Abstract
B cell receptor (BCR) signalling is an important pathway in diffuse large B cell lymphoma (DLBCL). In response to BCR triggering, normal and malignant B cells secrete the chemokines CCL3 and CCL4 to attract accessory cells to the tissue microenvironment. We measured CCL3 and CCL4 serum concentrations in 102 patients with newly diagnosed DLBCL by enzyme-linked immunosorbent assay (ELISA), investigated their prognostic impact and validated our findings in an independent cohort of 51 patient samples. We also tested CCL3 and CCL4 secretion by DLBCL cells, and the influence of BTK inhibitors on the secretion of these chemokines. High CCL3 (≥40 pg/ml) serum concentrations correlated with higher international prognostic index, lactate dehydrogenase and β2 microglobulin, as did CCL4 (≥180 pg/ml) with advanced Ann Arbor stages. High CCL3 levels correlated with significantly shorter progression-free and overall survival. The in vitro studies demonstrated that activated B cell-like (ABC), but not germinal centre B cell-like (GCB) DLBCL cells, secrete high levels of CCL3 and CCL4 after BCR triggering, which was exquisitely sensitive to BCR pathway inhibition. These findings support CCL3 and CCL4 protein concentrations as biomarkers for BCR pathway activation and prognosis in DLBCL.
Keywords: DLBCL, chemokines, lymphoma
Introduction
Diffuse large B-cell lymphoma (DLBCL) comprises a heterogeneous group of diseases with various outcomes which depend on clinical and biological features (Wilson 2013). Given the disease heterogeneity and the emergence of new targeted therapies, such as the PI3Kδ inhibitor, idelalisib (Furman, et al 2014, Gopal, et al 2014), and the BTK inhibitor, ibrutinib (Byrd, et al 2013, Wang, et al 2013), effective risk stratification and therapy selection based on predictive biomarkers are needed. Gene expression profiling (GEP) has been extremely valuable for dissecting the molecular heterogeneity and for predicting outcome in DLBCL(Alizadeh, et al 2000, Rosenwald, et al 2002, Shipp, et al 2002). GEP can distinguish two prognostic subtypes, germinal centre B cell-like (GCB) and activated B cell-like (ABC) DLBCL(Alizadeh, et al 2000), among whose functional differences are activity and importance of B cell receptor (BCR) signalling. ABC DLBCL cells have chronic active BCR signalling, upon which their survival is highly dependent (Davis, et al 2010).
In response to BCR stimulation, normal (Krzysiek, et al 1999) and malignant B cells (Burger, et al 2009) secrete the chemokines CCL3 and CCL4 to attract accessory cells, such as T cells (Bystry, et al 2001, Castellino, et al 2006), for cognate interactions in the tissue microenvironment. CCL3 and CCL4, previously called macrophage inflammatory protein-1α (MIP-1α) and MIP-1β, are chemokines of the CC subfamily and inducible in a number of haematopoietic cells, particularly those involved in adaptive immune responses (macrophages, dendritic cells, and B and T lymphocytes)(Eberlein, et al 2010). CCL3 signals through the chemokine receptors CCR1 and CCR5, whereas CCL4 signals only through CCR5. Previous studies highlighted CCL3 as a key response gene in B cells, which is up-regulated by BCR signalling (Burger, et al 2009, Herishanu, et al 2011), and repressed by BCL6(Shaffer, et al 2000). We previously reported that CLL patients have elevated CCL3 and CCL4 plasma levels, and multivariate analysis revealed high CCL3 levels as an independent prognostic marker (Sivina, et al 2011). Importantly, elevated CCL3 and CCL4 levels in patients with CLL rapidly normalized after pharmacological inhibition of BCR signalling with idelalisib (Hoellenriegel, et al 2011) or ibrutinib (Ponader, et al 2012, Wang, et al 2013). In DLBCL, the gene encoding CCL3 (CLL3; previously termed SCYA3) was identified as a signature gene for the ABC subtype (Alizadeh, et al 2000, Rosenwald, et al 2002) and was validated as one of the six most powerful independent predictors for survival in DLBCL(Lossos, et al 2004). Despite this evidence from GEP studies, CCL3 and CCL4 protein concentrations have not yet been explored as biomarkers of BCR activation or as prognostic markers in DLBCL. Gene expression does not always translate into protein expression, and secretory proteins, such as CCL3 and CCL4, may or may not be released into DLBCL culture supernatants or into the plasma in a fashion that correlates with gene expression. Given these potential variables, we conducted a series of experiments to characterize CCL3 and CCL4 protein levels in DLBCL. There are obvious clinical and translational advantages of using these markers, such as easy access to samples, given that CCL3/CCL4 can reliably be quantified in plasma and serum samples (Sivina, et al 2011), low costs of analyses and rapid modulation (normalization within days) by therapies targeting the BCR, based on the data in chronic lymphocytic leukaemia (CLL) and mantle cell lymphoma (MCL)(Hoellenriegel, et al 2011, Ponader, et al 2012, Wang, et al 2013). We therefore conducted a series of studies to explore the potential of CCL3 and CCL4 protein concentrations as BCR-related biomarkers in DLBCL.
Methods
DLBCL cell lines and reagents
The DLBCL cell lines TMD8, HBL-1, DB and OCI-Ly19 were grown in RPMI 1640 medium (HyClone Laboratories, Logan, UT) supplemented with glutamine, beta-mercaptoethanol, penicillin/streptomycin and 10% fetal bovine serum (FBS). Other DLBCL cell lines, OCI-Ly3 and OCI-Ly10, were maintained in Iscove’s modified Dulbecco’s medium (Life Technologies [Gibco], Grand Island, NY) supplemented with beta-mercaptoethanol, penicillin/streptomycin and 20% heparinized human plasma. All cell lines were grown in a humidified 5% CO2 incubator at 37°C. Ibrutinib (PCI-32765), was provided by Pharmacyclics, Inc. (Sunnyvale, CA) and Idelalisib and P505-15 were purchased from Selleckchem (Houston, TX). The kinase inhibitors were stored as stock solutions of 10 mM in 100 % dimethyl sulfoxide at −20 °C. These stock solutions were diluted in complete RPMI medium with 10% FBS, L-glutamine (HyClone Laboratories) and penicillin-streptomycin (Cellgro, Hemdon, VA), and added to the assay medium to the indicated final concentrations. For BCR stimulation culture medium was supplemented with 10 μg/ml anti-IgM (polyclonal goat F(ab′)2 fragments to human IgM, MP Biomedicals, Santa Ana, CA) for the indicated time periods.
Quantification of serum and supernatant CCL3 and CCL4 concentrations
Peripheral blood serum samples were obtained from the tissue bank of the Department of Lymphoma and Myeloma at MD Anderson Cancer Center (MDACC) and from the University of Nebraska Medical Center. All serum samples were aliquoted and stored at the time of initial referral to each institution. CCL3 and CCL4 levels in serum and cell culture supernatants were quantified by enzyme-linked immunosorbent assay (ELISA) using Quantikine Kits (R&D Systems, Minneapolis, MN) as previously described (Sivina, et al 2011). The absorbance was recorded on a microplate reader (ELx808, Bio-Tek Instruments, Winooski, VT), and data collection and analysis were performed using Gen5 software Version 1.08 (Bio-Tek Instruments).
DLBCL patient samples and characteristics
We retrospectively analysed serum samples from 102 patients with newly diagnosed DLBCL who were seen at MDACC between January 2009 and February 2011 (test cohort). Another set of 51 patient samples from MDACC with newly diagnosed DLBCL was studied for validation (validation cohort). Samples from 19 patients with newly diagnosed DLBCL from the University of Nebraska were included in the correlation analysis between serum CCL3/CCL4 levels and GCB vs. non-GCB subtype (Nebraska cohort). This cohort did not have clinical annotation other than DLBCL subtype information. Demographics and other clinical parameters for MDACC test and validation cohort patients from the time of initial referral to MDACC were analysed. DLBCL diagnosis was established by institutional pathologists according to the World Health Organization classification (Swerdlow, et al 2008). Immunophenotypic classification (GCB vs. non-GCB subtype) of DLBCL was determined by Hans criteria (Hans, et al 2004). The protocol for specimen handling and analyses followed the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board at MDACC and the University of Nebraska Medical Center without releasing any subjects’ identifying information.
Statistical analysis
Differences among variables were evaluated by the chi-square test and Mann-Whitney U test for categorical and continuous variables, respectively. Receiver operating curve (ROC) analysis was conducted to evaluate correlation between serum CCL3/CCL4 levels and immunophenotypic signatures by Hans criteria. Progression-free survival (PFS) was defined as the time interval between date of initial treatment and date of disease progression or death, whichever occurred first. Disease progression was defined as radiologically or biopsy confirmed relapse or progression of DLBCL after or during initial therapies. Overall survival (OS) was defined as the time interval between date of initial treatment and date of death or last follow-up date. Survival data were plotted according to the Kaplan-Meier method and group comparison was made by log-rank test. Multivariate Cox proportional hazards regression models were fitted to adjust the prognostic effect of each covariate. In all analysis, P < 0.05 was considered as statistically significant. All statistical analyses were conducted by IBM SPSS version 21.0 (IBM Corp, Armonk, NY).
Results
BCR signalling triggers secretion of high levels of CCL3 and CCL4 by ABC DLBCL
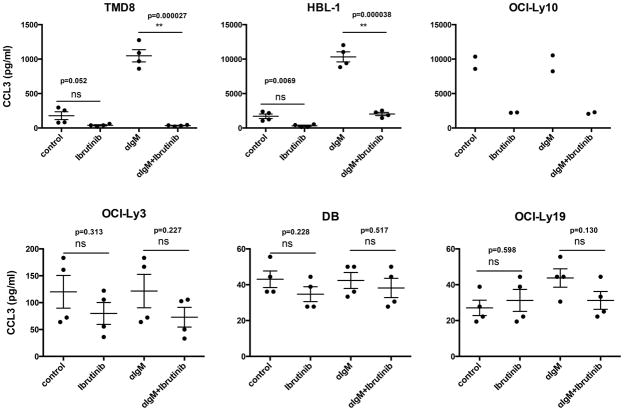
We measured the CCL3 and CCL4 secretion by DLBCL cells in the absence and presence of anti-IgM to trigger BCR signalling. Ibrutinib treatment was used to inhibit BCR signalling via blockade of BTK. We found that anti-IgM-induced BCR signalling triggered secretion of high concentrations of CCL3 and CCL4 by ABC DLBCL cell lines HBL-1 and TMD8, and that this was abrogated by ibrutinib (Figure 1, top panel). In contrast, OCI-Ly10 cells secrete high concentrations of CCL3 and CCL4 without stimulation, and there was no further increase in CCL3 or CCL4 concentrations following stimulation with anti-IgM. Nonetheless, CCL3 and CCL4 secretion by OCI-Ly10 cells was sensitive to inhibition by ibrutinib (Figure 1). OCI-Ly3 cells, as well as the tested GCB cell lines did not secrete CCL3 or CCL4 at high concentrations and did not respond to BCR stimulation or ibrutinib treatment, which is in line with the established BCR pathway alteration in these cells (Davis, et al 2010). Specifically, HBL-1 and TMD8 cells have mutations in CD79B, while OCI-Ly10 has a CD79A mutation; both of these genes are essential for transmission of BCR signals, and the mutations found are thought to contribute to the chronic active BCR signalling in these lines. In contrast, OCI-Ly3 cells do not display chronic active BCR signalling, although they have an ABC DLBCL phenotype, which is attributed to a gain-of-function mutation in CARD11, a downstream mediator of the effects of BCR signalling that is wild type in the other ABC DLBCL lines. Treatment with ibrutinib significantly decreased CCL3 (Figs. 1 and 2) and CCL4 (Figure S1) levels in ABC DLBCL cells with wild type CARD11. For example, in HBL-1 cells, anti-IgM stimulation significantly increased baseline CCL3 concentrations (± standard error, SE) from 1705.5 (± 27.5) pg/ml to 10324.3 (± 125.3) pg/ml; ibrutinib treatment of unstimulated cells decreased baseline CCL3 concentrations to 313.2 (± 6.8) pg/ml, and reduced anti-IgM-induced CCL3 to 2029.16 (± 26.9) pg/ml (Figure 1, top panel).
Figure 1. CCL3 secretion in DLBCL cell lines.
Secretion of CCL3 by ABC DLBCL cell lines (TMD8, HBL-1, OCI-Ly10 and OCI-Ly3) compared to the GCB DLBCL lines (DB, OCI-Ly19) at baseline and after anti-IgM stimulation with or without ibrutinib treatment. Each diagram represents the mean supernatant concentration of CCL3 from DLBCL cells cultured in complete medium (control), medium supplemented with 10 μg/ml of anti-IgM (αIgM), 1μM ibrutinib, or anti-IgM and ibrutinib. The diagrams are representative of 4 independent experiments.
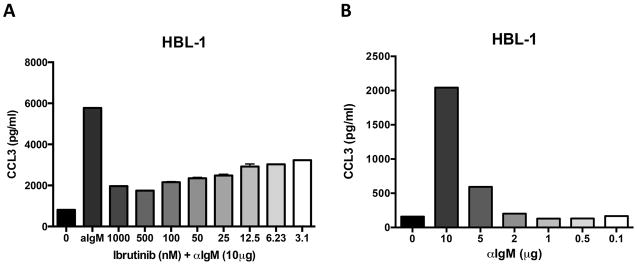
Figure 2. Effect of BCR stimulation and ibrutinib treatment on ABC DLBCL cells.
A. The bar diagram depicts the mean CCL3 secretion levels of the ABC DLBCL cell line HBL-1 cultured in complete medium, medium supplemented with 10 μg/ml of anti-IgM, or anti-IgM (αIgM) and different concentrations of ibrutinib. B. Effect of different anti-IgM concentration on CCL3 secretion of HBL-1 cells. The diagrams are representative of 3 independent experiments.
Sensitivity of ABC DLBCL cells to BCR stimulation and ibrutinib treatment
Titration experiments with different ibrutinib (Figure 2A) or anti-IgM (Figure 2B) concentrations revealed that CCL3 secretion by ABC DLBCL cells is highly sensitive to ibrutinib, with substantial (more than 50%) inhibition of CCL3 secretion even at low nanomolar ibrutinib concentrations. Similar inhibition of CCL3 and CCL4 secretion was noted when other inhibitors of BCR signalling pathway were explored, such as idelalisib and P505-15, a highly selective SYK inhibitor (Figure S2). Conversely, lower anti-IgM concentrations correlated with decreasing CCL3 and CCL4 concentrations (Figure 2B and Figure S3). These findings suggest that CCL3 and CCL4 secretion by ABC DLBCL cells is fine-tuned by BCR signalling.
DLBCL patient characteristics
We then evaluated CCL3 and CCL4 levels in pre-treatment serum samples obtained from DLBCL patients. Baseline demographics and clinical characteristics of the 102 patients from MDACC (test cohort) are summarized in Table S1. Thirty-nine patients had Ann Arbor Stage I or II, while 63 patients had stage III or IV disease. Sixty-five patients had lower International Prognostic Index (IPI) scores (0–2), and 37 had higher IPI (3–5). Mean and median levels of serum lactate dehydrogenase (LDH), β2 microglobulin (β2MG) and absolute lymphocyte count (ALC) are also displayed in Table S1. Based on the Hans criteria, 55 patients were classified as GCB subtype and 19 patients were classified as non-GCB subtype DLBCL but subtype classification was unknown in 28 patients. A majority of patients (93%) received rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone) or a CHOP-like chemotherapy regimen as frontline treatment, and 7 patients underwent high dose chemotherapy followed by autologous stem cell transplantation as a salvage regimen upon progression or disease relapse.
Serum CCL3 and CCL4 levels and correlation with other established prognostic factors in DLBCL
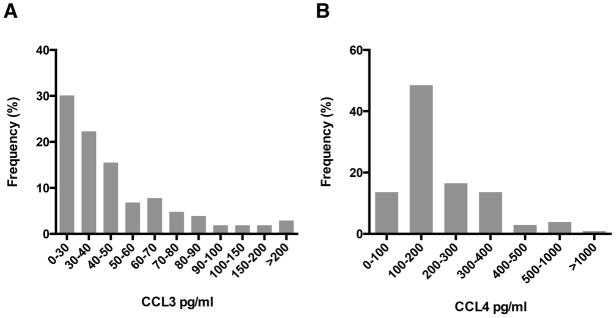
In the MDACC test cohort, median (range) serum CCL3 and CCL4 concentrations were 39.2 (20.5 – 224.7) pg/ml and 178.5 (56.9 – 1173.1) pg/ml, respectively. Distribution of serum CCL3 and CCL4 levels in the MDACC cohort is depicted in Figures 3A and 3B, respectively. Levels were dichotomized into high or low, based on the approximate median concentrations of CCL3 (40 pg/ml) and CCL4 (180 pg/ml). Table S2 shows correlation between serum CCL3 and CCL4 levels and other clinically relevant prognostic markers. High CCL3 (≥40 pg/ml) serum concentrations were associated with higher IPI scores (P = 0.03), higher LDH (P = 0.001), higher β2MG (P < 0.001) and lower ALC (P = 0.006). High serum CCL4 concentrations (≥180 pg/ml) were associated with advanced Ann Arbor stages (P < 0.001) and higher IPI scores (P = 0.06). Additionally, as seen before in CLL (Sivina, et al 2011), high CCL3 and high CCL4 levels correlated with each other (P = 0.003).
Figure 3.
Distribution of serum levels of (A) CCL3 and (B) CCL4 in the MD Anderson Cancer Center test cohort (N = 102).
Correlation between CCL3/CCL4 serum concentrations and DLBCL subtypes
A total of 93 patients (74 from the MDACC and 19 from the Nebraska cohort) had data available to classify immunophenotypic subtypes (GCB vs. non-GCB) based on Hans criteria. Serum levels of CCL3 or CCL4 were not statistically different between GCB versus non-GCB DLBCL (median [range]: CCL3: 37.9 [5.7 – 224.7] pg/ml for GCB vs. 27.6 [9.1 – 75.3] pg/ml for non-GCB, CCL4: 165.9 [56.1 – 1173.1] pg/ml for GCB vs. 164.1 [52.8 – 856.9] pg/ml for non-GCB). ROC analysis did not reveal any predictive value of serum CCL3 or CCL4 levels for predicting non-GCB subtype (see Figure S4).
Prognostic impact of serum CCL3 and CCL4 concentrations in DLBCL
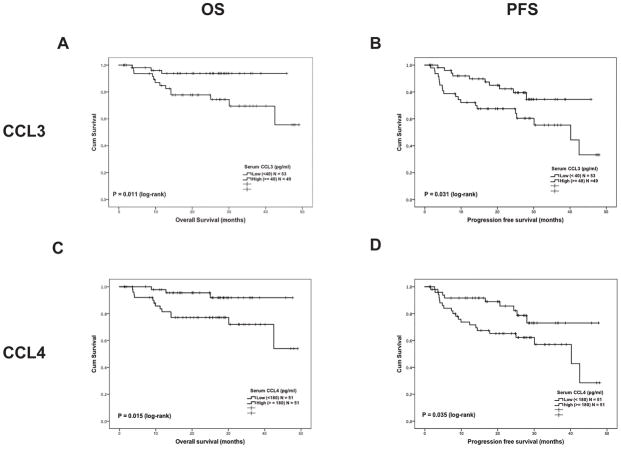
During the median follow-up period of 27.1 months (95% confidence interval [CI]; 25.5–28.7 months), 16 patients (16%) died and 30 patients (29%) suffered disease progression or death in the MDACC test cohort (N = 102). The MDACC test cohort did not reach the median for OS during the follow-up period, while median PFS was 42.5 months (95% CI; 27.7–52.3 months). The 2-year OS of the MDACC test cohort was 77% and 2-year PFS was 56%. The log-rank test demonstrated that high CCL3 (≥40 pg/ml) serum concentrations were associated with significantly shorter OS (P = 0.011) and PFS (P = 0.031) (Figure 4 A, and B). Similarly, high CCL4 (≥180 pg/ml) serum concentrations were also associated with shorter OS (P = 0.015) and PFS (P = 0.035) (Figure 4 C, and D). When both concentrations were taken into consideration, the CCL3high/CCL4high patient group (N = 29) had the worst PFS and OS (P = 0.002 and P = 0.008, CCL3high/CCL4high vs. CCL3low/CCL4low group, overall comparison P = 0.003 for OS and P = 0.038 for PFS, Figure S5A, B).
Figure 4.
Kaplan-Meier curve for overall survival (OS) and progression-free survival (PFS) in the MD Anderson Cancer Center test cohort (N = 102) based on the serum levels of CCL3 and CCL4. Survival comparison between two groups was tested by log-rank test. (A) OS difference between low (<40 pg/ml) and high (≥40 pg/ml) serum CCL3 level, (B) PFS difference between low (<40 pg/ml) and high (≥40 pg/ml) serum CCL3 level, (C) OS difference between low (<180 pg/ml) and high (≥180 pg/ml) serum CCL4 level, (D) PFS difference between low (≥180 pg/ml) and high (≥180 pg/ml) serum CCL4 level.
Results of the univariate log-rank test for other potential prognostic factors for OS and PFS in the MDACC test cohort are listed in Table S3. Because serum CCL3 and CCL4 concentrations were strongly correlated with IPI score (Table S2), we adjusted the prognostic effect of serum CCL3 and CCL4 with IPI score by multivariate Cox proportional hazards regression models. After adjusting the prognostic effect for IPI score, serum CCL3 concentrations had statistically significant prognostic impact on OS but not in PFS (Table I). On the other hand, the prognostic impact of serum CCL4 for PFS and OS was not statistically significant after adjusting for an effect of the IPI (Table I). Because CCL3high/CCL4high group had the worst prognosis (Figure S5), we adjusted the prognostic value of this cohort with IPI score. This model showed a strong prognostic value of CCL3high/CCL4high patients (Table I).
Table I.
Result of multivariate Cox proportional hazards regression model for OS and PFS in the MDACC cohort.
| OS
|
PFS
|
|||||||
|---|---|---|---|---|---|---|---|---|
| p | HR | 95% CI | p | HR | 95% CI | |||
| Low | High | Low | High | |||||
| Model 1 | ||||||||
| CCL3high | 0.049 | 3.62 | 1.005 | 13.004 | 0.127 | 1.85 | 0.840 | 4.075 |
| IPI 3–5 | 0.051 | 2.93 | 0.995 | 8.623 | 0.017 | 2.543 | 1.181 | 5.474 |
| Model 2 | ||||||||
| CCL4high | 0.061 | 3.39 | 0.943 | 12.171 | 0.137 | 1.842 | 0.824 | 4.122 |
| IPI 3–5 | 0.050 | 2.96 | 0.999 | 8.769 | 0.017 | 2.55 | 1.184 | 5.504 |
| Model 3 | ||||||||
| CCL3high/CCL4high | 0.019 | 3.91 | 1.251 | 12.230 | 0.082 | 2.023 | 0.914 | 4.477 |
| IPI 3–5 | 0.156 | 2.28 | 0.731 | 7.099 | 0.051 | 2.245 | 0.998 | 5.052 |
OS, overall survival; PFS, progression-free survival; MDACC, MD Anderson Cancer Center; HR, hazard ratio; 95% CI, 95% confidence interval; IPI, International Prognostic Index.
Findings in a validation cohort
Serum CCL3 and CCL4 concentrations in DLBCL were evaluated in an independent validation cohort (N = 51). Demographics and clinical characteristics of the validation cohort were similar to that of the test cohort (Table S4). Correlation to other prognostic markers in DLBCL confirmed a similar pattern of correlations as demonstrated in the test cohort (Table S4). Due to short follow-up and low event counts in the validation cohort (median follow up: 19.7 months, 95% CI: 18.0–21.4 months), prognostic association with OS and PFS did not reach statistical significance (Figure S6).
Effect of treatment on CCL3 and CCL4 serum concentrations
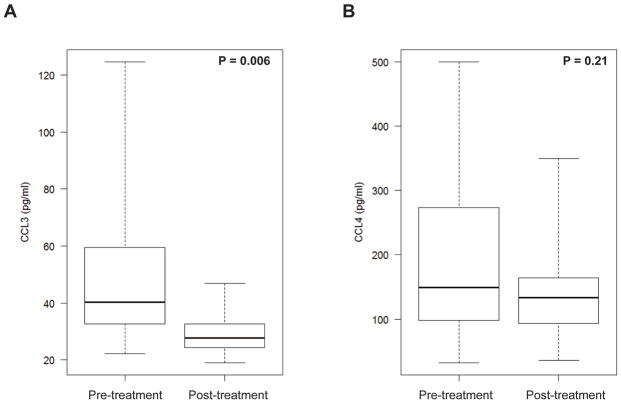
Nineteen patients had serial samples collected at pre- and post-treatment time points. The median time between pre- and post-treatment was 7.1 months (range: 4.1–18.4 months). At the time of post-treatment sample collection, 17 patients were in complete remission (CR) and 2 had partial responses (PR). Median level of pre- and post-treatment CCL3 serum concentrations was 40.3 pg/ml (range: 22.1–124.7) and 27.7 pg/ml (range: 18.9–46.8), respectively. Median level of pre- and post-treatment CCL4 serum concentrations was 149.4 pg/ml (range: 31.9 – 500) and 133.5 pg/ml (range: 36.3–349.4), respectively. The median reduction in serum CCL3 and CCL4 concentrations in matched pre- and post-treatment samples was 13.7 pg/ml and 20.6 pg/ml, respectively. Serum CCL3 and CCL4 concentrations decreased in 15 out of 18 patients (Figure 5A, B; Figure S7). Applying the same cut-off value used in the previous analysis to define high CCL3 (>40 pg/ml) and CCL4 (>180 pg/ml) concentrations, post-treatment CCL3 level became “low” in 7 out of 9 patients who had “high” pre-treatment CCL3, whereas post-treatment CCL4 became “low” in 4 out of 8 patients who had “high” pre-treatment CCL4. All 15 patients who achieved low CCL3 levels post-treatment maintained their CR or PR without progression of the disease.
Figure 5.
Serial quantification of serum (A) CCL3 and (B) CCL4 levels pre- and post-treatment in 19 patients with DLBCL.
Discussion
Kinase inhibitors targeting components of the BCR signalling pathway, such as SYK (Friedberg, et al 2010), BTK (Byrd, et al 2013, Wang, et al 2013) and PI3Kδ (Furman, et al 2014, Gopal, et al 2014), are a new class of highly successful targeted agents for treatment of patients with B cell malignancies. In patients with DLBCL, overall response rates to these agents have been lower than those in other diseases (CLL, MCL)(Advani, et al 2013, Byrd, et al 2014, Byrd, et al 2013, Friedberg, et al 2010, O’Brien, et al 2014, Wang, et al 2013), presumably due to greater disease heterogeneity. Specifically, differences in BCR signalling-dependency of individual tumours probably explain the heterogeneous responses in DLBCL. This is supported by data from a recent phase 2 study of ibrutinib in relapsed/refractory DLBCL, in which 29 ABC, 20 GCB and 21 unclassified/unknown DLBCL patients were enrolled (Wilson 2013). This study revealed significantly higher response rates in ABC DLBCL (41% responders) when compared to GCB DLBCL (5% responders, P = 0.007) (Wilson 2013), corroborating the preclinical finding that ABC DLBCL cells depend on BCR signalling for survival and growth (Davis, et al 2010). However, the predictive value of ABC versus GCB classification for responsiveness to these therapies appears to be not entirely accurate, and ABC versus GCB sub-classification may not always be available for patients requiring therapy. Therefore, additional biomarkers for predicting responsiveness to therapy and/or for early response assessment are urgently needed.
The data here suggest that CCL3 and CCL4 protein concentrations, which can be easily and reliably measured in patients’ plasma/serum samples by ELISA, may fulfil this requirement with high sensitivity and specificity, particularly in vitro. BCR-inducible CCL3 and CCL4 secretion was observed in ABC DLBCL cells, but not those of GCB type, as was profound inhibition by ibrutinib of BCR-inducible and baseline secretion of these chemokines (Figure 1), even at low nanomolar drug concentrations. The studies of DLBCL plasma samples demonstrated that high serum concentrations of CCL3 and CCL4 were associated with other poor prognostic features (advanced disease stage, high IPI, LDH and β2MG) and function as robust prognostic markers even after adjusted with IPI, in patients with DLBCL (Figure 4 and Table I). Furthermore, in most patients, high concentrations of serum CCL3 and CCL4 returned to low levels after therapy. Of particular interest, although anecdotal, one patient in whom CCL3 levels did not return to low levels later experienced relapse of his disease, suggesting that CCL3 and/or CCL4 might become useful as biomarkers for residual disease. However, validation of these possibilities in a larger cohort of serial samples is required.
The association of CCL3 and CCL4 with BCR signalling and the ABC DLBCL subtype is consistent with previous gene expression data and known mechanisms. CCL3 is an ABC DLBCL signature gene (Alizadeh, et al 2000, Rosenwald, et al 2002) and one of the most important independent predictors for survival in DLBCL (Lossos, et al 2004). An underlying molecular mechanism is supported by the finding that a combination of low BCL6 and high CCL3 expression is a clear indicator of poor prognosis (Lossos, et al 2004), given that BCL6 binds to cis-elements in the CCL3 promoter, where it functions as a critical repressor of CCL3 expression (Shaffer, et al 2000). However, serum CCL3 concentrations did not correlate with immunophenotypic sub-classification by Hans criteria. The Hans classifier has been widely used to distinguish GCB subtype vs. non-GCB subtype by 3 immunostains (CD10, BCL6, and MUM1) and can reproduce microarray-based classification in about 71% of GCB and 88% of non-GCB cases (Davis, et al 2010). The non-GCB subtype by the Hans classifier (Hans et al 2004) is not completely equivalent to the GEP-based ABC subtype, and includes other non-GCB, non-ABC cases of the “type 3” subtype (Rosenwald, et al 2002). Therefore, it is possible that non-GCB cases determined by the Hans criteria in our study included a significant number of non-GCB, non-ABC cases. Furthermore, immunohistochemical staining of DLBCL samples and its scoring have been reported to have significant inter- and intra-observer variability (de Jong, et al 2007). These factors may have contributed to the absence of significant correlation between high serum CCL3 level and non-GCB subtype in our study. Ideally, correlation of serum CCL3 level and DLBCL subtype should be re-evaluated in a cohort with GEP-based or Nanostring-based DLBCL subtype classification (Veldman-Jones, et al 2015).
In summary, ABC DLBCL cells secrete high CCL3 and CCL4 protein concentrations in response to BCR activation and/or at baseline, which correlates with underlying BCR pathway responsiveness and dependency. Secretion of these chemokines by DLBCL cells is exquisitely sensitive to inhibition by BCR pathway inhibition, indicating that CCL3 and CCL4 could become valuable biomarkers for risk stratification and/or response assessment in DLBCL. Further validation of the current findings with larger samples from DLBCL patients with GEP-/Nanostring-based subtype classification, and in serial samples from patients undergoing therapy with the new kinase inhibitors, are warranted.
Supplementary Material
Acknowledgments
The study was supported by a Leukemia & Lymphoma Society Scholar Award in Clinical Research (to J.A.B.), and in part by the MD Anderson Cancer Center Support Grant CA016672. The University of Texas MD Anderson Cancer Center Lymphoma Tissue Bank is supported by the National Institutes of Health Lymphoma SPORE grant P50CA136411 and Fredrick B. Hagemeister Research Fund.
Footnotes
Author contributions
K.T. collected and analysed data, M.S. performed serum CCL3/CCL4 assay and analysed data, J.H. performed DLBCL in vitro cell assays, Y.O., F.B.H, L.F., J.E.R., N.F., M.A.F., L.W.K., F.S., S. N., K.F., W.C.C. and J.M.V. provided patient samples and reviewed the manuscript, L.X. and X. H. performed statistical analyses, H.K., S.O.B. and M.J.K. helped with the study design and reviewed the manuscript, R.E.D. analysed data, helped with the study design, provided DLBCL cell lines and reviewed the manuscript, and J.A.B. designed the research, supervised the study, analysed the data and wrote the paper with K.T., M.S. and J.H.
Competing interests: J.A.B. and S.O.B. received research funding from Pharmacyclics.
References
- Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, Sukbuntherng J, Izumi R, Hamdy A, Hedrick E, Fowler NH. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ, Rosenwald A. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood. 2009;113:3050–3058. doi: 10.1182/blood-2008-07-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Sukbuntherng J, Chang BY, Clow F, Hedrick E, Buggy JJ, James DF, O’Brien S. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, Devereux S, Barr PM, Furman RR, Kipps TJ, Cymbalista F, Pocock C, Thornton P, Caligaris-Cappio F, Robak T, Delgado J, Schuster SJ, Montillo M, Schuh A, de Vos S, Gill D, Bloor A, Dearden C, Moreno C, Jones JJ, Chu AD, Fardis M, McGreivy J, Clow F, James DF, Hillmen P. Ibrutinib versus of atumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, Xu W, Shaffer AL, Wright G, Xiao W, Powell J, Jiang JK, Thomas CJ, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Connors JM, Johnson NA, Rimsza LM, Campo E, Jaffe ES, Wilson WH, Delabie J, Smeland EB, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Pierce SK, Staudt LM. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong D, Rosenwald A, Chhanabhai M, Gaulard P, Klapper W, Lee A, Sander B, Thorns C, Campo E, Molina T, Norton A, Hagenbeek A, Horning S, Lister A, Raemaekers J, Gascoyne RD, Salles G, Weller E. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications--a study from the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol. 2007;25:805–812. doi: 10.1200/JCO.2006.09.4490. [DOI] [PubMed] [Google Scholar]
- Eberlein J, Nguyen TT, Victorino F, Golden-Mason L, Rosen HR, Homann D. Comprehensive assessment of chemokine expression profiles by flow cytometry. J Clin Invest. 2010;120:907–923. doi: 10.1172/JCI40645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP, Cripe LD, Gregory SA, Sterba MP, Lowe AM, Levy R, Shipp MA. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, Ghia P, Eradat H, Ervin T, Lamanna N, Coiffier B, Pettitt AR, Ma S, Stilgenbauer S, Cramer P, Aiello M, Johnson DM, Miller LL, Li D, Jahn TM, Dansey RD, Hallek M, O’Brien SM. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A, Blum KA, Goy AH, Davies AJ, Zinzani PL, Dreyling M, Johnson D, Miller LL, Holes L, Li D, Dansey RD, Godfrey WR, Salles GA. PI3Kdelta Inhibition by Idelalisib in Patients with Relapsed Indolent Lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, Gibellini F, Njuguna N, Lee E, Stennett L, Raghavachari N, Liu P, McCoy JP, Raffeld M, Stetler-Stevenson M, Yuan C, Sherry R, Arthur DC, Maric I, White T, Marti GE, Munson P, Wilson WH, Wiestner A. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, Giese N, O’Brien S, Yu A, Miller LL, Lannutti BJ, Burger JA. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzysiek R, Lefevre EA, Zou W, Foussat A, Bernard J, Portier A, Galanaud P, Richard Y. Antigen receptor engagement selectively induces macrophage inflammatory protein-1 alpha (MIP-1 alpha) and MIP-1 beta chemokine production in human B cells. J Immunol. 1999;162:4455–4463. [PubMed] [Google Scholar]
- Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, Botstein D, Levy R. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, Grant B, Richards DA, Coleman M, Wierda WG, Jones JA, Zhao W, Heerema NA, Johnson AJ, Izumi R, Hamdy A, Chang BY, Graef T, Clow F, Buggy JJ, James DF, Byrd JC. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O’Brien S, Chiorazzi N, Burger JA. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM, Hurt EM, Zhao H, Averett L, Yang L, Wilson WH, Jaffe ES, Simon R, Klausner RD, Powell J, Duffey PL, Longo DL, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Montserrat E, Lopez-Guillermo A, Grogan TM, Miller TP, LeBlanc M, Ott G, Kvaloy S, Delabie J, Holte H, Krajci P, Stokke T, Staudt LM. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, Gaasenbeek M, Angelo M, Reich M, Pinkus GS, Ray TS, Koval MA, Last KW, Norton A, Lister TA, Mesirov J, Neuberg DS, Lander ES, Aster JC, Golub TR. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- Sivina M, Hartmann E, Kipps TJ, Rassenti L, Krupnik D, Lerner S, LaPushin R, Xiao L, Huang X, Werner L, Neuberg D, Kantarjian H, O’Brien S, Wierda WG, Keating MJ, Rosenwald A, Burger JA. CCL3 (MIP-1alpha) plasma levels and the risk for disease progression in chronic lymphocytic leukemia. Blood. 2011;117:1662–1669. doi: 10.1182/blood-2010-09-307249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. 4. International Agency for Research on Cancer Press; Lyon, France: 2008. [Google Scholar]
- Veldman-Jones MH, Lai Z, Wappett M, Harbron CG, Barrett JC, Harrington EA, Thress KS. Reproducible, Quantitative, and Flexible Molecular Subtyping of Clinical DLBCL Samples Using the NanoString nCounter System. Clin Cancer Res. 2015;21:2367–2378. doi: 10.1158/1078-0432.CCR-14-0357. [DOI] [PubMed] [Google Scholar]
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, Barrientos JC, Chmielowska E, Radford J, Stilgenbauer S, Dreyling M, Jedrzejczak WW, Johnson P, Spurgeon SE, Li L, Zhang L, Newberry K, Ou Z, Cheng N, Fang B, McGreivy J, Clow F, Buggy JJ, Chang BY, Beaupre DM, Kunkel LA, Blum KA. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WH. Treatment strategies for aggressive lymphomas: what works? Hematology Am Soc Hematol Educ Program. 2013;2013:584–590. doi: 10.1182/asheducation-2013.1.584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.