Abstract
Noninvasive imaging of kidney clearance kinetics (KCK) of renal clearable probes is key to studying unilateral kidney functional diseases but is highly challenging to be achieved with in vivo fluorescence imaging. While this long-standing challenge is often attributed to the limited light penetration depth, we found that rapid and persistent accumulation of conventional dyes in the skin “shadowed” real fluorescence signals from the kidneys and prevented noninvasive imaging of KCK, which, however, can be addressed with renal clearable nanofluorophores. By integrating near infrared emission with efficient renal clearance and ultralow background interference, the nanofluorophores can increase kidney-contrast enhancement and imaging-time window by ~50 and ~1000 times over conventional dyes and significantly minimize deviation between noninvasive and invasive KCK, laying down a foundation for translating in vivo fluorescence imaging in preclinical noninvasive kidney function assessment.
Keywords: Renal Clearable, Kidney Clearance Kinetics, Fluorescence imaging, Imaging agents, Nanoparticles
Noninvasive assessment of single kidney function is a key approach for understanding unilateral kidney diseases that are affecting a large population of adults worldwide.[1] To quantify individual kidney function, noninvasive imaging of kidney clearance kinetics (KCK) of renal clearable probes is an essential step.[2] Since accuracy of kidney functional evaluation requires dynamic imaging of kidneys at high contrast and high temporal resolution, nuclear imaging,[2a] magnetic resonance imaging (MRI)[2b] and computed tomography (CT)[2c] currently are major tools for both clinical diagnosis and preclinical kidney functional studies. However, because of high cost, limited access, and potential radiation exposure of current radiology-based kidney functional imaging, low-cost and high-sensitivity kidney functional imaging tools are highly desired for preclinical kidney research.[3]
In vivo near-infrared (NIR) fluorescence imaging has been widely used in preclinical disease studies due to its low cost, high sensitivity and no risk of radiation exposure.[4] Because of these merits, fluorescence imaging is also been translated into the clinical practices to improve surgical accuracy[4a, 5] and minimize potential damage to healthy tissues.[6] However, translation of fluorescence techniques into noninvasive preclinical kidney functional imaging remains highly challenging because very few fluorescent contrast agents can noninvasively report KCK.
Herein we report that this long-standing challenge in noninvasive fluorescence imaging of KCK is not due to the limited penetration of light but rapid and persistent accumulation of conventional fluorophores in the skin tissues after intravenous (iv) injection. Due to their high lipophilicity, conventional organic fluorophores tend to accumulate in the skin lipid membranes.[7a, 7b] Amphiphilic fluorescent NPs such as quantum dots,[7c] dye-conjugated silica NPs,[7a] and non-luminescent plasmonic AuNPs[7c] also have high accumulation in the skin. Although skin accumulation of organic dyes and amphiphilic NPs are offering exciting opportunities for detection of skin diseases,[7c, 8] such high accumulation of the fluorophores in the skin background is a roadblock for accurate quantification of KCK.
This challenge can be readily addressed with a renal clearable inorganic nanofluorophore, NIR-emitting glutathione-coated gold nanoparticle (GS-AuNP), which integrates deep tissue-penetration of NIR light with desired in vivo behaviors of contrast agents into a synergy to achieve high-contrast noninvasive fluorescence kidney imaging. More importantly, with assistance of GS-AuNPs, the kidney time-fluorescence intensity curves (TFICs) obtained from noninvasive florescence imaging can report the clearance kinetics of the GS-AuNPs via the kidney, laying down a foundation for future noninvasive fluorescence kidney functional imaging.
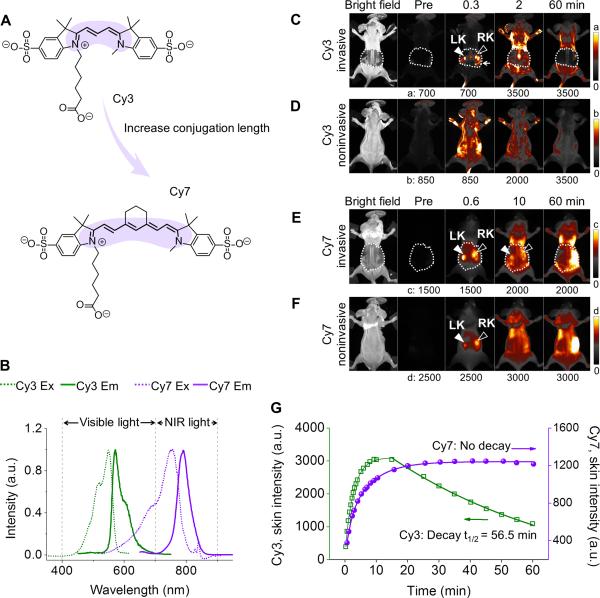
While the low contrast-enhancement in noninvasive fluorescence kidney imaging is often attributed to the limited tissue penetration of the light,[9] a comparison of kidney imaging in visible region and NIR window revealed that light penetration depth is not the only factor that governs kidney-contrast enhancements. Fig. 1 shows fluorescence images of mice injected with two Cyanine dyes, Cy3 and Cy7 (Fig. 1A,1B; Supporting Information, Fig. S1). While Cy3 rapidly accumulated in the kidneys within 1 min post injection (p.i.) as shown in invasive image (Fig. 1C,S2,S3), Cy3 failed to enhance the kidney contrast noninvasively (Fig. 1D). On the other hand, NIR-emitting Cy7 only transiently enhanced kidney contrast for less than 10 min (Fig. 1F). At 10 min p.i., the kidney became undetectable noninvasively, even though Cy7 remained in the kidneys as shown in invasive image (Fig. 1E,S3). Quantitative analysis shows that the Cy7 has even longer skin retention time than Cy3 (Fig. 1G), implying the enhanced hydrophobicity of NIR dyes significantly increased the affinity of molecules to tissues, consistent with previous findings.[10]
Figure 1.
Fluorescence kidney imaging of mice after intravenous (iv) injection of visible emitting Cy3 and NIR-emitting Cy7 dyes. (A) Chemical structures of Cy3 and Cy7. (B) Excitation and emission spectra of Cy3 and Cy7. The increase in conjugation length (highlighted in color) results in a red shift in excitation and emission maxima from the visible region (Cy3, 550/570 nm) to the NIR optical window (Cy7, 754/790 nm). (C-F) Whole-body invasive (C,E) and noninvasive (D,F) fluorescence images of mice before and after iv injection of Cy3 (C,D; Ex/Em filters: 550/600 nm) and Cy7 (E,F; Ex/Em filters: 710/790 nm). In C and E, the left and right kidneys (LK, RK) were exposed by partially removing the back skin (marked by dashed white line). (G) Time-dependent fluorescence intensity curves (TFICs) of the mouse skin following dye injection.
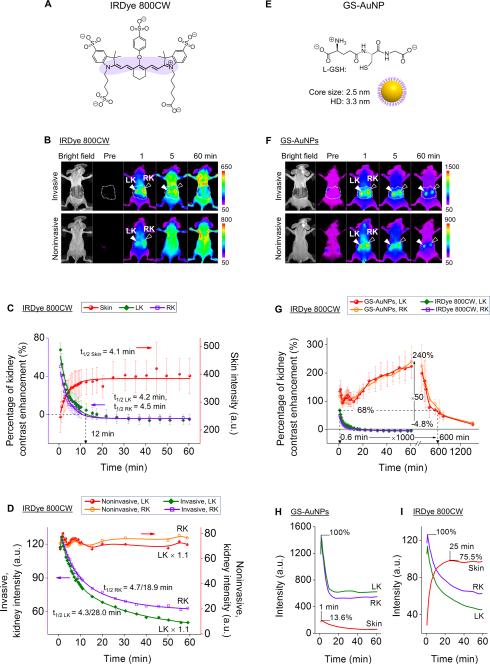
Not limited to Cy7, the well-known renal clearable fluorophore IRDye 800CW also only transiently lighted up the kidneys noninvasively for less than 5 min with 710 nm excitation and 790 nm emission filters (Fig. 2A,2B,S4). IRDye 800CW also exhibited persistent accumulation in the skin background and rapid kidney clearance (Fig. S5), which is independent of detection windows (790 nm vs. 830 nm) (Fig. S5), indicating that fluorescence images truly reflect behaviors of IRDye 800 CW in vivo. Quantitative studies on the contrast enhancements show that the percentages of kidney-contrast enhancement ([(mean intensity of kidney/mean intensity of surrounding tissue)-1]×100%) increased to 67.6 ± 7.1% and 49.9 ± 9.0% for left kidney (LK) and right kidney (RK) at 0.6 min p.i., respectively, then rapidly dropped to 0 at ~12 min p.i. with a single exponential decay kinetics (Fig. 2C). The decrease half-lives of kidney-contrast enhancements of noninvasive imaging were 4.2 min and 4.5 min for LK and RK respectively, comparable to the increasing half-life (t1/2) of skin intensity of 4.1 min (Fig. 2C), but faster than the dye clearance half-lives (4.3 min (50.1%)/28.0 min (49.9%) and 4.7 min (46.0%)/18.9 min (54.0%) for LK and RK, respectively) obtained from invasive imaging (Fig. 2D). Due to such strong skin interference, noninvasive time-dependent fluorescence intensities of kidney areas were maintained at a constant high level during 0-60 min p.i., and failed to report the actual process of dye uptake and clearance from the kidney as shown by invasive imaging (Fig. 2D). These results further confirmed that rapid accumulation and long retention of conventional organic fluorophores in the skin is a general cause for their failure in the noninvasive kidney imaging. As a result, applying conventional organic fluorophores for noninvasive imaging of KCK fell into a dilemma: while increasing conjugation length shifts the excitation and emission maxima from the visible region to NIR optical window, the large hydrophobic π-conjugated systems of NIR dyes lead to a high skin accumulation and thus generate a strong persistent fluorescence background, preventing noninvasive fluorescence imaging of KCK (Fig. 2D,S6).
Figure 2.
Comparison of renal clearable NIR emitting IRDye 800CW and GS-AuNPs in noninvasive kidney imaging. (A) Chemical structure of IRDye 800CW. (B) Whole-body invasive and noninvasive fluorescence images of mice before and after iv injection of IRDye 800CW (Ex/Em filters: 710/790 nm). The skin-removed areas were marked by dashed white line. LK, left kidney, RK, right kidney. (C) Time-course changes in skin intensity and percentage of kidney-contrast enhancements of mice after iv Injection of IRDye 800CW. N = 3, mean ± s.d. (D) Time-fluorescence intensity curves (TFICs) of kidneys obtained from noninvasive and invasive fluorescence imaging of mice after iv injection of IRDye 800CW. (E) Schematic representation of GS-AuNPs. (F) Whole-body invasive and noninvasive fluorescence images of mice before and after iv injection of GS-AuNPs (Ex/Em filters: 710/830 nm). (G) Comparison of time-dependent kidney contrast enhancements in percentage after GS-AuNPs and IRDye 800CW injection, respectively. N = 3, mean ± s.d. (H,I) TFICs of kidneys and skin obtained from invasive imaging after iv injection of GS-AuNPs (H) and IRDye 800CW (I).
Unlike organic fluorophores, luminescent inorganic nanoparticles can exhibit NIR emissions due to quantum size effects without sacrificing their hydrophilicity.[11] Among those known renal clearable inorganic nanoparticles,[12] luminescent GS-AuNPs (Fig. 2E,S7,S8) are a class of ultrasmall inorganic nanofluorophores that can be efficiently excreted through the kidney,[13] exhibit intrinsic NIR emission without conjugation of dyes,[14] have hydrophilic surface[15] and show faster clearance from normal tissues than dye molecules.[16]
In contrast to organic dyes, NIR-emitting GS-AuNPs can substantially enhance kidney contrast and extend detection-time window noninvasively. The noninvasive kidney images obtained with GS-AuNPs were very similar to the invasive ones (Fig. 2F). Right after injection of GS-AuNPs, the percentages of kidney contrast enhancement reached 90-150% within 12 min p.i., and gradually increased to maximum values of 240.0 ± 55.3% and 223.2 ± 41.5% for LK and RK at 60 min p.i. respectively, which were ~50 times higher than those obtained with IRDye 800 CW at 60 min p.i. (−4.7 ± 0.8% and −4.8 ± 5.1% for LK and RK) (Fig. 2G). The kidneys remained detectable at 10 h p.i with a contrast enhancement of ~68% (Fig. S9), which is the maximum enhancement that IRDye 800 can reach at 0.6 min p.i. (Fig. 2G, green and purple), suggesting the detection time window of GS-AuNPs was ~1000 times longer than that of IRDye 800CW.
The dramatic improvement in kidney contrast and detection time window achieved was attributed to the low skin accumulation of the hydrophilic GS-AuNPs and subsequent rapid clearance from the skin through the kidney. The maximum intensity of skin after GS-AuNPs injection was only ~13.6% of the maximum kidney intensity (Fig. 2H), much lower than the skin-to-kidney ratios of Cy7 (95.3%, Fig. S3) and IRDye 800CW (~75.5%, Fig. 2I). Additionally, accumulation in and clearance from skin of GS-AuNPs were much faster than those of NIR organic dyes. The skin intensity after GS-AuNPs injection (Fig. 2H) reached its maximum value at as early as ~1 min p.i., 20~30 times earlier than that of Cy7 (35 min p.i., Fig. S3) and IRDye 800CW (25 min p.i., Fig. 2I), and then immediately decreased and followed a single exponential decay kinetics with a half-life of 12.6 min. Therefore, the interference from the skin fluorescence on kidney imaging was incredibly reduced.
Besides low skin affinity, the prolonged detection time window of the kidneys was also contributed from the long retention of GS-AuNPs in the blood because blood flow to the two kidneys is normally ~22% of cardiac output[17] and renal vascular supply is up to 47% of kidneys[6a]. The elimination half-life (t1/2β) of GS-AuNPs (8.5 ± 2.1 h) was 9 times longer than that of IRDye 800CW (0.98 ± 0.08 h), which is responsible for the detection of kidneys for 10 h after injection of GS-AuNPs (Fig. S9).[16]
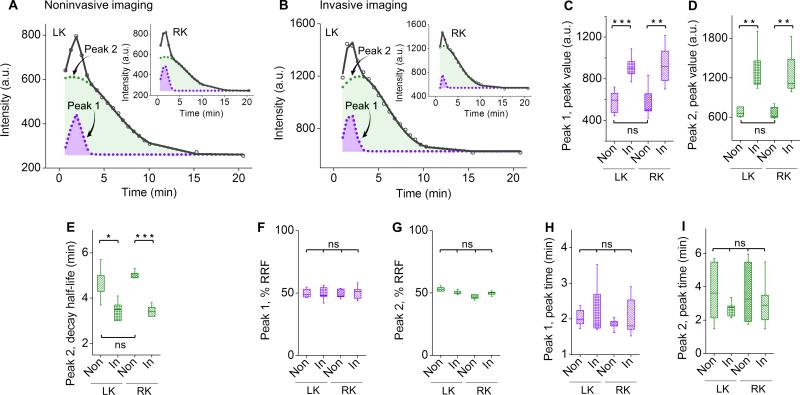
To determine whether the kidney TFICs obtained noninvasively can truly report the clearance kinetics of the GS-AuNPs via the kidney, we quantitatively compared the kidney TFICs measured noninvasively and invasively from the same group of mice (N = 6). Noninvasive kidney TFIC obtained with GS-AuNPs is very close to the invasive one in profile: a rapid ascending segment in the fluorescence signal followed by a descending segment with two distinct decay kinetics (Fig. 3A,3B). Further deconvolution of these kidney TFICs revealed two closely located peaks (peak 1 and 2), which were very similar to vascular phase and tubular phase as previously identified in kidney time-intensity curves of Gd-DTPA.[18]
Figure 3.
Quantitative comparison of noninvasive and invasive kidney imaging with GS-AuNPs. (A,B) Representative time-fluorescence intensity curves (TFICs) of kidneys obtained from noninvasive (A) and invasive (B) imaging of the same mouse after GS-AuNPs injection (0-20 min). The curves (in black) can be deconvoluted to peak 1 and peak 2 (in purple and green, respectively). (C-I) Statistical analysis of peak parameters extracted from noninvasive (“Non”) and invasive (“In”) kidney TFICs. The parameters included peak value (C,D), decay half-life (E), percentage of relative renal function (% RRF; F,G) and peak time (H,I), which were used to evaluate kidney function in clinical practice. LK, left kidney, RK, right kidney, N = 6 for C-I, mean ± s.d., *P < 0.05, **P < 0.01, ***P < 0.001; ns, no significant difference.
To gain more quantitative understandings of the similarity and differences in kidney TFICs measured noninvasively and invasively after injection of GS-AuNPs, we extracted and compared some key parameters from the two peaks such as peak value, peak time, decay half-life and percentage of relative renal function (% RRF), which are often used in radiology-based kidney function imaging.[19] Due to small light absorption of skin, the peak values detected in invasive imaging were slightly higher than those measured with noninvasive imaging (Fig. 3C,3D). In addition, decay half-lives of peak 2 obtained from noninvasive imaging were just one minute longer than those from invasive imaging (Fig. 3E), dramatically different from those with IRDye 800CW (Fig. 2D). On the other hand, regarding %RRF ([peak value of peak 1 or 2/(peak value of peak 1 + peak value of peak 2)]×100%) and peak times, no significant differences were observed between noninvasive and invasive kidney TFICs (Fig. 3F-3I). In addition, LK and RK exhibited no differences in all the parameters (Fig. 3F-3I and Table S1), consistent with the previous observations.[19] These results suggest that noninvasive kidney TFICs indeed reflect how the GS-AuNPs pass through the kidneys.
In summary, while NIR-emitting organic dyes meet the requirement on the light penetration depth for kidney imaging in small animals, their intrinsic large π-conjugation also induced rapid and persistent fluorescence background in the skin, making it highly challenging to noninvasively image KCK. With renal clearable NIR-emitting GS-AuNPs, this long-standing challenge in noninvasive fluorescence kidney functional imaging can be addressed: we observed ~50 and ~1000 times increases in kidney-contrast enhancement and imaging-time window over IRDye 800CW because of their desired in vivo behaviors (low skin distribution, rapid clearance from the skin, high kidney accumulation and long retention in the blood). As a result, high contrast noninvasive fluorescence imaging of KCK can be achieved, laying down a foundation for future fluorescence kidney functional imaging.
It should also be noted, low tissue penetration depth of the light, an intrinsic limitation of fluorescence imaging, will remain a roadblock for future clinical translation of these renal clearable luminescent AuNPs in kidney functional imaging. One potential solution is to incorporate radioisotopes such as 198[Au] into these GS-AuNPs,[14] so that they can serve as radiotracers for single-photon emission computed tomography (SPECT) imaging of kidney function. The related studies will be reported in our future work.
Supplementary Material
Footnotes
This study was supported by the NIH (1R01DK103363), CPRIT (RP120588 and RP140544) and the start-up fund from the University of Texas at Dallas. We thank C. Mohan and Y. Du for discussions.
Supporting information for this article is given via a link at the end of the document.
References
- 1.a Star RA. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]; b Chawla LS, Eggers PW, Star RA, Kimmel PL. N. Engl. J. Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Taylor AT. J. Nucl. Med. 2014;55:608–615. doi: 10.2967/jnumed.113.133447. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Grenier N, Basseau F, Ries M, Tyndal B, Jones R, Moonen C. Abdom. Imaging. 2003;28:164–175. doi: 10.1007/s00261-001-0183-8. [DOI] [PubMed] [Google Scholar]; c Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Am. J. Physiol-Renal. 2001;281:F630–F638. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 3.Penna FJ, Chow JS, Minnillo BJ, Passerotti CC, Barnewolt CE, Treves ST, Fahey FH, Dunning PS, Freilich DA, Retik AB, Nguyen HT. J. Urol. 2011;185:2405–2413. doi: 10.1016/j.juro.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 4.a Hyun H, Park MH, Owens EA, Wada H, Henary M, Handgraaf HJM, Vahrmeijer AL, Frangioni JV, Choi HS. Nat. Med. 2015;21:192–197. doi: 10.1038/nm.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F, Hyun H, Park G, Xie Y, Bae S, Henary M, Frangioni JV. Nat. Biotechnol. 2013;31:148–153. doi: 10.1038/nbt.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Cui L, Lin Q, Jin CS, Jiang W, Huang H, Ding L, Muhanna N, Irish JC, Wang F, Chen J, Zheng G. ACS Nano. 2015;9:4484–4495. doi: 10.1021/acsnano.5b01077. [DOI] [PubMed] [Google Scholar]; d Perrault SD, Chan WCW. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11194–11199. doi: 10.1073/pnas.1001367107. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Xiong LQ, Shuhendler AJ, Rao JH. Nat. Commun. 2012;3:1193. doi: 10.1038/ncomms2197. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Hong G, Diao S, Chang J, Antaris AL, Chen C, Zhang B, Zhao S, Atochin DN, Huang PL, Andreasson KI, Kuo CJ, Dai H. Nature Photon. 2014;8:723–730. doi: 10.1038/nphoton.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gioux S, Choi HS, Frangioni JV. Mol. Imaging. 2010;9:237–255. [PMC free article] [PubMed] [Google Scholar]
- 6.a Tobis S, Knopf JK, Silvers CR, Marshall J, Cardin A, Wood RW, Reeder JE, Erturk E, Madeb R, Yao J, Singer EA, Rashid H, Wu G, Messing E, Golijanin D. Urology. 2012;79:958–964. doi: 10.1016/j.urology.2011.10.016. [DOI] [PubMed] [Google Scholar]; b Tobis S, Knopf J, Silvers C, Yao J, Rashid H, Wu G, Golijanin D. J. Urol. 2011;186:47–52. doi: 10.1016/j.juro.2011.02.2701. [DOI] [PubMed] [Google Scholar]
- 7.a Kumar R, Roy I, Ohulchanskky TY, Vathy LA, Bergey EJ, Sajjad M, Prasad PN. ACS Nano. 2010;4:699–708. doi: 10.1021/nn901146y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhang XL, Tian YL, Zhang HB, Kavishwar A, Lynes M, Brownell AL, Sun HB, Tseng YH, Moore A, Ran CZ. Sci. Rep. 2015;5:13116. doi: 10.1038/srep13116. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sykes EA, Dai Q, Tsoi KM, Hwang DM, Chan WCW. Nat. Commun. 2014;5:3796. doi: 10.1038/ncomms4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a Sheridan RL, Schomaker KT, Lucchina LC, Hurley J, Yin LM, Tompkins RG, Jerath M, Torri A, Greaves KW, Bua DP. J. Burn Care Rehabil. 1995;16:602–604. doi: 10.1097/00004630-199511000-00007. [DOI] [PubMed] [Google Scholar]; b Ra H, González-González E, Uddin MJ, King BL, Lee A, Ali-Khan I, Marnett LJ, Tang JY, Contag CH. Neoplasia. 2015;17:201–207. doi: 10.1016/j.neo.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowe CK, Franco FB, Barbosa JABA, Minnillo BJ, Chow JS, Treves T, Retik AB, Nguyen HT. J. Urol. 2012;188:1978–1985. doi: 10.1016/j.juro.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Hyun H, Owens EA, Wada H, Levitz A, Park G, Park MH, Frangioni JV, Henary M, Choi HS. Angew. Chem. Int. Ed. 2015;54:8648–8652. doi: 10.1002/anie.201502287. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015;127:8772–8776. [Google Scholar]
- 11.a Gao XH, Cui YY, Levenson RM, Chung LWK, Nie SM. Nat. Biotechnol. 2004;22:969–976. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]; b Jin R. Nanoscale. 2010;2:343–362. doi: 10.1039/b9nr00160c. [DOI] [PubMed] [Google Scholar]; c Conroy CV, Jiang J, Zhang C, Ahuja T, Tang ZH, Prickett CA, Yang JJ, Wang GL. Nanoscale. 2014;6:7416–7423. doi: 10.1039/c4nr00827h. [DOI] [PubMed] [Google Scholar]; d Zheng J, Zhou C, Yu M, Liu J. Nanoscale. 2012;4:4073–4083. doi: 10.1039/c2nr31192e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Nat. Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Burns AA, Vider J, Ow H, Herz E, Penate-Medina O, Baumgart M, Larson SM, Wiesner U, Bradbury M. Nano Lett. 2008;9:442–448. doi: 10.1021/nl803405h. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Chen H, Wang GD, Tang W, Todd T, Zhen Z, Tsang C, Hekmatyar K, Cowger T, Hubbard RB, Zhang W, Stickney J, Shen B, Xie J. Adv. Mater. 2014;26:6761–6766. doi: 10.1002/adma.201402964. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Tang S, Chen M, Zheng N. Small. 2014;10:3139–3144. doi: 10.1002/smll.201303631. [DOI] [PubMed] [Google Scholar]; e Yu M, Zheng J. ACS Nano. 2015;9:6655–6674. doi: 10.1021/acsnano.5b01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou C, Long M, Qin Y, Sun X, Zheng J. Angew Chem Int Edit. 2011;50:3168–3172. doi: 10.1002/anie.201007321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou C, Hao GY, Thomas P, Liu JB, Yu MX, Sun SS, Oz OK, Sun XK, Zheng J. Angew Chem Int Edit. 2012;51:10118–10122. doi: 10.1002/anie.201203031. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012;124:10265–10269. [Google Scholar]
- 15.Yu M, Zhou C, Liu J, Hankins JD, Zheng J. J. Am. Chem. Soc. 2011;133:11014–11017. doi: 10.1021/ja201930p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Yu M, Zhou C, Yang S, Ning X, Zheng J. J. Am. Chem. Soc. 2013;135:4978–4981. doi: 10.1021/ja401612x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall JE. Guyton and Hall Textbook of Medical Physiology. 12 ed. Saunders; 2011. p. 304. [Google Scholar]
- 18.Radiological imaging of the kidney. 1 ed. Springer; Berlin Heidelberg: 2011. p. 849. [Google Scholar]
- 19.a Hao G, Du Y, Zhou XJ, Guo J, Sun X, Mohan C, Oez OK. PLoS One. 2013;8:e57418. doi: 10.1371/journal.pone.0057418. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Roberts J, Chen B, Curtis LM, Agarwal A, Sanders PW, Zinn KR. Am. J. Physiol-Renal. 2007;293:F1408–F1412. doi: 10.1152/ajprenal.00083.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tantawy MN, Jiang R, Wang F, Takahashi K, Peterson TE, Zemel D, Hao CM, Fujita H, Harris RC, Quarles CC, Takahashi T. BMC Nephrol. 2012;13:168. doi: 10.1186/1471-2369-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.