compiled here. In this study, carbon nanotubes (CNT) were incorporated into photo-cross-linkable gelatin methacrylate (GelMA) hydrogels. The composite material resulted in cardiac tissue constructs that showed excellent mechanical integrity, as well as advanced electrophysiological functions. Specifically, myocardial tissues cultured on 50 μm thick CNT-GelMA showed 3 times higher spontaneous synchronous beating rates and 85% lower excitation threshold, compared to cells cultured on plain GelMA hydrogels. This parameter, it is discussed, is important when engineering tissues with particularly important conductive ability, such as heart muscle and nervous tissue.[63]
4.4.2. Mechanical Properties
The driving reason to alter an ECM based hydrogel is to incorporate additional strength, shape integrity, or resistance to rapid degradation. Softness of hydrogels made from matrix materials have been reported around 5–10 Pa at 1Hz (specifically, a gel formed from myocardial matrix).[56] This significant decrease from native heart muscle, in this particular example, can negatively affect cell adhesion, cell migration and signal transport. Mechanical comparisons, conducted by Christman et al., found that the addition of PEG to the hydrogel with either NHS, star PEG arcylate, or PEG diacrylate, significantly increased the storage modulus of the gel (5–30 Pa in PEG NHS or PEG diacrylate, 719 Pa in the star PEG acrlyate system) over the ECM gel alone (5 Pa). The incorporation of PEG variations allowed for tunable degradation, with the ECM gel degrading two to three times faster than all of the hybrid variations. Cell studies indicate that adhesion and migration through myocardial matrix-PEG-NHS and acrylate hydrogels was possible, and that cells could be efficiently encapsulated, a process impossible in the weaker pure ECM gel.[56]
These results are echoed in studies investigating other combinations of materials. An interpenetrating network of gelatin methacrylimide polymerized within a tetrafunctional PEG framework resulted in unique properties. Crosslinking ECM proteins within a synthetic matrix created a stable scaffold with tunable properties and with long term cell anchorage points supporting cell attachment and proliferation in the 3D environment.[54] Cryogels of glycosaminoglycan-PEG were determined to have varied stiffness between the struts of the scaffold and the less stiff corresponding bulk hydrogel, suggesting this method can be utilized in design goals.[62] This control over mechanical properties is useful; however applications for hydrogel based tissue may be limited. If the polymer component could be incorporated to a point that made it possible to attain tensile and elastic properties, the composite could be used more broadly.
4.4.3. Biological Properties
In several studies, cell viability and function was seen to increase over the plain polymer controls. Cardiac cells improved cellular adhesion, organization, and cell–cell coupling on CNT-GelMA compared to GelMA alone.[63] Chondrocytes exhibited improved colonization and adhesion on ECM-PVA scaffolds compared with plain PVA as well as plain articular cartilage derived matrix, suggesting it is the combination of two materials that is most beneficial.[55]
Jung et al noted that hMSC viability was enhanced by the addition of exogenous ECM to PEG frameworks. In addition to viability, a glimpse of cell behavioral changes was observed in direct response to scaffold composition. For example, In the presence of exogenous laminin, hMSCs produced reticulate structures, as opposed to fibrillar masses in the presence of collagen I. In addition, miPSCs were able to form beating areas in composites containing no ECM, but to a lesser extent in composites containing either collagen I or laminin. Thus, this PEG-NCL approach provides an opportunity to independently examine the biochemical impact of ECM on stem cell differentiation in 3D environments.[32]
5. Other Considerations: Inflammatory Response
As ECM-based biohybrid materials continue to be designed, an in depth analysis of immune response to the materials could provide illuminating information on the mechanisms that contribute to successful reconstruction. Moving beyond basic cell viability and material biodegradability, advanced studies can start to investigate and learn the complex role of the immune response to these scaffolds, and how that plays a role in long term functionality and constructive remodeling.
Considering the complexity of the interaction between the immune response and other physiological systems, initial studies that have begun to look at this relationship are just beginning to graze the surface. The majority of biohybrid materials investigated in this report are at their furthest point tested in animal models. Some earlier reports use histological techniques to identify “immune” cells. Although this gives some measure of immune activation, it does not give any insights into the complicated interactions at play. Additionally, evaluating the materials in immunosuppressed animals artificially removes elements that could significantly change the course of remodeling of the tissue[3]
A large presence of foreign material can cause a foreign body response resulting from overlapping acute and chronic inflammation, and subsequent fibrotic and collagen encapsulation [64], which has historically been the assessment for “success” or “failure” of an implant. However, before that severe endpoint, there exists several mechanisms that can determine the fate of an implanted material. Although the initial events are not confirmed, one theory suggests that the foreign material will activate inflammasomes, which recruit macrophages as well as other leukocytes to the region, which secrete additional signal and activate successive pathways.[64, 65] Soon after arrival at the injured site, macrophages are expected to polarize, into a classic (M1) or an alternate (M2) phenotype.[66] The classic phenotype is associated with inflammatory related events, including tissue destruction and activation of Th1 T cells. The alternate phenotype, on the other hand, stimulates healing events, including tissue remodeling and inflammatory cytokine suppression.[66–68] Although it seems clear that activation towards an M2 phenotype in macrophages together with Th2 T cell activation would be desired, the interaction between the two activation pathways and local signaling events needs to be further investigated. An outline by Faulk suggests three mechanisms to include when studying this interaction. First to consider are the signals and environment created by the bioactive peptide fragments generated through scaffold degradation. Second, the recruitment of endogenous stem and progenitor stem cells to the site for ECM remodeling. And third, the modulation of the host macrophage response away from a proinflammatory phenotype, and towards an M2-TH2, pro-healing phenotype.[47]
Without intervention, ECM-based materials are most often degraded in vivo, and are associated with constructive tissue remodeling and minimal fibrosis.[64, 66] As of yet, the specific cell signaling events by which ECM biomaterials modulate the host macrophage population toward a more constructive remodeling phenotype are not fully understood.[51] In several examples, the presence of ECM components was shown to influence the initial response of the immune system to prohealing, despite the polymer components that would be expected to individually illicit an inflammatory response.[3, 22, 27, 69] The abdominal wall injury model comparing ECM-coated and uncoated polypropylene mesh devices showed that ECM coating decreased the inflammatory response as characterized by the number and distribution of M1 macrophages (CD86+/CD68+) around mesh fibers when compared to the uncoated mesh devices at 14 days post implantation.[47] Furthermore, the local response was characterized as having less scar tissue, resulting from the ECM mitigated immune response. Interestingly, other studies reference reduced fibrotic capsule,[3] an indicator of reduced inflammation, and production of functional ECM.[21, 27] Although these results do not provide insights into the intermediate steps, the end results do compel future studies on the material. Expanding the understanding of how the immune system interacts with the implants can provide tools to assure or even manipulate healing of the implant.
As an example, other studies investigating materials in subdermal implants have had success comparing the local cytokines belonging to either a Th1 (interleukin (IL) 2, interferon-γ) or Th2 (IL-4, IL-10, IL-13) profile. In a study comparing porcine dermal collagenous membranes to PTFE in Sprague Dawley rats, a higher ratio of Th1 cytokines was found surrounding the porcine dermal tissue, where an increase in Th2 cytokines was measured surrounding PTFE implants. This result agrees with the inverse relationship of the Th1/Th2 cell mediated and humoral immune response [67]. Although this study did not involve biohybrid materials, a similar protocol would provide new information regarding the intermediate steps in response to these materials.
Crosslinking, or other altering steps to the natural tissue component, threatens to negatively impact this positive remodeling. Crosslinking or otherwise altering the tissue affects the functional design, manifested in changes to rigidity, porosity, insolubility and topography of the matrix. These characteristics determine the mechanical profile of each individual tissue.[70, 71] Scaffold materials with chemical crosslinking agents may delay or prevent macrophage mediated degradation, but they also inhibit the formation of the M2-type response. The altered characteristics caused by crosslinking fibers result in decreased hydrophilicity and recognition of the scaffold, which can result in slowed cellular adhesion and proliferation in or near the scaffold. In fact, some crosslinked tissue shows resistance to cellular infiltration, and is characterized by a dense cell lining around the surface.[35] The lack of healing and remodeling can result in downstream scar tissue formation and a persistent foreign body response.[47]
In a less drastic way, the choice to use gelatin instead of collagen could also affect the matrix presentation to cells. Gelatin, being chemically similar, but structurally different from collagen, does partially contribute towards unfamiliar environments in the form of physical recognition. In some cases, the comparison between gelatin and collagen in the same biohybrid scaffold shows significant differences in cellular response. In the development of funnel-like collagen or gelatin sponges on one side of a PLLA woven mesh, the PLLA–collagen scaffolds supported cells that more strongly promoted rebuilding ECM than did the PLLA–gelatin scaffolds.[3]
6. Conclusion
A significant struggle in the development of tissue engineered constructs is remodeling and permanent maintenance by the body. This review considered the use of ECM-based biohybrid materials as tools to promote constructive remodeling through natural physiological functions. Keeping the scaffold as close as possible to the natural environment has been shown to significantly improve cellular infiltration, inhabitation, and constructive remodeling. Using natural tissue components is a direct way to incorporate natural signaling domains, recognition, attachment points to a scaffold. Incorporating polymer components can help recapitulate the bulk environment, which is also an important indicator of lasting success. Expanding the understanding of how the immune response interacts with the implants will help us determine how to use those systems to encourage success and integration of the scaffold. The quick recognition by native cells has been shown to be important in modulating immune response and contributing to lasting mechanical properties of the constructs.
Contributor Information
Laura G. Bracaglia, Fischell Department of Bioengineering, University of Maryland, College Park, MD.
John P. Fisher, Fischell Family Distinguished Professor and Associate Chair, Director of Graduate Studies, Fischell Department of Bioengineering, University of Maryland, 3238 Jeong H. Kim Engineering Building, College Park, Maryland 20742.
References
- 1.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu H, Oh HH, Kawazoe N, Yamagishi K, Chen G. PLLA–collagen and PLLA–gelatin hybrid scaffolds with funnel-like porous structure for skin tissue engineering. Sci Technol Adv Mater. 2012;13(6) doi: 10.1088/1468-6996/13/6/064210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, Turner NJ, Weber DJ, Simpson TW, Wyse A, Brown EH, Dziki JL, Fisher LE, Brown S, Badylak SF. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med. 2014;6(234):234ra58. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 2014;163(4):268–85. doi: 10.1016/j.trsl.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown BN, Badylak SF. Expanded applications, shifting paradigms and an improved understanding of host-biomaterial interactions. Acta Biomater. 2013;9(2):4948–55. doi: 10.1016/j.actbio.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Nimni ME, Cheung D, Strates B, Kodama M, Sheikh K. Chemically modified collagen: a natural biomaterial for tissue replacement. J Biomed Mater Res. 1987;21(6):741–71. doi: 10.1002/jbm.820210606. [DOI] [PubMed] [Google Scholar]
- 8.Escande Rémi NK, Di Centa Isabelle, Roques Caroline, Ba Maguette, Medjahed-Hamidi Fatima, Chaubet Frederic, Letourneur Didier, Lansac Emmanuel, Meddahi-Pelle Anne. Pericardial Processing: Challenges, Outcomes and Future Prospects. In: Pignatello PR, editor. Biomaterials Science and Engineering. In Tech; 2011. pp. 437–457. [Google Scholar]
- 9.Hansen KC, Kiemele L, Maller O, O’Brien J, Shankar A, Fornetti J, Schedin P. An in-solution ultrasonication-assisted digestion method for improved extracellular matrix proteome coverage. Mol Cell Proteomics. 2009;8(7):1648–57. doi: 10.1074/mcp.M900039-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Patnaik S, Guo X, Li Z, Lo W, Butler R, Claude A, Liu Z, Zhang G, Liao J, Anderson PM, Guan J. Cardiac differentiation of cardiosphere-derived cells in scaffolds mimicking morphology of the cardiac extracellular matrix. Acta Biomater. 2014;10(8):3449–62. doi: 10.1016/j.actbio.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guaccio A, Guarino V, Perez MA, Cirillo V, Netti PA, Ambrosio L. Influence of electrospun fiber mesh size on hMSC oxygen metabolism in 3D collagen matrices: experimental and theoretical evidences. Biotechnol Bioeng. 2011;108(8):1965–76. doi: 10.1002/bit.23113. [DOI] [PubMed] [Google Scholar]
- 12.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, Kwan OL, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach JA, Preul M, Kinsey AM, DeMaria AN, Dib N, Christman KL. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med. 2013;5(173):173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89(3):621–30. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 14.Zhu C, Ma X, Xian L, Zhou Y, Fan D. Characterization of a co-electrospun scaffold of HLC/CS/PLA for vascular tissue engineering. Biomed Mater Eng. 2014;24(6):1999–2005. doi: 10.3233/BME-141009. [DOI] [PubMed] [Google Scholar]
- 15.Kumbar SG, James R, Nukavarapu SP, Laurencin CT. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater. 2008;3(3):034002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- 16.Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007;59(14):1413–33. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Tatekawa Y, Kawazoe N, Chen G, Shirasaki Y, Komuro H, Kaneko M. Tracheal defect repair using a PLGA-collagen hybrid scaffold reinforced by a copolymer stent with bFGF-impregnated gelatin hydrogel. Pediatr Surg Int. 2010;26(6):575–80. doi: 10.1007/s00383-010-2609-2. [DOI] [PubMed] [Google Scholar]
- 18.Wolf MT, Carruthers CA, Dearth CL, Crapo PM, Huber A, Burnsed OA, Londono R, Johnson SA, Daly KA, Stahl EC, Freund JM, Medberry CJ, Carey LE, Nieponice A, Amoroso NJ, Badylak SF. Polypropylene surgical mesh coated with extracellular matrix mitigates the host foreign body response. J Biomed Mater Res A. 2014;102(1):234–46. doi: 10.1002/jbm.a.34671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JG, Mo XM. Current research on electrospinning of silk fibroin and its blends with natural and synthetic biodegradable polymers. Frontiers of Materials Science. 2013;7(2):129–142. [Google Scholar]
- 20.Jung SM, Kim DS, Ju JH, Shin HS. Evaluation of EPS-PCL Nanofibers as a Nanobiocomposite for Artificial Skin Based on Dermal Fibroblast Culture. Journal of Nanomaterials. 2013;2013(2013):1–6. [Google Scholar]
- 21.Koch S, Flanagan TC, Sachweh JS, Tanios F, Schnoering H, Deichmann T, Ella V, Kellomaki M, Gronloh N, Gries T, Tolba R, Schmitz-Rode T, Jockenhoevel S. Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials. 2010;31(17):4731–9. doi: 10.1016/j.biomaterials.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 22.Jang YS, Jang CH, Cho YB, Kim M, Kim GH. Tracheal regeneration using polycaprolactone/collagen-nanofiber coated with umbilical cord serum after partial resection. Int J Pediatr Otorhinolaryngol. 2014;78(12):2237–43. doi: 10.1016/j.ijporl.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Song JEL, Yujung, Lee Yun Me, Cho Sun Ah, Jang Ji Eun, Lee Dongwon, Khang Gilson. Effects of PLGA/Fibrin Scaffolds on Attachment and Proliferation of Costal Cartilage Cells. Polymer-Korea. 2013;37(2):141–147. [Google Scholar]
- 24.Hong S, Kim GH. Electrospun Polycaprolactone/Silk Fibroin/Small Intestine Submucosa Composites for Biomedical Applications. Macromolecular Materials and Engineering. 2010;295(6):529–534. [Google Scholar]
- 25.Cai C, Chen C, Chen G, Wang F, Guo L, Yin L, Feng D, Yang L. Type I collagen and polyvinyl alcohol blend fiber scaffold for anterior cruciate ligament reconstruction. Biomed Mater. 2013;8(3):035001. doi: 10.1088/1748-6041/8/3/035001. [DOI] [PubMed] [Google Scholar]
- 26.Han J, Gerstenhaber JA, Lazarovici P, Lelkes PI. Tissue factor activity and ECM-related gene expression in human aortic endothelial cells grown on electrospun biohybrid scaffolds. Biomacromolecules. 2013;14(5):1338–48. doi: 10.1021/bm400450m. [DOI] [PubMed] [Google Scholar]
- 27.Hong Y, Huber A, Takanari K, Amoroso NJ, Hashizume R, Badylak SF, Wagner WR. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber-extracellular matrix hydrogel biohybrid scaffold. Biomaterials. 2011;32(13):3387–94. doi: 10.1016/j.biomaterials.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ekaputra AK, Prestwich GD, Cool SM, Hutmacher DW. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (epsilon-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials. 2011;32(32):8108–17. doi: 10.1016/j.biomaterials.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 29.Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials. 2008;29(19):2899–2906. doi: 10.1016/j.biomaterials.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 30.Chong EJ, Phan TT, Lim IJ, Zhang YZ, Bay BH, Ramakrishna S, Lim CT. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007;3(3):321–330. doi: 10.1016/j.actbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29(34):4532–4539. doi: 10.1016/j.biomaterials.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Jung JP, Sprangers AJ, Byce JR, Su J, Squirrell JM, Messersmith PB, Eliceiri KW, Ogle BM. ECM-incorporated hydrogels cross-linked via native chemical ligation to engineer stem cell microenvironments. Biomacromolecules. 2013;14(9):3102–11. doi: 10.1021/bm400728e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang D, Hsiao BS, Chu B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv Drug Deliv Rev. 2007;59(14):1392–1412. doi: 10.1016/j.addr.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan JF, Liu NH, Sun H, Xu F. Preparation and characterization of electrospun PLCL/Poloxamer nanofibers and dextran/gelatin hydrogels for skin tissue engineering. PLoS One. 2014;9(11):e112885. doi: 10.1371/journal.pone.0112885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bracaglia LG, Yu L, Hibino N, Fisher JP. Reinforced pericardium as a hybrid material for cardiovascular applications. Tissue Eng Part A. 2014;20(21–22):2807–16. doi: 10.1089/ten.tea.2014.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guldner NW, Jasmund I, Zimmermann H, Heinlein M, Girndt B, Meier V, Fluss F, Rohde D, Gebert A, Sievers HH. Detoxification and endothelialization of glutaraldehyde-fixed bovine pericardium with titanium coating: a new technology for cardiovascular tissue engineering. Circulation. 2009;119(12):1653–60. doi: 10.1161/CIRCULATIONAHA.108.823948. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Guo Y, Ziegler KR, Model LS, Eghbalieh SD, Brenes RA, Kim ST, Shu C, Dardik A. Current usage and future directions for the bovine pericardial patch. Ann Vasc Surg. 2011;25(4):561–8. doi: 10.1016/j.avsg.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loke WK, Khor E, Wee A, Teoh SH, Chian KS. Hybrid biomaterials based on the interaction of polyurethane oligomers with porcine pericardium. Biomaterials. 1996;17(22):2163–72. doi: 10.1016/0142-9612(96)00053-1. [DOI] [PubMed] [Google Scholar]
- 39.Mendoza-Novelo B, Alvarado-Castro DI, Mata-Mata JL, Cauich-Rodriguez JV, Vega-Gonzalez A, Jorge-Herrero E, Rojo FJ, Guinea GV. Stability and mechanical evaluation of bovine pericardium cross-linked with polyurethane prepolymer in aqueous medium. Mater Sci Eng C Mater Biol Appl. 2013;33(4):2392–8. doi: 10.1016/j.msec.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Nogueira GM, Rodas ACD, Weska RF, Aimoli CG, Higa OZ, Maizato M, Leiner AA, Pitombo RNM, Polakiewicz B, Beppu MM. Bovine pericardium coated with biopolymeric films as an alternative to prevent calcification: In vitro calcification and cytotoxicity results. Materials Science and Engineering: C. 2010;30(4):575–582. [Google Scholar]
- 41.Mathapati S, Bishi DK, Venugopal JR, Cherian KM, Guhathakurta S, Ramakrishna S, Verma RS. Nanofibers coated on acellular tissue-engineered bovine pericardium supports differentiation of mesenchymal stem cells into endothelial cells for tissue engineering. Nanomedicine (Lond) 2014;9(5):623–34. doi: 10.2217/nnm.13.76. [DOI] [PubMed] [Google Scholar]
- 42.Ricchetti ET, Aurora A, Iannotti JP, Derwin KA. Scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2012;21(2):251–65. doi: 10.1016/j.jse.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Wojak-Cwik IM, Hintze V, Schnabelrauch M, Moeller S, Dobrzynski P, Pamula E, Scharnweber D. Poly(L-lactide-co-glycolide) scaffolds coated with collagen and glycosaminoglycans: impact on proliferation and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A. 2013;101(11):3109–22. doi: 10.1002/jbm.a.34620. [DOI] [PubMed] [Google Scholar]
- 44.Kawazoe N, Lin Xiaoting, Tateishi T, Chen Guoping. Three-dimensional Cultures of Rat Pancreatic RIN-5F Cells in Porous PLGA-collagen Hybrid Scaffolds. Journal of Bioactive and Compatible Polymers. 2009;24(1):25–42. [Google Scholar]
- 45.Martinez-Ramos C, Rodriguez-Perez E, Garnes MP, Chachques JC, Moratal D, Valles-Lluch A, Monleon Pradas M. Design and assembly procedures for large-sized biohybrid scaffolds as patches for myocardial infarct. Tissue Eng Part C Methods. 2014;20(10):817–27. doi: 10.1089/ten.tec.2013.0489. [DOI] [PubMed] [Google Scholar]
- 46.Chachques JC, Pradas MM, Bayes-Genis A, Semino C. Creating the bioartificial myocardium for cardiac repair: challenges and clinical targets. Expert Rev Cardiovasc Ther. 2013;11(12):1701–11. doi: 10.1586/14779072.2013.854165. [DOI] [PubMed] [Google Scholar]
- 47.Faulk DM, Londono R, Wolf MT, Ranallo CA, Carruthers CA, Wildemann JD, Dearth CL, Badylak SF. ECM hydrogel coating mitigates the chronic inflammatory response to polypropylene mesh. Biomaterials. 2014;35(30):8585–95. doi: 10.1016/j.biomaterials.2014.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang CC, Boland ED, Williams SK, Hoying JB. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater. 2011;98(1):160–70. doi: 10.1002/jbm.b.31831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mintz BR, Cooper JA., Jr Hybrid hyaluronic acid hydrogel/poly(varepsilon-caprolactone) scaffold provides mechanically favorable platform for cartilage tissue engineering studies. J Biomed Mater Res A. 2014;102(9):2918–26. doi: 10.1002/jbm.a.34957. [DOI] [PubMed] [Google Scholar]
- 50.Danti S, Stefanini C, D’Alessandro D, Moscato S, Pietrabissa A, Petrini M, Berrettini S. Novel biological/biohybrid prostheses for the ossicular chain: fabrication feasibility and preliminary functional characterization. Biomed Microdevices. 2009;11(4):783–93. doi: 10.1007/s10544-009-9293-9. [DOI] [PubMed] [Google Scholar]
- 51.Shtrichman R, Zeevi-Levin N, Zaid R, Barak E, Fishman B, Ziskind A, Shulman R, Novak A, Avrahami R, Livne E, Lowenstein L, Zussman E, Itskovitz-Eldor J. The generation of hybrid electrospun nanofiber layer with extracellular matrix derived from human pluripotent stem cells, for regenerative medicine applications. Tissue Eng Part A. 2014;20(19–20):2756–67. doi: 10.1089/ten.TEA.2013.0705. [DOI] [PubMed] [Google Scholar]
- 52.Antebi B, Zhang Z, Wang Y, Lu Z, Chen XD, Ling J. Stromal-cell-derived extracellular matrix promotes the proliferation and retains the osteogenic differentiation capacity of mesenchymal stem cells on three-dimensional scaffolds. Tissue Eng Part C Methods. 2015;21(2):171–81. doi: 10.1089/ten.tec.2014.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31(17):4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daniele MA, Adams AA, Naciri J, North SH, Ligler FS. Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds. Biomaterials. 2014;35(6):1845–56. doi: 10.1016/j.biomaterials.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Stocco E, Barbon S, Dalzoppo D, Lora S, Sartore L, Folin M, Parnigotto PP, Grandi C. Tailored PVA/ECM scaffolds for cartilage regeneration. Biomed Res Int. 2014;2014:762189. doi: 10.1155/2014/762189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grover GN, Rao N, Christman KL. Myocardial matrix-polyethylene glycol hybrid hydrogels for tissue engineering. Nanotechnology. 2014;25(1):014011. doi: 10.1088/0957-4484/25/1/014011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao W, He J, Nichol JW, Wang L, Hutson CB, Wang B, Du Y, Fan H, Khademhosseini A. Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomater. 2011;7(6):2384–93. doi: 10.1016/j.actbio.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutson CB, Nichol JW, Aubin H, Bae H, Yamanlar S, Al-Haque S, Koshy ST, Khademhosseini A. Synthesis and characterization of tunable poly(ethylene glycol): gelatin methacrylate composite hydrogels. Tissue Eng Part A. 2011;17(13–14):1713–23. doi: 10.1089/ten.tea.2010.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Vlierberghe S, Samal SK, Dubruel P. Development of Mechanically Tailored Gelatin-Chondroitin Sulphate Hydrogel Films. Macromolecular Symposia. 2011;309–310(1):173–181. [Google Scholar]
- 60.Haroun AA, Gamal-Eldeen A, Harding DR. Preparation, characterization and in vitro biological study of biomimetic three-dimensional gelatin-montmorillonite/cellulose scaffold for tissue engineering. J Mater Sci Mater Med. 2009;20(12):2527–40. doi: 10.1007/s10856-009-3818-x. [DOI] [PubMed] [Google Scholar]
- 61.Samal SK, Dash M, Chiellini F, Wang X, Chiellini E, Declercq HA, Kaplan DL. Silk/chitosan biohybrid hydrogels and scaffolds via green technology. RSC Advances. 2014;4(96):53547–53556. [Google Scholar]
- 62.Welzel PB, Friedrichs J, Grimmer M, Vogler S, Freudenberg U, Werner C. Cryogel micromechanics unraveled by atomic force microscopy-based nanoindentation. Adv Healthc Mater. 2014;3(11):1849–53. doi: 10.1002/adhm.201400102. [DOI] [PubMed] [Google Scholar]
- 63.Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim SB, Nikkhah M, Khabiry M, Azize M, Kong J, Wan KT, Palacios T, Dokmeci MR, Bae H, Tang XS, Khademhosseini A. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7(3):2369–80. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malik AF, Hoque R, Ouyang X, Ghani A, Hong E, Khan K, Moore LB, Ng G, Munro F, Flavell RA, Shi Y, Kyriakides TR, Mehal WZ. Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response. Proc Natl Acad Sci U S A. 2011;108(50):20095–100. doi: 10.1073/pnas.1105152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Avula MN, Rao AN, McGill LD, Grainger DW, Solzbacher F. Foreign body response to subcutaneous biomaterial implants in a mast cell-deficient Kit(w-Sh) murine model. Acta Biomater. 2014;10(5):1856–63. doi: 10.1016/j.actbio.2013.12.056. [DOI] [PubMed] [Google Scholar]
- 66.Wolf MT, Vodovotz Y, Tottey S, Brown BN, Badylak SF. Predicting in vivo responses to biomaterials via combined in vitro and in silico analysis. Tissue Eng Part C Methods. 2015;21(2):148–59. doi: 10.1089/ten.tec.2014.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu HK, Ko MT, Wu MF. Comparison of Th1/Th2 cytokine profiles of initial wound healing of rats induced by PDCM and e-PTFE. J Biomed Mater Res B Appl Biomater. 2004;68(1):75–80. doi: 10.1002/jbm.b.10081. [DOI] [PubMed] [Google Scholar]
- 68.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–91. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahoo S, Toh SL, Goh JC. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;31(11):2990–8. doi: 10.1016/j.biomaterials.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 70.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121(Pt 3):255–64. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 71.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12) doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han J, Lazarovici P, Pomerantz C, Chen X, Wei Y, Lelkes PI. Co-electrospun blends of PLGA, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules. 2011;12(2):399–408. doi: 10.1021/bm101149r. [DOI] [PubMed] [Google Scholar]