Abstract
The superior fronto‐occipital fasciculus (SFOF), a long association bundle that connects frontal and occipital lobes, is well‐documented in monkeys but is controversial in human brain. Its assumed role is in visual processing and spatial awareness. To date, anatomical and neuroimaging studies on human and animal brains are not in agreement about the existence, course, and terminations of SFOF. To clarify the existence of the SFOF in human brains, we applied deterministic fiber tractography to a template of 488 healthy subjects and to 80 individual subjects from the Human Connectome Project (HCP) and validated the results with white matter microdissection of post‐mortem human brains. The imaging results showed that previous reconstructions of the SFOF were generated by two false continuations, namely between superior thalamic peduncle (STP) and stria terminalis (ST), and ST and posterior thalamic peduncle. The anatomical microdissection confirmed this finding. No other fiber tracts in the previously described location of the SFOF were identified. Hence, our data suggest that the SFOF does not exist in the human brain. Hum Brain Mapp 36:4964–4971, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: diffusion tensor imaging, connectome, white matter, magnetic resonance imaging, microdissection
Abbreviations
- DSI
diffusion spectrum imaging
- DTI
diffusion tensor imaging
- HCP
Human Connectome Project
- HCP‐488
Human Connectome Project 488‐subjects template
- IFOF
inferior fronto‐occipital fasciculus
- MB
Muratoff's bundle
- PTP
posterior thalamic peduncle
- ROI
region of interest
- SFOF
superior fronto‐occipital fasciculus
- SLF
superior longitudinal fasciculus
- ST
stria terminalis
- STP
superior thalamic peduncle
INTRODUCTION
The frontal and occipital lobes are at the opposite sides of the human brain hemispheres. Nonetheless, they have been found to be highly integrated through different anatomical pathways, such as the superior longitudinal fasciculus (SLF), the cingulum, and the fronto‐occipital fasciculi. The fronto‐occipital connection represented by the inferior fronto‐occipital fasciculus (IFOF) is undisputed in humans but not present in monkeys [Forkel et al., 2014]. The superior fronto‐occipital fasciculus (SFOF) has been shown initially in pathological brains affected by acallosal agenesis by Echler in 1878, in dogs by Muratoff in 1893 [Forkel et al., 2014], and finally in monkeys in vivo [Schmahmann and Pandya, 2007; Yakovlev and Locke, 1961; Yeterian and Pandya, 2010]. Dejerine (1895) provided the original description of the location and trajectory of the SFOF based on post‐mortem dissection studies of human healthy brains [Forkel et al., 2014].
The SFOF has been considered a potential anatomical corridor for the diffusion of visual information to the anterior frontal lobes and for the immediate top‐to‐down control of visual processing [Forkel et al., 2014]. Impairment of visual processing in alcohol‐dependent subjects has been correlated with radiological microstructural abnormalities of the SFOF [Bagga et al., 2014]. Furthermore, it has been involved in spatial awareness and visuospatial neglect [Karnath et al., 2009; Thiebaut de Schotten et al., 2011]. Nonetheless, the most recent study of post‐mortem healthy human brains [Ture et al., 1997] has failed to reveal the SFOF, challenging Dejerine's century‐long description, and the in vivo diffusion tensor imaging (DTI) studies of human brains do not agree on its existence, course, and terminations [Catani et al., 2002; Catani and Thiebaut de Schotten, 2012; Forkel et al., 2014; Jellison et al., 2004; Makris et al., 2007; Mori et al., 2002; Wakana et al., 2004].
The Human Connectome Project (HCP) represents the first large‐scale attempt to collect and share data of a scope and detail sufficient to begin the process of addressing deeply fundamental questions about human connectional anatomy and variation [Van Essen et al., 2013]. Thus, in order to solve the controversy of SFOF in humans, here we have applied advanced deterministic fiber tractography to a template of 488 subjects from the HCP and to 80 subjects from the same sample (Q1–Q3 release, WU‐Minn HCP consortium).
METHODS
The Human Connectome Project 488‐Subjects Template
The WU‐Minn HCP consortium is an ongoing project led by Washington University, University of Minnesota and Oxford University, aimed to define in detail a “map” of the human brain connectivity and function. It will allow analyzing and comparing brain circuits, behavioral features, and genetic tracts within the same subject and between subjects. A total of 500 subjects’ data were released for the first three quarters (Q1–Q3, June 2014) and 488 subjects had diffusion scans (289 females, 199 males; average age 29.15, SD ± 3.47). The diffusion data were acquired in a Siemens 3T Skyra scanner using a 2D spin‐echo single‐shot multiband EPI sequence with a multi‐band factor of 3 and monopolar gradient pulse [Sotiropoulos et al., 2013]. The spatial resolution was 1.25 mm isotropic, repetition time (TR) = 5,500 ms, echo time (TE) = 89 ms. A multishell diffusion scheme was used. The b‐values were 1,000, 2,000 and 3,000 s/mm2. The total number of diffusion sampling directions was 270. The total scanning time was around 55 min. The diffusion data were reconstructed using q‐space diffeomorphic reconstruction [Yeh et al., 2010], a method that reconstructs spin distribution functions in a stereotaxic space. The reconstructed data of the 488 subjects were averaged to create a representative template (HCP 488‐subjects template is freely downloadable at: http://dsi-studio.labsolver.org/download-images). Whole brain fiber tracking was conducted using a deterministic fiber tracking algorithm [Yeh et al., 2013]. Because the 488‐subjects template represents an “averaged map” created by the contribution of individual connection maps, it can be argued that the SFOF might be an inconsistent anatomical feature in the general healthy population that could be absent in some subjects. Hence, we performed fiber tracking on a sample of 80 individual connection maps (subject‐based approach) randomly selected from the maps forming the HCP‐488, in order to verify the results of the template‐based approach.
Fiber Tractography
According to its anatomical description by Dejerine (1895), the SFOF is located on the superolateral aspect of the caudate nucleus, between the ependyma of the lateral ventricle and the internal capsule and under the corpus callosum fibers [Schmahmann and Pandya, 2007]. Several other authors have described it as a bundle running parallel and superolateral to the caudate nucleus [Catani et al., 2002; Catani and Thiebaut de Schotten, 2012; Jellison et al., 2004; Makris et al., 2007; Wakana et al., 2004]. Thus, in our study we used the previous information to attempt the reconstruction of the SFOF. The caudate nucleus was easily identified in the T1‐weighted sequence of the template (MNI space) and the region of interest (ROI) was tailored to enclose the caudate nucleus, extending to the white matter immediately beyond its anatomical borders in all three dimensions. This ROI was invariably located under the corpus callosum fibers, lateral to the lateral ventricle and medial to the internal capsule fibers.
We then selected all the fibers with a predominant anteroposterior orientation and analyzed their trajectory and connections. The spatial relationships of the fibers with surrounding structures related to their course (thalamus, caudate nucleus, lateral ventricle, fornix, hippocampus) were examined in a three dimensional MRI environment, as displayed by DSI Studio (freely downloadable at: http://dsi-studio.labsolver.org/). Furthermore, we retrieved three‐dimensional models of the same structures from neuroanatomical atlases integrated with DSI Studio, in order to facilitate the visualization of the gross anatomical relationships of the fibers (Fig. 1).
Figure 1.
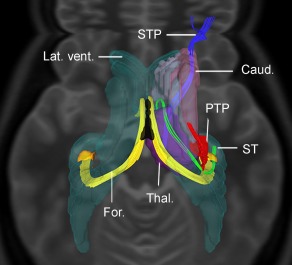
Top‐to‐down view of the fiber tractographic reconstruction of the STP, ST, PTP, and caudate nucleus (Caud.). The ST is a C‐shaped bundle arising from the hypothalamic area, runs in the groove between the inferomedial aspect of caudate nucleus and the superolateral aspect of the thalamus and then, it arches inferiorly and laterally on the posterolateral aspect of the thalamus (Thal.) and finally courses anterolaterally on the roof of the temporal horn of the lateral ventricle (Lat. vent.). The sole other C‐shaped white matter tract originating in the temporal lobe and lining the lateral ventricle is the fornix (For.) which, differently from the ST, reaches the midline to join the contralateral bundle and to form the body of the fornix (in black). The PTP reaches the thalamus without joining the ST. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Fiber Dissection Technique
Ten normal brains (five females) obtained at routine autopsy were fixed in 10% formalin aqueous solution for 4 weeks. Then, the specimens were frozen for 2 weeks at −16°C, according to the Klingler's method [Ludwig and Klingler, 1956]. Progressive dissection of the white matter tracts was performed by peeling off the gray matter and isolating the fiber bundles in their glial sheets. We undertook the fiber dissection studies in the Surgical Neuroanatomy Laboratory at the University of Pittsburgh School of Medicine, with the aid of microsurgical instrumentation and surgical microscope (6–40 magnification; Carl Zeiss, OPMI CS‐NC) as previously reported [Fernandez‐Miranda et al., 2008]. Twenty hemispheres were dissected from both the lateral to the medial side, and vice versa. Briefly, on the lateral side of the hemisphere, we removed the gray matter of the superior, middle, and inferior frontal gyri, precentral, postcentral and supramarginal gyri, superior and inferior parietal lobule. The short associative fibers connecting adjacent gyri, also known as U‐fibers, were exposed and dissected. Then, the superior longitudinal fascicle and the arcuate fascicle were exposed and removed. The insular cortex, the external capsule, and the claustrum were sequentially shown and resected. We exposed the internal capsule, whose fibers converge radially toward the central core of the brain, leaving the putamen and the globus pallidus on its lateral aspect. At this level, medial to the internal capsule and corona radiata, we searched for the SFOF.
The dissection was then performed from the medial to the lateral side of the same hemisphere. We removed the medial part of the genu and anterior half of the body of the corpus callosum. The underlying portion of fornix was stripped off in order to show the superior aspect of the thalamus, the superomedial surface of the head and body of caudate nucleus, and the lateral border of the lateral ventricle (Fig. 2A). The ependyma was removed from the floor and lateral wall of the lateral ventricle to reach the area where the SFOF has been previously located (Fig. 2B).
Figure 2.
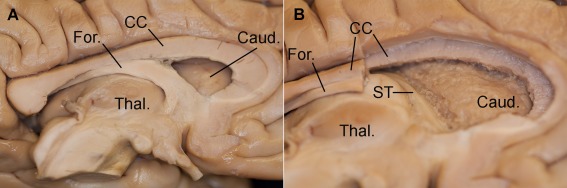
Stepwise dissection of medial surface of the left hemisphere. A: The medial surface of the left hemisphere after sagittal section through the midline. From top‐to‐down, corpus callosum (CC), fornix (For.), and thalamus (Thal.) can be distinguished. Behind the corpus callosum there is the caudate nucleus (Caud.). B: The medial part of the genu and of the anterior half of the body of the corpus callosum (CC) have been removed along with the underlying portion of fornix (For.), in order to expose the superomedial surface of the head and body of caudate nucleus (Caud.) and the superior aspect of the thalamus (Thal.). The ependyma was removed from the floor and lateral wall of the lateral ventricle, exposing the ST. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
RESULTS
Tractography of the SFOF
Our tractographic study (performed both on the template HCP‐488 and on 80 single individual connection maps) could not identify any continuous tract from frontal to parieto‐occipital areas running above the caudate nucleus, under the corpus callosum fibers and medial to the internal capsule. We found essentially three groups of fibers on the anteroposterior axis in the presumed location of the SFOF: first, a bundle connecting the frontal lobe and the thalamus that corresponds to the superior thalamic peduncle (STP) (Figs. 1, 3B, and 4B); second, a C‐shaped fascicle, the stria terminalis (ST) that runs in the groove between the inferomedial aspect of caudate nucleus and the superolateral aspect of the thalamus; third, and most posterior, a fascicle connecting the parieto‐occipital region with the thalamus corresponding to the posterior thalamic peduncle (PTP). Both the STP and PTP fibers appeared intermingled with those of the internal capsule, and none of them showed true continuation with the fibers of the ST.
Figure 3.
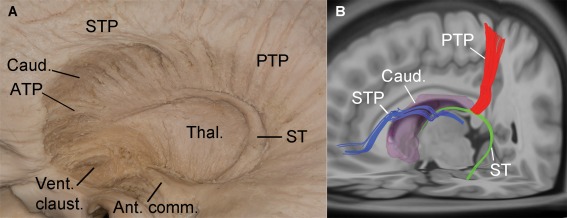
Lateral view of the STP, ST, and PTP of the left hemisphere, depicted by anatomical dissection (A) and fiber tracking reconstruction (B). (A) and (B) show that the STP knees posteriorly on the superolateral aspect of the caudate nucleus (Caud.), and then arches inferiorly on the inferolateral side of the head and body of the caudate nucleus, reaching the thalamus (Thal.). Posteriorly and medially to the STP, the ST runs in the groove between the inferomedial aspect of caudate nucleus and superolateral aspect of the thalamus, then it knees inferiorly on the lateral aspect of the thalamus and finally courses anterolaterally toward the temporal horn of the lateral ventricle. (A) and (B) show no continuation between the fibers of the STP, ST, and PTP (ATP, anterior thalamic peduncle; Vent. claus., ventral claustrum; Ant. comm., anterior commissure). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 4.
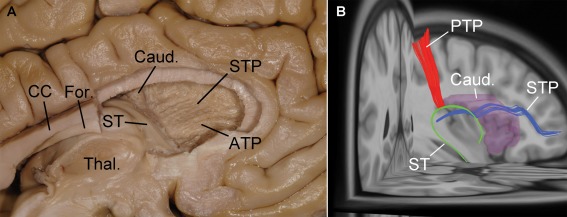
Medial view of the STP, ST, and PTP of the same left hemisphere as in Figure 3, using anatomical dissection (A) and fiber tracking reconstruction (B). A: After the removal of the anterior half of the fornix (For.) and corpus callosum (CC), and of the head and anterior part of the body of the caudate nucleus (Caud.), the STP and the anterior thalamic peduncle (ATP) are evident. There is no continuation between ST and STP. B: The STP, ST, and PTP are seen to be close but without continuation (Thal., thalamus). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Fiber Dissection
Our imaging results were complemented and confirmed by the results of white matter microdissection of ten post‐mortem human brains (Figs. 2, 3A, and 4A). The anatomical microdissection showed that the fibers of the STP diverge from the main radial orientation of internal capsule fibers at the level of the superolateral margin of caudate nucleus, assuming an anteroposterior orientation, in agreement with a previous anatomical study [Ture et al., 1997] (Fig. 3A). The C‐shaped white matter bundle running on the inferomedial aspect of caudate nucleus and superolateral to the thalamus, courses on the roof of the temporal horn of the lateral ventricle and corresponds to the ST. The internal capsule fibers, coming from the frontal, parietal, and occipital lobes, were observed crossing lateral to this structure, rather than continuing with it. In a similar fashion, fibers coming from the parieto‐occipital region reach the thalamus without joining the ST. They form the PTP. Furthermore, the anatomical dissection confirmed the close proximity of the STP and PTP with ST without true continuation (Supporting Information Movie 1). Thus, on coronal sections, the STP fibers grouping together on the superolateral aspect of caudate nucleus may be regarded erroneously as the SFOF according to Dejerine's description.
DISCUSSION
A detailed history of the discoveries and denials of the existence of SFOF, also called the occipitofrontal fascicle, has been recently reviewed by different Authors [Forkel et al., 2014; Schmahmann and Pandya, 2007]. Briefly, in the late 19th century, the SFOF had been erroneously identified as a “pathological” tract in autopsies of human subjects affected by callosal agenesis. In the absence of corpus callosum, the tapetum of the corpus callosum and the lateral appendix of its forceps were interpreted as long association fiber systems. Other studies correctly interpreted this bundle as aberrant fibers of the corpus callosum that failed to cross to the opposite hemisphere. This view was embraced later by Probst who correctly regarded it as misplaced callosal fibers directed in the rostral‐caudal plane and was often referred to as “Probst bundle” [Schmahmann and Pandya, 2007]. Finally, in 1895, Dejerine considered the SFOF as a distinct 2–3 mm wide fiber bundle located medial to the corona radiata and SLF, running on the superolateral aspect of the caudate nucleus, between the ependyma of the lateral ventricle and the internal capsule that corresponds to the modern description of the tract [Nieuwenhuys et al., 1988; Schmahmann and Pandya, 2007].
Unequivocally, a true SFOF was identified in monkeys on serial sections and in vivo isotope tract‐tracing studies and confirmed by diffusion spectrum imaging (DSI) as a distinct tract situated above the caudate nucleus that continues rostrally to the dorso‐lateral prefrontal cortex, between the corona radiata laterally and the corpus callosum medially [Schmahmann et al., 2007; Yakovlev and Locke, 1961; Yeterian and Pandya, 2010]. Conversely, in humans the most recent examination using fiber dissection technique on post‐mortem healthy brains has failed to reveal the SFOF [Ture et al., 1997].
The history of the SFOF is related with another tract, the Muratoff's bundle (MB). It was firstly described in dogs as a “subcallosal bundle,” lying between corpus callosum and corona radiata, but without any detail about its terminations. In monkeys, the existence of a distinct MB was confirmed [Schmahmann and Pandya, 2006; Van Essen et al., 2013; Yakovlev and Locke, 1961; Yeterian and Pandya, 2010] and shown to separate the SFOF from the body and head of the caudate nucleus [Schmahmann et al., 2007]. In humans, Meynert (1887) described the MB as a fronto‐striatal component of the SFOF [Forkel et al., 2014]. Thus, in post‐mortem studies performed on different species, the MB and the SFOF were viewed as a single or two separate entities.
The use of modern imaging methodologies has not yet succeeded to solve the disagreements on SFOF existence, pathway, and terminations. Most of the DTI studies reported direct connections between frontal lobe and parietal regions [Catani et al., 2002; Catani and Thiebaut de Schotten, 2012; Forkel et al., 2014; Jellison et al., 2004; Makris et al., 2007; Mori et al., 2002; Wakana et al., 2004]. Mori et al. [2002] were not able to reveal the SFOF by using a tract‐reconstruction approach, although they found a bundle of substantial size where the SFOF is generally described, but its terminations were not specified.
Conversely, other studies [Catani and Thiebaut de Schotten, 2012; Jellison et al., 2004; Makris et al., 2007; Wakana et al., 2004] supported its existence, though several discrepancies in their descriptions can be found. Thus, the DTI study by Makris et al. [2007] described the SFOF as distinct from the subcallosal fasciculus (MB), and suggested its connection with both parietal lobule and the occipital lobe. Catani et al. [2002], based on DTI, reported that the SFOF appears to connect mainly dorsolateral prefrontal cortex with the superior parietal gyrus in accordance with some earlier representations of the tract [Nieuwenhuys et al., 1988]. Later, Catani and Thiebaut de Schotten [2012] described the SFOF as a bundle originating in the frontal lobe, running above the caudate nucleus, and terminating at three different areas: the thalamus, the head of the caudate nucleus, and the parietal lobe. Based on these terminations, three subcomponents can be identified, namely thalamic fibers, the frontostriatal fibers, and the fronto‐parietal fibers. However, the existence of the latter bundle was questioned. Recently, Catani's group [Forkel et al., 2014] using a more advanced fiber tractography technique (spherical deconvolution) could not confirm the fronto‐occipital connections running in the subcallosal area where SFOF was traditionally located, and showed that the fronto‐occipital connections overlapped with the course of the horizontal branch of the SLF that is lateral to the corona radiata. Thus, although most DTI studies reported some type of fronto‐occipital connections, none of them found SFOF in humans as originally described by Dejerine.
In this study, both our tractography and anatomical data deny the existence of the SFOF. The high‐quality dataset obtained from the HCP and the advanced fiber tractography technique we applied clearly suggest that the SFOF can easily be generated by two false continuations, namely between the STP and the ST, and between the ST and the PTP (Supporting Information Movie 1). The classic description in humans (by Dejerine) and in monkeys [Schmahmann and Pandya, 2006] of an anteroposterior bundle running on the superolateral aspect of the caudate nucleus, medial to internal capsule and ventrolateral to the corpus callosum, may simply correspond to the STP fibers that, diverging from the main radial orientation of internal capsule fibers, assume an anteroposterior orientation on the superolateral aspect of the caudate nucleus, as previously suggested [Ture et al., 1997]. Then, the STP fibers bend inferiorly to reach the thalamus, crossing lateral to the ST fibers. Thus, the brain coronal sections performed by Dejerine revealed some fibers on the superolateral aspect of the caudate nucleus, belonging to the STP, and erroneously attributed to the SFOF.
Remarkably, we analyzed a template of 488 subjects and 80 individual subjects and in none of them were we able to identify the SFOF. This represents the largest study ever done on the SFOF, and our results point out the inability of previous DTI studies to solve the crossing of fibers (the crossing problem) and to identify accurately the origin and termination of fibers (the termination problem), resulting in artifacts and false tracts [Alexander et al., 2001; Le Bihan et al., 2006]. Although the advanced deterministic fiber tractography performed in the present study remarkably improved the results of traditional DTI‐based techniques [Fernandez‐Miranda et al., 2012], still cannot be considered the gold standard for human brain anatomy studies [Thomas et al., 2014]. While the crossing and termination problems are largely improved, the fiber tracking algorithm still cannot differentiate crossing and branching patterns. Consequently, the tractography derived may have false connections or premature terminations. This limitation, however, can be better addressed by post‐mortem white matter fiber dissection technique, which is an excellent method for validation of radiological studies.
Nevertheless, the fiber dissection results are contingent on the individual skills and anatomy knowledge of the investigator. Whether the dissection findings (negative for SFOF) are due to individual difference cannot be completely justified because it is impractical to perform it on a large group of specimens. This limitation can be complemented by diffusion MRI fiber tracking of 488 subjects in our study. A combination of group diffusion MRI fiber tracking and post‐mortem fiber dissection provides complementary strength in validating fiber pathways.
Although our tractographic and anatomic studies are in agreement about the morphology and the relationship between the STP, ST, and PTP, different theories on the existence of SFOF still should be considered. In particular, as previously described by Ture et al. [1997], between the ependyma of the lateral surface of the lateral ventricle and the thalamic peduncles, there is a “layered, sheet‐like mass of substance of nearly uniform thickness,” namely the subcallosal stratum that, as indicated by the name, is not a white matter fascicle. Nonetheless, further radiological and anatomical–hystological studies should investigate this area for the existence of a few anteroposterior fibers intermingled in the subcallosal stratum, whose actual function would remain questionable. Another hypothesis is that a scant contingent of fibers, undetected by our methods, might run along with STP, ST, and PTP, with a very kinky course from frontal to parieto‐occipital areas.
Assuming that the SFOF does not exist, the anatomical basis of highly complex neurological functions previously attributed at least in part to the SFOF (visual processing and spatial awareness), and the consequent deficits related to psychiatric and neurological diseases, such as alcoholism, schizophrenia, and stroke, remain obscure and require further investigations. In particular, further comparative anatomy and functional studies are required to evaluate which fascicles in humans carry the function of SFOF in monkeys. Previous whole‐brain neuroanatomical dissection studies [Fernandez‐Miranda et al., 2008] did not reveal any other longitudinal connection of the parieto‐occipital region with dorsolateral prefrontal cortices in the human brain other than the SLF and IFOF. In fact, the theoretical posterior termination of the SFOF is the upper and medial portion of the parieto‐occipital area, while the subcomponent of the SLF connecting the frontal region with parietal lobe, terminates in the inferior parietal lobule [Wang et al., in press] and not the parieto‐occipital region. The IFOF, however, interconnects the orbito‐frontal and prefrontal region with the occipito‐temporal and parieto‐occipital areas [Fernandez‐Miranda et al., 2008].
From the functional viewpoint, the dominant IFOF might play a role in controlling nonarticulatory linguistic functions such as syntax and grammar, and has been described as the ventral semantic pathway [Duffau et al., 2005]. It is unknown whether the IFOF might also represent a potential corridor for the diffusion of visual information to the anterior frontal lobes and for the immediate top‐to‐down control of visual processing, as it has been suggested for SFOF. On the other hand, the SLF function might include visuospatial processing [Schmahmann and Pandya, 2006] as demonstrated by clinical observation of patients with symptoms of left spatial hemineglect caused by injury in the non‐dominant supramarginal gyrus (rostral inferior parietal lobule) or underlying frontoparietal white matter [Doricchi and Tomaiuolo, 2003]. Thus, we can postulate that SLF in humans may include the function of SFOF in monkeys.
Nonetheless, our anatomo‐radiological study neither allows making any conclusion about the function of the SFOF in humans, nor to perform a comparative study between monkeys and humans.
CONCLUSION
It can be concluded that not all the findings in the non‐primate brain can be extrapolated to human brain.
Using a large sample of high‐quality datasets, we could not find the SFOF. Its presence in humans could represent only a pathological feature of acallosal brains, as initially reported by anatomists in 19th century. This finding is in contrast with that in non‐human primates where the presence of SFOF is well established. Previous imaging studies demonstrating the anatomy of the SFOF were possibly affected by artifacts secondary to technical and study design limitations, although we acknowledge some limitations of our methods, too. Our results could open the way to rethink the anatomical substrate of neurological functions previously attributed at least in part to the SFOF (visual processing and spatial awareness), and of the consequent deficits related to psychiatric and neurological diseases, such as alcoholism, schizophrenia, and stroke.
Supporting information
Supporting Information Movie 1.
ACKNOWLEDGMENTS
Data were provided (in part) by the Human Connectome Project, WU‐Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
REFERENCES
- Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL (2001): Analysis of partial volume effects in diffusion‐tensor MRI. Magn Reson Med 45:770–780. [DOI] [PubMed] [Google Scholar]
- Bagga D, Sharma A, Kumari A, Kaur P, Bhattacharya D, Garg ML, Khushu S, Singh N (2014): Decreased white matter integrity in fronto‐occipital fasciculus bundles: Relation to visual information processing in alcohol‐dependent subjects. Alcohol 48:43–53. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M (2012): Atlas of Human Brain Connections. Oxford, New York: Oxford University Press; xii, 519 p. [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK (2002): Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage 17:77–94. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Tomaiuolo F (2003): The anatomy of neglect without hemianopia: A key role for parietal‐frontal disconnection? Neuroreport 14:2239–2243. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio‐Mazoyer N, Capelle L (2005): New insights into the anatomo‐functional connectivity of the semantic system: A study using cortico‐subcortical electrostimulations. Brain 128:797–810. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Miranda JC, Rhoton AL Jr, Alvarez‐Linera J, Kakizawa Y, Choi C, de Oliveira EP (2008): Three‐dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery 62:989–1026. discussion 1026–1028. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Miranda JC, Pathak S, Engh J, Jarbo K, Verstynen T, Yeh FC, Wang Y, Mintz A, Boada F, Schneider W, Friedlander R (2012): High‐definition fiber tractography of the human brain: Neuroanatomical validation and neurosurgical applications. Neurosurgery 71:430–453. [DOI] [PubMed] [Google Scholar]
- Forkel SJ, Thiebaut de Schotten M, Kawadler JM, Dell'Acqua F, Danek A, Catani M (2014): The anatomy of fronto‐occipital connections from early blunt dissections to contemporary tractography. Cortex 56:73–84. [DOI] [PubMed] [Google Scholar]
- Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL (2004): Diffusion tensor imaging of cerebral white matter: A pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol 25:356–369. [PMC free article] [PubMed] [Google Scholar]
- Karnath HO, Rorden C, Ticini LF (2009): Damage to white matter fiber tracts in acute spatial neglect. Cereb Cortex 19:2331–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Poupon C, Amadon A, Lethimonnier F (2006): Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging 24:478–488. [DOI] [PubMed] [Google Scholar]
- Ludwig, E , Klingler, J (1956): Atlas cerebri humani. Der innere Bau des Gehirns dargestellt auf Grund makroskopischer Präparate. The Inner Structure of the Brain Demonstrated on the Basis of Macroscopical Preparations. Boston: Little Brown; 36 p. [Google Scholar]
- Makris N, Papadimitriou GM, Sorg S, Kennedy DN, Caviness VS, Pandya DN (2007): The occipitofrontal fascicle in humans: A quantitative, in vivo, DT‐MRI study. NeuroImage 37:1100–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, Pearlson GD, Melhem ER, Solaiyappan M, Raymond GV, Moser HW, van Zijl PC (2002): Imaging cortical association tracts in the human brain using diffusion‐tensor‐based axonal tracking. Magn Reson Med 47:215–223. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, Huijzen CV (1988): The Human Central Nervous System: A Synopsis and Atlas. Berlin; New York: Springer‐Verlag; xii, 437 p. [Google Scholar]
- Schmahmann JD, Pandya DN (2006): Fiber Pathways of the Brain. Oxford, New York: Oxford University Press; xviii, 654 p.. [Google Scholar]
- Schmahmann JD, Pandya DN (2007): The complex history of the fronto‐occipital fasciculus. J Hist Neurosci 16:362–377. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ (2007): Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain 130:630–653. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, Glasser MF, Hernandez M, Sapiro G, Jenkinson M, Feinberg DA, Yacoub E, Lenglet C, Van Essen DC, Ugurbil K, Behrens TE, Consortium WUMH (2013): Advances in diffusion MRI acquisition and processing in the Human Connectome Project. NeuroImage 80:125–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, Catani M (2011): A lateralized brain network for visuospatial attention. Nat Neurosci 14:1245–1246. [DOI] [PubMed] [Google Scholar]
- Thomas C, Ye FQ, Irfanoglu MO, Modi P, Saleem KS, Leopold DA, Pierpaoli C (2014): Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci USA 111:16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ture U, Yasargil MG, Pait TG (1997): Is there a superior occipitofrontal fasciculus? A microsurgical anatomic study. Neurosurgery 40:1226–1232. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K, Consortium WUMH (2013): The WU‐Minn Human Connectome Project: An overview. NeuroImage 80:62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PC, Mori S (2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230:77–87. [DOI] [PubMed] [Google Scholar]
- Wang X, Pathak S, Stefaneanu L, Yeh FC, Li S, Fernandez‐Miranda JC (2015): Subcomponents and connectivity of the superior longitudinal fasciculus in the human brain. Brain Struct Funct 220:1665–1680. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Locke S (1961): Limbic nuclei of thalamus and connections of limbic cortex. III. Corticocortical connections of the anterior cingulate gyrus, the cingulum, and the subcallosal bundle in monkey. Arch Neurol 5:364–400. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Wedeen VJ, Tseng WY (2010): Generalized q‐sampling imaging. IEEE Trans Med Imaging 29:1626–1635. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Verstynen TD, Wang Y, Fernandez‐Miranda JC, Tseng WY (2013): Deterministic diffusion fiber tracking improved by quantitative anisotropy. PloS One 8:e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN (2010): Fiber pathways and cortical connections of preoccipital areas in rhesus monkeys. J Comp Neurol 518:3725–3751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Movie 1.


