Abstract
FOXG1-related disorders are caused by heterozygous mutations in FOXG1 and result in a spectrum of neurodevelopmental phenotypes including postnatal microcephaly, intellectual disability with absent speech, epilepsy, chorea, and corpus callosum abnormalities. The recurrence risk for de novo mutations in FOXG1-related disorders is assumed to be low. Here, we describe three unrelated sets of full siblings with mutations in FOXG1 (c.515_577del63, c.460dupG, and c.572T>G), representing familial recurrence of the disorder. In one family, we have documented maternal somatic mosaicism for the FOXG1 mutation, and all of the families presumably represent parental gonadal (or germline) mosaicism. To our knowledge, mosaicism has not been previously reported in FOXG1-related disorders. Therefore, this report provides evidence that germline mosaicism for FOXG1 mutations is a likely explanation for familial recurrence and should be considered during recurrence risk counseling for families of children with FOXG1-related disorders.
Keywords: FOXG1, 14q12, gonadal mosaicism, familial recurrence
INTRODUCTION
The FOXG1 gene, on chromosome 14q12, encodes the transcription factor forkhead box G1 that is expressed in the fetal and adult brain. FOXG1 is critically important in the regulation of neurogenesis in the telencephalon [Martynoga et al., 2005]. FOXG1-related disorders are a spectrum of neurodevelopmental phenotypes [Papa et al., 2008; Jacob et al., 2009; Florian et al., 2012], caused by de novo heterozygous abnormalities in FOXG1. Intragenic mutations of FOXG1 and deletions of 14q12 encompassing FOXG1 result in postnatal microcephaly, intellectual disability with absent speech, variable epilepsy, corpus callosum abnormalities, generalized hypotonia, and choreiform movements [Bisgaard et al., 2006; Ariani et al., 2008; Papa et al., 2008; Jacob et al., 2009; Bahi-Buisson et al., 2010; Mencarelli et al., 2010; Philippe et al., 2010; Kortüm et al., 2011; Le Guen et al., 2011; Seltzer et al., 2014]. Additionally, larger deletions of 14q12 involving FOXG1 have been associated with dysmorphic facial features [Bisgaard et al., 2006; Papa et al., 2008; Jacob et al., 2009]. Duplications of 14q12 involving FOXG1, on the other hand, result in developmental delay/intellectual disability with absent speech, infantile spasms, and an autism phenotype [Yeung et al., 2009; Brunetti-Pierri et al., 2011; Paciorkowski et al., 2011; Striano et al., 2011; Tohyama et al., 2011; Bertossi et al., 2013; Seltzer et al., 2014].
Mosaicism is described as more than one genetically or cytogenetically discrete cell lines within an organism [Youssoufian and Pyeritz, 2002]. Mosaicism can be present either in the somatic cells and gonadal cells, giving an offspring recurrence risk of up to 50% or confined to the gonadal cells (or germline), in which case it typically involves a subset of the gonadal cells depending on the gonadal generation in which the mutation occurred [van der Meulen et al., 1995]. Individuals with somatic mosaicism may be unaffected or mildly affected, depending on the tissue distribution and burden of mutation in a given tissue, while individuals with gonadal mosaicism are generally unaffected [Hall, 1988]. Familial recurrence of a presumed de novo mutation in a child [Wijsman, 1991] has been reported in several conditions in which gonadal mosaicism has been well described, including Duchenne muscular dystrophy [Bakker et al., 1987], osteogenesis imperfecta type II [Byers et al., 1988], and neurofibromatis type I [Lázaro et al., 1994].
Here, we describe three unrelated sets of full siblings with recurrence of FOXG1 mutation. In one family, with three affected children, the FOXG1 mutation was documented in maternal DNA from blood, skin, and saliva implicating germline mosaicism as the likely disease mechanism. The recurrence in the other two families also likely resulted from parental germline mosaicism, although somatic mosaicism was not evident on parental testing. Two of the sibling pairs were published previously [Seltzer et al., 2014], and now we discuss the implications for genetic counseling. To our knowledge, gonadal mosaicism has not previously been reported in FOXG1-related disorders.
METHODS
Patients
Patients were ascertained through the Genetic Studies of Developmental Brain Disorders research program (Rochester) and the Study of Inherited Metabolic Diseases program (London, UK). Informed consent was obtained with approval of the University of Rochester Research Studies Review Board and the National Research Ethics Service (NRES) in the UK (NRES Committee: London – Bloomsbury, REC reference: 13/LO/0168, IRAS project ID: 95005). Retrospective clinical records and brain MRI scans were reviewed by the investigators.
FOXG1 Sequencing
Targeted single gene FOXG1 sequencing (NM_005249) was performed on families DB12-017 and DB13-029 as part of their routine clinical evaluations. Skin, saliva, and blood DNA samples were obtained from family DBL01-010 and FOXG1 sequencing was performed at the Great Ormond Street Hospital Genetics laboratory using the diagnostic multiple gene panel test targeted for disorders causing early infantile epilepsy and developmental delay.
RESULTS
Clinical Report
Family 1
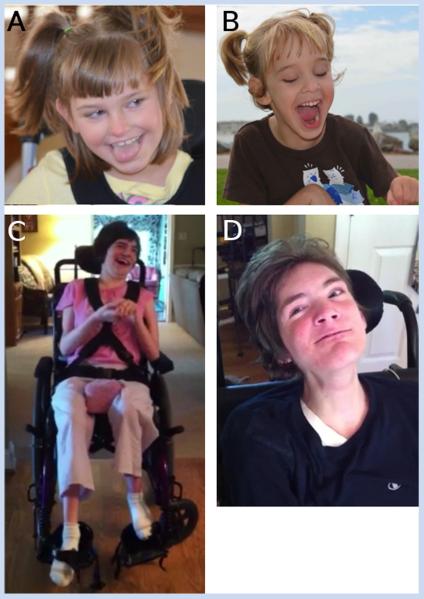
DB12-017a1, a female (Fig. 1A), was the first child born to healthy, non-consanguineous parents with an unremarkable family history. Pregnancy was uncomplicated and she was delivered by cesarean section due to failure to progress. Birth weight was 3.3 kg with no postnatal complications. At age 9 years, head circumference was −4 SD. She could crawl and sit with assistance but was not able to walk independently. She had constipation, gastroesophageal reflux, pain insensitivity, and sensitivity to heat. She had strabismus surgically corrected and required G-tube placement for feeding. She had limited purposeful hand movements and was nonverbal. She was diagnosed with complex partial seizures at 18 months and was on one anti-seizure medication.
FIG. 1.
Subject DB12-017a1 at 9 years of age (A) and sibling DB12-017a2 at 6 years of age (B). Subject DB13-029a1 at 25 years of age (C) and sibling DB13-029a2 at 21 years of age (D).
DB12-017a2, a female (Fig. 1B), was the second child born to the same parents. Pregnancy was uncomplicated. She was born full term by scheduled repeat cesarean section. She weighed 3.8 kg at birth and had jaundice requiring phototherapy. At age 6 years, head circumference was −4 SD; she could roll, scoot, and walk with assistance. She was nonverbal but could use pictures for communication. She had constipation, pain insensitivity, and strabismus. She could feed herself by mouth with assistance and did not require a G-tube for feeding. She had limited purposeful hand movements. Unlike her sibling, DB12-017a1, she has never had seizures. The FOXG1 mutation c.515_577del63/p.Gly172_Met192del was identified in both siblings in this family by clinical testing.
Family 2
DB13-029a1, a female (Fig. 1C), was the first child born to healthy, consanguineous parents with an unremarkable family history. Pregnancy was uncomplicated. She was born at full-term by spontaneous vaginal delivery and weighed 3.4 kg. She remained in the neonatal intensive care unit (NICU) for difficulty eating and failure to thrive. A patent ductus arteriosus (PDA) was subsequently identified and closed on its own without surgical intervention. At age 25 years, she was in an adult day care program. She could not walk independently and had limited purposeful hand movements. She had constipation, gastroesophageal reflux, scoliosis, strabismus, and cold extremities. She could eat pureed food by mouth with assistance but primarily required a G-tube for feeding. She was nonverbal and laughed and smiled inappropriately. She was diagnosed with generalized tonic–clonic seizures at 2 years old, was treated with valproic acid, had her last seizure at 15 years of age, and no longer required anti-seizure drugs.
DB13-029a2, a male (Fig. 1D), was the third child born to the same parents. There is one unaffected sibling between DB13-029a1 and DB13-029a2. Pregnancy was uncomplicated. He was born full-term by spontaneous vaginal delivery and weighted 4.1 kg with no postnatal complications. At age 22 years, he cannot walk independently. He had constipation, gastroesophogeal reflux, strabismus, and scoliosis. He required a G-tube for feeding. He was nonverbal, had limited purposeful hand movements, and laughed and smiled inappropriately. He was diagnosed with generalized tonic–clonic seizures at 2 years old and continued to require two anti-seizure drugs. The FOXG1 mutation c.460dupG p.Glu154GlyfsX301 was identified in both siblings in this family by clinical testing.
Family 3
Subject DBL01-010a1, a female, was born at term by spontaneous vaginal delivery. Pregnancy was unremarkable. She had low muscle tone as an infant and febrile convulsions at 4 months of age, followed by absence seizures at 3 years. She is currently 18 years old and on lamotrigine monotherapy for seizures (facial grimacing and perioral cyanosis followed by limb jerking) occurring infrequently, every few months. There have been no hospitalizations for complications of epilepsy. She sat at 18 months and walked independently at 2 years of age. She can speak in sentences and is in a special needs school. She is able to feed and dress herself, can play basic games, but has limited eye contact and a high pain threshold. Head circumference is currently 57.5 cm (91st–98th centile).
Subject DBL01-010a2, a female and the second child born to the same parents, was born at 42 weeks gestation through spontaneous vaginal delivery following an uncomplicated pregnancy. The neonatal and early infantile period was unremarkable. During childhood, she showed delay in achieving milestones, sitting at 1 year and walking at 2 years of age. She had three episodes of absence seizures associated with fever (two in early childhood and one at 11 years of age) but was never diagnosed with epilepsy and at 13 years of age is not on anti-seizure medication. Head circumference is 56 cm (between the 75th and 91st centile). She has better language skills than her other affected siblings and is in a mainstream classroom with educational support. She is able to perform activities of daily living with minimum assistance.
Subject DBL01-010a3, a male, is the youngest of the three siblings. He was born at term by normal vaginal delivery following an uncomplicated pregnancy. He developed seizures at 4 years of age, which are primarily absences in semiology. At the age of 11 years, he is currently well-controlled on valproic acid, and his last seizure was over 2 years ago. Head circumference is 55 cm (just above 50th centile). He walked at 3.5 years of age, has around 20 words that are used consistently, but his receptive language skills are described as better than his expressive language skills. He requires help with dressing and other fine motor tasks. The FOXG1 mutation c.572T > G/p.Met191Arg was identified in all three siblings in this family. Additionally, parental studies revealed the same mutation in maternal DNA extracted from blood, skin, and saliva. The chromatographic tracing of the variant allele in all maternal samples was consistently lower than expected for a heterozygote and in keeping with somatic mosaicism.
Brain Imaging
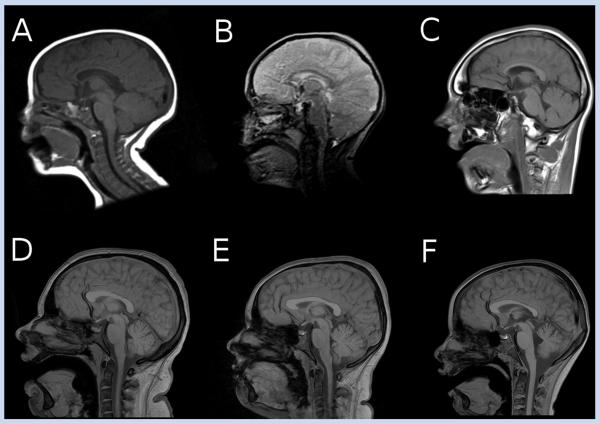
Brain MRI scans performed clinically were reviewed for subjects DB12-017a1, DB12-017a2, DB13-029a2, DBL01-010a1, DBL01-010a2, and DBL01-010a3. Hypoplasia of the genu of the corpus callosum was seen in subjects DB12-017a1, DB12-017a2, and DB13-029a2, along with mild underdevelopment of the frontal lobes (Fig. 2A–C). Subjects DBL01-010a1 and DBL01-010a2 had normal callosal and frontotemporal structure appearances but mild prominence of the cerebellar fissures. Subject DBL01-010a3 had mild hypoplasia in the posterior part of the corpus callosum associated with parietal underdevelopment, as well as a greater degree of cerebellar hypoplasia which was more pronounced in the cerebellar vermis. DB13-029a1 did not have scans available for review. The genotypes and phenotypes of subjects in this report are summarized in Table I.
FIG. 2.
Brain MRI of subjects DB12-017a1 (A), DB12-017a2 (B), and DB13-029a2 (C) demonstrating hypoplasia of genu of the corpus callosum. MRI scan of subjects DBL01-010a1 (D) and DBL01-010a2 (E) show mild prominence of the cerebellar fissures, whereas in DBL01-010a3 (F) mild hypoplasia of the splenium of the corpus callosum, along with a greater degree of cerebellar hypoplasia, are seen. All subjects in family DBL01-010 had normal anterior corpus callosum morphology.
TABLE I.
Genomic and Clinical Features of Subjects With Familial Recurrences of FOXG1-Related Disorder
| Subject | FOXG1 genotype | Sex | Age at time of study | Epilepsy | ID | Walking | Movements |
|---|---|---|---|---|---|---|---|
| DB12-017a1 | c.515_577del63/p.Gly172_Met192del | Female | 9 years 8 months | Complex partial | Yes | No | Hyperkinesis, with choreiform movements |
| DB12-017a2 | c.515_577del63/p.Gly172_Met192del | Female | 6 years 19 months | None | Yes | No | Hyperkinesis, with choreiform movements |
| DB13-029a1 | c.460dupG p.Glu154GlyfsX301 | Female | 25 years 10 months | Generalized tonic-clonic | Yes | No | Hypokinetic, with hand stereotypies and increased tone. |
| DB13-029a2 | c.460dupG p.Glu154GlyfsX301 | Male | 22 years | Generalized tonic-clonic | Yes | No | Hypokinetic, with hand stereotypies and increased tone. |
| DBL01-010a1 | c.572T > G p.Met191Arg | Female | 18 years | Absence | Yes | Yes | Hyperkinesis, with choreiform movements, dystonia |
| DBL01-010a2 | c.572T > G p.Met191Arg | Female | 13 years | None | Mild | Yes | Hyperkinesis, with choreiform movements, dystonia |
| DBL01-010a3 | c.572T > G p.Met191Arg | Male | 11 years | Absence | Yes | Yes | Hyperkinesis, with choreiform movements dystonia, orofacial dyskinesia |
DISCUSSION
FOXG1 Familial Occurrence
In males, spermatogenesis begins at puberty and continues throughout adult life. In contrast, oogonesis in females begins during embryogenesis during which oogonia differentiate into primary oocytes, which then enter and arrest in prophase of Meiosis I until ovulation [Chandley, 1991]. It is estimated that the number of gonadal generations is approximately 23 in females and 30 in males followed by an additional 23 divisions per year following puberty in males [Wijsman, 1991]. The distinction between gonadal and somatic mosaicism is difficult [Youssoufian and Pyeritz, 2002] because mosaicism in tissues is random and the tissues sampled by any given method or study may not be mosaic. The parents of all siblings we report here were clinically unaffected. Somatic mosaicism in the parents of the DB12-017 and DB13-029 families was not detected by Sanger sequencing of both blood and saliva-derived DNA. We did find the FOXG1 mutation present in the mother of the DBL01-010 family, indicating both gonadal and somatic mosaicism. Somatic mosaicism has not been described in FOXG1-related disorders before.
The recurrence risk for a single occurrence of a heterozygous de novo mutation, with unaffected parents, is generally assumed to be low [Emery, 1986]. However, as more evidence emerges that gonadal mosaicism is not as uncommon as once thought, recurrence risks should be adjusted [Hall, 1988]. While the number of gonadal cells containing a mutation depends on the stage at which the mutation occurred [Zlotogora, 1998], this cannot be accurately predicted. Recurrence risks for unaffected couples with disorders can be calculated and are dependent on parental age, sex, and number of unaffected offspring [Campbell et al., 2014; Hartl, 1971; van der Meulen et al., 1995]. In practice, these factors are difficult to model for individual genes and empiric data are generalizations at best. Additional factors influence recurrence risk, such as parental origin of mutation, particularly if the condition is known to have a strong paternal age effect [Wijsman, 1991; Campbell et al., 2014], and evidence of reduced penetrance [Emery, 1986]. Reduced penetrance and paternal age effect have not been demonstrated in FOXG1 mutations.
Siblings DB12-017a1 and DB12-017a2 were the only children born to their unaffected parents. Using the empiric recurrence risks for gonadal mosaics available, it can be predicted that recurrence risk for an additional child is approximately 33% [van der Meulen et al., 1995]. Siblings DB13-029a1 and DB13-029a2 have one unaffected sibling. Recurrence risks with two affected children and one unaffected children, without known parental origin, are approximately 28% (van der Meulen et al., 1995). Siblings DBL01-010a1, DBL01-010a2, DBL01-010a3 were the only children born to this family, and given the documented maternal mosaicism, the recurrence risk could be as high as 50%.
It is also remarkable that the three siblings in family DBL01-010 have the most mild neurocognitive phenotypes described to date in FOXG1-related disorder. All three were able to walk independently. Two of the three had epilepsy, with seizures well-controlled on monotherapy in both cases. All three had verbal language, although dysarthria and speech delay was more prominent in the youngest. Interestingly, head circumferences were within normal limits and MRI brain findings were also mild in comparison to those with classical FOXG1 syndrome.
In conclusion, we describe here three families with recurrence of intragenic FOXG1 mutations out of 38 families with related phenotypes reported to date. This is evidence that germline mosaicism for FOXG1 mutations is a likely explanation for familial recurrence in a child with clinically unaffected parents, and, therefore, has implications for recurrence risk counseling. The overall prevalence of FOXG1-related disorders is unknown, and, therefore, we cannot predict how common or uncommon this event may be. Given the number of cases reported to date, our data suggest gonadal mosaicism is present in 8% (3/38) of families currently reported. Most children with a FOXG1 mutation and unaffected parents will be a result of a de novo mutation; however, parental mosaicism should be kept in mind during counseling for recurrence risks of FOXG1-related disorders.
ACKNOWLEDGMENTS
We gratefully acknowledge our research families and the FOXG1 Foundation. This work was supported by the National Institutes of Health, National Institute of Neurologic Disorders and Stroke under award number K08NS078054 (to ARP). AP receives funding from Actelion to study undiagnosed neurometabolic disorders, and also from the NBIA Disorders association and Child Brain Research (a charity operating under the parent organization UK Children's Neurological Research Campaign). MAK is funded by a Wellcome Trust Intermediate Clinical Fellowship and receives funding from the Rosetrees Trust and Gracious Heart Charity Foundation.
Grant sponsor: National Institutes of Health, National Institute of Neurologic Disorders and Stroke; Grant number: K08NS078054; Grant sponsor: Actelion; Grant sponsor: NBIA Disorders association and Child Brain Research; Grant sponsor: Wellcome Trust Intermediate Clinical Fellowship; Grant sponsor: Rosetrees Trust and Gracious Heart Charity Foundation.
REFERENCES
- Ariani F, Hayek G, Rondinella D, Artuso R, Mencarelli MA, Spanhol-Rosseto A, Pollazzon M, Buoni S, Spiga O, Ricciardi S, Meloni I, Longo I, Mari F, Broccoli V, Zappella M, Renieri A. FOXG1 is responsible for the congenital variant of Rett syndrome. Am J Hum Genet. 2008;83:89–93. doi: 10.1016/j.ajhg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi-Buisson N, Nectoux J, Girard B, Van Esch H, De Ravel T, Boddaert N, Plouin P, Rio M, Fichou Y, Chelly J, Bienvenu T. Revisiting the phenotype associated with FOXG1 mutations: Two novel cases of congenital Rett variant. Neurogenetics. 2010;11:241–249. doi: 10.1007/s10048-009-0220-2. [DOI] [PubMed] [Google Scholar]
- Bakker E, Van Broeckhoven C, Bonten EJ, van de Vooren MJ, Veenema H, Van Hul W, Van Ommen GJ, Vandenberghe A, Pearson PL. Germline mosaicism and Duchenne muscular dystrophy mutations. Nature. 1987;329:554–556. doi: 10.1038/329554a0. [DOI] [PubMed] [Google Scholar]
- Bertossi C, Cassina M, De Palma L, Vecchi M, Rossato S, Toldo I, Donà M, Murgia A, Boniver C, Sartori S. 14q12 duplication including FOXG1: Is there a common age-dependent epileptic phenotype? Brain Dev. 2013;36:402–407. doi: 10.1016/j.braindev.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Bisgaard A-M, Kirchhoff M, Tümer Z, Jepsen B, Brøndum-Nielsen K, Cohen M, Hamborg-Petersen B, Bryndorf T, Tommerup N, Skovby F. Additional chromosomal abnormalities in patients with a previously detected abnormal karyotype, mental retardation, and dysmorphic features. Am J Med Genet A. 2006;140:2180–2187. doi: 10.1002/ajmg.a.31425. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Paciorkowski AR, Ciccone R, Mina ED, Bonaglia MC, Borgatti R, Schaaf CP, Sutton VR, Xia Z, Jelluma N, Ruivenkamp C, Bertrand M, de Ravel TJ, Jayakar P, Belli S, Rocchetti K, Pantaleoni C, D'Arrigo S, Hughes J, Cheung SW, Zuffardi O, Stankiewicz P. Duplications of FOXG1 in 14q12 are associated with developmental epilepsy, mental retardation, and severe speech impairment. Eur J Hum Genet EJHG. 2011;19:102–107. doi: 10.1038/ejhg.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers PH, Tsipouras P, Bonadio JF, Starman BJ, Schwartz RC. Perinatal lethal osteogenesis imperfecta (OI type II): A biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988;42:237–248. [PMC free article] [PubMed] [Google Scholar]
- Campbell IM, Stewart JR, James RA, Lupski JR, Stankiewicz P, Olofsson P, Shaw CA. Parent of origin, mosaicism, and recurrence risk: Probabilistic modeling explains the broken symmetry of transmission genetics. Am J Hum Genet. 2014;95:345–359. doi: 10.1016/j.ajhg.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandley AC. On the parental origin of de novo mutation in man. J Med Genet. 1991;28:217–223. doi: 10.1136/jmg.28.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery AE. Risk estimation in autosomal dominant disorders with reduced penetrance. J Med Genet. 1986;23:316–318. doi: 10.1136/jmg.23.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian C, Bahi-Buisson N, Bienvenu T. FOXG1-related disorders: From clinical description to molecular genetics. Mol Syndromol. 2012;2:153–163. doi: 10.1159/000327329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guen T, Bahi-Buisson N, Nectoux J, Boddaert N, Fichou Y, Diebold B, Desguerre I, Raqbi F, Daire VC, Chelly J, Bienvenu T. A FOXG1 mutation in a boy with congenital variant of Rett syndrome. Neurogenetics. 2011;12:1–8. doi: 10.1007/s10048-010-0255-4. [DOI] [PubMed] [Google Scholar]
- Hall JG. Review and hypotheses: Somatic mosaicism: Observations related to clinical genetics. Am J Hum Genet. 1988;43:355–363. [PMC free article] [PubMed] [Google Scholar]
- Hartl DL. Recurrence risks for germinal mosaics. Am J Hum Genet. 1971;23:124–134. [PMC free article] [PubMed] [Google Scholar]
- Jacob FD, Ramaswamy V, Andersen J, Bolduc FV. Atypical Rett syndrome with selective FOXG1 deletion detected by comparative genomic hybridization: Case report and review of literature. Eur J Hum Genet EJHG. 2009;17:1577–1581. doi: 10.1038/ejhg.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortüm F, Das S, Flindt M, Morris-Rosendahl DJ, Stefanova I, Goldstein A, Horn D, Klopocki E, Kluger G, Martin P, Rauch A, Roumer A, Saitta S, Walsh LE, Wieczorek D, Uyanik G, Kutsche K, Dobyns WB. The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis. J Med Genet. 2011;48:396–406. doi: 10.1136/jmg.2010.087528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázaro C, Ravella A, Gaona A, Volpini V, Estivill X. Neurofibromatosis type 1 due to germ-line mosaicism in a clinically normal father. N Engl J Med. 1994;331:1403–1407. doi: 10.1056/NEJM199411243312102. [DOI] [PubMed] [Google Scholar]
- Martynoga B, Morrison H, Price DJ, Mason JO. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev Biol. 2005;283:113–127. doi: 10.1016/j.ydbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Mencarelli MA, Spanhol-Rosseto A, Artuso R, Rondinella D, De Filippis R, Bahi-Buisson N, Nectoux J, Rubinsztajn R, Bienvenu T, Moncla A, Chabrol B, Villard L, Krumina Z, Armstrong J, Roche A, Pineda M, Gak E, Mari F, Ariani F, Renieri A. Novel FOXG1 mutations associated with the congenital variant of Rett syndrome. J Med Genet. 2010;47:49–53. doi: 10.1136/jmg.2009.067884. [DOI] [PubMed] [Google Scholar]
- Van der Meulen MA, van der Meulen MJ, te Meerman GJ. Recurrence risk for germinal mosaics revisited. J Med Genet. 1995;32:102–104. doi: 10.1136/jmg.32.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorkowski AR, Thio LL, Rosenfeld JA, Gajecka M, Gurnett CA, Kulkarni S, Chung WK, Marsh ED, Gentile M, Reggin JD, Wheless JW, Balasubramanian S, Kumar R, Christian SL, Marini C, Guerrini R, Maltsev N, Shaffer LG, Dobyns WB. Copy number variants and infantile spasms: Evidence for abnormalities in ventral forebrain development and pathways of synaptic function. Eur J Hum Genet. 2011;19:1238–1245. doi: 10.1038/ejhg.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa FT, Mencarelli MA, Caselli R, Katzaki E, Sampieri K, Meloni I, Ariani F, Longo I, Maggio A, Balestri P, Grosso S, Farnetani MA, Berardi R, Mari F, Renieri A. A 3 Mb deletion in 14q12 causes severe mental retardation, mild facial dysmorphisms and Rett-like features. Am J Med Genet A. 2008;146A:1994–1998. doi: 10.1002/ajmg.a.32413. [DOI] [PubMed] [Google Scholar]
- Philippe C, Amsallem D, Francannet C, Lambert L, Saunier A, Verneau F, Jonveaux P. Phenotypic variability in Rett syndrome associated with FOXG1 mutations in females. J Med Genet. 2010;47:59–65. doi: 10.1136/jmg.2009.067355. [DOI] [PubMed] [Google Scholar]
- Seltzer LE, Ma M, Ahmed S, Bertrand M, Dobyns WB, Wheless J, Paciorkowski AR. Epilepsy and outcome in FOXG1-related disorders. Epilepsia. 2014;55:1292–1300. doi: 10.1111/epi.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striano P, Paravidino R, Sicca F, Chiurazzi P, Gimelli S, Coppola A, Robbiano A, Traverso M, Pintaudi M, Giovannini S, Operto F, Vigliano P, Granata T, Coppola G, Romeo A, Specchio N, Giordano L, Osborne LR, Gimelli G, Minetti C, Zara F. West syndrome associated with 14q12 duplications harboring FO XG1. Neurology. 2011;76:1600–1602. doi: 10.1212/WNL.0b013e3182194bbf. [DOI] [PubMed] [Google Scholar]
- Tohyama J, Yamamoto T, Hosoki K, Nagasaki K, Akasaka N, Ohashi T, Kobayashi Y, Saitoh S. West syndrome associated with mosaic duplication of FOXG1 in a patient with maternal uniparental disomy of chromosome 14. Am J Med Genet A. 2011;155A:2584–2588. doi: 10.1002/ajmg.a.34224. [DOI] [PubMed] [Google Scholar]
- Wijsman E. Recurrence risk of a new dominant mutation in children of unaffected parents. Am J Hum Genet. 1991;48:654–661. [PMC free article] [PubMed] [Google Scholar]
- Yeung A, Bruno D, Scheffer IE, Carranza D, Burgess T, Slater HR, Amor DJ. 4.45 Mb microduplication in chromosome band 14q12 including FOXG1 in a girl with refractory epilepsy and intellectual impairment. Eur J Med Genet. 2009;52:440–442. doi: 10.1016/j.ejmg.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Youssoufian H, Pyeritz RE. Mechanisms and consequences of somatic mosaicism in humans. Nat Rev Genet. 2002;3:748–758. doi: 10.1038/nrg906. [DOI] [PubMed] [Google Scholar]
- Zlotogora J. Germ line mosaicism. Hum Genet. 1998;102:381–386. doi: 10.1007/s004390050708. [DOI] [PubMed] [Google Scholar]