Abstract
Scope
UVB exposure, a major factor in the development of skin cancer, has differential sex effects. Tomato product consumption reduces the intensity of UVB-induced erythema in humans, but the mechanisms are unknown.
Methods and results
Four week old SKH-1 hairless mice (40 females, 40 males) were divided into two feeding groups (control or with 10% tangerine tomatoes naturally rich in UV-absorbing phytoene and phytofluene) and two UV exposure groups (with or without UV). After 10 weeks of feeding, the UV group was exposed to a single UV dose and sacrificed 48 hours later. Blood and dorsal skin samples were taken for carotenoid analysis. Dorsal skin was harvested to assess sex and UV effects on carotenoid deposition, inflammation (skinfold thickness, myeloperoxidase levels) and DNA damage (cyclobutane pyrimidine dimers, p53). Females had significantly higher levels of both skin and blood carotenoids relative to males. UV exposure significantly reduced skin carotenoid levels in females but not males. Tomato consumption attenuated acute UV-induced increases in CPD in both sexes, and reduced myeloperoxidase activity and % p53 positive epidermal cells in males.
Conclusion
Tangerine tomatoes mediate acute UV-induced skin damage in SKH-1 mice via reduced DNA damage in both sexes, and through reduced inflammation in males.
Keywords: tomato carotenoids, skin, myeloperoxidase, cyclobutane pyrimidine dimers, acute UV exposure
1. INTRODUCTION
As highlighted by the recent Surgeon General’s Call to Action, skin cancer is the most common form of cancer in humans, with over 3.5 million new cases identified in the United States each year[1, 2]. In fact each year there are more new cases of skin cancer diagnosed than the combined incidence of cancers of the breast, prostate, lung and colon[3]. Ultraviolet light B (UVB; 290–320 nm) is a major environmental carcinogen that has been implicated in the development of both basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), collectively known as keratinocyte cancers [3]. Since most Americans do not undergo regular skin cancer screening, the incidence of skin cancer continues to increase annually. However, there is a distinct sex imbalance in risk. Men are twice as likely to be diagnosed with BCC and 3 times as likely to be diagnosed with SCC as compared to women[4, 5]. While traditionally attributed to lifestyle differences [6, 7], recent data published by our group and others now suggests that this sex disparity in skin cancer incidence may have biological origin[8–10].
The solar ultraviolet (UV) rays which reach the earth’s surface are sub-divided into two categories: higher energy UVB radiation (electromagnetic waves emitted from 280–315 nm) and lower energy UVA radiation (320–400 nm). UVA causes DNA damage[11] and produces singlet oxygen[12] in human skin cells. UVB is considered to be the major cause of sunburn and sun-induced damage to DNA and proteins in skin tissue [13]. Furthermore, UVB exposure has been implicated as a major factor in the development of both BCC and SCC. Surprisingly little is known about sex differences in response to acute UV exposure. In humans, sex-specific differences in skin barrier function have been demonstrated after exposure to varying doses of sunlight[14]. Studies in the SKH-1 mouse model have shown that acute UVB exposure induces higher levels of inflammation and lower levels of DNA damage in females relative to males[10], male SKH-1 mice have a reduced capacity to eliminate reactive oxygen species produced after UVB exposure [15], and a reduction of the immunoprotective response after UVA exposure[16] relative to females. Likewise, natural[17] or artificial[18] reduction in estrogen receptor expression in SKH-1 females results in a reduced immunoprotective response after UVA exposure.
Consumption of dietary compounds that may inhibit acute UV-induced skin damage presents a novel approach to reducing skin cancer risk. Carotenoids are a class of lipophilic dietary compounds which impart the yellow, orange, and red hue associated with many fruits and vegetables and have been shown to deposit in human skin. Carotenoids have been proposed to 1) act as antioxidants in vivo by scavenging free-radicals (reviewed by Cantrell et al., [19]), and 2) be metabolized into nuclear receptor agonists/antagonists to influence gene expression[20–22]. Carotenoids may further confer photoprotection against UV-induced damage via direct absorption of high-energy UV light[23], or by physical quenching of singlet oxygen generated from the donation of energy from a photosensitized species[24, 25].
Previous studies in animals and humans support the effectiveness of the tomato carotenoid lycopene in skin protection from ultraviolet light exposure. Female SKH-1 mice treated topically with lycopene and exposed to a single UVB dose had a step-wise reduction in markers of inflammation with increasing lycopene dose[27]. Furthermore, multiple human studies feeding lycopene alone[26] or in a tomato product[26, 27] for ≥ 10 weeks have found a significant reduction in UV-induced erythema (i.e. redness)[26, 27][27, 28]. However, a more pronounced decrease in UV-induced erythema has been observed for tomato products or tomato extracts as compared to lycopene alone[26]. The authors suggested that the carotenoids phytoene and phytofluene found in tomato products and extracts may be responsible for this greater decrease in erythema formation. Phytoene and phytofluene, most abundant in apricots, tomatoes and tomato products[28], absorb light from 255–315 nm and 285–385 nm, respectively, making them ideal candidates for photoprotection. Although evidence is sparse, the skin protective effect of phytoene is further supported by a previous animal study. Hairless mice injected intraperitoneally with phytoene developed no skin tumors after UV exposure, while 64% of animals injected with vehicle alone developed tumors during 24 weeks of follow-up[29].
Red tomatoes contain all-trans lycopene, which confers the red color. In contrast, tangerine tomatoes are a unique varietal of tomato which contain tetra-cis lycopene, a geometrical isomer of all-trans lycopene, which imparts the “tangerine” hue from whence the name is derived[30]. These varieties contain one of several mutations that disrupt the isomerase which converts cis-lycopene into all-trans lycopene[31]. The cis-isomers are not accessible to conversion into β-carotene, and thus biochemical precursors accumulate. As a result, tangerine tomatoes contain 2 ½ to 3 times more phytoene and phytofluene than red tomatoes and high levels of the carotenoids ζ-carotene, and neurosporene in addition to to tetra-cis lycopene.
The objective of this study was to determine if SKH-1 mice would serve as a viable model to evaluate the effects of dietary tomato consumption on UVB-induced skin damage. We expected tangerine tomato carotenoids to be deposited in the dorsal skin, and we expected there to be a reduction in skin carotenoid levels in animals exposed to UV light. We also hypothesized that mice consuming the phytoene and phytofluene-rich tangerine tomato diet would have a reduction in skin inflammation and DNA damage as compared to control diet animals. Finally, we explored the effect of sex on both carotenoid accumulation and biological markers of skin damage.
2. MATERIALS AND METHODS
Materials
HPLC grade methyl tert-butyl ether (MTBE), dichloromethane, ethanol, and Optima grade hexane, water, and methanol were purchased from Fisher Scientific (Pittsburg, PA, USA). High purity formic acid (≥95%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Ammonium acetate was obtained from J.T. Baker (Phillipsburg, NJ, USA). All-trans-lycopene was purified and crystallized as described previously[32]. Phytoene, phytofluene, ζ-carotene, and neurosporene were isolated from a tangerine tomato extract using a C18 preparatory HPLC method, adapted from an analytical HPLC method by Isaacson et al., [33]. ζ-Carotene and neurosporene required further purification with a C30 preparatory column and a MeOH/MTBE gradient. The isolated carotenoids were individually tested for purity with an analytical C30 HPLC-PDA method which separates all of the carotenoids, and were found to be ≥ 95% pure.
Tomatoes
Tangerine tomato (S. lycopersicum, L.) variety FG04-169 was developed at The Ohio State University to facilitate nutritional and medical trials. FG04-169 has a compact plant habit, determinate vine, and firm “roma” type fruit, a combination of traits that favors high density cultivation, mechanical harvest, and processing into juice and sauce. Plants were grown and harvested at the OSU North Central Agricultural Research Station in Fremont, Ohio following conventional practices[34]. Following harvest, the tomatoes were diced into cubes and frozen. Frozen samples were then lyophilized in a Virtis Ultra 35LE lyophilizer (SP Industries/SP Scientific, Warminster, PA) in the Food Industries Center at the Ohio State University, and powdered in a food processor (Cuisinart, East Windsor, NJ). The powder was stored frozen until it was sent by express delivery to Research Diets, Inc. (New Brunswick, NJ) to be incorporated into an AIN-93G based diet at a 10% level and pelleted. The breakdown of the control diet and the 10% tangerine tomato powder diet is detailed in Table 1. The diets were stored at 4°C throughout the duration of the study. Carotenoids were extracted from the finished 10% tomato diet pellets using the method previously published [35], and analyzed as described below in the HPLC-MS/MS Analysis of Carotenoids
Table 1.
Composition of AIN-93G based diets (in g per kg diet).
Animal Treatment
Animals were housed in a facility approved by the American Association for the Accreditation of Laboratory Animal Care, and the Ohio State University Institutional Animal Care and Use Committee approved all procedures under protocol number 2007A0099. SKH-1 hairless mice (n = 40 females, 40 males) were purchased from Charles River Laboratories (Wilmington, MA) and were received at 4 weeks of age. Animals of the same sex were randomized into different cages, with 5 animals housed per cage. Each cage was assigned to a specific feeding group, and each group was maintained on their respective diet (either control diet or 10% tomato powder diet) for 10 weeks during which time they were allowed to consume both food and water ad libidum. Ten weeks of feeding was chosen as previous studies in humans have suggested that at least 10 weeks of daily carotenoid consumption is required to reduce erythema induced by UVB exposure [26, 36]. Within a sex, 20 animals received the control diet, and 20 animals received the 10% tomato powder diet. Animals were weighed weekly. The feed was changed every 2–3 days to minimize carotenoid degradation.
UV Irradiation
At week 10, a subset of each sex-feeding group (n=15) was irradiated once with Phillips FS40 UVB bulbs (American Ultraviolet Company, Murray Hill, NJ) covered by Kodacel filters (Eastman Kodak, Rochester, NY) to filter out UVC and some very high energy UVB light[37]. UVB intensity was determined by a UVR meter equipped with a UVB sensor (UVP Inc., Upland, CA), and exposure time was calculated to deliver 2240 J/m2 UVB. The remaining 5 animals in each sex-feeding group received no UV exposure.
Sacrifice and Sample Collection
All animals were sacrificed 48 hrs post-a single UV exposure. Animals were sacrificed by lethal inhalation of carbon dioxide. Blood and skin samples for carotenoid analysis, myeloperoxidaise (MPO) assay, cyclobutane pyrimidine dimer (CPD) dot blot analysis, and p53 immunohistochemical analysis were taken immediately afterward as described below.
Skinfold Thickness
Just before sacrifice, i.e. 48 hrs post-UV exposure, skinfold thickness was determined using metric calipers with submillimeter measurement capacity (Bel-Art Products, Pequannock, NJ).
p53, MPO, and CPD assays
Dorsal skin samples for p53 analysis were fixed in 10% neutral buffered formalin for immunohistochemical analysis as previously described [38]. Dorsal skin samples for the myeloperoxidase and cyclobutane pyrimidine dimer assays were snap frozen in liquid nitrogen and analyzed following previously developed methods [10, 39].
Blood Carotenoid Levels
Blood samples were taken via cardiac puncture and placed into a microcentrifuge tube. The tubes were stored on ice, and then centrifuged at 11,000 rcf at 4 °C for 20 minutes. The supernatant was collected and centrifuged for an additional10 minutes, to ensure removal of all red blood cells. The upper serum layer was collected and stored at −80 °C for analysis of carotenoids.
Skin Carotenoid Levels
All remaining dorsal skin not allocated for the assays listed above was snap frozen in liquid nitrogen and stored at −80°C for carotenoid analysis. Carotenoids were extracted from blood serum following the method previously published [40] scaled to a 300 μL sample. Carotenoids from dorsal skin were extracted by first grinding the frozen skin tissue into a powder with liquid nitrogen in a mortar and pestle. Next, 400 mg of tissue was weighed into a glass vial and 1 mL of ethanol containing 0.1% BHT (w/v), 1 mL of water, and 5 mL of 5:1 hexane/dichloromethane (the extraction solvent previously used by Stahl et al., [41]) was added to the vial. The sample was probe sonicated for 30 seconds using a medium intensity setting. Next, the sample was centrifuged at 300 × g for 2 minutes to induce phase separation. The upper layer of solvent was removed with a glass pipette, and placed into a clean glass vial. The extraction procedure with hexane/dichloromethane was repeated, and the extracts were pooled together. Extracts were dried under nitrogen gas, and stored at −80°C until analysis.
HPLC-MS/MS Analysis of Carotenoids
Extracts were reconstituted in 100 μL of 1:1 MTBE/MeOH and a 10μL injection volume was used. Carotenoids in the sample were separated using reverse phase chromatography with a Zorbax C18 column, 4.6 mm × 50 mm, 1.8 μm particle size (Agilent Technologies, Santa Clara, CA, USA) on a 1200 series system HPLC with an HP 1200 series diode array detector (Agilent Technologies, Santa Clara, CA, USA). The column heater was set at 30 °C. Total run time was 8.5 min at a flow rate of 2.33 mL/min. The following gradient was employed to separate the compounds in the sample: 0% B with a linear gradient to 25% B over 3 minutes, holding at 25% B for 2 minute, increasing to 100% B over one minute and holding for 0.5 minutes, then returning to 0% B over 0.1 minutes and holding for 1.9 minutes. Solvent A = 20:80 water/MeOH (v/v) and solvent B = 2:20:78 water/MeOH/MTBE (v/v/v).
Mass spectrometry was necessary for quantitation as the carotenoid level in the skin tissue of some animals was below the photodiode array detection limit. The eluent from the HPLC was interfaced with a QTrap 5500 mass spectrometer (AB Sciex, Foster City, CA, USA) via an atmospheric pressure chemical ionization (APCI) probe operated in positive ion mode. Additional parameters were as follows: curtain gas = 10 psi, source temperature = 550 °C, ion source gas 1 = 60 psi, declustering potential = 100 V, entrance potential = 10 V, collision cell exit potential = 7 V. Parent > daughter ions for each analyte, which were selective for the compounds of interest and for which background interference did not co-elute, were chosen for carotenoid quantitation (Table 2), and a summation of the transitions for each carotenoid was used for quantitation. Molar extinction coefficients were used to determine phytoene, phytofluene, and total lycopene concentration, and external calibration curves for quantitation were generated using MS/MS peak area. Identity of ζ-carotene and neurosporene were confirmed with isolated standards. The MS/MS response of ζ-carotene, neurosporene, and lycopene standard was normalized to the PDA peak area adjusted to their respective λmax. The slope of the lycopene standard curve was then adjusted using the relative response factors calculated for ζ-carotene and neurosporene to quantitate the levels of these two carotenoids in the samples. Data acquisition was carried out using multiple reaction monitoring windows with dwell times of 15 msec for each channel, and 3 msec pauses between channels. Analyst 1.5.1 software (AB Sciex, Foster City, CA, USA) was used for data acquisition and analysis.
Table 2.
Analyte-specific parameters for HPLC-MS/MS analysis of carotenoids
| Peak | Retention time (min) | Compound | HPLC-PDA λmax (nm)a | HPLC-APCI(+)-MS/MS m/z parent ion > m/z daughter ions | Collision energy MS/MSb |
|---|---|---|---|---|---|
| 1 | 3.88 | lycopene | 472 | 537.5 > 455.6, 269.3 | 22, 20 |
| 2 | 3.91 | neurosporene isomer | 440 | 539.5 > 457.6, 415.4, 389.6, 269.3 | 22, 29, 29, 20 |
| 3 | 4.08 | neurosporene isomer | 440 | 539.5 > 457.6, 415.4, 389.6, 269.3 | 22, 29, 29, 20 |
| 4 | 4.29 | neurosporene isomer | 440 | 539.5 > 457.6, 415.4, 389.6, 269.3 | 22, 29, 29, 20 |
| 5 | 4.29 | ζ-carotene | 400 | 541.5 > 457.6, 349.4, 271.3 | 22, 22, 20 |
| 6 | 4.42 | phytofluene isomer | 348 | 543.5 > 393.6, 325.4 | 29, 22 |
| 7 | 4.55 | phytofluene isomer | 348 | 543.5 > 393.6, 325.4 | 29, 22 |
| 8 | 4.56 | phytoene | 286 | 545.5 > 463.6, 421.6, 395.6, 327.4 | 22, 22, 29, 25 |
Value used for relative ratio calculation – see HPLC-MS/MS Analysis of Carotenoids in Methods section
Collision energy used for the respective MS/MS is displayed in the same order as the parent ion/daughter ions (MRM).
Statistical Analysis
Statistical analysis was performed using SAS (SAS Institute, Inc., Cary, NC, USA). Final body weights for each diet were assessed by sex using a 2-sample t-test, and p < 0.05 was considered statistically significant. Blood carotenoid levels, differences in skinfold thickness, and % p53 immunoreactivity were analyzed directly, while skin carotenoid levels, MPO, and CPD levels were log-transformed to meet the assumptions of normality. For differences in % p53 immunoreactivity, only UV-treated animals were considered. For difference in skinfold thickness, MPO, CPD, and skin carotenoid levels, a linear model was fit with sex, feed, and UV exposure as well as considering all interactions of the three. Differences in blood carotenoid levels were performed as just described but excluding UV exposure.
3. RESULTS
Animals
Animals consumed both diets well, and normal weight gain was observed. There was no significant difference between final body weights for female mice consuming the control diet as compared to the tangerine tomato diet (24.0 ± 1.95 vs. 25.1 ± 2.02). In contrast, the average body weight of a male mouse consuming the tangerine tomato diet was 8% heavier than a male mouse consuming the control diet (33.4 ± 2.15 vs. 30.7 ± 2.23, respectively, p = 0.001).
Skin Carotenoid Levels
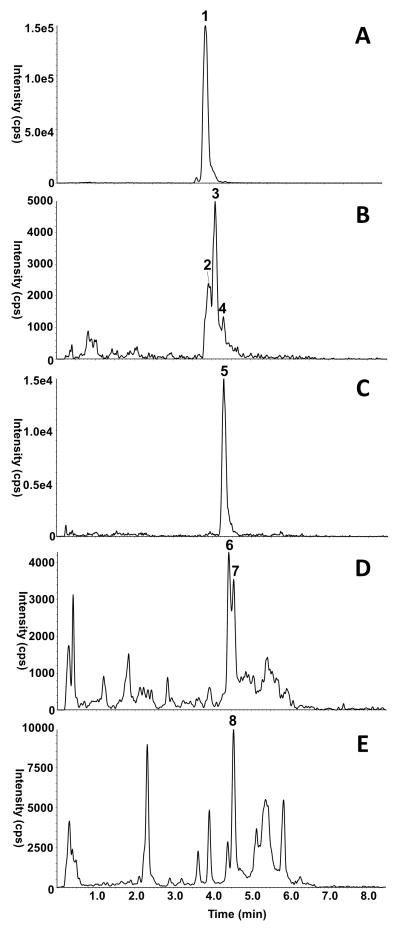
Figure 1 is a representative HPLC-MS/MS chromatogram of carotenoids extracted from dorsal skin. All carotenoids observed in the tangerine tomato diet were also present in the dorsal skin of mice fed the tomato diet. A significant number of geometrical isomers of each carotenoid were observed in the tomato diet, consistent with expectations for the tangerine tomato. Thus, carotenoid profiles of the tomato diet were used to assist in identification of all carotenoids present in the skin samples. Furthermore, dorsal tissue from control animals was used to identify tissue matrix present in the extract which produced the same parent > daughter ion combination(s) as the carotenoid of interest, but which also appeared in some or all of the control animal tissue.
Figure 1.
HPLC-MS/MS Chromatograms of Carotenoids in Mouse Skin. A = lycopene, B = neurosporene, C = ζ-carotene, D = phytofluene, E = phytoene. Each trace represents the sum of the MRM’s listed in Table 2 for each individual carotenoid. Carotenoids are denoted with the corresponding number listed in Table 2, and non-denoted peaks in the traces represent background matrix.
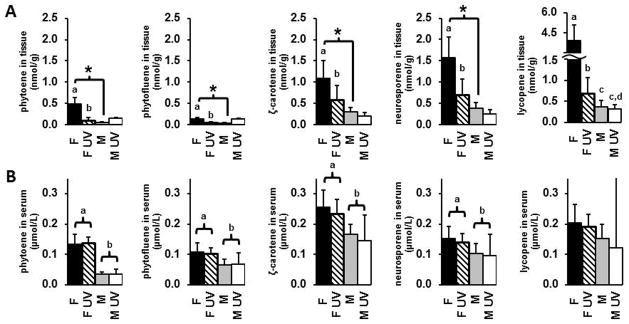
Figure 2A displays the mean dorsal skin level of carotenoids. The amount of carotenoid deposited in the tissue increased from phytofluene < phytoene < ζ-carotene < neurosporene < total lycopene (left to right, Figure 2A). There was a significant sex difference between skin carotenoid deposition in non-irradiated animals. Non UV exposed females had significantly higher levels of phytoene (10.7 fold, p = 0.0001), phytofluene (4.1 fold, p = 0.0156), ζ-carotene (3.6 fold, p = 0.0006), neurosporene (4.3 fold, p<0.001), and total lycopene (10.9 fold, p = 0.0089) as compared to no UV males. UV irradiation resulted in significantly reduced dorsal skin levels of of phytoene (80% decrease, p = 0.0012), phytofluene (68% decrease, p = 0.0166), ζ-carotene (49% decrease, p = 0.0182), neurosporene (60% decrease, p = 0.0013), and total lycopene (84% decrease, p < 0.0001) in females. In contrast, UV exposure did not significantly decrease dorsal skin carotenoid levels in males. The impact of UV exposure on lycopene levels was significantly different for females as compared to males (interaction p=0.0079), but not for the other carotenoids.
Figure 2.
Mean level of carotenoid ± standard deviation in A) dorsal skin tissue and B) serum of animals consuming the tomato-containing diet. Different letters indicate statistically significant differences (p < 0.05). An asterisk indicates a statistically significant difference in dorsal tissue carotenoid levels between sexes without UV irradiation (p < 0.05).
Blood Carotenoid Levels
Blood serum levels of carotenoids in mice consuming the tangerine tomato diet are shown in Figure 2B. Comparing blood levels between the sexes, females had significantly higher levels of phytoene (3.8 fold, p<0.0001), phytofluene (1.5 fold, p = 0.0002), ζ-carotene (1.6 fold, p = 0.0001), and neurosporene (1.4 fold, p = 0.0072) as compared to males. No significant difference was observed for total lycopene. It should be noted that one male UV mouse had blood lycopene levels 10× higher than the other animals in the same group (while blood levels of all other carotenoids were within the range of the other animals), and the data from this animal is likely responsible for not finding any significant differences for lycopene. Indeed, when we performed the same statistical analysis omitting this animal, a significant difference in blood lycopene was observed by sex (p = 0.0027, data not shown). UV exposure did not have a significant impact on blood carotenoid levels. Likewise, no significant sex × UV interaction effects were observed.
Skinfold Thickness
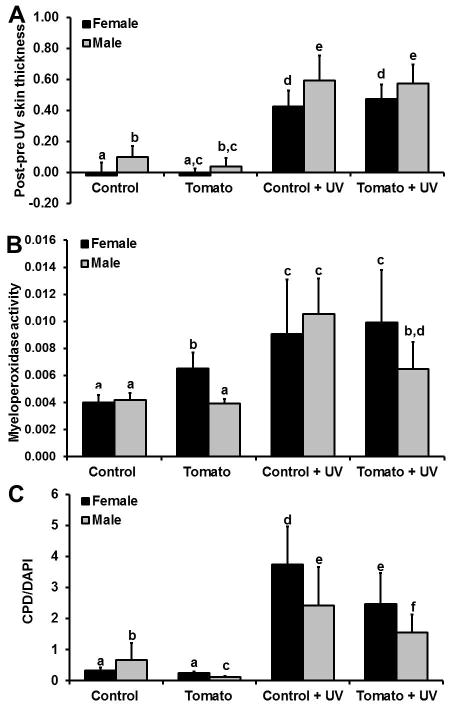
Exposure to UV increased skinfold thickness in all animals regardless of diet or sex (Figure 3A). Dietary tomato consumption did not impact UV-induced changes in skinfold thickness.
Figure 3.
Biomarkers by diet, sex, and UV exposure. A) Skinfold thickness changes before and 48 hours after (control and tomato groups) or 48 hours after UV exposure (control + UV and tomato + UV groups) B) Mean units of myeloperoxidase activity (± standard deviation) and C) ratio of cyclobutanepyrimidine dimer to 4′,6-diamidino-2-phenylindole (DAPI) stain. Different letters denote statistically significant differences (p < 0.05).
MPO Assay
Results of the myeloperoxidase assay are shown in Figure 3B. The tomato diet increased baseline levels of MPO activity by 66% in female skin as compared to the control diet (p = 0.002). No difference in MPO activity was observed in male tomato vs. male control mice. UV irradiation induced a significant increase in MPO activity in control animals compared to no UV in both sexes (p<0.0001 for both). Male tomato + UV mice had a 39% decrease in MPO activity as compared to male control + UV mice (p < 0.0001). In contrast, there was no significant difference in MPO activity in female tomato + UV mice as compared to female control + UV mice. There was a differential effect of sex between the diets (p < 0.0001) and effect of UV between the diets (p = 0.0153), but no differential effect of UV between the sexes. The three-way interaction of diet × sex × UV exposure was not significant.
Dot blot analysis of CPD adducts in epidermal DNA
Results of the CPD dot blot assay are shown in Figure 3C. Control males had a 177% higher base level of CPD as compared to control females (p = 0.0297, without UV exposure). In contrast, males fed the tangerine tomato diet had a 56% lower baseline level of CPD as compared to females fed the tangerine tomato diet (p = 0.0020). UV exposed females fed the tangerine tomato diet had 36% lower CPD levels than UV exposed females fed the control diet (p = 0.004). Likewise, males fed the tangerine tomato diet and exposed to UV had 33% lower CPD levels than males fed the control diet and exposed to UV (p = 0.0075). There was a significant 3-way interaction between sex × diet × UV (p = 0.0011).
Immunohistochemical localization of p53 in the epidermis
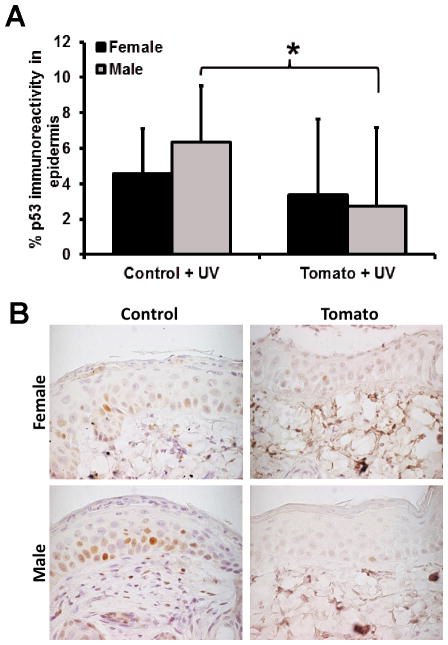
Only UV-treated animals were considered as p53 positive epidermal cells were not visible in most non-treated animals. A statistically significant difference was observed between control diet + UV males as compared to tangerine tomato diet + UV males (p = 0.0122). The decrease in females was not statistically significant, though there was no evidence of a differential effect between sexes (interaction p = 0.27) suggesting the same trend was present in the females despite a lack of statistical significance.
4. DISCUSSION
To the best of our knowledge, we are the first group to demonstrate the deposition of carotenoids in mouse skin. Furthermore, the immunocompetent, hairless SKH-1 model we have chosen is commonly used to study UV-induced skin damage and skin cancer. Based upon the results presented herein, SKH-1 mice appear to be a viable model to study various mechanisms of dietary tomato carotenoid photoprotection.
Lycopene was clearly the most abundant carotenoid in the dorsal tissue of our animals, with levels comparable to those reported previously in humans[42, 43], and with a significantly greater level than that reported in Mongolian gerbils [44]. We hypothesize that this difference between our study and that of the Mongolian gerbils was due to the higher bioavailability of tetra-cis and other-cis isomers of lycopene found in tangerine tomatoes [45, 46] as compared to the all-trans lycopene found in red tomatoes[44]. Likewise, phytoene, phytofluene, and ζcarotene levels were approximately 10-fold higher than levels previously reported in free-living humans[47]. The 10-week feeding of the tangerine tomato, which contains higher levels of these carotenoids than a free-living diet[28], is likely responsible for this effect. These carotenoids have also been detected in Mongolian gerbil skin but were below the PDA limit of quantitation[44]. Skin levels of neurosporene have not previously been reported in rodents or humans.
Females had dramatically higher levels of skin and blood carotenoids as compared to males. This intriguing sex difference further supports a previous observational study where significantly higher levels of total skin carotenoids, as measured by resonance Raman spectroscopy (RSS), were observed in free-living women as compared to men[48]. Similarly, some epidemiological [49, 50] and prospective feeding studies[51, 52][52], have reported higher levels of plasma carotenoids in women as compared to men, although sex differences have not been well investigated. Furthermore, we observed that UV exposure significantly reduced skin carotenoid levels in females. Our results lend evidence to support recent observations in human skin where UV exposure was inversely correlated with skin carotenoid status as measured by RRS[53].
Comparing circulating blood levels of a carotenoid (Figure 2B) to dorsal tissue levels of the same carotenoid (Figure 2A) reveals some surprising observations. Dorsal tissue phytoene levels are approximately 10× lower than dorsal tissue lycopene levels. In contrast, blood phytoene levels are only 1.5× lower than blood lycopene levels. What is perhaps most remarkable is that 3.6× more phytoene was fed than lycopene. Taken together, this data demonstrates that there must be inequality in 1) the uptake of these carotenoids from the diet into the body, 2) the uptake from the circulation system into the dorsal skin tissue, and/or 3) the rate of metabolism and excretion. Overall, our data suggests that the more unsaturated carotenoids are taken up more efficiently and/or excreted less rapidly. Furthermore, relative levels of circulating blood carotenoids may not directly reflect the distribution of carotenoids in skin, and careful consideration should be taken with regards to both UV exposure and gender before extrapolating skin carotenoid values to carotenoid consumption and/or carotenoid status.
Levels of carotenoid in the UV exposed female skin, as well as both non-exposed and UV exposed male skin, were below PDA levels of detection, requiring the use of the MS for quantitation. C30 carotenoid columns are traditionally used to separate and identify various carotenoid isomers[54]. However, dorsal skin tissue matrix suppression was observed in the MS during preliminary testing with tomato and control skin extracts. Thus, a C18 column was necessary for proper quantitation of phytoene and phytofluene in these samples. Carotenoid isomers typically collapse into fewer peaks with a C18 column, so the exact isomer profile is not known, although some partial separation of isomers is observed in our method (as indicated in Table 2). It should also be noted that we did not remove subcutaneous fat from the dorsal skin tissue before pulverizing the samples for carotenoid analysis. Previous reports assessing skin carotenoid levels have not reported removal of this layer before extraction[41, 43, 44, 55], thus, levels measured in our study and others reflect carotenoid in both tissues.
In contrast to previous studies of carotenoids in SKH-1 mice[56, 57], we observed no difference in UV-induced edema (i.e. skinfold thickness) in mice consuming the tangerine tomato diet regardless of sex or feeding group. Besides differences in mode of carotenoid administration, type of carotenoid, dose, and chow diets, these studies assessed skinfold changes at 24h post-UV dose while we measured skinfold thickness at 48h. Thus, it is possible that we would have observed greater changes if we had assessed skinfold thickness earlier.
UVB-induced inflammation also results in the recruitment of neutrophils to the site of damage. Active neutrophils release MPO, and thus MPO activity is assessed as a surrogate marker of neutrophil infiltration. In our study, UV-induced MPO activity was mitigated with tomato consumption in males. Similarly, a dose-wise reduction in UV-induced MPO activity was previously reported in female SKH-1 mice treated with topical lycopene [58].
Surprisingly, female mice fed the tomato diet had increased MPO activity relative to female controls, and no difference in MPO activity was observed between female control diet + UV vs. female tomato diet + UV. Thus, the tomato diet appeared to increase baseline levels of inflammation in females, but did not impact inflammation after UV exposure. It is not clear why this result was observed. Previous in vitro work demonstrated that mixtures of β-carotene oxidation products (COP) decreased the lag time of phorbol myristate acetate-induced neutrophil activation, and increased the velocity of superoxide formation when administered at a 0.1–10 μM COP dose[59]. Thus, we could speculate that the higher levels of skin carotenoids in non-UV treated female mice consuming the tomato diet were sufficient to produce oxidative metabolites which increased baseline MPO activity, but clearly more experiments would need to be done to test this hypothesis. Furthermore, it remains to be determined if increased levels of baseline inflammation cause harm in females, or if they might serve a protective role[10].
Decreases in levels of epidermal p53 (as markers of DNA damage) in both male and female skin were also observed, however the only statistically significant difference was between males consuming the control diet + UV vs. the tomato diet + UV. The greatest difference between % p53 positive cells is usually observed at 24 hours post-UV exposure [38] while we sacrificed our animals at 48 hours to optimally measure other biological outcomes. We speculate that if we had sacrificed the animals at 24 hours, we would have also seen a statistically significant protective effect of the tomato diet in females.
CPDs are the result of direct DNA interaction with UV light[60]. Thus, the decreases in both male and female tomato diet + UV as compared to control diet + UV suggests that the tangerine tomato diet may be providing “sunscreen” like protection against UVB damage. This hypothesis is further supported by the significant decrease in dorsal skin tissue carotenoid in female tomato diet + UV animals as compared to females consuming the tomato diet in the absence of UV exposure, suggesting that the high energy UV is being absorbed by and destroying carotenoids preferentially over DNA. Alternatively, tomato consumption may increase the rate of CPD excision and repair. In conclusion, SKH-1 mice absorb tangerine tomato carotenoids, but demonstrate selective uptake and dorsal skin tissue deposition of the various carotenoid species. Females had significantly higher levels of both skin and blood carotenoids relative to males. Furthermore, exposure to UV significantly reduced skin carotenoid levels in females. Based upon the results presented herein, SKH-1 mice appear to be a viable model to study various mechanisms of dietary tomato carotenoid photoprotection. Our data support the hypothesis that tangerine tomatoes attenuate UV-induced DNA damage in both males and females, and through reduced inflammation in males. Future studies investigating the effects of tangerine tomato consumption on chronic UV exposure will reveal whether attenuation of acute UV response ultimately results in a reduction in SCC incidence and severity in this model.
Figure 4.
Influence of diet and sex on UV-induced epidermal p53 expression. A) Bars represent average % p53 immunoreactivity ± standard deviation. The asterisk represents a statistically significant difference. B) Formalin-fixed, paraffin-embedded tissue sections were immunohistochemically stained for p53 in UV exposed epidermis. Original magnification × 100.
Acknowledgments
Funding: The authors would like to thank Dr. Jessica Cooperstone for her critical review of the manuscript. This research was supported with seed funds from the Center of Advanced Functional Foods Research and Entrepreneurship (CAFFRE) and from The Ohio State University Comprehensive Cancer Center (NIH/NCI P30 016058) Nutrient and Phytochemical Analytic Shared Resource (S.J.S.) and Molecular Carcinogenesis and Chemoprevention program (T.M.O.), and NIH/NCI R21 CA158625.
Abbreviations
- APCI
atmospheric pressure chemical ionization
- BHT
butylated hydroxytoluene
- CPD
cyclobutane pyrimidine dimer
- MeOH
methanol
- MPO
myeloperoxidase
- MTBE
methyl tert-butyl ether
- UVB
ultraviolet B light
Footnotes
Author Contributions: REK, KLT, DMF, SJS, and TMO designed research; DMF produced the tomatoes for the study; REK, JS, KLT, TMO conducted the animal study and tissue analyses; REK, KMR, SJS conducted skin carotenoid analysis; REK and GSY analyzed data; REK wrote paper; TMO had primary responsibility for final content.
The authors have declared no conflict of interest.
References
- 1.U.S. Department of Health and Human Services, Office of the Surgeon General. The surgeon general’s call to action to prevent skin cancer. 2014. [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.Cancer facts & figures 2014. American Cancer Society; n.d. [Google Scholar]
- 4.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B, Biol. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 5.Foote JA, Harris RB, Giuliano AR, Roe DJ, et al. Predictors for cutaneous basal- and squamous-cell carcinoma among actinically damaged adults. Int J Cancer. 2001;95:7–11. doi: 10.1002/1097-0215(20010120)95:1<7::aid-ijc1001>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy EM, Ethridge KP, Wagner RF. Beach holiday sunburn: the sunscreen paradox and gender differences. Cutis. 1999;64:37–42. [PubMed] [Google Scholar]
- 7.Hall HI, May DS, Lew RA, Koh HK, et al. Sun protection behaviors of the U.S. white population. Prev Med. 1997;26:401–407. doi: 10.1006/pmed.1997.0168. [DOI] [PubMed] [Google Scholar]
- 8.Black HS, deGruijl FR, Forbes PD, Cleaver JE, et al. Photocarcinogenesis: an overview. J Photochem Photobiol B, Biol. 1997;40:29–47. doi: 10.1016/s1011-1344(97)00021-3. [DOI] [PubMed] [Google Scholar]
- 9.Scrivener Y, Grosshans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41–47. doi: 10.1046/j.1365-2133.2002.04804.x. [DOI] [PubMed] [Google Scholar]
- 10.Thomas-Ahner JM, Wulff BC, Tober KL, Kusewitt DF, et al. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer Res. 2007;67:3468–3474. doi: 10.1158/0008-5472.CAN-06-3798. [DOI] [PubMed] [Google Scholar]
- 11.Kielbassa C, Roza L, Epe B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis. 1997;18:811–816. doi: 10.1093/carcin/18.4.811. [DOI] [PubMed] [Google Scholar]
- 12.Baier J, Maisch T, Maier M, Landthaler M, et al. Direct detection of singlet oxygen generated by UVA irradiation in human cells and skin. J Invest Dermatol. 2007;127:1498–1506. doi: 10.1038/sj.jid.5700741. [DOI] [PubMed] [Google Scholar]
- 13.De Gruijl FR. Skin cancer and solar UV radiation. Eur J Cancer. 1999;35:2003–2009. doi: 10.1016/s0959-8049(99)00283-x. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Fluhr JW, Song SP, Sun Z, et al. Sun-induced changes in stratum corneum function are gender and dose dependent in a Chinese population. Skin Pharmacol Physiol. 2010;23:313–319. doi: 10.1159/000314138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan NJ, Tober KL, Burns EM, Schick JS, et al. UV light B-mediated inhibition of skin catalase activity promotes Gr-1+ CD11b+ myeloid cell expansion. J Invest Dermatol. 2012;132:695–702. doi: 10.1038/jid.2011.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeve VE, Allanson M, Domanski D, Painter N. Gender differences in UV-induced inflammation and immunosuppression in mice reveal male unresponsiveness to UVA radiation. Photochem Photobiol Sci. 2012;11:173–9. doi: 10.1039/c1pp05224a. [DOI] [PubMed] [Google Scholar]
- 17.Cho JL, Allanson M, Domanski D, Arun SJ, et al. Estrogen receptor-beta signaling protects epidermal cytokine expression and immune function from UVB-induced impairment in mice. Photochem Photobiol Sci. 2008;7:120–5. doi: 10.1039/b709856a. [DOI] [PubMed] [Google Scholar]
- 18.Widyarini S, Domanski D, Painter N, Reeve VE. Estrogen receptor signaling protects against immune suppression by UV radiation exposure. Proc Natl Acad Sci USA. 2006;103:12837–42. doi: 10.1073/pnas.0603642103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantrell A, Truscott TG. Carotenoids and radicals: Interactions with other nutrients. In: Krinsky NI, Mayne ST, Sies H, editors. Carotenoids in Health and Disease. CRC Press; 2004. pp. 31–52. [Google Scholar]
- 20.Linnewiel K, Ernst H, Caris-Veyrat C, Ben-Dor A, et al. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic Biol Med. 2009;47:659–667. doi: 10.1016/j.freeradbiomed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Lian F, Wang XD. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int J Cancer. 2008;123:1262–1268. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, et al. Naturally occurring eccentric cleavage products of provitamin A β-carotene function as antagonists of retinoic acid receptors. J Biol Chem. 2012;287:15886–15895. doi: 10.1074/jbc.M111.325142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stahl W, Sies H. β-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr. 2012;96:1179S–84S. doi: 10.3945/ajcn.112.034819. [DOI] [PubMed] [Google Scholar]
- 24.Foote CS, Denny RW. Chemistry of singlet oxygen. VII. Quenching by β-carotene. J Am Chem Soc. 1968;90:6233–6235. [Google Scholar]
- 25.Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- 26.Aust O, Stahl W, Sies H, Tronnier H, et al. Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Int J Vitam Nutr Res. 2005;75:54–60. doi: 10.1024/0300-9831.75.1.54. [DOI] [PubMed] [Google Scholar]
- 27.Stahl W, Heinrich U, Wiseman S, Eichler O, et al. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. J Nutr. 2001;131:1449–1451. doi: 10.1093/jn/131.5.1449. [DOI] [PubMed] [Google Scholar]
- 28.Biehler E, Alkerwi A, Hoffmann L, Krause E, et al. Contribution of violaxanthin, neoxanthin, phytoene and phytofluene to total carotenoid intake: Assessment in Luxembourg. J Food Comp Anal. 2012;25:56–65. [Google Scholar]
- 29.Mathews-Roth MM. Carotenoid pigment administration and delay in development of UV-B-induced tumors. Photochem Photobiol. 1983;37:509–511. doi: 10.1111/j.1751-1097.1983.tb04509.x. [DOI] [PubMed] [Google Scholar]
- 30.Zechmeister L, LeRosen AL, Went FW, Pauling L. Prolycopene, a naturally occuring stereoisomer of lycopene. Proc Natl Acad Sci. 1941;27:468–474. doi: 10.1073/pnas.27.10.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kachanovsky DE, Filler S, Isaacson T, Hirschberg J. Epistasis in tomato color mutations involves regulation of phytoene synthase 1 expression by cis-carotenoids. Proc Natl Acad Sci US A. 2012;109:19021–19026. doi: 10.1073/pnas.1214808109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopec RE, Riedl KM, Harrison EH, Curley RW, Jr, et al. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem. 2010;58:3290–3296. doi: 10.1021/jf100415z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isaacson T, Ronen G, Zamir D, Hirschberg J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of β-carotene and xanthophylls in plants. Plant Cell. 2002;14:333–342. doi: 10.1105/tpc.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Percheur RJ. Ohio Vegetable Production Guide. Bulletin. 2009;672 [Google Scholar]
- 35.Garzón GA, Narváez-Cuenca CE, Kopec RE, Barry AM, et al. Determination of carotenoids, total phenolic content, and antioxidant activity of Arazá (Eugenia stipitata McVaugh), an Amazonian fruit. J Agric Food Chem. 2012;60:4709–4717. doi: 10.1021/jf205347f. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich U, Tronnier H, Stahl W, Béjot M, et al. Antioxidant supplements improve parameters related to skin structure in humans. Skin Pharmacol Physiol. 2006;19:224–231. doi: 10.1159/000093118. [DOI] [PubMed] [Google Scholar]
- 37.Brown DB, Peritz AE, Mitchell DL, Chiarello S. Uitto, Jouni, Gasparro, Francis P, Common fluorescent sunlamps are an inappropriate substitute for sunlight. Photochem Photobiol. 2000;72:340–344. [PubMed] [Google Scholar]
- 38.Wilgus TA, Koki AT, Zweifel BS, Kusewitt DF, et al. Inhibition of cutaneous ultraviolet light B-mediated inflammation and tumor formation with topical celecoxib treatment. Mol Carcinog. 2003;38:49–58. doi: 10.1002/mc.10141. [DOI] [PubMed] [Google Scholar]
- 39.Nagarajan P, Tober KL, Riggenbach JA, Kusewitt DF, et al. MIF Antagonist (CPSI-1306) Protects against UVB-Induced Squamous Cell Carcinoma. Mol Cancer Res. 2014;12:1292–1302. doi: 10.1158/1541-7786.MCR-14-0255-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barona J, Jones JJ, Kopec RE, Comperatore M, et al. A Mediterranean-style low-glycemic-load diet increases plasma carotenoids and decreases LDL oxidation in women with metabolic syndrome. J Nutr Biochem. 2012;23:609–615. doi: 10.1016/j.jnutbio.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stahl W, Schwarz W, Sundquist AR, Sies H. Cis-trans isomers of lycopene and β-carotene in human serum and tissues. Arch Biochem Biophys. 1992;294:173–177. doi: 10.1016/0003-9861(92)90153-n. [DOI] [PubMed] [Google Scholar]
- 42.Ribaya-Mercado JD, Garmyn M, Gilchrest BA, Russell RM. Skin lycopene is destroyed preferentially over β-carotene during ultraviolet irradiation in humans. J Nutr. 1995;125:1854–1859. doi: 10.1093/jn/125.7.1854. [DOI] [PubMed] [Google Scholar]
- 43.Mayne ST, Cartmel B, Scarmo S, Lin H, et al. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am J Clin Nutr. 2010;92:794–800. doi: 10.3945/ajcn.2010.29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conlon LE, King RD, Moran NE, Erdman JW. Coconut oil enhances tomato carotenoid tissue accumulation compared to safflower oil in the Mongolian gerbil ( Meriones unguiculatus ) J Agric Food Chem. 2012;60:8386–8394. doi: 10.1021/jf301902k. [DOI] [PubMed] [Google Scholar]
- 45.Unlu NZ, Bohn T, Francis D, Clinton SK, et al. Carotenoid absorption in humans consuming tomato sauces obtained from tangerine or high-β-carotene varieties of tomatoes. J Agric Food Chem. 2007;55:1597–1603. doi: 10.1021/jf062337b. [DOI] [PubMed] [Google Scholar]
- 46.Cooperstone JL, Ralston RA, Riedl KM, Haufe TC, et al. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol Nutr Food Res. 2015;59:658–669. doi: 10.1002/mnfr.201400658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hata TR, Scholz TA, Ermakov IV, McClane RW, et al. Non-invasive raman spectroscopic detection of carotenoids in human skin. J Invest Dermatol. 2000;115:441–448. doi: 10.1046/j.1523-1747.2000.00060.x. [DOI] [PubMed] [Google Scholar]
- 48.Meinke MC, Lauer A, Taskoparan B, Gersonde I, et al. Influence on the carotenoid levels of skin arising from age, gender, body mass index in smoking/non-smoking individuals. Free Rad Antiox. 2011;1:15–20. [Google Scholar]
- 49.Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr. 1996;126:129–137. doi: 10.1093/jn/126.1.129. [DOI] [PubMed] [Google Scholar]
- 50.Tucker KL, Chen H, Vogel S, Wilson PWF, et al. Carotenoid intakes, assessed by dietary questionnaire, are associated with plasma carotenoid concentrations in an elderly population. J Nutr. 1999;129:438–445. doi: 10.1093/jn/129.2.438. [DOI] [PubMed] [Google Scholar]
- 51.Nierenberg DW, Stukel TA, Baron JA, Dain BJ, et al. Determinants of increase in plasma concentration of β-carotene after chronic oral supplementation. The Skin Cancer Prevention Study Group. Am J Clin Nutr. 1991;53:1443–1449. doi: 10.1093/ajcn/53.6.1443. [DOI] [PubMed] [Google Scholar]
- 52.Edwards AJ, Vinyard BT, Wiley ER, Brown ED, et al. Consumption of watermelon juice increases plasma concentrations of lycopene and beta-carotene in humans. J Nutr. 2003;133:1043–1050. doi: 10.1093/jn/133.4.1043. [DOI] [PubMed] [Google Scholar]
- 53.Scarmo S, Cartmel B, Lin H, Leffell DJ, et al. Single v. multiple measures of skin carotenoids by resonance Raman spectroscopy as a biomarker of usual carotenoid status. Br J Nutr. 2013;110:911–917. doi: 10.1017/S000711451200582X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emenhiser C, Simunovic N, Sander LC, Schwartz SJ. Separation of geometrical carotenoid isomers in biological extracts using a polymeric C30 column in reversed-phase liquid chromatography. J Agric Food Chem. 1996;44:3887–3893. [Google Scholar]
- 55.Peng YM, Peng YS, Lin Y. A nonsaponification method for the determination of carotenoids, retinoids, and tocopherols in solid human tissues. Cancer Epidemiol Biomarkers Prev. 1993;2:139–144. [PubMed] [Google Scholar]
- 56.Astner S, Wu A, Chen J, Philips N, et al. Dietary lutein/zeaxanthin partially reduces photoaging and photocarcinogenesis in chronically UVB-irradiated SKH-1 hairless mice. Skin Pharmacol Physiol. 2007;20:283–291. doi: 10.1159/000107576. [DOI] [PubMed] [Google Scholar]
- 57.González S, Astner S, An W, Goukassian D, et al. Dietary lutein/zeaxanthin decreases ultraviolet B-induced epidermal hyperproliferation and acute inflammation in hairless mice. J Invest Dermatol. 2003;121:399–405. doi: 10.1046/j.1523-1747.2003.12355.x. [DOI] [PubMed] [Google Scholar]
- 58.Fazekas Z, Gao D, Saladi RN, Lu Y, et al. Protective effects of lycopene against ultraviolet B-induced photodamage. Nutr Cancer. 2003;47:181–187. doi: 10.1207/s15327914nc4702_11. [DOI] [PubMed] [Google Scholar]
- 59.Siems W, Capuozzo E, Crifò C, Sommerburg O, et al. Carotenoid cleavage products modify respiratory burst and induce apoptosis of human neutrophils. Biochem Biophys Res Commun. 2003;1639:27–33. doi: 10.1016/s0925-4439(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 60.Mouret S, Bogdanowicz P, Haure MJ, Castex-Rizzi N, et al. Assessment of the photoprotection properties of sunscreens by chromatographic measurement of DNA damage in skin explants. Photochem Photobiol. 2011;87:109–116. doi: 10.1111/j.1751-1097.2010.00834.x. [DOI] [PubMed] [Google Scholar]
- 61.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: Final report of the American institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]