Abstract
Objective
To support efforts to address vaccine hesitancy, we sought to validate a brief measure of vaccination confidence using a large, nationally representative sample of parents.
Methods
We analyzed weighted data from 9,018 parents who completed the 2010 National Immunization Survey-Teen, an annual, population-based telephone survey. Parents reported on the immunization history of a 13- to 17-year-old child in their households for vaccines including tetanus, diphtheria, and acellular pertussis (Tdap), meningococcal, and human papillomavirus (HPV) vaccines. For each vaccine, separate logistic regression models assessed associations between parents’ mean scores on the 8-item Vaccination Confidence Scale and vaccine refusal, vaccine delay, and vaccination status. We repeated analyses for the scale’s 4-item short form.
Results
One quarter of parents (24%) reported refusal of any vaccine, with refusal of specific vaccines ranging from 21% for HPV to 2% for Tdap. Using the full 8-item scale, vaccination confidence was negatively associated with measures of vaccine refusal and positively associated with measures of vaccination status. For example, refusal of any vaccine was more common among parents whose scale scores were medium (odds ratio [OR] = 2.08, 95% confidence interval [CI], 1.75–2.47) or low (OR = 4.61, 95% CI, 3.51–6.05) versus high. For the 4-item short form, scores were also consistently associated with vaccine refusal and vaccination status. Vaccination confidence was inconsistently associated with vaccine delay.
Conclusions
The Vaccination Confidence Scale shows promise as a tool for identifying parents at risk for refusing adolescent vaccines. The scale’s short form appears to offer comparable performance.
Keywords: adolescent health, vaccine hesitancy, meningococcal vaccine, human papillomavirus vaccine, tetanus vaccine, immunization
INTRODUCTION
A sizeable minority of parents in the United States have concerns that lead them to refuse or intentionally delay certain vaccines for their children.1 Although forgone vaccination has been studied most extensively with regard to vaccines in the early childhood schedule,1–5 the problem is also highly relevant to the adolescent platform: tetanus, diphtheria, and acellular pertussis (Tdap); meningococcal; and human papillomavirus (HPV) vaccines. For example, almost one-third of parents (31%) report having refused or delayed HPV vaccine for an age-eligible daughter, and not surprisingly, parental refusal and delay are associated with lower HPV vaccination coverage.6 The most common reasons for refusing or delaying HPV vaccine are concerns about long-term side effects, believing the vaccine is not needed, and uncertainty about vaccine effectiveness.6 National prevalence estimates are not currently available for the refusal and delay for Tdap and meningococcal vaccines. However, parents of unvaccinated children commonly report that they have not gotten these vaccines for their children due to lack of information or believing the vaccines are not needed.7 Taken together, these findings suggest that parents’ vaccination beliefs are important for understanding their participation in adolescent immunization programs.
Efforts to intervene on parents’ vaccination beliefs so as to prevent refusal and delay of adolescent vaccines are currently hindered by a lack of valid and reliable measures for identifying populations most at risk for these behaviors. Although researchers, including this study team, have developed scales to assess vaccination beliefs with regard to early childhood vaccines or HPV vaccine specifically,8–9 the field currently lacks a composite measure capable of characterizing adolescent vaccination beliefs more holistically across vaccine types. To be most useful, a measure would be validated with regard to adolescents’ vaccination status as well as with the specific behaviors of parental vaccine refusal and delay. The ideal measure would also be very brief so as to minimize participant burden and the considerable expense that large surveys typically incur.
To develop such a tool, we sought to validate the Vaccination Confidence Scale, an 8-item, 3-factor measure of vaccination beliefs that our prior research has shown to be highly reliable across diverse populations.10 Using a nationally representative sample of parents of adolescents, this study aimed to assess associations between Vaccination Confidence Scale scores and vaccine refusal, vaccine delay, and vaccination status. To increase the utility of the scale, we also sought to establish meaningful thresholds for categorizing scale scores as indicating low, medium, or high vaccination confidence. Finally, because of the premium placed on scale length, we assessed the performance of each of the scale’s three factors to identify possible “short forms” of our measure. By creating a brief, validated measure of vaccination beliefs, this study aims to provide a practical tool for understanding and intervening on forgone vaccination among parents of adolescents.
METHODS
Participants and Data Source
Data came from the 2010 National Immunization Survey (NIS)-Teen, an annual, population-based survey involving two phases of data collection. In an initial household telephone survey, parents and guardians contacted through random digit dialing provided immunization-related information about a randomly selected 13- to 17-year-old child in their household. Because most respondents reported being a biological parent of the child in question, we refer to these respondents collectively as “parents.” For children whose parents gave consent, a follow-up, mail-based survey of healthcare providers assessed vaccination status.
The household response rate for the 2010 NIS-Teen was 58%.11 We drew our sample from 11,754 parents who completed the “Parental Attitudes Module,” a special set of questions included in the 2010 NIS-Teen for two quarters of data collection. We excluded parents who had missing data on parental attitudes or vaccination behaviors (n=2,129) or who completed the survey in a language other than English (n=607). Our primary analytic sample consisted of the remaining 9,018 parents. For analyses involving vaccination status, we used a secondary analytic sample consisting of the subset of 7,173 parents with provider-reported vaccination status.
The National Center for Health Statistics (NCHS) Research Ethics Review Board approved data collection for the 2010 NIS-Teen. Analysis of de-identified data from the survey is exempt from the federal regulations for the protection of human research participants. We accessed data from the Parental Attitudes Module through the NCHS Research Data Center because these restricted variables are not included in the public-use dataset. Analysis of restricted data through the NCHS Research Data Center was approved by the NCHS Ethics Review Board. The University of North Carolina Institutional Review Board determined that this study was exempt from further review.
Measures
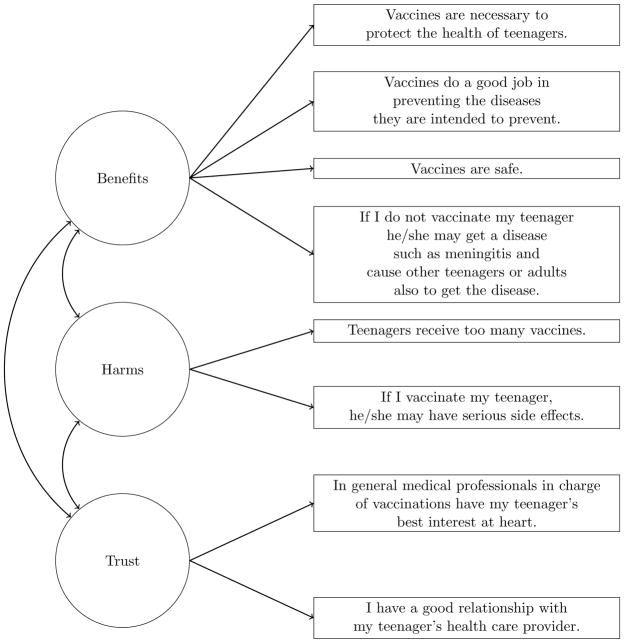
The Parental Attitudes Module assessed parents’ beliefs about vaccination with survey items conceptualized using the Health Belief Model.12 Items used an 11-point response scale ranging from 0 (“strongly disagree”) to 10 (“strongly agree”). In a prior study, we used 8 of 11 available items to develop the Vaccination Confidence Scale (Figure 1). Consisting of three factors assessing the benefits of vaccination (i.e., “Benefits”), the harms of vaccination (“Harms”), and trust in healthcare providers (“Trust”), the scale showed good fit both overall (comparative fit index = 0.97; root mean square error of approximation = 0.06) and across subgroups of seven demographic factors, including race/ethnicity, poverty status, and child’s age.10
Figure 1.
Factor structure of Vaccination Confidence Scale
Additional items in the Parental Attitudes Module assessed parents’ history of refusing or delaying vaccines. Parents first indicated whether they had ever “refused or decided not to get a vaccination” for their child (i.e., “any vaccine refusal”); this item was not specific to adolescent vaccination. For those reporting any refusal, separate items assessed whether parents had refused Td/Tdap, a meningitis shot, or an HPV shot. Parents next reported whether they had ever “delayed or put off getting a vaccination” for their child (i.e., “any vaccine delay”). For those reporting any delay, parents indicated which vaccines they had delayed as for vaccine refusal. All items on refusal and delay used yes/no response options.
The 2010 NIS-Teen household survey assessed participant characteristics including the child’s age, sex, race/ethnicity, and eligibility for the Vaccines for Children (VFC) program (Table 1). VFC is a federally-funded program which provides free vaccines to vulnerable populations, including uninsured and Medicaid-eligible youth.13 Respondents indicated their relationship to the child, the age and educational attainment of the child’s mother, and the annual income and geographic location of the household. NCHS analysts classified households as urban, suburban, or rural based on metropolitan statistical areas.14
Table 1.
Sample characteristics (n = 9,018).
| n (%) | |
|---|---|
| Child characteristics | |
| Age | |
| 13 | 1,755 (20) |
| 14 | 1,800 (20) |
| 15 | 1,843 (20) |
| 16 | 1,878 (21) |
| 17 | 1,742 (19) |
| Sex | |
| Male | 4,726 (51) |
| Female | 4,292 (49) |
| Race/ethnicity | |
| Non-Hispanic white | 6,418 (65) |
| Non-Hispanic black | 1,068 (16) |
| Hispanic | 800 (11) |
| Other | 732 (7) |
| Vaccines for Children Eligibility | |
| Yes | 1,856 (23) |
| No | 5,303 (55) |
| Not reported | 1,859 (21) |
| Parent characteristics | |
| Relationship to child | |
| Mother/female guardian | 7,073 (77) |
| Father/male guardian | 1,503 (17) |
| Other | 442 (6) |
| Mother’s age | |
| ≤ 34 years | 621 (7) |
| 35–44 years | 3,617 (43) |
| ≥ 45 years | 4,780 (50) |
| Mother’s education | |
| 12 years or less | 2,409 (35) |
| Some college, no degree | 2,696 (27) |
| College degree or more | 3,913 (39) |
| Household characteristics | |
| Region | |
| Northeast | 1,783 (19) |
| Midwest | 1,952 (23) |
| South | 3,360 (38) |
| West | 1,923 (21) |
| Annual incomea | |
| Below poverty level | 1,024 (14) |
| Above poverty level, ≤$75,000 | 4,017 (42) |
| >$75,000 | 3,619 (39) |
| Not reported | 358 (4) |
| MSA status | |
| Urban | 3,452 (34) |
| Suburban | 3,448 (48) |
| Rural | 2,118 (18) |
Note. Table shows raw frequencies and weighted percentages. Percentages may not total 100% due to rounding.
MSA: metropolitan statistical area.
Poverty level based on 2009 U.S. Census poverty threshold
The 2010 NIS-Teen provider survey assessed the child’s vaccination status. Providers used medical records to indicate the dates on which the child received vaccine doses, including doses of Td/Tdap, meningococcal, and HPV vaccines. NCHS analysts then used vaccination dates to determine whether the child was up-to-date for Td/Tdap, meningococcal vaccine, and HPV vaccine initiation (≥1 dose) and completion (3 doses). Because data collection for the 2010 NIS-Teen occurred before the addition of HPV vaccine to boys’ routine immunization schedule, we limited all analyses related to HPV vaccine to female children.15
Statistical Analyses
Using the Vaccination Confidence Scale, we reverse-coded negative attitudes in the Harms factor and calculated mean scores for each parent by averaging responses for all 8 items. The resulting scores had a possible range of 0 to 10 with higher scores indicating more positive attitudes about vaccination. To investigate the relationship between overall vaccination confidence and vaccination behavior, we used separate bivariate logistic regression models to assess the association between mean scale scores and vaccine refusal or delay reported for any vaccine, or Td/Tdap, meningococcal, or HPV vaccines specifically. We also used bivariate logistic regression to assess the association between mean scale scores and vaccination status for Td/Tdap, meningococcal vaccine, and HPV vaccine initiation and completion. For statistically significant (p<.05) associations, we re-ran each model controlling for demographic factors that we found were associated with vaccine refusal or delay: child’s race/ethnicity, mother’s educational attainment, and annual household income.
We calculated mean scores for the Benefits, Harms, and Trust factors from the scale by averaging the item responses within each factor. We used logistic regression to assess the association between mean factor scores and vaccine refusal, vaccine delay, and vaccination status in the manner described above. We used the findings of these analyses to identify those factors most strongly and consistently associated with refusal, delay, and vaccination status; we considered these factors as candidates for creating a short form of our scale.
We next established thresholds for our scale with regard to vaccine refusal. The purpose of this analysis was to provide cut-points for researchers wishing to use our scale to stratify analyses based on risk of refusal. We graphed the percentage of parents reporting any vaccine refusal within each one-point interval in mean scores, using all 8 items. We visually inspected the graph to identify changes in slope that may indicate natural cut-points. Using the resulting cut-points, we categorized mean scale and factor scores into low, medium, and high values. We used logistic regression to assess associations between these categories and any vaccine refusal. We repeated these procedures for the factors previously identified as promising candidates for a short form.
Our analyses used survey weights developed by the NCHS for the primary and secondary samples to obtain nationally representative estimates. We report raw frequencies and weighted means, percentages, and odds ratios. Conducted in SAS 9.3 (Cary, NC), all statistical tests were 2-tailed with a critical alpha of 0.05.
RESULTS
Sample Characteristics
Most parents reported on children who were non-Hispanic white (65%), non-Hispanic black (16%), or Hispanic (11%) (Table 1). The sample included similar numbers of children by age (mean: 15.1 years) and sex (51% male). Most respondents were mothers or female guardians (77%). On indicators of socioeconomic status, about one-third of children had mothers with a high school degree or less education (35%), and over one-tenth (14%) lived in poverty.
Scale Validation
Vaccination confidence
Using response scales of 0 to 10, parents reported high overall vaccination confidence. The mean score for the full, 8-item scale was 8.19 (standard error [SE] = 0.03) after reverse-coding for Harms. Factor score means were 8.49 (SE = 0.03) for Benefits, 3.31 (SE = 0.04) for Harms (without reverse coding), and 9.06 (SE = 0.03) for Trust.
Vaccine refusal
About one-quarter of parents (24%) reported having refused any vaccine for their child, with the prevalence of vaccine-specific refusal being 21% for HPV vaccine (females only), 5% for meningococcal vaccine, and 2% for Tdap (Table 2). For the overall scale, vaccination confidence was negatively associated with refusal of any vaccine, such that every one point increase in mean score corresponded with a 4% decrease in the odds of refusal (odds ratio [OR] = 0.96, 95% confidence interval [CI], 0.95–0.96). Vaccination confidence was also negatively associated with refusal of Tdap, meningococcal, and HPV vaccines.
Table 2.
Parent-reported vaccine refusal: Multivariable associations with scale and factor score means (n = 9,018).
| Refused n (%) |
Scale (8 items) | Benefits (4 items) | Factors Harms (2 items) | Trust (2 items) | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Mean (SE) | OR (95% CI) | Mean (SE) | OR (95% CI) | Mean (SE) | OR (95% CI) | Mean (SE) | OR (95% CI) | ||
| Any vaccine | |||||||||
| Yes | 2,186 (24%) | 7.62 (0.05) | 0.96 (0.95, 0.96) | 7.84 (0.06) | 0.94 (0.93, 0.95) | 3.99 (0.08) | 1.04 (1.02, 1.06) | 8.76 (0.06) | -- |
| No | 6,832 (76%) | 8.36 (0.03) | Reference | 8.70 (0.03) | Reference | 3.10 (0.05) | Reference | 9.16 (0.03) | |
| Tdap | |||||||||
| Yes | 174 (2%) | 6.39 (0.22) | 0.93 (0.92, 0.94) | 6.39 (0.26) | 0.91 (0.89, 0.94) | 5.49 (0.25) | 1.09 (1.04, 1.14) | 8.28 (0.27) | -- |
| No | 8,844 (98%) | 8.21 (0.03) | Reference | 8.53 (0.03) | Reference | 3.28 (0.04) | Reference | 9.08 (0.03) | |
| Meningococcal | |||||||||
| Yes | 424 (5%) | 6.87 (0.13) | 0.94 (0.93, 0.95) | 6.81 (0.16) | 0.90 (0.88, 0.91) | 4.63 (0.21) | -- | 8.50 (0.16) | -- |
| No | 8,594 (95%) | 8.24 (0.03) | Reference | 8.56 (0.03) | Reference | 3.26 (0.04) | 9.09 (0.03) | ||
| HPVa | |||||||||
| Yes | 741 (21%) | 7.58 (0.08) | 0.96 (0.94, 0.97) | 7.83 (0.11) | 0.94 (0.92, 0.96) | 4.07 (0.13) | 1.04 (1.01, 1.07) | 8.72 (0.13) | -- |
| No | 2,806 (79%) | 8.34 (0.05) | Reference | 8.70 (0.06) | Reference | 3.20 (0.08) | Reference | 9.17 (0.04) | |
Note: CI: confidence interval. HPV: human papillomavirus. OR: odds ratio. SE: standard error. Tdap: tetanus, diphtheria, and acellular pertussis. Table shows raw frequencies and weighted estimates. Models controlled for child’s race/ethnicity, mother’s educational attainment, and annual household income. Dashes (--) indicate that multivariable findings are not presented because bivariate analyses did not yield statistically significant associations.
Human papillomavirus vaccine refusal was assessed only for females (n =3,547). The model excluded parents (n=745) who had missing data on HPV vaccine refusal.
Compared to overall confidence scores, scores for the Benefits factor alone were somewhat more strongly associated with any vaccine refusal (OR = 0.94, 95% CI, 0.93–0.95) as well as with refusal of Tdap, meningococcal, and HPV vaccines. Factor scores for Harms were positively associated with refusal of any vaccine, Tdap, and HPV vaccine, but not meningococcal vaccine. Factor scores for Trust were not associated with any of the four refusal measures.
Vaccine delay
Over one-fifth of parents (22%) reported having delayed any vaccine for their child, with vaccine-specific delay ranging from 11% for HPV vaccine (females only) to 7% for meningococcal vaccine to 4% for Tdap. Overall confidence scores were weakly associated with delay of any vaccine (OR = 0.99, 95% CI, 0.99–1.00), Tdap (OR = 0.97, 95% CI, 0.96–0.98), and meningococcal vaccines (OR = 0.98, 95% CI, 0.97–0.98), but not HPV vaccine. Factor scores for Benefits were also associated with delay of any vaccine (OR = 0.99, 95% CI, 0.98–1.00) and Tdap (OR = 0.95, 95% CI, 0.93–0.97). Factor scores for Harms were associated with delay of meningococcal vaccine only (OR = 1.06, 95% CI, 1.04–1.09). Factor scores for Trust were not associated with any of the four delay measures.
Vaccination status
Provider-reported vaccination coverage was highest for Tdap (83%) and meningococcal vaccines (66%), with a smaller proportion of females having initiated (52%) or completed (37%) the HPV vaccine series (Table 3). Overall confidence scores were positively associated with all four measures of vaccination status, with the magnitude of the association being highest for HPV vaccine initiation (OR = 1.52, 95% CI, 1.31–1.68) and lowest for Tdap vaccination (OR = 1.20, 95% CI, 1.11–1.30). Factor scores for Benefits were also associated with each measure of vaccination status, although not as strongly as overall scores. Factor scores for Harms were associated with meningococcal vaccination and HPV vaccine initiation. Factor scores for Trust were associated with meningococcal vaccination only.
Table 3.
Provider-reported vaccination status: Multivariable associations with scale and factor score means (n = 7,173).
| Vaccinated n (%) |
Scale (8 items) | Benefits (4 items) | Factors Harms (2 items) | Trust (2 items) | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Mean (SE) | OR (95% CI) | Mean (SE) | OR (95% CI) | Mean (SE) | OR (95% CI) | Mean (SE) | OR (95% CI) | ||
| Tdap | |||||||||
| Yes | 5,969 (83%) | 8.30 (0.03) | 1.20 (1.11, 1.30) | 8.63 (0.04) | 1.20 (1.12, 1.28) | 3.19 (0.06) | -- | 9.16 (0.03) | -- |
| No | 1,204 (17%) | 7.92 (0.08) | Reference | 8.13 (0.10) | Reference | 3.53 (0.13) | 8.97 (0.06) | ||
| Meningococcal | |||||||||
| Yes | 4,766 (66%) | 8.41 (0.04) | 1.31 (1.22, 1.39) | 8.72 (0.04) | 1.17 (1.09, 1.26) | 3.05 (0.07) | 0.96 (0.92, 1.00) | 9.24 (0.04) | 1.09 (1.01, 1.18) |
| No | 2,407 (34%) | 7.89 (0.05) | Reference | 8.17 (0.07) | Reference | 3.65 (0.09) | Reference | 8.89 (0.05) | Reference |
| HPV (≥1 dose)a | |||||||||
| Yes | 1,778 (52%) | 8.57 (0.05) | 1.52 (1.38, 1.68) | 8.94 (0.05) | 1.36 (1.24, 1.49) | 2.95 (0.10) | 0.93 (0.88, 0.99) | 9.34 (0.04) | -- |
| No | 1,641 (48%) | 7.87 (0.06) | Reference | 8.16 (0.08) | Reference | 3.79 (0.10) | Reference | 8.96 (0.07) | |
| HPV (3 doses)a | |||||||||
| Yes | 1,253 (37%) | 8.61 (0.06) | 1.46 (1.31, 1.64) | 9.01 (0.06) | 1.41 (1.28, 1.55) | 2.93 (0.12) | -- | 9.38 (0.05) | -- |
| No | 2,166 (63%) | 8.01 (0.05) | Reference | 8.30 (0.07) | Reference | 3.60 (0.09) | 9.03 (0.06) | ||
Note: CI: confidence interval. HPV: human papillomavirus. OR: odds ratio. SE: standard error. Tdap: tetanus, diphtheria, and acellular pertussis. Table shows raw frequencies and weighted estimates. Models controlled for child’s race/ethnicity, mother’s educational attainment, and annual household income. Dashes (--) indicate that multivariable findings are not presented because bivariate analyses did not yield statistically significant associations.
Human papillomavirus vaccination coverage assessed only for females (n=3,419).
Factor Comparison and Thresholds
Factor comparison
Based on the validation analyses, we identified the Benefits factor as having the strongest factor-specific performance. Compared to scores on the full scale, which were associated with 11 of the 12 validation measures of vaccine refusal, delay, and vaccination status, scores on the Benefits factor alone were associated with 10 measures, and the magnitudes of the associations were comparable in most cases. By comparison, scores on Harms were associated with 6 validation measures, and scores on Trust were associated with only 1 measure. Based on these findings, we assessed the Benefits factor in subsequent analyses as a short form of our scale.
Thresholds
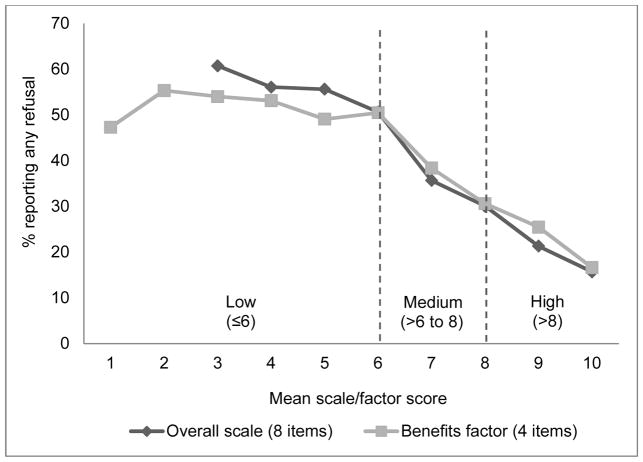
A graph of prevalence of any vaccine refusal by confidence scale scores suggested a sharp decrease in prevalence for scores greater than 6 with a smaller decrease for scores greater than 8 (Figure 2). A graph of factors scores for the 4-item Benefits factor followed a similar pattern. Based on this analysis, we categorized overall and individual factor scores as low (≤6), medium (>6 to 8), or high (>8).
Figure 2.
Vaccination confidence thresholds for mean scale and factor scores
Confidence categories were associated with vaccine refusal in the expected order. Parents with medium versus high confidence had about two times higher odds of reporting any refusal (OR = 2.08, 95% CI, 1.75–2.47) (Table 4). Parents with low versus high confidence had over four times higher odds of reporting any refusal (OR = 4.61, 95% CI, 3.51–6.05). Categories for the Benefits factor alone demonstrated similar, although slightly weaker, associations such that refusal of any vaccine was more common among parents whose scale scores were medium (OR = 1.99, 95% CI, 1.65–2.40) or low (OR = 4.07, 95% CI, 3.11–5.34) versus high.
Table 4.
Parent-reported refusal of any vaccine: Bivariate associations with scale and factor categories n = 9,018).
| Scale (8 items)
|
Benefits Factor (4 items)
|
|||||
|---|---|---|---|---|---|---|
| Overall N (%) |
Refused n (%) |
OR (95% CI) | Overall N (%) |
Refused n (%) |
OR (95% CI) | |
| Mean scores | ||||||
| Low (≤6) | 667 (8) | 318 (49) | 4.61 (3.51, 6.05) | 757 (9) | 358 (47) | 4.07 (3.11, 5.34) |
| Medium (>6 to 8) | 2,902 (32) | 861 (30) | 2.08 (1.75, 2.47) | 2,114 (23) | 641 (31) | 1.99 (1.65, 2.40) |
| High (>8) | 5,449 (60) | 1,007 (17) | Reference | 6,147 (68) | 1,187 (18) | Reference |
Note: CI: confidence interval. OR: odds ratio. Table shows raw frequencies and weighted estimates.
DISCUSSION
Using data from a large, population-based sample of parents, we found that mean scores on the Vaccination Confidence Scale were consistently associated with vaccine refusal and vaccination status across the adolescent platform. Associations were larger in magnitude for vaccination status than vaccine refusal, with vaccination confidence showing a particularly strong association with having initiated or completed the HPV vaccine series. For example, for every one point increase in parents’ mean scale scores, adolescents had over 50% greater odds of having received at least one dose of HPV vaccine. Our threshold analyses further demonstrated a gradient between confidence and prevalence of vaccine refusal, with parents in the “low” versus “high” confidence category having over four times the odds of reporting any refusal. Overall, these findings provide strong support for the validity of the Vaccination Confidence Scale as a measure of vaccination beliefs associated with vaccine refusal and vaccination status among adolescents.
We found that mean scale scores were only weakly and inconsistently associated with measures of vaccine delay. This finding may reflect a shortcoming of our measures of delay, which did not distinguish between intentional delays and those that were medically indicated. Alternatively, compared to vaccine refusal, vaccine delay may simply be less closely linked with parents’ confidence in adolescent vaccines; issues such as cost, convenience, or strength of a provider’s recommendation may instead be more salient factors.6,16–17 Given that vaccine delay is associated with under-immunization and is particularly common with regard to HPV vaccination,6 future studies should seek to better understand beliefs associated with this behavior.
In terms of applications, our findings provide support for using the Vaccination Confidence Scale to identify populations of parents at risk for refusing adolescent vaccines. Such a tool is useful based on research indicating that parents’ informational needs vary according to whether and how much they are hesitant to vaccinate their children.18–19 Using the cut-points we have established for our scale, researchers can stratify study samples to assess the differential impact of messages and other interventions by vaccination confidence. In contrast to these and other population-level research applications, the utility of our scale as a clinical screening tool for identifying individual parents at-risk for vaccine refusal is less clear; before the scale could be used in this way, additional research will be needed to assess its sensitivity and specificity as well as the feasibility of integrating such a measure into clinical care. Future work is also needed to further establish the predictive validity of the Vaccination Confidence Scale by prospectively assessing the relationship between vaccination confidence and subsequent behavior.
We were interested to find that the 4-item Benefits factor demonstrated comparable performance to the full, 8-item scale. Compared to the full scale, mean scores for Benefits were slightly more strongly associated with measures of vaccine refusal and less strongly associated with vaccination status. In contrast, the Harms and Trust factors were inconsistently associated with these measures. These findings suggest that perceived benefits are particularly important to understanding parents’ vaccination behavior.20–22 Indeed, prior studies in health communication have found that messages about benefits can increase parents’ intentions to vaccinate, particularly when those messages emphasize the potential loss of benefits.23–24 From a measurement perspective, the successful validation of the Benefits factor suggests that it can be used as a short form of the Vaccination Confidence Scale, thereby increasing the utility of our measure in the context of national surveys or other research activities for which cost and participant burden must be strictly managed.
Strengths of our study include the use of a large, nationally representative sample of parents and provider-reported data on vaccination status. To our knowledge, this study is the first to validate a measure of vaccination beliefs with regard to the behavior of vaccine refusal, which is important as a specific and potentially modifiable antecedent to vaccination status. Limitations to this study include its cross-sectional design, which prevents us from assessing the directionality of the relationship between vaccination confidence and behavior. In addition, our sample consisted of parents of 13- to 17-year-old children, whereas practice guidelines recommend 11- to 12-year-old children for the routine administration of adolescent vaccines. Although our analyses yielded no evidence to suggest that the relationship between vaccination confidence and refusal, delay, or vaccination status varied by child’s age, our findings will need to be replicated with regard to younger children. Future studies should also investigate subgroup variation in the association between vaccination confidence and vaccine refusal as well as the potential for using the Vaccination Confidence Scale to identify populations at risk for refusing other vaccines, including those administered in early childhood and HPV vaccine when administered to boys.
Conclusion
The Vaccination Confidence Scale shows promise as a tool for identifying parents at risk for refusing adolescent vaccines. Mean scores on the 8-item scale were associated with vaccine refusal and vaccination status across the adolescent platform, and items assessing the perceived benefits of vaccination performed especially well. Indeed, our findings suggest that the 4-item Benefits factor can serve as a short form for our scale, thereby halving its length, with only small trade-offs in performance. As a very brief measure validated with respect to vaccine refusal, the Vaccination Confidence Scale can usefully extend our arsenal of measurement tools for assessing and, in turn, intervening to improve vaccination beliefs. Given that almost one-quarter of parents in our sample reported having refused vaccines for their children, our findings underscore the importance of these efforts.
What’s New.
Using data from a nationally representative sample of parents, we found that mean scores on the Vaccination Confidence Scale were consistently associated with vaccine refusal and vaccination status across the adolescent platform. The scale’s 4-item short form demonstrated comparable performance.
Acknowledgments
Funding sources: This study was supported by an Academic Pediatric Association Young Investigator Award, the Cancer Control Education Program at UNC Lineberger Comprehensive Cancer Center (R25 CA57726), and a career development award from the National Cancer Institute (K22 CA186979). The funders did not play a role in study design, data analysis, report writing, or the decision to submit the article for publication.
The authors wish to thank Frances McCarty at the National Center for Health Statistics for facilitating access to the dataset. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Research Data Center, the National Center for Health Statistics, or the Centers for Disease Control and Prevention.
Footnotes
Conflicts of interest: PR has received HPV vaccine-related grants from Merck Sharp & Dohme Corp. and from Cervical Cancer-Free America, via an unrestricted educational grant from GlaxoSmithKline. AD serves on advisory boards for Merck and Pfizer, but has not received any research funding from these companies. NB has received HPV vaccine-related grants from or been on advisory boards for GlaxoSmithKline and Merck. MG, BM, and ALM have no disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Melissa B. Gilkey, Email: gilkey@email.unc.edu.
Paul L. Reiter, Email: paul.reiter@osumc.edu.
Brooke E. Magnus, Email: brooke.magnus@unc.edu.
Annie-Laurie McRee, Email: mcree.1@osu.edu.
Amanda F. Dempsey, Email: amanda.dempsey@ucdenver.edu.
Noel T. Brewer, Email: ntb@unc.edu.
References
- 1.Smith PJ, Humiston SG, Marcuse EK, et al. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Public Health Rep. 2011;126 (Suppl 2):135–146. doi: 10.1177/00333549111260S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008;122:718–725. doi: 10.1542/peds.2007-0538. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey AF, Schaffer S, Singer D, Butchart A, et al. Alternative vaccination schedule preferences among parents of young children. Pediatrics. 2011;128:848–856. doi: 10.1542/peds.2011-0400. [DOI] [PubMed] [Google Scholar]
- 4.Kempe A, Daley MF, McCauley MM, et al. Prevalence of parental concerns about childhood vaccines. Am J Prev Med. 2011;40:548–555. doi: 10.1016/j.amepre.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy A, LaVail K, Nowak G, et al. Confidence about vaccines in the Unites States: understanding parents’ perceptions. Health Aff. 2011;6:1151–1159. doi: 10.1377/hlthaff.2011.0396. [DOI] [PubMed] [Google Scholar]
- 6.Dorell C, Yankey D, Jeyarajah J, et al. Delay and refusal of human papillomavirus vaccine for girls, National Immunization Survey-Teen, 2010. Clin Pediatr (Phila) 2014;53(3):261–269. doi: 10.1177/0009922813520070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darden PM, Thompson DM, Roberts JR, et al. Reasons for not vaccinating adolescents: National Immunization Survey of Teens, 2008–2010. Pediatrics. 2013;131(4):645–651. doi: 10.1542/peds.2012-2384. [DOI] [PubMed] [Google Scholar]
- 8.McRee AL, Brewer NT, Reiter PL, et al. The Carolina HPV Immunization Attitudes and Beliefs Scale (CHIAS): scale development and associations with intentions to vaccinate. Sex Transm Dis. 2010;37(4):234–239. doi: 10.1097/OLQ.0b013e3181c37e15. [DOI] [PubMed] [Google Scholar]
- 9.Opel DJ, Taylor JA, Zhou C, et al. The relationship between parent attitudes about childhood vaccines survey scores and future child immunization status: a validation study. JAMA Pediatr. 2013;167(11):1065–1071. doi: 10.1001/jamapediatrics.2013.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilkey MB, Magnus BE, Reiter PL, et al. The Vaccination Confidence Scale: a brief measure of parents’ vaccination beliefs. Vaccine. 2014;32(47):6259–6265. doi: 10.1016/j.vaccine.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. [Accessed February 6, 2015];About the National Immunization Survey. 2015 Available at: http://www.cdc.gov/nchs/nis/about_nis.htm.
- 12.Rosenstock IM. Historical origins of the Health Belief model. Health Educ Behav. 1974;2(4):328–335. [Google Scholar]
- 13.Centers for Disease Control and Prevention. [Accessed February 6, 2015];Vaccines for Children (VFC) Program. 2015 Available at: http://www.cdc.gov/vaccines/programs/vfc/index.html.
- 14.U.S. Census Bureau. [Accessed February 6, 2015];Metropolitan and micropolitan statistical areas. 2015 Available at: http://www.census.gov/population/metro/
- 15.Centers for Disease Control and Prevention (CDC). . Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–8. [PubMed] [Google Scholar]
- 16.Hughes CC, Jones AL, Feemster KA, Fiks AG. HPV vaccine decision making in pediatric primary care: a semi-structured interview study. BMC Pediatr. 2011;11:74. doi: 10.1186/1471-2431-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamlish T1, Clarke L, Alexander KA. Barriers to HPV immunization for African American adolescent females. Vaccine. 2012;30(45):6472–6476. doi: 10.1016/j.vaccine.2012.07.085. [DOI] [PubMed] [Google Scholar]
- 18.Leask J, Kinnersley P, Jackson C, et al. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12:154. doi: 10.1186/1471-2431-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyhan B1, Reifler J, Richey S, Freed GL. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133(4):e835–42. doi: 10.1542/peds.2013-2365. [DOI] [PubMed] [Google Scholar]
- 20.Dempsey AF, Butchart A, Singer D, et al. Factors associated with parental intentions for male human papillomavirus vaccination: results of a national survey. Sex Transm Dis. 2011;38(8):769–76. doi: 10.1097/OLQ.0b013e318211c248. [DOI] [PubMed] [Google Scholar]
- 21.Tickner S, Leman PJ, Woodcock A. The Immunisation Beliefs and Intentions Measure (IBIM): predicting parents’ intentions to immunise preschool children. Vaccine. 2010;28(19):3350–62. doi: 10.1016/j.vaccine.2010.02.083. [DOI] [PubMed] [Google Scholar]
- 22.Brewer NT, Fazekas KO. Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Prev Med. 2007;45:107–114. doi: 10.1016/j.ypmed.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Gerend MA, Shepherd JE. Using message framing to promote acceptance of the human papillomavirus vaccine. Health Psychol. 2007;26(6):745–52. doi: 10.1037/0278-6133.26.6.745. [DOI] [PubMed] [Google Scholar]
- 24.Abhyankar P, O’Connor DB, Lawton R. The role of message framing in promoting MMR vaccination: evidence of a loss-frame advantage. Psychol Health Med. 2008;13(1):1–16. doi: 10.1080/13548500701235732. [DOI] [PubMed] [Google Scholar]