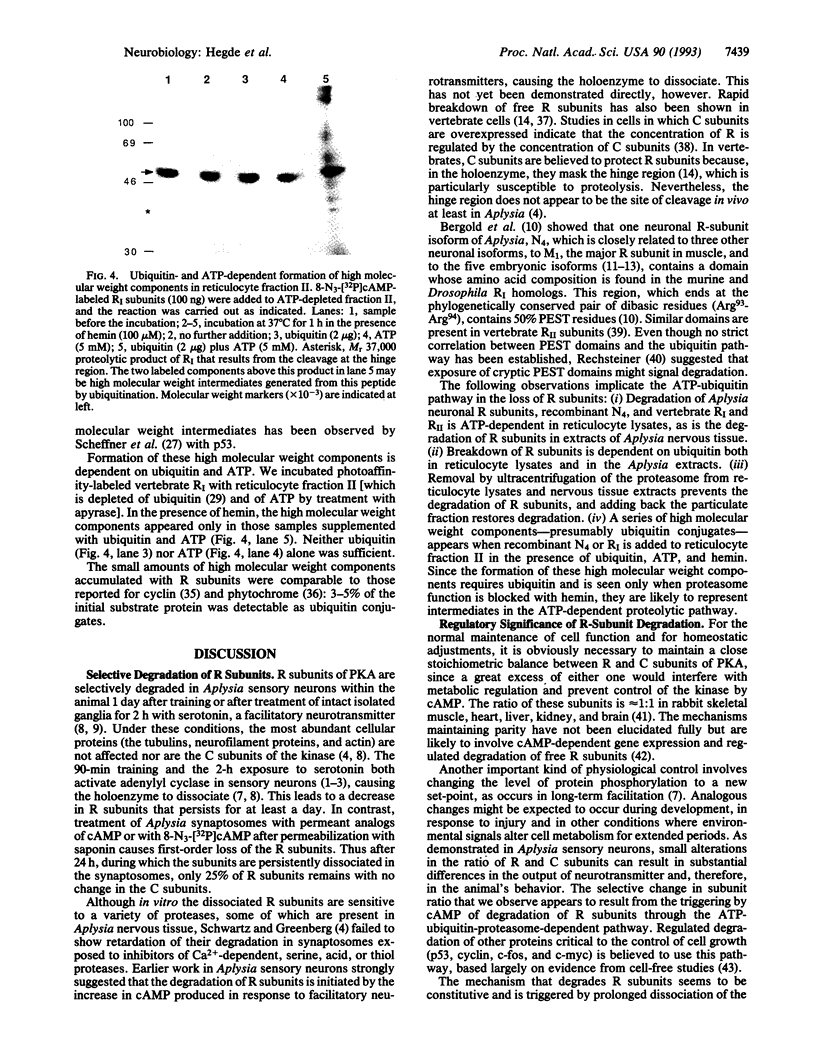
Abstract
In Aplysia, behavioral sensitization of defensive reflexes and the underlying presynaptic facilitation of sensory-to-motor neuron synapses lasts for several minutes (short term) or days to weeks (long term). Short-term sensitization has been explained by modulation of ion-channel function through cAMP-dependent protein phosphorylation. Long-term facilitation requires additional molecular changes including protein synthesis. A key event is the persistent activation of the cAMP-dependent protein kinase at baseline concentrations of cAMP. This activation is due to selective loss of regulatory (R) subunits of PKA without any change in catalytic (C) subunits. To understand the molecular mechanisms that produce the loss of R subunits in long-term facilitation, we investigated how R subunits are degraded in extracts of Aplysia nervous tissue and in rabbit reticulocyte lysates. Degradation of Aplysia R subunits requires ATP, ubiquitin, and a particulate component that appears to be the proteasome complex. Degradation is blocked by hemin, which causes the accumulation of high molecular weight derivatives of R subunits that are likely to be ubiquitin conjugates of R subunits and intermediates in the degradative pathway. We also show that vertebrate RI and RII subunits can be degraded through the ubiquitin pathway. We suggest that degradation is initiated by cAMP, which causes the holoenzyme to dissociate and, further, that the altered R-to-C ratio in Aplysia sensory neurons is maintained in long-term facilitation by newly synthesized proteins that help target R subunits for accelerated degradation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beavo J. A., Bechtel P. J., Krebs E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Bergold P. J., Beushausen S. A., Sacktor T. C., Cheley S., Bayley H., Schwartz J. H. A regulatory subunit of the cAMP-dependent protein kinase down-regulated in aplysia sensory neurons during long-term sensitization. Neuron. 1992 Feb;8(2):387–397. doi: 10.1016/0896-6273(92)90304-v. [DOI] [PubMed] [Google Scholar]
- Bergold P. J., Sweatt J. D., Winicov I., Weiss K. R., Kandel E. R., Schwartz J. H. Protein synthesis during acquisition of long-term facilitation is needed for the persistent loss of regulatory subunits of the Aplysia cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1990 May;87(10):3788–3791. doi: 10.1073/pnas.87.10.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., DiGiuseppe J. A., Bercovich B., Orian A., Richter J. D., Schwartz A. L., Brodeur G. M. Degradation of nuclear oncoproteins by the ubiquitin system in vitro. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):139–143. doi: 10.1073/pnas.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciehanover A., Hod Y., Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1100–1105. doi: 10.1016/0006-291x(78)91249-4. [DOI] [PubMed] [Google Scholar]
- Eppler C. M., Bayley H., Greenberg S. M., Schwartz J. H. Structural studies on a family of cAMP-binding proteins in the nervous system of Aplysia. J Cell Biol. 1986 Jan;102(1):320–331. doi: 10.1083/jcb.102.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppler C. M., Palazzolo M. J., Schwartz J. H. Characterization and localization of adenosine 3':5'-monophosphate-binding proteins in the nervous system of Aplysia. J Neurosci. 1982 Dec;2(12):1692–1704. doi: 10.1523/JNEUROSCI.02-12-01692.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D., Chau V. Ubiquitination. Annu Rev Cell Biol. 1991;7:25–69. doi: 10.1146/annurev.cb.07.110191.000325. [DOI] [PubMed] [Google Scholar]
- Fraser J., Luu H. A., Neculcea J., Thomas D. Y., Storms R. K. Ubiquitin gene expression: response to environmental changes. Curr Genet. 1991 Jul;20(1-2):17–23. doi: 10.1007/BF00312760. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Rock K. L. Proteolysis, proteasomes and antigen presentation. Nature. 1992 Jun 4;357(6377):375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. The mechanism and functions of ATP-dependent proteases in bacterial and animal cells. Eur J Biochem. 1992 Jan 15;203(1-2):9–23. doi: 10.1111/j.1432-1033.1992.tb19822.x. [DOI] [PubMed] [Google Scholar]
- Greenberg S. M., Castellucci V. F., Bayley H., Schwartz J. H. A molecular mechanism for long-term sensitization in Aplysia. Nature. 1987 Sep 3;329(6134):62–65. doi: 10.1038/329062a0. [DOI] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Hershko A., Heller H. Occurrence of a polyubiquitin structure in ubiquitin-protein conjugates. Biochem Biophys Res Commun. 1985 May 16;128(3):1079–1086. doi: 10.1016/0006-291x(85)91050-2. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Hough R., Pratt G., Rechsteiner M. Purification of two high molecular weight proteases from rabbit reticulocyte lysate. J Biol Chem. 1987 Jun 15;262(17):8303–8313. [PubMed] [Google Scholar]
- Jabben M., Shanklin J., Vierstra R. D. Ubiquitin-phytochrome conjugates. Pool dynamics during in vivo phytochrome degradation. J Biol Chem. 1989 Mar 25;264(9):4998–5005. [PubMed] [Google Scholar]
- Jack D. L., Chakraborty G., Ingoglia N. A. Ubiquitin is associated with aggregates of arginine modified proteins in injured nerves. Neuroreport. 1992 Jan;3(1):47–50. doi: 10.1097/00001756-199201000-00012. [DOI] [PubMed] [Google Scholar]
- Johnston N. L., Cohen R. E. Uncoupling ubiquitin-protein conjugation from ubiquitin-dependent proteolysis by use of beta, gamma-nonhydrolyzable ATP analogues. Biochemistry. 1991 Jul 30;30(30):7514–7522. doi: 10.1021/bi00244a021. [DOI] [PubMed] [Google Scholar]
- Kanayama H. O., Tamura T., Ugai S., Kagawa S., Tanahashi N., Yoshimura T., Tanaka K., Ichihara A. Demonstration that a human 26S proteolytic complex consists of a proteasome and multiple associated protein components and hydrolyzes ATP and ubiquitin-ligated proteins by closely linked mechanisms. Eur J Biochem. 1992 Jun 1;206(2):567–578. doi: 10.1111/j.1432-1033.1992.tb16961.x. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Clegg C. H., Uhler M. D., Chrivia J. C., Cadd G. G., Correll L. A., Otten A. D. Analysis of the cAMP-dependent protein kinase system using molecular genetic approaches. Recent Prog Horm Res. 1988;44:307–335. doi: 10.1016/b978-0-12-571144-9.50014-4. [DOI] [PubMed] [Google Scholar]
- Montarolo P. G., Goelet P., Castellucci V. F., Morgan J., Kandel E. R., Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986 Dec 5;234(4781):1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Palazzolo M., Katz F., Kennedy T. E., Schwartz J. H. Multiple cAMP-binding proteins in Aplysia tissues. J Neurobiol. 1989 Dec;20(8):746–761. doi: 10.1002/neu.480200807. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. Natural substrates of the ubiquitin proteolytic pathway. Cell. 1991 Aug 23;66(4):615–618. doi: 10.1016/0092-8674(91)90104-7. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. PEST sequences are signals for rapid intracellular proteolysis. Semin Cell Biol. 1990 Dec;1(6):433–440. [PubMed] [Google Scholar]
- Rechsteiner M. Ubiquitin-mediated pathways for intracellular proteolysis. Annu Rev Cell Biol. 1987;3:1–30. doi: 10.1146/annurev.cb.03.110187.000245. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Scott J. D., Glaccum M. B., Zoller M. J., Uhler M. D., Helfman D. M., McKnight G. S., Krebs E. G. The molecular cloning of a type II regulatory subunit of the cAMP-dependent protein kinase from rat skeletal muscle and mouse brain. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5192–5196. doi: 10.1073/pnas.84.15.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert W., Jentsch S. Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J. 1990 Feb;9(2):543–550. doi: 10.1002/j.1460-2075.1990.tb08141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Agard D. A. Turnover of regulatory subunit of cyclic AMP-dependent protein kinase in S49 mouse lymphoma cells. Regulation by catalytic subunit and analogs of cyclic AMP. J Biol Chem. 1981 Nov 10;256(21):10731–10734. [PubMed] [Google Scholar]
- Sweatt J. D., Kandel E. R. Persistent and transcriptionally-dependent increase in protein phosphorylation in long-term facilitation of Aplysia sensory neurons. Nature. 1989 May 4;339(6219):51–54. doi: 10.1038/339051a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Buechler J. A., Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Uhler M. D., McKnight G. S. Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1987 Nov 5;262(31):15202–15207. [PubMed] [Google Scholar]
- Walters E. T., Alizadeh H., Castro G. A. Similar neuronal alterations induced by axonal injury and learning in Aplysia. Science. 1991 Aug 16;253(5021):797–799. doi: 10.1126/science.1652154. [DOI] [PubMed] [Google Scholar]
- Waxman L., Fagan J. M., Goldberg A. L. Demonstration of two distinct high molecular weight proteases in rabbit reticulocytes, one of which degrades ubiquitin conjugates. J Biol Chem. 1987 Feb 25;262(6):2451–2457. [PubMed] [Google Scholar]
- Weber W., Vogel C. W., Hilz H. A new cAMP affinity matrix for the rapid purification of protein kinase regulatory subunits. FEBS Lett. 1979 Mar 1;99(1):62–66. doi: 10.1016/0014-5793(79)80249-5. [DOI] [PubMed] [Google Scholar]