Abstract
The hippocampal complex is affected early in Alzheimer's disease (AD). Increasingly, altered functional connectivity of the hippocampus is recognized as an important feature of preclinical AD. Carriers of the APOEɛ4 allele are at an increased risk for AD, which could lead to altered hippocampal connectivity even in healthy older adults. To test this hypothesis, we used a paired‐associates memory task to examine differences in task‐dependent functional connectivity of the anterior and posterior hippocampus in nondemented APOEɛ4 carriers (n = 34, 18F) and noncarriers (n = 46, 31F). We examined anterior and posterior portions of the hippocampus separately to test the theory that APOEɛ4‐mediated differences would be more pronounced in the anterior region, which is affected earlier in the AD course. This study is the first to use a psychophysiological interaction approach to query the context‐dependent connectivity of subregions of the hippocampus during a memory task in adults at increased genetic risk for AD. During encoding, APOEɛ4 carriers had lower functional connectivity change compared to baseline between the anterior hippocampus and right precuneus, anterior insula and cingulate cortex. During retrieval, bilateral supramarginal gyrus and right precuneus showed lower functional connectivity change with anterior hippocampus in carriers. Also during retrieval, carriers showed lower connectivity change in the posterior hippocampus with auditory cortex. In each case, APOEɛ4 carriers showed strong negative connectivity changes compared to noncarriers where positive connectivity change was measured. These differences may represent prodromal functional changes mediated in part by APOEɛ4 and are consistent with the anterior‐to‐posterior theory of AD progression in the hippocampus. Hum Brain Mapp 37:366–380, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: aging, APOE, connectivity, fMRI, preclinical Alzheimer's disease, psychophysiological interaction
INTRODUCTION
Alzheimer's disease (AD) is the most common cause of dementia and currently affects more than five million Americans. The illness is unique among polygenic human neurological diseases because there is a single genetic risk factor, APOE, which accounts for a relatively large portion of the variation in heritability, yet is not a causative gene. Specifically, twin studies reveal that the heritability of AD may exceed 60 to 80% [Bergem et al., 1997; Gatz et al., 2006]. APOE was identified as a susceptibility gene for AD over 20 years ago and has been studied extensively since [Corder et al., 1993; Schmechel et al., 1993; Strittmatter et al., 1993]. APOE allele status accounts for about 50% of the variation in heritability estimates [Waring and Rosenberg, 2008]. A single copy of the ɛ4 allele of APOE (APOEɛ4) increases lifetime risk for AD fourfold, and two copies of the allele confer a 10‐fold increase [Bertram and Tanzi, 2012]. Here, we examined the effect of APOEɛ4 on the functional connectivity of the anterior and posterior hippocampus during encoding and retrieval. This design allowed us to interrogate group differences while also testing the theory that APOEɛ4‐mediated differences in an asymptomatic cohort would be more severe in the anterior hippocampus, the region of the structure where AD pathology first occurs [Braak et al., 1993].
One popular method for studying the effects of APOE allele status in humans is task‐based functional magnetic resonance imaging (fMRI). Task‐based fMRI allows investigators to localize significant increases in blood‐oxygen‐level dependent (BOLD) signal associated with particular cognitive processes. Because the APOEɛ4 allele is a strong risk factor for AD, there is particular interest in how the neural substrates of memory function are modulated by APOE. Since 2000 investigators have attempted to characterize the neural signature of the risk conferred by the APOEɛ4 allele, but results have been contradictory (for a review see Trachtenberg et al., 2012). Roughly half of memory task‐based fMRI studies describe significant increases in activity (BOLD signal) in carriers of the APOEɛ4 allele compared to noncarriers, while the other half report the opposite effect. This may be due to the heterogeneity of the tasks used in these studies [Trachtenberg et al., 2012]. In addition, differences in other non‐APOE genetic risk factors (including family history) may affect results, especially in small cohorts [Burggren et al., 2002].
In contrast to task‐based fMRI, resting state fMRI (rs‐fMRI) measures fluctuations in BOLD signal while the subject is at rest, as opposed to performing a specific cognitive task [Damoiseaux et al., 2006]. rs‐fMRI studies have revealed complex differences in functional connectivity mediated by APOE allele status in healthy older adults [Damoiseaux et al., 2012; Heise et al., 2014; Machulda et al., 2011; Sheline et al., 2010]. These network‐based alterations have been suggested as a potential early endophenotype for AD [Sperling, 2011]. This, as well as the inconsistent findings in task‐based fMRI, has led to the idea that functional connectivity alterations capture more of the complex interaction between APOE and brain function than task‐induced activations. As task‐based fMRI analysis methods continue to be improved and refined, we have an opportunity to resolve the conflicts in the APOE‐fMRI literature. One way to tease out the complex relationship between APOEɛ4 allele and memory function is to measure the context‐dependent functional connectivity of an anatomical region (seed) and a specific task phase using a psychophysiological interaction (PPI) model [Friston et al., 1997]. This approach allows investigators to examine functional connectivity in the context of specific cognitive processes. In addition, PPI modeling requires differences between groups to be limited to the connectivity relationships between an a priori seed and regions where activity is mediated or modified by that seed in certain behavioral contexts, such as memory encoding or retrieval. Thus, differences between groups are differences in functional connectivity of the seed during the particular phase of the task that is being modeled. Here, we employ a method of modeling PPIs that has been shown to increase the sensitivity and specificity of findings [McLaren et al., 2012].
Focusing on subregions of the hippocampus during an associative memory task allows us to sensitively interrogate the effect of APOEɛ4 allele on connectivity alterations in functionally distinct regions of the hippocampus during specific task phases. One reason we chose to examine the anterior portion and the posterior portion of the hippocampus separately is because of the known functional and anatomical segregation of the hippocampus along the longitudinal axis [Salami et al., 2012; Schacter and Wagner, 1999; Strange et al., 1999; Strange and Dolan, 1999]. In general, anterior regions of the hippocampal complex, including the entorhinal cortex, are the main input regions and are involved in encoding new memories while posterior regions are output regions involved in memory retrieval and consolidation [Eldridge et al., 2005; Strange et al., 2014; Zeineh et al., 2003]. At the cellular level, the entorhinal cortex is the first area to be affected by AD pathology so we might expect that there would be early functional changes in anterior hippocampus before posterior regions [Braak et al., 1993; Small et al., 2011; Thal et al., 2002]. In fact, structural imaging has revealed that entorhinal cortex is significantly thinner in healthy, older APOEɛ4 carriers than noncarriers [Burggren et al., 2008]. Therefore, we were interested in interrogating the two active phases of the memory task, encoding and retrieval, and the phase‐dependent functional connectivity of the anterior and posterior portions of the hippocampus in order to better understand memory‐induced connectivity of functional subregions of the hippocampus.
This study is the first to examine differences in context‐dependent functional connectivity of subregions of the hippocampus during the performance of a complex memory task in healthy adults. Our participants were non‐demented older adults who generally have a high incidence of family history of AD and a high carriage rate of AD risk variants such as APOEɛ4. This allows us to examine differences in task‐related hippocampal functional connectivity changes between well‐matched groups of APOEɛ4 carriers and noncarriers. We specifically compare the hippocampal connectivity that is related to either encoding or retrieval processes in APOEɛ4 carriers and noncarriers. Recent work at the molecular level has suggested that AD pathology moves in a trans‐synaptic fashion [Harris et al., 2010; Liu et al., 2012]. One of the earliest sites of neurofibrillary tangle deposition is the entorhinal cortex, adjacent to the anterior hippocampus [Braak et al., 1993; Frankó and Joly, 2013]. Thus, our study design was based on a pair of nested hypotheses: first, that carriers in of the APOEɛ4 allele would show decreased context‐dependent functional connectivity of the hippocampus with cortical regions during a memory task and second, that these differences would be more pronounced when interrogating the anterior subregion of the hippocampus. Our findings provide evidence from functional imaging in humans that supports the hypothesis that anterior regions of the hippocampus are more susceptible to differences in function based on APOEɛ4. We believe these findings highlight a susceptibility in APOEɛ4 carriers to AD‐related hippocampal functional changes [Reinvang et al., 2013]. Our focus on genetic risk for AD is motivated by the need to better understand how risk factors like APOEɛ4 affect brain function before the onset of symptoms. The effects of genetic risk for AD on functional endophenotypes for AD may help to define preclinical AD patients who are candidates for preventative therapies.
MATERIALS AND METHODS
Participants
Participants were recruited by the UCLA Longevity Center as part of an ongoing initiative to study aging, AD genetic risk, and dementia. Recruitment efforts included posting flyers in older adult communities and adult day care centers, the local Alzheimer's Association chapter, memory groups, and other groups catering to older adults with age‐related memory concerns. This strategy enabled the recruitment of approximately 40 to 50% of participants carrying at least one copy of the APOEɛ4 allele, as opposed to the 20 to 25% that would be expected from a purely random recruitment [Bookheimer et al., 2000; Small et al., 2000]. In the present study, all participants were healthy and cognitively intact at the time of imaging acquisition. Participants are defined as nondemented in our study if they are cognitively intact based on the results of the Mini Mental State Exam (MMSE; for gross cognition, threshold ≥26) and standard criteria for AAMI (Age Associated Memory Impairment); that is, participants were excluded if they had scores more than two standard deviations below normal on two or more of the memory tests described below. Finally, participants with clinical anxiety, depression or any neuropsychiatric or neurological illness were excluded. This study was performed in compliance with the UCLA Institutional Review Board (IRB) protocols and approved by the UCLA Human Subjects Protection Committee. All participants gave written informed consent in order to enroll in this study.
Neuropsychological Assessment
Participants performed a neuropsychological battery including tests of the following: General Intelligence (Subtests of the WAIS‐III) [Wechsler, 1997], Fluency (Fruits and Vegetables) [Cauthen, 1978], Attention (Digits Forward and Backward) [Wechsler, 1997], Language (Boston Naming Test) [Goodglass and Kaplan, 2001], Verbal Memory (Buschke‐Fuld Selective Reminding Task) [Buschke and Fuld, 1974], WMS‐III Logical Memory and Verbal Paired Associates learning [Wechsler, 1997], and Visual Memory (Rey‐Osterrieth Figure test) [Osterrieth, 1944]. Participants also completed the following: family history questionnaire [Breitner and Folstein, 1984], memory complaints self‐report questionnaire [Gilewski et al., 1990], Hamilton Depression and Anxiety Inventories [Hamilton, 1959, 1960], Neuropsychiatric Inventory [Cummings et al., 1994], and the MMSE [Folstein et al., 1983].
Genotyping
A blood sample was drawn from each participant by a trained phlebotomist at the UCLA Clinical and Translational Research Laboratory. Leukocytes from 10 ml of the sample were frozen and stored at −80°C. Two hundred microgram genomic DNA was isolated from the remaining 10ml and screened using a PCR‐based mutation detection assay and a microsatellite marker based genotyping. APOE SNP (rs429358 and rs7412) genotyping was carried out by real‐time PCR on an Applied Biosystems 7900HT Real Time PCR machine. In addition to a standard curve amplification protocol, an allelic discrimination step was added to facilitate the contrast between the two alleles and their respective reporter dyes. These dyes are incorporated into a Taqman SNP Genotyping Assay with identification numbers C_3084793_20 and C_904973_10 for rs429358 and rs7412, respectively (Applied Biosystems, Foster City, CA). The experiment was performed in duplicate to confirm results. SDS software (version 2.3, Applied Biosystems) was used to analyze the SNP genotyping data. This program calculates the affinity of the sample to one of the two reporter dyes that, in turn, represents one allele over the other. The results of these tests are strictly confidential and are never made available to the research participant.
Imaging Acquisition
MRI scanning was conducted using a Siemens 3T Trio magnet located at the UCLA Center for Cognitive Neuroscience in the Semel Institute. Whole‐brain, structural MRI was collected using a 3D T1‐weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) volumetric scan sequence with axial slicing, TR = 1,900 ms, TE = 2.26 ms, FOV = 250 mm × 218 mm, flip angle = 9°, matrix = 256 × 215, 176 slices, slice thickness = 1 mm, zero‐filled to a matrix of 256 × 224 resulting in a voxel size = 1 × 0.976 × 0.976 mm3. To facilitate registration of functional images, co‐planar, T2‐weighted structural images were also acquired in axial slices with TR = 5,000 ms, TE = 34 ms, FOV = 200 mm × 200 mm, flip angle = 90°, matrix = 128 × 128, 28 slices, slice thickness = 3 mm, interslice gap = 1 mm and voxel size = 1.6 × 1.6 × 4 mm. Whole‐brain, functional MRI scans were acquired using a sequence with the following parameters: interleaved axial slices, TR = 2,500 ms, TE = 21 ms, FOV = 200 mm × 200 mm, flip angle = 75°, matrix = 64 × 64, 33 slices, slice thickness = 3 mm, interslice gap = 0.75 mm, voxel size = 3.125 × 3.125 × 3.75 mm. This acquisition sequence was designed to minimize signal drop‐out caused by susceptibility artifact in the medial temporal lobes, an area of particular interest in older participants and in the analyses described here. The functional imaging data acquired during the course of this study have not been analyzed in other publications. Participants were also scanned using a high‐resolution hippocampal structural sequence that was not analyzed as a part of this study. Some participants' structural imaging data have been used in previous publications [Brown et al., 2011; Burggren et al., 2011; Burggren and Brown, 2013; Donix et al., 2010a,2010b,2013]. Previous work from our group on the effect of the APOEɛ4 allele on brain function using whole‐brain fMRI was completed with a separate, older dataset. The current dataset was collected from Spring 2006 to Fall 2012.
Memory Task
During the functional scan participants completed a paired‐associates memory task that has been previously shown to be sensitive to subtle memory impairment in disease and normal aging and to differentiate across APOEɛ4 carriers and noncarriers [Bookheimer et al., 2000; Persson et al., 2011; Sperling et al., 2002; Suthana et al., 2010]. Participants were presented with seven pairs of unrelated words that had to be learned and then recalled (Fig. 1). The task includes six blocks each of alternating encoding and retrieval phases (30 s each) separated by a baseline condition (20 s). During encoding, seven unrelated word pairs (e.g., clock/green, jazz/beast) were presented sequentially and participants were asked to learn the word pairs. Words were presented as simultaneous auditory and visual stimuli. Following each encoding block participants completed a baseline control task in which they were instructed to fixate on a symbol in the center of the screen (“+” or “o”) and press a button every time the symbol changed [Stark and Squire, 2001]. Next, participants completed a retrieval block in which they saw and heard the first word of each pair and were asked to silently recall the second word of the pair. Because the retrieval phase of the task requires a spontaneous recall response, all participants completed an alternate form of the task outside the scanner where we assessed performance using the WMS‐III Verbal Paired Associates. This generates a valid proxy of in‐scanner performance, which is preferable to using a recognition‐based response that would fundamentally change the nature of the memory task; prior work in our lab has verified the comparability of performance in and outside the scanner using this approach [Bookheimer et al., 2000].
Figure 1.
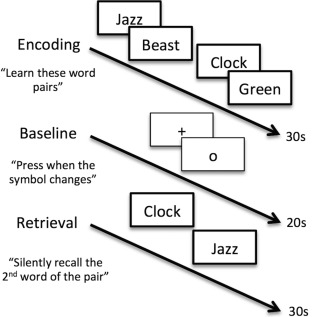
Unrelated words, paired‐associates memory task design. This is a block design task that includes six blocks each of alternating encoding and retrieval phases separated by a baseline condition. During encoding (30‐s block), seven unrelated word pairs (e.g., jazz/beast, clock/green) are presented sequentially using both audio and visual stimuli and participants are asked to learn the word pairs. Next, during the baseline block (20‐s block), participants are instructed to fixate on a symbol in the center of the screen (“+” or “o”) and press a button every time the symbol changes. Finally, during the retrieval phase (30‐s block) participants see and hear the first word of each pair and are asked to silently recall the second word of the pair. s = seconds.
Statistical and Imaging Analyses
Neuropsychological performance
To test whether the APOEɛ4 carrier and noncarrier groups differed in cognitive ability, scores on each neuropsychological test were compared using two‐sample, two‐tailed t‐tests. Fisher's exact tests were used to test for group differences in the categorical variables of sex and family history of AD. These tests were completed using tools from R Project for Statistical Computing (http://www.r-project.org).
Hippocampal seeds
A mask of the left hippocampus in each participant's high resolution structural space was created using FSL's FIRST and a hippocampal model based on 336 subjects as a prior [Patenaude et al., 2011]. We focused our analysis on the left hippocampus because of the preferential engagement of left‐lateralized hippocampal complex areas during verbal memory tasks [Ryan et al., 2008]. Masks were checked manually for accuracy, eroded and binarized. Next, for each participant's unique hippocampal mask, the anterior and posterior thirds of the structure were identified using custom code in MATLAB (version R2012a) (Fig. 2). Specifically, the length of the volumetric hippocampal mask in the anterior‐posterior plane was determined and then used to generate coordinates demarking the anterior and posterior thirds of this plane for each participant. Next, using FSL tools, we generated anterior and posterior hippocampal mask images based on these coordinates. Finally, we transformed the anterior and posterior hippocampal masks into native functional space. Using the anterior and posterior thirds prevented signal blurring across the two hippocampal seeds after registration to functional space while still allowing us to include the majority of the hippocampus in our study. Also, the anterior third of the hippocampus is perfused by a different arterial supply (anterior choroidal) than the posterior two thirds (posterior cerebral) which may affect BOLD signal [Duvernoy, 2005]. We follow the example of previous studies that have also examined the anterior and posterior thirds of the hippocampus for these reasons [Duarte et al., 2014; Greicius et al., 2003a].
Figure 2.
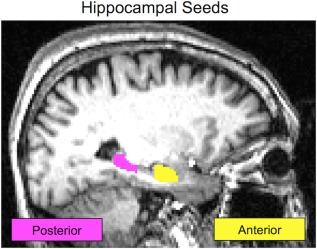
Hippocampal seeds. In native space, a single participant's anterior hippocampus seed is shown in yellow. The posterior hippocampus seed for the same participant is shown in pink. Seeds are defined in each participant's unique structural image and then registered to their functional scan. Seeds are never in a standardized space which improves the accuracy of the hippocampal segmentation.
Structural imaging
Differences in cortical integrity caused by atrophy can confound functional imaging studies in older subjects, especially when one group is at increased risk for a neurodegenerative disease like AD. To ensure that are were no differences in gray matter thickness between APOEɛ4 carriers and noncarriers in this study, whole‐brain structural MRI scans were processed using Freesurfer (version 5.1.0 available at freesurfer.net). This computational neuroanatomy software suite uses tissue contrast to determine the boundary between gray and white matter as well as delineate the pial surface of the brain. A mesh of vertices is plotted across each of these boundaries or surfaces. The software calculates the distance between each pair of vertices to measure cortical thickness. The details of the FreeSurfer pipeline are described in previous publications [Fischl and Dale, 2000]. After completing the FreeSurfer automated pipeline, each participant's scan was visually checked for accuracy. Minimal manual edits were completed when necessary by a single individual (TMH). Vertex‐wise general linear models (GLMs) were used to compare cortical thickness across groups with a statistical threshold set at false discovery rate (FDR) of P < 0.05. We also examined differences thresholded at P < 0.01, uncorrected to check for regions trending toward differences.
Functional imaging
First‐level analysis: Preprocessing and task activation model
Functional imaging preprocessing was completed using FSL (version 6.0: http://fsl.fmrib.ox.ac.uk). Preprocessing included skull‐stripping and head motion correction [Jenkinson et al., 2002; Smith, 2002]. A Gaussian kernel of FWHM 5 mm was applied to the data for spatial smoothing. This kernel size is slightly below the 6 mm kernel that is recommended based on the Nyquiest theorem. However, we chose to use a 5 mm kernel due to concern about over‐smoothing in the hippocampus, which is a structure with a small diameter and very intricate anatomy. Images were high‐pass filtered at sigma = 100 s and prewhitened [Woolrich et al., 2001]. The functional data was registered to co‐planar T2 structural images with 6 degrees of freedom. The co‐planar structural images were then registered to each participant's high‐resolution structural image using boundary‐based registration [Greve and Fischl, 2009]. Finally, each high‐resolution structural scan was registered to the MNI152 standard using 12 parameter affine transformation. A linear transformation was used because this method produced more accurate alignment results than the more common non‐linear approach. Within‐subjects analysis was completed with a GLM including the two active phases of the functional task, six motion parameters as well as a regressor for each motion outlier volume, as determined by frame displacement (FD) calculations and standard outlier identification (75th percentile + 1.5 times the interquartile range [Power et al., 2014]). After these preprocessing steps were completed, the denoised average time series from both hippocampal seeds were extracted for each participant.
Midlevel analysis: gPPI
A generalized psychophysiological interaction (gPPI) analysis strategy was used to interrogate functional coupling of the hippocampus with the rest of the brain during the active phases of the paired‐associates task. Separate gPPI analyses were run for the anterior and posterior hippocampus seeds. A GLM which included regressors for the encoding and retrieval phases of the task, a regressor for the denoised, average timeseries of either the left anterior or posterior hippocampal seed and a PPI regressor for each phase of the task was used to analyze activation in individual participants. These models also included the motion parameters and motion outlier regressors from the first‐level analyses. Standard PPI includes a single PPI regressor in each GLM. However, by more comprehensively modeling the entire task the gPPI method has been shown to more accurately fit the data, leading to improvements in sensitivity and specificity [McLaren et al., 2012].
Second‐level analysis: Group comparisons
To compare the context‐dependent functional connectivity of the two seeds of interest between APOEɛ4 carriers and noncarriers, individual contrast of parameter estimates maps for each of the two PPI regressors in each of the two PPI models were registered from native space to MNI space using the registration parameters from the first‐level analyses. The PPI regressors were seed x encoding and seed x retrieval, the two PPI models were anterior seed and posterior seed, and the registration to MNI space used 2 mm isotropic voxels. Thus, for each participant, four statistical maps were examined: anterior seed × encoding, anterior seed × retrieval, posterior seed × encoding and posterior seed × retrieval. Unpaired t‐tests, with memory performance included as regressor, were run in SPM8 comparing APOEɛ4 carriers to noncarriers.
Significance thresholding for group analyses was carried out using tools available in the AFNI software suite. First, spatial smoothness was estimated on the residuals across the whole cohort. Smoothness estimates were extremely similar for each gPPI model and did not differ based on the seed included. Thus, for simplicity, a single average smoothness estimate (FWHM (x,y,z) = 7.06, 7.11, 6.50) was used in Monte Carlo simulations to estimate cluster extent minimums at uncorrected voxel thresholds. After simulations, 3dClustSim creates a table with cluster extent estimates at different voxel‐wise P values and cluster‐wise alpha values. Thus, rather than testing many voxel and cluster threshold combinations, 3dClustSim minimizes guesswork and allows the investigators' hypotheses about cluster size to guide significance testing. In the present study, results were thresholded to reveal clusters significant at α <0.05 with a voxelwise threshold of P < 0.005. Using this method and these thresholds, the significant cluster size minimum was 108 contiguous voxels. Masks were created from all significant clusters in each analysis in order to extract summary statistics from each participant to illustrate the shape of the effect.
RESULTS
Participants
For this study 93 nondemented adults aged 55 and older were recruited. Of the 93 participants, 9 were excluded because they carried at least one ɛ2 allele (2 ɛ2/ɛ2, 5 ɛ2/ɛ3, and 2 ɛ2/ɛ4). Another four participants were excluded because they were homozygous for the ɛ4 allele. The remaining cohort included 34 APOEɛ4 carriers (all ɛ3/ɛ4) and 46 noncarriers (all ɛ3/ɛ3). Across the two experimental groups, APOEɛ4 carriers and noncarriers, there were no significant differences in age, sex, education, or family history of AD (Table 1). Two‐sample, two‐tailed t‐tests revealed that the groups did not differ in cognitive ability except in two measures of verbal memory: Logical Memory Delay and Verbal Paired Associates Delay. These two measures were highly correlated across the entire sample (r = 0.43, P < 0.0001). To control for the differences between groups in verbal memory, performance on Verbal Paired Associates was included as a regressor in all higher‐level functional analyses. We ran group comparisons without controlling for verbal memory performance in order to determine how performance differences might influence the results (Supporting Information Fig. S4). We also tested for correlations between memory performance and the four PPIs that we examined (Supporting Information Fig. S5).
Table 1.
Cohort characteristics
| Characteristic/test | APOEɛ4 carriers (n = 34) | Noncarriers (n = 46) | P |
|---|---|---|---|
| Age (yr) | 68.1 | 66.7 | 0.470 |
| Sex (M/F) | 16/18 | 15/31 | 0.247 |
| Family history (yes/no) | 26/8 | 30/16 | 0.330 |
| Education (yr) | 17.0 | 17.2 | 0.593 |
| MMSE (0–30) | 28.6 | 28.9 | 0.390 |
| Boston naming (0–60) | 56.1 | 56.0 | 0.973 |
| WMS LM delay total (0–50) | 23.4 | 28.9 | 0.007** |
| WMS VP delay (0–10) | 6.1 | 7.1 | 0.024* |
| Buschke CLTR (0–144) | 58.2 | 60.9 | 0.742 |
| WAIS digit span | 18.4 | 17.6 | 0.399 |
| WAIS digit symbol | 64.1 | 63.0 | 0.780 |
| Fluency: fruits and vegs | 18.4 | 19.6 | 0.294 |
APOEɛ4 carriers and noncarriers do not significantly differ in age, sex, family history of AD, or education. Measures of intelligence and cognition did not differ between groups, except on two verbal memory tests. As a result, verbal memory performance was regressed out of imaging analyses. APOEɛ4 = apolipoprotein E ɛ4 MMSE = Mini Mental State Exam; WMS = Wechsler Memory Scale; LM = Logical Memory; VP = Verbal Paired Associates; CLTR = Consistent Long‐Term Retrieval; WAIS = Wechsler Adult Intelligence Scale.
*P < 0.05;
**P < 0.01.
Hippocampal Seeds Volume
We calculated the volume of both the anterior and posterior hippocampal seeds in each participant. Two‐sample t‐tests revealed that there was no significant difference in seed volume between APOEɛ4 carriers and noncarriers for either the anterior (carriers average [SD] = 1,946.6 mm3 [311.0], noncarriers = 1,949.8 mm3 [302.6], P = 0.96) or posterior hippocampus (carriers average [SD] = 1,446.6 mm3 [244.3], noncarriers = 1,437.3 mm3 [211.1], P = 0.86).
Cortical Thickness
After visual inspection and manual intervention, one participant's FreeSurfer‐processed structural scan did not meet our accuracy standards (female, 65‐year‐old APOEɛ4 noncarrier). This left 79 subjects with usable FreeSurfer data. Cortical thickness did not differ in any region of the cortex between the APOEɛ4 carrier and noncarrier groups at FDR of P < 0.05 or at P < 0.01 uncorrected. Additional models were evaluated that accounted for sex and that examined differences in age‐cortical thickness correlations between APOEɛ4 carriers and noncarriers. There were no significant differences in cortical thickness in any region in these two models at either of the two statistical thresholds that were employed.
Head Motion
Differences in head motion between experimental groups may lead to spurious results [Power et al., 2012]. To ensure that the APOEɛ4 carriers and noncarriers in this study do not differ in head motion estimates, we calculated the average FD for each participant's functional scan. A two‐sample t‐test revealed that there was no significant difference in FD between APOEɛ4 carriers and noncarriers (carriers average [SD] = 0.21 mm [0.09], noncarrier = 0.20 mm [0.10], P = 0.45).
Univariate Task Activation
There were no significant differences between APOEɛ4 carriers and noncarriers in task activation during encoding or retrieval. The within‐group task activation maps show that the occipital lobe, auditory cortex, large regions of parietal lobe, frontal language areas, superior temporal gyrus, and caudate (more pronounced during retrieval) show significant BOLD signal increases during encoding and retrieval in both experimental groups (Supporting Information Fig. S1).
Task‐Dependent Connectivity (PPI): Anterior Seed
Using the anterior left hippocampus as a seed, significant differences between APOEɛ4 carriers and noncarriers were found for both encoding and retrieval phases of the task, such that APOEɛ4 noncarriers had more positive task‐dependent connectivity change than carriers in several cortical regions (Figs. 3 and 4). In contrast, there were no cortical regions in which connectivity change was significantly more positive for APOEɛ4 carriers compared to noncarriers in either task phase. Three clusters in the right hemisphere including the precuneus, the anterior insula and an area of anterior middle cingulate differed significantly between APOEɛ4 carriers and noncarriers for the PPI of the encoding phase with the anterior hippocampus seed (Fig. 3). Each of these clusters was examined as a region of interest (ROI) in order to better characterize group differences. The average parameter estimate from every participant was extracted from each ROI and then plotted by group (Fig. 3). These plots show that the direction of the difference between APOEɛ4 carriers and noncarriers is consistent across clusters. Specifically, APOEɛ4 noncarriers on average have a greater‐than‐baseline relationship between BOLD activity and the PPI, while APOEɛ4 carriers have a lower‐than‐baseline relationship between BOLD activity and the PPI. This means that in APOEɛ4 noncarriers during encoding anterior hippocampus activity predicts higher activity in precuneus, anterior insula, and a region of the cingulate, while in APOEɛ4 carriers anterior hippocampus activity during encoding predicts lower activity in these regions. One sample t‐tests showed that within each group these activity‐PPI relationships are significantly different from zero (Table 2). In other words, in the regions where significant differences between groups were found, the APOEɛ4 noncarriers show significant increases in activity while APOEɛ4 carriers show significant decreases in activity. The within‐group functional connectivity maps show that there are no significant increases in functional connectivity of the hippocampal seeds in either APOEɛ4 carriers or noncarriers (Supporting Information Fig. S2), but there are significant decreases in functional connectivity in APOEɛ4 carriers in each condition and in APOEɛ4 noncarriers only for posterior hippocampus during encoding (Supporting Information Fig. S3). These maps, in contrast to the univariate activation maps which showed no differences, show a divergence between APOEɛ4 carriers and noncarriers in how hippocampal functional connectivity changes during a memory task. This divergence can be measured as a significant difference in the precuneus, anterior insula and the cingulate, as discussed above.
Figure 3.
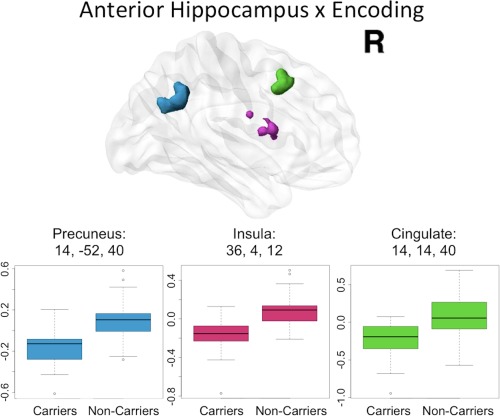
Anterior hippocampal seed connectivity differences in APOEɛ4 carriers and noncarriers during encoding. During encoding, significant differences in anterior hippocampus connectivity between APOEɛ4 carriers and noncarriers were found in right precuneus (blue), right anterior insula (pink) as well as right middle cingulate cortex (green). The peak coordinate for each cluster is reported in Montreal Neurological Institute (MNI) space, in x, y, z planes (mm). For illustration of the direction and magnitude of the difference between groups, contrasts of parameter estimates from each cluster are plotted by group in boxplots. The band within the box represents the median while the upper and lower edges of the box represent the first and third quartiles, respectively. The whiskers extend up to 1.5 times the interquartile range. Data points outside this range are plotted as outliers.
Figure 4.
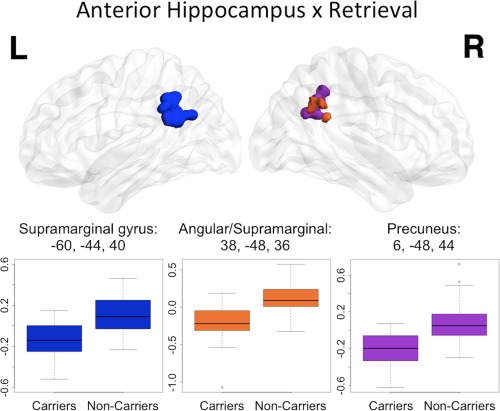
Anterior hippocampal seed connectivity differences in APOEɛ4 carriers and noncarriers during retrieval. During retrieval, significant differences between APOEɛ4 carriers and noncarriers were found in left supramarginal gyrus (dark blue), right supramarginal/angular junction (orange) as well as right precuneus (purple). The peak coordinate for each cluster is reported in MNI space, in x, y, z planes (mm). For illustration of the direction and magnitude of the difference between groups, contrasts of parameter estimates from each cluster are plotted by group. The band within the box represents the median while the upper and lower edges of the box represent the first and third quartiles, respectively. The whiskers extend up to 1.5 times the interquartile range. Data points outside this range are plotted as outliers.
Table 2.
ROI analyses of significant clusters
| PPI | Cluster peak MNI coordinates (mm) | APOEɛ4 carriers | APOEɛ4 noncarriers | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | Average contrast PE | One sample t‐test | Average contrast PE | One sample t‐test | |
| Anterior × encoding | 14 | 14 | 40 | −0.237 | 0.000*** | 0.079 | 0.030* |
| 14 | −52 | 40 | −0.162 | 0.000*** | 0.086 | 0.001** | |
| 36 | 4 | 12 | −0.163 | 0.000*** | 0.080 | 0.001** | |
| Anterior × retrieval | 38 | −48 | 36 | −0.201 | 0.000*** | 0.124 | 0.000*** |
| −60 | −44 | 40 | −0.158 | 0.000*** | 0.128 | 0.000*** | |
| 6 | −48 | 44 | −0.218 | 0.000*** | 0.078 | 0.008** | |
| Posterior × retrieval | −38 | −28 | 10 | −0.220 | 0.000*** | 0.077 | 0.004** |
One sample t‐tests show that for each region where significant differences between groups were observed APOEɛ4 carriers' contrasts of parameter estimates were significantly less than 0 while noncarriers' contrasts of parameter estimates were significantly greater than 0. PPI = psychophysiological interaction; MNI = Montreal Neurological Institute; PE = parameter estimate.
*P < 0.05;
**P < 0.01;
***P < 0.001.
The retrieval phase PPI with anterior hippocampus revealed significant group differences in three clusters located in bilateral supramarginal (with some angular gyrus in the right hemisphere) and right precuneus (Fig. 4). ROI analyses of these clusters showed an effect of APOEɛ4 carrier status similar to the encoding phase PPI with anterior hippocampus. Specifically, in APOEɛ4 noncarriers activity in the anterior hippocampus positively predicts BOLD signal in bilateral supramarginal gyri and right precuneus while in APOEɛ4 carriers the anterior hippocampus shows lower‐than‐baseline functional connectivity to these regions during retrieval. Once again, one sample t‐tests showed that within each group these BOLD signal‐PPI relationships are significantly different from zero indicating that the parameter estimates represent a significant change from baseline in these regions (Table 2).
Although there were no group differences in age, we did test the main effect of age on functional connectivity changes of the anterior hippocampus during encoding and retrieval. There were no regions where an effect of age was significant in either phase. We also tested for correlations between memory performance and task‐related functional connectivity changes and found no significant results (Supporting Information Fig. S5).
Task‐Dependent Connectivity (PPI): Posterior Seed
Using the posterior left hippocampus as a seed, significant group differences were found for only the retrieval phase of the unrelated words task. Similar to the results from the anterior hippocampus seed, differences were found such that APOEɛ4 noncarriers had significantly higher retrieval‐dependent posterior hippocampal connectivity change to cortical areas compared to APOEɛ4 carriers. There were no cortical regions in which connectivity change was significantly more positive for APOEɛ4 carriers compared to noncarriers. The significant cluster, in left auditory cortex (transverse temporal gyri) and superior temporal gyrus, was examined as an ROI (Fig. 5). As with the anterior hippocampus seed, APOEɛ4 noncarriers on average have a higher‐than‐baseline relationship between the PPI of the retrieval phase with the posterior hippocampus and BOLD activity in the ROI. In contrast, APOEɛ4 carriers have a lower‐than‐baseline relationship between the PPI of the retrieval phase with the posterior hippocampus and BOLD activity in the ROI. One sample t‐tests showed that within each group these BOLD signal‐PPI relationships are significantly different from zero (Table 2). Finally, there were no main effects of age or memory performance on functional connectivity changes of the posterior hippocampus during either the encoding or retrieval phase of the memory task.
Figure 5.
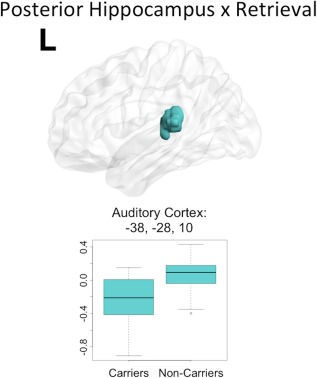
Posterior hippocampal seed connectivity differences in APOEɛ4 carriers and noncarriers during retrieval. During retrieval, significant differences in posterior hippocampus connectivity between APOEɛ4 carriers and noncarriers were found in a single cluster including left auditory cortex and some superior temporal gyrus (teal). The peak coordinate for the cluster is reported in MNI space, in x, y, z planes (mm). For illustration of the direction and magnitude of the difference between groups, contrasts of parameter estimates from each cluster are plotted by group in boxplots. The band within the box represents the median while the upper and lower edges of the box represent the first and third quartiles, respectively. The whiskers extend up to 1.5 times the interquartile range. Data points outside this range are plotted as outliers.
DISCUSSION
This study identified differences in task‐dependent functional connectivity between APOEɛ4 carriers and noncarriers during memory encoding and retrieval. During both encoding and retrieval of word pair associate learning we found significant differences in task‐related functional connectivity of the hippocampus and several cortical regions. Group differences, regardless of task phase or hippocampal seed, were consistent in both direction and magnitude. Specifically, the relationship between the PPI regressor (the interaction of the task phase and activity in the hippocampal seed) and cortical activity was higher‐than‐baseline in APOEɛ4 noncarriers and lower‐than‐baseline in carriers. This consistency across task phase and hippocampal seed indicates that there is a characteristic difference between APOEɛ4 carriers and noncarriers in memory‐related functional connectivity of the hippocampus and cortex. We found evidence of active disengagement in APOEɛ4 carriers of memory and language cortical regions that were positively modulated by the hippocampus in APOEɛ4 noncarriers during the memory task. These regions included right precuneus, right anterior insula, right middle cingulate cortex, and bilateral supramarginal gyri. Our data suggest that a different functional network could be mediating memory performance in APOEɛ4 carriers compared to noncarriers. Furthermore, APOE group differences in task‐dependent functional connectivity change of the anterior hippocampus were present in both encoding and retrieval phases of the task. However, the posterior hippocampus functional connectivity change was only different between groups during the retrieval phase, indicating that the severity of APOEɛ4 carrier effects is greater in the anterior hippocampus.
rs‐fMRI studies suggest that an early endophenotype of AD that is detectable even before the onset of clinical symptoms is dysfunction of the default mode network (DMN) [Fleisher et al., 2009b; Hafkemeijer et al., 2013; Sperling, 2011]. Activity within the DMN is relatively increased when the brain is not engaged in a specific cognitive task. The DMN has been linked to introspective processes and includes the hippocampus as one the nodes in the network [Greicius et al., 2003b]. One of the key functions of the hippocampus is consolidation, which is a process that occurs when the brain is in a “resting state.” This is likely to be one reason why hippocampal activity is correlated with the DMN, as measured with rs‐fMRI. In healthy older APOEɛ4 carriers, decreased DMN connectivity has been described in several studies [Damoiseaux et al., 2012; Heise et al., 2014; Machulda et al., 2011; Sheline et al., 2010]. One theory explaining this DMN dysfunction in APOEɛ4 carriers states that the genetic vulnerability for AD may cause a loss of appropriate hippocampal decoupling from cortical DMN regions during active states, like when completing a task [Westlye et al., 2011]. This theory is supported by a negative correlation between hippocampus‐DMN synchronization and performance on a memory test that has been reported [Westlye et al., 2011]. It has also been shown that greater resting hippocampal connectivity is associated with cognitive decline in normal aging [Salami et al., 2014]. Thus, it may be that impairment in switching hippocampal network engagement from resting functional connectivity state to task‐based functional connectivity state recruiting memory‐relevant regions underlies the apparent disengagement results described in the present study. Dynamic connectivity of hippocampal complex regions and DMN mediated by behavior has also been reported in other studies not specifically interested in APOE [McLaren et al., 2014; Ward et al., 2014].
The strong associations to memory, language and early AD‐related changes of the regions identified as significantly different between groups in this study converge on the potential importance of these regions and the effect of APOEɛ4 on their function. Specifically, we found lower task‐dependent connectivity change among APOEɛ4 carriers between the anterior hippocampus and right precuneus, anterior insula and a region of the cingulate during encoding. The precuneus is part of the DMN and, like other regions of this network, has high metabolic activity at rest [Raichle et al., 2001]. In addition, the precuneus is one of the first cortical regions to be affected by AD, showing decreased glucose metabolism and amyloid deposition in the earliest phases of the disease and in those at increased risk [Buckner et al., 2005; Reiman et al., 1996]. We also found a significant difference between APOEɛ4 carriers and noncarriers in the right precuneus when we examined change in functional connectivity of the anterior hippocampus during retrieval. Given these findings, it may be that APOEɛ4 carriers have a strong negative change in task‐dependent connectivity in this region because of some early AD‐related process or a baseline susceptibility in this region conferred by APOEɛ4. The anterior insula, another region where group differences were identified for the anterior hippocampus and encoding interaction, is a key region of the salience network [Seeley et al., 2007]. The anterior insula and its functional network have been previously associated with episodic memory decline in patients with mild cognitive impairment [Xie et al., 2012]. Similarly, the cingulate has been implicated as a crucial region for normal memory function, especially the posterior portion [Maddock et al., 2001]. Lastly, in addition to right precuneus, during the retrieval phase, we found significant differences in task‐dependent functional connectivity changes of the anterior hippocampus and bilateral parietal language areas, including supramarginal gyrus. These areas are responsible for aspects of language comprehension and repetition [Damasio and Damasio, 1980; Paulesu et al., 1993; Rogalski et al., 2011]. These regions must work in concert with memory systems in order to complete verbal memory tasks, like the paradigm used in this study.
The posterior hippocampus is important for episodic memory retrieval. We found no significant differences in APOEɛ4 carriers and noncarriers when we examined coupling of the posterior hippocampus and whole cortex during encoding. This is not surprising given that encoding processes have been linked primarily in the anterior portions of the structure [Strange et al., 2014]. However, there was a significant difference between groups when we examined change in functional connectivity of the posterior hippocampus during retrieval. Specifically, we found lower connectivity change of posterior hippocampus with left primary auditory cortex in APOEɛ4 carriers. This difference in primary auditory cortex, located along the transverse temporal gyri, may be related to the effort of recalling the second word of a word pair (words are simultaneously presented as both visual and auditory stimuli). We posit that this area may be involved in the active recalling of the spoken word pairs in order to select the appropriate word that paired with the retrieval stimulus. This finding, in contrast to those we reported using the anterior hippocampus seed, is unique as it involves a primary sensory cortical region, as opposed to higher order sensory integration regions. It is also important to note that the difference between groups in this region is not significant when verbal memory performance is not statistically controlled in the model (Supporting Information Fig. S4). Thus, the difference between groups in this region may be related to accuracy and performance, but further studies are needed to formally test this hypothesis in a new cohort. Within our cohort, we found no significant association between memory performance and the PPI of either seed in either encoding or retrieval (Supporting Information Fig. S5).
A possible limitation of this study is the lack of significant within‐group increases in functional connectivity of the hippocampal seeds to cortical regions during encoding and retrieval (Supporting Information Fig. S2). However, we do see significant decreases in functional connectivity of the hippocampal seeds within group, especially for APOEɛ4 carriers (Supporting Information Fig. S3). Certainly, if these significant effects were in the positive direction interpretation of the results would be more straightforward. However, we believe these results show that there is a disconnection phenotype of the hippocampus from cortical regions during active memory function in APOEɛ4 carriers and that this finding is valuable in itself. We argue that this might be part of an overall disruption of normal functional connectivity both in resting networks and in response to task demands.
The participants in this study are older adults and it is likely that some of them have begun the process of hippocampal atrophy and dysfunction that is associated with normal aging (Small et al. 2011). However, because none of the participants exhibited clinical features of cognitive dysfunction, we believe that they are an ideal group in which to examine the effects of the APOEɛ4 allele. Because of our unique recruitment strategy, our APOEɛ4 noncarrier group may be enriched for other genetic risk factors for AD, such as family history of AD, despite their lack of an APOEɛ4 allele. We consider this a strength because our results can be more confidently attributed to APOEɛ4 carrier status because of how closely matched our groups are on other factors, including family history of AD, which is usually higher in APOEɛ4 carriers than noncarriers. It is possible that some of our results may be related to amyloid deposition, especially in the APOEɛ4 carriers, but a large portion of our cohort is young enough (average age = 67.3) that severe amyloid deposition is not a primary concern. In future follow‐up studies of these participants as they age, it will be critically important to acquire amyloid imaging. It is not known whether or not the results described here are evidence of a compensatory strategy in APOEɛ4 carriers that affects BOLD activity, nor is there sufficient information to determine whether the findings are related to baseline perfusion differences [Fleisher et al., 2009a; Wierenga et al., 2010].
The cortical regions where we identified differences between APOEɛ4 carriers and noncarriers are all putatively related to task‐performance, which indicates our approach was strong and our findings are valid. It is also important to note that in this study no masking procedures were used to amplify the power of the PPI to detect differences between groups in specific areas. While a masking approach is sound and supported when there is a strong hypothesis about a specific cortical area, we chose to interrogate the whole brain in order to elucidate robust differences between groups without restriction.
CONCLUSION
There is an increasing emphasis on the development of neuroimaging endophenotypes for AD. The ultimate goal is to use neuroimaging biomarkers to detect preclinical AD on the individual level in order to ensure that preclinical patients receive available interventions or are invited to enroll in treatment trials. One way to identify potential neuroimaging endophenotypes is to examine groups of participants at increased genetic risk for AD. Our findings suggest that there are cortical regions in which APOEɛ4 carriers and noncarriers show consistent differences in task‐based hippocampal connectivity. The consistency of these findings across memory task phases and hippocampal subregion seeds suggests that task‐based hippocampal functional connectivity changes differ between APOEɛ4 carriers and noncarriers at the network level, as opposed to in specific, homogenous functional regions. This may be related to the well‐validated dysfunction of the DMN in preclinical AD, as well as cohorts of healthy APOEɛ4 carriers [Chhatwal et al., 2013; Damoiseaux et al., 2012; Heise et al., 2014; Machulda et al., 2011; Sheline et al., 2010]. The results described here are consistent with neuropathological evidence suggesting that anterior hippocampus is affected earlier in the course of AD pathophysiology and thus may be more susceptible to the earliest preclinical changes. Future studies linking task‐based functional connectivity changes and rs‐fMRI cognitive networks in healthy older APOEɛ4 carriers and noncarriers are necessary to better understand how alterations in network connectivity at “rest” influence functional connectivity alterations during a memory task.
Supporting information
Supporting Information
ACKNOWLEDGMENT
The authors thank Ms. Jacqueline Martinez for her help in participant recruitment, data management, and study coordination.
REFERENCES
- Bergem AL, Engedal K, Kringlen E (1997): The role of heredity in late‐onset Alzheimer disease and vascular dementia. A twin study. Arch Gen Psychiatry 54:264–270. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE (2012): The genetics of Alzheimer's disease. Prog Mol Biol Transl Sci 107:79–100. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak‐Vance MA, Mazziotta JC, Small GW (2000): Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med 343:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J (1993): Staging of Alzheimer‐related cortical destruction. Eur Neurol 33:403–408. [DOI] [PubMed] [Google Scholar]
- Breitner JC, Folstein MF (1984): Familial Alzheimer Dementia: A prevalent disorder with specific clinical features. Psychol Med 14:63–80. [DOI] [PubMed] [Google Scholar]
- Brown JA, Terashima KH, Burggren AC, Ercoli LM, Miller KJ, Small GW, Bookheimer SY (2011): Brain network local interconnectivity loss in aging APOE‐4 allele carriers. Proc Natl Acad Sci USA 108:20760–20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA (2005): Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25:7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren AC, Renner B, Jones M, Donix M, Suthana NA, Martin‐Harris L, Ercoli LM, Miller KJ, Siddarth P, Small GW, Bookheimer SY (2011): Thickness in entorhinal and subicular cortex predicts episodic memory decline in mild cognitive impairment. Int J Alzheimers Dis 2011:956053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY (2008): Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage 41:1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren A, Brown J (2013): Imaging markers of structural and functional brain changes that precede cognitive symptoms in risk for Alzheimer's disease. Brain Imaging Behav. 8:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren AC, Small GW, Sabb FW, Bookheimer SY (2002): Specificity of brain activation patterns in people at genetic risk for Alzheimer disease. Am J Geriatr Psychiatry 10:44–51. [PubMed] [Google Scholar]
- Buschke H, Fuld PA (1974): Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 24:1019–1025. [DOI] [PubMed] [Google Scholar]
- Cauthen NR (1978): Verbal fluency: Normative data. J Clin Psychol 34:126–129. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Schultz AP, Johnson K, Benzinger TLS, Jack C, Ances BM, Sullivan CA, Salloway SP, Ringman JM, Koeppe RA, Marcus DS, Thompson P, Saykin AJ, Correia S, Schofield PR, Rowe CC, Fox NC, Brickman AM, Mayeux R, McDade E, Bateman R, Fagan AM, Goate AM, Xiong C, Buckles VD, Morris JC, Sperling RA (2013): Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology 81:736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak‐Vance MA (1993): Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261:921–923. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J (1994): The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44:2308–2314. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio AR (1980): The anatomical basis of conduction aphasia. Brain 103:337–350. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Rosen HJ, Miller BL, Kramer JH, Greicius MD (2012): Gender modulates the APOE ɛ4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci 32:8254–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M, Burggren AC, Scharf M, Marschner K, Suthana NA, Siddarth P, Krupa AK, Jones M, Martin‐Harris L, Ercoli LM, Miller KJ, Werner A, von Kummer R, Sauer C, Small GW, Holthoff VA, Bookheimer SY (2013): APOE associated hemispheric asymmetry of entorhinal cortical thickness in aging and Alzheimer's disease. Psychiatry Res 214:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M, Burggren AC, Suthana NA, Siddarth P, Ekstrom AD, Krupa AK, Jones M, Martin‐Harris L, Ercoli LM, Miller KJ, Small GW, Bookheimer SY (2010a): Family history of Alzheimer's disease and hippocampal structure in healthy people. Am J Psychiatry 167:1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donix M, Burggren AC, Suthana NA, Siddarth P, Ekstrom AD, Krupa AK, Jones M, Rao A, Martin‐Harris L, Ercoli LM, Miller KJ, Small GW, Bookheimer SY (2010b): Longitudinal changes in medial temporal cortical thickness in normal subjects with the APOE‐4 polymorphism. Neuroimage 53:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte IC, Ferreira C, Marques J, Castelo‐Branco M (2014): Anterior/posterior competitive deactivation/activation dichotomy in the human hippocampus as revealed by a 3D navigation task. PLoS One 9:e86213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernoy HM (2005): The Human Hippocampus. Berlin/Heidelberg: Springer‐Verlag. [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ (2005): A dissociation of encoding and retrieval processes in the human hippocampus. J Neurosci 25:3280–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Podraza KM, Bangen KJ, Taylor C, Sherzai A, Sidhar K, Liu TT, Dale AM, Buxton RB (2009a): Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiol Aging 30:1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Sherzai A, Taylor C, Langbaum JBS, Chen K, Buxton RB (2009b): Resting‐state BOLD networks versus task‐associated functional MRI for distinguishing Alzheimer's disease risk groups. Neuroimage 47:1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Robins LN, Helzer JE (1983): The Mini‐Mental State Examination. Arch Gen Psychiatry 40:812. [DOI] [PubMed] [Google Scholar]
- Frankó E, Joly O (2013): Evaluating Alzheimer's disease progression using rate of regional hippocampal atrophy. PLoS One 8:e71354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL (2006): Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 63:168–174. [DOI] [PubMed] [Google Scholar]
- Gilewski MJ, Zelinski EM, Schaie KW (1990): The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging 5:482–490. [DOI] [PubMed] [Google Scholar]
- Goodglass HP, Kaplan EP (2001): Boston Naming Test, 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Greicius MD, Krasnow B, Boyett‐Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V (2003a): Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus 13:164–174. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003b): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B (2009): Accurate and robust brain image alignment using boundary‐based registration. Neuroimage 48:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkemeijer A, Altmann‐Schneider I, Oleksik AM, van de Wiel L, Middelkoop HAM, van Buchem MA, van der Grond J, Rombouts SARB (2013): Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connect 3:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1959): The assessment of anxiety states by rating. Br J Med Psychol 32:50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Devidze N, Verret L, Ho K, Halabisky B, Thwin MT, Kim D, Hamto P, Lo I, Yu G‐Q, Palop JJ, Masliah E, Mucke L (2010): Transsynaptic progression of amyloid‐β‐induced neuronal dysfunction within the entorhinal‐hippocampal network. Neuron 68:428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise V, Filippini N, Trachtenberg AJ, Suri S, Ebmeier KP, Mackay CE (2014): Apolipoprotein E genotype, gender and age modulate connectivity of the hippocampus in healthy adults. Neuroimage 98:23–30. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, Duff K (2012): Trans‐synaptic spread of tau pathology in vivo. PLoS One 7:e31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda MM, Jones DT, Vemuri P, McDade E, Avula R, Przybelski S, Boeve BF, Knopman DS, Petersen RC, Jack CR (2011): Effect of APOE ɛ4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch Neurol 68:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock R, Garrett A, Buonocore M (2001): Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104:667–676. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012): A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Sperling RA, Atri A (2014): Flexible modulation of network connectivity related to cognition in Alzheimer's disease. Neuroimage 100:544–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth PA (1944): Le test de copie d'une figure complex: Contribution à l'étude de la perception et de la memoir. Arch Psychol (Geneve) 286–356. [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011): A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS (1993): The neural correlates of the verbal component of working memory. Nature 362:342–345. [DOI] [PubMed] [Google Scholar]
- Persson J, Kalpouzos G, Nilsson L‐G, Ryberg M, Nyberg L (2011): Preserved hippocampus activation in normal aging as revealed by fMRI. Hippocampus 21:753–766. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014): Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D (1996): Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 334:752–758. [DOI] [PubMed] [Google Scholar]
- Reinvang I, Espeseth T, Westlye LT (2013): APOE‐related biomarker profiles in non‐pathological aging and early phases of Alzheimer's disease. Neurosci Biobehav Rev 37:1322–1335. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, Mesulam M‐M (2011): Anatomy of language impairments in primary progressive aphasia. J Neurosci 31:3344–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan L, Cox C, Hayes SM, Nadel L (2008): Hippocampal activation during episodic and semantic memory retrieval: Comparing category production and category cued recall. Neuropsychologia 46:2109–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A, Eriksson J, Nyberg L (2012): Opposing effects of aging on large‐scale brain systems for memory encoding and cognitive control. J Neurosci 32:10749–10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A, Pudas S, Nyberg L (2014): Elevated hippocampal resting‐state connectivity underlies deficient neurocognitive function in aging. Proc Natl Acad Sci USA 111:201410233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD (1999): Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 9:7–24. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak‐Vance MA, Goldgaber D, Roses AD (1993): Increased amyloid beta‐peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late‐onset Alzheimer disease. Proc Natl Acad Sci USA 90:9649–9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D'Angelo G, Liu C, Dixit S, Benzinger T, Fagan A, Goate A, Mintun MA (2010): APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J Neurosci 30:17035–17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, Mazziotta JC, Saxena S, Wu HM, Mega MS, Cummings JL, Saunders AM, Pericak‐Vance MA, Roses AD, Barrio JR, Phelps ME (2000): Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA 97:6037–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011): A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R (2011): Potential of functional MRI as a biomarker in early Alzheimer's disease. Neurobiol Aging 32:S37–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Greve D, Dale A, Killiany R, Holmes J, Rosas HD, Cocchiarella A, Firth P, Rosen B, Lake S, Lange N, Routledge C, Albert M (2002): Functional MRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci USA 99:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR (2001): When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci USA 98:12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ (1999): Segregating the functions of human hippocampus. Proc Natl Acad Sci USA 96:4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange B, Dolan R (1999): Functional segregation within the human hippocampus. Mol Psychiatry 4:508–511. [DOI] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI (2014): Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 15:655–669. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak‐Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD (1993): Binding of human apolipoprotein E to synthetic amyloid beta peptide: Isoform‐specific effects and implications for late‐onset Alzheimer disease. Proc Natl Acad Sci USA 90:8098–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana NA, Krupa A, Donix M, Burggren A, Ekstrom AD, Jones M, Ercoli LM, Miller KJ, Siddarth P, Small GW, Bookheimer SY (2010): Reduced hippocampal CA2, CA3, and dentate gyrus activity in asymptomatic people at genetic risk for Alzheimer's disease. Neuroimage 53:1077–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, Braak H (2002): Phases of A beta‐deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800. [DOI] [PubMed] [Google Scholar]
- Trachtenberg AJ, Filippini N, Mackay CE (2012): The effects of APOE‐ɛ4 on the BOLD response. Neurobiol Aging 33:323–334. [DOI] [PubMed] [Google Scholar]
- Ward AM, Schultz AP, Huijbers W, Van Dijk KRA, Hedden T, Sperling RA (2014): The parahippocampal gyrus links the default‐mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp 35:1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring SC, Rosenberg RN (2008): Genome‐wide association studies in Alzheimer disease. Arch Neurol 65:329–334. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997): Wecshler Adult Intelligence Scale, 3rd ed San Antonio, TX: Harcourt Assessement. [Google Scholar]
- Westlye ET, Lundervold A, Rootwelt H, Lundervold AJ, Westlye LT (2011): Increased hippocampal default mode synchronization during rest in middle‐aged and elderly APOE ɛ4 carriers: Relationships with memory performance. J Neurosci 31:7775–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Stricker NH, McCauley A, Simmons A, Jak AJ, Chang Y‐L, Delano‐Wood L, Bangen KJ, Salmon DP, Bondi MW (2010): Increased functional brain response during word retrieval in cognitively intact older adults at genetic risk for Alzheimer's disease. Neuroimage 51:1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM (2001): Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14:1370–1386. [DOI] [PubMed] [Google Scholar]
- Xie C, Bai F, Yu H, Shi Y, Yuan Y, Chen G, Li W, Chen G, Zhang Z, Li S‐J (2012): Abnormal insula functional network is associated with episodic memory decline in amnestic mild cognitive impairment. Neuroimage 63:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY (2003): Dynamics of the hippocampus during encoding and retrieval of face‐name pairs. Science 299:577–580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


