Abstract
Rationale
Obliterative bronchiolitis in former coffee workers prompted a cross-sectional study of current workers. Diacetyl and 2,3-pentanedione levels were highest in areas for flavoring and grinding/packaging unflavored coffee.
Methods
We interviewed 75 (88%) workers, measured lung function, and created exposure groups based on work history. We calculated standardized morbidity ratios (SMRs) for symptoms and spirometric abnormalities. We examined health outcomes by exposure groups.
Results
SMRs were elevated 1.6-fold for dyspnea and 2.7-fold for obstruction. The exposure group working in both coffee flavoring and grinding/packaging of unflavored coffee areas had significantly lower mean ratio of forced expiratory volume in 1 s to forced vital capacity and percent predicted mid-expiratory flow than workers without such exposure.
Conclusion
Current workers have occupational lung morbidity associated with high diacetyl and 2,3-pentanedione exposures, which were not limited to flavoring areas.
Keywords: obliterative bronchiolitis; diacetyl; 2,3-pentanedione; flavorings; coffee; asthma
INTRODUCTION
In the last 15 years, occupational obliterative bronchiolitis with indolent onset has been recognized in workers manufacturing microwave popcorn [Kanwal et al., 2006], cookie dough [Cavalcanti et al., 2012], and butter and other flavorings used in such food manufacture [NIOSH, 2011]. A common exposure across these industries has been diacetyl (2,3-butanedione). Given concerns about diacetyl's respiratory toxicity, manufacturers have sought out diacetyl substitutes, often similar alpha-diketones, such as 2,3-pentanedione [Day et al., 2011; Cummings et al., 2014]. However, animal studies indicate that 2,3-pentanedione also is toxic to the respiratory epithelium [Hubbs et al., 2012; Morgan et al., 2012].
Recently, two cases of biopsy-proven obliterative bronchiolitis occurred in former workers of a coffee processing facility [CDC, 2013]. Three additional cases in former workers who had worked in the flavoring room subsequently were diagnosed on the basis of clinical presentation and results of non-invasive testing.
This paper describes the respiratory morbidity of current workers in this coffee plant, derived from a 2012 public health investigation conducted by the National Institute for Occupational Safety and Health (NIOSH), in response to employee concerns about respiratory symptoms, lung disease, and eye irritation related to substances used in the manufacturing of coffee products, including ingredients mixed in the flavoring process. Our specific aim was to identify whether the sentinel case patients reflected a risk of occupational lung disease among current workers and to identify possible risk factors to guide intervention priorities, including alpha-diketone exposure–response relations. Given the occurrence of lung disease cases who had worked in the flavoring room, we expected high exposures in that area. However, many fermentation and pyrolysis products also generate diacetyl exposures as in the manufacture of beer, wine, dairy products, and roasted coffee [Akiyama et al., 2003; Daglia et al., 2007; HSDB, 2007]. Even the tobacco in cigarettes generates diacetyl during combustion [Polzin et al., 2007]. Thus, we recognized that flavoring chemical exposures and lung disease risk might not be limited to the flavoring room. Furthermore, the coffee industry has a known risk of occupational asthma in relation to green and roasted coffee dusts [Karr et al., 1978; Zuskin et al., 1979, 1985; Jones et al., 1982; Thomas et al., 1991] and castor bean dusts from contaminated shipping bags [Figley and Rawling, 1950; Thomas et al., 1991]. We therefore considered work-related asthma, in addition to flavoring-related lung disease, as a possible health outcome.
Workforce, Facility, and Exposures
In September 2012, the plant had approximately 85 employees, producing flavored and unflavored whole bean and ground roasted coffee and packaging some tea, largely for commercial consumers. The current facility, occupied in 2003, consisted of a one-story steel industrial-style building that contained the production operations, directly attached to a two-story office space. Trucks delivered green coffee beans in burlap bags to the facility. Workers known as greens unloaders used forklifts to transfer the bags onto pallets stacked in the greens warehouse. Material handlers moved the pallets to dumping stations for each roasting line. Workers known as greens dumpers emptied the burlap bags of green coffee beans into hoppers in the floor. The beans were automatically fed into a gas-fired coffee roaster. Workers known as roasters monitored the process and took samples of roasted coffee beans to the quality control room to evaluate the color of the roasted coffee. After the roasting process, the beans were cooled and then transferred by a bucket elevator system to hoppers on a mezzanine above the packaging lines in the grinding/packaging room. In the grinding/packaging room, unflavored whole bean coffee was packaged or ground and then packaged. Grinding and packaging machine operators frequently ascended the mezzanine stairs to inspect hopper material levels and dislodge material. Packers and helpers placed cardboard boxes at the end of the packaging lines to catch bags from the conveyer belt and taped full cardboard boxes and stacked them on pallets which were transported by material handlers to the finished products warehouse. For small batches, hand packers manually filled and labeled bags with roasted whole bean or ground coffee. Hand packers also performed rework, where coffee with faulty packaging was manually redirected into the packaging process. Coffee designated for flavoring was transported from the roasting room mezzanine level by elevators to hoppers in the flavoring room. Workers known as flavoring specialists and mixers flavored ground or whole bean coffee as described in Duling et al. [in clearance for submission]. Flavored coffee was packaged in the flavoring room. Quality control technicians performed quality checks throughout the production process.
Many unflavored coffee production areas (warehouse, roasting room, grinding/packaging room, hold room, production support corridor) were not completely isolated, as the walls did not reach to the ceiling, and large curtained openings between the spaces existed to facilitate forklift traffic. The flavoring room was isolated with walls to the ceiling and had a strip curtain door separating it from unflavored coffee production. The flavoring room was kept under negative air pressure with respect to the other production areas, to prevent contamination of unflavored coffee with flavorings. Respiratory protection had not been commonly used in the facility prior to NIOSH involvement in August 2012 and then was introduced in the flavoring room.
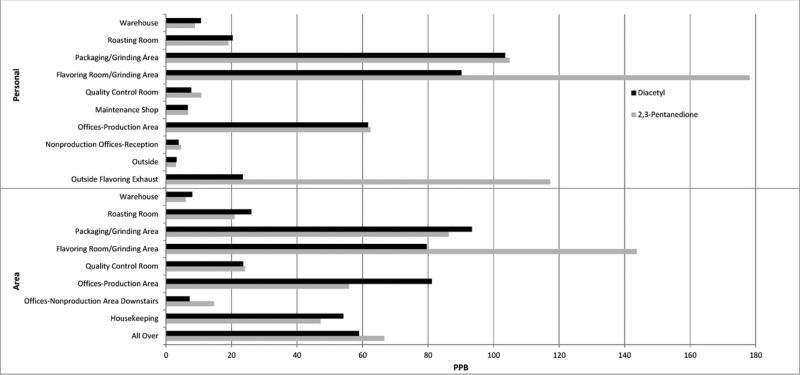
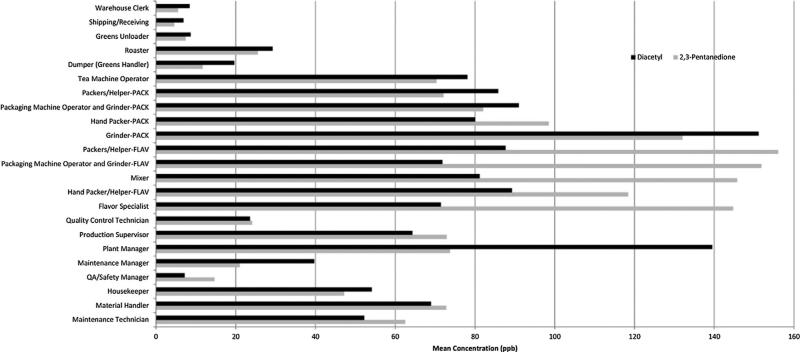
The facility had requested diacetyl-free flavorings beginning in the fall of 2011, and quantitative measurements by NIOSH of alpha-diketones in headspace of bulk samples of flavorings being used in July/August 2012 suggested that 2,3-pentanedione may have been substituted for diacetyl in several flavorings [Duling et al., in clearance for submission]. The highest mean area concentrations of diacetyl and 2,3-pentanedione occurred in the grinding/packaging room and flavoring room (Fig. 1). Similarly, the highest personal sample concentrations, averaged over job titles within a location, were highest for workers in the grinding/packaging room and flavoring room. The sum of the means of the two alpha-diketones for grinders in grinding/packaging room was 283 ppb, similar to the sum for mixers of 227 ppb and packer/helpers of 244 ppb in the flavoring room (Fig. 2). A 15-min air sample collected at the open hatch of a grinding/packaging room mezzanine hopper holding unflavored ground coffee above an active packaging line measured concentrations of 14,300 ppb diacetyl and 18,400 ppb 2,3-pentanedione; workers opening these hatches momentarily to monitor coffee levels in the hoppers throughout their shifts would be exposed to such levels. The roasting room was unique in having higher total inhalable particulate concentrations than other areas, with a mean of 0.78 milligrams per cubic meter (mg/m3), ranging up to 1.24 mg/m3, for the 17 out of 20 samples with detectable results.
FIGURE 1.
Mean 2,3-pentanedione and diacetyl air sampling results (OSHA method 1012) by location at coffee processing facility, NIOSH industrial hygiene survey, November 2012. Offices-nonproduction includes reception area; warehouse includes the green coffee warehouse and finished goods warehouse; offices-production category also includes corridor, break room, and maintenance room air samples; grinding/packaging room category also includes the tea machine and hold room air samples; all-over includes material handlers and maintenance technicians. LEV, local exhaust ventilation; ppb, parts per billion.
FIGURE 2.
Mean 2,3-pentanedione and diacetyl personal air sampling results (OSHA method 1012) by job title at coffee processing facility, NIOSH industrial hygiene survey, November 2012. Note: PACK, grinding/packaging room; FLAV, flavoring room; ppb, parts per billion.
METHODS
Participants
We invited all 85 current employees, including on-site temporary workers, to participate in the medical survey at the workplace, after written informed consent approved by the NIOSH Institutional Review Board. We consented and tested the five sentinel former workers with physician-diagnosed obliterative bronchiolitis off-site, as well as eight other former workers with chest symptoms. The interviewer-administered computerized medical and work history questionnaire had questions derived from the European Community Respiratory Health Survey (ECRHS) [Grassi et al., 2003], the American Thoracic Society (ATS) adult respiratory questionnaire (ATS-DLD-78) [Ferris, 1978], and the Third National Health and Nutrition Examination Survey (NHANES III) questionnaire [CDC, 1996], supplemented with questions on respiratory, dermatological, and systemic symptoms and current and past work areas and jobs.
On the basis of industrial hygiene monitoring [Duling et al., in clearance for submission], we classified workers who worked in both the grinding/packaging area for unflavored coffee and the flavoring area to be the highest alpha-diketone-exposed group, and compared them to the remainder of employees, as well as to workers who worked in neither of these high exposure areas. Most workers had intermittent duties apart from the department area to which they were assigned, precluding estimation of quantitative individual alpha-diketone exposure based on job title.
Spirometry
We used a volume spirometer, ATS criteria for acceptability and repeatability [Miller et al., 2005], and equations for predicted values and lower limits of normal (LLN) derived from NHANES III data to define abnormal spirometry [Hankinson et al., 1999]. We defined obstruction as a forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) and FEV1 less than the LLN; restrictive pattern as an FVC less than the LLN; and mixed obstruction and restrictive pattern as having FEV1, FVC, and FEV1/FVC all less than the LLN. We used the FEV1 percent predicted to categorize abnormalities as mild, moderate, moderately severe, severe, or very severe [Pellegrino et al., 2005]. In participants with these abnormal spirometric measurements, we tested for reversibility of at least 12% and 200 milliliters (ml) for either FEV1 or FVC with albuterol as bronchodilator. We defined abnormal mid-expiratory flow (FEF25–75) as less than the LLN.
Diffusing Capacity and Alveolar Volume
We used the single breath technique with helium as the tracer gas to measure diffusing capacity of the lung for carbon monoxide (DLCO) and alveolar volume, ATS criteria for acceptability and repeatability [MacIntyre et al., 2005], and reference values for the LLN generated from a stratified random sample of the general population of an entire state [Miller et al., 1983]. From the same test, we estimated total lung capacity using the calculated alveolar volume.
Mannitol Challenge Test for Bronchial Hyperresponsiveness
In workers with normal spirometry, we measured the FEV1 following successive doses of mannitol (0, 5, 10, 20, 40, 80, 160, 160, 160 milligrams [mg]). The maximum cumulative dose of mannitol was 635 mg. Airways hyperreactivity was defined as a 15% fall in FEV1 from baseline (0 mg dose) or a 10% fall in FEV1 between two successive doses [Porsbjerg et al., 2009].
Total and Specific Immunoglobulin E and G
We tested participants’ serum to determine if they had immunoglobulin G (IgG) and/or immunoglobulin E (IgE) antibodies that reacted to coffee (Rf221), green coffee beans (k70), or castor beans (k71) allergens, as well as total IgE by fluoroenzymeimmunoassay using the ImmunoCAP100 (Phadia AB, Uppsala, Sweden). We defined atopy as having total IgE serum levels equal to or greater than 100 kilounits per liter (kU/L). Sensitivity to a specific allergen was considered positive when IgE titers exceeded 0.35 kUA/L. Exposure to a specific antigen was considered present when antigen-specific IgG was 0.02 milligrams per liter (mg/L) or higher.
Lung Disease Case Definitions
Suspect obliterative bronchiolitis was defined as: shortness of breath on exertion (a report of shortness of breath walking with people of his/her own age on level ground; of having to stop for breath when walking at his/her own pace on level ground; or having to stop for breath after walking about 100 yards or after a few minutes); cough; chest wheeze/whistling; regular trouble with breathing; abnormal spirometry; decreased diffusing capacity; and/or decreased alveolar volume. Thus, suspect obliterative bronchiolitis could be based solely on reported chest symptoms, as lung function can be normal or have abnormalities in any pattern in biopsy-proven cases of this disease [Markopoulou et al., 2002; Ghanei et al., 2008; King et al., 2011]. However, we did not require symptoms, as pulmonary function abnormalities consistent with obliterative bronchiolitis may occur in asymptomatic flavoring-exposed workers [Kreiss et al., 2002]. We accepted physician diagnosis of obliterative bronchiolitis in the former worker sentinel cases.
Suspect asthma was defined as any of the following: awoken by an attack of shortness of breath or a feeling of tightness in the chest in the past 12 months; cough; chest wheeze/whistling in the past 12 months; and regular trouble with breathing. Probable asthma was defined as the presence of asthma symptoms and either obstruction on spirometry with a bronchodilator response or positive response to mannitol. We defined suspect occupational asthma as that arising during employment and probable occupational asthma as having a positive IgE response to coffee, green coffee beans, or castor bean allergens. We defined physician-diagnosed pre-employment asthma with symptoms that improved when away from work as work-exacerbated asthma.
Statistical Analysis
Using indirect standardization, we calculated standardized morbidity ratios (SMRs) by comparing the observed numbers of participants with symptoms and spirometric obstructive and restrictive abnormalities and abnormal FEF25-75 to the expected numbers calculated using gender, race (black, Hispanic, white), age (≤39 years and ≥40 years), and smoking (ever/never) specific prevalences from a sample of the general population reflected in the NHANES III [CDC 1996]. For restrictive pattern on spirometry, we also included body mass index (BMI: <25, >25, and <30; >30 kilograms per meter squared) specific prevalences in the calculation of expected values. We derived 95% confidence intervals for a SMR assuming that the observed data were from a Poisson distribution.
We conducted analyses using SAS version 9.3 (SAS Institute, Cary, North Carolina). We ran linear regressions using the GLM procedure on percent predicted FEV1, FVC, FEF25-75, DLCO, and alveolar volume, and on the FEV1/FVC ratio in relation to currently and ever having any work time in the grinding/packaging room and flavoring room (high exposure areas), as well as for currently and ever having any work time in the roasting room (higher particulate exposure). We used logistic regression models to evaluate prevalence of upper and lower respiratory symptoms, sinus symptoms, systemic symptoms, and eye irritation in relation to currently having worked in the grinding/packaging room and/or flavoring room, and currently having worked in the roasting room. The comparison group for having work time in the grinding/packaging room or flavoring room was composed of participants who did not have work time in either. We used two comparison groups for having work time in both the grinding/packaging room and flavoring room: participants who did not have work time in both; and participants who did not have work time in either. The comparison group for working in the roasting room was those who did not have work time in the roasting room. The models for symptoms and the FEV1/FVC ratio were adjusted for age and smoking pack-years, while the percent predicted lung function parameter models were adjusted for smoking pack-years only. We chose a probability of less than or equal to 0.05 as a criterion for statistical significance. In view of the small size of the worker population and the curtailed distribution of exposure indices, we report a probability >0.05 and <0.1 as marginally significant.
RESULTS
Former Workers
We tested 13 former workers, five of whom reported physician-diagnosed obliterative bronchiolitis. At the time of our testing, the five ranged in age from 25 to 42 years. All five reported post-hire onset of lower respiratory symptoms and had abnormal spirometry (FEV1 range: 22.4–41.9% of predicted; FVC range: 73.1–88.3% of predicted; FEV1/FVC ratio range: 25.9–43.6%; FEF25-75 range: 6.1–12.3% of predicted). Two had obstructive abnormalities, and three had mixed abnormalities with reduction in FEV1/FVC ratio, FEV1, and FVC. None had reversibility with bronchodilator. The two participants with the lowest percent predicted FEV1s (22.4% and 31.9%) had low alveolar volumes.
All five who reported physician-diagnosed obliterative bronchiolitis had worked in the flavoring room (as a mixer, flavor specialist, hand packer/helper, packaging machine operator, and/or grinder); four reported working in the grinding/packaging room (as a helper, packaging machine operator, and/or grinder). Some reported that in addition to their primary job, they sometimes had secondary duties helping out in other areas including grinding coffee or performing re-works in the flavoring room. Two of the five had high levels of total IgE; none of the five had specific IgE to coffee, green coffee beans, or castor beans. Only one of five had ever smoked, although an age less than 40 years indicated that smoking was not an etiology for the severe spirometric abnormality.
The remaining eight former workers tested reported chest symptoms and ranged in age from 22 to 51 years. One had mild restriction on spirometry without bronchodilator response, and the remainder had normal spirometry and other lung function tests. Another had probable occupational asthma with specific IgE to green coffee, coffee, and castor bean allergens, as well as an elevated total IgE. Two others also had high total IgE.
Current Workers
Of 75 current employee participants (88% response rate), the majority were male (68%), Hispanic (69%), and current (31%) or ex-smokers (23%), with an average age of 35 years (range 18–67 years). The mean duration of employment at the current facility was 2.9 years, with a median of 1.3 years, and a range of 0.04-9.3 years. Forty-one percent of participants had worked one year or less. Of the 75 employees, 12 had also worked at the previous coffee processing facility. Mean years employed at the current and the previous plant was 4.3 years and ranged to 25.0 years.
At the time of the survey, 67% of the 75 participants reported currently spending work time in the grinding/packaging room (n = 50) and a similar percentage reported work time in the flavoring room (n = 50). The number currently spending work time in either area was 63 (84%). The number spending work time in both areas was 37 (49.3%). During their tenure at the current facility, 58 (77.3%) participants had ever spent work time in the grinding/packaging room, and 54 (72%) had ever spent work time in the flavoring room, with 66 (88%) having ever spent work time in one or the other high alpha-diketone exposure areas, and 46 (61.3%) having ever concurrently spent work time in both high alpha-diketone exposure areas. The number of workers reporting currently spending work time in the roasting area was 33 (44%), and 43 (57.3%) reported ever spending work time in the roasting area during their tenure.
Medical Tests
Of 69 participants with spirometry results, five had obstructive abnormalities (four mild and one moderate); two had restrictive abnormalities; and eight had low FEF25-75 (Table I). The five with obstructive abnormalities constituted a statistically significant 2.7-fold SMR (95% confidence interval [CI]: 1.2–6.4) in comparison to the general U.S. population sample. The SMRs for abnormal restrictive spirometry (0.4; CI: 0.1–1.6) and FEF25-75 (1.1; CI: 0.6–2.2) were not elevated. Of three participants with mild obstruction, one met the definition of reversibility with bronchodilator, and the others were not administered bronchodilator. All five participants with obstruction had normal diffusing capacity and alveolar volumes. The two with a restrictive pattern on spirometry had a low DLCO and/or alveolar volume. Four other participants with low DLCO and/or alveolar volume had normal spirometry.
TABLE I.
Results of Lung Function Testing for Current Coffee Processing Workers, September 2012
| Spirometry (n = 69) | |
|---|---|
| FEV1% predicted, mean (range) | 97.6 (55.4–130.9) |
| FVC % predicted, mean (range) | 99.9 (60.8–136.9) |
| FEV1/FVC %, mean (range) | 80.5 (51.1–91.6) |
| FEF25–75% predicted, mean (range) | 94.4 (27.1–163.0) |
| Abnormally low FEF25–75, n (%) | 8 (11.6%) |
| Obstruction, n (%) | 5 (7.2%) |
| Restriction, n (%) | 2 (2.9%) |
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEF25-75, forced expiratory flow between 25% and 75% of exhaled volume (forced mid-expiratory flow).
Of the 45 participants who completed the mannitol challenge test, five had abnormal tests reflecting bronchial hyperreactivity. All five had normal spirometry, diffusing capacity, and alveolar volume. Two of those with abnormal mannitol tests reported no respiratory symptoms, excluding a diagnosis of clinical asthma. Four of the five with an abnormal mannitol challenge test participated in the blood draw, and three had elevated total IgE reflecting atopy, but none had detectable levels of specific IgE to allergens from green coffee beans, coffee, or castor bean.
Of 60 current worker participants with ImmunoCAP blood tests, all had evidence of having been exposed to green coffee beans by their IgG levels (mean 1.54 mg/L). The great majority also had evidence of exposure to coffee (n = 57, 95%, mean 0.95 mg/L) and castor beans (n = 52, 87%, mean 0.60 mg/L). Of the 60 participants, 22 (36.6%) had high levels of total IgE; however, no current worker had specific IgE to coffee or green coffee beans. One participant had specific IgE to castor beans. This participant reported no physician-diagnosed asthma; he/she reported waking up with chest tightness in the past 12 months but not in the previous 4 weeks. The participant's spirometry was normal; the participant did not complete the mannitol challenge test.
Lung Function by Work Area
When we ran models on the spirometry data for all 69 participants (Table II), we found that participants currently spending time in both high exposure areas had significantly lower mean FEV1/FVC ratios compared to those who did not currently spend time in both areas (77.9% vs. 82.9%, P = 0.0004). Participants who currently spent work time in either the grinding/packaging room or the flavoring room had marginally significantly lower FEV1/FVC ratio compared to participants who currently did not spend work time in either area (80.0% vs. 83.4%, P = 0.08). Participants currently spending time in both high exposure areas had significantly lower percent predicted FEF25-75 than those who did not currently spend time in both areas (83.1% vs. 104.1%, P = 0.002). A logistic regression of abnormal FEF25-75 indicated a marginally significant effect of currently spending time in both high exposure areas (OR 8.8, CI = 0.98–79.7, P = 0.052).
TABLE II.
Adjusted Means of Lung Function Parameters Versus Work in High Alpha-Diketone Areas Currently or Ever During Tenure, From Regression Models, Coffee Processing Facility, 2012
| Work Area | % predicted FEV1a | % predicted FVCa | FEV1/FVC %b | % predicted FEF25–75a |
|---|---|---|---|---|
| Currently in Grinding/Packaging or Flavoring | ||||
| Yes (n = 57) | 97.9 | 100.8 | 80.0* | 93.3 |
| No (n = 12) | 96.5 | 95.5 | 83.4 | 99.8 |
| Currently in Grinding/Packaging and Flavoring | ||||
| Yes (n = 32) | 94.9 | 100.2 | 77.9** | 83.1** |
| No (n = 37)c | 100.0 | 99.7 | 82.9 | 104.1 |
| Currently in Grinding/Packaging and Flavoringd | ||||
| Yes (n = 32) | 94.4 | 100.1 | 77.3** | 81.9* |
| No (n = 12)e | 95.9 | 95.5 | 82.7 | 98.3 |
| Ever in Grinding/Packaging and Flavoringc | ||||
| Yes (n = 40) | 96.1 | 100.3 | 79.0** | 87.1** |
| No (n = 29) | 99.7 | 99.3 | 82.7 | 104.4 |
0.05 < P ≤ 0.1.
P ≤ 0.05.
Models adjusted for smoking pack-years.
Models adjusted for age and smoking pack-years.
Model with different comparison group from above.
Comparison group are those not spending work time in both grinding/packaging and flavoring areas; this includes workers spending work time in one or the other of these areas.
Comparison group are those not spending work time in either grinding/packaging or flavoring areas.
No other lung function parameter was associated with major work area or with time spent in these two high alpha-diketone exposure areas. No lung function parameter was associated with spending work time (either currently or ever) in the roasting room.
Symptoms and Diagnoses
Of the 75 participants, 21 (28%) reported shortness of breath hurrying on level ground or walking up a slight hill, which represented a SMR of 1.6 in comparison to the U.S. population sample (95%CI: 1.0–2.4). Of these 21 participants, nine reported being short of breath walking with people of his or her own age on level ground, and five reported having to stop for breath after walking about 100 yards or after a few minutes on level ground. Of the 12 who provided a start date for onset of shortness of breath, half reported that their onset was after or the same month as hire date. The only other symptom that approached a statistically significant SMR was wheeze (SMR 1.5, 95%CI: 0.9–2.5).
Mucous membrane irritation of eyes, nose, or sinuses was common, and the majority reported that such symptoms were better away from work or that something at work caused or aggravated the symptoms (data not shown). At the time of the survey, 26% (20/75) of participants reported one or more of the following symptoms in the past 12 months: wheezing or whistling in their chest, waking up with chest tightness, or awakening by an attack of shortness of breath. Of the 20, one reported current asthma with physician diagnosis after hire, and one reported a past physician diagnosis of asthma with diagnosis after hire. No current workers reported a diagnosis of emphysema, bronchiolitis obliterans, interstitial lung disease, hypersensitivity pneumonitis, or chemical pneumonitis. No current employee participants reported having changed job duties because of breathing difficulties.
Symptoms by Work Area
In comparison to working in other areas, currently spending work time in the roasting room was significantly associated with the following symptoms in the last 12 months: having trouble with breathing (OR 3.8, CI = 1.0–14.0, P = 0.044); wheeze (OR 3.4, CI = 1.0–11.3, P = 0.047); sinus symptoms (OR 4.2, CI = 1.2–14.6, P = 0.025); burning eyes (OR 4.4, CI = 1.3–15.2, P = 0.020), and flu-like achiness or achy joints (OR 4.9, CI = 1.6–15.6, P = 0.007). There were no significant associations between any symptoms and currently spending work time in the flavoring and/or grinding/packaging rooms (Table III).
TABLE III.
Prevalence of Health Conditions Reported by Participants During the Previous 12 Months, Coffee Processing Facility, September 2012
| Currently in grinding/packaging or flavoring |
Currently in grinding/packaging and flavoring |
Currently in roasting |
||||
|---|---|---|---|---|---|---|
| Symptom in the last 12 months | Yes (%) n = 63 | No (%) n=12 | Yes (%) n = 37 | No (%) n = 38 | Yes (%) n=33 | No (%) n = 42 |
| Stuffy, itchy, or runny nose | 42.9 | 58.3 | 43.2 | 47.4 | 54.6 | 38.1 |
| Stinging, burning, irritated nose | 11.1 | 16.7 | 13.5 | 10.5 | 12.1 | 11.9 |
| Watery, itchy eyes | 38.1 | 41.7 | 40.5 | 36.8 | 42.2 | 35.7 |
| Stinging, burning, irritated eyes | 23.8 | 8.3 | 21.6 | 21.1 | 33.3 | 11.9** |
| Sinusitis or sinus problems | 27.0 | 16.7 | 32.4 | 18.4 | 36.4 | 16.7** |
| Usual Cough | 17.5 | 8.3 | 16.2 | 15.8 | 18.2 | 14.3 |
| Awoke with chest tightness | 11.1 | 8.3 | 8.1 | 13.2 | 15.2 | 7.1 |
| Awoke with shortness of breath | 12.7 | 0.0 | 8.1 | 13.2 | 9.1 | 11.9 |
| Breathing trouble | 15.9 | 25.0 | 16.2 | 18.4 | 27.3 | 9.5** |
| Shortness of breath on level ground or walking up a slight hill | 28.6 | 25.0 | 27.0 | 29.0 | 36.4 | 21.4 |
| Shortness of breath walking with people of own age on level or worsea | 14.3 | 16.7 | 16.2 | 13.2 | 18.2 | 11.9 |
| Chest wheezing or whistling | 17.5 | 33.3 | 16.2 | 23.7 | 30.3 | 11.9** |
| Flu-like achiness or achy joints | 25.4 | 33.3 | 35.1 | 18.4 | 42.4 | 14.3** |
| Fever or chills | 12.7 | 25.0 | 13.5 | 15.8 | 15.2 | 14.3 |
| Usual tiredness or fatigue | 27.0 | 41.7 | 29.7 | 29.0 | 33.3 | 26.2 |
| Skin rash or skin problems | 26.6 | 8.3 | 21.6 | 15.8 | 21.2 | 16.7 |
P ≤ 0.05.
Three participants answered “yes” to the main question (shortness of breath on level ground or walking up a slight hill) and the third sub-question (Stop for breath after walking about a 100 yards or a few minutes) but “no” to one or both of the first two sub-questions.
Suspect Work-Related Lung Disease
Six participants, who had spent part of their tenure in the flavoring room or the grinding/packaging room, met our criteria for suspect obliterative bronchiolitis, of whom five had shortness of breath on exertion. Three of the five reported post-hire onset of the shortness of breath, and the other two did not give a date of onset. Four of the six reported other lower respiratory symptoms. Three of the six had spirometric obstructive, restrictive, or mixed abnormalities. Four of the six had never smoked, and two had a minor smoking history of less than one pack-year. One of the six reported a diagnosis of walking pneumonia that did not respond to antibiotics, and one had a post-hire diagnosis of asthma but did not report current asthma.
In addition to the worker mentioned above with suspect obliterative bronchiolitis and a post-hire diagnosis of asthma, five other workers met our criteria for suspect work-related asthma, including two with probable or suspect occupational asthma. One had specific IgE to castor beans; the second had mild obstruction and post-hire diagnosis of asthma. Three participants had suspect work-exacerbated asthma based on asthma symptoms that improved away from work. One had pre-hire onset of symptoms; two were mannitol-sensitive and had elevated total IgE.
In summary, eleven current employees had medical test abnormalities or symptoms consistent with suspect occupational lung disease, either obliterative bronchiolitis or work-related asthma.
DISCUSSION
The current workforce of a coffee plant had evidence of occupational respiratory health effects in addition to the five sentinel cases of physician-diagnosed obliterative bronchiolitis and probable case of occupational asthma among former workers. Our findings consistent with this conclusion follow. (1) A statistically significant 2.7-fold excess of measured spirometric obstruction and a 1.6-fold excess of shortness of breath (when hurrying on level ground or walking up a slight hill) existed in the current coffee plant workers compared to the U.S. noninstitutionalized population of the same age, race/ethnic, sex, and cigarette smoking distribution. (2) Several workers had a severe degree of exertional shortness of breath (without diagnoses), which is unusual in working populations. (3) Participants currently spending work time in both grinding/packaging and flavoring rooms (the two high alpha-diketone exposure areas) had a 5.4 percentage point decrease in mean FEV1/FVC ratio in comparison to workers that had not worked in either area, adjusted for age and smoking. (4) Workers currently spending work time in both of the high alpha-diketone exposure areas had a mean FEF25–75% predicted that was 21 percentage points lower than workers without such exposure. (5) We identified six workers with production area alpha-diketone exposures whose shortness of breath and/or abnormalities on the battery of medical tests suggested undiagnosed obliterative bronchiolitis and were unlikely to be explained by cigarette smoking. In addition, we identified five current workers with suspect work-related asthma, of whom two had likely occupational asthma.
The current worker participants had a 2.7-fold excess of obstructive spirometry. The five former workers with physician-diagnosed obliterative bronchiolitis had marked obstructive or mixed obstructive and restrictive spirometric abnormalities on our testing, consistent with their physician diagnoses. We found no current workers with that degree of impairment on spirometry, although five current workers reported comparable degrees of severe shortness of breath with exertion. One purpose of spirometric measurement is to identify workers with mild or moderate impairment so that they can be prevented from developing severe impairment, precluding their employment and many activities of daily living, as in those former workers with diagnoses. Three biopsied case series of obliterative bronchiolitis have shown that cases can have any pattern of spirometric abnormality or even normal spirometry [Markopoulou et al., 2002, Ghanei et al., 2008, King et al., 2011]. For that reason, in addition to those with abnormal spirometry, we suggest exposure cessation and/or diagnostic testing based on the cardinal symptom, exertional shortness of breath, particularly in workers from workforces with sentinel cases.
We found that workers currently assigned to or spending time in both grinding and flavoring areas with high exposure to diacetyl and 2,3-pentanedione had a 5.4 percentage points lower average FEV1/FVC ratio, compared to other workers. This finding suggests that there may be a subclinical effect on spirometry in this group, which accounts for nearly half of the current production population. Our finding of a 21 percentage point difference in the mean FEF25–75% predicted in the high exposure group compared to all other workers is consistent with a small airways effect in employees who spent time in both high exposure areas, as was striking in the sentinel former worker obliterative bronchiolitis cases whose FEF25–75 measurements were all less than 12.3% predicted. Medical surveillance programs for alpha-diketone exposed workers, whether in flavoring production or food production that involves flavoring or pyrolizing exposures, as in roasting coffee, can identify workers who have excessive declines in FEV1 and FEV1/FVC ratio that, if continued, will result in their developing abnormal spirometry. Identifying such workers allows prevention of occupational lung disease through counseling regarding risks, engineering interventions to lower exposure, possible reassignment to lower exposure jobs, and motivating better adherence to respiratory protection.
We found that spending time in the roasting room was associated with sinus trouble, burning eyes, wheezing, and having trouble with breathing in the last 12 months, despite the comparison group that included those with high alpha-diketone exposures in grinding/packaging and/or flavoring areas. Mucous membrane irritation of eyes, nose, or sinuses was attributed by some participants to the burlap bags, green or roasted coffee dust, smoke, pallet dust/debris, or roasting room heat. However, irritant symptoms related to dust and smoke are more likely attributed to the workplace than the chronic exertional dyspnea associated with obliterative bronchiolitis which is not temporally related to work shift or employment cessation.
Twenty current worker participants had symptoms of wheezing or whistling in their chest, waking up with chest tightness, or awakening by an attack of shortness of breath, but only five current workers reported a physician diagnosis of asthma. Asthma symptoms have been reported among diagnosed cases of obliterative bronchiolitis [Akpinar-Elci et al., 2004; Modi et al., 2008; Cavalcanti et al., 2012]. A previous case series concentrating on occupational asthma has documented a worker in coffee processing with shortness of breath and fixed pulmonary function abnormality who may have been unrecognized case of obliterative bronchiolitis [Karr et al., 1978]. The absence of physician diagnosis of asthma or objective tests consistent with asthma are worrisome in the presence of excess exertional shortness of breath, which is the predominant symptom of obliterative bronchiolitis.
This study had several limitations. The first is that the workforce was small, and the second is that there was not an optimal internal unexposed group with which we could compare the alpha-diketone exposed workers. We had initially planned to compare flavoring workers with other production workers. However, the environmental data collected by NIOSH showed that alpha-diketone exposures were similar for workers in the flavoring room and workers in the grinding and packaging area of unflavored coffee. Furthermore, the questionnaire responses indicated that workers from a number of different departments spent at least some time in the high exposure areas, although we did not have sufficient information to estimate the proportion of work time spent in different locations. We contend that the human body cannot differentiate between chemicals that are created by roasting green coffee and grinding roasted unflavored coffee (so-called natural chemicals) and the same chemicals that are present in chemical flavors added to coffee. Both diacetyl and 2,3-pentanedione have comparable toxicity to the airway epithelium in animals exposed to the chemicals [Hubbs et al., 2012; Morgan et al., 2012], and NIOSH's draft recommendation is for similar exposure limits for both (5 ppb and 9.3 ppb, respectively) [NIOSH, 2011]. Apart from the adverse effect of working in the two high alpha-diketone exposure areas on FEV1/FVC ratio and percent predicted FEF25-75, our inability to show other exposure–response relationships for alpha-diketone exposure associated with adverse respiratory outcomes is likely explained by most employees’ (including those in production offices adjacent to the grinding/packaging room) having similar exposures at some time during their work tenure. With little range in exposures, it is possible that nearly the entire workforce had some exposures sufficient to have affected them to some degree. This is consistent with the comparison to national data which showed that the current workforce as a whole had excess abnormalities in obstruction and shortness of breath.
A third limitation was that historical alpha-diketone measurements did not exist when the five sentinel cases became ill enough to seek medical attention. One change that may have affected chemical exposures in the flavoring room was the substitution of diacetyl-containing flavorings with flavorings containing 2,3-pentanedione. However, since 2,3-pentanedione is no safer than diacetyl, this change is unlikely to have lowered risk to workers. Another change that may have lowered average levels of exposure to flavoring chemicals was the engineering controls introduced in the flavoring room, including exhaust hoods, ventilated storage cabinets, and changes in the process of flavoring coffee. Respiratory protection for volatile chemicals was not introduced until after our initial visit and increased in the flavoring room after the NIOSH medical survey. In any case, even powered air-purifying respirators with an assigned protection factor of 1,000 might be inadequate to lower the peak exposure risk of many thousands of ppb diacetyl and 2,3-pentanedione for a few seconds that were measured on the mezzanine over bins of unflavored ground coffee stored for off-gassing. Peak exposures appeared to be risk factors for obstructive spirometry in the microwave popcorn industry [Kreiss et al., 2002; Kanwal et al., 2006].
Despite its limitations, our study of the current coffee processing workforce strengthens the previous sentinel case reports by epidemiologically establishing unexpected respiratory morbidity in a new industry related to chemicals also present in butter and nut flavorings. Our finding of high diacetyl and 2,3-pentanedione exposures in the grinding of unflavored coffee beans merits consideration by clinicians seeing patients working in the coffee industry, whether flavored or unflavored, as well as further systematic study of coffee processing workforces and their exposures.
ACKNOWLEDGMENTS
We thank the worker participants and management of the coffee facility, as well as the NIOSH medical and environmental field teams who administered tests, translated study materials into Spanish, and/or assisted with data analyses, including Chris Piacitelli, Nicole Edwards, Diana Freeland, Mike Beaty, Elizabeth Garza, Diana Cale, Cammie Chaumont Menendez, PhD and Drs. Kristin Cummings, Anna-Binney McCague, and Walter A. Alarcon. Don Beezhold, PhD, contributed to discussions with the laboratory co-authors regarding allergen study design and serum results.
Footnotes
AUTHORS’ CONTRIBUTION
All authors made substantial contributions to the conception or design of the paper; or the acquisition, analysis, or interpretation of data for the paper. All authors drafted the paper or revised it critically for important intellectual content. All authors provided final approval of the version to be published. All authors agree be accountable for all aspects of the paper in ensuring that questions related to the accuracy or integrity of any part of the paper are appropriately investigated and resolved.
Disclosure Statement: The authors report no conflicts of interest. This paper has been reviewed and approved at NIOSH as a work product conducted by the authors as part of their employment without external funding.
Written informed consent approved by the NIOSH Institutional Review Board was obtained from all the participants.
REFERENCES
- Akiyama M, Murakami K, Ohtani N, Iwatsuki K, Sotoyama K, Wada A, Tokuno K, Iwabuchi H, Tanaka K. Analysis of volatile compounds released during the grinding of roasted coffee beans using solid-phase microextraction. J Agric Food Chem. 2003;51:1961–1969. doi: 10.1021/jf020724p. [DOI] [PubMed] [Google Scholar]
- Akpinar-Elci M, Travis WD, Lynch DA, Kreiss K. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur Respir J. 2004;24:298–302. doi: 10.1183/09031936.04.00013903. [DOI] [PubMed] [Google Scholar]
- Cavalcanti Zdo R, Albuquerque Filho AP, Pereira CA, Coletta EN. Bronchiolitis associated with exposure to artificial butter flavoring in workers at a cookie factory in Brazil. J Bras Pneumol. 2012;38:395–399. doi: 10.1590/s1806-37132012000300016. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Third National Health and Nutrition Examination Survey, 1988-1994, NHANES III Examination Data File [CDROM] U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, Maryland: 1996. (Public use data file documentation No. 76300) [Google Scholar]
- Centers for Disease Control and Prevention Obliterative bronchiolitis in workers in a coffee-processing facility - Texas, 2008–2012. MMWR Morb Mortal Wkly Rep. 2013;62:305–307. [PMC free article] [PubMed] [Google Scholar]
- Cummings KJ, Boylstein R, Stanton M, Piacitelli C, Edwards N, LeBouf R, Kreiss K. Respiratory symptoms and lung function abnormalities related to work at a flavoring manufacturing facility. Occup Environ Med. 2014;71:549–554. doi: 10.1136/oemed-2013-101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daglia M, Papetti A, Aceti C, Sordelli B, Spini V, Gazzani G. Isolation and determination of alpha-dicarbonyl compounds by RP-HPLC-DAD in green and roasted coffee. J Agric Food Chem. 2007;55:8877–8882. doi: 10.1021/jf071917l. [DOI] [PubMed] [Google Scholar]
- Day G, LeBouf R, Grote A, Pendergrass S, Cummings K, Kreiss K, Kullman G. Identification and measurement of diacetyl substitutes in dry bakery mix production. J Occup Environ Hyg. 2011;8:93–103. doi: 10.1080/15459624.2011.547148. [DOI] [PubMed] [Google Scholar]
- Duling MG, LeBouf RF, Cox-Ganser JM, Kreiss K, Martin S, Bailey RL. Environmental characterization of a coffee processing workplace with obliterative bronchiolitis risk. 2015 doi: 10.1080/15459624.2016.1177649. In clearance for submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris BG. Epidemiology standardization project. Am Rev Respir Dis. 1978;118(Suppl):1–53. [PubMed] [Google Scholar]
- Figley KD, Rawling FF. Castor bean: an industrial hazard as a contaminant of green coffee dust and used burlap bags. J Allergy. 1950;21:545–553. doi: 10.1016/0021-8707(50)90106-7. [DOI] [PubMed] [Google Scholar]
- Ghanei M, Tazelaar HD, Chilosi M, Harandi AA, Peyman M, Akbari HM, Shamsaei H, Bahadori M, Aslani J, Mohammadi A. An international collaborative pathologic study of surgical lung biopsies from mustard gas-exposed patients. Respir Med. 2008;102:825–830. doi: 10.1016/j.rmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Grassi M, Rezzani C, Biino G, Marinoni A. Asthma-like symptoms assessment through ECRHS screening questionnaire scoring. J Clin Epidemiol. 2003;56:238–247. doi: 10.1016/s0895-4356(02)00613-3. [DOI] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Hazardous Substances Data Bank . Diacetyl CASRN: 431- 03-8. U.S. National Library of Medicine, National Institutes of Health, U.S. Department of Health & Human Services; 2007. [September 25, 2015]. [Available at http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?/temp/~u34jo5:1] [Google Scholar]
- Hubbs AF, Cumpston AM, Goldsmith WT, Battelli LA, Kashon ML, Jackson MC, Frazer DG, Fedan JS, Goravanahally MP, Castranova V, et al. Respiratory and olfactory cytotoxicity of inhaled 2,3-pentanedione in Sprague-Dawley rats. Am J Pathol. 2012;181:829–844. doi: 10.1016/j.ajpath.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Hughes JM, Lehrer SB, Butcher BT, Glindmeyer HW, Diem JE, Hammad YY, Salvaggio J, Weill H. Lung function consequences of exposure and hypersensitivity in workers who process green coffee beans. Am Rev Respir. 1982;125:199–202. doi: 10.1164/arrd.1982.125.2.199. [DOI] [PubMed] [Google Scholar]
- Kanwal R, Kullman G, Piacitelli C, Boylstein R, Sahakian N, Martin S, Fedan K, Kreiss K. Evaluation of flavorings-related lung disease risk at six microwave popcorn plants. J Occup Environ Med. 2006;48:149–157. doi: 10.1097/01.jom.0000194152.48728.fb. [DOI] [PubMed] [Google Scholar]
- Karr RM, Davies RJ, Butcher BT, Lehrer SB, Wilson MR, Dharmarajan V, Salvaggio JE. Occupational asthma. J Allergy Clin Immunol. 1978;61:54–65. doi: 10.1016/0091-6749(78)90474-8. [DOI] [PubMed] [Google Scholar]
- King MS, Eisenberg R, Newman JH, Tolle JJ, Harrell FE, Jr, Nian H, Ninan M, Lambright ES, Sheller JR, Johnson JE, Miller RF. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365:222–230. doi: 10.1056/NEJMoa1101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347:330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardization of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- Markopoulou KD, Cool CD, Elliot TL, Lync DA, Newell JD, Jr, Hale VA, Brown KK, Schwarz MI, Tuder RM. Obliterative bronchiolitis: Varying presentations and clinicopathological correlation. Eur Respir J. 2002;19:20–30. doi: 10.1183/09031936.02.00282001. [DOI] [PubMed] [Google Scholar]
- Miller A, Thornton J, Warshaw R, Andrson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state. Am Rev Respir Dis. 1983;127:270–277. doi: 10.1164/arrd.1983.127.3.270. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Modi P, Yadava V, Sreedhar R, Khasawaneh F, Balk RA. A case of flavor-induced lung disease. South Med J. 2008;101:541–542. doi: 10.1097/SMJ.0b013e31816bead7. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Jokinen MP, Price HC, Gwinn WM, Palmer SM, Flake GP. Bronchial and bronchiolar fibrosis in rats exposed to 2,3-pentanedione vapors: Implications for bronchiolitis obliterans in humans. Toxicol Pathol. 2012;40:448–465. doi: 10.1177/0192623311431946. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational and Health . Draft criteria for a recommended standard: occupational exposure to diacetyl and 2,3-pentanedione. 2011; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; [September 25, 2015]. DHHS (NIOSH) Publication No. 20XX-XXX. August 12, 2011 External Review Draft. [Available at http://www.cdc.gov/niosh/docket/archive/pdfs/NIOSH-245/DraftDiacetylCriteriaDocument081211.pdf] [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- Polzin GM, Kosa-Maines RE, Ashley DL, Watson CH. Analysis of volatile organic compounds in mainsteam cigarette smoke. Environ Sci Technol. 2007;41:1297–1302. doi: 10.1021/es060609l. [DOI] [PubMed] [Google Scholar]
- Porsbjerg C, Backer V, Joos G, Kerstjens HA, Rodriguez-Roisin R. Current and future use of the mannitol bronchial challenge in everyday clinical practice. Clin Respir J. 2009;3:189–197. doi: 10.1111/j.1752-699X.2009.00161.x. [DOI] [PubMed] [Google Scholar]
- Thomas KE, Trigg CJ, Baxter PJ, Topping M, Lacey J, Crook B, Whitehead P, Bennett JB, Davies RJ. Factors relating to the development of respiratory symptoms in coffee process workers. Br J Ind Med. 1991;48:314–322. doi: 10.1136/oem.48.5.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuskin E, Valić F, Skurić Z. Respiratory function in coffee workers. Br J Ind Med. 1979;36:117–122. doi: 10.1136/oem.36.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuskin E, Kanceljak B, Skurić Z, Butković D. Bronchial reactivity in green coffee exposure. Br J Ind Med. 1985;42:415–420. doi: 10.1136/oem.42.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]