Abstract
Amide proton transfer (APT) imaging may potentially detect mobile proteins/peptides non-invasively in vivo, but its specificity may be reduced by contamination from other confounding effects such as asymmetry of non-specific magnetization transfer (MT) effects and spin-lattice relaxation with rate R1 (=1/T1). Previously reported spillover, MT and R1 correction methods were based on a two-pool model, in which the existence of multiple water compartments with heterogeneous relaxation properties in real tissues was ignored. Such simple models may not adequately represent real tissues and thus such corrections may be unreliable. The current study investigated the effectiveness and accuracy of correcting for R1 in APT imaging via simulations and in vivo experiments using tumor-bearing rats subjected to serial injections of Gd-DTPA that produced different tissue R1 values in regions of blood-brain-barrier breakdown. The results suggest that conventional measurements of APT contrast (such as APT* and MTRasym) may be significantly contaminated by R1 variations, while the R1-corrected metric AREX* was found to be relatively unaffected by R1 changes over a broad range (0.4 – 1 Hz). Our results confirm the importance of correcting for spin-lattice relaxation effects in quantitative APT imaging, and demonstrate the reliability of using the observed tissue R1 for corrections to obtain more specific and accurate measurements of APT contrast in vivo. The results also indicate that, due to relatively fast transcytolemmal water exchange, the influence of intra- and extracellular water compartments on CEST measurements with seconds long saturation time may be ignored in tumors.
Keywords: R1, APT, CEST, AREX, Gd-DTPA, tumor, transcytolemmal water exchange
Graphical Abstract
The influence of multiple water compartments and heterogeneous relaxation on APT imaging in real tissues was investigated with simulations and in vivo experiments with serial Gd-DTPA injections. The results confirm that the spin-lattice relaxation rate R1 significantly confounds conventional APT measures; and suggest that the R1-corrected AREX metrics based on the 1/Z method is an appropriate means to remove the influences of spin-lattice relaxation on APT measurements.
Introduction
Chemical exchange saturation transfer (CEST) imaging can measure the concentrations of relatively small solutes indirectly by detecting the attenuation of water signals induced by chemical exchange (1,2). Compared with direct MR measurements (e.g. using high resolution magnetic resonance spectroscopy) of pools of solute protons at low concentrations (typically millimolar or lower) in biological tissues, the detection of changes in the background water signal caused by saturation transfer significantly enhances the sensitivity (by up to 500,000 (3)) for detecting low levels of exchanging compounds. Thus CEST provides an attractive means to image distributions of molecules such as peptides and metabolites with potentially higher signal-to-noise ratios and higher spatial resolutions. During CEST experiments, saturated water signals (Msat(Δω)) are usually acquired over a range of irradiation offset frequencies (Δω) around the water resonance and normalized by the corresponding unsaturated water signal M0. The Z-spectrum (Z(Δω) = Msat (Δω)/M0) is then used to quantify the CEST contrast at different offsets. Amide proton transfer (APT), a specific form of CEST at Δω = 3.6 ppm relative to water, has been suggested as a surrogate biomarker of endogenous mobile proteins and peptides as well as a pH-dependent indicator of amide proton exchange rates in biological tissues. APT has been widely implemented for characterizing abnormal tissues such as tumors (3–6) and stroke (7–10).
Unfortunately, APT imaging in practice may be significantly influenced by factors other than chemical exchange, including effects caused by B0 inhomogeneities, non-specific magnetization transfer (MT) and asymmetric MT effects, water longitudinal relaxation rate (R1), and direct water saturation (RF spillover). Several approaches have been developed to reduce these confounding effects. For example, the WASSR method corrects for spatial B0 field variations (11). The magnetization transfer asymmetry (MTRasym) metric corrects for direct water saturation by subtracting the signals acquired with irradiation on the solute of interest (the label scan) from those on the corresponding other side of water (the reference scan). However, in most biological tissues the background MT effects are themselves asymmetric, and nuclear Overhauser effects (NOE) also can contribute, so that MTRasym is still influenced by processes that are not specific for chemical exchange of amides. These significantly reduce the specificity and quantitative accuracy of APT for detecting and measuring mobile proteins/peptides, and complicate the interpretation of APT data. Furthermore, MTRasym makes no correction for R1 contributions.
Several refinements have been proposed to further reduce the effects of asymmetric MT (12–16). For example, Jin et al. proposed to exploit the wide spectral separation available at high field strength (e.g. 9.4T) and interpolate measurements made at three offset frequencies to better approximate APT, denoted as APT* (16). Different acquisition strategies, such as the SAFARI (13), CERT (17), and VDMP-CEST (15), have also been developed to eliminate some confounding effects. However, these methods (like MTRasym) do not incorporate a correction for R1 effects on CEST measurements. Recently, Zaiss et al. analyzed the behaviors of CEST measurements and developed a reciprocal Z-spectrum analysis (denoted as the 1/Z method) to eliminate RF spillover and MT effects (18–20). Moreover, this analysis indicates a simple way that the influence of R1 on APT measurements can be eliminated. By combining the three-offset and the 1/Z methods, a new metric AREX* (apparent exchange dependent relaxation), can be obtained, which is an exchange rate-weighted APT contrast with much reduced influence from other confounding effects. This method has been successfully implemented to characterize brain cancer in rats (21) and humans (22), and stroke in rats (19,23), resulting in very different estimates of APT effects compared with more conventional methods such as MTRasym and APT*. These results suggest strongly that the influence of R1 plays an important role in estimates of APT contrast.
Like most other CEST models, the 1/Z analysis was originally developed based on a simple two-pool (water and amide protons) model, in which a single, measured average R1 of water is used in corrections (19,21,23,24). Although a recent study extended the 1/Z method to a three-pool model to include the semi-solid MT pool (25), the complex arrangement of multiple water pools in real biological tissues is still not considered. It is well known that water may exist in multiple compartments such as intra- and extracellular spaces, and the relaxation properties in each compartment are likely different from each other. Moreover, not all pools necessarily have large numbers of exchanging protons, so the assumption of a single relaxation rate to represent all pools may introduce inaccuracies, especially if the water compartment fractions and relaxation rates change in pathologies such as stroke (26). There are therefore reasons to question whether R1 correction approaches based on simple two-pool models are appropriate, and whether they can introduce extra uncertainty into estimates of APT effects.
In principle, the potentially confounding influences of water compartmentation and heterogeneous relaxation in real tissues on APT measurements may be significantly reduced if transcytolemmal water exchange occurs rapidly compared with the long (several seconds) duration of the saturation phase. For example, the apparent mean lifetime of intracellular water has been reported as 625 ± 43 ms in frontal human white matter and 344.8 ± 95.1 ms in human solid brain tumors (27). Moreover, the apparent mean lifetime of intracellular water in tumors can decrease further to 147 ± 84 ms during apoptosis (28). For a comparison, the total duration of saturation pulse(s) is on the order of several seconds, at least several times larger than the typical intracellular water lifetime. If the water molecules inside tissues can diffuse long enough so that they are well mixed at the end of the saturation phase, all the distinct water compartments can be approximated as a single mixed one, and hence a single water relaxation rate may be sufficient to describe all water molecules in the APT models. If true, this can simplify the analysis of APT data from real biological tissues, and the previously reported R1 correction methods based on two-pool models can be applied in clinical practice.
Unfortunately, the influences of multiple water pools, heterogeneous relaxation and transcytolemmal water exchange on CEST measurements have not previously been fully investigated. Therefore in this study computer simulations and measurements in vivo were performed to evaluate such effects. Specifically, a more general four-pool model consisting of intracellular water, extracellular water, exchanging protons and an MT pool, was examined using computer simulations. Furthermore, the hypothesis of relaxation influence and compensation was directly tested in vivo: tumor-bearing rats with regions of blood-brain-barrier breakdown received serial injections of Gd-DTPA while measuring CEST signals. By such a means, the extracellular water relaxation rate was selectively altered as tracked by R1 mapping, and hence the effectiveness and accuracy of R1 corrections were investigated. In addition to the R1-corrected AREX contrast, the conventional MTRasym and APT* metrics were also calculated and compared to quantify the influence of R1 variations on APT contrast.
Methods
Quantification of APT
For the simple two-pool (water and amide protons) model, the CEST effect is defined as a function of two Z-spectral values: the label scan Zlab=Mlab/M0, acquired at the amide proton frequency (3.6 ppm in biological tissues) and a reference scan Zref that has no contribution of amide. The conventional MTRasym uses the opposite frequency as a reference scan Zref=Z(−3.6 ppm), acquired at the amide proton frequency (3,16), namely
| [1] |
However, Z(−3.6 ppm) is contaminated by asymmetric MT and NOE effects in biological tissues. Jin et al. (2013) found that Z values at 3.0 and 4.2 ppm of rodent brain tissues on 9.4T appeared to have minimal APT saturation effect, and hence defined the apparent APT contrast APT* using the three-offset method as
| [2] |
Zaiss et al. defined the apparent exchange-dependent relaxation (AREX) using the 1/Z analysis as (19)
| [3] |
However, Eq.[3] was derived based on a simple two-pool model without the consideration of other confounding effects, e.g. asymmetric MT and NOE, which may play an important role in biological tissues. Specifically, Zref(−3.6 ppm) may suffer the contaminations from these effects and may bias the estimation of AREX(APT). To reduce these contaminations, we previously proposed to use in the three-offset method to replace Zref and obtained (21,23)
| [4] |
The detailed derivations of Eq.[3] and [4] have been reported before (19,20,25), and were already applied in previous studies (19,21,23,29). The quantity AREX* corrects for spillover, R1 and asymmetric MT effects, and hence should provide an exchange rate-weighted APT measurement relatively free of other influences (21). Note that, in principle, the AREX method is independent on how the reference value is obtained.
Numerical simulations
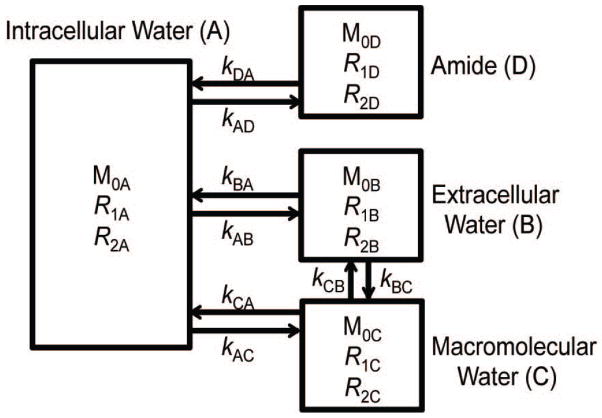
Numerical simulations based on a four-pool model were performed by solving Bloch-McConnell equations using in-house scripts written in Matlab (Mathworks, Natick, MA). The four pools were denoted as intracellular water (“A”), extracellular water (“B”), macromolecular MT (“C”), and amide (“D”) protons. Proton exchange was allowed between any two pools except that the amide (“D”) pool could only exchange with intracellular water “A”. Note that a separate work found that distinct macromolecular pools exchanging with the intra and extra cellular water pools were not necessary when fitting qMT data with rapid transcytolemmal exchange (30). Hence, we use only one macromolecular pool here. The schematic diagram with corresponding exchange rate constants is illustrated in Figure 1. The parameters used in the simulations were (7,31): M0A = 0.6888, R1A = 0.4 s−1, R2A = 20 s−1, M0B = 0.25, R2B = 20 s−1, M0C = 0.06, R1C = 1 s−1, R2C = 105 s−1, M0D = 0.0012, R1D = 1 s−1, R2D = 66.67 s−1, and the extracellular water R1B varied from 0.3 to 3.3 s−1 to mimic the contrast agent induced R1 variations 0.4 – 1 Hz observed in the experiments in vivo (see Figure 6). Other parameters were: amide water exchange rate constant kDA = 30 s−1, macromolecular water exchange rate constant kCA = kCB = 20 s−1, transcytolemmal water exchange rate constant kAB = 0, 1, 2, 4, or 6 s−1. The observed R1 of the whole system was simulated with a selective inversion recovery (SIR) method as described previously (31–33). MTRasym, APT*, and AREX* were simulated and calculated according to Eqs. [1], [3] and [4]. The MR sequence parameters (TR, TE, RF duration and power) were the same as those used in the in vivo experiments (see below).
Figure 1.
Schematic diagram of a four-pool model comprised of intracellular water (A), extracellular water (B), macromolecular water (C), and amide proton (D) pools. Arrows indicate possible magnetization exchanges between pools.
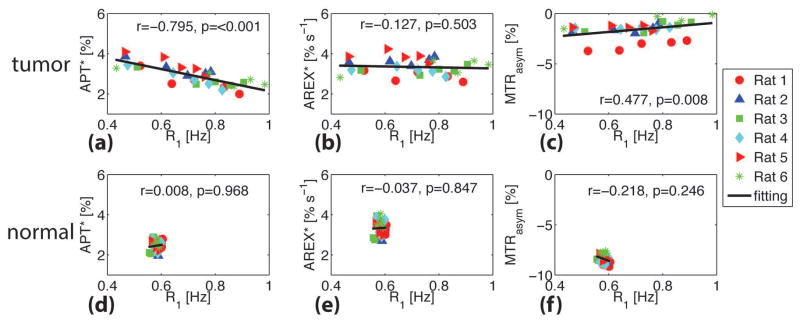
Figure 6.
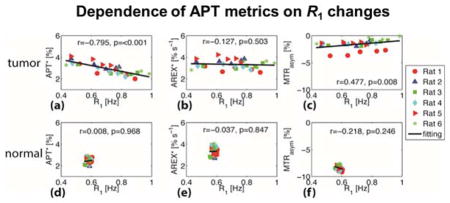
Correlations of tumor APT* (a), AREX* (b), and MTRasym (c) with R1 for six rats. The corresponding correlations for the contralateral normal brain tissues are shown in (d), (e), and (f). The Spearman’s coefficient r and p values are provided for each correlation. The full lines represent the linear regression of all data points in each subfigure.
MR imaging of animals
All animal-related procedures were approved by the Institutional Animal Care and Use Committee at Vanderbilt University. Six male Fisher 344 rats (280–310 g) bearing 9L brain tumors were scanned. MR images were acquired on a 9.4T Varian 21-cm-bore horizontal imaging system with a 38-mm RF volume coil for both transmission and reception. During MRI experiments, the rat rectal temperature was maintained at around 37 °C using a warm-air feedback system.
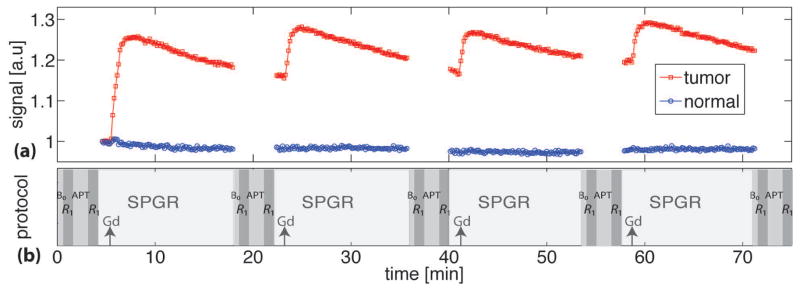
Figure 2b shows the in vivo experimental protocol of the current study. The intravenous injections of Gd-DTPA (0.083 mmol kg−1) were repeated four times to obtain five (including the baseline) different accumulated Gd-DTPA concentrations as well as five different R1 values. The measurements of B0 field map, R1, APT, and spoiled-gradient echo (SPGR) signals were interleaved and repeated five times to obtain multiple MR parametric maps with five different R1’s. To assist determining the delay time between each Gd-DTPA injection and each APT measurement, the SPGR sequence was used starting from 1 minute before through 13 minutes after each Gd-DTPA injection to monitor the time course of R1 variations caused by Gd-DTPA. Figure 2a shows the SPGR signals of the tumor (red squares) and contralateral normal brain tissue (blue circles) from a representative rat. The SPGR signals reached a relatively flat plateau after 13 minutes of Gd-DTPA injections, indicating R1 was relatively stable after that time. Therefore, except for the baseline, all the acquisitions of multiple MR parametric maps were performed after 13 minutes of each Gd-DTPA injection. By such a means, the rapid variations of R1 were avoided during all APT measurements. Furthermore, to quantify the R1 changes, two R1 maps were acquired before and after each repeated APT measurement, respectively, and hence the percentage R1 variation δR1 (δR1%= 200•|R1before−R1after|/|R1before+R1after|) can be obtained showing the percentage R1 change during each APT measurement. In order to monitor possible B0 shifts during the whole experiments, a B0 map was acquired before each of the five APT measurements.
Figure 2.
(a) The time course of spoiled-gradient echo (SPGR) signals of a tumor (red squares) and contralateral normal brain tissue (blue circles) from a representative rat. (b) Schematic diagram of the data acquisition protocol. The acquisitions of B0, R1, APT, R1, and SPGR signals were interleaved and repeated five times. The black arrows indicate the time when Gd-DTPA was injected.
Specifically, B0 field maps were reconstructed from four complex gradient echo images with TE = 3, 5, 7, and 9 ms. R1 was mapped using a seven-point selective inversion recovery sequence specifically optimized for cancer imaging(30). APT measurements were acquired with 5-sec cw saturation pulses with B1 = 1 μT. Five frequency offsets (300, 4.2, 3.6, 3, −3.6 ppm) were acquired in each APT measurement. Note that B0 variations were monitored during experiments (see Figure 4). R1 and APT images were acquired on a single slice of 2 mm thickness using a single-shot spin-echo echo-planar imaging (EPI) sequence (FOV = 32 × 32 mm2; matrix size = 64 × 64). After the pixel-wise mapping of B0, R1, MTRasym, APT*, and AREX*, quantitative analyses were performed on regions of interest (ROIs) of the tumors and the corresponding contralateral normal tissues.
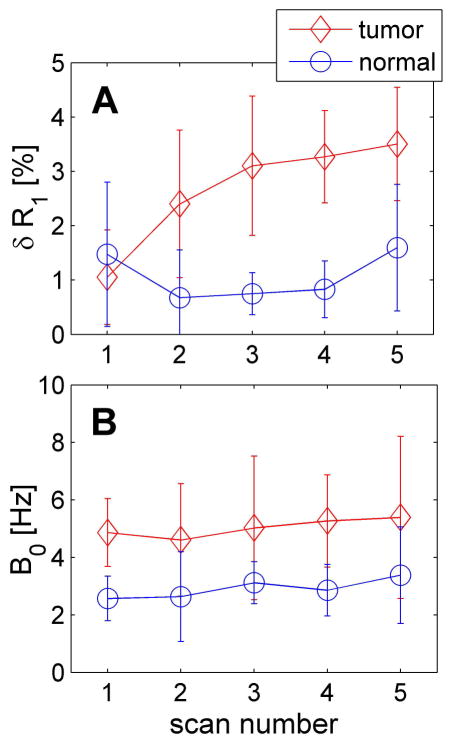
Figure 4.
The δR1 variation between before and after each repeated APT measurement (A) and B0 field shift at the starting point of each APT measurement (B). Scan number represents the repeated scans, and scan 1 represents the baseline acquisition.
Results
Numerical simulations
Figure 3 shows the simulated dependence of MTRasym, APT* and AREX* on the average R1 for the four-pool model. The change of R1 was achieved via adjusting R1b of the extracellular water only, mimicking the effects of injections of Gd-DTPA. The transcytolemmal water exchange rate constant kAA was allowed to vary from 0 to 6 Hz, with corresponding intracellular water lifetime from infinity to 167 ms. MTRasym and APT* were very dependent on R1 at all values of kAB e.g. ~ 41% change when R1 changed from 0.4 to 1 Hz. The values from both methods are highly affected by R1 no matter how fast the transcytolemmal water exchange. By contrast, although the R1-corrected AREX* showed slight variations (~ 10%) when kAB < 2 Hz, it became relatively independent of R1 (< 5%) over a broad range of R1 values from 0.4 to 1.2 Hz when transcytolemmal water exchange was faster (kAB > 2 Hz). This suggests that R1 effects can be eliminated in R1-corrected AREX* if kAB is fast enough. Even if kAB is relatively slow (< 2 Hz), the R1 effects are still small (~ 10%) in AREX*. In contrast, both MTRasym and APT* are significantly influenced (~ 40%) by R1 effects even with large kAB values.
Figure 3.
Simulated dependence of MTRasym (a), APT* (b), and R1-corrected AREX* (c) on R1 with different transcytolemmal water exchange rate constants kAB.
In vivo MRI experiments
Figure 4 shows the δR1 variation and B0 field shift during the MRI scans of a representative animal. Recall that δR1 is the percentage change of R1 before and after each of the five APT measurements. Although δR1 of the tumors increased slightly with the accumulation of injected Gd-DTPA, simulations indicated that the variations of APT* < 1% and the variations of MTRasym < 5 % for δR1 < 3.5% (data not shown). Thus the variations of R1 that occurred during each APT measurement were ignored in the current study. Figure 4B shows that the ΔB0 was constant in both tumors and contralateral normal tissues throughout the whole experiments. Therefore, the B0 field shift was not considered in the data analyses of the current study since the maximum B0 shift was only ~ 5 Hz (0.0125 ppm) in the regions of interest.
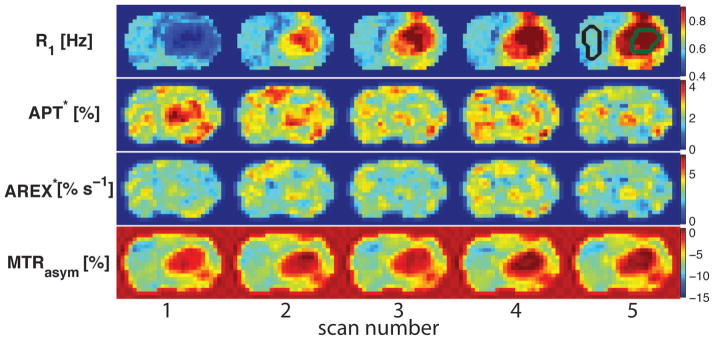
Figure 5 shows the multi-parametric maps of a representative rat brain for each of the dynamic scans. The R1 maps confirm that the injections of Gd-DTPA affected the tumors only, as expected. The 5th R1 map (upper-right) shows the ROIs manually selected on the tumor (green) and contralateral normal tissues (black). Consistent with the numerical simulations (see above), APT* values were lower in tumors after the Gd-DTPA injections. By contrast, MTRasym in tumors increased gradually with Gd-DTPA injections, which is different from the predicted results that MTRasym should decrease with higher R1 values. This discrepancy may be due to other effects such as the presence of NOE contributions. The R1-corrected AREX* was constant throughout all scans, indicating it was independent on the R1 variations caused by the Gd-DTPA injections.
Figure 5.
Temporal evolution of R1, APT*, AREX*, and MTRasym maps acquired from a representative rat before and after Gd-DTPA injections. The 5th R1 map shows the ROIs of the tumor (green) and contralateral normal tissues (black) used in the data analysis.
Figure 6 summarizes the correlations between APT measures (APT*, AREX*, and MTRasym) and R1 obtained in vivo. For the tumors, APT* appears to be significantly inversely correlated with R1 (Spearman’s correlation r = −0.795 and p < 0.001), but R1-corrected AREX* showed no significant correlation with R1 (p = 0.503). Note also that, consistent with previous reports (21), R1-corrected AREX* in tumors (3.34 ± 0.40 % s−1) is closely similar to that in normal tissues (3.33 ± 0.35 % s−1). Although MTRasym showed a slightly positive correlation with R1 (r = 0.477), which is different from the stronger positive correlations predicted by the simulations, the dependence of MTRasym on R1 is clear (p = 0.008). The predicted decrease of MTRasym with increasing R1 agrees with previous simulations based on a simple two-pool model (water and amide) (34), but is at variance with the experimental results found here. This may be due to the influence of NOE effects that were not considered in the simulations or to differences between the parameter values used in the simulations and the actual values present in vivo. Nevertheless, these results confirm again that MTRasym and APT* are significantly affected by R1 values, and hence their accuracy for quantifying mobile proteins/peptides is compromised. By contrast, R1-corrected AREX* is immune to the large variations of R1 (from 0.4 to 1.0 Hz) in real tissues in which multiple water compartments exist. This suggests that R1-corrected AREX* is a more reliable indicator of levels of mobile proteins/peptides compared with other APT methods. For reference, the correlations of APT values with R1 in contralateral normal tissues are also provided in Figure 6.
Discussion
In order to obtain reliable measurements of APT, it is necessary to remove or correct for possible influences other than chemical exchange with amides in mobile peptides and proteins. Effects such as the presence of asymmetric MT, variations in R1, RF spillover, and NOEs can reduce the accuracy and specificity of APT in practice, and lessen its value as a molecular imaging technique. For example, any detected APT changes without corrections for confounding effects could be due to changes in R1, MT, amide proton concentrations or combinations of these effects. This will increase the difficulty to interpret APT data and hinder its application in practice. We have previously proposed to use AREX* to correct other confounding effects and achieved a relatively “clean” exchange-rate-dependent metric. However, previous studies using AREX* are all based a simple two-pool model and the accuracy used in biological tissues with multiple physical compartments have not been fully investigated before. The current study aimed to evaluate whether the existence of multiple water compartments in real tissues with heterogeneous relaxation rates could affect the measurements of APT by different methods. The results suggest that both conventional magnetization transfer asymmetry MTRasym and the three-offset APT* methods may be strongly affected by values of R1, while R1-corrected AREX* is independent of R1 over a broad physiologically relevant range (0.4 – 1.0 Hz). This indicates that R1 significantly confounds conventional APT measures; and the R1-corrected AREX metrics based on the 1/Z method is an appropriate means to remove R1 influences on APT measurements.
Note that after a single bolus injection of Gd-DTPA, tumor R1 could change significantly during the wash-in and wash-out processes. However, such a R1 change is relatively too fast for APT measurements especially in the first ~ 10 minutes after an injection. Note that although R1 values were very different between different APT measurements in the current study, R1 should be relatively stable during the acquisition of each APT measurement. Otherwise, the different R1 weighting e.g. at control and label scans may cause a significant biased estimation of APT. The same strategy has been used to map water exchange rates using multiple bolus injections of contrast agents (35). Therefore, APT measurements were performed only when R1 changes reached a relatively flat plateau (after 13 minutes) in the current study. Moreover, R1 mapping was performed immediately before and after each APT measurement in order to confirm a relatively stable R1 change during each APT measurement. Our simulations showed that the variations of APT* < 1% and the variations of MTRasym < 5 % for δR1 < 3.5% during each APT measurement.
A smaller value of R1 implies a slower recovery from saturation, which should result in a larger value of MTRasym (34). However, the observed MTRasym in tumors showed a slight increase with increase of R1. In biological tissues, MTRasym may also be strongly affected by asymmetric MT and NOE effects. MTRasym can be approximated as ≈ APTR – NOER, where APTR is the proton transfer ratio for the amide protons, and NOER is the NOE-based MT ratio (36). Both APTR and NOER should decrease with increasing R1, but the slight increase of MTRasym with R1 may suggest a stronger dependence of NOER on R1 than APTR. In addition, the variation of R1 during the acquisitions of APT images can also slightly bias the dependence of MTRasym on R1 (~5% shown in simulations). A different study also observed that MTRasym changed significantly after Gd administration to patients who were to undergo carotid endarterectomy (37). This suggests that MTRasym is not a reliable measure of mobile proteins/peptides and may be significantly affected by variations in R1.
The apparent dependence of APT* and independence of AREX* on R1 demonstrates the importance of R1 corrections for interpreting APT changes. In our previous studies, it was shown that corrections for RF spillover, MT and R1 effects contributed differently in tumors (21) and stroke (23). APT* in tumors was higher than that in normal tissues, while R1-corrected AREX* was similar in tumors and normal tissues (21), which was consistent with an independent study using a different approach (38). However, R1-corrected AREX* showed a more pronounced contrast between ischemic and normal brain than APT* (23). Thus R1 corrections may strongly affect inferences about changes within tissues in pathological conditions. Note that although AREX* significantly reduces the contrast between brain tumors and normal brain tissues, it provides unique information on mobile proteins/peptides that are not achievable by other conventional MRI methods. Moreover, considering the potentially strong influence of other variables on APT measurements, other MR parameters (R1, R2, quantitative MT (e.g. the pool size ratio of macromolecular vs water protons) should be measured to avoid misinterpretation of APT variations.
The amide proton pool is usually believed to be mainly within the intracellular space (7), so in our simulations we considered amide proton transfer only between amide protons and intracellular water. However, the situation when both intra- and extracellular water protons exchange with amide protons has also been simulated, and the conclusion is qualitatively the same (specific data not shown): APT* and MTRasym would decrease with increasing R1, but AREX* stays almost constant.
Both simulations and experiments show that R1-corrected AREX* is independent of R1. The intracellular exchange lifetime is much shorter than the total duration of saturation pulse(s) used in APT imaging (e.g. 5 sec in the current study). The integrated water signal from all compartments may then be approximately regarded as from a single water pool. Therefore, though Gd-DTPA selectively alters the extracellular water R1, the overall observed R1 is still suitable for R1 correction of APT imaging in biological tissues. Note that this conclusion may also hold for other exchange sites, e.g. amine. Therefore, under the circumstances when intracellular water life time is much shorter than the total duration of saturation pulse(s), i.e. fast transcytolemmal water exchange rate, the influences of different water compartmentation (i.e. intra- and extracellular spaces) and relaxation properties can be ignored because all water molecules can be considered well-mixed at the end of saturation pulse(s). This may assist better data interpretation of not only APT but also other types of CEST measurements.
The present work not only represents a verification of the proposed relaxation-compensated features of the APT evaluation method AREX*, but also has practical implications. Gadolinium-based contrast agents have been widely used in clinical MRI. However, due to its strong influence on R1 relaxation, CEST measurements were not recommended with gadolinium injections (37). The current study shows that R1-corrected AREX* can compensate the influences caused by variations in R1 relaxation, and hence can be measured anytime including after the gadolinium injections. This can not only increase the accuracy of APT imaging when contrast agent is present, but also increase the management flexibility of patient imaging in clinical practice.
Conclusion
The effectiveness and accuracy of R1 correction in APT imaging has been investigated via simulations and in vivo experiments. The time courses of APT*, MTRasym, and AREX* were measured in tumors following serial injections of Gd-DTPA to result in different R1 values. Different from conventional APT* and MTRasym contrasts, R1-corrected AREX* was found independent of R1 changes. This study establishes the importance of R1 corrections for accurate APT imaging, and confirmed the reliability of using the overall observed tissue R1 for R1-correction in vivo. Our results suggest an appropriate means to correct for R1 and MT effects in CEST imaging, and may also assist in better understanding the contrast mechanisms of CEST imaging in biological tissues.
Acknowledgments
This work was funded by National Institutes of Health (NIH) K25CA168936, R01CA109106, R01CA173593, R01EB000214, P50CA128323, R21EB017873, R01EB017767, and R01CA184693.
Abbreviations
- APT
amide proton transfer
- MTRasym
magnetization transfer ratio obtained using asymmetric analysis
- APT*
amide proton transfer obtained using the three-offset method
- AREX
apparent exchange dependent relaxation obtained using the 1/Z method
- AREX*
AREX obtained using the 1/Z method and three-offset method
- kAB
transcytolemmal exchange rate from intra- to extracellular spaces
Appendix
The detailed description of the four-pool model (see Figure 1) and parameters were presented in the text. The corresponding modified Bloch-Mcconnell equations including the transcytolemmal exchange between the intracellular and extra cellular compartments can be expressed as
| [S1] |
where r2A = R2A + kAB + kAD, r1A = R1A + kAB + kAC + kAD, r1C = R1C + kCA + RRFB,C, r2B = R2B + kBA, r1B = R1B + kBA, r2D = R2D + kDA, r1D = R1D + kDA, where is the saturation rate of the macromolecular pool, and g is the super-Lorentzian lineshape (39)
| [S2] |
where T2B is the transverse relaxation time of the macromolecular protons, Δ is the frequency offset. Note that only continuous-wave APT experiments were considered in the current study, so that Eq.[S1] was directly adopted and matrix operations were performed to simulate the signals. All simulations were based on in-house written scripts with Matlab (Mathworks, Natick, MA). It took ~ 4 sec on an i5-3210M 2.5GHz processor to complete one set of simulations (i.e. 4 kAB values, 14 R1 values, and 121 frequency offsets).
References
- 1.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143:79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 2.Van Zijl PCM, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn’t? Magn Reson Med. 2011;65:927–48. doi: 10.1002/mrm.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003;50:1120–1126. doi: 10.1002/mrm.10651. [DOI] [PubMed] [Google Scholar]
- 4.Jones CK, Schlosser MJ, Van Zijl PCM, Pomper MG, Golay X, Zhou J. Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med. 2006;56:585–592. doi: 10.1002/mrm.20989. [DOI] [PubMed] [Google Scholar]
- 5.Salhotra A, Lal B, Laterra J. Amide proton transfer imaging of 9L gliosarcoma and human glioblastoma xenografts. NMR Biomed. 2008;21:489–497. doi: 10.1002/nbm.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia G, Abaza R, Williams JD, et al. Amide proton transfer MR imaging of prostate cancer: a preliminary study. J Magn Reson imaging. 2011;33:647–654. doi: 10.1002/jmri.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Payen J-F, Wilson Da, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9:1085–90. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 8.Jokivarsi KT, Gröhn HI, Gröhn OH, Kauppinen Ra. Proton transfer ratio, lactate, and intracellular pH in acute cerebral ischemia. Magn Reson Med. 2007;57:647–653. doi: 10.1002/mrm.21181. [DOI] [PubMed] [Google Scholar]
- 9.Sun PZ, Zhou J, Huang J, Van Zijl P. Simplified quantitative description of amide proton transfer (APT) imaging during acute ischemia. Magn Reson Med. 2007;57:405–410. doi: 10.1002/mrm.21151. [DOI] [PubMed] [Google Scholar]
- 10.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PCM. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb blood flow Metab. 2007;27:1129–36. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- 11.Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009;61:1441–1450. doi: 10.1002/mrm.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JS, Regatte RR, Jerschow A. Isolating chemical exchange saturation transfer contrast from magnetization transfer asymmetry under two-frequency rf irradiation. J Magn Reson. 2011;215:56–63. doi: 10.1016/j.jmr.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheidegger R, Vinogradov E, Alsop DC. Amide proton transfer imaging with improved robustness to magnetic field inhomogeneity and magnetization transfer asymmetry using saturation with frequency alternating RF irradiation. Magn Reson Med. 2011;66:1275–1285. doi: 10.1002/mrm.22912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zu Z, Xu J, Li H, Chekmenev EY, Quarles CC, Does MD, Gore JC, Gochberg DF. Imaging amide proton transfer and nuclear overhauser enhancement using chemical exchange rotation transfer (CERT) Magn Reson Med. 2014;72:471–476. doi: 10.1002/mrm.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Yadav NN, Bar-Shir A, Jones CK, Chan KWY, Zhang J, Walczak P, McMahon MT, Van Zijl PCM. Variable delay multi-pulse train for fast chemical exchange saturation transfer and relayed-nuclear overhauser enhancement MRI. Magn Reson Med. 2014;71:1798–1812. doi: 10.1002/mrm.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin T, Wang P, Zong X, Kim SG. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn Reson Med. 2013;69:760–770. doi: 10.1002/mrm.24315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zu Z, Janve VA, Xu J, Does MD, Gore JC, Gochberg DF. A new method for detecting exchanging amide protons using chemical exchange rotation transfer. Magn Reson Med. 2013;69:637–647. doi: 10.1002/mrm.24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaiss M, Bachert P. Exchange-dependent relaxation in the rotating frame for slow and intermediate exchange - modeling off-resonant spin-lock and chemical exchange saturation transfer. NMR Biomed. 2013;26:507–518. doi: 10.1002/nbm.2887. [DOI] [PubMed] [Google Scholar]
- 19.Zaiss M, Xu J, Goerke S, Khan IS, Singer RJ, Gore JC, Gochberg DF, Bachert P. Inverse Z-spectrum analysis for spillover-, MT-, and T1 -corrected steady-state pulsed CEST-MRI--application to pH-weighted MRI of acute stroke. NMR Biomed. 2014;27:240–52. doi: 10.1002/nbm.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaiss M, Bachert P. Chemical exchange saturation transfer (CEST) and MR Z-spectroscopy in vivo: a review of theoretical approaches and methods. Phys Med Biol. 2013;58:R221–69. doi: 10.1088/0031-9155/58/22/R221. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Zaiss M, Zu Z, Li H, Xie J, Gochberg DF, Bachert P, Gore JC. On the origins of chemical exchange saturation transfer (CEST) contrast in tumors at 9.4 T. NMR Biomed. 2014;27:406–416. doi: 10.1002/nbm.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaiss M, Windschuh J, Paech D, et al. Relaxation-compensated CEST-MRI of the human brain at 7T: Unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Zu Z, Zaiss M, Khan IS, Singer RJ, Gochberg DF, Bachert P, Gore JC, Xu J. Imaging of amide proton transfer and nuclear Overhauser enhancement in ischemic stroke with corrections for competing effects. NMR Biomed. 2015;28:200–209. doi: 10.1002/nbm.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun PZ, Wang E, Cheung JS. Imaging acute ischemic tissue acidosis with pH-sensitive endogenous amide proton transfer (APT) MRI-Correction of tissue relaxation and concomitant RF irradiation effects toward mapping quantitative cerebral tissue pH. Neuroimage. 2012;60:1–6. doi: 10.1016/j.neuroimage.2011.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaiss M, Zu Z, Xu J, Schuenke P, Gochberg DF, Gore JC, Ladd ME, Bachert P. A combined analytical solution for chemical exchange saturation transfer and semi-solid magnetization transfer. NMR Biomed. 2015;28:217–230. doi: 10.1002/nbm.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syková E, Svoboda J, Polák J, Chvátal A. Extracellular volume fraction and diffusion characteristics during progressive ischemia and terminal anoxia in the spinal cord of the rat. J Cereb blood flow Metab. 1994;14:301–311. doi: 10.1038/jcbfm.1994.37. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson M, Lätt J, van Westen D, Brockstedt S, Lasič S, Ståhlberg F, Topgaard D. Noninvasive mapping of water diffusional exchange in the human brain using filter-exchange imaging. Magn Reson Med. 2013;69:1573–81. doi: 10.1002/mrm.24395. [DOI] [PubMed] [Google Scholar]
- 28.Bailey C, Giles A, Czarnota GJ, Stanisz GJ. Detection of apoptotic cell death in vitro in the presence of Gd-DTPA-BMA. Magn Reson Med. 2009;62:46–55. doi: 10.1002/mrm.21972. [DOI] [PubMed] [Google Scholar]
- 29.Wu R, Xiao G, Zhou IY, Ran C, Sun PZ. Quantitative chemical exchange saturation transfer (qCEST) MRI - RF spillover effect-corrected omega plot for simultaneous determination of labile proton fraction ratio and exchange rate. NMR Biomed. 2015;28:376–383. doi: 10.1002/nbm.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li K, Li H, Zhang X, Stokes AM, Kang H, Zu Z, Quarles CC, Gochberg DF, Gore JC, Xu J. Influence of water compartmentation and heterogeneous relaxation on quantitative magnetization transfer imaging in rodent brain tumors. Magn Reson Med. doi: 10.1002/mrm.25893. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Li K, Zu Z, Li X, Gochberg DF, Gore JC. Quantitative magnetization transfer imaging of rodent glioma using selective inversion recovery. NMR Biomed. 2014;27:253–260. doi: 10.1002/nbm.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li K, Zu Z, Xu J, Janve VA, Gore JC, Does MD, Gochberg DF. Optimized inversion recovery sequences for quantitative T1 and magnetization transfer imaging. Magn Reson Med. 2010;64:491–500. doi: 10.1002/mrm.22440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gochberg DF, Gore JC. Quantitative imaging of magnetization transfer using an inversion recovery sequence. Magn Reson Med. 2003;49:501–505. doi: 10.1002/mrm.10386. [DOI] [PubMed] [Google Scholar]
- 34.Liu G, Song X, Chan KWY, Mcmahon MT. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed. 2013;26:810–828. doi: 10.1002/nbm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey C, Moosvi F, Stanisz GJ. Mapping water exchange rates in rat tumor xenografts using the late-stage uptake following bolus injections of contrast agent. Magn Reson Med. 2013;71:1874–1887. doi: 10.1002/mrm.24847. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Hong X, Zhao X, Gao JH, Yuan J. APT-weighted and NOE-weighted image contrasts in glioma with different RF saturation powers based on magnetization transfer ratio asymmetry analyses. Magn Reson Med. 2013;70:320–327. doi: 10.1002/mrm.24784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tee YK, Donahue MJ, Harston GWJ, Payne SJ, Chappell MA. Quantification of amide proton transfer effect pre- and post-gadolinium contrast agent administration. J Magn Reson Imaging. 2014;40:832–8. doi: 10.1002/jmri.24441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheidegger R, Wong ET, Alsop DC. Contributors to contrast between glioma and brain tissue in chemical exchange saturation transfer sensitive imaging at 3Tesla. Neuroimage. 2014;99:256–268. doi: 10.1016/j.neuroimage.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison C, Stanisz G, Henkelman RM. Modeling magnetization transfer for biological-like systems using a semi-solid pool with a super-Lorentzian lineshape and dipolar reservoir. J Magn Reson B. 1995;108:103–113. doi: 10.1006/jmrb.1995.1111. [DOI] [PubMed] [Google Scholar]