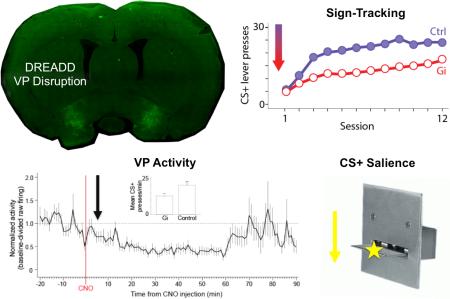
Abstract
Cues associated with rewarding events acquire value themselves as a result of the incentive value of the reward being transferred to the cue. Consequently, presentation of a reward-paired cue can trigger reward-seeking behaviors towards the cue itself (i.e., sign-tracking). The ventral pallidum (VP) has been demonstrated to be involved in a number of motivated behaviors, both conditioned and unconditioned. However, its contribution to the acquisition of incentive value is unknown. Using a discriminative autoshaping procedure with levers, we investigated the effects of disrupting VP activity in rats on the emergence of sign-tracking using chemogenetics (Designer Receptors Exclusively Activated by Designer Drugs; DREADDs). Transient disruption of VP neurons (activation of the inhibitory hM4D(Gi) DREADD through systemic injections of clozapine N-oxide (CNO) prior to each autoshaping session) impaired acquisition of sign-tracking (lever press rate) without having any effect on approach to the site of reward delivery (i.e., goal-tracking) nor on the expression of sign-tracking after it was acquired. In addition, we conducted electrophysiological recordings in freely behaving rats following VP DREADD activation. The majority of VP units that were responsive to CNO injections exhibited rapid inhibition relative to baseline, a subset of CNO-responsive units showed delayed excitation, and a smaller subset displayed a mixed response of inhibition and excitation following CNO injections. We argue that disruption of VP during autoshaping specifically disrupted the transfer of incentive value that was attributed to the lever cue, suggesting a surprisingly fundamental role for the VP in acquiring compared to expressing Pavlovian incentive values.
Graphical Abstract
Introduction
Presentation of a cue that has been paired with a rewarding event can trigger behavior that is directed towards the cue itself (i.e., sign-tracking; Brown and Jenkins, 1968) rather than the site of reward delivery (i.e., goal-tracking; Boakes, 1977). The emergence of sign-tracking has been argued to be a paradigmatic example of how reward-paired cues can acquire incentive salience, a process by which the incentive motivational value of the reward is transferred to the cue (Berridge, 2004; Robinson and Berridge, 2003). Previous research has demonstrated that the nucleus accumbens (NAc) and its dopaminergic input are critical for sign-tracking (Flagel et al, 2011; Saunders and Robinson, 2012; Chang et al, 2012b). However, incentive salience is thought to arise through larger circuit operations between NAc and other brain regions involved in reward learning.
One such region is the ventral pallidum (VP), which receives projections from NAc shell and core (Zahm, 2000; Heimer et al, 1991). Once regarded as a site for motivation expression (Mogenson et al, 1980), the VP has recently been argued to serve as a central hub for hedonic and motivational processes (Smith et al, 2009; Root et al, 2015). Across rodent and primate species (including humans), VP activation correlates with, and is necessary for, a range of motivational processes that include learned effort to obtain reward, reinstatement of reward seeking, conditioned place preference for reward, and eating behavior (Mahler et al, 2014; Perry and McNally, 2013; Pessiglione et al, 2007; Beaver et al, 2006; Gong et al, 1996, 1997; Cromwell and Berridge, 1993; Ho and Berridge, 2013; Tachibana and Hikosaka, 2012). Notably, activation of VP μ-opioid receptors or blockade of GABAA receptors enhances food seeking and consumption (Smith et al, 2005), and VP firing becomes aligned to reward-predictive cues with learning and is modulated by both motivational states (e.g., appetites) (Tindell et al, 2009) and by levels of opioid and dopamine signaling in the NAc (Smith et al, 2011). Finally, the VP has been shown to interact with NAc in generating motivated behaviors, including eating (Smith and Berridge, 2007) and Pavlovian-instrumental transfer (PIT) (Leung and Balleine, 2013).
Nevertheless, the role of VP has not been evaluated with regard to behavioral attraction to cues themselves, a hallmark of incentive salience (Berridge, 2004). Moreover, technological limitations have made it difficult to transiently perturb activity in reward regions like VP over the length of conditioning without compromising the integrity of neurons in that region. It thus remains unknown whether VP is causally involved in the acquisition of incentive salience, or whether its role is more restricted to the expression of reward-seeking behaviors. To resolve this issue, the present set of experiments investigated the effects of perturbing VP activity on sign-tracking acquisition and expression using Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), a technology that allows for repeated activation of engineered receptors by systemic injection of the otherwise inert ligand clozapine N-oxide (CNO) (Armbruster et al, 2007). Additionally, neural recordings of VP were conducted to assess CNO effects in freely behaving rats.
Materials and Methods
Three behavioral experiments were conducted to investigate the effects of disrupting VP activity with DREADDs on sign-tracking. Experiment 1 controlled for non-specific effects from infusing DREADDs into VP, and Experiments 2 and 3 controlled for non-specific effects from injecting CNO into rats. Electrophysiological recordings of VP in behaving animals were made separately to establish DREADD-evoked modulation of firing activity.
Animals
The subjects were male Long-Evans rats (n = 16 for each Experiment; Harlan Laboratories, Indianapolis, IN), which weighed 250-300 g on arrival. Rats were housed in a climate controlled colony room that was illuminated from 7:00 AM to 7:00 PM. Rats were initially pair-housed but were then individually-housed following surgery for the entirety of the experiment. Rats were given ad libitum access to food and water before and continuing two weeks after surgery. Rats were then placed on a food restriction schedule in which they were maintained at 85% of their ad libitum weights for the duration of the experiment. Experiments were carried out in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals, and protocols were approved by the Dartmouth College Animal Care and Use Committee.
Surgical procedures
Surgery was performed under aseptic conditions with isoflurane anesthesia, and all infusions were made with a 10-μl syringe equipped with a 33-gauge beveled needle (World Precision Instruments, Inc., Sarasota, FL) and a Quintessential Stereotaxic Injector (Stoelting Inc., Kiel, WI). Infusions were made into the VP at 0.12 mm anterior from bregma, 2.40 mm from the midline, and 8.20 mm ventral from the skull surface. Each infusion was 0.80 μl in volume and was made at a rate of 0.15 μl/min. Following infusion, the syringe was left in place for 3 min to allow for diffusion. In Experiment 1, rats in Group Gi-CNO (n = 8) and Group Control (n = 8) received infusions of the inhibitory hM4D(Gi) DREADD (AAV8-hSyn-Gi-hM4Di-mCitrine; UNC vector core). In Experiments 2 and 3, rats in Group Gi-CNO (n = 8) received infusions of the hM4D(Gi) DREADD, and rats in Group Control (n = 8) received infusions of a control virus that contained DNA for green fluorescent protein but not the hM4D(Gi) receptor (GFP; AAV8-hSyn-GFP; UNC vector core). Expression of the transgenes was allowed to take place over the course of 3 weeks before the beginning of behavioral training.
Apparatus
Behavioral procedures were carried out in 8 identical standard conditioning chambers (24 × 30.5 × 29 cm; Med Associates, Georgia, VT) enclosed in sound-attenuating chambers (62 × 56 × 56 cm) outfitted with an exhaust fan to provide airflow and background noise (~68 dB). The conditioning chambers consisted of aluminum front and back walls, clear acrylic sides and top, and grid floors. Each chamber was outfitted with a food cup recessed in the center of the front wall. Retractable levers (Med Associates model: ENV-112CM) were positioned to the left and right of the food cup. These levers were 4.8 cm long and positioned 6.2 cm above the grid floor. The levers protruded 1.9 cm when extended. The chambers were illuminated by a house light mounted 15 cm above the grid floor on the back wall of the chamber. The unconditioned stimulus was the presentation of two 45-mg grain-based rodent food pellets (Bioserv, Flemington, NJ). Task events were controlled by computer equipment located in an adjacent room.
Behavioral training
Rats first received a single 30-min session of magazine training during which food pellets were delivered freely on a random time 30 s (RT 30 s) schedule resulting in approximately 60 pellets being delivered. This schedule was programmed by delivering a pellet in a given second with a 1-in-30 probability.
Prior to each session of Experiment 1, rats in Group Gi-CNO (n = 8) received injections of CNO (1 mg/ml/kg in water, i.p.; National Institute of Mental Health's Chemical Synthesis and Drug Supply Program or Sigma Aldrich, St. Louis, MO), while rats in Group Control (n = 8) received injections of sterile water (1 mg/kg, i.p.; Baxter, Deerfield, IL). The CNO dosage was chosen based on prior work showing that 1 mg/kg is an effective dose for observing specific behavioral deficits in learning (Robinson et al, 2014; Ferguson et al, 2011). For Experiments 2 and 3, rats in Group Gi-CNO and Group Control received infusions of CNO. Thus, VP activity was expected to be disrupted for Group Gi-CNO and normal for Group Control in each Experiment. For each Experiment, following the injections, rats were left in transport carts for 30 min to allow for CNO to activate hM4D(Gi) receptors before they were placed in the conditioning chambers (e.g., Robinson et al, 2014; Ferguson et al, 2011; Mahler et al, 2014; Yau and McNally, 2015). Within each 60 min session, there were 25 CS+ and 25 CS− trials ordered so that no more than two of the same trial type occurred in a sequence. The intertrial interval was variable, averaging 60 sec (with a min/max of ± 15 sec). On CS+ trials, one lever was extended for 10 sec and reinforced with two food pellets upon retraction. On CS− trials, the other lever was extended for 10 sec, but the reinforcer was not delivered. The identities of the CS+ and CS− (left vs. right lever) were counterbalanced across animals and within groups.
After training, rats in Experiment 1 were re-assigned for an expression test, such that half of the Control rats received CNO (half maintained on water) and half of the Gi-CNO rats received water (half maintained on CNO). This test was conducted in extinction and included 4 CS+ and CS− presentations (order counterbalanced). Although this re-assignment separated rats into n's of 4, this CNO/water reassignment procedure maintained n's of 8 for the main comparisons, which were the focus of analyses for this aspect of the study. In Experiment 3, rats were given two expression test sessions. In contrast to Experiment 1, the expression test sessions in Experiment 3 included US delivery (i.e., animals received reward as during training). This was done to evaluate the consequence of removing VP disruption on sign-tracking expression in a reinforced context. For this experiment, all rats were given an additional training day with CNO injections (Last Acq), and then two additional training days with injections of water (Test 1, 2).
Data analysis
The rate of lever pressing to the CS+ and CS− was analyzed over the course of acquisition. In addition, percentage of time spent in the food cup before, during, and after CS presentations was analyzed. Each measure was subjected to a 3-way mixed ANOVA with between-subjects variables of Group (Gi-CNO, Control) and Cue (CS+, CS−) and a within-subjects variable of Session (12 days) using a rejection criterion of p < 0.05. Subsequent Group × Session ANOVAs were conducted for each level of Cue (CS+, CS−) to assess the source of significant three-way Group × Cue × Session interactions. In Experiment 3, differences between Gi-CNO and Control rats in sign-tracking were further analyzed using three-session block ANOVAs with a Bonferroni correction for multiple comparisons. In order to assess if our data were normally distributed at the end of training, and prior to group re-assignment, Shapiro-Wilk tests were carried out for CS+ responding on Day 12 for each group of each Experiment. Effect sizes measured by partial eta squared values () from ANOVAs of Days 1-12 of each Experiment were assessed as well.
Histological procedures
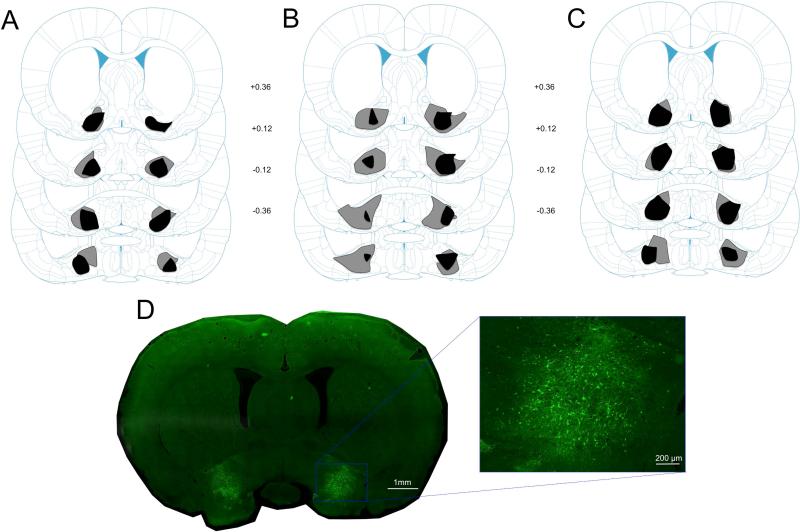
After behavioral testing, rats were anesthetized with sodium pentobarbital (100 mg/kg) and perfused intracardially with 0.9% saline, followed by 10% Formalin. Brains were removed and stored in 20% sucrose, and then sectioned at 40 μm. Sections were then mounted on microscope slides and coverslipped with a DAPI-containing hardset mounting medium (Vectashield, Vector Laboratories, Burlingame, CA) for verification of hM4D(Gi)-mCitrine or GFP expression in VP using a fluorescent microscope (Olympus, Center Valley, PA). To assess for bilateral expression of hM4D(Gi)-mCitrine in VP, areas of expression were mapped onto structural boundaries in the Paxinos & Watson (2009) atlas (Figure 1) which accord extremely well with VP immunostains (e.g., Leu Enkephalin) at this anteroposterior level (Smith & Berridge, 2005). Only rats with bilateral VP hM4D(Gi)-mCitrine expression were included in the analyses.
Figure 1.
Histological results. (A) Schematic representation of hM4D(Gi)-mCitrine expression in the VP showing the minimum (black) and maximum (gray) amount of expression in rats from Group Gi-CNO (n = 7) from Experiment 1. (B) Schematic representation of hM4D(Gi)-mCitrine expression in the VP showing the minimum (black) and maximum (gray) amount of expression in rats from Group Gi-CNO (n = 7) from Experiment 2. (C) Schematic representation of hM4D(Gi)-mCitrine expression in the VP showing the minimum (black) and maximum (gray) amount of expression in rats from Group Gi-CNO (n = 6) from Experiment 3. (D) Representative brain slice showing hM4D(Gi)-mCitrine expression in the VP. Numbers represent the number of mm from bregma. Coronal slices adapted from Paxinos & Watson (2009).
Electrophysiological recordings
Animals
Thirty-six VP units were recorded from two male Long-Evans rats (n = 2; Harlan Laboratories), which weighed 250-300 g on arrival. Rats were housed as in Experiments 1 and 2, but had ad libitum access to food and water throughout the entire experiment.
Surgical procedures
Surgery for hM4Di vector infusion into the VP was performed under the same conditions as in Experiments 1 and 2. After 3 weeks of incubation, rats were implanted with a head-stage consisting of 12 individually-drivable tetrodes (four 12.5 μm nichrome wires at 150-200 kΩ impedance) positioned above the VP using the same coordinates that were used for DREADD injections. The head-stage was anchored to the skull using cranial screws and cement. Tetrodes were gradually lowered to VP over the ensuing week.
Apparatus and recording procedures
Recordings were made in a conditioning chamber (31 × 33 × 34.5 cm; Med Associates) to which rats were pre-exposed for familiarity. The rats underwent multiple recording sessions in which they were allowed to freely explore the chamber as electrophysiological activity was acquired using a 96-channel digital Neuralynx system and Cheetah acquisition software. Electrical signals were amplified at 100-1000, sampled at 32 kHz, filtered for 600-6000 Hz, and recorded to a computer. Each session began with a rat being handheld as preamplifiers were connected to the implanted head-stage. After adjusting recording parameters (ca. 15 min), a 20 min baseline recording session commenced. Rats then received an i.p. injection of CNO (1 mg/kg) and were immediately returned to the chamber for another 1.5 hr of VP recording. Consistent recording quality and waveform stability across the brief period of CNO injection was confirmed offline by comparing waveform shape and amplitude. Experimenters observed rats during recording sessions. After each session, rats were returned to their home-cages. The potential of repeatedly sampling the same units across sessions was small due to our lowering most tetrodes in ~40 μm increments prior to each session to acquire new units. In all cases, recorded units were assessed online, and later offline, to confirm distinctive waveform and firing rate characteristics compared to previously recorded units from that tetrode.
Data analysis
Recorded waveforms were sorted into separate units using Plexon Offline Sorter. Units were analyzed for differences in mean firing rates before CNO injection compared to after CNO injection using NeuroExplorer, Microsoft Excel, and MATLAB. A unit was considered responsive to CNO if activity in five consecutive 1-min time bins was above or below a 99% confidence limit derived from firing activity during the baseline period. The latency for each responsive unit to change activity after CNO injection was calculated as the time bin in which activity rose above or fell beyond the min/max of baseline firing and beyond the 99% baseline confidence limit. Per-unit normalized activity was calculated by dividing each time bin by average baseline activity to assess average response magnitudes and durations.
Histological procedures
Following the completion of electrophysiological recordings, current (25 μA, 10 s) was passed through each tetrode to create small lesions for later localization. Brains were then removed, sectioned, and analyzed as in Experiments 1-3.
Results
Histological results
The use of transgenes tagged to fluorescent markers makes it possible to estimate the zone of likely affected areas in the brain more readily than prior inactivation methods. Figures 1A-C show schematic representations of hM4D(Gi)-mCitrine expression of rats in Group Gi-CNO from Experiments 1-3 (minimum: black; maximum: gray). Rats with acceptable expression (Experiment 1, n = 7; Experiment 2, n = 7; Experiment 3, n = 6) had bilateral hM4D(Gi)-mCitrine expression in the VP. Rats from each Experiment were removed from the data analysis due to missed placements of the hM4D(Gi) DREADD (total n = 4). These excluded rats had either unilateral VP hM4D(Gi) expression (n =3) or bilateral VP hM4D(Gi) expression that also included unilateral expression in the nucleus accumbens shell (n = 1). Importantly, rats that were removed showed no differences in sign-tracking compared to Control rats from all 3 experiments (Figure S1). Although there was some inconsistent spread to adjacent areas in some rats, the area of maximal expression was confined to VP. We did not observe spread of DREADDs along the injection tract above the VP. In addition, one Control rat was excluded from the data analysis due to acquiring goal-tracking instead of sign-tracking. This rat exhibited less than 1 lever press on average to the CS+ and showed levels of food cup behavior that were 2 standard deviations above the mean of the Control group for both the CS+ and CS−. This was the only Control rat excluded from the data analysis (Experiment 1, final n = 8; Experiment 2, n = 8; Experiment 3, n = 7). Recording locations were confirmed to be in the VP for all analyzed units.
Sign-tracking results
Experiment 1
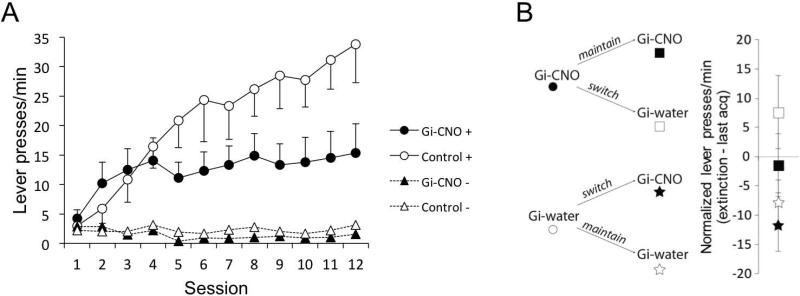
The mean number of lever presses per minute over the course of training is presented in Figure 2A. Perturbing the activity of VP neurons produced substantial deficits in levels of sign-tracking. Gi-CNO and Control rats acquired comparable levels of sign-tracking over the first 4 days of training, pressing more to the CS+ than the CS−. However, as training progressed, Control rats continued to increase responding to the CS+ while Gi-CNO rats maintained lower levels of CS+ responding. Gi-CNO and Control rats showed comparable and minimal levels of responding to the CS− over the course of training. A Shapiro-Wilk test of CS+ responding of each group on Day 12 confirmed that responding was normally distributed at the end of training (lowest p = 0.53). A 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA confirmed a significant main effect of Cue, F(1, 13) = 44.52, p < 0.001, , Session, F(11, 143) = 8.19, p < 0.001, and significant interactions between Session and Group, F(11, 143) = 3.97, p < 0.001, , and Session and Cue, F(11, 143) = 10.54, p < 0.001, . The three-way interaction between Cue, Session, and Group was also significant, F(11, 143) = 3.23, p = 0.001, .
Figure 2.
Behavioral results. (A) Gi-CNO rats showed attenuated levels of sign-tracking compared to Control rats as measured by lever presses per minute in Experiment 1. (B) Switching rats from Experiment 1 onto or off of CNO had no effect on expression of sign-tracking. This CNO/water reassignment procedure maintained n's of 8 for the main comparisons. Error bars represent ±SEM.
To assess the source of the three-way interaction, we conducted separate Group × Session ANOVAs within each level of Cue (CS+ or CS−). For the CS+, this analysis revealed a main effect of Session, F(11, 143) = 9.78, p < 0.001, , and a significant interaction between Session and Group, F(11, 143) = 3.79, p < 0.001, , indicating that groups’ CS+ responding changed differentially over sessions, as can be seen in Figure 2A. The main effect of Group was not significant as a result (p = 0.11, ). These results suggested that daily disruption of normal VP activity selectively impaired the ability of salient reward-paired cues to acquire motivational value.
If instead incentive salience had been acquired normally in CNO-treated rats but could not be maximally expressed, then switching CNO trained rats to vehicle (water) would be expected to result in an immediate increase in responding. Conversely, switching vehicle (water)-treated rats to CNO would cause an immediate decrease. To test this possibility, treatment conditions were reversed for half of the rats in each group and behavior was tested under extinction conditions (Figure 2B), and behavior assessed with a distinct ad hoc ANOVA. Importantly, this CNO/water reassignment procedure maintained n's of 8 for the main comparisons. An ANOVA with acquisition treatment (CNO vs. Water) and test treatment (CNO vs. Water) as factors failed to reveal a significant effect of the test factor (p = 0.25), or an interaction between the acquisition and test factor (p = 0.6). Thus, following acquisition, a switch onto or off of CNO did not cause a significant decrease or increase of responding, respectively. Further, neither group differed in sign-tracking from their last acquisition session. This outcome indicates that perturbation of VP activity resulted in a specific decrement in acquisition of sign-tracking that was not explainable by a deficit in performance.
Experiment 2
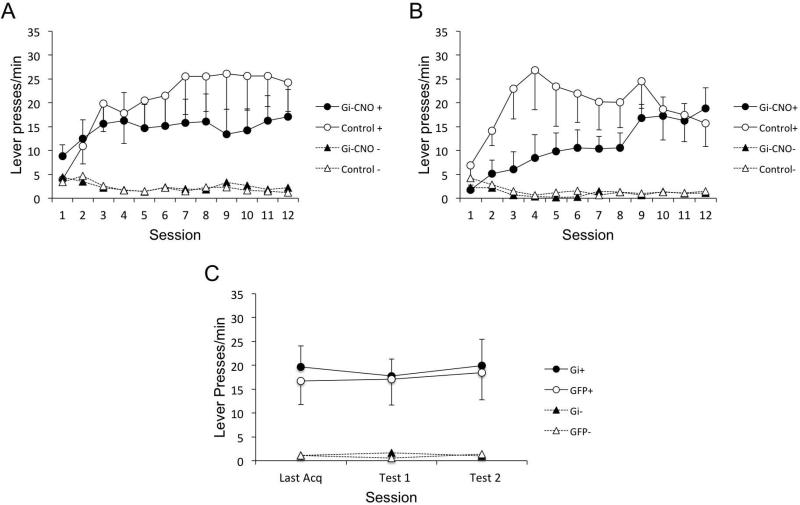
The mean number of lever presses per minute, over the course of training is presented in Figure 3A. As in Experiment 1, Gi-CNO and Control rats acquired sign-tracking at different rates over the course of training. Both groups showed comparable and minimal levels of CS− responding. A Shapiro-Wilk test of CS+ responding of each group on Day 12 confirmed that responding was normally distributed at the end (lowest p = 0.32). A 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA revealed a main effect of Cue F(1, 13) = 17.14, p = 0.001, , Session, F(11, 143) = 3.63, p < 0.001, , and an interaction between Session and Cue, F(11, 143) = 7.06, p < 0.001, . The interaction between Session and Group approached significance, F(11, 143) = 1.73, p = 0.07, , and the three-way interaction between Cue, Session, and Group was significant, F(11, 143) = 2.1, p = 0.024, . This suggested that acquisition of responding to CS+ or CS− differed between Gi-CNO and Control rats, as it had in Experiment 1.
Figure 3.
Behavioral results. (A) Gi-CNO rats showed attenuated levels of sign-tracking compared to Control rats as measured by lever presses per minute in Experiment 2. (B) Gi-CNO rats show slower acquisition of sign-tracking compared to Control rats in Experiment 3. (C) Switching rats from Experiment 3 off of CNO had no effect on expression of sign-tracking. Error bars represent ±SEM.
To again assess the source of the three-way interaction, we conducted separate Group × Session ANOVAs within each level of Cue (CS+ or CS−). For the CS+, there was a main effect of Session, F(11, 143) = 5.41, p < 0.001, and a significant interaction between Session and Group, F(11, 143) = 1.98, p = 0.03, , indicating that the change in the rate of lever pressing over sessions was not equal between groups: rats with VP disruption showed attenuated levels of CS+ responding compared to Control rats. However, the main effect of Group was not significant (p = 0.44, ), nor was it significant for any particular session. This suggested that Gi-CNO and Control rats did not differ significantly overall, but CS+ responding was differentially affected by VP manipulation across sessions (as in Experiment 1). The same analysis for the CS− data revealed a significant main effect of Session, F(11, 143) = 3.74, p < 0.001, . Neither the main effect of Group nor the interaction between Session and Group were significant. Overall, these results show that VP perturbation specifically affected CS+ responding only.
Experiment 3
The prior experiments left unresolved whether removing animals from VP perturbation after sign-tracking acquisition would affect performance in the context of reinforcement feedback. Thus we repeated Experiment 2, but included an additional expression test (i.e., when water was given instead of CNO) for two test sessions after an acquisition period. The mean number of lever presses per minute, over the course of training is presented in Figure 3B. As in Experiments 1 and 2, Gi-CNO and Control rats differed in the rate of acquiring sign-tracking over the course of training. However, deficits in sign-tracking in Gi-CNO rats were observed early rather than late in training in this cohort of animals, which we attribute to across-subject variations in learning this conditioned response (e.g., the control group this time reached their sign-tracking peak early). Control rats rapidly acquired sign-tracking over the first 6 days of training, whereas Gi-CNO rats showed lower levels of CS+ responding than Control rats. Over the course of training, Gi-CNO rats were able to acquire comparable rates of CS+ responding to Control rats. Both groups showed minimal levels of CS− responding. A Shapiro-Wilk test of CS+ responding of each group on Day 12 confirmed that responding was normally distributed (lowest p = 0.45). A 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA revealed a main effect of Cue F(1, 11) = 29.78, p < 0.001, , but no effect of Session F(11, 121) = 1.68, p = 0.086, or Group F(1, 11) = 2.89, p = 0.12, . In addition, there was no Cue × Group F(1, 11) = 2.41, p = 0.15, or Cue × Group × Session F(11, 121) = 1.51, p = 0.14, interaction. Although we did not observe the 3-way interaction as in earlier experiments, the source of this was due to the differential early versus late effects of VP disruption on sign-tracking when all sessions were incorporated. To assess this, we analyzed responding within each level of Cue (CS+ or CS−) in subsequent ANOVAs that were separated into 3-session blocks (rejection criterion after Bonferroni correction = 0.0127). For the CS+, these ANOVAs confirmed a main effect of Group for days 1-3 (F(1, 38) = 9.25, p = 0.004) and days 4-6 (F(1, 38) = 8.28, p = 0.007) but not for any other 3-day block (largest F(1, 38) = 5.82, p = 0.021). For the CS−, there was a group effect for days 4-6 only (F(1,38) = 10.73, p = 0.002) indicating lower CS− responding for Gi-CNO rats, though response levels were minimal; other day blocks being not significantly different (largest F(1,38) = 1.87, p = 0.18).
Finally, comparison was made of the three-session block containing the last acquisition day (Last Acq) and the two post-acquisition expression tests (Test 1, 2) in which water was given instead of CNO revealed no differences. A distinct ad hoc Group × Session ANOVA of this block confirmed no effect of Group F(1, 11) = 0.06, p = 0.81, Session F(2, 22) = 0.71, p = 0.50, or Group × Session interaction F(2, 22) = 0.27, p = 0.76. The lack of a Group × Session interaction within this 3-day block demonstrates that both groups performed similarly by the end of training and their performance did not change (e.g., did not show sudden sign-tracking inflation) when CNO was then removed. This expression test result suggested that rats’ deficit in sign-tracking with VP disruption had likely not been just a consequence of differently processing the US. This conclusion is further supported by the similar levels of sign-tracking observed, the identical US consumption levels between groups (i.e., all pellets were consumed), and by the fact that rats with VP disruption in this cohort spent if anything more time in the food cup than control rats in the post-CS period (food cup responding below). Overall, these results show that disrupting VP activity disrupted acquisition of sign-tracking, albeit early rather than late in training.
Food cup responding
The mean percent time spent in the food cup, during 3 different periods, is presented in Table 1. The data is averaged over all 12 acquisition sessions. Behavior directed towards the food cup was not affected by manipulation of VP activity, despite sign-tracking being markedly affected in the same sessions.
Table 1.
Temporal distribution of food cup responding
| Experiment 1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| Measure | Gi-CNO | Gi-H2O | Gi-CNO | GFP-CNO | Gi-CNO | GFP-CNO |
| Pre CS+ | 11.33 (2.77) | 3.84 (0.52) | 6.11 (1.61) | 10.35 (2.91) | 5.15 (1.73) | 2.66 (0.88) |
| CS+ | 20.84 (7.73) | 13.82 (4.40) | 6.85 (5.68) | 8.29 (2.84) | 10.84 (2.99) | 10.55 (3.35) |
| Post CS+ | 52.50 (5.47) | 42.28 (4.48) | 46.50 (6.75) | 39.14 (3.52) | 49.06 (4.86) | 36.59 (4.43) |
| Pre CS− | 11.52 (2.84) | 3.88 (0.52) | 6.12 (1.51) | 9.13 (2.22) | 4.70 (1.28) | 3.09 (0.94) |
| CS− | 12.59 (2.87) | 5.78 (0.88) | 5.10 (0.99) | 8.83 (2.40) | 7.23 (1.53) | 6.50 (1.29) |
| Post CS− | 13.89 (3.14) | 7.16 (1.23) | 6.99 (1.39) | 10.54 (2.57) | 7.05 (1.43) | 4.69 (1.19) |
Experiment 1
There were no differences in food cup behavior during CS presentations. Food cup responding decreased as training progressed for both groups. A 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA revealed a main effect of Cue, F(1, 13) = 4.97, p < 0.05, , and a Cue by Session interaction, F(11, 143) = 2.73, p < 0.01, . No other main effects or interactions were significant, largest F(1, 13) = 1.73, p = 0.21, .
For pre-CS behavior, a 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA revealed a main effect of Session, F(11, 143) = 2.33, p = 0.012, , due to pre-CS behavior decreasing over training. There was also an unexpected main effect of Cue, F(1, 13) = 8.24, p = 0.013, , with higher responding prior to CS− than CS+. However, the numerical difference between CS+ (7.59) and CS− (7.71) was minimal. The main effect of Group was also significant, F(1, 13) = 7.89, p = 0.014, , with Gi-CNO rats spending more time in the food cup (11.43 sec) than Control rats (3.86 sec). Although the Group and Cue effects were unexpected, this pattern was not evident for the rate measure (not shown), nor did we observe this effect in Experiment 2.
For post-CS behavior, rats spent more time in the food cup after the CS+ than the CS− as training progressed, presumably due to the presence of food. There were no differences between groups. A 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA revealed a main effect of Cue, F(1, 13) = 130.70, p < 0.001, and Session, F(11, 143) = 2.37, p = 0.01, , as well as an interaction between Cue and Session, F(11, 143) = 2.50, p < 0.01, .
Experiment 2
There were no differences in food cup behavior during CS presentations. A 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA did not reveal any significant main effects or interactions, largest F(11, 143) = 1.65, p = 0.09, .
For pre-CS behavior, both groups spent less time in the food cup as training progressed. However, there were no group differences. A 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA confirmed a main effect of Session, F(11, 143) = 2.58, p < 0.01, .
For post-CS behavior, both groups spent more time in the food cup after the CS+ than the CS−, as they had in Experiment 1. A 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA revealed a main effect of Cue, F(1, 13) = 66.01, p < 0.001, , as well as an interaction between Cue and Session, F(11, 143) = 4.34, p < 0.001, .
Experiment 3
There were no differences in food cup behavior during CS presentations. A 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA revealed only a main effect of Session, F(11, 121) = 12.70, p < 0.001, . There were no other main effects or interactions, largest F(1, 11) = 3.67, p = 0.08, .
For pre-CS behavior, both groups spent less time in the food cup as training progressed. However, there were no group differences. A 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA confirmed a main effect of Session, F(11, 121) = 26.68, p < 0.001, . There were no other main effects or interactions, largest F(1, 11) = 2.91, p = 0.12, .
For post-CS behavior, both groups spent more time in the food cup after the CS+ than the CS−, as in Experiments 1 and 2. However, Gi-CNO rats spent more time in the food cup than Control rats after CS+ presentations throughout the course of training. A 2 (Group: Gi-CNO, Control) × 2 (Cue: CS+, CS−) × 12 (Session) ANOVA confirmed main effects of Cue, F(1, 11) = 300.96, p < 0.001, , Session, F(11, 121) = 2.73, p = 0.004, , and Group, F(1, 11) = 6.57, p = 0.03, . Additionally, there was a Group × Cue interaction, F(1, 11) = 5.62, p = 0.04, and Cue × Session interaction, F(11, 121) = 7.13, p < 0.001, . There were no other interactions, largest F(11, 121) = 1.17, p = 0.32, .
Collectively, these analyses confirm that VP disruption did not decrease food cup behavior, and if anything increased it in Experiment 3 (post-CS period). Thus, VP disruption effects were specific to the incentive value attributed to the lever CS.
VP Neural Recordings
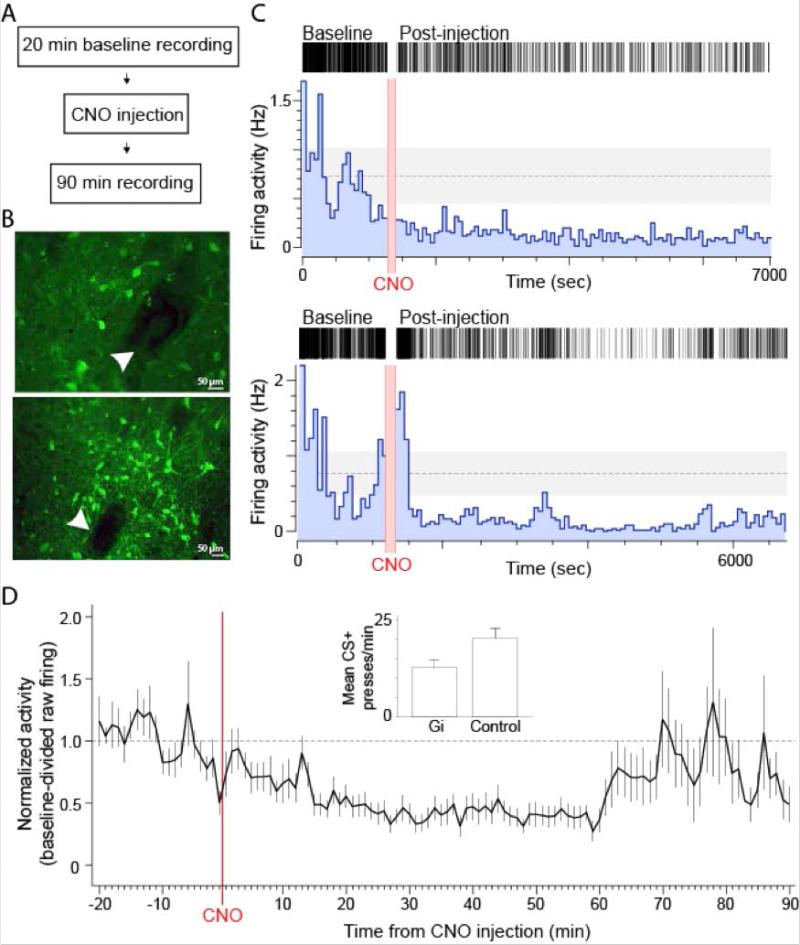
A remaining question concerned the extent to which the DREADD approach was affecting ongoing VP neuronal activity given the suppression, but not elimination, of sign-tracking behavior. In order to best estimate the effects during sign-tracking sessions, we evaluated the effect of CNO on hM4D(Gi)-expressing VP neurons in freely behaving rats using tetrode recording procedures before and after CNO injection (Figures 4-5). Thirty-six VP units were recorded from tetrodes confirmed to be adjacent to hM4D(Gi)-expressing neurons (Figure 4B).
Figure 4.
CNO-evoked inhibition of VP activity in behaving rats. (A) Recording timeline. (B) Two example photomicrographs showing VP neurons expressing hM4D(Gi)-mCitrine adjacent to tetrode lesion marks (white arrowheads). (C) Two example raster and histogram plots of VP units exhibiting a suppression of firing activity after CNO injection. (D) Baseline-divided activity (mean ±SEM) of the population of inhibited units. Insert graph: average CS+ lever presses for all Gi and Control rats combined (comparison: F(1, 42) = 5.25, p = 0.027) for reference to recording data.
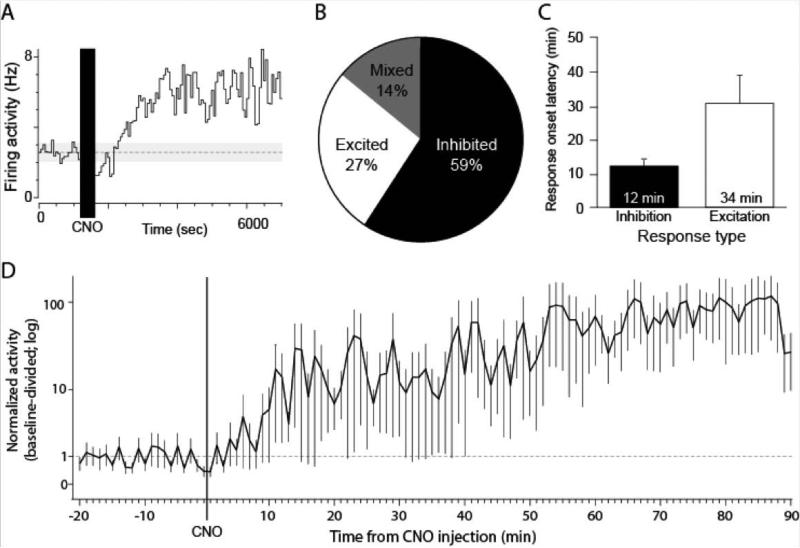
Figure 5.
Mixed effects of CNO on VP activity. (A) Example VP unit exhibiting long-latency firing excitation after CNO injection. (B) Population distribution of VP units responsive to CNO. (C) Average response latency after CNO in inhibited and excited units. (D) Baseline-divided activity (mean ±SEM) of the population of excited units.
Of the recorded VP units, 22 (61%) exhibited a significant change in firing rate during the 90 min recording session after CNO injection (Figures 4-5). Within this population of responsive units, the activity of 13 (59%) were inhibited, 6 (27%) were excited, and 3 (14%) exhibited complex inhibitory and excitatory responses after CNO (Figure 4). Within the inhibited population, the average time from CNO injection to the onset of a firing inhibition was 11.85 min (Figure 4C). Firing inhibition was consistent until ca. 65 min post-CNO. The firing of some units remained inhibited in firing through the 90 min recording period (Figure 4B), while the firing inhibition of others was not as long lasting, resulting in average activity that returned to about baseline levels by the 70 min mark (Figure 4D). Concerning inhibition magnitude, during the 90 min post-CNO period activity was suppressed to 59% of baseline (41% inhibition) while during the peak 15-60 min window it was suppressed to 42% of baseline (58% inhibition) (Figure 4D). In all, we estimated that CNO consistently quieted hM4D(Gi)-expressing VP neurons from 12-70 min after injection.
The fewer VP units that exhibited firing excitation after CNO exhibited starkly different response dynamics (Figure 5). The average latency for firing increases in the excited units was 33.63 min, about three-times the time it took for inhibition to occur (Figure 5C). This highly delayed excitatory response could reflect the engagement of larger circuits following initial VP inhibition. Firing excitation in those responsive populations was variable in magnitude but lasted for the duration of the recording session once it began (Figure 5A,D).
Baseline firing rates, assessed over a 20 min period, were variable across units, ranged from <1 Hz to >12 Hz, and did not appear to distinguish the types of responses to CNO (mean Hz: inhibited, 1.2; excited, 0.8). We also did not detect a clear demarcation of waveform shape or firing rate among the units that might reflect distinct cell types.
Discussion
Adaptive behavior that is associated with achieving goals like obtaining food has been thought to result from motivational value or incentive salience being attributed not only to the goal but also to the environmental stimuli that predict it. Research on this process has uncovered key brain areas and transmitter systems including NAc-VP-amygdala and midbrain dopamine input that are important for the expression of motivated behaviors. However, it has been difficult to study how motivational value is attributed to reward cues (sign-tracking) over the course of learning, as traditional lesion methods can induce compensatory brain changes and repeated intracranial injection of silencing agents such as muscimol cause damage. To circumvent this issue, the DREADD approach was used to target a key but understudied substrate for motivation, the VP. VP activity becomes time-locked to reward-predictive cues with learning, and the activity closely tracks motivational states, making it a prime candidate for mediating incentive salience acquisition despite traditional notions that it regulates the performance of motivated behaviors (Mogenson et al, 1980).
Our DREADD approach to this issue revealed a critical and strikingly focused role for the VP: VP disruption suppressed the acquisition of incentive salience reflected in sign-tracking behavior, but did not show generalized effects on primary motivation as measured by eating (food cup approach and food consumption) and the responding to stimuli that were not paired with reward. Despite some variation in sign-tracking rates and asymptotic levels between Experiments 1-3 likely due to inherent variance in behavior on this task, we observed deficits in the acquisition of sign-tracking in Gi-CNO rats with respect to each of their within-experiment control group. Specifically, VP disruption in Experiment 1 reduced and lowered the asymptote of responding in relation to a higher and potentially still-rising control responding, in Experiment 2 reduced and lowered the asymptote of responding in relation to a higher asymptotic control level, and in Experiment 3 reduced responding early in relation to controls but with similar terminal levels (this cohort reached asymptote more rapidly, revealing the early acquisition effect). Although our sample sizes for each group (n = 6-8) may increase the potential for type I or II errors, we are confident in the behavioral effects we observed following VP disruption given that we observed a deficit in sign-tracking in all 3 experiments with reasonable effect sizes. As with most Pavlovian appetitive behaviors, we suggest these data reflect that the task involves an ongoing mix of learning, US feedback, and expression. Nevertheless, our results within each Experiment suggest a consistent dampening of sign-tracking by VP disruption. The lack of positive results during the expression tests lend credence to the possibility that this deficit was primarily due to sign-tracking acquisition, rather than expression or US-related processing variables. This suppression of sign-tracking acquisition, but not its expression, by DREADDs supports a causal role for the VP in assigning value to reward cues and contradicts the common view that the VP serves chiefly as a behavior expression area (Mogenson et al, 1980; Smith et al, 2009; Root et al, 2015). Coupled with a novel assessment of the physiological consequences of DREADDs, the data instead suggest that dampening but not eliminating VP activity can selectively dampen the attribution of motivational value to Pavlovian reward cues.
Notably, all rats here exhibited sign-tracking, in contrast to some studies showing a split of sign-trackers and goal-trackers (e.g., Flagel et al, 2011) using a single CS paradigm. Prior studies using a CS+ and CS− design have also found nearly unanimous sign-trackers (e.g., Chang et al, 2012a,b), raising the potential that the present design biases sign-tracking behavior. It is also possible that the animals used in these sets of studies are from breeding lines of sign-trackers and thus have a genetic bias to do so (Fitzpatrick et al, 2013). Regardless, the results of this novel approach to studying motivational signals in the brain indicate that there is a surprising differential sensitivity of behavior directed towards cues versus rewards even at the level of VP.
All other behaviors remained intact during VP disruption. There were no consistent differences in food cup behavior between Gi-CNO and Control rats during cue presentations. In addition, Gi-CNO and Control rats spent comparable amounts of time in the food cup once the reward was delivered (even more so for Gi-CNO rats in Experiment 3), providing evidence that both groups were equivalently motivated to consume the reward. Finally, the expression tests from Experiments 1 and 3 show that the deficits we observed in sign-tracking produced by disruption of VP activity were not due to the inability of Gi-CNO rats to express sign-tracking (Experiment 1) or from a reduction in US value (Experiment 3), as rats showed no differences in performance when taken off of CNO either under extinction or when USs were delivered. If performance or impairment of US value were driving the sign-tracking deficits, then we would instead have predicted a sudden rise in sign-tracking when rats were removed from VP disruption. Finally, rats tested under the extinction conditions did not show any behavior change, as they might if the lack of US feedback were being processed differently between groups. Thus, the effects on sign-tracking cannot parsimoniously be attributed to an impairment in food-consuming behavior, an ability to express sign-tracking, or a reduction in US value. Notably, activation of mu-opioid receptors within the posterior VP has been shown to enhance hedonic reactions to oral infusions of sucrose solution (Smith & Berridge, 2005, 2007). Because we did not observe a deficit in US responding, we suggest that hedonics were likely not affected given that increases in US hedonics always precede increases in CS-evoked approach and consumption measures in our and others’ experience (Smith and Berridge, 2005, 2007; Berridge et al, 2009). However, it is possible that deficits in US responding/hedonics may occur with higher doses of CNO, and were thus only minimally affected using the current procedure. Although future investigation into the effects of disrupting VP activity on reward processing using higher doses of CNO is needed, our findings indicate at least a greater sensitivity to VP disruption of sign-tracking compared to US valuation. We also suggest these findings indicate that results are not explainable by a generalized deficit in motor behavior for the following similar reasons: (1) in the expression tests, control rats given CNO did not drop in performance, nor did Gi-CNO rats show a rise in performance when CNO was removed; (2) food cup behavior and reward consumption were unaffected; (3) rats in Experiment 3 could eventually reach normal terminal sign-tracking levels despite continued VP disruption.
The present findings contribute to a growing literature that suggests that VP is a critical brain region involved in appetitive motivation. VP neurons fire selectively during presentation of reward-paired cues (Tindell et al, 2004) as well as to cues that have been paired with previously aversive outcomes (salt solution) following appetite shifts (sodium depletion) (Tindell et al, 2009). Furthermore, activation of NAc opioid or dopamine receptors enhances VP activity to presentation of reward-paired cues (Smith et al, 2011). VP has also been shown to be involved in the reinstatement of reward-seeking behaviors. For example, microinjections of a mu opioid antagonist impair context-induced reinstatement of alcohol seeking (Perry and McNally, 2013). In addition, inactivation of medial VP or disconnection of the medial VP and NAc shell blocks outcome-specific PIT (Leung and Balleine, 2013), and inhibition of rostral VP neurons or disconnection of rostral VP and VTA dopamine neurons impairs cue-induced reinstatement of cocaine-seeking (Mahler et al, 2014). While these results suggest a key role for the VP in cue-evoked reward seeking, it had remained unclear whether VP participated in the attribution of motivational value to the reward cues themselves, argued to be a critical motivational process underlying reward seeking (Berridge, 2004). The present results now indicate that it does. Similar causal roles for cue-directed behavior and sign-tracking have been noted for dopaminergic innervation of the NAc, NAc neurons, and the basolateral amygdala (Flagel et al, 2011; Saunders and Robinson, 2012; Chang et al, 2012b). Given the connectivity between these areas, these findings suggest that the VP could be part of a larger circuit that contributes to different aspects of the sign-tracking response. However, generally, prior loss-of-function studies have not distinguished acquisition versus expression aspects of sign-tracking, leaving open the question of what neural circuits may regulate incentive salience attributions as we demonstrate for the VP. We note that the VP has anatomical heterogeneity along the anterior-posterior and medial-lateral axes. Here we targeted manipulations to cover them all, following the logic of the functional homology of the VP observed in terms of appetitive behavior using GABAergic manipulations (Smith and Berridge, 2005). Future experiments built on these findings will be important to dissect the roles of the inputs/outputs of VP subregions with respect to sign-tracking roles. It is possible that other brain areas, even those near the VP, could similarly contribute to sign-tracking. However, the DREADD expression being circumscribed nearly entirely to the VP, and lack of sign-tracking changes from rats with missed placements, gives us confidence in the effects being related to VP function.
The VP recording data provide insight into why sign-tracking behavior was suppressed rather than eliminated, which is notable compared to the more general disruption of motivated behavior and hedonic processing in rats with permanent lesions of VP (Cromwell and Berridge, 1993). The VP recording data and histological analyses of DREADD expression suggest that not all cells expressed the hM4D(Gi) receptor. In the recordings, about one-third of isolated VP units responded with inhibition of firing activity after CNO injection. These units exhibited a 58% reduction in activity compared to pre-CNO baseline, which is similar to inhibition levels described in a recent report conducting recordings of hM4D(Gi)-expressing VP cells in anesthetized rats (Mahler et al, 2014). A smaller group of recorded units exhibited firing excitation after a long delay. Such mixed effects are characteristic of methods that do not disrupt the activity of every neuron in a target area, including optogenetics (Smith et al, 2012; Anikeeva et al, 2011), and indicate that the disruption involves disinhibition of some cells lacking hM4D(Gi) receptors as a result of changes in local microcircuitry or wider networks. We note that our VP neural recordings took place in a context that was different from the experimental task itself. Future work investigating task-relevant VP activity with respect to sign-tracking would be useful in future work to resolve the in-task effects of DREADD manipulations on task-related VP activity.
The combination of behavioral electrophysiology with DREADD manipulations provides new opportunities to assess how neural activity is changing locally and in larger circuits. This approach carries a distinct advantage over traditional methods for transient neural intervention due to the feasibility of directly assessing the extent of transgene expression and CNO-induced changes in firing activity. For example, the suppression of sign-tracking behavior along with the incomplete inhibition of VP activity implies that VP contributions to motivated behavior can be graded. Future studies using recordings while titrating the level of inhibition or leveraging cell type-specific targeting strategies to disrupt VP activity could help resolve if the relationship of VP activity and motivated behavior is a linear or more complex one.
In conclusion, our results provide the first evidence for the involvement of the VP in sign-tracking and the first characterization of changes in neural activity following activation of hM4D(Gi) receptors in freely behaving rats. It will be important to investigate the role of the VP in sign-tracking with respect to the other regions mediating incentive learning including NAc and VTA. This new generation of tools to suppress activity allows us to dissect the circuits and neural dynamics responsible for incentive salience acquisition in sign-tracking and other instances of goal-directed behavior. Future investigations focused on the interplay between these regions in sign-tracking may provide constructive insights into understanding the neural basis of maladaptive reward-seeking behaviors such as drug relapse. Specific dampening of regions involved in cue-directed behavior could be of use in treating excessive attraction to drug cues without, potentially, disturbing other aspects of goal-directed behavior. We argue that disrupting VP activity specifically reduced the incentive value attributed to the lever cue, as measured by lever press rate. To our knowledge, these findings are the first to demonstrate a role for the VP in sign-tracking and suggest that there may be dissociable processes governing the assignment of value to conditioned cues and the motivation to approach and consume the resulting rewards.
Supplementary Material
Acknowledgements:
This work was supported by funding from the Whitehall Foundation (KSS), NIDA Grant R01DA02768 (DJB), NIH Grant F32MH105125 (TPT), and NIH Grant F32MH106178 (SEC).
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, et al. Optetrode: A multichannel readout for optogenetic control in freely moving mice. Nat Neurosci. 2011;15:163–170. doi: 10.1038/nn.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–5166. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. Auto-shaping of the pigeon's key-peck. J Exp Anal Behav. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian interactions. Lawrence Erlbaum Associates; Hillsdale, NJ: 1977. pp. 67–97. [Google Scholar]
- Chang SE, Wheeler DS, Holland PC. Effects of lesions of the amygdala central nucleus on autoshaped lever pressing. Brain Res. 2012a;1450:49–56. doi: 10.1016/j.brainres.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Wheeler DS, Holland PC. Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing. Neurobiol Learn Mem. 2012b;97:441–451. doi: 10.1016/j.nlm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: The ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V, et al. Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PLoS One. 2013;8:e75042. doi: 10.1371/journal.pone.0075042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Neill D, Justice JB., Jr Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res. 1996;707:64–74. doi: 10.1016/0006-8993(95)01222-2. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill D, Justice JB., Jr 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain Res. 1997;754:103–112. doi: 10.1016/s0006-8993(97)00059-0. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Ho CY, Berridge KC. An orexin hotspot in ventral pallidum amplifies hedonic ‘liking’ for sweetness. Neuropsychopharmacology. 2013;38:1655–1664. doi: 10.1038/npp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung BK, Balleine BW. The ventral striato-pallidal pathway mediates the effect of predictive learning on choice between goal-directed actions. J Neurosci. 2013;33:13848–13860. doi: 10.1523/JNEUROSCI.1697-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, et al. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: Functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 2009. [Google Scholar]
- Perry CJ, McNally GP. A role for the ventral pallidum in context-induced and primed reinstatement of alcohol seeking. Eur J Neurosci. 2013;38:2762–2773. doi: 10.1111/ejn.12283. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, et al. How the brain translates money into force: A neuroimaging study of subliminal motivation. Science. 2007;316:904–906. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, Napier TC. The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol. 2015;130:29–70. doi: 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: Neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: Interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci USA. 2011;108:E255–E264. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc Natl Acad Sci USA. 2012;109:18932–18937. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana Y, Hikosaka O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron. 2012;76:826–837. doi: 10.1016/j.neuron.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of Pavlovian cues and reward: Population and rate codes. J Neurosci. 2004;24:1058–1069. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Berridge KC, Aldridge JW. Dynamic computation of incentive salience: “Wanting” what was never “liked”. J Neurosci. 2009;29:12220–12228. doi: 10.1523/JNEUROSCI.2499-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JO, McNally GP. Pharmacogenetic excitation of dorsomedial prefrontal cortex restores fear prediction error. J Neurosci. 2015;35:74–83. doi: 10.1523/JNEUROSCI.3777-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.